Abstract
Many organisms can survive and proliferate in changing environmental temperatures. Here, we introduce a molecular physiological mechanism for cold tolerance and acclimation of the nematode Caenorhabditis elegans on the basis of previous reports and a new result. Three types of thermosensory neurons located in the head, ASJ, ASG, and ADL, regulate cold tolerance and acclimation. In ASJ, components of the light-signaling pathway are involved in thermosensation. In ASG, mechanoreceptor DEG-1 acts as thermoreceptor. In ADL, transient receptor potential channels are thermoreceptors; however, the presence of an additional unidentified thermoreceptor is also speculated. ADL thermoresponsivity is modulated by oxygen sensory signaling from URX oxygen sensory neurons via hub interneurons. ASJ releases insulin and steroid hormones that are received by the intestine, which results in lipid composition changing with cold tolerance. Additionally, the intestinal transcriptional alteration affects sperm functions, which in turn affects the thermosensitivity of ASJ; thus, the neuron–intestine–sperm–neuron tissue circuit is essential for cold tolerance.
Keywords: cold tolerance, thermoreceptor, DEG/ENaC, trimeric G-protein, sperm, oxygen
Introduction
Many organisms can adapt to environmental changes when they are consistently exposed to environmental stimuli. Among various environmental factors, temperature is one of the critically important factors. Modern living organisms are experiencing global warming, and it is expected that 15–37% of species will be close to extinction in 2050, because of increased ambient temperature.3) Therefore, elucidation of the mechanism of temperature tolerance and acclimation in organisms is an important topic.
In homeothermic animals, severe body temperature alterations trigger illness. Heatstroke or hypothermia is caused by abnormal alterations of body temperature in high or low temperatures, respectively. Rapid temperature change triggers acute blood pressure elevation and can cause brain or heart infarction. We therefore have a thermoregulation system to maintain general health. Moreover, temperature is directly involved in biological reactions. Enzyme activity generally increases in association with body temperature elevation. Consequently, homeothermic animals maintain appropriate body temperatures to maintain enzyme activity.
In animals, environmental temperature is received by thermoreceptor proteins in sensory neurons4) that transmit the information to the brain or other parts of the nervous system, which then send appropriate instructions to various tissues or organs, such as brown adipose tissue and skeletal muscles, throughout the body.5) Although the brain and other parts of nervous system play critically important roles in processing temperature information, the associated mechanisms remain unknown. Humans have an extremely complex brain comprised of approximately 86 billion neurons.6) In the human neocortex, there are more than 100 trillion synapses.7) Therefore, model animals such as fruit flies and nematodes are studied to reveal molecular physiological mechanisms involved in temperature sensing.8–10)
The nematode Caenorhabditis elegans has a simple neural circuit comprising only 302 neurons. All 302 neurons of C. elegans have been identified and their positions are almost identical between individuals. Complete neural connections have also been revealed by electron microscopy.1,2) Caenorhabditis elegans are therefore used as a model animal to analyze neural circuit systems. Previous studies indicated that C. elegans has various traits related to temperature response, such as thermotaxis behavior and tolerance to cold and heat.8,9,11–13) The molecular mechanisms and neuronal circuits have been gradually revealed by previous studies of these experimental systems. In this review, we discuss recent and previous findings on cold tolerance and acclimation that were revealed by analyzing C. elegans.
Temperature responses of worm and fly larvae
Although humans have various systems of thermoregulation to maintain general health, these mechanisms remain unclear. The nematode C. elegans is a model animal that is used to reveal thermal response dynamics. In addition to C. elegans, the fruit fly Drosophila melanogaster is classically used to analyze temperature sensation; therefore, we also introduce its thermosensory behavior here. Drosophila melanogaster larvae have the ability to sense thermal differences and can recognize their optimal temperature (18 ℃).10) Various molecules underlying thermosensory signaling cascades have been identified by examining temperature selection behavior of fly larvae. A transient receptor potential (TRP) channel, TRPA1, functions as a warm receptor in thermosensory neurons.14) Moreover, a gustatory receptor (Gr), Gr28b(D), has been identified as a candidate thermosensory molecule. Gr28b(D) is activated by temperature stimuli in D. melanogaster and is required for rapid warmth avoidance independent of TRPA1.15) A photoreceptor protein, rhodopsin, is also involved in temperature-seeking behavior of fly larvae.16,17)
Temperature response of C. elegans can be observed during various phenomena, such as thermotaxis behavior, cold-avoidance behavior, cold tolerance, and dauer larva formation. After cultivation with food, C. elegans migrates to the past cultivated temperature on a temperature gradient; this is defined as thermotaxis.8,9) In thermotaxis, environmental temperature information is received by AFD thermosensory neurons in the head, in which receptor-type guanylyl cyclases (GCs) encoded by three genes, gcy-8, gcy-18, and gcy-23, act as thermoreceptors.18) Cold-avoidance behavior is a phenomenon where cooling induces turning behavior during swimming. In cold avoidance, deleterious cold stimuli are received by GLR-3—a kainate-type glutamate receptor encoded by glr-3, which may activate Go-type trimeric G proteins without cation channel function.19)
The growing temperature of C. elegans ranges from 13 ℃ to 27 ℃. Above 27 ℃, the worm larvae become thin, black, and closed-mouth. These tolerant larvae are called dauer larvae; they can survive at high temperatures and starvation conditions for up to approximately 4 months by decreasing metabolic activity.20) At low temperatures, C. elegans can adapt to decreasing ambient temperature without dauer formation by changing cell membrane fluidity and metabolism. Worms exposed to low temperatures change lipid composition; in particular, they increase the unsaturated fatty acid percentage in the body.21)
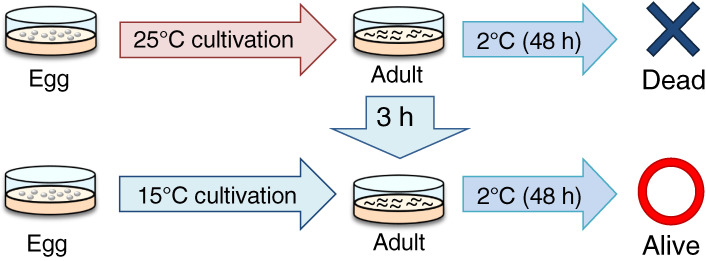
Caenorhabditis elegans shows cold tolerance that depends on cultivation temperature. For example, a worm grown at 25 ℃ cannot survive at 2 ℃ even though a worm grown at 15 ℃ can survive at 2 ℃ (Fig. 1).12,22) Worms can acclimate to new temperature after only a few hours and develop tolerance to cold, which is defined as cold acclimation. For example, after 25 ℃-grown worms were transferred to 15 ℃ for only 3–8 h, these worms could survive at 2 ℃ (Fig. 1).12,23,24)
Figure 1.
(Color online) Caenorhabditis elegans cold tolerance. The 25 ℃-cultivated wild-type individuals cannot survive at 2 ℃, whereas 15 ℃-cultivated wild-type individuals can survive at 2 ℃. However, 25 ℃-cultivated wild-type individuals can survive at 2 ℃ after cultivation at 15 ℃ for 3 h.
When we started studying cold tolerance of C. elegans, we initially considered two possibilities for associated molecular mechanisms. First, “all the somatic cells, not any specialized cell such as neurons, in the worms are affected by temperature stimuli”, by which they alter the ratio of saturated and unsaturated fatty acids, as previously known in plant and insects. The second possibility is that “specific thermosensory neurons or cells sense temperature” and transmit their information to other tissues or cells, thereby changing the composition of fatty acids in the body to acquire cold tolerance. If the cold tolerance of C. elegans is due to the latter system, the mechanisms underlying thermal processing of the nervous system could be clarified in a relatively short time using a simple experimental system that does not rely on behavior.
ASJ sensory neurons required for cold tolerance in C. elegans
Because of their cold tolerance and temperature acclimation, recent studies stated that C. elegans represents a useful experimental model to analyze the molecular physiology of thermosensation in sensory neurons and temperature signaling in tissue networks.13) However, whether specific cell(s) can determine cold tolerance status of the body had not been established when we started our C. elegans cold tolerance study. Therefore, we wanted to elucidate whether specific tissues are required for cold tolerance and whether known stress response molecules are required for cold tolerance.12) First, we measured cold tolerance of various mutants with specific impaired tissues, such as neurons, cuticle, and muscle cells,12) and we speculated that abnormality in any of the tissues could reduce cold tolerance. However, we unexpectedly found that the unc-104 mutant defective in kinesin expressed in almost all neurons showed abnormal enhancement of cold tolerance.12) Because the mutant defective in OSM-6, a cilium component expressed in almost all sensory neurons in the head, also showed enhanced cold tolerance, we proposed that specific sensory neurons negatively regulate cold tolerance.12) Additionally, the mutants defective in AFD and AWC thermosensory neurons, which are essential for thermotaxis behavior, showed normal cold tolerance. Therefore, we suggested that temperature sensation is probably performed by different sensory neuron(s) independent of the thermotaxis neural circuit.9,25,26)
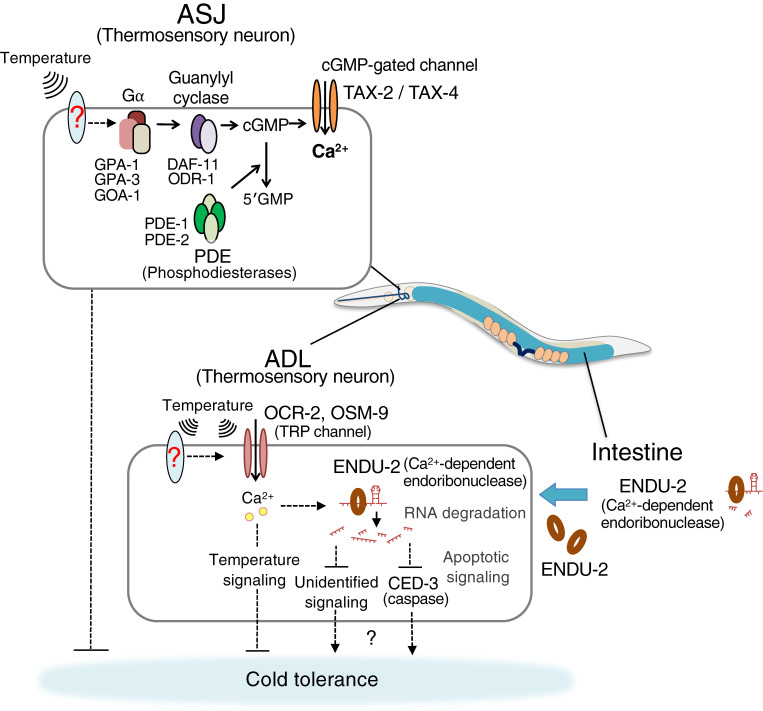
To identify specific sensory neurons that regulate cold tolerance, cold tolerance was analyzed in various sensory neuron mutants. It was found that 20 ℃-grown wild-type individuals cannot survive at 2 ℃; in contrast, tax-4 and tax-2 mutants impairing alpha and beta subunits, respectively, of cGMP-gated channel (CNGC) expressed in head sensory neurons showed abnormally enhanced cold tolerance.12) The enhanced cold tolerance of the tax-4 mutant was restored by expressing wild-type tax-4 in a pair of ASJ head sensory neurons, while the abnormality was not restored by expressing wild-type tax-4 in ASI or AWC sensory neurons involved in thermotaxis.12) ASJ sensory neurons are known to be light-sensing neurons27); therefore, we then measured cold tolerance of many mutants defective in the light-sensing pathway, such as the light receptor (LITE-1), trimeric G protein alpha subunits (Gα) (GPA-1, GPA-3, and GOA-1), GCs (DAF-11 and ODR-1), or phosphodiesterases (PDEs; PDE-1 and PDE-2) (Fig. 2). The Gα, GC, and PDE mutants showed abnormally enhanced cold tolerance, but the light receptor mutant lite-1 did not show any abnormality.12,28) We recognized that other receptor proteins independent of light receptors sense temperature, but ASJ has common sensory signaling molecules for temperature and light.
Figure 2.
(Color online) A model of temperature signaling in two head thermosensory neurons, ASJ and ADL. These neurons have different mechanisms underlying temperature response. In ASJ, Gα (GOA-1, GPA-1, and GPA-3), Guanylyl cyclase (DAF-11 and ODR-1), Phosphodiesterase (PDE-1 and PDE-2), and the cGMP-gated channel (TAX-4 and TAX-2) are required for cold tolerance. In ADL, TRP channels (OCR-2 and OSM-9) are required; moreover, ENDU-2, which is secreted from the intestine, may regulate neural activity of ADL. The TRP channels and other thermoreceptor molecule DEG-1 are not expressed in ASJ, suggesting that unidentified temperature receptors, such as GPCRs, act upstream of G protein-mediated signaling in ASJ.
The physiology of ASJ sensory neurons was analyzed by opto-genetics with genetically encodable calcium indicators, cameleon and GCaMP.12,28,29) Intracellular calcium concentration in ASJ increases with warming and decreases with cooling. ASJ thermal response was normal in a mutant with impaired SNB-1/synaptobrevin essential for synaptic transmission, indicating that ASJ thermal response does not require neurochemical input from other neurons.12) Moreover, ASJ temperature response in wild-type is altered by changing cultivation temperature; the ASJ thermal response of 25 ℃-grown worms is higher than that of 15 ℃-grown worms.12) This temperature dependency could be a modulation of thermal sensitivity in ASJ, but the underlying molecular mechanism is still unknown.
ASJ temperature response strongly decreased in tax-4 mutants defective in the cGMP-gated channel, and partially decreased in Gα, GC, and PDE mutants.12,28) Three Gα proteins, GOA-1, GPA-1, and GPA-3, are additively required for temperature response of ASJ; this was determined because the goa-1; gpa-3 gpa-1 triple mutant showed stronger abnormality in ASJ thermal response than that of each single mutant.28) In GCs and PDEs, multiple molecules are additively required in ASJ temperature signaling (Fig. 2).28) However, the light receptor LITE-1 is not required for ASJ thermosensation.12) Therefore, it is plausible that an unidentified temperature receptor upstream of Gα may sense temperature and sequentially activate Gα (GOA-1, GPA-1, and GPA-3), GC (DAF-11, and ODR-1), and CNGC (TAX-4/TAX-2), and PDEs (PDE-1, PDE-2) act as negative regulators (Fig. 2). Because the common upstream molecule for G protein-coupled signaling is thought to be a G protein-coupled receptor (GPCR), we speculate that an unidentified GPCR acts as a thermoreceptor in ASJ.
ADL thermosensory neurons involved in cold tolerance
DNA microarray analysis using mRNA of wild-type individuals upon temperature changes from 23 ℃ to 17 ℃ revealed that the expression levels changed for approximately 80 genes. Of these, the mutant of endu-2/M60.2, which encodes calcium-dependent endoribonuclease, showed strongly enhanced cold tolerance after cultivation at 20 ℃ or 25 ℃.12,30) ENDU-2 is homologous to human EndoU and Xenopus XendoU, which degrades RNA to generate small nucleolar RNA (snoRNA) that regulates ribosome biosynthesis.31,32) XendoU is located in the cytosol and on the surface of the endoplasmic reticulum (ER) and is required for nuclear-envelope assembly and formation of the tubular ER network.31) C. elegans ENDU-2 also functions as an endoribonuclease and regulates the volume of the ER.30) We reported that an ENDU-2::GFP fusion protein is located in intestinal cells, head sensory neurons, and various muscle cells, and enhanced cold tolerance of the endu-2 mutant was rescued by expressing wild-type endu-2cDNA in either muscle cells or ADL sensory neurons that received aversive chemical stimuli such as 1-octanol.30)
Calcium imaging with cameleon clarified that ADL acts as a temperature-sensing neuron, and the intracellular calcium concentration in ADL was found to increase with warming and decrease with cooling.30) Similar to ASJ thermosensory neurons, thermal calcium response of ADL is altered by changing cultivation temperature24,30); the ADL thermal response of 25 ℃-grown worms was higher than that of 15 ℃-grown worms, which indicated that the temperature sensitivity of ADL may be modulated depending on the cultivation temperature. The endu-2 mutant was defective in ADL temperature response.30) Transcriptome analysis of the endu-2 mutant and epistasis studies demonstrated that ENDU-2 inhibits apoptotic signaling via CED-3 caspase,30) and defective function of ADL in endu-2 mutants may be caused by hyperactivation of apoptotic signaling in ADL (Fig. 2).
We unexpectedly found that defective ADL thermal response of endu-2 mutants was rescued by expression of wild-type endu-2 in either ADL neurons or muscle cells.30) We then proposed that both cell autonomous and non-autonomous control of RNase ENDU-2 regulates ADL thermal sensitivity (Fig. 2).30) Qi et al. recently noted that RNase ENDU-2 behaves as a secretory protein, and that the secretion signal peptide comprised of the first 19 amino acids in ENDU-2 is essential for this phenomenon.33) Although the detailed molecular machinery involved in ENDU-2 secretion, such as packaging in exosomes or lysosomes, is unclear, ENDU-2 has been shown to be taken up by tissues other than the expressing cells.33) In the report by Qi et al., endu-2 is mainly expressed in intestinal cells.33) Then, the secreted RNase ENDU-2 from intestinal cells may non-autonomously affects ADL thermosensitivity.
The thermosensory mechanism in ADL neurons has been gradually revealed. Two TRP channels, OSM-9 and OCR-2, cooperatively function as a temperature receptor in ADL (Fig. 2),34) and another TRP, OCR-1, could be a negative regulator of OSM-9/OCR-2 in ADL (Fig. 3C).24) Ectopic expression of OSM-9 and OCR-2 in non-warm sensitive ASER gustatory neuron confers temperature responsiveness to ASER.34) A thermal stimuli-induced OSM-9/OCR-2-dependent current is detectable in Xenopus oocytes at the electrophysiological level,34) but this current is substantially smaller than that of the common thermosensitive TRP channel. Additionally, ADL of osm-9 ocr-2 double mutants respond to thermal stimuli including both warming and cooling34); therefore, unidentified temperature signaling probably acts in ADL. We propose an interesting model in which OSM-9/OCR-2 channel activity could be enhanced by unknown upstream molecules because various TRP channels are controlled by upstream G protein-mediated signaling through second messengers.35) The common upstream molecule for trimeric G proteins is thought to be a G protein-coupled receptor; therefore, unidentified temperature receptors could enhance OSM-9/OCR-2 temperature signaling in ADL (Fig. 2).
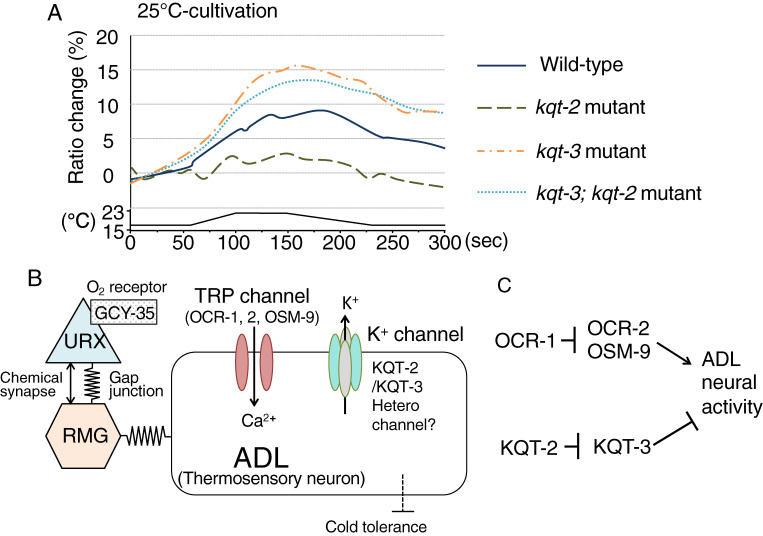
Figure 3.
(Color online) A molecular and neural circuit models for integrating temperature and oxygen signaling in cold acclimation. (A): Ca2+ imaging for ADL thermosensitivity in the mutants impairs potassium channels, KQT-2 and KQT-3. The relative increase or decrease in Ca2+ concentration in ADL of each strain is measured as a changing the YFP/CFP fluorescence ratio of the cameleon protein (Ratio change). These channels act in ADL thermal responsiveness. Data source: Okahata et al. (2019).24) (B): A neuronal circuit model for integrating temperature signaling of ADL with oxygen signaling from URX visceral oxygen sensory neurons via RMG hub interneurons. (C): ADL activity is positively regulated by TRP channels OCR-2 or OSM-9, and is negatively regulated by the potassium channel KQT-3. OSM-9/OCR-2 and KQT-3 are genetically inhibited by OCR-1 and KQT-2, respectively.
Potassium channels required for ADL thermal responsiveness have been identified. KQT-3, a KCNQ-type potassium channel, negatively regulates ADL activity as a well-known potassium channel function, and ADL thermal response of kqt-3 mutants was increased (Fig. 3A, 3B).24) KQT-2, which is another potassium channel in ADL, and ADL thermal response of kqt-2 mutants was unexpectedly decreased at the optical calcium imaging level (Fig. 3A).24) The decreased thermal response of kqt-2 mutants was suppressed by kqt-3 mutation, and excess production of KQT-2 in wild-type induced supra-normal ADL thermal response.24) Therefore, KQT-2 could act as a negative regulator of KQT-3 channel function with respect to thermosensitivity in ADL thermosensory neurons (Fig. 3C).
Head sensory neurons are surrounded by glial cells, and impaired glial function affects sensory neuronal function. PROS-1, a homeodomain transcription factor homologous to Drosophila prospero/mammalian Prox1, is required for glial development and normal morphology of sensory neurons in C. elegans.36) Because pros-1-knockdown worms showed abnormally enhanced-cold tolerance but ASJ thermal response was almost normal in pros-1-knockdown animals,36) it is possible that ADL thermal response is abnormal in pros-1-knockdown animals.
Cold acclimation is affected by oxygen signaling via KQT-type potassium channels
Previous DNA microarray analysis indicated that the expression levels of 79 genes changed as temperature changed.12) Among them, expression of the kqt-2 gene, which encodes a KCNQ-type potassium channel, increased when temperature decreased.12) The C. elegans genome contains three KQT-type potassium channels. KQT-2 and KQT-3 are expressed in neural cells, including ADL thermosensory neurons (Fig. 3B).24,37) Both kqt-2 and kqt-3 mutants showed abnormal cold acclimation and ADL thermoresponsivity. Moreover, the abnormal cold acclimation and ADL thermoresponsivity of kqt-2 mutants was suppressed by kqt-3 mutation.24) In mammals, KCNQ2 co-assembles with KCNQ3 as a heteromeric potassium channel.38) Similar to mammals, C. elegans KQT-2 may co-assemble with KQT-3 to modulate potassium channel activity (Fig. 3B).24)
The abnormal cold acclimation of kqt-2 mutants was stronger when kqt-2 mutants were cultivated in medium-size agar plates (diameter 6 cm) compared with smaller plates (diameter 3.5 cm). ADL receives information for both temperature and ascaroside, which is known as nematode pheromone. The ADL responsivity to ascaroside is modulated by oxygen concentration from URX oxygen sensory neurons that are located upstream of ADL.39) gcy-35 and gcy-36 encode guanylate cyclase, which function as oxygen receptors in URX.40,41) The oxygen information received by GCY-35 and GCY-36 is transmitted to RMG interneurons that function as the hub of the C. elegans neural circuit by connecting seven classes of sensory neurons, including URX and ADL.42) gcy-35 mutants showed decreased cold acclimation, and this mutation can suppress both abnormal cold acclimation and ADL thermoresponsivity in kqt-2 mutants. Oxygen and temperature signaling are integrated in ADL and determine the thermosensitivity and cold acclimation (Fig. 3B). This neural circuit could be a model for studying neural processing in integration and discrimination of multiple forms of sensory information.24)
DEG-1 is a temperature receptor in ASG sensory neurons
DNA microarray analysis using mRNA from C. elegans wild-type animals exposed to warming showed that flp-17 expression changed with warming.43) The flp-17(ok3587) null mutant showed severely decreased cold tolerance; however, another knockout mutation of flp-17 did not induce any cold tolerance abnormality.44) Moreover, the defective cold tolerance in flp-17(ok3587) was not restored by expressing wild-type flp-17 gene. We then proposed that any background mutation except ok3587 in flp-17(ok3587) mutants probably causes cold tolerance defects. Deep DNA sequencing and genetic analysis revealed that a chr1 mutation in xdh-1 caused cold tolerance defects.44) Actually, decreased cold tolerance abnormality of flp-17(ok3587) mutants was rescued by expressing wild-type xdh-1 gene, and the xdh-1 deletion mutant showed defective cold tolerance.44) We sometimes encountered cases in which one knockout mutant showed abnormal cold tolerance but another knockout mutant of the same gene did not. Therefore, we suggest that identification of the background mutation in such mutants is a useful strategy for isolating novel genes involved in cold tolerance because current deep DNA sequencing methods can easily provide the whole genome sequence for any mutant.
The XDH-1 protein, which is encoded by xdh-1 gene, is homologous to mammalian xanthine dehydrogenase (XDH), which catalyzes the hydroxylation of xanthine and its subsequent conversion to uric acid.44) Defective cold tolerance of xdh-1 mutants was rescued by expressing wild-type xdh-1 gene under simultaneously driven by the inx-17 and hlh-34 promoters. The inx-17 promoter drives gene expression in AIN interneurons. When we reported this result, hlh-34 promoter was thought to specifically drive the gene expression in AVJ interneurons45); however, Cook et al. recently reported that hlh-34 can be used to exclusively drive gene expression in the AVH interneuron pair.46) Therefore, we revised our model by the result in the hlh-34 promoter drives gene expression in the AVH interneurons, not AVJ interneurons (Fig. 4A).
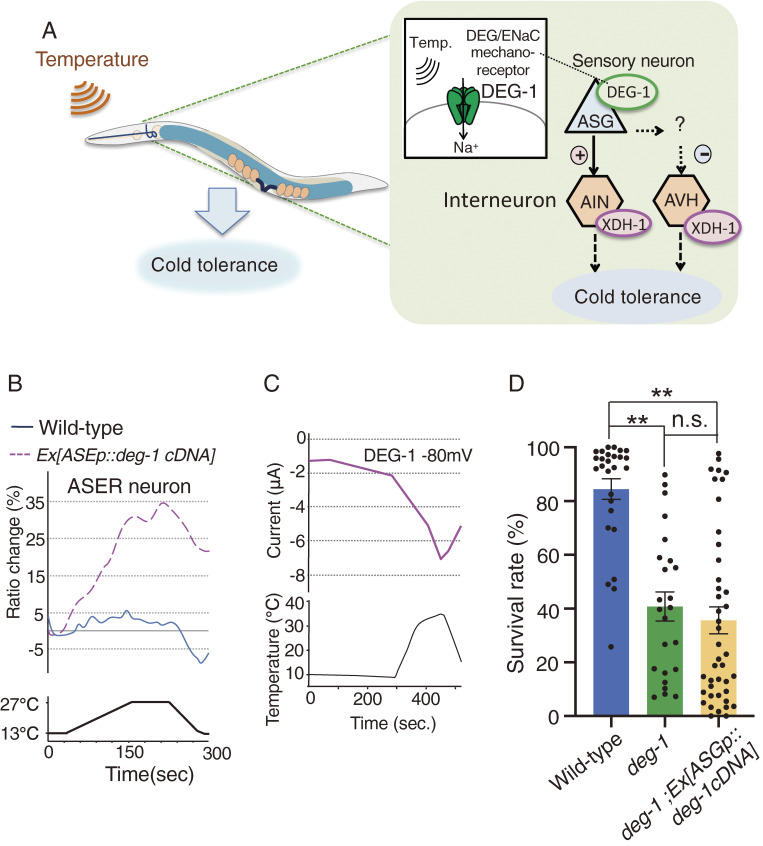
Figure 4.
A neural circuit model for positively regulated cold tolerance. (A): ASG thermosensory neurons positively and negatively control their downstream interneurons AIN and AVH, respectively. ASG receives temperature by DEG/ENaC mechanoreceptor DEG-1 and transmits temperature signaling to AIN and AVH interneurons, where xanthine dehydrogenase (XDH) positively regulates cold tolerance. (B): ASER neuron ectopically expressing DEG-1 responds to temperature stimuli, while ASER in wild-type did not responds to warming.44) The relative increase or decrease in Ca2+ concentration in ASER of each strain is measured as a change in the GCaMP8/RFP fluorescence ratio (Ratio change). Data source: Takagaki et al. (2020).44) (C): Temperature stimulus evoked internally directed Na+ current in Xenopus oocytes expressing DEG-1.44) Two-electrode voltage clamp method was used. Data source: Takagaki et al. (2020).44) (D): The data in this paper show that abnormal cold tolerance of the deg-1 mutant was not rescued by expressing deg-1cDNA in ASG neurons. 15 ℃-cultivated animals were transferred to 2 ℃ and stayed for 96 h. Number of assays ≥24. Error bar indicates SEM. **P < 0.01. n.s., not significant (P > 0.05). Comparison was performed using one-way ANOVA followed by Tukey–Kramer test.
Calcium imaging revealed that calcium concentrations of AIN and AVH interneurons changed in wild-type animals exposed to temperature changes. Activities are decreased in AIN and increased in AVH; then, XDH-1 plays opposite roles in regulating AIN and AVH activities (Fig. 4A).44) However, how xanthine metabolism induces these opposite neural regulations via XDH-1 is not yet clear.
Because the role of XDH-1 in regulating the activities of the specific interneurons was not clarified, we analyzed the neural circuits upstream of these interneurons from a different perspective. We speculated that temperature signaling from any upstream thermosensory neuron affects the interneuron activities. There were nine such neurons, five of which are mechanosensory neurons. We hypothesized that mechanosensory neurons act as thermosensory neurons, and that mechanosensory molecules are involved in cold tolerance. As expected, mutant animals with impaired mechanoreceptor proteins of the degenerin/epithelial Na+ channel (DEG/ENaC) demonstrated abnormally decreased cold tolerance.44) Among them, deg-1 mutants showed particularly severe cold tolerance dysfunction.
DEG-1 is expressed in at least ASG sensory neurons, which are the sensory neurons upstream of AIN or AVH interneurons (Fig. 4A). Calcium imaging indicated that ASG acts as thermosensory neurons, and the ASG temperature response is defective in deg-1 mutants.44) We hypothesized that the mechanoreceptor DEG-1 senses temperature in ASG sensory neurons. To determine whether DEG-1 senses temperature, DEG-1 was ectopically expressed in non-warm-sensitive ASER gustatory neurons. Calcium imaging with suggested that ASER lacking DEG-1 expression did not respond to warming, whereas ASER ectopically expressing DEG-1 strongly responded to temperature changes (Fig. 4B).44) Other DEG-1 expressing neurons AVG and PVC also responded to temperature changes.44) Although DEG-1 is a sodium channel, activation of DEG-1 probably induces calcium current via any voltage-gated calcium channels (VGCC) UNC-2 and/or UNC-36 which are expressed in most of C. elegans neurons including ASG, ASER, AVG and PVC. Consequently, ectopic expression of DEG-1 enabled temperature detection by otherwise non-warm-sensitive ASER neurons. Electrophysiological analysis using the two-electrode voltage clamp method with Xenopus oocytes demonstrated that thermal stimulus generated a sodium current in oocytes expressing DEG-1 (Fig. 4C), whereas a DEG/ENaC inhibitor, amiloride, inhibits DEG-1 currents.44) Surprisingly, the DEG-1 human homolog, MDEG1, similarly responds to temperature stimuli, resulting in sodium currents in Xenopus oocytes.44) Thus, the human MDEG1 and DEG-1 are proteins that are capable of detecting temperature (Fig. 4A).44)
Although DEG-1 probably acts as a temperature receptor, we describe new results here in which abnormal cold tolerance of deg-1 mutants was not rescued by expressing wild-type deg-1 gene in ASG of deg-1 mutants (Fig. 4D). Because DEG-1 is expressed in both ASG and various mechanosensory cells, it is probable that thermosensory function of DEG-1 in both other cell(s) and ASG is required for cold tolerance in wild-type individuals.
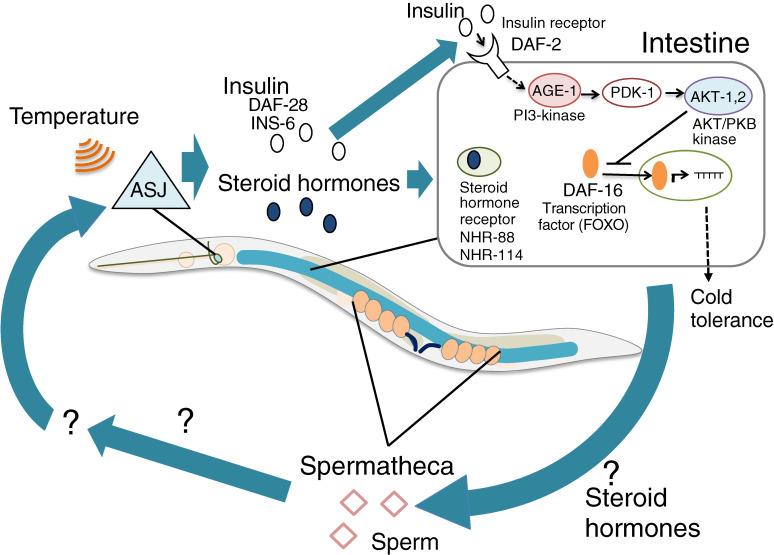
Insulin-like signaling from neurons to the intestine in cold tolerance
In addition to the analysis of thermosensory neurons, we investigated whether stress response signaling, such as insulin signaling and HSPs, are also involved in cold tolerance when we started our cold tolerance study in 2010. HSPs, the stress-responsive MAP kinase cascade, and HSFs are not yet known to be involved in cold tolerance.12) Savory et al. reported that cold tolerance is regulated by the molecules involved in an aging pathway that contains FOXO-type transcription factor DAF-16 and PI3 kinase AGE-1.47) As Ohta et al. described, the mutant with impaired insulin-like molecules DAF-28 and INS-6 showed enhanced cold tolerance, and this abnormality was restored by expressing DAF-28 in ASJ thermosensory neurons.12) Because DAF-28 is localized in presynaptic vesicles, DAF-28 insulin is probably secreted from ASJ synapses depending on thermosensation (Fig. 5).12) Longer calcium imaging of ASJ thermosensory neurons for 5 h exhibited a prolonged response of ASJ neurons to higher temperatures for over 30 min after warming.12) Because cold tolerance can change within 3 h after altering the cultivation temperature,24) insulin secretion from ASJ may continue for at least 30 min.
Figure 5.
(Color online) A model of the tissue network required for cold tolerance. ASJ sensory neurons receive temperature and release insulin and steroid hormones that are received by the intestine. In the intestine, the insulin signaling pathway containing AGE-1, PDK-1 and AKT-1, 2 negatively regulates the nuclear shuttling DAF-16/FOXO transcriptional factor that induces expression of cold tolerance genes. The intestine affects sperm-related tissues, which in turn positively control ASJ thermosensitivity, as a feedback network when developing cold tolerance.
The sole insulin receptor encoded by daf-2 gene in C. elegans is expressed in various tissues in the body, and daf-2 mutants showed abnormally enhanced cold tolerance after cultivation at both 20 ℃ and 25 ℃.12,43) Enhanced cold tolerance of daf-2 mutants cultivated at 25 ℃ was restored by expressing normal DAF-2 in both the intestine and neurons; however, the abnormality of daf-2 mutants was not restored after cultivation at 20 ℃.12) Therefore, DAF-2 in other tissue(s) is required for normal cold tolerance.
DAF-2 sequentially activates PI3 kinase AGE-1, PDK kinase PDK-1, and AKT kinase AKT-1,2, which probably negatively regulate nuclear shuttling of FOXO transcriptional factor DAF-16, resulting in the inhibition of cold tolerance (Fig. 5). Our previous report show that insulin secreted from the thermosensory neuron ASJ is received by the intestine, and that the fatty acid composition of the intestinal insulin receptor mutant daf-2 is slightly different from that of the wild-type strain,12) but it is not yet known what causes the difference between life and death in low-temperature conditions. Subsequently, downstream events of insulin signaling, such as gene expression and changing cell metabolism in the intestine and neurons, may determine cold tolerance status within 3 h. Because accumulation of unsaturated fatty acids in the intestine and glycerol accumulation are involved in cold tolerance via a desaturase and a hormone-sensitive lipase HOSL-1, respectively,21,47,48) these phenomena could be regulated by insulin signaling in the intestine. Intestinal TRP channel (TRPA-1) and its downstream protein kinase C (PKC-2) are involved in cold sensation in the aging,49) but these mutant animals showed normal cold tolerance after cultivation at 15 ℃ or 20 ℃.12)
Sperm affects temperature sensing in thermosensory neurons
To identify downstream molecules of insulin signaling in cold tolerance, temperature-dependent gene expression changes in daf-2 mutants were measured with DNA microarray. Expression levels of over 1500 genes were altered, and the majority of genes are expressed in neurons, the intestine, and reproductive tissues. In the reproductive tissue genes, the expression levels of sperm-related genes were mainly altered, rather than genes associated with oocytes and gonads.43) Lack of gsp-3 and gsp-4 genes, which encode sperm-specific protein phosphatase PP1, resulted in abnormally enhanced cold tolerance after cultivation at 20 ℃. This abnormality in hermaphrodite gsp-4 mutants was restored by mating with wild-type males, which indicated that normal sperm are required for cold tolerance.43) Genetic epistasis and quantitative PCR studies revealed that sperm gsp-4 is downstream of daf-2-mediated insulin signaling and steroid hormonal signaling from ASJ to the intestine through NHR-88 and NHR-114 (Fig. 5).43)
Tissue networks associated with cold tolerance have been gradually revealed by genetic analysis. The gsp-4 supra-normal cold tolerance was suppressed by mutations in thermosensation of head thermosensory neurons, such as Gα, GPA-3, and GC ODR-1, which are required for thermosensory signaling in ASJ.28,43) ASJ thermosensitivity was severely decreased in gsp-4 mutants, and this abnormality was rescued by specifically expressing wild-type gsp-4 gene in sperm. These findings indicate that GSP-4 in sperm affects thermosensory activity of ASJ neurons in the head.43) Thus, temperature information received by ASJ is transmitted to the intestine via insulin and steroid hormones; then, the information is sent from the intestine to the sperm, after which the sperm in turn influences the neural activity of ASJ as feedback machinery via an unidentified secretory pathway (Fig. 5).
How sperm is involved in cold tolerance is still unknown, but sperm motility or development maybe essential for establishing cold tolerance. PP1s, GSP-3 and GSP-4, regulate sperm motility and development via the major sperm proteins (MSPs), and knockdown of MSPs induced supra-normal cold tolerance.43) MSPs are cytoskeletal elements that are abundantly contained in the pseudopod, which is critical for the flagella/actin-independent motility of C. elegans sperm. We hypothesize that MSP-dependent sperm motility or development could affect tissue around the sperm, such as the sperm sac, which could release secretory signaling to the thermosensory neurons in the head.
Diversity of cold tolerance and acclimation in various nematode species
Nematodes live in various regions worldwide and are known to be the most populous animal on Earth. The northern root-knot nematode, Meloidogyne hapla, is widely distributed in cold regions, in which the average monthly temperature ranges from −15 ℃ to 27 ℃.50) They are parasitic on plants and survive the winter in soil or diseased roots. Meloidogyne hapla can withstand cold temperatures as second-stage juveniles. Moreover, M. hapla eggs can survive down to −18 ℃.51) An Antarctic nematode, Panagrolaimus sp. DAW1, is a unique multicellular organism that is known to tolerate intracellular freezing on a routine basis.52,53) Cold-acclimated DAW1 animals store trehalose by upregulating tps-2 and lea-1, which are trehalose synthesis genes.54) Additionally, a parasitic nematode, Marshallagia marshalli, infects reindeer during the winter season in subfreezing conditions. Their eggs are able to live and develop even when they are exposed to subfreezing conditions for 28 months. Their infective-stage larvae can survive even if they are exposed to −30 ℃ or cultivated at 5 ℃ for 80 days.55) Moreover, an entomopathogenic nematode, Steinernema feltiae, is also freezing-tolerant and able to withstand extensive intracellular freezing56); they can survive intracellular freezing by increasing trehalose and glycerol levels.56)
Caenorhabditis briggsae, which is closely related to C. elegans, exhibits different temperature responses based on locality. Wild C. briggsae isolated from temperate localities have more cold tolerance than strains isolated from tropical localities.57) Moreover, C. briggsae isolated from tropical localities have greater fecundity when cultivated at high temperatures and lower fecundity at lower temperatures than strains isolated temperate localities.58)
Natural wild-type C. elegans variants also have various cold tolerance and acclimation phenotypes, which is probably caused by their gene polymorphisms.23) AB1 strain from Australia rapidly acclimates to lower temperatures, whereas CB4856 strain from Hawaii requires more time to acclimate to lower temperatures.23) The gene polymorphisms responsible for rapid cold acclimation in AB1 have been mapped onto the middle of chromosome I.23) Identification of the gene polymorphism(s) responsible for cold acclimation diversity of C. elegans should be useful for investigating the mechanisms underlying the diversity of temperature responses among animal species.
Future perspectives
Caenorhabditis elegans cold tolerance and acclimation has become a high-throughput experimental system for analyzing thermo-sensation and animal temperature response. Initially, the system was designed to analyze metabolism, but it is now expected to become useful for studying biological systems from the perspective of multi-organ cooperation, which is currently an important topic in life sciences. The 2021 Nobel Prize in Physiology and Medicine was awarded for the discovery of temperature-sensitive TRP channels. We expect to find more novel thermoreceptors using this experimental system of C. elegans cold tolerance and acclimation. In addition, this system may lead to the discovery of new molecular physiological mechanisms involved in temperature adaptation in the body. Thus, the dynamic field of discovering novel molecular physiological mechanisms in animal cryobiology, starting with C. elegans, provides a thought-provoking paradigm for understanding animal biology as a whole, including human biology. Moreover, such information can also provide a molecular basis for new medical fields, such as long-term cold preservation of organs for transplant, which is desperately needed.
Methods
Ethical issues and approval.
All animal treatments in this research were performed in accordance with the Japanese Act on Welfare and Management of Animals (Act No. 105 of October 1, 1973; latest revisions, Act No. 51 of June 2, 2017, effective June 1, 2018). All experimental protocols were approved by the Institutional Animal Care and Use Committees of Konan University.
Caenorhabditis elegans strains.
The wild-type N2 (Bristol) and deg-1(u38) mutant strains were used in the experiment shown in Fig. 4D.
Statistical analysis.
Statistical analyses were performed using ANOVA followed by Tukey–Kramer post-hoc test for multiple comparisons (Fig. 4D). See Supplemental Dataset S1 for further details on raw data and statistical figures.
Cold tolerance assay.
The cold tolerance assay was performed according to previous reports.12,22,44) In the cold tolerance assay, we cultivated well-fed young adult C. elegans on 2% (w/v) agar nematode growth medium, which was seeded with Escherichia coli OP50, for 16–24 h at 15 ℃; then, they were removed. Subsequently, the progenies were cultivated to maturity for 120–130 h at 15 ℃. These plates were placed on ice for 20 min and transferred to a 2 ℃ refrigerated cabinet (CRB-41A Hitachi, Japan) for 96 h. After applying the cold stimulus, plates were stored at 15 ℃ overnight. We then counted living and dead worms on the plate to calculate survival rates.
Molecular biology.
pNTN126 (gcy-21p::deg-1cDNA) was created by replacing the gcy-5 promoter in gcy-5p::deg-1cDNA (pNTN106) with the gcy-21 promoter, which contains a 1,403-bp upstream sequence for the initial start codon of gcy-21.
Data availability
The datasets generated during in this study are available from the corresponding author on reasonable request.
Acknowledgements
We thank Natsune Takagaki for providing supporting experiments. We thank Mallory Eckstut, PhD, from Edanz for editing a draft of this manuscript.
Funding
A.K. and A.O. were supported by the Kinoshita Memorial Foundation, Naito Foundation, Takeda Science Foundation, Suzuken Memorial Foundation, and Hirao Taro Foundation of KONAN GAKUEN for Academic Research. A.K. was supported by the Asahi Glass Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, NOVARTIS Foundation for the Promotion of Science, AMED PRIME (21gm6510004h0001), and JSPS KAKENHI [21H02534, 21K19279, 20H05074 (Brain information dynamics)]. A.O., M.O., and H.M. were supported by JSPS KAKENHI [18K06344, 19J40017, 21K06275 (A.O.), 20J30004 (M.O.), 21J20026 (H.M.)]. A.O. and M.O. was supported by the Hyogo Science and Technology Association.
Non-standard abbreviation list
- AGE
ageing alteration
- AKT
AKT serine/threonine kinase
- C. elegans
Caenorhabditis elegans
- cDNA
complementary DNA
- CED
cell death abnormality
- CFP
cyan fluorescent protein
- cGMP
cyclic guanosine monophosphate
- CNGC
cyclic nucleotide gated channel
- DAF
abnormal Dauer Formation
- DEG/ENaC
degenerin/epithelial sodium channel
- EndoU
Endoribonuclease
- ENDU
ENDonuclease, poly(U) specific
- ER
endoplasmic reticulum
- FLP
FMRF-like peptide
- FOXO
forkhead box-containing protein O sub-family
- Gα
G protein alpha subunit
- GC
guanylyl cyclase
- GCaMP
GFP-calmodulin fusion protein
- GCY
guanylyl cyclase
- GFP
green fluorescent protein
- GLR
glutamate receptor
- GMP
guanosine monophosphate
- GOA
G protein O alpha subunit
- Gr28b(D)
gustatory receptor 28b (Drosophila)
- GPA
G protein alpha subunit
- GPCR
G protein coupled receptor
- GSP
GLC7 (yeast Glc seven) like phosphatase
- HLH
helix loop helix
- HOSL
hormone-sensitive lipase homolog
- HSF
heat shock factor
- HSP
heat shock protein
- INS
insulin related
- INX
innexin
- KQT
K+ channel related to QT interval
- LEA
plant late embryo abundant (LEA) related
- LITE
high-energy light unresponsive
- MAP kinase
mitogen-activated protein kinase
- MSPs
major sperm proteins
- NHR
nuclear hormone receptor
- OCR
Osm-9 and capsaicin receptor-Related
- ODR
odorant response abnormal
- OSM
osmotic avoidance abnormal
- PCR
polymerase chain reaction
- PDE
phosphodiesterase
- PDK
3-phosphoinositede-dependent protein kinase1
- PI3 kinase
phosphoinositide 3-kinase
- PKC
protein kinaseC
- PP
protein phosphatase
- PROS
prospero homeobox homolog
- SNB
synaptobrevin related
- snoRNA
small nucleolar RNA
- TAX
abnormal chemotaxis
- TPS
trehalose 6-phosphate synthase
- TRP
transient receptor potential
- TRPA
TRPA cation channel homolog
- UNC
uncoordinated
- XDH
xanthine dehydrogenase
- YFP
yellow fluorescent protein
- ADL, AFD, AIN, ASER, ASG, ASJ, ASI, AVG, AVH, AVJ, AWC, PVC, RMG, URX
these indicate identical neurons of C. elegans that are designated by a three-letter code with additional letters added to denote positional differences between otherwise identical cells, e.g., R for right and L for left.1,2)
Profile
Atsushi Kuhara was born in Nagoya in 1976. He graduated from Nagoya University and the Graduate School of Science, Nagoya University. He studied the molecular mechanism of thermotaxis behavior of C. elegans. After being awarded his Ph.D. degree in 2002, he became an Assistant Professor in the Department of Life Science at the Graduate School of Science, Nagoya University. He explored the molecules and physiological system of temperature signaling in thermotaxis of C. elegans. Since 2011, he has served as a Lecturer (2011–2013), Associate Professor (2013–2017) and Professor (2017–2022), Department of Biology, Graduate School of Natural Science and Faculty of Science and Engineering, Konan University, focusing on cold tolerance and temperature acclimation of C. elegans. He revealed that the thermo-receptor DEG/ENaC and trimeric G protein-coupled temperature signaling in thermo-sensation of cold tolerance and temperature acclimation. He was awarded the JSPS Prize in 2018 and Kao Science Award in 2021.
References
- 1).White J.G., Southgate E., Thomson J.N., Brenner S. (1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340. [DOI] [PubMed] [Google Scholar]
- 2).Cook S.J., Jarrell T.A., Brittin C.A., Wang Y., Bloniarz A.E., Yakovlev M.A., et al. (2019) Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 571, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Thomas C.D., Cameron A., Green R.E., Bakkenes M., Beaumont L.J., Collingham Y.C., et al. (2004) Extinction risk from climate change. Nature 427, 145–148. [DOI] [PubMed] [Google Scholar]
- 4).Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- 5).Nakamura K. (2011) Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1207–R1228. [DOI] [PubMed] [Google Scholar]
- 6).Azevedo F.A., Carvalho L.R., Grinberg L.T., Farfel J.M., Ferretti R.E., Leite R.E., et al. (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532–541. [DOI] [PubMed] [Google Scholar]
- 7).Tang Y., Nyengaard J.R., De Groot D.M., Gundersen H.J. (2001) Total regional and global number of synapses in the human brain neocortex. Synapse 41, 258–273. [DOI] [PubMed] [Google Scholar]
- 8).Hedgecock E.M., Russell R.L. (1975) Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 72, 4061–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Mori I., Ohshima Y. (1995) Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376, 344–348. [DOI] [PubMed] [Google Scholar]
- 10).Sayeed O., Benzer S. (1996) Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 93, 6079–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Ohta A., Kuhara A. (2013) Molecular mechanism for trimeric G protein-coupled thermosensation and synaptic regulation in the temperature response circuit of Caenorhabditis elegans. Neurosci. Res. 76, 119–124. [DOI] [PubMed] [Google Scholar]
- 12).Ohta A., Ujisawa T., Sonoda S., Kuhara A. (2014) Light and pheromone-sensing neurons regulates cold habituation through insulin signalling in Caenorhabditis elegans. Nat. Commun. 5, 4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Takeishi A., Takagaki N., Kuhara A. (2020) Temperature signaling underlying thermotaxis and cold tolerance in Caenorhabditis elegans. J. Neurogenet. 34, 351–362. [DOI] [PubMed] [Google Scholar]
- 14).Viswanath V., Story G.M., Peier A.M., Petrus M.J., Lee V.M., Hwang S.W., et al. (2003) Opposite thermosensor in fruitfly and mouse. Nature 423, 822–823. [DOI] [PubMed] [Google Scholar]
- 15).Ni L., Bronk P., Chang E.C., Lowell A.M., Flam J.O., Panzano V.C., et al. (2013) A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 500, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Shen W.L., Kwon Y., Adegbola A.A., Luo J., Chess A., Montell C. (2011) Function of rhodopsin in temperature discrimination in Drosophila. Science 331, 1333–1336. [DOI] [PubMed] [Google Scholar]
- 17).Sokabe T., Chen H.C., Luo J., Montell C. (2016) A switch in thermal preference in Drosophila larvae depends on multiple rhodopsins. Cell Rep. 17, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Takeishi A., Yu Y.V., Hapiak V.M., Bell H.W., O’Leary T., Sengupta P. (2016) Receptor-type guanylyl cyclases confer thermosensory responses in C. elegans. Neuron 90, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Gong J., Liu J., Ronan E.A., He F., Cai W., Fatima M., et al. (2019) A cold-sensing receptor encoded by a glutamate receptor gene. Cell 178, 1375–1386.e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Cassada R.C., Russell R.L. (1975) The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46, 326–342. [DOI] [PubMed] [Google Scholar]
- 21).Murray P., Hayward S.A., Govan G.G., Gracey A.Y., Cossins A.R. (2007) An explicit test of the phospholipid saturation hypothesis of acquired cold tolerance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 104, 5489–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Ohta A., Kuhara A., Ujisawa T., Okahata M., Sonoda S. (2014) Cold tolerance assay for studying cultivation-temperature-dependent cold habituation in C. elegans. Protocol Exchange , doi:10.1038/protex.2014.032. [Google Scholar]
- 23).Okahata M., Ohta A., Mizutani H., Minakuchi Y., Toyoda A., Kuhara A. (2016) Natural variations of cold tolerance and temperature acclimation in Caenorhabditis elegans. J. Comp. Physiol. B 186, 985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Okahata M., Wei A.D., Ohta A., Kuhara A. (2019) Cold acclimation via the KQT-2 potassium channel is modulated by oxygen in Caenorhabditis elegans. Sci. Adv. 5, eaav3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Kuhara A., Okumura M., Kimata T., Tanizawa Y., Takano R., Kimura K.D., et al. (2008) Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 320, 803–807. [DOI] [PubMed] [Google Scholar]
- 26).Kuhara A., Ohnishi N., Shimowada T., Mori I. (2011) Neural coding in a single sensory neuron controlling opposite seeking behaviours in Caenorhabditis elegans. Nat. Commun. 2, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Liu J., Ward A., Gao J., Dong Y., Nishio N., Inada H., et al. (2010) C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat. Neurosci. 13, 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Ujisawa T., Ohta A., Uda-Yagi M., Kuhara A. (2016) Diverse regulation of temperature sensation by trimeric G-protein signaling in Caenorhabditis elegans. PLoS One 11, e0165518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Zhang B., Gong J., Zhang W., Xiao R., Liu J., Xu X.Z.S. (2018) Brain-gut communications via distinct neuroendocrine signals bidirectionally regulate longevity in C. elegans. Genes Dev. 32, 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Ujisawa T., Ohta A., Ii T., Minakuchi Y., Toyoda A., Ii M., et al. (2018) Endoribonuclease ENDU-2 regulates multiple traits including cold tolerance via cell autonomous and nonautonomous controls in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 115, 8823–8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Schwarz D.S., Blower M.D. (2014) The calcium-dependent ribonuclease XendoU promotes ER network formation through local RNA degradation. J. Cell Biol. 207, 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Laneve P., Gioia U., Ragno R., Altieri F., Di Franco C., Santini T., et al. (2008) The tumor marker human placental protein 11 is an endoribonuclease. J. Biol. Chem. 283, 34712–34719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Qi W., Gromoff E.D.V., Xu F., Zhao Q., Yang W., Pfeifer D., et al. (2021) The secreted endoribonuclease ENDU-2 from the soma protects germline immortality in C. elegans. Nat. Commun. 12, 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Ohnishi K., Saito S., Miura T., Ohta A., Tominaga M., Sokabe T., et al. (2020) OSM-9 and OCR-2 TRPV channels are accessorial warm receptors in Caenorhabditis elegans temperature acclimatisation. Sci. Rep. 10, 18566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Minke B., Cook B. (2002) TRP channel proteins and signal transduction. Physiol. Rev. 82, 429–472. [DOI] [PubMed] [Google Scholar]
- 36).Kage-Nakadai E., Ohta A., Ujisawa T., Sun S., Nishikawa Y., Kuhara A., et al. (2016) Caenorhabditis elegans homologue of Prox1/Prospero is expressed in the glia and is required for sensory behavior and cold tolerance. Genes Cells 21, 936–948. [DOI] [PubMed] [Google Scholar]
- 37).Wei A.D., Butler A., Salkoff L. (2005) KCNQ-like potassium channels in Caenorhabditis elegans. Conserved properties and modulation. J. Biol. Chem. 280, 21337–21345. [DOI] [PubMed] [Google Scholar]
- 38).Wang H.S., Pan Z., Shi W., Brown B.S., Wymore R.S., Cohen I.S., et al. (1998) KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282, 1890–1893. [DOI] [PubMed] [Google Scholar]
- 39).Fenk L.A., de Bono M. (2017) Memory of recent oxygen experience switches pheromone valence in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 114, 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Cheung B.H., Arellano-Carbajal F., Rybicki I., de Bono M. (2004) Soluble guanylate cyclases act in neurons exposed to the body fluid to promote C. elegans aggregation behavior. Curr. Biol. 14, 1105–1111. [DOI] [PubMed] [Google Scholar]
- 41).Gray J.M., Karow D.S., Lu H., Chang A.J., Chang J.S., Ellis R.E., et al. (2004) Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322. [DOI] [PubMed] [Google Scholar]
- 42).Macosko E.Z., Pokala N., Feinberg E.H., Chalasani S.H., Butcher R.A., Clardy J., et al. (2009) A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458, 1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Sonoda S., Ohta A., Maruo A., Ujisawa T., Kuhara A. (2016) Sperm affects head sensory neuron in temperature tolerance of Caenorhabditis elegans. Cell Rep. 16, 56–65. [DOI] [PubMed] [Google Scholar]
- 44).Takagaki N., Ohta A., Ohnishi K., Kawanabe A., Minakuchi Y., Toyoda A., et al. (2020) The mechanoreceptor DEG-1 regulates cold tolerance in Caenorhabditis elegans. EMBO Rep. 21, e48671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Cunningham K.A., Hua Z., Srinivasan S., Liu J., Lee B.H., Edwards R.H., et al. (2012) AMP-activated kinase links serotonergic signaling to glutamate release for regulation of feeding behavior in C. elegans. Cell Metab. 16, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Cook S.J., Vidal B., Hobert O. (2021) The bHLH-PAS gene hlh-34 is expressed in the AVH, not AVJ interneurons. MicroPubl. Biol. 2021, doi:10.17912/micropub.biology.000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Savory F.R., Sait S.M., Hope I.A. (2011) DAF-16 and Delta9 desaturase genes promote cold tolerance in long-lived Caenorhabditis elegans age-1 mutants. PLoS One 6, e24550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Liu F., Xiao Y., Ji X.L., Zhang K.Q., Zou C.G. (2017) The cAMP-PKA pathway-mediated fat mobilization is required for cold tolerance in C. elegans. Sci. Rep. 7, 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Xiao R., Zhang B., Dong Y., Gong J., Xu T., Liu J., et al. (2013) A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152, 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Taylor, A.L., Sasser, J.N., Nelson, L.A. and International Meloidogyne Project (1982) Relationship of Climate and Soil Characteristics to Geographical Distribution of Meloidogyne Species in Agricultural Soils. International Meloidogyne Project. Available from Dept. of Plant Pathology, North Carolina State University, Raleigh, N.C., U.S.A.
- 51).Wu X., Zhu X., Wang Y., Liu X., Chen L., Duan Y. (2018) The cold tolerance of the northern root-knot nematode, Meloidogyne hapla. PLoS One 13, e0190531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Wharton D.A., Brown I.M. (1991) Cold-tolerance mechanisms of the Antarctic nematode Panagrolaimus davidi. J. Exp. Biol. 155, 629–641. [DOI] [PubMed] [Google Scholar]
- 53).Wharton D., Ferns D. (1995) Survival of intracellular freezing by the Antarctic nematode Panagrolaimus davidi. J. Exp. Biol. 198, 1381–1387. [DOI] [PubMed] [Google Scholar]
- 54).Seybold A.C., Wharton D.A., Thorne M.A.S., Marshall C.J. (2017) Investigating trehalose synthesis genes after cold acclimation in the Antarctic nematode Panagrolaimus sp. DAW1. Biol. Open 6, 1953–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Carlsson A.M., Irvine R.J., Wilson K., Coulson S.J. (2013) Adaptations to the Arctic: low-temperature development and cold tolerance in the free-living stages of a parasitic nematode from Svalbard. Polar Biol. 36, 997–1005. [Google Scholar]
- 56).Ali F., Wharton D.A. (2015) Infective juveniles of the entomopathogenic nematode, Steinernema feltiae produce cryoprotectants in response to freezing and cold acclimation. PLoS One 10, e0141810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Wang W., Flury A.G., Garrison J.L., Brem R.B. (2021) Cold survival and its molecular mechanisms in a locally adapted nematode population. Genome Biol. Evol. 13, evab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Prasad A., Croydon-Sugarman M.J., Murray R.L., Cutter A.D. (2011) Temperature-dependent fecundity associates with latitude in Caenorhabditis briggsae. Evolution 65, 52–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during in this study are available from the corresponding author on reasonable request.