Abstract
Background
Doublet combination therapies targeting immune checkpoints have shown promising efficacy in patients with advanced solid tumors, but it is unknown if rational triplet combinations will be well tolerated and associated with improved antitumor activity. The objective of this trial was to determine the recommended phase 2 doses (RP2Ds) and to assess the safety and efficacy of the programmed cell death protein 1 (PD-1) inhibitor dostarlimab in combination with (1) the poly(ADP-ribose) polymerase inhibitor niraparib with or without vascular endothelial growth factor inhibitor bevacizumab or (2) carboplatin–paclitaxel chemotherapy with or without bevacizumab, in patients with advanced cancer.
Methods
IOLite is a multicenter, open-label, multi-arm clinical trial. Patients with advanced solid tumors were enrolled. Patients received dostarlimab in combination with niraparib with or without bevacizumab or in combination with carboplatin–paclitaxel with or without bevacizumab until disease progression, unacceptable toxicity, or withdrawal from the study. Prespecified endpoints in all parts were to evaluate the dose-limiting toxicities (DLTs), RP2Ds, pharmacokinetics (PKs), and preliminary efficacy for each combination.
Results
A total of 55 patients were enrolled; patients received dostarlimab and: (1) niraparib in part A (n=22); (2) carboplatin–paclitaxel in part B (n=14); (3) niraparib plus bevacizumab in part C (n=13); (4) carboplatin–paclitaxel plus bevacizumab in part D (n=6). The RP2Ds of all combinations were determined. All combinations were safe and tolerable, with no new safety signals observed. DLTs were reported in 2, 1, 2, and 0 patients, in parts A–D, respectively. Preliminary antitumor activity was observed, with confirmed Response Evaluation Criteria in Solid Tumors v1.1 complete/partial responses reported in 4 of 22 patients (18.2%), 6 of 14 patients (42.9%), 4 of 13 patients (30.8%), and 3 of 6 (50.0%) patients, in parts A–D, respectively. Disease control rates were 40.9%, 57.1%, 84.6%, and 83.3%, in parts A–D, respectively. Dostarlimab PK was unaffected by any combinations tested. Coadministration of bevacizumab showed no impact on niraparib PKs. The overall mean PD-1 receptor occupancy was 99.0%.
Conclusions
Dostarlimab was well tolerated in both doublet and triplet regimens tested, with promising antitumor activity observed with all combinations. We observed higher disease control rates in the triplet regimens than in doublet regimens.
Trial registration number
Keywords: Clinical Trials as Topic; Drug Therapy, Combination; Immunotherapy; Programmed Cell Death 1 Receptor
Introduction
Cancer immunotherapy agents are designed to utilize both adaptive and innate immunity to attack tumor cells. Most therapies that target adaptive immunity rely on T cells, whose functions are controlled by a series of costimulatory and coinhibitory signals.1 2 In malignancies, immune suppression can occur, preventing T-cell activation. Cancer immunotherapy has seen significant clinical success driven by immune checkpoint blockades (ICBs) that restore T-cell activation. ICBs act in multiple ways to alter T-cell function, including the downregulation of inhibitory signaling. One target of ICBs is programmed cell death protein 1 (PD-1).
PD-1 is a negative regulator of T-cell activity, and when bound to its ligands, programmed cell death ligand 1 (PD-L1) and PD-L2, T-cell activity is limited. PD-L1 and PD-L2 are overexpressed in multiple cancers, leading to T-cell repression. Targeting of PD-1 or PD-L1 (PD-[L]1) by monoclonal antibodies blocks the interaction between PD-1 on T cells and PD-L1 on cancer cells, leading to a restoration of T-cell activity. Several PD-(L)1 inhibitors have been approved as immunotherapeutic agents for various cancers.
Preclinical research has provided the rationale for novel antitumor therapeutic approaches for PD-(L)1 inhibitor combination strategies. Platinum-based chemotherapies, in addition to their direct cytotoxic effects, activate tumor-targeted immune responses, such as the reduction of tumor immunosuppression and T-cell activation in dendritic cells through the downregulation of PD-L2.3 4 Angiogenic factors, such as vascular endothelial growth factor (VEGF), have shown an immunosuppressive effect via inhibition of dendritic cell maturation and antigen presentation.5 6 VEGF inhibitors, such as bevacizumab, have increasingly demonstrated immunomodulatory properties due to their induction of immunological changes in the tumor microenvironment.7 The immunomodulatory effects of platinum-based chemotherapy and VEGF inhibitors may potentially be enhanced by the addition of PD-(L)1 inhibitors.
Clinical trials assessing the synergistic effects of ICB combinations with other cancer therapies have led to the approvals of combination therapies in the USA and/or Europe. In squamous non-small cell lung cancer, pembrolizumab combined with carboplatin–paclitaxel chemotherapy in the KEYNOTE-407 trial improved overall survival compared with placebo plus chemotherapy in patients regardless of PD-L1 expression and is now approved in this indication.8 The Food and Drug Administration (FDA) also granted accelerated approval for pembrolizumab and lenvatinib combination for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability high or mismatch repair deficient based on trial data demonstrating an objective response rate (ORR) of 38.3% (95% CI, 29% to 49%).9 Although now FDA approved, these data indicate that the majority of patients still do not benefit from this doublet combination and that there is therefore potential for further improvement in patient outcomes. One strategy may be to develop rational triplet combinations involving these different classes of antitumor agents. However, it is unknown if such triplet combinations will be safe and more efficacious than doublet combinations.
The PD-1 inhibitor dostarlimab (TSR-042) has demonstrated clinical activity in multiple tumor types, including non-small cell lung cancer and endometrial cancer.10–12 Additionally, dostarlimab has shown a pharmacokinetics (PK) profile that allows the dosing interval to expand from 3 to 6 weeks.11 Dostarlimab (JEMPERLI) is approved in the USA as a monotherapy in adults with mismatch repair-deficient recurrent or advanced endometrial cancer that has progressed on or following prior treatment with a platinum-containing regimen, or solid tumors that have progressed on or following prior treatment and who have no satisfactory alternative treatment options.13
Recent preclinical evidence has indicated a link between the DNA damage response and inflammation that may be exploited through ICB and poly(ADP-ribose) polymerase (PARP) inhibitor combination. PARP inhibition has been shown to upregulate PD-L1 expression, immunogenicity, and the immune response.14 15 Tumors treated with the PARP inhibitor niraparib showed activation of the cyclic GMP-AMP synthase-stimulator of interferon genes pathway, with increased immune cell penetration into intratumoral compartments.16 These cellular effects synergized with PD-(L)1 inhibitors in BRCA1 and BRCA2 (BRCA1/2) mutated and BRCA wild-type tumor models.16 Niraparib and pembrolizumab doublet was assessed in the phase 1/2 TOPACIO study (NCT02657889) among patients with recurrent, platinum-resistant/refractory ovarian cancer (OC) or triple-negative breast cancer.17 18 Niraparib plus pembrolizumab was well tolerated and showed promising clinical activity independent of platinum sensitivity, BRCA1/2 mutation, or homologous recombination deficiency status.
Based on supporting preclinical and early clinical data, we conducted a dose-finding phase 1b study of dostarlimab doublet and triplet combinations with niraparib or carboplatin–paclitaxel, with or without bevacizumab.
Methods
Study design and patients
IOLite (NCT03307785) was a multicenter, open-label, multi-arm phase 1b study designed to determine the recommended phase 2 dose (RP2D), safety, PK, and preliminary efficacy of dostarlimab in combination with approved cancer therapies for patients (≥18 years) with advanced or metastatic cancer. Parts A (dostarlimab plus niraparib) and C (part A regimen plus bevacizumab) enrolled patients who received no more than four lines of previous treatment for advanced or metastatic cancer. Patients who had received prior treatment with a PARP inhibitor were excluded. Parts B (dostarlimab plus carboplatin–paclitaxel) and D (part B regimen plus bevacizumab) enrolled patients who received no more than one line of previous chemotherapy in the metastatic setting and for whom treatment with carboplatin and paclitaxel was indicated. In all parts, patients who had received a prior PD-(L)1 inhibitor or any drug that targets checkpoint pathways were excluded. Patients on this trial were required to have measurable disease according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1), an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. All patients provided written, informed consent before participation. This study was conducted in compliance with Good Clinical Practice and all applicable local laws.
Objectives
The primary objective was to evaluate dose-limiting toxicities (DLTs) of each combination and establish an RP2D schedule for each part. Secondary objectives were to assess ORR, disease control rate (DCR), duration of treatment of each combination, and PK and pharmacodynamics.
Procedures and assessments
Patients in all parts received intravenous dostarlimab, 500 mg every 3 weeks (Q3W) for four cycles, followed by 1000 mg every 6 weeks for up to 2 years or until disease progression, unacceptable toxicity, investigator’s decision, withdrawal of consent, or death. In parts A and C, patients also received niraparib (either 200 mg or 300 mg once daily (QD), orally) (figure 1). Niraparib doses were selected based on a retrospective analysis of the pivotal ENGOT-OV16/NOVA trial.19 Patients could remain on niraparib for up to 2 years or until disease progression, unacceptable toxicity, investigator’s decision, withdrawal of consent, or death. In parts B and D, patients also received carboplatin (area under the curve (AUC) five or six, determined by the investigator, Q3W) and paclitaxel (175 mg/m2 Q3W) combination for four to six cycles, as indicated. In parts C and D, patients also received bevacizumab (15 mg/kg Q3W) for up to 22 cycles.
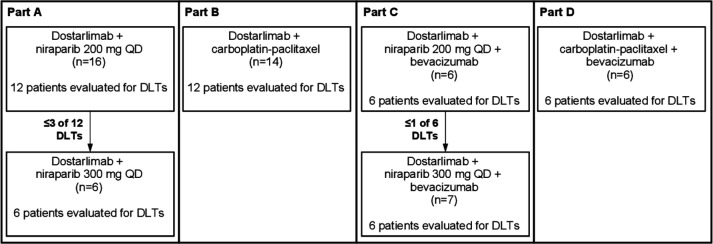
Figure 1.
Patient enrollment by trial part. Parts A and C show the enrollment of new patients for niraparib dose escalation from 200 mg QD to 300 mg QD per 6+6 dose escalation rules. DLT, dose-limiting toxicity; QD, once daily.
Parts A–D followed a 6+6 dose escalation enrollment format to confirm that doses for each combination were tolerable. Full details are available in the online supplemental appendix.
jitc-2021-003924supp001.pdf (21.5MB, pdf)
Plasma and serum samples were collected for niraparib and dostarlimab, respectively, to determine the drug concentrations by LC/MS/MS and ELISA and receptor occupancy as described in the online supplemental appendix. Non-compartmental PK analysis was done for dostarlimab and niraparib (Phoenix WinNonlin, V.6.4, Certara).
Statistical analysis
Demographics, baseline characteristics, safety, and efficacy data were summarized for patients who received at least one dose of study treatments using descriptive statistics. Response-evaluable patients were defined as those patients who received at least one dose of study medication, had measurable disease at baseline, and had one of the following: at least one postbaseline tumor assessment, clinical progression of disease, or death before the first tumor evaluation during treatment. A patient was considered non-evaluable for DLTs if, for any reason other than safety, the patient was unable to complete the 21-day combination treatment DLT observation period or was unable to take >80% of the intended dose of either agent. The data cut-off date was June 1, 2020. No formal statistical comparisons were performed. All statistics were performed using SAS software (V.9.4; SAS Institute).
Results
Patient characteristics
A total of 55 patients with advanced solid tumors were enrolled from October 2017 through September 2018: 22 patients in part A, 14 in part B, 13 in part C, and 6 in part D (table 1; figure 1).
Table 1.
Demographics and baseline characteristics
| Characteristic | Part A | Part B | Part C | Part D | ||
| DOS+NIR 200 mg (n=16) |
DOS+NIR 300 mg (n=6) |
DOS+C–P (n=14) |
DOS+NIR 200 mg +BEV (n=6) |
DOS+NIR 300 mg +BEV (n=7) |
DOS+C–P+BEV (n=6) |
|
| Age, years | ||||||
| Median (mean) | 63.5 (60.9) | 61.5 (60.5) | 70.5 (67.6) | 59.0 (57.0) | 46.0 (49.4) | 65.0 (66.8) |
| Range | 39–85 | 40–79 | 41–82 | 37–74 | 35–66 | 60–80 |
| Age group, years, n (%) | ||||||
| <65 | 8 (50.0) | 3 (50.0) | 5 (35.7) | 4 (66.7) | 6 (85.7) | 2 (33.3) |
| ≥65 | 8 (50.0) | 3 (50.0) | 9 (64.3) | 2 (33.3) | 1 (14.3) | 4 (66.7) |
| Body weight, kg | ||||||
| Mean | 82.0 | 96.6 | 81.6 | 85.1 | 88.6 | 80.5 |
| Range | 47.7–131.3 | 78.3–126.2 | 49.9–135.0 | 59.9–114.1 | 76.4–121.9 | 42.3–108.0 |
| Sex, n (%) | ||||||
| Female | 9 (56.3) | 2 (33.3) | 8 (57.1) | 4 (66.7) | 7 (100.0) | 5 (83.3) |
| Male | 7 (43.8) | 4 (66.7) | 6 (42.9) | 2 (33.3) | 0 | 1 (16.7) |
| ECOG performance status, n (%) | ||||||
| 0 | 8 (50.0) | 3 (50.0) | 8 (57.1) | 2 (33.3) | 4 (57.1) | 3 (50.0) |
| 1 | 8 (50.0) | 3 (50.0) | 6 (42.9) | 4 (66.7) | 3 (42.9) | 3 (50.0) |
| Prior regimens | ||||||
| Median (mean) | 2.0 (2.2) | 2.5 (2.3) | 1.0 (1.6) | 1.5 (2.7) | 2.0 (3.0) | 1.0 (1.3) |
| Primary tumor site at first diagnosis, n (%) | ||||||
| Bladder | 1 (6.3) | 0 | 1 (7.1) | 0 | 0 | 0 |
| Breast | 3 (18.8) | 0 | 2 (14.3) | 1 (16.7) | 2 (28.6) | 0 |
| Cholangiocarcinoma | 0 | 0 | 0 | 0 | 2 (28.6) | 1 (16.7) |
| Colorectal | 2 (12.5) | 4 (66.7) | 1 (7.1) | 0 | 0 | 0 |
| Endometrium | 1 (6.3) | 0 | 0 | 0 | 0 | 1 (16.7) |
| Gastric | 1 (6.3) | 0 | 1 (7.1) | 0 | 0 | 0 |
| Gastrointestinal stromal tumor | 2 (12.5) | 2 (33.3) | 0 | 1 (16.7) | 0 | 0 |
| Head and neck | 1 (6.3) | 0 | 2 (14.3) | 0 | 0 | 1 (16.7) |
| Liver | 1 (6.3) | 1 (16.7) | 0 | 0 | 0 | 0 |
| Lung, small cell | 0 | 0 | 3 (21.4) | 0 | 0 | 0 |
| NSCLC | 1 (6.3) | 0 | 1 (7.1) | 0 | 0 | 1 (16.7) |
| Ovarian | 1 (6.3) | 0 | 0 | 1 (16.7) | 1 (14.3) | 1 (16.7) |
| Pancreas | 1 (6.3) | 1 (16.7) | 0 | 1 (16.7) | 0 | 0 |
| Prostate | 1 (6.3) | 1 (16.7) | 1 (7.1) | 1 (16.7) | 0 | 0 |
| Other* | 1 (6.3) | 0 | 2 (14.3) | 1 (16.7) | 2 (28.6) | 1 (16.7) |
*Other includes appendix, squamous cell carcinoma, vulva, leiomyosarcoma, fallopian tube, uterus, and esophageal cancers.
BEV, bevacizumab; C–P, carboplatin–paclitaxel; DOS, dostarlimab; ECOG, Eastern Cooperative Oncology Group; NIR, niraparib; NSCLC, non-small cell lung cancer.
Safety
Dosing
In part A, 16 patients were enrolled to receive dostarlimab plus 200 mg of niraparib QD. Among the first enrolled patients, four patients were considered non-evaluable for DLTs. After two of the six patients had DLTs (grade 3 mucosal inflammation and hypertension), six additional patients were enrolled. No DLTs were reported in the additional six patients and the dose was considered safe (table 2). After the niraparib dose was escalated to 300 mg QD, zero of six patients experienced a DLT and the RP2D was confirmed. In part B, 14 patients were enrolled to receive dostarlimab plus carboplatin (AUC five or six, Q3W) plus paclitaxel (175 mg/m2 Q3W). Two patients were considered non-evaluable. One of 12 evaluable patients experienced a DLT (grade 3 aspartate aminotransferase increased) and the RP2D was confirmed. In part C, six patients were enrolled to receive dostarlimab plus niraparib 200 mg QD plus bevacizumab (15 mg/kg Q3W). One of six patients experienced a DLT (grade 3 vertebral artery dissection associated with hypertension, which were considered related to both niraparib and bevacizumab per investigator); the dose was considered safe, and the niraparib dose was escalated to 300 mg. Seven additional patients were enrolled to receive dostarlimab plus niraparib 300 mg QD plus bevacizumab (15 mg/kg Q3W). One patient was considered non-evaluable. The RP2D was confirmed when one of six evaluable patients experienced a DLT (grade 4 neutrophil count decreased). In part D, six patients were enrolled to receive dostarlimab plus carboplatin (AUC five or six, Q3W) plus paclitaxel (175 mg/m2 Q3W) plus bevacizumab (15 mg/kg Q3W). The RP2D was confirmed when zero of six patients experienced a DLT.
Table 2.
Safety summary for parts A–D
| Preferred term, n (%) | Part A DOS+NIR |
Part B DOS+C–P |
Part C DOS+NIR+BEV |
Part D DOS+C–P+BEV |
||||
| Niraparib 200 mg QD (n=16) |
Niraparib 300 mg QD (n=6) |
Overall (n=22) |
Overall (n=14) |
Niraparib 200 mg QD (n=6) |
Niraparib 300 mg QD (n=7) |
Overall (n=13) |
Overall (n=6) |
|
| Any-grade TEAEs* | 16 (100) | 6 (100) | 22 (100) | 14 (100) | 6 (100) | 7 (100) | 13 (100) | 6 (100) |
| Nausea | 8 (50) | 4 (67) | 12 (55) | 4 (29) | 3 (50) | 4 (57) | 7 (54) | 3 (50) |
| Anemia | 4 (25) | 3 (50) | 7 (32) | 10 (71) | 2 (33) | 5 (71) | 7 (54) | 5 (83) |
| Fatigue | 4 (25) | 3 (50) | 7 (32) | 7 (50) | 2 (33) | 4 (57) | 6 (46) | 4 (67) |
| Vomiting | 5 (31) | 2 (33) | 7 (32) | 2 (14) | 1 (17) | 4 (57) | 5 (39) | 2 (33) |
| Cough | 4 (25) | 3 (50) | 7 (32) | 3 (21) | 1 (17) | 2 (29) | 3 (23) | 2 (33) |
| Dyspnea | 5 (31) | 1 (17) | 6 (27) | 7 (50) | 2 (33) | 3 (43) | 5 (39) | 3 (50) |
| Thrombocytopenia | 5 (31) | 2 (33) | 6 (27) | 2 (14) | 2 (33) | 2 (29) | 4 (31) | 2 (33) |
| Constipation | 5 (31) | 2 (33) | 7 (32) | 4 (29) | 4 (67) | 4 (57) | 8 (62) | 3 (50) |
| Headache | 3 (19) | 1 (17) | 4 (18) | 3 (21) | 4 (67) | 1 (14) | 5 (39) | 1 (17) |
| Alopecia | 0 | 0 | 0 | 8 (57) | 0 | 1 (14) | 1 (8) | 5 (83) |
| Decreased appetite | 2 (13) | 0 | 2 (9) | 6 (43) | 0 | 3 (43) | 3 (23) | 1 (17) |
| Neutropenia | 1 (6) | 1 (17) | 2 (9) | 6 (43) | 1 (17) | 1 (14) | 2 (15) | 4 (67) |
| Diarrhea | 0 | 0 | 0 | 6 (43) | 2 (33) | 4 (57) | 6 (46) | 4 (67) |
| Weight decrease | 4 (25) | 1 (17) | 5 (23) | 1 (7) | 1 (17) | 2 (29) | 3 (23) | 3 (50) |
| Grade ≥3 TEAEs† | 12 (75) | 5 (83) | 17 (77) | 10 (71) | 5 (83) | 7 (100) | 12 (92) | 6 (100) |
| Thrombocytopenia | 2 (13) | 1 (17) | 3 (14) | 0 | 1 (17) | 1 (14) | 2 (15) | 2 (33) |
| Anemia | 3 (19) | 0 | 3 (14) | 3 (21) | 1 (17) | 2 (29) | 3 (23) | 0 |
| Neutropenia | 1 (6) | 1 (17) | 2 (9) | 4 (29) | 0 | 1 (14) | 1 (8) | 3 (50) |
| Pneumonia | 1 (6) | 1 (17) | 2 (9) | 0 | 1 (17) | 0 | 1 (8) | 0 |
| ALT increase | 1 (6) | 0 | 1 (5) | 2 (14) | 0 | 1 (14) | 1 (8) | 0 |
| AST increase | 1 (6) | 0 | 1 (5) | 2 (14) | 0 | 1 (14) | 1 (8) | 0 |
| Asthenia | 0 | 1 (17) | 1 (5) | 2 (14) | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 1 (17) | 1 (5) | 3 (21) | 0 | 0 | 0 | 1 (17) |
| Vomiting | 0 | 0 | 0 | 1 (7) | 0 | 0 | 0 | 0 |
| Dose-limiting toxicity‡ | 2 (13) | 0 | 2 (9) | 1 (7) | 1 (17) | 1 (14) | 2 (15) | 0 |
| AST increase | 0 | 0 | 0 | 1 (7) | 0 | 0 | 0 | 0 |
| Vertebral artery dissection | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (7) | 0 |
| Hypertension | 1 (6) | 0 | 1 (5) | 0 | 0 | 0 | 0 | 0 |
| Mucosal inflammation | 1 (6) | 0 | 1 (5) | 0 | 0 | 0 | 0 | 0 |
| Neutrophil count decreased§ | 0 | 0 | 0 | 0 | 0 | 1 (14) | 1 (7) | 0 |
| TEAE leading to treatment discontinuation | 3 (19) | 1 (17) | 4 (18) | 4 (29) | 2 (33) | 1 (14) | 3 (23) | 1 (17) |
*Any-grade TEAEs are listed for AEs that occurred in >25% of patients overall in any part.
†Grade ≥3 TEAEs and SAEs are listed for AEs that occurred in ≥2 patients overall in any part.
‡DLTs were grade 3 unless otherwise noted.
§Grade 4.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BEV, bevacizumab; C–P, carboplatin–paclitaxel; DLT, dose-limiting toxicitie; DOS, dostarlimab; NIR, niraparib; QD, once daily; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Adverse events
Grade ≥3 treatment-emergent adverse events (TEAEs, adverse events (AEs) regardless of causality) were reported in 17 of 22 (77%) patients in part A, 10 of 14 (71%) in part B, 12 of 13 (92%) in part C, and 6 of 6 (100%) in part D. Nausea, anemia, and fatigue were the most common any-grade TEAEs across all parts of the study (table 2). In parts A and C, nausea was the common TEAE (55% and 54%, respectively). In parts B and D, anemia (71% and 83%, respectively) and alopecia (57% and 83%, respectively) were the most common TEAEs. The most common grade ≥3 TEAEs across all parts were anemia, thrombocytopenia, and neutropenia (table 2). TEAEs leading to niraparib dose reductions occurred in 2 of 22 (9%) and 4 of 13 (31%) patients in parts A and C, respectively. Anemia was responsible for discontinuations in both patients in part A. In part C, patients discontinued because of anemia, thrombocytopenia, neutropenia, and diarrhea. TEAEs leading to treatment discontinuation occurred in 4 of 22 (18%) patients in part A, 4 of 14 (29%) in part B, 3 of 13 (23%) in part C, and 1 of 6 (17%) in part D (table 2).
In part A, two on-treatment deaths were reported; both were due to disease progression. In part B, three on-treatment deaths were reported: one was due to disease progression and two were due to unrelated TEAEs leading to death (sudden death and pneumonia aspiration). Parts C and D each had one on-treatment death due to disease progression.
Efficacy
In part A, 4 of 22 response-evaluable patients had confirmed RECIST v1.1 partial response (ORR, 18.2%; 90% CI, 6.5% to 36.9%), and 5 of 22 had stable disease (DCR, 40.9%; 90% CI, 23.3% to 60.5%) as their best radiological response. In part B, 6 of 14 response-evaluable patients had confirmed RECIST v1.1 complete or partial response (ORR, 42.9%; 90% CI, 20.6% to 67.5%), and 2 of 14 had stable disease (DCR, 57.1%; 90% CI, 32.5% to 79.4%) as their best radiological response. In part C, 4 of 13 patients had confirmed RECIST v1.1 partial response (ORR, 30.8%; 90% CI, 11.3% to 57.3%), and 7 of 13 had RECIST v1.1 stable disease (DCR, 84.6%; 90% CI, 59.0% to 97.2%) as their best radiological response. In part D, 3 of 6 patients had confirmed RECIST v1.1 complete or partial response (ORR, 50.0%; 90% CI, 15.3% to 84.7%), and 2 of 6 had RECIST v1.1 stable disease (DCR, 83.3%; 90% CI, 41.8% to 99.1%).
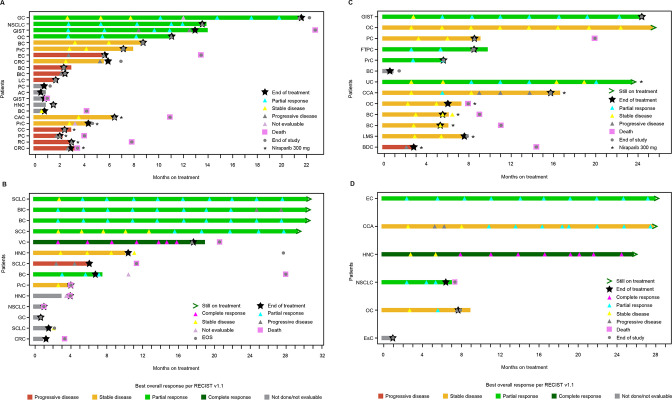
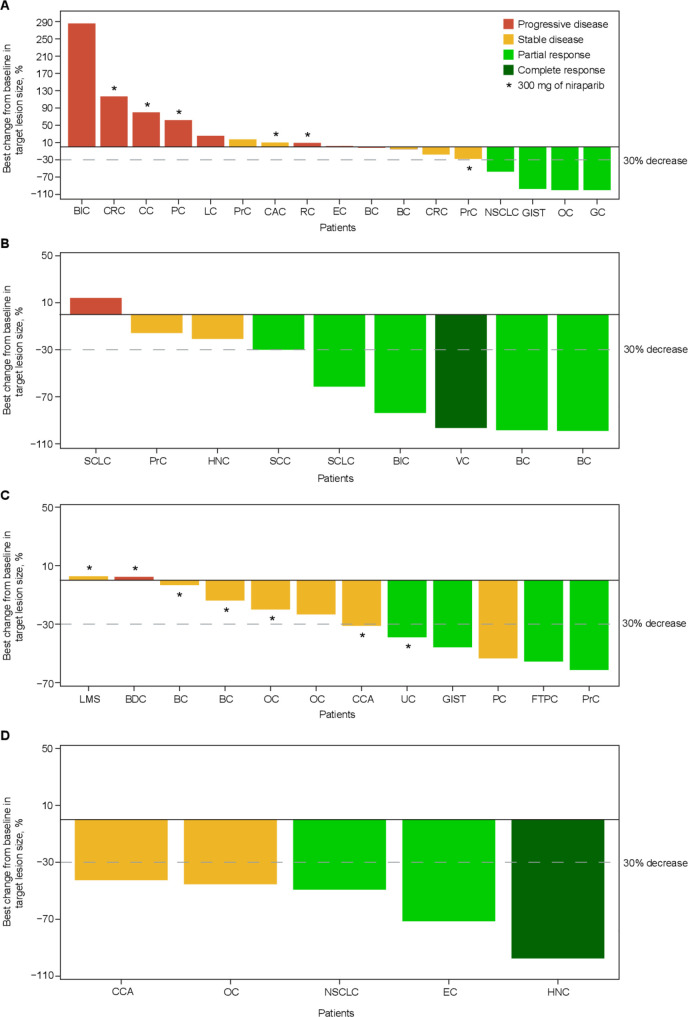
In part A, no patients were ongoing treatment at the time of data cut, and the median duration of response was 7.6 months (range, 5.8–10.9 months) (figure 2). For all patients in part A, the progression-free survival (PFS) rate was 43.2% (95% CI, 20.2% to 64.4%) at 6 months and 12.3% (95% CI, 2.1% to 32.3%) at 12 months. In part B, 4 of 14 patients were ongoing treatment, and the median duration of response was not reached (range, 4.2+ to 25.1+ months). PFS rate was 58.7% (95% CI, 27.4% to 80.4%) at 6 months and was unchanged at 12 months. In part C, 3 of 13 patients were still receiving treatment, and the median duration of response was not reached (2.9+ to 16.9+ months). For all patients in part C, PFS rate was 91.7% (95% CI, 53.9% to 98.8%) at 6 months and 30.6% (95% CI, 7.3% to 58.5%) at 12 months. In part D, 2 of 6 patients were still receiving treatment, and the median duration of response was not reached (range, 5.0–25.0+ months). PFS rate was 80.0% (95% CI, 20.4% to 96.9%) at 6 months and 40.0% (95% CI, 5.2% to 75.3%) at 12 months. Figure 3 shows best change in tumor volume.
Figure 2.
Duration of response and treatment in part A (A), part B (B), part C (C), and part D (D). AC, appendix cancer; BC, breast cancer; BDC, bile duct cancer; BLC, bladder cancer; CAC, colon adenocarcinoma; CC, colon cancer; CCA, cholangiocarcinoma; CRC, colorectal cancer; EC, endometrial cancer; EOS, end of study; ESC, esophageal cancer; FTPC, fallopian tube papillary carcinoma; GC, gastrointestinal cancer; GIST, gastrointestinal stromal tumor; HNC, head and neck cancer; LC, liver cancer; LMS, leiomyosarcoma; NSCLC, non-small cell lung carcinoma; OC, ovarian cancer; PC, pancreatic cancer; PRC, prostate cancer; RC, rectal cancer; RECIST, Response Evaluation Criteria in Solid Tumors; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; UC, uterine cancer; VC, vulvar cancer.
Figure 3.
Best change in target lesion size in part A (A), part B (B), part C (C), and part D (D). AC, appendix cancer; BC, breast cancer; BDC, bile duct cancer; BLC, bladder cancer; CC, colon cancer; CCA, cholangiocarcinoma; CRC, colorectal cancer; EC, endometrial cancer; FTPC, fallopian tube papillary carcinoma; GC, gastrointestinal cancer; GIST, gastrointestinal stromal tumor; HNC, head and neck cancer; LC, liver cancer; LMS, leiomyosarcoma; NSCLC, non-small cell lung carcinoma; OC, ovarian cancer; PC, pancreatic cancer; PRC, prostate cancer; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; UC, uterine cancer; VC, vulvar cancer.
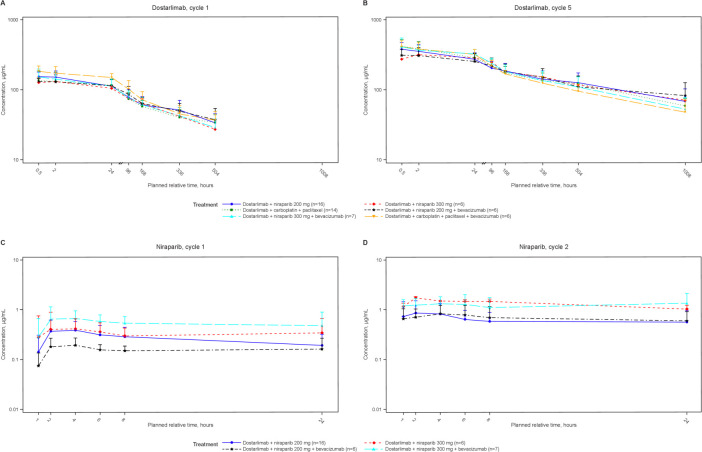
Dostarlimab PKs
The first-dose PKs of dostarlimab at 500 mg Q3W were evaluated and compared with monotherapy dostarlimab from the ongoing GARNET trial.20 In all parts (figure 4; online supplemental etable 1), maximum observed plasma concentration (Cmax), observed plasma concentration at 3 weeks (Clast), time to reach maximum observed plasma concentration (tmax), and AUC during 3 weeks (AUClast) were consistent and similar to PK data for dostarlimab monotherapy.
Figure 4.
Time plots of mean dostarlimab and niraparib serum pharmacokinetic concentration by treatment and cycle. Mean (±SD) dostarlimab serum concentration by treatment as semi-logarithmic curves for dostarlimab cycle 1 (A), dostarlimab cycle 5 (B), niraparib cycle 1 (C), and niraparib cycle 2 (D).
jitc-2021-003924supp002.pdf (151.5KB, pdf)
Niraparib PKs
First-dose PKs of niraparib at 200 mg and 300 mg QD for parts A and C were evaluated (figure 4; online supplemental etable 2) and compared with monotherapy data from published studies.21 At the 300 mg dose, Cmax, observed plasma concentration at last time point, nominal at 24 hours (Clast), and tmax were consistent across both parts and similar to PK data for niraparib monotherapy.21 The AUCs during time zero and last time point, nominal of 24 hours (AUClast), from parts A and C were comparable to PK data for niraparib monotherapy (9.6% lower from part A and 31.6% higher from part C).21 At the 200 mg dose, exposures (Cmax and AUClast) in part A were comparable to monotherapy. In part C, niraparib exposures (Cmax and AUClast) were lower (47.1% for Cmax and 42.9% for AUClast) than in part A.
Discussion
This study showed that doublet and triplet combination of dostarlimab with niraparib or carboplatin–paclitaxel, with or without bevacizumab, was safe and tolerable with promising evidence of antitumor activity in patients with advanced solid tumors. No new safety signals were observed at the RP2D for any combination tested. There was no observed impact to dostarlimab PK from the coadministration of niraparib, carboplatin–paclitaxel, or bevacizumab. We assessed the combinations in the PARP inhibitor-naive and PD-1/L1 inhibitor-naive settings to avoid the potential impact of prior therapies on the efficacy of the combinations. This is also a potential registration setting in different cancers and the combination is now being assessed in a similar treatment-naive setting in OC and showing potential.22 Furthermore, these combinations are now being validated in larger, randomized, double-blind, adaptive phase 3 studies with appropriate comparator arms to formally determine the treatment potential of the combinations studied in this trial.
The RP2D for each part was established and all doses were safe. In the dostarlimab plus carboplatin–paclitaxel parts B and D (plus bevacizumab), the most common grade ≥3 TEAE was neutropenia (35.0% of patients from both parts). In the KEYNOTE-189 trial of pembrolizumab combination with chemotherapy, neutropenia was one of the most frequently reported grade ≥3 TEAEs (15.8% of patients).23 The IMpower150 study of atezolizumab and bevacizumab reported a similar incidence of related grade ≥3 neutropenia (13.7%).24 In the dostarlimab plus niraparib parts A and C (plus bevacizumab), hematologic toxicities such as thrombocytopenia (14% and 15%, respectively) and neutropenia (9% and 8%, respectively) were the most common grade ≥3 TEAEs reported. No difference in hematologic toxicity rates was observed between patients receiving either 200 mg or 300 mg of niraparib QD.
Tumor response rates for dostarlimab plus carboplatin–paclitaxel in parts B and D (plus bevacizumab) are similar to what has been reported for other PD-(L)1 inhibitor and chemotherapy combinations (ORR, 48%–58%).8 23 25 Tumor response rates for dostarlimab plus niraparib in parts A and C (plus bevacizumab) were similar to the TOPACIO study of niraparib plus pembrolizumab combination for the treatment of molecularly unselected ovarian (ORR, 18%) or triple-negative breast cancer (ORR, 21%).17 18 Such chemotherapy-free PARP inhibitor combinations may allow more heavily pretreated patients to be treated and are an attractive option for patients.
PARP inhibitor combinations with VEGF blockade, such as bevacizumab plus niraparib, significantly improved PFS in platinum-sensitive recurrent OC compared with niraparib alone.26 Likewise, the VEGF inhibitor cediranib plus olaparib improved PFS in women with platinum-sensitive high-grade serous and endometrioid OC.27 Here, the addition of bevacizumab to the chemo-free regimen of dostarlimab and niraparib in part C was associated with a DCR of 84.6%, while the dostarlimab and niraparib combination in part A was associated with a DCR of 40.9%. The trial was not powered to assess direct comparison between patient groups, however, these data are supported by another study combining olaparib, durvalumab, and bevacizumab in advanced OC that reported a dramatic difference in 24-week DCR between doublet and triplet therapies (28.1% and 77.4%, respectively).28
Results from clinical trials assessing the combination of VEGF blockade and PD1/PD-L1 inhibitors have been inconsistent across tumor types, with the best results in non-small cell lung cancer, some gastric cancers, and hepatocellular cancer.29 Here, the addition of bevacizumab in parts C and D was associated with an increased DCR (84.6% and 83.3%, respectively) compared with parts A and B (40.9% and 57.1%, respectively).
The PKs of the first dose of dostarlimab 500 mg Q3W were similar to monotherapy PK data. Niraparib (300 mg QD) PKs were consistent in parts A and C for Cmax, Clast, and tmax and comparable to monotherapy for AUClast. The variability of niraparib exposure was ~50% regardless of dose and cohort, consistent with monotherapy data (up to 66%).21 Bevacizumab, an anti-VEGF monoclonal antibody, was reported to have reduced tumor vascular permeability without affecting plasma exposure.30 The first-dose niraparib PKs with or without bevacizumab did not show consistent impact. Although the mean exposure (AUClast) from patients in part C who received 200 mg of niraparib QD was approximately 43% lower than that of part A data, the mean exposure (AUClast) from patients in part C who received 300 mg of niraparib QD was approximately 46% higher than part A. Further comprehensive longitudinal analysis is required to determine bevacizumab’s effect on niraparib exposure.
Promising safety and preliminary efficacy data were observed for the doublet and triplet combinations tested in this phase 1b clinical trial. These data, including the contribution of each drug component, are now being validated in larger, randomized, double-blind, adaptive, phase 3 studies with appropriate comparator arms to formally determine the treatment potential of the combinations studied here in different cancers, including recurrent or primary advanced endometrial cancer and in the first-line treatment of stage 3/4 non-mucinous epithelial OC.31 32
Acknowledgments
Medical writing and editorial support, coordinated by Hasan H. Jamal, MSc, of GlaxoSmithKline (Waltham, Massachusetts), was provided by Eric Scocchera, PhD, and Jennifer Robertson, PhD, of Ashfield MedComms, an Ashfield Health company (Middletown, Connecticut).
Footnotes
Presented at: Data were presented at the American Society of Clinical Oncology (ASCO) annual meeting; May 31–June 4, 2019; Chicago, Illinois (abstract #2560).
Contributors: TAY: Study conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. AB: Collection and assembly of data, data analysis and interpretation, final approval of manuscript. EPH: Collection and assembly of data, data analysis and interpretation, final approval of manuscript. JS: Collection and assembly of data, data analysis and interpretation, final approval of manuscript. MRP: Collection and assembly of data, data analysis and interpretation, final approval of manuscript. JRA: Collection and assembly of data, data analysis and interpretation, final approval of manuscript. LE: Study conception and design, collection and assembly of data, data analysis and interpretation, final approval of manuscript. MD: Collection and assembly of data, data analysis and interpretation, final approval of manuscript. WG: Collection and assembly of data, data analysis and interpretation, final approval of manuscript. SK: Collection and assembly of data, data analysis and interpretation, final approval of manuscript. SL: Collection and assembly of data, data analysis and interpretation, final approval of manuscript, final approval of manuscript. BJD: Study conception and design, collection and assembly of data, data analysis and interpretation, final approval of manuscript. NG: Study conception and design, collection and assembly of data, data analysis and interpretation, final approval of manuscript. TAY is responsible for the overall content as guarantor.
Funding: This work was supported by GlaxoSmithKline (Waltham, Massachusetts). Trademarks are owned by or licensed to the GSK group of companies. Drs Yap and Rodon acknowledge the MD Anderson Cancer Center support grant (P30 CA016672). Dr Yap is a V Foundation V Clinical Scholar (VC2020-001), which supports a program of clinical trials targeting the DNA damage response.
Competing interests: TAY has received research support to the institution from Artios, AstraZeneca, Bayer, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F-Star, Genentech, GlaxoSmithKline, ImmuneSensor, Ipsen, Jounce, Karyopharm, Kyowa, Merck, Novartis, Pfizer, Regeneron, Repare, Ribon Therapeutics, Sanofi, Scholar Rock, Seattle Genetics, Tesaro, and Vertex Pharmaceuticals; and has served as a consultant for Aduro, Almac, AstraZeneca, Atrin, Axiom, Bayer, Bristol Myers Squibb, Calithera, Clovis, Cybrexa, EMD Serono, F-Star, Guidepoint, Ignyta, I-Mab, Janssen, Merck, Pfizer, Repare, Roche, Rubius, Schrodinger, Seattle Genetics, Varian, and Zai Labs. AB has nothing to disclose. EPH reports institutional research or clinical trial support from Seattle Genetics, Puma, AstraZeneca, Hutchinson MediPharma, OncoMed, MedImmune, StemCentrx, Genentech/Roche, Curis, Verastem, Zymeworks, Syndax, Lycera, Rgenix, Novartis, Mersana, Millenium, TapImmune, Lilly, BerGenBio, Medivation, Pfizer, Tesaro, Boehringer Ingelheim, Eisai, H3 Biomedicine, Radius Health, Acerta, Takeda, Macrogenics, AbbVie, Immunomedics, FujiFilm, Effector, Merus, Nucana, Regeneron, Leap Therapeutics, Taiho Pharmaceutical, EMD Serono, Daiichi Sankyo, ArQule, Syros, Clovis, Cytomx, InventisBio, Deciphera, Unum Therapeutics, Sermonix Pharmaceuticals, Sutro, Aravive, Zenith Epigenetics, Arvinas, Torque, Harpoon, Fochon, Black Diamond, Orinove, Molecular Templates, Silverback Therapeutics, Compugen, G1Therapeutics, Karyopharm Therapeutics and Torque Therapeutics, outside the submitted work. JS reports grants from Pfizer, Celgene, Genentech; personal fees from Celgene, PUMA, TTC Oncology, Pfizer, Novartis, TapImmune, Ipsen, Tempus, and AstraZeneca; and honoraria from Ipsen, Celgene, PUMA, Novartis, Pfizer, Tempus, and AstraZeneca; institutional research support from Pfizer, AbbVie, AstraZeneca, Verastem, Leap therapeutics, Endocyte, Cleave, Merck, Bayer, Exelixis, Medivation, Biomarin, Tesaro, TTC-Oncology, Genentech/Roche, Arqule, Syros, Sermonix, Black Diamond, Fujifilm, Arcus, Corcept, ImmuneSensor, Aduro, Agenus, Five Prime, Gilead, CytRx, Merrimack, Ipsen, Plexxikon, Proacta, Esanex, MiRNA, and BIND outside the submitted work. MRP reports funding from Tesaro/GSK during the conduct of the study; consulting fees from Janssen, EMD serono, Pfizer, Pharmacyclics, Bayer, and Genentech outside the submitted work. JRA reports personal fees from Novartis, Eli Lilly, Orion Pharmaceuticals, Servier Pharmaceuticals, Peptomyc, Merck Sharp & Dohme, Kelun Pharmaceuticals/Klus Pharma, Spectrum Pharmaceuticals, Pfizer, Roche Pharmaceuticals, Ellipses Pharma, Certera, Bayer, Molecular Partners, NovellusDX, IONCTURA SA; grants from Bayer, Novartis, Blueprint Pharmaceuticals, Spectrum Pharmaceuticals, Tocagen, Symphogen, BioAlta, Pfizer, GenMab, CytomX, Kelun-Biotech, Takeda-Millenium, GlaxoSmithKline, Ipsen; travel reimbursement from ESMO, Department of Defense, Merck Sharp & Dohme, Louisiana State University, Kelun Pharmaceuticals/Klus Pharma, Huntsman Cancer Institute, Cancer Core Europe, Karolinska Cancer Institute, King Abdullah International Medical Research Center, Bayer, WIN Consortium, Jansen, Molecular Partners; and other compensation from European Journal of Cancer, VHIO/Ministero De Empleo Y Seguridad Social, Chinese University of Hong Kong, SOLTI, Elsevier, GlaxoSmithKline, outside the submitted work. LE was an employee of GSK at the time the study was done. MD was an employee of GSK at the time the study was done. WG was an employee of GSK at the time the study was done. SK was an employee of GSK at the time the study was done. SL was an employee of GSK at the time the study was done. BJD was an employee of GSK at the time the study was done. NG has nothing to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All studies received institutional review board approvals at the participating centers and were conducted in accordance with Good Clinical Practice guidelines consistent with the Declaration of Helsinki as well as applicable national and local regulatory requirements. Written informed consent was obtained for all participants. Participants gave informed consent to participate in the study before taking part.
References
- 1.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016;15:235–47. 10.1038/nrd.2015.35 [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205–14. 10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesterhuis WJ, Punt CJA, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011;121:3100–8. 10.1172/JCI43656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, Buqué A, Kepp O, et al. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015;28:690–714. 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 5.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res 2001;23:263–72. 10.1385/IR:23:2-3:263 [DOI] [PubMed] [Google Scholar]
- 6.Oyama T, Ran S, Ishida T, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 1998;160:1224–32. [PubMed] [Google Scholar]
- 7.de Aguiar RB, de Moraes JZ, Barbosa de Aguiar R. Exploring the immunological mechanisms underlying the anti-vascular endothelial growth factor activity in tumors. Front Immunol 2019;10:1023. 10.3389/fimmu.2019.01023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040–51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 9.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20:711–8. 10.1016/S1470-2045(19)30020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oaknin A, Ellard SL, Leath III C, et al. Preliminary safety, efficacy, and PK/PD characterization from GARNET, a phase I clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-H endometrial cancer. Annals of Oncology 2018;29:viii334. 10.1093/annonc/mdy285.144 [DOI] [Google Scholar]
- 11.Wu X, Snir O, Rottmann D, et al. Minimal microsatellite shift in microsatellite instability high endometrial cancer: a significant pitfall in diagnostic interpretation. Mod Pathol 2019;32:650–8. 10.1038/s41379-018-0179-3 [DOI] [PubMed] [Google Scholar]
- 12.Subramanian J, Moreno V, Bosch-Barrera J, et al. 1399P safety and efficacy of dostarlimab in patients (PTS) with recurrent/advanced non-small cell lung cancer (NSCLC). Annals of Oncology 2020;31:S886–7. 10.1016/j.annonc.2020.08.1713 [DOI] [Google Scholar]
- 13.Jemperli (dostarlimab) . Prescribing information. GlaxoSmithKline, 2021. Available: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Jemperli/pdf/JEMPERLI-PI-MG.PDF [Accessed November 12, 2021].
- 14.Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 2017;23:3711–20. 10.1158/1078-0432.CCR-16-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen J, Zhao W, Ju Z, et al. PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res 2019;79:311–9. 10.1158/0008-5472.CAN-18-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Sun K, Xiao Y, et al. Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models. Sci Rep 2019;9:1853. 10.1038/s41598-019-38534-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol 2019;5:1132–40. 10.1001/jamaoncol.2019.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konstantinopoulos PA, Waggoner S, Vidal GA, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol 2019;5:1141–9. 10.1001/jamaoncol.2019.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berek JS, Matulonis UA, Peen U, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol 2018;29:1784–92. 10.1093/annonc/mdy181 [DOI] [PubMed] [Google Scholar]
- 20.Oaknin A. Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase 1/2 clinical trial of the anti-PD-1 monoclonal antibody, dostarlimab, in patients with recurrent or advanced MSI-H and MSS endometrial cancer (EC). SGO Annual Meeting 2019. [Google Scholar]
- 21.Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol 2013;14:882–92. 10.1016/S1470-2045(13)70240-7 [DOI] [PubMed] [Google Scholar]
- 22.Berberabe T. Dostarlimab triplet shows antitumor activity, tolerability in ovarian cancer. OncLive, 2021. Available: https://www.onclive.com/view/dostarlimab-triplet-shows-antitumor-activity-tolerability-in-ovarian-cancer [Accessed 20 Dec 2021].
- 23.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 24.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 25.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 26.Mirza MR, Åvall Lundqvist E, Birrer MJ, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol 2019;20:1409–19. 10.1016/S1470-2045(19)30515-7 [DOI] [PubMed] [Google Scholar]
- 27.Liu JF, Barry WT, Birrer M, et al. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann Oncol 2019;30:551–7. 10.1093/annonc/mdz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drew Y, Penson RT, O'Malley DM, et al. 814MO phase II study of olaparib (o) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): initial results in patients (PTS) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Annals of Oncology 2020;31:S615–6. 10.1016/j.annonc.2020.08.953 [DOI] [Google Scholar]
- 29.Gao F, Yang C. Anti-VEGF/VEGFR2 monoclonal antibodies and their combinations with PD-1/PD-L1 inhibitors in clinic. Curr Cancer Drug Targets 2020;20:3–18. 10.2174/1568009619666191114110359 [DOI] [PubMed] [Google Scholar]
- 30.Abuqayyas L, Balthasar JP. Pharmacokinetic mAb-mAb interaction: anti-VEGF mAb decreases the distribution of anti-CEA mAb into colorectal tumor xenografts. AAPS J 2012;14:445–55. 10.1208/s12248-012-9357-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirza MR, Coleman RL, Hanker LC, et al. ENGOT-EN6/NSGO-RUBY: a phase III, randomized, double-blind, multicenter study of dostarlimab + carboplatin-paclitaxel versus placebo + carboplatin-paclitaxel in recurrent or primary advanced endometrial cancer (EC). JCO 2020;38:TPS6107. 10.1200/JCO.2020.38.15_suppl.TPS6107 [DOI] [Google Scholar]
- 32.Hardy-Bessard A-C, Moore KN, Mirza MR, et al. ENGOT-OV44/FIRST study: a randomized, double-blind, adaptive, phase III study of platinum-based therapy with dostarlimab (TSR-042) + niraparib versus standard-of-care (soc) platinum-based therapy as first-line treatment of stage 3/4 non-mucinous epithelial ovarian cancer (OC). JCO 2019;37:TPS5600. 10.1200/JCO.2019.37.15_suppl.TPS5600 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-003924supp001.pdf (21.5MB, pdf)
jitc-2021-003924supp002.pdf (151.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.