Abstract
The aac(6′)-Iz gene of Stenotrophomonas maltophilia BM2690 encoding an aminoglycoside 6′-N-acetyltransferase was characterized. The gene was identified as a coding sequence of 462 bp corresponding to a protein with a calculated mass of 16,506 Da, a value in good agreement with that of ca. 16,000 found by in vitro coupled transcription-translation. Analysis of the deduced amino acid sequence indicated that the protein was a member of the major subfamily of aminoglycoside 6′-N-acetyltransferases. The enzyme conferred resistance to amikacin but not to gentamicin, indicating that it was an AAC(6′) of type I. The open reading frame upstream from the aac(6′)-Iz gene was homologous to the fprA gene of Myxococcus xanthus (61% identity), which encodes a putative pyridoxine (pyridoxamine) 5′-phosphate oxidase. Pulsed-field gel electrophoresis of total DNA from BM2690 and S. maltophilia ATTC 13637 digested with XbaI, DraI, and SpeI followed by hybridization with rRNA and aac(6′)-Iz-specific probes indicated that the gene was located in the chromosome. The aac(6′)-Iz gene was detected by DNA-DNA hybridization in all 80 strains of S. maltophilia tested. The MICs of gentamicin against these strains of S. maltophilia were lower than those of amikacin, netilmicin, and tobramycin, indicating that production of AAC(6′)-Iz contributes to aminoglycoside resistance in S. maltophilia.
Stenotrophomonas maltophilia (formerly Xanthomonas maltophilia) is a free-living, ubiquitous, nonfermentative gram-negative bacillus. It is an important opportunistic nosocomial pathogen in neutropenic, immunocompromised, and debilitated patients. S. maltophilia is associated with a wide variety of clinical infections, including bacteremia, endocarditis, meningitis, respiratory and urinary tract infections, and wound sepsis (13). More recently, increasing chronic colonization by this bacterial species of the lower respiratory tract in patients with cystic fibrosis has been reported (2, 12).
S. maltophilia is characteristically resistant to most broad-spectrum antimicrobial agents, including aminoglycosides, carbapenems, cephalosporins, penicillins, and quinolones. These antibiotics are seldom used alone (13), and synergism in vitro has been observed for certain combinations (5, 29, 49). The resistance of S. maltophilia is mainly attributed to permeability barriers (8, 25, 47). For instance, growth temperature-dependent variation in susceptibility to aminoglycosides, between 30 and 37°C, could be due to changes in the conformation of the outer membrane (30, 31, 46). Efflux pumps could also play a role in multiple resistance, but this mechanism has not yet been documented in this species. In addition to intrinsic resistance by impermeability, S. maltophilia can inactivate antibiotics by synthesis of detoxifying enzymes. Metallo-β-lactamases and cephalosporinases have been extensively investigated (9, 26, 36), and modification of aminoglycosides by O-nucleotidylation and N-acetylation has been reported, but the responsible genes have not been identified (20, 45). Among the various aminoglycoside-modifying enzymes, the AAC(6′) family is of particular interest, since it modifies antibiotics of therapeutic importance, such as amikacin, isepamicin, gentamicin, netilmicin, and tobramycin. The sequences of 22 aac(6′) genes encoding type I enzymes which modify amikacin but not gentamicin have been determined. Certain genes are associated with mobile elements, including plasmids, transposons, and integron cassettes (4, 40), whereas others are species specific, such as aac(6′)-Ic of Serratia marcescens (41); aac(6′)-Ii of Enterococcus faecium (6); and aac(6′)-Ig, -Ij, -Ik, and -Ir to -Iw of proteolytic genomospecies of Acinetobacter (22, 23, 34, 35). In this work, we describe the aac(6′)-Iz gene indigenous to S. maltophilia which contributes to aminoglycoside resistance in the species.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Epidemiologically unrelated clinical isolates of S. maltophilia from different sources, including strain BM2690 isolated from the sputum of a patient with cystic fibrosis, were used. The strains were isolated between 1992 and 1998 at the Hopital Saint Michel in Paris, France. Eight additional strains isolated from cystic fibrosis patients were from Hopital Robert Debré in Paris. S. maltophilia ATCC 13637 was also included in this study. Certain strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| E. coli JM83 | araΔ(lac mutant proAB) rpsL [Φ80Δ(lacZ)M15] | 48 |
| S. maltophilia | ||
| BM2690 | Ak Gm Is Km Nt Tm | Clinical isolate |
| ATCC 13637 | Ak Gm Is Km Nt Tm | Type strain |
| Plasmids | ||
| pUC18 | Tra− Mob− Ap | 48 |
| pAT680 | Tra− Mob− Ak Is Km Nt Tm | 1.1-kb PstI fragment from BM2690 into pUC18 |
| pAT681 | Tra− Mob− Ak Is Km Nt Tm | 903-bp EcoRI-PstI fragment from pAT680 into pUC18 |
Tra−, non-self-transferable; Mob−, non mobilizable; Ak, amikacin resistance; Ap, ampicillin resistance; Is, isepamicin resistance; Gm, gentamicin resistance; Km, kanamycin resistance; Nt, netilmicin resistance; Tm, tobramycin resistance.
Strain identification and growth conditions.
Identification was performed with API 20 NE strips (bioMérieux, La-Balme-les-Grottes, France). All the isolates were resistant to imipenem. The strains were grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) or on Mueller-Hinton (MH) agar (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France). MH medium supplemented with 0.005% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (IPTG) was used to detect production of β-galactosidase. MICs on MH agar containing serially twofold-diluted aminoglycosides were determined by the method of Steers et al. (42) with ca. 104 CFU per spot. The plates were incubated at 37°C for 18 h. The activity of 2′- and 6′-N-ethylnetilmicin was studied by diffusion on MH agar at 37°C with disks containing 100 μg of antibiotic.
Assay for aminoglycoside-modifying enzymes.
The activity of aminoglycoside-modifying enzymes was detected in bacterial extracts by the phosphocellulose paper-binding technique (17). The reaction was allowed to proceed for 30 min at 30°C.
Genetic techniques.
Transformation of Escherichia coli JM83 was performed as described previously (38). The antibiotics for selection were ampicillin (100 μg/ml) and tobramycin (5 μg/ml).
Preparation and analysis of DNA.
Isolation of total DNA and small- and large-scale preparation of plasmid DNA was done as described previously (38). Electrophoresis was performed in 0.8% agarose gels (Sigma Chemical Co., St. Louis, Mo.) with a Tris-borate buffer system.
For the analysis of total DNA by pulsed-field gel electrophoresis (PFGE), strains were grown in 2-ml volumes of brain heart infusion broth. After centrifugation at 32 × g for 10 min, the cell pellet was suspended in 750 μl of 10 mM Tris base, 10 mM EDTA, 10 mM EGTA, 1 M NaCl (pH 7.5). Agarose plugs were made from a 1:1 mixture of 1.8% insert agarose (FMC BioProducts, Rockland, Maine) and the cell suspension. Each plug was placed in 1 ml of lysis buffer (6 mM Tris-HCl, 1 M NaCl, 100 mM EDTA, 0.5% Brij 58 [Sigma], 0.2% deoxycholic acid [Sigma], 0.5% N-lauroyl sarcosine [Sigma], 1 mg of lysozyme [Sigma]/ml) for 1 h at 37°C. Genomic DNA in agarose plugs was then treated for 16 h at 55°C with 1 ml of TE buffer (10 mM Tris base, 1 mM EDTA) containing 100 μg of proteinase K (Sigma). After three 1-h washes with TE buffer, the plugs were stored in TE buffer at 4°C. The plugs were digested with 20 U of restriction enzyme DraI, XbaI, or SpeI (Boehringer, Mannheim, Germany) for 20 h at 37°C. They were then loaded into the wells of a 0.8% agarose gel in 45 mM Tris-borate, 1 mM EDTA (pH 8.0), and genomic DNA was separated by PFGE at 4.5 V/cm for 21 h at 14°C with a Chef DRIII apparatus (Bio-Rad, Ivry-sur-Seine, France). The pulse time was 5 to 50 s with linear ramping. Lambda ladder PFGE I (Boehringer) was incorporated as a size standard. The electrophoresis products were visualized by ethidium bromide staining for 30 min.
DNA techniques.
For dot blotting and Southern hybridization, DNA was immobilized on Nytran membranes (Schleicher & Schuell, Dassel, Germany). Prehybridization and hybridization were carried out as described previously (38). The aac(6′)-Iz-specific probe was obtained by amplification of a 367-bp fragment by PCR from plasmid DNA obtained from E. coli JM83/pAT680 with primers 5′ TGTACCCGTGATCGCCA and 5′ ACTGTCCGAAGCCAGTT deduced from the sequence shown in Fig. 1. PCR was performed in a DNA Thermal Cycler 480 (Perkin-Elmer Cetus, Norwalk, Conn.) with primer annealing at 55°C, as described previously (28). The 367-bp fragment was separated by electrophoresis in low-temperature-gelling agarose type VII (Sigma), extracted and purified with a Qiagen (Chatsworth, Calif.) kit, and radiolabeled with [α-32P]dCTP with the nick translation kit from Bethesda Research Laboratories Inc. (Gaithersburg, Md.) as described previously (38). The cDNA of the 16S and 23S rRNA of E. coli MRE 600 (Boehringer) was obtained with avian myeloblastosis virus reverse transcriptase (Boehringer) and radiolabeled with [α-32P]dCTP according to the recommendations of the manufacturer. The physical link between aac(6′)-Iz and the pdxH-like gene was studied with primers C (5′ AGCCAGATTGGCGCGTGG) and D (5′ TAGACGACCCGTTCGGTCTC) located in these genes, respectively (Fig. 1), under PCR conditions similar to those indicated above.
FIG. 1.
Sequence of S. maltophilia aac(6′)-Iz and part of pdxH-like gene and predicted amino acid sequences. The putative ribosome binding site (RBS) and potential −35 and −10 promoter sequences are underlined. The start and stop codons are depicted in boldface, with the stop codons indicated by asterisks.
DNA sequencing.
The 1.1-kb PstI fragment was cloned into bacteriophage M13 derivatives (Boehringer) and sequenced by the dideoxynucleotide chain terminator technique with synthetic oligonucleotides and the Sequenase version 2.0 DNA-sequencing kit (United States Biochemical Corp., Cleveland, Ohio) according to the recommendations of the manufacturer. DNA fragments were resolved by electrophoresis on 8% polyacrylamide gels containing 8 M urea. Nucleotide and amino acid sequences were analyzed and compared by using the GenBank, EMBL, and Swiss-Prot databases with the FASTA program (Genetics Computer Group, Madison, Wis.).
Analysis of plasmid-encoded proteins.
The proteins specified by the recombinant plasmids were synthesized in a coupled in vitro transcription-translation system (50). The proteins were labeled with l-[35S]methionine and processed for electrophoresis in a sodium dodecyl sulfate-polyacrylamide gel as described previously (38).
Enzymes and chemicals.
T4 DNA ligase was from Amersham (Little Chalfont, Buckinghamshire, England), lysozyme was from Sigma Chemical Co., and RNaseA (bovine pancreas) was from Calbiochem-Behring (La Jolla, Calif.). [α-32P]dCTP, [1-14C]acetyl coenzyme A, and [α-35S]dATP (400 Ci/mmol) were obtained from the Radiochemical Centre, Amersham. Antibiotics were provided by the following laboratories: amikacin, ampicillin, and kanamycin B, Bristol-Myers Squibb (Princeton, N.J.); tobramycin, Eli Lilly & Co. (Indianapolis, Ind.); gentamicin, gentamicins C1a, C1, and C2, isepamicin, netilmicin, 2′-N-ethylnetilmicin, and 6′-N-ethylnetilmicin, Schering-Plough Research Institute (Kenilworth, N.J.); and nalidixic acid, Sterling Winthrop (New York, N.Y.).
Nucleotide sequence accession number.
The nucleotide sequence of aac(6′)-Iz has been deposited in the GenBank data library under accession no. AF140221.
RESULTS AND DISCUSSION
Aminoglycoside resistance of S. maltophilia BM2690.
The MICs of aminoglycosides against S. maltophilia BM2690 indicated that amikacin, isepamicin, netilmicin, and tobramycin were slightly less active against this strain than gentamicin (Table 2). Similar results were observed for most S. maltophilia strains (Table 3). The variability in aminoglycoside MICs could be due to alterations in penetration of the antibiotics in the cells, as has been observed in Pseudomonas spp. Disk-agar diffusion tests indicated that the activity of 2′-N-ethylnetilmicin against S. maltophilia BM2690 was diminished compared with that of 6′-N-ethylnetilmicin. These two compounds have similar intrinsic activities against aminoglycoside-susceptible strains, and the difference observed can be taken as evidence for production of a 6′-N-acetyltransferase.
TABLE 2.
MICs of various aminoglycosides against S. maltophilia BM2690 and E. coli JM83 with and without the aac(6′)-Iz gene
| Strain | MIC (μg/ml)a
|
||||
|---|---|---|---|---|---|
| Amikacin | Gentamicin | Isepamicin | Netilmicin | Tobramycin | |
| S. maltophilia BM2690 | 256 | 16 | 64 | 64 | 64 |
| E. coli JM83 | 0.5 | 0.5 | 0.5 | 0.25 | 0.5 |
| E. coli JM83/pAT680 | 4 | 1 | 2 | 4 | 16 |
MICs determined on MH agar at 37°C.
TABLE 3.
Susceptibility of 80 S. maltophilia strains to aminoglycosides
| Aminoglycoside | MIC (μg/ml)a
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Amikacin | 4→256 | 128 | >256 |
| Gentamicin | 1→256 | 16 | 128 |
| Isepamicin | 4→256 | 64 | 256 |
| Netilmicin | 1→256 | 64 | 256 |
| Tobramycin | 1→256 | 32 | 256 |
MICs determined on MH agar at 37°C.
Aminoglycoside-modifying enzyme in S. maltophilia BM2690.
Extracts of S. maltophilia BM2690 were shown to contain aminoglycoside acetyltransferase activity. The substrate profile of the enzyme was consistent with an AAC(6′)-I, since amikacin, gentamicins C1a and C2, isepamicin, kanamycin B, netilmicin, tobramycin, and 2′-N-ethylnetilmicin were modified whereas gentamicin C1 and 6′-N-ethylnetilmicin were not (data not shown).
Cloning and sequencing of the aac(6′)-I gene.
Total DNA from BM2690 and pUC18 DNA were digested with PstI, mixed, ligated, and introduced by transformation into E. coli JM83. Transformants selected on medium containing ampicillin plus tobramycin were screened for their plasmid content by agarose gel electrophoresis of crude bacterial lysates. The smallest recombinant plasmid, pAT680, contained a 1.1-kb PstI fragment that conferred aminoglycoside resistance by synthesis of an AAC(6′)-I. Analysis of the insert in pAT680 revealed two open reading frames (ORFs) homologous to sequences in the GenBank sequence library. Nucleotides 1 to 393 were 69% identical to the 3′ terminus of the fprA gene, which encodes a putative pyridoxaminephosphate oxidase in Myxococcus xanthus (18). Nucleotides 411 to 849 were 59% identical to the aac(6′)-Ic gene of S. marcescens. The search for stop codons indicated that the aac(6′)-Iz gene was included in an ORF located between the TGA codons at coordinates 335 and 849 (Fig. 1). Two potential translation initiation codons, CTG and GTG, were located at positions 366 and 390, respectively, but neither was preceded by a typical ribosome binding site. This may account for the low-level aminoglycoside resistance observed in E. coli JM83/pAT680 (Table 1). Promoter sequences were not readily apparent by analysis of the region upstream from aac(6′)-Iz; a putative promoter may consist of −35 (TTGGCG) (position 149 to 154) and −10 (TCGAAG) motifs separated by 17 nucleotides (the underlined nucleotides indicate identity with the consensus −35 and −10 promoter elements recognized by the E. coli ς70 factor). It is also possible that aac(6′)-Iz and a pdxH-like gene form a single transcription unit, similar to aacC3 and cysC in Pseudomonas aeruginosa (44).
Analysis of the AAC(6′)-Iz protein.
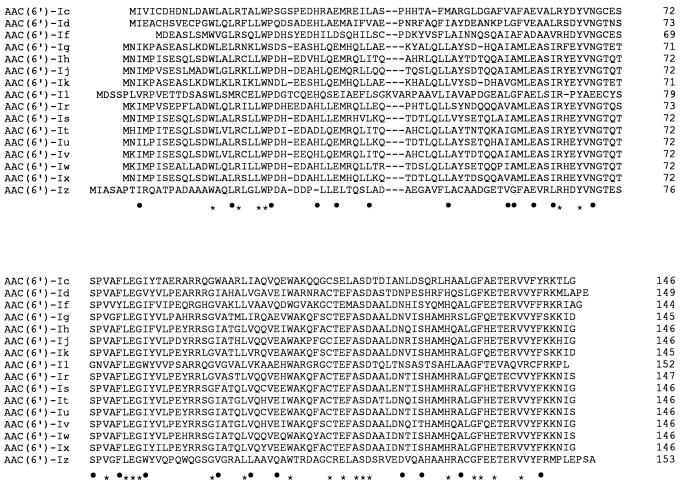
To confirm that the ORF between nucleotides 335 and 849 encodes the AAC(6′)-Iz protein, a 903-bp EcoRI-PstI fragment was subcloned into pUC18, generating pAT681. The proteins specified by pUC18 and pAT681 were characterized in an E. coli in vitro coupled transcription-translation system. A band of approximately 16,000 Da, which should correspond to the resistance protein, was encoded by pAT681 but not by the pUC18 control (data not shown). This apparent molecular mass was in good agreement with the 16,506 Da calculated for the predicted amino acid sequence. On the basis of sequence relationships, three subfamilies of AAC(6′) have been distinguished. AAC(6′)-Iz is a member of the major subfamily which contains AAC(6′)-Ic specific for S. marcescens (41) (46% identity); AAC(6′)-Id found in Klebsiella pneumoniae (39) (42% identity); AAC(6′)-If from Enterobacter cloacae (43) (45% identity); AAC(6′)-Il from Enterobacter aerogenes (4) (44% identity); AAC(6′)-Ih from Acinetobacter baumannii (23) (42% identity); and AAC(6′)-Ig, -Ij, -Ik, -Ir, -Is, -It, -Iu, -Iv, -Iw, and -Ix specific for the various species of proteolytic Acinetobacter (22, 23, 34, 35) (42 to 46% identity) (Fig. 2). The relatedness of the major subfamily AAC(6′)-I is shown in Fig. 3.
FIG. 2.
Alignment of the deduced amino acid sequences of AAC(6′)-I. In addition to S. maltophilia AAC(6′)-Iz, the sequences are from S. marcescens [AAC(6′)-Ic] (41), K. pneumoniae [AAC(6′)-Id] (39), E. cloacae [AAC(6′)-If] (43), Acinetobacter haemolyticus [AAC(6′)-Ig] (22), A. baumannii [AAC(6′)-Ih] (23), Acinetobacter sp. 13 [AAC(6′)-Ij] (23), Acinetobacter sp. 6 [AAC(6′)-Ik] (34), the pBWH301 plasmid of E. aerogenes [AAC(6′)-Il] (4), Acinetobacter sp. 14 [AAC(6′)-Ir] (35), Acinetobacter sp. 15 [AAC(6′)-Is] (35), Acinetobacter sp. 16 [AAC(6′)-It] (35), Acinetobacter sp. 17 [AAC(6′)-Iu] (35), Acinetobacter ungrouped 631 [AAC(6′)-Iv] (35), Acinetobacter ungrouped 640 [AAC(6′)-Iw] (35), and Acinetobacter ungrouped BM2722 [AAC(6′)-Ix] (35). The dashes indicate gaps introduced to optimize sequence similarity. The alignments were derived with the Clustal V program (19). Identical amino acids are indicated by asterisks. The dots indicate conservative amino acid substitutions corresponding to the following exchange groups: A, G, P, S, and T; H, K, and R; F, W, and Y; D, E, N, and Q; I, L, M, and V; and C (11).
FIG. 3.
Phylogenetic tree of the major subfamily AAC(6′)-I constructed with the Phylip computer program package (15) by the neighbor-joining method (37). The topology of the tree was compared to that obtained by the maximum-parsimony method (16). The numbers shown next to the nodes indicate percent bootstrap values of the 100 replicates (14). The line below the alignment indicates the distances that corresponds to approximately 5% divergence. In addition to S. maltophilia AAC(6′)-Iz, proteins are from S. marcescens [AAC(6′)-Ic] (41), K. pneumoniae [AAC(6′)-Id] (39), E. cloacae [AAC(6′)-If] (43), Acinetobacter haemolyticus [AAC(6′)-Ig] (22), A. baumannii [AAC(6′)-Ih] (23), Acinetobacter sp. 13 [AAC(6′)-Ij] (23), Acinetobacter sp. 6 [AAC(6′)-Ik] (34), the pBWH301 plasmid of E. aerogenes [AAC(6′)-Il] (4), Acinetobacter sp. 14 [AAC(6′)-Ir] (35), Acinetobacter sp. 15 [AAC(6′)-Is] (35), Acinetobacter sp. 16 [AAC(6′)-It] (35), Acinetobacter sp. 17 [AAC(6′)-Iu] (35), Acinetobacter ungrouped 631 [AAC(6′)-Iv] (35), Acinetobacter ungrouped 640 [AAC(6′)-Iw] (35), and Acinetobacter ungrouped BM2722 [AAC(6′)-Ix] (35).
Analysis of the protein deduced from the second ORF.
The deduced C-terminal portion of the putative protein encoded by the truncated DNA sequence upstream from the aac(6′)-Iz gene had homology with pyridoxine (pyridoxamine) 5′-phosphate oxidase proteins found in M. xanthus (18) (61% identity), Saccharomyces cerevisiae (24) (43% identity), and E. coli (21) (39% identity). It is likely that this DNA corresponds to the analogous gene in S. maltophilia. Pyridoxine (pyridoxamine) 5′-phosphate oxidase is a key enzyme in biosynthesis of the essential coenzyme pyridoxal 5′-phosphate, which participates in many aspects of amino acid metabolism. The fact that, in S. maltophilia BM2690, aac(6′)-Iz was adjacent to a gene involved in essential host metabolism strongly suggests a chromosomal location for that gene. Furthermore, the G+C content of the aac(6′)-Iz was 69% similar to that of S. maltophilia, in agreement with an indigenous origin of this gene.
Distribution and location of the aac(6′)-Iz gene.
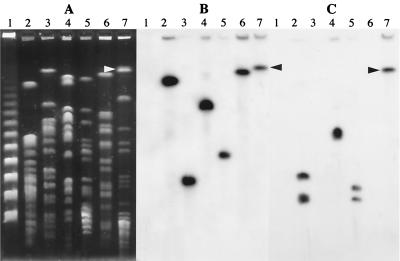
Total DNA from 80 S. maltophilia strains was spotted on a Nytran membrane and hybridized with an intragenic aac(6′)-Iz probe. The gene was found to be present in all the strains (data not shown). To determine the location of the aac(6′)-Iz gene, PFGE was performed with total DNA of S. maltophilia BM2690 and ATCC 13637 digested with XbaI, DraI, and SpeI. The fragments were transferred to Nytran membranes and hybridized successively with an rRNA and an aac(6′)-Iz probe (Fig. 4). The aac(6′)-Iz gene was located in a ca. 510-kb XbaI fragment in S. maltophilia BM2690 and in a ca. 680-kb SpeI fragment in S. maltophilia ATCC 13637 which cohybridized with the rRNA probe. These results confirmed that the aac(6′)-Iz gene was located in the chromosome of S. maltophilia. In addition, the adjacent location of aac(6′)-Iz and pdxH-like genes in the remaining strains of S. maltophilia was demonstrated by amplification of a 676-bp fragment with primers C, specific for pdxH, and D, specific for aac(6′)-Iz (data not shown).
FIG. 4.
Analysis of total DNA by PFGE (A) and by hybridization with probes made from aac(6′)-Iz (B) and rRNA (C). Total DNA was digested with XbaI, DraI, or SpeI, and the resulting fragments were separated by PFGE, transferred to a Nytran filter, and hybridized with the 32P-labeled probes. Lanes: 1, PFGE I ladder (Boehringer Mannheim); 2, 3, and 4, S. maltophilia BM2690 digested with XbaI, DraI, and SpeI, respectively; 5, 6, and 7, S. maltophilia ATCC 13637 digested with XbaI, DraI, and SpeI, respectively. The arrowheads indicate the S. maltophilia ATCC 13637 fragment containing the aac(6′)-Iz which hybridized with the rRNA probe. The lack of DraI fragments hybridizing with the rRNA probe was due to their migration out of the gel.
Conclusions.
Like S. marcescens (41), E. faecium (6), and the various species of proteolytic Acinetobacter (35), S. maltophilia harbors a “housekeeping” aac(6′)-I. This gene, probably weakly expressed, contributes to aminoglycoside resistance in this species as indicated by the slight difference in susceptibility to gentamicin and to the other aminoglycosides (Table 3). The origin of aminoglycoside resistance genes is still a matter of controversy (33). A possibility is that they derive from antibiotic-producing microorganisms in which they are required for self-protection against the toxic aminoglycosides (3, 7, 10). Alternatively, they could derive from housekeeping genes involved either in cellular metabolism or in peptidoglycan or lipopolysaccharide metabolism (27). These two hypotheses are not mutually exclusive, and there are strong arguments in favor of each proposal, depending on the bacterial species considered. It has been established that the resident AAC(2′)-Ia in Providencia stuartii can O-acetylate peptidoglycan (27, 32), and a similar function for the AAC(2′)-Id of Mycobacterium segmatis is possible (1). The functional role, if any, of AAC(6′)-Iz in S. maltophilia remains unknown.
ACKNOWLEDGMENTS
This work was supported by a grant from the Association Française de Lutte contre la Mucoviscidose and by a Bristol-Myers Squibb Unrestricted Biomedical Grant in Infectious Diseases.
We thank E. Bingen for the gift of strains.
REFERENCES
- 1.Ainsa J A, Pérez E, Pelicic V, Berthet F-X, Gicquel B, Martin C. Aminoglycoside 2′-N-acetyltransferase genes are universally present in mycobacteria: characterization of the aac(2′)-Ic gene from Mycobacterium tuberculosis and the aac(2′)-Id gene from Mycobacterium smegmatis. Mol Microbiol. 1997;24:431–441. doi: 10.1046/j.1365-2958.1997.3471717.x. [DOI] [PubMed] [Google Scholar]
- 2.Ballestero S, Virseda I, Escobar H, Suarez L, Baquero F. Stenotrophomonas in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 1995;14:728–729. doi: 10.1007/BF01690887. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste R, Davies J. Aminoglycoside inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic resistant bacteria. Proc Natl Acad Sci USA. 1973;70:2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunny K L, Hall R M, Stokes H W. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob Agents Chemother. 1995;39:686–693. doi: 10.1128/AAC.39.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow A W, Wong J, Barlett K H. Synergistic interactions of ciprofloxacin and extended spectrum beta-lactams or aminoglycosides against multiply drug-resistant Pseudomonas maltophilia. Antimicrob Agents Chemother. 1988;32:782–784. doi: 10.1128/aac.32.5.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa Y, Galimand M, Leclercq R, Duval J, Courvalin P. Characterization of the chromosomal aac(6′)-Ii gene specific for Enterococcus faecium. Antimicrob Agents Chemother. 1993;37:1896–1903. doi: 10.1128/aac.37.9.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courvalin P, Weisblum B, Davies J. Aminoglycoside-modifying enzyme of an antibiotic-producing bacterium acts as a determinant of antibiotic resistance in Escherichia coli. Proc Natl Acad Sci USA. 1977;74:999–1003. doi: 10.1073/pnas.74.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullmann W. Antibiotic susceptibility and outer membrane proteins of clinical Xanthomonas maltophilia isolates. Chemotherapy. 1991;37:246–250. doi: 10.1159/000238862. [DOI] [PubMed] [Google Scholar]
- 9.Cullmann W, Dick W. Heterogeneity of beta-lactamase production in Pseudomonas maltophilia: a nosocomial pathogen. Chemotherapy. 1990;36:117–126. doi: 10.1159/000238757. [DOI] [PubMed] [Google Scholar]
- 10.Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- 11.Dayhoff M O, Schwartz R M, Orcott B L. A model of evolutionary change in proteins. In: Dayhoff M O, editor. Atlas of protein sequence and structure. Vol. 5. 1978. pp. 345–352. , suppl. 3. National Biomedical Research Foundation, Washington, D.C. [Google Scholar]
- 12.Denton M. Stenotrophomonas maltophilia: an emerging problem in cystic fibrosis patients. Rev Med Microbiol. 1997;8:15–19. [Google Scholar]
- 13.Denton M, Kerr K G. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev. 1998;11:57–80. doi: 10.1128/cmr.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 15.Felsenstein J. Phylip version 3.5c. Seattle: University of Washington; 1993. [Google Scholar]
- 16.Fitch W. Toward defining the course of evolution: minimum change for a specified tree topology. Syst Zool. 1971;20:496–512. [Google Scholar]
- 17.Haas M J, Dowding J E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- 18.Hagen T J, Shimkets L J. Nucleotide sequence and transcriptional products of the csg locus of Myxococcus xanthus. J Bacteriol. 1990;172:15–23. doi: 10.1128/jb.172.1.15-23.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins D G, Bleasby A J, Futchs R. Clustal V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 20.King B A, Shannon K P, Phillips I. Aminoglycoside-6′-N-acetyltransferase production by an isolate of Pseudomonas maltophilia. J Antimicrob Chemother. 1978;4:467–468. doi: 10.1093/jac/4.5.467-a. [DOI] [PubMed] [Google Scholar]
- 21.Lam H-M, Wincler M E. Characterization of the complex pdxH-tyrS operon of Escherichia coli K-12 and pleiotrophic phenotypes caused by pdxH insertion mutations. J Bacteriol. 1992;174:6033–6045. doi: 10.1128/jb.174.19.6033-6045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert T, Gerbaud G, Courvalin P. Characterization of Acinetobacter haemolyticus aac(6′)-Ig gene encoding an aminoglycoside 6′-N-acetyltransferase which modifies amikacin. Antimicrob Agents Chemother. 1993;37:2093–2100. doi: 10.1128/aac.37.10.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert T, Gerbaud G, Courvalin P. Characterization of the chromosomal aac(6′)-Ij gene of Acinetobacter sp. 13 and the aac(6′)-Ih plasmid gene of Acinetobacter baumannii. Antimicrob Agents Chemother. 1994;38:1883–1889. doi: 10.1128/aac.38.9.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loubbardi A, Marcireau C, Karst F, Guilloton M. Sterol uptake induced by an impairment of pyridoxal phosphate synthesis in Saccharomyces cerevisiae: cloning and sequencing of the PDX3 gene encoding pyridoxine (pyridoxamine) phosphate oxidase. J Bacteriol. 1995;177:1817–1823. doi: 10.1128/jb.177.7.1817-1823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mett H, Rosta S, Schacher B, Frei R. Outer membrane permeability and beta-lactamase content in Pseudomonas maltophilia clinical isolates and laboratory mutants. Rev Infect Dis. 1988;10:765–769. doi: 10.1093/clinids/10.4.765. [DOI] [PubMed] [Google Scholar]
- 26.Paton R, Miles R S, Amyes S G B. Biochemical properties of inductible β-lactamases produced from Xanthomonas maltophilia. Antimicrob Agents Chemother. 1994;38:2143–2149. doi: 10.1128/aac.38.9.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payie K G, Clarke A J. Characterization of gentamicin 2′-N-acetyltransferase from Providencia stuartii: its use of peptidoglycan metabolites for acetylation of both aminoglycosides and peptidoglycan. J Bacteriol. 1997;179:4106–4114. doi: 10.1128/jb.179.13.4106-4114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ploy, M.-C., H. Giamarellou, P. Bourlioux, P. Courvalin, and T. Lambert. 1994. Detection of aac(6′)-I genes in amikacin-resistant Acinetobacter spp. by PCR. 38:2925–2928. [DOI] [PMC free article] [PubMed]
- 29.Poulos C D, Matsumura S O, Willey B M, Low D E, McGeer A. In vitro activities of antimicrobial combinations against Stenotrophomonas (Xanthomonas) maltophilia. Antimicrob Agents Chemother. 1995;39:2220–2223. doi: 10.1128/aac.39.10.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahmati-Bahram A, Magee J T, Jackson S K. Temperature-dependent aminoglycoside resistance in Stenotrophomonas (Xanthomonas) maltophilia: alterations in protein and lipopolysaccharide with growth temperature. J Antimicrob Chemother. 1996;37:665–676. doi: 10.1093/jac/37.4.665. [DOI] [PubMed] [Google Scholar]
- 31.Rahmati-Bahram A, Magee J T, Jackson S K. Effect of temperature on aminoglycoside binding sites in Stenotrophomonas maltophilia. J Antimicrob Chemother. 1997;39:19–24. doi: 10.1093/jac/39.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Rather P N, Orosz E, Shaw K J, Hare R S, Miller G H. Characterization and transcriptional regulation of the 2′-N-acetyltransferase gene from Providencia stuartii. J Bacteriol. 1993;175:6492–6498. doi: 10.1128/jb.175.20.6492-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rather P N. Origins of the aminoglycoside modifying enzymes. Drug Resistance Updates. 1998;I:285–291. doi: 10.1016/s1368-7646(98)80044-7. [DOI] [PubMed] [Google Scholar]
- 34.Rudant E, Bourlioux P, Courvalin P, Lambert T. Characterization of the aac(6′)-Ik gene of Acinetobacter sp. 6. FEMS Microbiol Lett. 1994;124:49–54. doi: 10.1016/0378-1097(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 35.Rudant E, Bouvet P, Courvalin P, Lambert T. Phylogenetic analysis of proteolytic Acinetobacter strains based on the sequence of genes encoding aminoglycoside 6′-N-acetyltransferases. Syst Appl Microbiol. 1999;22:59–67. doi: 10.1016/S0723-2020(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 36.Saino Y, Inoue M, Mitsuhashi S. Purification and properties of inducible penicillin β-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1984;22:564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 39.Schmidt F R J, Nüken E J, Henschke R B. Nucleotide sequence analysis of 2"-aminoglycoside nucleotidyl-transferase ANT(2") from Tn4000: its relationship with AAD(3") and impact on Tn21 evolution. Mol Microbiol. 1988;2:709–717. doi: 10.1111/j.1365-2958.1988.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 40.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw K J, Rather P N, Sabatelli F J, Mann P, Munayyer H, Mierzwa R, Petrikkos G L, Hare R S, Miller G H, Bennett P, Downey P. Characterization of the chromosomal aac(6′)-Ic gene from Serratia marcescens. Antimicrob Agents Chemother. 1992;36:1447–1455. doi: 10.1128/aac.36.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steers E, Foltz E L, Graves B S, Riden J. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot Chemother (Basel) 1959;9:307–311. [PubMed] [Google Scholar]
- 43.Teran F J, Suarez J E, Mendoza M C. Cloning, sequencing, and use as a molecular probe of a gene encoding an aminoglycoside 6′-N-acetyltransferase of broad substrate profile. Antimicrob Agents Chemother. 1991;35:714–719. doi: 10.1128/aac.35.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Boxtel R A J, van de Klundert J A M. Expression of the Pseudomonas aeruginosa gentamicin resistance gene aacC3 in Escherichia coli. Antimicrob Agents Chemother. 1998;42:3173–3178. doi: 10.1128/aac.42.12.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanhoof R, Sonk P, Hannecart-Pokorni E. The role of lipopolysaccharide anionic binding sites in aminoglycoside uptake in Stenotrophomonas (Xanthomonas) maltophilia. J Antimicrob Chemother. 1995;35:167–171. doi: 10.1093/jac/35.1.167. [DOI] [PubMed] [Google Scholar]
- 46.Wilcox M H, Winstanley T G, Spencer R C. Outer membrane protein profiles of Xanthomonas maltophilia isolates displaying temperature-dependent susceptibility to gentamicin. J Antimicrob Chemother. 1994;33:663–666. doi: 10.1093/jac/33.3.663. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki E, Ishii J, Sato K, Nakae T. The barrier function of the outer membrane of Pseudomonas maltophilia in the diffusion of saccharides and beta-lactam antibiotics. FEMS Microbiol Lett. 1989;60:85–88. doi: 10.1016/0378-1097(89)90082-7. [DOI] [PubMed] [Google Scholar]
- 48.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 49.Yu V L, Felegie T P, Yee R B, Pasculle A W, Taylor F H. Synergistic interaction in vitro with use of three antibiotics simultaneously against Pseudomonas maltophilia. J Infect Dis. 1980;142:602–607. doi: 10.1093/infdis/142.4.602. [DOI] [PubMed] [Google Scholar]
- 50.Zubay G. The isolation and properties of CAP, the catabolite gene activator. Methods Enzymol. 1980;65:856–877. doi: 10.1016/s0076-6879(80)65079-4. [DOI] [PubMed] [Google Scholar]