Abstract
We present a case of a thoracic aortic mural thrombus (AMT) in a non-atherosclerotic and non-aneurysmal aorta that presented with acute limb ischaemia. AMT in a non-diseased aorta without an underlying hypercoagulable disorder is rare. The AMT in our patient was managed with anticoagulation, which resolved on a 5-month follow-up CT scan. This provided us an opportunity to discuss the successful medical management of an AMT, to review the literature on the management of AMT and to add to the literature on a rare presentation of an AMT.
Keywords: Cardiovascular system, Haematology (drugs and medicines), Vascular surgery
Background
Aortic mural thrombus (AMT) is usually identified incidentally or can present with acute limb ischaemia or stroke.1 It is a rare occurrence if identified in a non-atherosclerotic aorta and in patients without a hypercoagulable disorder. Anticoagulation and endovascular or surgical intervention are the management options, but there are no consensus guidelines.2
Case presentation
The patient was a woman in her 40s who presented with a 2-hour history of generalised abdominal pain, emesis and left lower extremity pain. She presented with tachypnoea and altered mental status. The patient reported severe rest pain. The left foot was cool with non-pitting oedema. The dorsalis pedis and popliteal pulses were non-palpable without Doppler signals, and there was diminished sensation to the palmar aspect of the left foot. The left lower limb strength was 0/5 in plantarflexion and dorsiflexion compared with 5/5 in the right lower limb. The reflexes of the right and left knees were 2+. No skin changes were observed during the examination.
Her medical history was significant for insulin-dependent diabetes mellitus (unknown haemoglobin A1c), morbid obesity (body mass index of 52 kg/m2) and cervical cancer status after a loop electrosurgical excision procedure. Before the onset of symptoms, the patient was fully functional and independent, without an assistant device. She was a current smoker with a 10-pack-year history. The patient endorsed occasional alcohol use but denied illicit drug use. She had gravida 9 para 5 (G9P54144) with two spontaneous abortions and one preterm delivery at 20 weeks. The patient denied any prior vascular intervention. She was not on anticoagulation but took aspirin 81 mg/day. Family history is significant for cerebrovascular accidents and hypertension.
Investigations
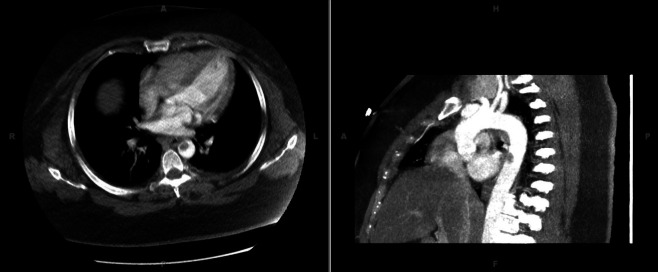
A stroke workup was initially pursued because of her altered mental status. Her complete blood count and basic metabolic panel demonstrated leucocytosis but were otherwise unremarkable. Electrocardiography revealed sinus tachycardia without ischaemic changes. A head CT scan did not reveal an ischaemic or haemorrhagic infarct. CT angiography of the head and neck demonstrated right vertebral artery stenosis. CT angiography of the chest, abdomen and pelvis showed a 14×11 mm thrombus in the descending thoracic aorta without dissection or aneurysm (figure 1). Lower extremity runoff demonstrated an abrupt filling defect within the left common femoral artery with complete occlusion in the distal superficial femoral artery extending into the popliteal artery and the arteries below the trifurcation. Her symptoms were consistent with Rutherford stage III acute limb ischaemia.
Figure 1.
Descending thoracic aortic mural thrombus.
Differential diagnosis
Our initial clinical suspicion was a cerebrovascular accident, given the patient’s history and clinical presentation with altered mental status and left lower limb weakness and numbness. However, the stroke workup was negative, which led to the concern that the patient’s lower extremity symptoms could be due to acute limb ischaemia, prompting the CT angiogram. Once acute limb ischaemia was identified, the next question was whether it was due to thrombosis or an embolic event. Given the lack of history of peripheral vascular disease or disease in other vascular beds and the mural thrombus in the aorta, the most likely cause was embolus from the mural thrombus. Electrocardiography did not demonstrate atrial fibrillation; therefore, a cardiac source was less likely.
Treatment
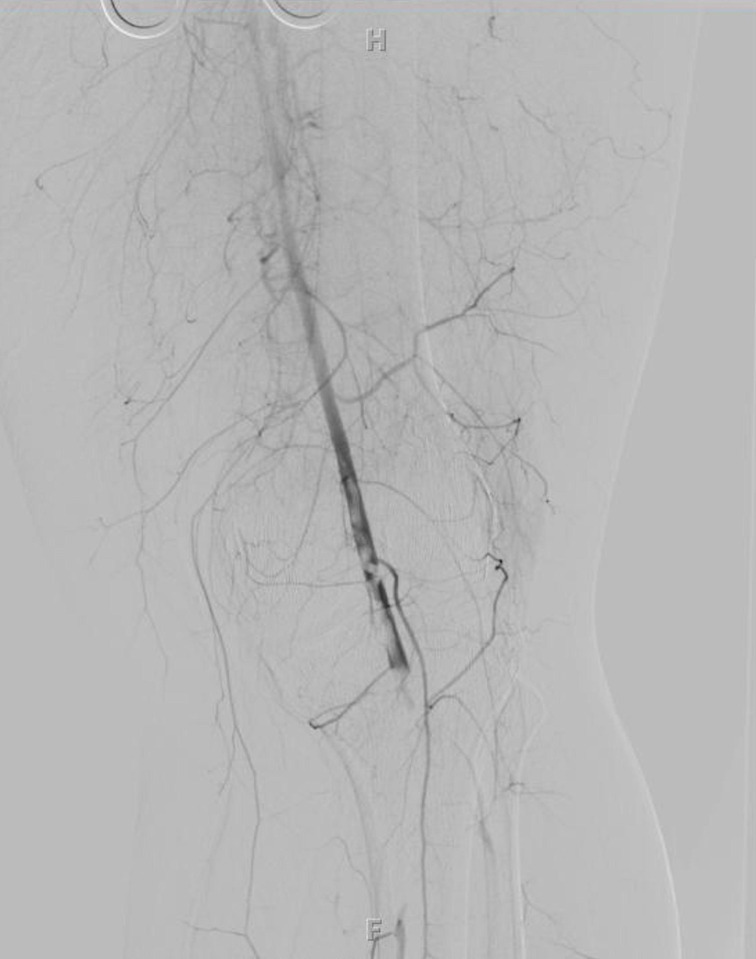
The patient was started on a heparin drip with a goal partial thromboplastin time of 70–100 s. Three hours after presentation, the patient was taken to the operating room for a left iliofemoral embolectomy via a common femoral artery cutdown. During the femoral cutdown, a large inguinal lymph node was encountered and excised, which was negative for malignancy. Following the embolectomy, an angiogram was performed, which demonstrated residual embolus at the popliteal artery (figure 2). A popliteal cutdown was used to perform a popliteal embolectomy using Fogarty catheters. A four-compartment fasciotomy was performed. Postoperatively, the patient had a transthoracic and transoesophageal echocardiogram (TEE), which ruled out a cardiac source of embolism. On postoperative day 4, the patient had no return of sensation or motor function; therefore, she underwent an above-knee amputation. The remainder of her postoperative course was significant for rhabdomyolysis that caused an acute kidney injury, which was managed with resuscitative fluids.
Figure 2.
Angiogram demonstrating popliteal artery embolism.
A hypercoagulable workup was performed. She also had an elevated prothrombin time and partial thromboplastin time. The JAK2 V617F gene mutation, which is present in nearly 100% of patients with polycythemia vera and in approximately 50% of patients with essential thrombocytosis and primary myelofibrosis, was not detected.3 Lupus anticoagulant was not detected. Antiphospholipid syndrome was ruled out by testing for antibodies against beta-2 glycoprotein 1 and cardiolipin.4 Paroxysmal nocturnal haemoglobinuria (PNH) was ruled out by testing for the percentage of PNH red blood cells, PNH polymorphonuclear leucocytes and PNH monocytes. PNH is known for its complications of intravascular haemolysis, and thrombosis was negative.5 COVID-19 was negative. HIV and rapid plasma reagin tests were non-reactive, and the patient had rubella immunity. The folate and cobalamin levels were normal. Vitamin B12 deficiency-induced thrombotic microangiopathy, which can mimic thrombotic thrombocytopenic purpura,6 was ruled out by normal vitamin B12 levels. Severe folate and vitamin B12 deficiency can present as hyperhomocysteinaemia, leading to thrombosis. Folate levels were also normal.
Outcome and follow-up
The heparin drip was continued postoperatively until transitioning the patient to apixaban 5 mg two tiimes per day on hospital day 10. The patient was discharged to inpatient rehabilitation on hospital day 10. She spent 14 days in inpatient rehabilitation before being discharged home. Of note, the patient was prescribed 30 days of anticoagulation therapy and had a gap in treatment of 1–2 months before resuming therapy. A follow-up CT angiogram was obtained at approximately 5 months posthospitalisation, which demonstrated complete resolution of her AMT.
Discussion
AMT was first described by Weismann and Tobin in 1958.7 AMT is most commonly seen in younger women in the abdominal aorta, with occurrence in the thoracic aorta largely limited to case reports.8 The most common predisposing factor in the formation of an AMT is atherosclerosis or aneurysm. However, they can also occur in a non-diseased aorta, which may be from minor intimal injuries caused by turbulent flow.1 Herein, we present a case of an AMT in a non-diseased aorta and provide a brief review of literature on management trends.
AMTs are usually identified incidentally, but if symptomatic, they typically present with acute limb ischaemia, visceral organ ischaemia or stroke.9 If the site of attachment of the thrombus is close to the aortic valve cusps or coronary sinus, the patient may present with decreased ejection fraction or acute coronary syndrome.10
Workup includes ruling out a hypercoagulable state, most commonly malignancy, antiphospholipid syndrome, factor V Leiden mutation, prothrombin gene G20210A mutation, elevated factor VIII and hyperhomocysteinaemia, antithrombin deficiency, and protein C or S deficiency.11 If it has not been performed, a CT or MRI angiography is recommended to define the complete extent of the thrombus.11 Transthoracic and TEE should be used to rule out intracardiac thrombus.12
Anticoagulation and surgical intervention are the mainstays of therapy; however, there are no consensus guidelines on the optimal management of AMT.2 The therapeutic strategy is influenced by the location of the thrombus, associated clinical presentation, inherent patient comorbidities and individual clinician preference.13 Historically, therapeutic anticoagulation was proposed as first-line therapy, and surgical intervention was reserved for mobile thrombus, recurrent embolism and contraindication for anticoagulation.14
Endovascular therapy is gaining popularity in the management of AMT. It appears to have a lower rate of recurrent embolisation when compared with anticoagulation alone and lower perioperative morbidity when compared with surgical thrombectomy.15 Endovascular therapy should be avoided in ascending AMT as there is a risk of embolic events or even thrombus migration during manipulation while placing the endovascular prosthesis.9 In acute limb ischaemia, endovascular management of the AMT can be concomitant or after the peripheral embolectomy.16
Endovascular therapies include stent placement over the AMT, catheter-directed thrombolysis and thrombus aspiration.17 Endovascular management is associated with a 5.7% failure or recurrence rate.1 Catheter aspiration and thrombolysis have varying success rates with an increased risk of iatrogenic embolism. Stent grafts should have at least a 2 cm overlap proximally and distally from the thrombus. To limit the chance of distal embolus, oversizing of the stent should be limited to not more than 5%, and no poststent balloon angioplasty should be used.9 Techniques suggested to minimise morbidity include the use of intravascular ultrasound and/or intraoperative TEE to correctly identify the location of the thrombus and select the minimum length necessary of a stent graft, minimise the use of contrast during delivery, temporary balloon occlusion of downstream visceral arteries and handling of catheters/wires and avoiding balloon dilation.18 Despite the theoretical risk, there have been no reports of distal embolus from placing a stent graft. Therefore, it seems to be an effective method for managing AMT with low morbidity.2
Open surgical intervention may be appropriate in otherwise healthy patients with an AMT at high risk of distal embolisation, such as in the ascending aorta, or a substantial clot burden that predisposes a significant risk of embolisation during endovascular management or would require an excessive length of coverage.2 9 Surgical approaches include aortic thrombectomy with primary suture or patch closure, thrombectomy using the ‘trapdoor’ technique in the visceral aorta, replacement of a segment of aorta and aortoiliac embolectomy by femoral cutdowns.19 Some authors suggest that an infrarenal AMT be managed with embolectomy, unless the thrombus was juxtaposed to the renal arteries, in which case a blind embolectomy risks renal artery embolism.9 Surgical thrombectomy has the advantage of providing a histopathological diagnosis into the aetiology of the thrombus. Unsurprisingly, surgical intervention has the highest morbidity and mortality with an estimated mortality of 2.6%–5.0% and periopera
tive complications in the order of 30%–71%.1
Fayad et al published a meta-analysis in 2013 comparing 88 patients who underwent surgical intervention to 112 patients treated with anticoagulation alone. Surgery was associated with lower recurrence of recurrent embolic event (9% vs 26%) and less amputations (2.5% vs 9.0%) than anticoagulation therapy. Importantly, a majority of the patients who underwent surgery (57%), had an AMT in the ascending aorta or aortic arch. The complication rate (stroke, limb loss and bowel resections) was non-significantly higher in the anticoagulation group (28% vs 17%, p=0.07) with a 6% mortality in each group. The authors suggest a primary surgical approach, especially in the low-risk group and one or more risk factors for recurrence.20
Alternatively, medical management with anticoagulation may be considered and may be preferable in cases of a normal aorta.11 The duration of oral anticoagulation therapy varies between publications, described between until there is complete resolution of the thrombus and indefinite dosing.21 Recurrence with anticoagulation alone ranges from 0% to 50%. Most occur within the first 4 weeks. In the absence of hypercoagulable disorders, the duration of anticoagulation should be until resolution of the thrombus, generally 4–12 months.19 Dual therapy was also reported in several cases in which oral anticoagulation was used with open thrombectomy or endovascular revascularisation. It is unknown, however, if the benefit is solely due to surgery or anticoagulation therapy, or if both benefits are additive.19 Patient selection is paramount in anticoagulation alone as they need to be compliant with taking the medication and strict follow-up. Antiplatelets are associated with a lower risk of AMT; however, they are not used in the management of AMT.12 One proposed option is 6 months of anticoagulation with a repeat CT angiography to demonstrate thrombus resolution before transitioning from anticoagulation to aspirin 75 mg/day.9
There is a paucity of data on thrombolytic therapy. Kruger et al described the successful use of systemic alteplase with distal aortic mural thrombi in three cases: one patient presented with stroke symptoms and the other two with upper extremity ischaemia and mesenteric ischaemia.22 The concern with thrombolytic therapy is of the thrombus dislodging if a pedicle exists since the pedicle will lyse before the corpus.1
AMT tends to occur in atherosclerotic or aneurysmal aortas; however, they can present in non-diseased aortas, as in our patient. They either present with embolic complications or are identified incidentally. Anticoagulation is the mainstay of therapy. Endovascular intervention is becoming more popular especially with certain thrombus morphologies. Surgical intervention is typically reserved for ascending aorta and aortic arch thrombus, as well as healthy patients at a high risk of embolisation using endovascular methods. We present a case of an AMT in a non-diseased aorta that presented with acute limb ischaemia. AMT in non-diseased aortas in a patient without a hypercoagulable disorder is rare. This case provided us with an opportunity to discuss the successful medical management of our patient, review the literature on the management of AMTs and add to the literature on a rare presentation of an AMT.
Learning points.
Aortic mural thrombus (AMT) is rare in non-atherosclerotic and non-aneurysmal aortas.
No consensus guidelines on the management of AMT.
Mainstay of management of AMT is anticoagulation.
Footnotes
Contributors: Research idea and study design: AK and JJK. Supervision and mentorship: DL. Each author contributed important intellectual content during manuscript drafting or revision and agreed to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including documentation in the literature if appropriate.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Verma H, Meda N, Vora S, et al. Contemporary management of symptomatic primary aortic mural thrombus. J Vasc Surg 2014;60:1524–34. 10.1016/j.jvs.2014.08.057 [DOI] [PubMed] [Google Scholar]
- 2.Meyermann K, Trani J, Caputo FJ, et al. Descending thoracic aortic mural thrombus presentation and treatment strategies. J Vasc Surg 2017;66:931–6. 10.1016/j.jvs.2017.05.109 [DOI] [PubMed] [Google Scholar]
- 3.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61. 10.1016/S0140-6736(05)71142-9 [DOI] [PubMed] [Google Scholar]
- 4.Miyakis S, Giannakopoulos B, Krilis SA. Beta 2 glycoprotein I--function in health and disease. Thromb Res 2004;114:335–46. 10.1016/j.thromres.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 5.Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood 2014;124:2804–11. 10.1182/blood-2014-02-522128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keskin EY, Keskin M. Severe vitamin B12 deficiency in a 15-year-old boy: presentation with haemolysis and pancytopenia. BMJ Case Rep 2015;2015. 10.1136/bcr-2015-209718. [Epub ahead of print: 14 May 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weismann RE, Tobin RW. Arterial embolism occurring during systemic heparin therapy. AMA Arch Surg 1958;76:219–27. 10.1001/archsurg.1958.01280200041005 [DOI] [PubMed] [Google Scholar]
- 8.Hassan I, Zehr KJ, Freeman WK. A case of asymptomatic thoracic aorta mural thrombi. Ann Thorac Surg 2001;72:1735–7. 10.1016/S0003-4975(01)02612-1 [DOI] [PubMed] [Google Scholar]
- 9.Verma H, Meda N, Vora S, et al. Contemporary management of symptomatic primary aortic mural thrombus. J Vasc Surg 2014;60:1524–34. 10.1016/j.jvs.2014.08.057 [DOI] [PubMed] [Google Scholar]
- 10.Nakashima MO, Rogers HJ. Hypercoagulable states: an algorithmic approach to laboratory testing and update on monitoring of direct oral anticoagulants. Blood Res 2014;49:85–94. 10.5045/br.2014.49.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad SN, Singh V, Selvamurugan V, et al. Vertebrobasilar dolichoectasia with typical radiological features. BMJ Case Rep 2021;14:e239866. 10.1136/bcr-2020-239866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai Z, Fukuda T, Shiratori Y, et al. Aortic mural thrombus visualised on transoesophageal echocardiography. BMJ Case Rep 2019;12:e229212. 10.1136/bcr-2019-229212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ungprasert P, Ratanapo S, Cheungpasitporn W. Manage-ment in thoracic aorta mural thrombi: evidence based medicine and controversy.. Emergency Medicine 2011;1:e104. [Google Scholar]
- 14.Varino J, Rodrigues R, Pereira B. Trombo aórtico mural [Aortic mural thrombus]. Rev Port Cir Cardiotorac Vasc 2019;26:19–26. [PubMed] [Google Scholar]
- 15.Reber PU, Patel AG, Stauffer E, et al. Mural aortic thrombi: an important cause of peripheral embolization. J Vasc Surg 1999;30:1084–9. 10.1016/S0741-5214(99)70047-9 [DOI] [PubMed] [Google Scholar]
- 16.Moris D, Karaolanis G, Schizas D, et al. eComment. mural thrombus in normal appearing aorta: unfinished SAGA in uncharted waters. Interact Cardiovasc Thorac Surg 2016;22:373.2–4. 10.1093/icvts/ivv407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnamoorthy V, Bhatt K, Nicolau R, et al. Transesophageal echocardiography-guided aortic thrombectomy in a patient with a mobile thoracic aortic thrombus. Semin Cardiothorac Vasc Anesth 2011;15:176–8. 10.1177/1089253211415123 [DOI] [PubMed] [Google Scholar]
- 18.Criado E, Wall P, Lucas P, et al. Transesophageal echo-guided endovascular exclusion of thoracic aortic mobile thrombi. J Vasc Surg 2004;39:238–42. 10.1016/j.jvs.2003.07.017 [DOI] [PubMed] [Google Scholar]
- 19.Boufi M, Mameli A, Compes P, et al. Elective stent-graft treatment for the management of thoracic aorta mural thrombus. Eur J Vasc Endovasc Surg 2014;47:335–41. 10.1016/j.ejvs.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 20.Fayad ZY, Semaan E, Fahoum B, et al. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg 2013;27:282–90. 10.1016/j.avsg.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 21.Giovanni N, Daniela M, Giovanni M, et al. Endovascular treatment of thoracic aortic floating thrombus in patients presenting with acute lower limb ischemia. Int J Vasc Med 2011;2011:604362 10.1155/2011/604362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krüger T, Liske B, Ziemer S, et al. Thrombolysis to treat thrombi of the aortic arch. Clin Appl Thromb Hemost 2011;17:340–5. 10.1177/1076029610364519 [DOI] [PubMed] [Google Scholar]