Abstract
Mechanical ventilation may cause ventilator-induced lung injury (VILI) in patients requiring ventilator support. Inhibition of autophagy is an important approach to ameliorate VILI as it always enhances lung injury after exposure to various stress agents. This study aimed to further reveal the potential mechanisms underlying the effects of geranylgeranyl diphosphate synthase large subunit 1 (GGPPS1) knockout and autophagy in VILI using C57BL/6 mice with lung-specific GGPPS1 knockout that were subjected to mechanical ventilation. The results demonstrate that GGPPS1 knockout mice exhibit significantly attenuated VILI based on the histologic score, the lung wet-to-dry ratio, total protein levels, neutrophils in bronchoalveolar lavage fluid, and reduced levels of inflammatory cytokines. Importantly, the expression levels of autophagy markers were obviously decreased in GGPPS1 knockout mice compared with wild-type mice. The inhibitory effects of GGPPS1 knockout on autophagy were further confirmed by measuring the ultrastructural change of lung tissues under transmission electron microscopy. In addition, knockdown of GGPPS1 in RAW264.7 cells reduced cyclic stretch-induced inflammation and autophagy. The benefits of GGPPS1 knockout for VILI can be partially eliminated through treatment with rapamycin. Further analysis revealed that Rab37 was significantly downregulated in GGPPS1 knockout mice after mechanical ventilation, while it was highly expressed in the control group. Simultaneously, Rab37 overexpression significantly enhances autophagy in cells that are treated with cyclin stretch, including GGPPS1 knockout cells. Collectively, our results indicate that GGPPS1 knockout results in reduced expression of Rab37 proteins, further restraining autophagy and VILI.
Keywords: GGPPS1, Ventilator-induced lung injury, Inflammation, Autophagy, Rab37
Introduction
Protective ventilation is currently applied in a one-size-fits-all manner, and although this practical strategy has reduced the incidence of death due to acute respiratory distress syndrome (ARDS), mortality is still high and improvements are at a standstill [1]. Furthermore, it remains poorly understood how ventilator-induced lung injury (VILI) can be minimized for any given lung, and the issue is, moreover, controversial. Mechanical ventilation in patients with acute lung injury can exacerbate lung dysfunction and damage through the inflammatory response pathway [2, 3]. The complexity of and various factors involved in the pathogenesis of ARDS make the development of effective targeted strategies for the control of further inflammation and damage extremely difficult. Recent studies have found that autophagy is closely related to the key processes underlying lung damage and inflammation, such as microvascular barrier dysfunction [4], endothelial cell barrier dysfunction [5], oxidative stress [6], and macrophage-mediated lung inflammation [7]. Autophagy may play a key role in the mechanisms underlying VILI and the autophagic machinery could potentially be targeted therapeutically [8].
Our previous studies revealed that geranylgeranyl diphosphate synthase (GGPPS) large subunit 1 (GGPPS1) contributes to pulmonary inflammation and disease progression in pulmonary fibrosis [9] and alleviates lung injury in VILI mice [10]. However, whether GGPPS1 exerts its biological function in VILI via regulation of autophagy is still unclear. Therefore, a better understanding of the mechanisms underlying GGPPS1-mediated inflammation and injury is crucial for the development of improved therapies for acute lung injury. Here, we demonstrate that GGPPS1-mediated autophagy is involved in VILI inflammation pathogenesis and could serve as a theoretical basis upon which minimally injurious mechanical ventilation strategies might be developed.
In the present study, we investigate the ventilator-triggered autophagy response both in cultured cells and in a VILI mouse model. This study also provides a foundation for further research on GGPPS1–Rab37-mediated and inflammation-related autophagy signaling pathways in VILI.
Materials and methods
Animals and VILI model
SPF-grade male C57BL/6 mice (10 weeks old, 22–25 g) were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). All animal experiments were approved by the Animal Care and Use Committee of the Affiliated Jiangning Hospital of Nanjing Medical University. Animal were fed as described in our previously published paper [10]. C57BL/6 mice with lung-specific GGPPS1 knockout (GGPPS1spc−rtTA−teTO−cre−floxP/floxP, GGPPS1−/−) were kindly provided by the Laboratory of Prof. Li Chaojun (Model Animal Research Center and the Medical School of Nanjing University) as a gift.
To construct the VILI animal model, mice were subjected to mechanical ventilation with prior anesthesia by intraperitoneal injections of pentobarbitone (90 mg/kg). In brief, the mice were fixed in a supine position, the neck skin was incised, and the trachea was exposed. Mice in the model group were ventilated with a tidal volume of 28 ml/kg for 4 h by the ventilator (ALC-V8, Alcott Biotech Co., Ltd., Shanghai, China). Mechanical ventilation was conducted with a respiratory rate of 60 breaths/min, an inspiratory:expiratory ratio of 1:1, an inspired O2 concentration of 0.21, and a positive end-expiratory pressure of 0 cm H2O. To evaluate the effects of autophagy on VILI, mice were intravenously injected with 30 mg/kg 3-MA (Sigma-Aldrich, St. Louis, MO, USA) or 8 mg/kg rapamycin (RAPA, Sigma-Aldrich) following 1 h of mechanical ventilation. An equal volume of saline was intravenously injected as a blank control. Finally, the mice were killed by cervical dislocation, and the lung tissues and the bronchoalveolar lavage fluid (BALF) were collected for the following experiments.
Tissue processing
The collected right lung upper lobe was fixed with 10% neutral formalin, dehydrated, and embedded. The tissues were sliced into 4-µm-thick sections and stained with hematoxylin and eosin (HE). The stained tissues were photographed under an optical microscope (Nikon, Tokyo, Japan). The lung injury was scored by a histologic scoring system as described in a previously published article [11].
For immunofluorescence, the dewaxed and dehydrated sections were washed with 0.01 M PBST three times (5 min per time). After blocking with 10% BSA for 30 min at 37 °C, sections were incubated with anti-LC3B (1:50, Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C. Cell nuclei were stained with DAPI. ImageJ software was used to count the LC3B-positive puncta per cell.
The left lung was used for BALF collection and the lung wet-to-dry ratio was calculated. The dry lung weight was recorded after incubation of the wet lung tissues for 72 h at 70 °C.
BALF analysis
The left lung was lavaged three times with 0.8 ml PBS. The collected lavage fluid was subjected to centrifugation for 10 min at 1000 g and 4 °C. The supernatant was collected for use as BALF, and the total protein content was measured using a BCA kit (KeyGen Biotech, Nanjing, China). To calculate the percentage of neutrophils in BALF, the collected BALF cells were resuspended in PBS supplemented with 10% fetal calf serum (Gibco, Grand Island, NY, USA). A sample of 5 × 105 cells was probed by a specific anti-neutrophil antibody (Abcam, Cambridge, MA, USA) for 30 min and then incubated with a FITC-labeled secondary antibody (Abcam) for another 30 min. The fluorescence intensity of each sample was analyzed by CytoFLEX flow cytometry (Beckman, CA, USA).
Transmission electron microscopy
The lung tissues of mice were mixed with 2% glutaraldehyde and then post-fixed with 1% osmium tetroxide for 1 h. The tissues were dehydrated in ethanol solutions and embedded in propylene oxide. Next, tissues were cut into ultrathin sections, which were stained by uranyl acetate (Ieda Chemicals, Tokyo, Japan) and lead citrate (Sigma-Aldrich). The stained sections were finally analyzed under by transmission electron microscopy (TEM, Servicebio, Wuhan, China).
Cell culture
RAW264.7 cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium containing 4 mM l-glutamine, 4500 mg/l glucose, 1 mM sodium pyruvate, and 1500 mg/l sodium bicarbonate (all from Sigma-Aldrich) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA). Cells were maintained at 37 °C in a humidified incubator with 5% CO2. For induction of autophagy, cells were treated with 50 nM RAPA for 30 min. The autophagic flux was measured by treating cells with 50 μM chloroquine (CQ, Sigma-Aldrich, USA), an inhibitor of lysosomal degradation, for 8 h [12].
shRNA transfection
LV12 (U6 /Luciferase05 & Puro) shGGPPS1 (5'-ACCTCGGGACAAGGCCTC GATATTTATCAAGAGTAAATATCGAGGCCTTGTCCCTT-3') and scrambled negative control shRNA (shNC; 5'-ACCTCGATGAACCAGAGCGTCTTAGTTCAAGAGACTAAGACGCTCTGGTT CATCTT-3') were purchased from GenePharma (cat. No.: C06006; Shanghai, China). RAW264.7 cells were infected with lentiviruses expressing shGGPPS1 or shNC for 72 h at 37 °C. Mouse GGPPS1 cDNA was amplified by PCR and cloned into pcDNA3.0 for constructing the Rab37 overexpression plasmid (pcRab37), which was bought from Ribobio (Guangzhou, China). Transient transfection was performed using Lipofectamine® 3000 (Invitrogen, Carlsbad, CA, USA) for 48 h at 37 °C.
Cyclic stretch
Transfected cells were subjected to cyclic stretching using an FX-5000 T Flexcell Tension system (Flexcell International, McKeesport, PA, USA) at 30 cycles/min and 30% amplitude. Cyclic stretch was set at 20% changes and the cells were stretched for 4 h at 37 °C in a cell culture incubator. Non-stretched cells served as the negative control.
ELISA
The concentrations of cytokines, including IL-6, IL-1β, IL-18, and TNF-α, in BALF and cell culture supernatants were analyzed by ELISA kits from eBioscience (San Diego, CA, USA), according to the manufacturer’s protocols.
qRT-PCR
Total RNA in lung tissues and RAW264.7 cells were extracted using TRIzol reagent with or without grinding the cells/tissues in liquid nitrogen. RNA was reverse transcribed into cDNA using TB Green Premix Ex Taq II (Takara, Dalian, China). qPCR analysis was conducted using an Mx-3000P Sequence Detection System (Agilent, CA, USA) with the SYBR Premix Ex Taq kit (Takara). The primers used in this study were synthesized by Takara Bio, Inc. The primer sequences can be found in our previously published article [10]. The expression levels of target genes were normalized to β-actin.
Western blot
Total protein was isolated from lung tissues and RAW264.7 cells using RIPA lysis buffer (Beyotime, Shanghai, China). The protein concentration was measured using a BCA kit (KeyGen Biotech). An equal volume per sample was subjected to 10% SDS-PAGE and the proteins were transferred onto PVDF membranes (Millipore, Bedford, MA). The membranes were blocked in 5% non-fat milk and probed with anti-GGPPS1 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), anti-IL-1β (1:1000, Abcam), anti-IL-6 (1:1000, Abcam), anti-IL-18 (1:200, Abcam), anti-TNF-α (1:1000, Abcam), anti-LC3B (1:200, Abcam), anti-ATG5 (1:1000, Cell Signaling Technology, Danvers, MA), anti-Beclin1 (1:1000, Cell Signaling Technology), anti-Rab37 (1:500, Abcam), and anti-Actin (1:1000, Santa Cruz Biotechnology). After washing, the membranes were probed with HRP-conjugated secondary antibodies (1:2000, Abcam) and the signal was detected by the classical ECL method.
Statistics
Data presented in this study are shown as mean ± SD. Animal experiments were performed using n = 8 mice per group. The in vitro data were obtained from three independent experiments. Statistical differences between groups were analyzed by ANOVA in SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered to indicate statistical significance.
Results
GGPPS1 knockout ameliorated VILI in mice
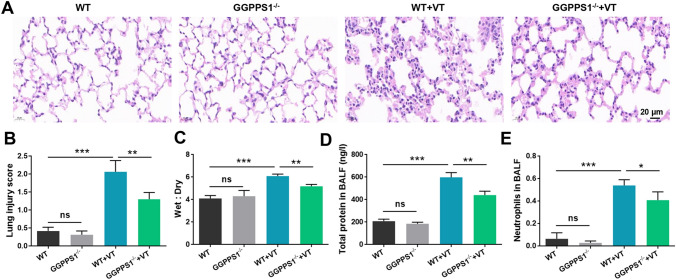
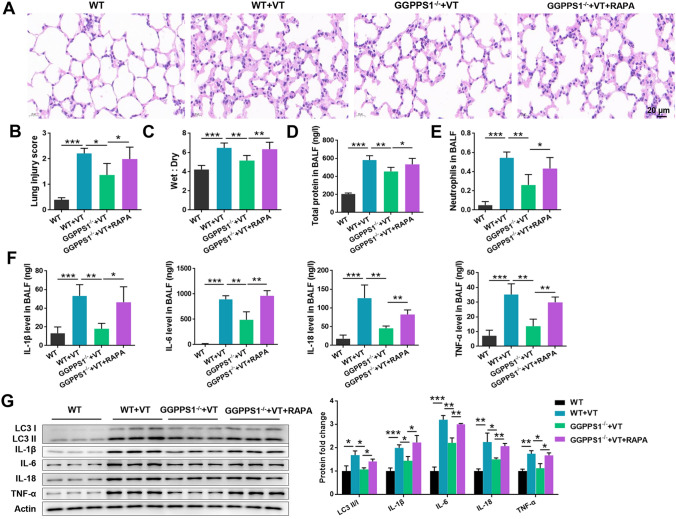
At first, an animal model of VILI was established by conducting mechanical ventilation in C57BL/6 mice. Obvious diffuse lung injury was observed in VT groups (rats receiving ventilation) (P < 0.05, Fig. 1a, b). The lung injury was accompanied by pulmonary edema, focal hemorrhage, disrupted alveolar structural integrity, alveolar epithelial cell necrosis, alveolar septum thickening, and interstitial hyperemia. GGPPS1 knockout significantly attenuated the severity of VILI (P < 0.05) while it had no significant effects on normal rats (P > 0.05). The lung wet-to-dry ratio was high in the VT group, and GGPPS1 knockout significantly reduced the ratio to normal levels (P < 0.05, Fig. 1c). In addition, the total protein levels and neutrophil counts in BALF were significantly higher in the VT group (P < 0.05, Fig. 1d, e). GGPPS1 knockout significantly reduced the total protein levels and neutrophil counts in VT mice (P < 0.05) but not in normal mice (P > 0.05).
Fig. 1.
GGPPS1 knockout ameliorated VILI in mice. C57BL/6 mice with lung-specific GGPPS1 knockout (GGPPS1−/−) and wild-type (WT) mice were subjected to mechanical ventilation (VT) or not. a Lung injury was analyzed by HE staining and b lung injury was scored. c Mouse lungs were collected for calculation of the wet-to-dry ratio. d Total protein levels in BALF were analyzed using a BCA kit. e Neutrophils in BALF were analyzed by flow cytometry. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001 vs. the indicated group
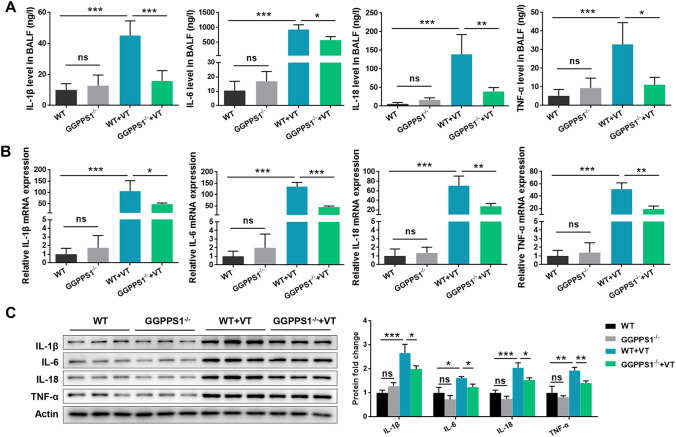
Since lung injury and neutrophil infiltration were observed in mice of the VT group, we then analyzed the production and release of inflammatory cytokines in different groups. As shown in Fig. 2a, the levels of inflammatory cytokines including IL-1β, IL-6, IL-18, and TNF-α in BALF were significantly higher in VT mice than in control mice (P < 0.05). Accordingly, in the lung tissues, the expression levels of these cytokines, at both mRNA and protein levels, were significantly higher in the VT group than in the control group (P < 0.05, Fig. 2b, c). GGPPS1 knockout reduced the production and release of these cytokines in VT mice, but had no significant effects on normal mice (P < 0.05). These results indicated that the VILI animal model was established successfully and GGPPS1 knockout ameliorated VILI in mice.
Fig. 2.
GGPPS1 knockout ameliorated mechanical ventilation-induced inflammation in mice. C57BL/6 mice with lung-specific GGPPS1 knockout (GGPPS1−/−) and wild-type (WT) mice were subjected to mechanical ventilation (VT) or not. a The contents of inflammatory cytokines, including IL-1β, IL-6, IL-18, and TNF-α, in BALF were measured by ELISA. b mRNA and c protein levels of the inflammatory cytokines were measured by qRT-PCR and western blot, respectively. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001 vs. the indicated group
GGPPS1 knockout inhibited autophagy in VILI mice
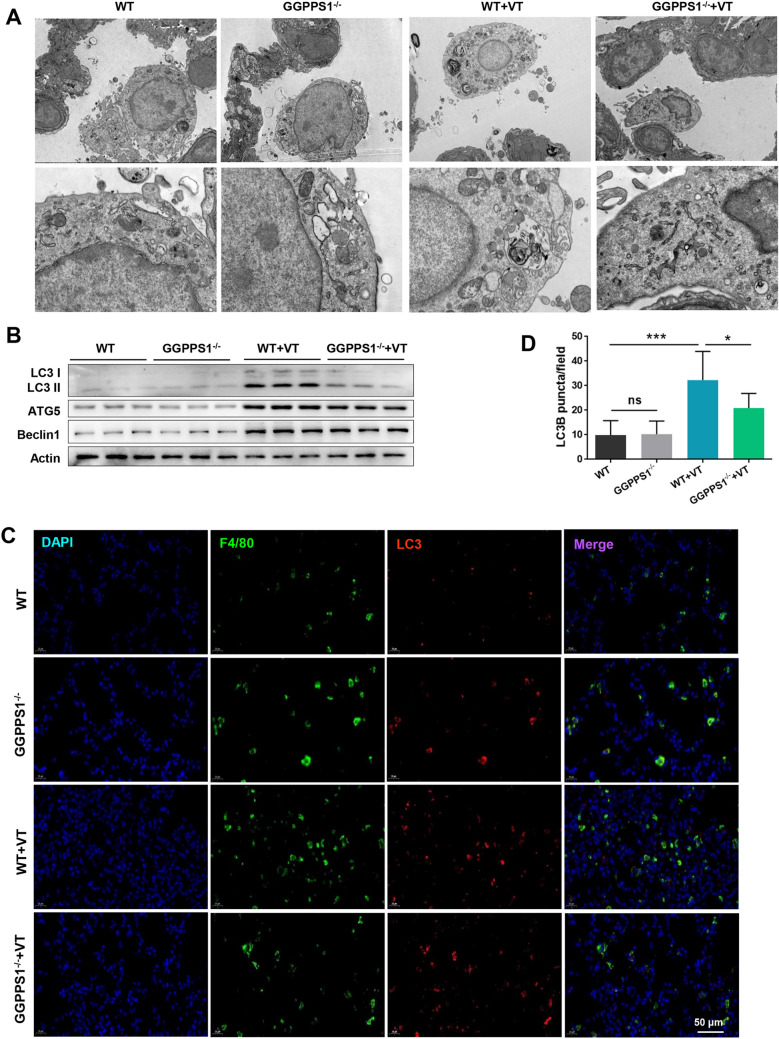
The impacts of GGPPS1 knockout on autophagy in VILI mice were then studied. The ultrastructural change of lung tissues was observed by TEM. The lung tissues in the VT group presented significant alveolar type II epithelial cell degeneration, vacuolated eosinophilic lamellar bodies, swollen mitochondria, increased matrix electron density, and disordered or missing cristae (Fig. 3a). GGPPS1 knockout remarkably attenuated these ventilation-induced manifestations, and it additionally reduced the number of autophagosomes. The expression levels of autophagy-related proteins, including LC3 I/II, ATG5, and Beclin1, were remarkably increased in VILI mice as compared with normal mice (Fig. 3b). It seems that GGPPS1 knockout downregulated these proteins in VILI mice but not in normal mice. The results for immunofluorescence experiments indicated that the number of LC3B-positive puncta was found to be higher in the VT group than in the control group (P < 0.05, Fig. 3c, d). GGPPS1 knockout significantly reduced the number of LC3B-positive puncta in the VT group (P < 0.05), but not in the control group (P > 0.05).
Fig. 3.
GGPPS1 knockout inhibited autophagy in VILI mice. C57BL/6 mice with lung-specific GGPPS1 knockout (GGPPS1−/−) and wild-type (WT) mice were subjected to mechanical ventilation (VT) or not. a The ultrastructural changes of lung tissues were observed by TEM. b Protein expression of autophagy markers, including LC3 I/II, ATG5, and Beclin1, was detected by western blot analysis. c, d LC3B-positive puncta in lung tissues were analyzed by immunofluorescence. ns, not significant; *P < 0.05; ***P < 0.001 vs. the indicated group
Inhibition of autophagy ameliorated VILI in mice
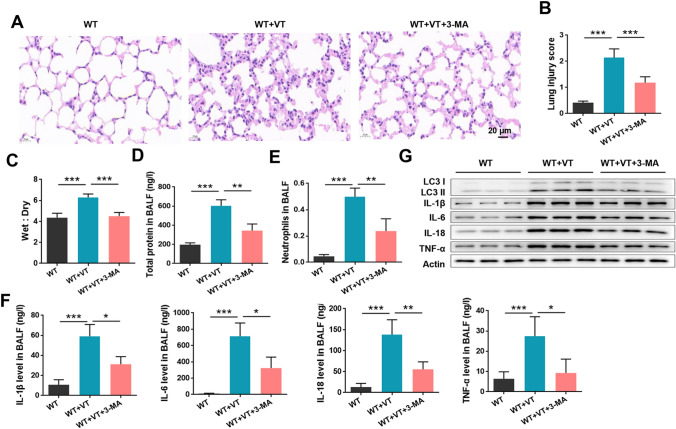
To identify the specific role of autophagy in the event of VILI, 3-MA (a key inhibitor of autophagy) was intravenously injected into mice. The severity of VILI was significantly reduced by 3-MA treatment (P < 0.05, Fig. 4a, b). In line with this, the lung wet-to-dry ratio (Fig. 4c), the total protein levels (Fig. 4d), neutrophil counts (Fig. 4e), and the concentrations of cytokines in BALF (Fig. 4f) in VILI mice were all significantly reduced by 3-MA treatment (all P < 0.05). The upregulation of LC3 I/II, IL-1β, IL-6, IL-18, and TNF-α proteins induced by ventilation in mouse lung tissues was also obviously attenuated by 3-MA (Fig. 4g). These data suggest that autophagy acts as a disease-promoting progress and that inhibition of autophagy effectively ameliorates VILI.
Fig. 4.
Inhibition of autophagy ameliorated VILI in mice. Wild-type (WT) C57BL/6 mice were subjected to mechanical ventilation (VT) and intravenously injected with 3-MA (a key inhibitor of autophagy). a Lung injury was analyzed by HE staining and b lung injury was scored. c Mouse lungs were collected for calculation of the wet-to-dry ratio. d Total protein levels in BALF were analyzed using a BCA kit. e Neutrophils in BALF were analyzed by flow cytometry. f The contents of inflammatory cytokines, including IL-1β, IL-6, IL-18, and TNF-α, in BALF were measured by ELISA. g Protein expression of LC3 I/II and cytokines was analyzed by western blot. *P < 0.05; **P < 0.01; ***P < 0.001 vs. the indicated group
GGPPS1 knockout ameliorated VILI in mice via inhibition of autophagy
GGPPS1−/− mice with VILI were treated with RAPA (a key activator of autophagy) to investigate whether GGPPS1 mediates VILI via regulation of autophagy. As shown in Fig. 5a, b, the protective function induced by GGPPS1 knockdown was significantly eliminated by intravenous injection of RAPA (P < 0.05). The lung wet-to-dry ratio (Fig. 5c), total protein levels (Fig. 5d), neutrophil counts (Fig. 5e), and cytokine levels in BALF (Fig. 5f), which were reduced by GGPPS1 knockout, were significantly increased by RAPA (P < 0.05). Protein expression of LC3 I/II and inflammatory cytokines that were downregulated by GGPPS1 knockout were reversed by RAPA (P < 0.05, Fig. 5g). Taken together, these results indicate that the beneficial function of GGPPS1 knockout could be eliminated by RAPA, indicating that GGPPS1 knockout ameliorates VILI via inhibition of autophagy.
Fig. 5.
GGPPS1 knockout ameliorated VILI in mice via inhibition of autophagy. C57BL/6 mice with lung-specific GGPPS1 knockout (GGPPS1−/−) and wild-type (WT) mice were subjected to mechanical ventilation (VT) or not. The GGPPS1−/− mice in the VT group were then intravenously injected with RAPA (a key activator of autophagy). a Lung injury was analyzed by HE staining and b lung injury was scored. c Mouse lungs were collected for calculation of the wet-to-dry ratio. d Total protein levels in BALF were analyzed using a BCA kit. e Neutrophils in BALF were analyzed by flow cytometry. f The contents of inflammatory cytokines, including IL-1β, IL-6, IL-18, and TNF-α, in BALF were measured by ELISA. g Protein expression of LC3 I/II and cytokines was analyzed by western blot. *P < 0.05; **P < 0.01; ***P < 0.001 vs. the indicated group
GGPPS1 knockdown ameliorated cyclic stretch-induced cytokine release from RAW264.7 cells
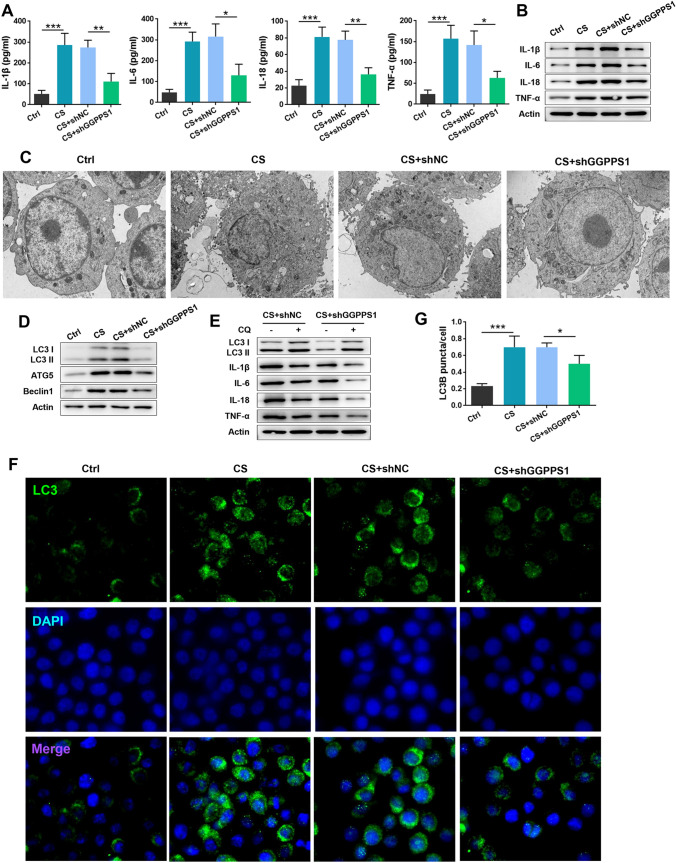
RAW264.7 cells were subjected to cyclic stretch to generate a cell model of VILI. The content of inflammatory cytokines in the cell supernatant was significantly increased in the cyclic stretch group (P < 0.05, Fig. 6a). In addition, the protein levels of these cytokines were upregulated by cyclic stretch (Fig. 6b), indicating that the VILI cell model was established successfully. In addition, GGPPS1 knockdown by shRNA significantly attenuated the inflammation induced by cyclic stretch, as evidenced by the reduced release and expression of IL-1β, IL-6, IL-18, and TNF-α. The number of autophagosomes was sharply increased by cyclic stretch, while it was reduced by GGPPS1 knockdown (P < 0.05, Fig. 6c). Protein expression of LC3 I/II, ATG5, and Beclin1, which was induced by cyclic stretch, was downregulated by GGPPS1 knockdown (Fig. 6d). To further confirm the effects of GGPPS1 knockdown on cyclic stretch-induced autophagy, CQ was used to treat cells for inhibiting lysosomal degradation of autophagosomes. As expected, the accumulation of LC3 II was higher in CQ-treated cells. GGPPS1 knockdown in CQ-treated cells remarkably downregulated LC3 II, which accompanied by the observed downregulation of IL-1β, IL-6, IL-18, and TNF-α (Fig. 6e). Moreover, the number of LC3B-positive puncta in cyclic stretch-treated cells was reduced by GGPPS1 knockdown (P < 0.05, Fig. 6f, g), which further confirmed the anti-inflammatory function of GGPPS1 knockdown in the event of VILI.
Fig. 6.
GGPPS1 knockdown ameliorated cyclic stretch-induced cytokine release from RAW264.7 cells. RAW264.7 cells were transfected with GGPPS1-specific shRNA (shGGPPS1) or negative control shRNA (shNC). The transfected cells were then subjected to cyclic stretch (CS) or not. a The content of inflammatory cytokines in the cell supernatant was analyzed by ELISA. b Protein expression of cytokines was analyzed by western blot. c Autophagosomes and autolysosomes were observed by TEM. d Protein expression of autophagy markers was detected by western blot. e Protein expression of LC3 II and cytokines in chloroquine (CQ)-treated cells was analyzed by western blot. f, g Intracellular LC3B-positive puncta were analyzed by immunofluorescence. *P < 0.05; **P < 0.01; ***P < 0.001 vs. the indicated group
GGPPS1 knockdown ameliorated cytokine release from RAW264.7 cells via regulation of Rab37-mediated autophagy
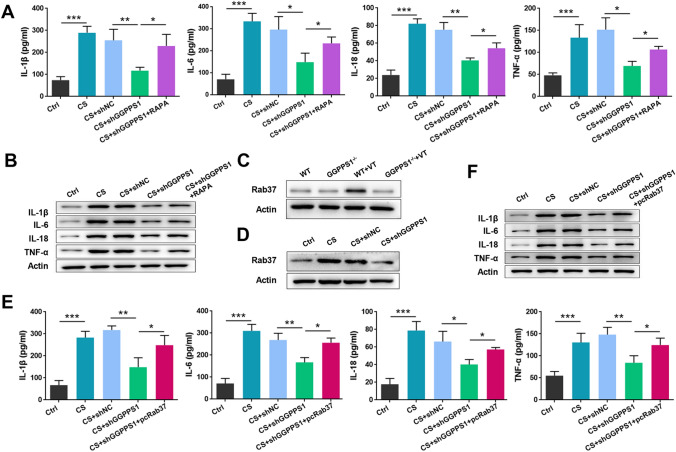
RAPA was then used to validate the role of autophagy in the cytoprotective effects of GGPPS1 knockdown. As shown in Fig. 7a, b, the increased release and expression of cytokines upon cyclic stretch were significantly eliminated by RAPA. These data suggest that GGPPS1 knockdown ameliorates cyclic stretch-induced cytokine release from RAW264.7 cells via inhibition of autophagy. Furthermore, we studied how GGPPS1 knockdown mediates autophagy inhibition. Rab37 has been recently identified as a pivotal organizer of autophagosomal membrane biogenesis [13]. Therefore, we detected the expression of Rab37 following GGPPS1 knockout. As shown in Fig. 7c, d, Rab37 was remarkably upregulated in both the mouse model and the cell model of VILI. GGPPS1 knockout in mice and GGPPS1 knockdown in RAW264.7 cells downregulated Rab37 expression to basic levels. Moreover, the release and expression of cytokines, which were reduced by GGPPS1 knockdown in RAW264.7 cells, were significantly increased upon Rab37 overexpression (P < 0.05, Fig. 7e, f). All these data indicate that GGPPS1 knockdown ameliorates cyclic stretch-induced cytokine release from RAW264.7 cells via regulation of Rab37-mediated autophagy.
Fig. 7.
GGPPS1 knockdown ameliorated cytokine release from RAW264.7 cells via regulation of Rab37-mediated autophagy. a RAW264.7 cells transfected with GGPPS1-specific shRNA (shGGPPS1) or negative control shRNA (shNC) were subjected to cyclic stretch (CS) or not. Cells were treated with RAPA to induce autophagy. The content of inflammatory cytokines in the cell supernatant was analyzed by ELISA. b Protein expression of cytokines was analyzed by western blot. Rab37 protein expression levels in c GGPPS1−/− mice subjected to mechanical ventilation (VT) and d GGPPS1 knockdown RAW264.7 cells subjected to CS were analyzed by western blot. e The Rab37 overexpression plasmid (pcRab37) was transfected into RAW264.7 cells. The content of inflammatory cytokines in the cell supernatant was analyzed by ELISA. f Protein expression of cytokines was analyzed by western blot. *P < 0.05; **P < 0.01; ***P < 0.001 vs. the indicated group
Discussion
It has been confirmed that long-term exposure of lungs to stretching leads to the recruitment of inflammatory cells (including neutrophils) and induces the production and release of cytokines, ultimately damaging alveolar epithelial cells [14]. Mechanical ventilation-induced inflammation and lung injury are major downsides of protective ventilation strategies. Although the inflammatory initiation process of VILI has been widely studied, the mechanisms underlying inflammation in VILI are not fully understood. GGPPS is a branch enzyme that converts farnesyl diphosphate to geranylgeranyl diphosphate in the mevalonate pathway [15].
GGPPS has been confirmed to be closely associated with inflammation and organ damage [16–18]. Our previous series of studies confirmed that GGPPS can regulate the inflammatory response induced by lipopolysaccharide(LPS), bleomycin, and mechanical ventilation and plays an important role in the pathogenesis of lung injury and pulmonary fibrosis [9, 19, 20]. In this study, we found that (1) GGPPS1 knockout inhibits autophagy in VILI mice, (2) GGPPS1 knockout ameliorates VILI in mice via inhibition of autophagy and (3) GGPPS1 knockdown ameliorates cytokine release from RAW264.7 cells via regulation of Rab37-mediated autophagy.
Several studies have confirmed that mechanical ventilation, based on the presence of pulmonary inflammation, is associated with a significant risk of lung injury with pulmonary edema and focal pulmonary hemorrhage [21, 22]. Mechanical ventilation increases the influx of neutrophils into BALF and augments the release of inflammatory cytokines, including IL-1β, IL-6, IL-18, and TNF-α. The protective effects of GGPPS1 knockout demonstrated here are consistent with our previous report [10]. Previous studies suggested that autophagy also plays an important role in inflammation and injury [23, 24]; for example, autophagy modulates endothelial junctions to restrain neutrophil diapedesis during inflammation[23], dysregulated autophagy contributes to the pathogenesis of acute kidney injury [24, 25] and myocardial ischemia–reperfusion injury [26], and autophagy is specifically dysregulated by SARS-CoV-2 ORF3 in COVID-19 pathophysiology [27]. Autophagy is an intracellular digestion system that acts as an inducible adaptive response to lung injury which is a result of exposure to various stress agents such as hypoxia, ischemia–reperfusion, and xenobiotics [28]. Therefore, what is the regulatory relationship between GGPPS and autophagy in VILI? Our study found that GGPPS inhibits inflammation and lung injury through inhibition of the autophagy signaling pathway. These phenomena have also been reported in other studies of autophagy, inflammation, and lung injury, which found that autophagy prevents increased lung permeability and hypoxemia by downregulating inflammasome activity, IL-1 expression, and endoplasmic reticulum stress in both LPS-induced inflammation and VILI [29, 30].
We further explored the specific regulators of autophagy in both the animal model and the cell model of VILI, and we discovered that the protein levels of LC3 I/II, ATG5, and Beclin1 were upregulated remarkably. In mammals, autophagy is controlled by Atg genes. The Atg12–Atg5 and Atg8/LC3 conjugation systems are required for the formation of autophagosomes [31]. The C-terminus of Atg8/LC3 can be cleaved by Atg4 proteases to generate cytoplasmic LC3 I. LC3 I is then converted to LC3 II by Atg7 and Atg3. The LC3 I/II level has been considered as a key marker of autophagy flux [32]. In addition to LC3, Beclin1 is also involved in the initial steps of autophagy by regulating the recruitment of ATG proteins [33]. This finding was in line with several previous studies [7, 34], evidencing autophagy is critical in the development of VILI. Of note, we for the first time report that GGPPS1 deficiency protects against VILI through inhibition of autophagy. As MV results in a variation in pulmonary lesions in a time- and tidal volume-dependent manner [35–37], further studies are required to uncover the expression of GGPPS1 and autophagy biomarkers in mouse lungs ventilated with different time and tidal volume. In addition, the effects of GGPPS1 knockout on mouse VILI models ventilated with different time and tidal volume should be confirmed.
Then, we observed that Rab37 was upregulated in VILI models and GGPPS1 deficiency remarkably decreased its expression. In addition, Rab37 overexpression in VILI cells significantly attenuated GGPPS1 knockdown-induced anti-inflammatory functions. Rab GTPases are proteins that are responsible for regulating intracellular membrane trafficking [38]. It has been reported that numerous Rab proteins, including Rab7, Rab8B, Rab24, and Rab37, are required for autophagy [13, 39, 40]. Suppression of Rab37 expression and activity is effective in controlling autophagy [41]. Our study suggests that GGPPS1 knockdown regulates autophagy in the lung by regulating the expression of rab37. However, further studies are needed to elucidate the complex relationship between GGPPS, rab37, and autophagy.
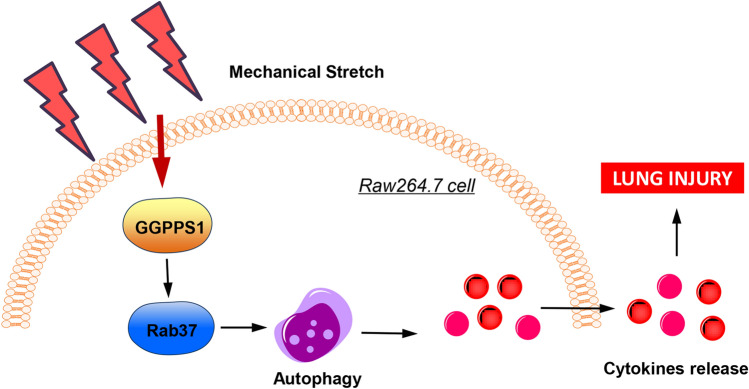
In conclusion, by establishing animal and cell models of VILI, the protective function of GGPPS1 deficiency against VILI was confirmed in this study. The protective effects of GGPPS1 deficiency against VILI might be mediated by inhibition of Rab37-mediated autophagy (Fig. 8). The findings of this study highlight GGPPS1 as a potential target for VILI treatment.
Fig. 8.
Schematic diagram of the effects of GGPPS1 on mechanical stretch-induced lung injury
Author contributions
WB and YLZ conceived and designed the research; ZXW, MZC, LW, CY, QQL and JJJ performed the experiments; BW and ZXW drafted the manuscript; WB, YLZ, ZXW, MZC, LW, QQL and JJJ approved the publication of the final version of manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81971726), the Natural science Foundation of Jiangsu Province (grant no. BK20191351), the Jiangsu Postdoctoral Research Fund (grant no. 2019k178), the Hunan Natural Science Foundation Youth Fund Project (grant no. 2019JJ50021), and the Project funded by China Postdoctoral Science Foundation (grant no. 2020M670091ZX).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
No conflict of interest, financial or otherwise, is declared by the authors.
Ethical approval
The experimental protocol of our study was performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Animal Care and Use Committee of the Affiliated Jiangning Hospital of Nanjing Medical University.
Consent for publication
No.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zexu Wang and Meizi Chen contributed equally to this study.
Contributor Information
Yunlei Zhang, Email: yunleizhang@njmu.edu.cn.
Bing Wan, Email: bingwan76@njmu.edu.cn.
References
- 1.Gaver DP, Nieman GF, Gatto LA, Cereda M, Habashi NM, Bates JHT. The poor get poorer: a hypothesis for the pathogenesis of ventilator-induced lung injury. Am J Respir Crit Care Med. 2020;202(8):1081–7. doi: 10.1164/rccm.202002-0453CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobba CM, Fei Q, Shukla V, Lee H, Patel P, Putman RK, et al. Nanoparticle delivery of microRNA-146a regulates mechanotransduction in lung macrophages and mitigates injury during mechanical ventilation. Nat Commun. 2021;12(1):289. doi: 10.1038/s41467-020-20449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curley GF, Laffey JG, Zhang H, Slutsky AS. Biotrauma and ventilator-induced lung injury: clinical implications. Chest. 2016;150(5):1109–1117. doi: 10.1016/j.chest.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Dong W, He B, Qian H, Liu Q, Wang D, Li J, Wei Z, Wang Z, Xu Z, Wu G, Qian G, Wang G. RAB26-dependent autophagy protects adherens junctional integrity in acute lung injury. Autophagy. 2018;14(10):1677–1692. doi: 10.1080/15548627.2018.1476811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slavin SA, Leonard A, Grose V, Fazal F, Rahman A. Autophagy inhibitor 3-methyladenine protects against endothelial cell barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2018;314(3):L388–L396. doi: 10.1152/ajplung.00555.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li T, Wu YN, Wang H, Ma JY, Zhai SS, Duan J. Dapk1 improves inflammation, oxidative stress and autophagy in LPS-induced acute lung injury via p38MAPK/NF-κB signaling pathway. Mol Immunol. 2020;120:13–22. doi: 10.1016/j.molimm.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Li Y, Zheng SF, Han YB, Bai QL, Zhao T. Autophagy in pulmonary macrophages mediates lung inflammatory injury via c-Src tyrosine kinase pathway activation during mechanical ventilation. Eur Rev Med Pharmacol Sci. 2019;23(4):1674–1680. doi: 10.26355/eurrev_201902_17129. [DOI] [PubMed] [Google Scholar]
- 8.Mandell EW, Savani RC. Ceramides, autophagy, and apoptosis mechanisms of ventilator-induced lung injury and potential therapeutic targets. Am J Respir Crit Care Med. 2019;199(6):687–689. doi: 10.1164/rccm.201810-1857ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M, Wan B, Zhu S, Zhang F, Jin J, Li X, Wang X, Lv Y, Chen C, Lv T, Song Y. Geranylgeranyl diphosphate synthase deficiency aggravates lung fibrosis in mice by modulating TGF-β1/BMP-4 signaling. Biol Chem. 2019;400(12):1617–1627. doi: 10.1515/hsz-2019-0168. [DOI] [PubMed] [Google Scholar]
- 10.Wan B, Xu WJ, Chen MZ, Sun SS, Jin JJ, Lv YL, Zhan P, Zhu SH, Wang XX, Lv TF, Song Y. Geranylgeranyl diphosphate synthase 1 knockout ameliorates ventilator-induced lung injury via regulation of TLR2/4-AP-1 signaling. Free Radic Biol Med. 2020;147:159–166. doi: 10.1016/j.freeradbiomed.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM. An official American thoracic society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salazar G, Cullen A, Huang J, Zhao Y, Serino A, Hilenski L, et al. SQSTM1/p62 and PPARGC1A/PGC-1alpha at the interface of autophagy and vascular senescence. Autophagy. 2020;16(6):1092–110. doi: 10.1080/15548627.2019.1659612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song Y, Shang D, Cheng H, Zhou R. The small GTPase RAB37 functions as an organizer for autophagosome biogenesis. Autophagy. 2018;14(4):727–729. doi: 10.1080/15548627.2018.1434374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minucci S. Mathematical modeling of ventilator-induced lung inflammation. bioRxiv (the preprint server for biology) 2020;17:132258. doi: 10.1016/j.jtbi.2021.110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghorbani P, Smith TK, Fullerton MD. Does prenylation predict progression in NAFLD? J Pathol. 2019;247(3):283–6. doi: 10.1002/path.5190. [DOI] [PubMed] [Google Scholar]
- 16.Jia WJ, Tang QL, Jiang S, Sun SQ, Xue B, Qiu YD, et al. Conditional loss of geranylgeranyl diphosphate synthase alleviates acute obstructive cholestatic liver injury by regulating hepatic bile acid metabolism. FEBS J. 2020;287(15):3328–45. doi: 10.1111/febs.15204. [DOI] [PubMed] [Google Scholar]
- 17.Wang XX, Ying P, Diao F, Wang Q, Ye D, Jiang C, Shen N, Xu N, Chen WB, Lai SS, Jiang S, Miao XL, Feng J, Tao WW, Zhao NW, Yao B, Xu ZP, Sun HX, Li JM, Sha JH, Huang XX, Shi QH, Tang H, Gao X, Li CJ. Altered protein prenylation in Sertoli cells is associated with adult infertility resulting from childhood mumps infection. J Exp Med. 2013;210(8):1559–1574. doi: 10.1084/jem.20121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Jiang S, Zhao Y, Sun Q, Zhang J, Shen D, et al. Geranylgeranyl diphosphate synthase (GGPPS) regulates non-alcoholic fatty liver disease (NAFLD)-fibrosis progression by determining hepatic glucose/fatty acid preference under high-fat diet conditions. J Pathol. 2018;246(3):277–88. doi: 10.1002/path.5131. [DOI] [PubMed] [Google Scholar]
- 19.Jin J, Qian H, Wan B, Zhou L, Chen C, Lv Y, Chen M, Zhu S, Ye L, Wang X, Xu W, Lv T, Song Y. Geranylgeranyl diphosphate synthase deficiency hyperactivates macrophages and aggravates lipopolysaccharide-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2021;320(6):L1011–l1024. doi: 10.1152/ajplung.00281.2020. [DOI] [PubMed] [Google Scholar]
- 20.Xu WJ, Wang XX, Jin JJ, Zou Q, Wu L, Lv TF, Wan B, Zhan P, Zhu SH, Liu HB, Zhao NW, Li CJ, Song Y. Inhibition of GGPPS1 attenuated LPS-induced acute lung injury and was associated with NLRP3 inflammasome suppression. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L567–l577. doi: 10.1152/ajplung.00190.2018. [DOI] [PubMed] [Google Scholar]
- 21.López-Alonso I, Blázquez-Prieto J, Amado-Rodríguez L, González-López A, Astudillo A, Sánchez M, et al. Preventing loss of mechanosensation by the nuclear membranes of alveolar cells reduces lung injury in mice during mechanical ventilation. Sci Transl Med. 2018;10:456. doi: 10.1126/scitranslmed.aam7598. [DOI] [PubMed] [Google Scholar]
- 22.Müller-Redetzky HC, Will D, Hellwig K, Kummer W, Tschernig T, Pfeil U, Paddenberg R, Menger MD, Kershaw O, Gruber AD, Weissmann N, Hippenstiel S, Suttorp N, Witzenrath M. Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: protection by adrenomedullin. Crit care (London, England) 2014;18(2):R73. doi: 10.1186/cc13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reglero-Real N, Pérez-Gutiérrez L, Yoshimura A, Rolas L, Garrido-Mesa J, Barkaway A, Pickworth C, Saleeb RS, Gonzalez-Nuñez M, Austin-Williams SN, Cooper D, Vázquez-Martínez L, Fu T, De Rossi G, Golding M, Voisin MB, Boulanger CM, Kubota Y, Muller WA, Tooze SA, Nightingale TD, Collinson L, Perretti M, Aksoy E, Nourshargh S. Autophagy modulates endothelial junctions to restrain neutrophil diapedesis during inflammation. Immunity. 2021;54(9):1989–2004.e1989. doi: 10.1016/j.immuni.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura S, Shigeyama S, Minami S, Shima T, Akayama S, Matsuda T, et al. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat cell biol. 2020;22(10):1252–63. doi: 10.1038/s41556-020-00583-9. [DOI] [PubMed] [Google Scholar]
- 25.Tang C, Livingston MJ, Liu Z, Dong Z. Autophagy in kidney homeostasis and disease. Nat Rev Nephrol. 2020;16(9):489–508. doi: 10.1038/s41581-020-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17(12):773–789. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 27.Stukalov A, Girault V, Grass V, Karayel O, Bergant V, Urban C, et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 2021;594(7862):246–52. doi: 10.1038/s41586-021-03493-4. [DOI] [PubMed] [Google Scholar]
- 28.Vishnupriya S, Priya Dharshini LC, Sakthivel KM, Rasmi RR. Autophagy markers as mediators of lung injury-implication for therapeutic intervention. Life Sci. 2020;260:118308. doi: 10.1016/j.lfs.2020.118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nosaka N, Martinon D, Moreira D, Crother TR, Arditi M, Shimada K. Autophagy protects against developing increased lung permeability and hypoxemia by down regulating inflammasome activity and IL-1β in LPS plus mechanical ventilation-induced acute lung injury. Front Immunol. 2020;11:207. doi: 10.3389/fimmu.2020.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge X, Sun J, Fei A, Gao C, Pan S, Wu Z. Hydrogen sulfide treatment alleviated ventilator-induced lung injury through regulation of autophagy and endoplasmic reticulum stress. Int J Biol Sci. 2019;15(13):2872–2884. doi: 10.7150/ijbs.38315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki K, Akioka M, Kondo-Kakuta C, Yamamoto H, Ohsumi Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J Cell Sci. 2013;126(Pt 11):2534–2544. doi: 10.1242/jcs.122960. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Dang Y, Su W, Liu C, Ma H, Shan Y, Pei Y, Wan B, Guo J, Yu L. Molecular cloning and characterization of rat LC3A and LC3B–two novel markers of autophagosome. Biochem Biophys Res Commun. 2006;339(1):437–442. doi: 10.1016/j.bbrc.2005.10.211. [DOI] [PubMed] [Google Scholar]
- 33.Wirawan E, Lippens S, Vanden Berghe T, Romagnoli A, Fimia GM, Piacentini M, Vandenabeele P. Beclin1: a role in membrane dynamics and beyond. Autophagy. 2012;8(1):6–17. doi: 10.4161/auto.8.1.16645. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Liu G, Dull RO, Schwartz DE, Hu G. Autophagy in pulmonary macrophages mediates lung inflammatory injury via NLRP3 inflammasome activation during mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):L173–185. doi: 10.1152/ajplung.00083.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieman G, Satalin J, Andrews P, Wilcox K, Aiash H, Baker S, Kollisch-Singule M, Madden M, Gatto L, Habashi N. Preemptive mechanical ventilation based on dynamic physiology in the alveolar microenvironment: novel considerations of time-dependent properties of the respiratory system. J Trauma Acute Care Surg. 2018;85(6):1081–1091. doi: 10.1097/ta.0000000000002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helmerhorst HJF, Schouten LRA, Wagenaar GTM, Juffermans NP, Roelofs J, Schultz MJ, de Jonge E, van Westerloo DJ. Hyperoxia provokes a time-and dose-dependent inflammatory response in mechanically ventilated mice, irrespective of tidal volumes. Intensive Care Med Exp. 2017;5(1):27. doi: 10.1186/s40635-017-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felix NS, Samary CS, Cruz FF, Rocha NN, Fernandes MVS, Machado JA, Bose-Madureira RL, Capelozzi VL, Pelosi P, Silva PL, Marini JJ, Rocco PRM. Gradually increasing tidal volume may mitigate experimental lung injury in rats. Anesthesiology. 2019;130(5):767–777. doi: 10.1097/aln.0000000000002630. [DOI] [PubMed] [Google Scholar]
- 38.Homma Y, Hiragi S, Fukuda M. Rab family of small GTPases: an updated view on their regulation and functions. FEBS J. 2021;288(1):36–55. doi: 10.1111/febs.15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21(3):348–358. doi: 10.1038/cdd.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng Y, Song Y, Li Z, Wang Y, Lin H, Cheng H, Zhou R. RAB37 interacts directly with ATG5 and promotes autophagosome formation via regulating ATG5-12-16 complex assembly. Cell Death Differ. 2018;25(5):918–34. doi: 10.1038/s41418-017-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin L, Li SH, Liu C, Zeng F, Cao JQ, Zhou LQ, Zhou Q, Yuan YW. Methionine represses the autophagy of gastric cancer stem cells via promoting the methylation and phosphorylation of RAB37. Cell cycle (Georgetown, Tex) 2020;19(20):2644–2652. doi: 10.1080/15384101.2020.1814044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.