Abstract
Introduction
Infection after stroke is associated with unfavorable outcome. Randomized controlled studies did not show benefit of preventive antibiotics in stroke but lacked power for subgroup analyses. Aim of this study is to assess whether preventive antibiotic therapy after stroke improves functional outcome for specific patient groups in an individual patient data meta-analysis.
Patients and methods
We searched MEDLINE (1946–7 May 2021), Embase (1947–7 May 2021), CENTRAL (17th September 2021), trial registries, cross-checked references and contacted researchers for randomized controlled trials of preventive antibiotic therapy versus placebo or standard care in ischemic or hemorrhagic stroke patients. Meta-analysis was performed by a one-step and two-step approach. Primary outcome was functional outcome adjusted for age and stroke severity. Secondary outcomes were infections and mortality.
Results
4197 patients from nine trials were included. Preventive antibiotic therapy was not associated with a shift in functional outcome (mRS) at 3 months (OR1.13, 95%CI 0.98–1.31) or unfavorable functional outcome (mRS 3–6) (OR0.85, 95%CI 0.60–1.19). Preventive antibiotics did not improve functional outcome in pre-defined subgroups (age, stroke severity, timing and type of antibiotic therapy, pneumonia prediction scores, dysphagia, type of stroke, and type of trial). Preventive antibiotics reduced infections (276/2066 (13.4%) in the preventive antibiotic group vs. 417/2059 (20.3%) in the control group, OR 0.60, 95% CI 0.51–0.71, p < 0.001), but not pneumonia (191/2066 (9.2%) in the preventive antibiotic group vs. 205/2061 (9.9%) in the control group (OR 0.92 (0.75–1.14), p = 0.450).
Discussion and conclusion
Preventive antibiotic therapy did not benefit any subgroup of patients with acute stroke and currently cannot be recommended.
Keywords: stroke, infection, antibiotic therapy
Introduction
Stroke is an important cause of death, accounting for 11.8% of deaths worldwide, and is the third most common cause of disability. 1 Infections occur frequently after stroke and have been associated with unfavorable disease outcome. 2 Several randomized clinical trials have investigated antibiotics to prevent infections after stroke.3–8 In a Cochrane systematic review, preventive antibiotic therapy compared to placebo or standard care did not reduce mortality or unfavorable outcome after stroke. 9 Preventive antibiotics do reduce the number of infections after stroke and it could well be that some patients still benefit but not others, and how to select patients who could benefit is unclear. Our aim was to address this question with a meta-analysis of data from all trials for which individual patient data were available.
Materials and methods
Study selection
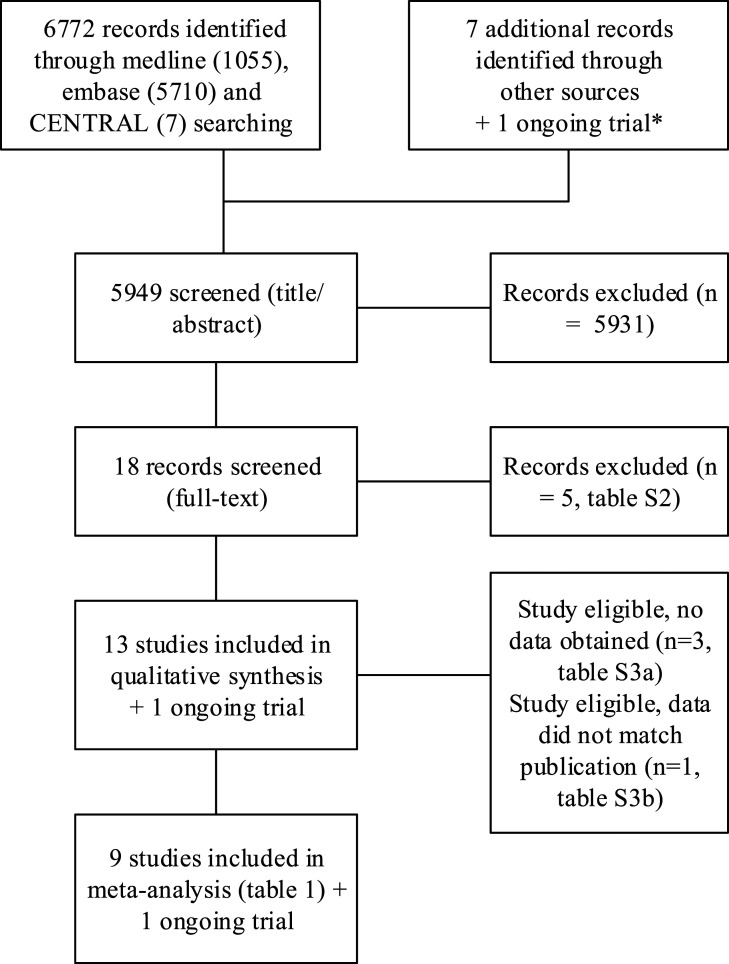
A systematic literature review was undertaken in accordance with Centre for Reviews and Dissemination and Cochrane Collaboration guidance (Higgins, 2009; Figure 1, Supplementary figure 1). Search strategies are shown in Online only Supplementary table 1. Searches were undertaken in multiple electronic databases using pre-defined search criteria and terms. No language restrictions were applied to the search. We searched Ovid MEDLINE, Embase (1946–2020) and CENTRAL (17th September 2021), for randomized clinical trials of preventive antibiotic therapy in stroke. We cross-checked references, contacted researchers in the field, and principal investigators of included clinical trials to identify any other or unpublished material. We searched trial and research registers to identify ongoing studies (ClinicalTrials.gov (www.clinicaltrials.gov); ISRCTN Registry (www.isrctn.com); Stroke Trials Registry (www.strokecenter.org/trials); and WHO Registry Platform (apps.who.int/trialsearch). Two reviewers (WFW, JDV) screened the titles, abstract, and fulltext of the articles for inclusion. Possible disagreements were resolved in discussion with a third study reviewer (PN). After defining eligible studies, we contacted the principal investigators and co-authors of the trials for the individual patient data.
Figure 1.
PRISMA flow-chart of the search.* Ongoing trial: www.precious-trial.eu. Search date: 7 May 2021.
We included randomized controlled trials of adult (age 18 years or older) stroke patients (ischemic, intracerebral hemorrhage (ICH), or both) that randomized between preventive antibiotic therapy (route of administration: oral/by nasogastric tube, systemic, or intramuscular) and placebo or a standard care treatment that reported at least one of the following: infection or pneumonia rate, mortality, functional outcome. We excluded studies on patients with subarachnoid hemorrhage and studies with exclusively intubated and mechanically ventilated patients.
Quality assessment
Study quality was assessed by two independent observers who had no role in the conduct of the included studies (AK, CS). The Cochrane Collaboration Tool was used for the assessment of study quality. The following items were evaluated: random sequence generation, (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias.
Definitions and outcome measurements
Data extraction and data definitions of variables for each trial are shown in Supplementary table 4 and 5.
General information was extracted for each trial: name of the author, year, and country of the study, type of stroke patients, number of patients included, whether placebo treatment or standard care was used as control group. For the individual patient analysis, we requested the following information from each trial (for each individual patient): sex; age; medical history (atrial fibrillation; chronic obstructive pulmonary disease (COPD); diabetes; baseline, pre-stroke disability (modified Rankin Scale [mRS])); stroke type; stroke severity (NIHSS); treatment with intravenous thrombolysis; use of urinary catheter; dysphagia; diagnosis of infection (pneumonia, urinary tract infection [UTI], other); time to diagnosis of infection; name, dosage, and frequency of preventive antibiotic therapy; time to first administration of preventive antibiotic therapy; functional outcome (mRS, Barthel Index [BI]) and mortality at discharge and at 3 months. Definition of variables across trials was investigated by completion of a questionnaire by the contact author of each trial. Data obtained from each trial were cross checked with the original publication.
Primary outcome was defined as functional improvement on the total range of the mRS. A secondary analysis of primary outcome was defined as the proportion of patients with unfavorable functional outcome at 3 months assessed on the mRS (mRS 3–6). Secondary outcomes were death, infection (any), and pneumonia during the follow-up period from each study. All analyses were adjusted for age and stroke severity and performed in the intention to treat populations of the included trials. Sensitivity analyses were performed for trials aimed at improving outcome by reducing infections (type 1 trial) and trials of neuroprotection with the antibiotic minocycline (type 2 trial).
For each of the primary and secondary outcomes, we performed prespecified subgroup analyses (for all trials and type 1 and 2 trials separate): age (≥/< 65 and 80 years), sex (male or female), stroke severity (NIHSS ≥ 5 and 10), type of stroke (ischemic vs hemorrhagic), treatment with thrombolysis (yes vs no), stratified risk of pneumonia (high risk patients vs low risk patients for pneumonia as defined by the externally validated ISAN-score 10 for all strokes and A2DS2 score 11 for ischemic stroke), dysphagia (based on initial swallow screening test), time to treatment (0–3 h, 3–6 h, 6–12 h, 12–24 h, >24 h), antibiotic class (tetracyclines, cephalosporins, fluoroquinolones, penicillins), quality of the study (placebo-controlled or open-label) and whether treatment was administered according to protocol.
We collected the following adverse events on trial level (as the type and definition of adverse events differed largely by trial): neurological (CT confirmed stroke extension, hemorrhagic stroke, recurrent stroke), general (cardiac, pulmonary, gastrointestinal events), development of antibiotic resistance (infections or colonization with resistant micro-organisms), and side-effects of medication (allergic reaction, diarrhea by Clostridium difficile, raised liver/plasma enzymes).
Statistical analysis
For ordinal analyses of the mRS, the proportional odds assumption was not met, for example, the odds ratio (OR) for one level and the next was not constant and could not be summarized with a common OR. Therefore, analysis was performed using a two-step approach with an assumption-free ordinal analysis: Agresti’s generalized ORs (R statistical software, GenOdds package). This method calculates the odds that, if a pair of observations are randomly selected from two groups (preventive antibiotic therapy or control) the outcome in one group is higher than the other. This method has the additional advantage that it takes tied observations (“ties”) into consideration: ties are observations that belong to the same group of the ordinal variable (mRS) and therefore none of the two is higher than the other. Since ignoring tied observations consistently overestimates treatment effect compared to splitting tied observations, we split tied observations.12,13 The ordinal overall, and subgroup analysis followed a two-step approach: the assumption-free ordinal analysis, stratified by age and NIHSS (in categories), was performed on individual trial level, followed by a random-effect inverse variance pooling across trials.
Meta-analysis of dichotomous outcomes (unfavorable outcome, death, infection, pneumonia rate) was performed using a one-step approach; data from all studies were pooled while accounting for the clustering of patients within trials 14 ). Logistic regression was used with “trial” and “trial*treatment” terms with adjustment for age and NIHSS (on a continuous scale). For subgroup analyses of dichotomous outcomes, the interaction term “treatment*pre-specified variable” and the prespecified variable separately were added to the model to test for statistically significant differences in treatment effects across the subgroups. SPSS version 26 and R statistical software were used for the analyses.
We assessed the amount of missing data for each outcome. Corresponding authors were contacted first for missing data. In case a certain variable was not collected in a trial, but was necessary for the analysis, this trial was excluded from the analysis. For each analysis, we describe the number of patients and trials on which this analysis was based. In case a variable was collected, but missing data occurred, the proportion of missing data was estimated. When this exceeded 5%, we analyzed whether data were missing at random or not, in case data were not missing at random the study was excluded from analysis. No data were imputed for this meta-analysis.
Role of the funding source
The study sponsors had no role in the study design, collection, analysis, and interpretation of the data, or the decision to submit the manuscript for publication. W.F. Westendorp had full access to all data in the study. All authors approved and were responsible for submission of the manuscript.
Results
In total, 18 publications were identified of which five were excluded (2 were not a randomized study, in 1 the randomization procedure was unclear, 1 study only included patients with indwelling catheters and in 1 study treatment with preventive antibiotic therapy was guided by procalcitonin levels; Supplementary table 2). Investigators of 13 trials were approached and 10 authors shared their data (2 studies were eligible for inclusion but we received no response from authors,6,15 1 study was eligible but an author responded that the database was no longer available 16 ; Supplementary table 3a). One additional trial was excluded because the received data did not match the original publication, leaving nine trials and 4197 stroke patients for the analysis (Table 1). Four trials were included as type 1 trials, aimed at improving outcome by reducing infections. These four trials included 3970 of 4197 (95%) of evaluated patients. Five trials were smaller type 2 trials, aimed at neuroprotection with the antibiotic minocycline. Risk of bias was generally low in type 1 studies and was moderate in some of the type 2 studies (Supplementary table 8).
Table 1.
Included studies.
| Author, year | Study population | Country | No. of patients | Antibiotic | Primary outcome | Secondary outcomes | Control group |
|---|---|---|---|---|---|---|---|
| Kalra et al., 2015 5 | Ischemic and hemorrhagic stroke patients with dysphagia | United Kingdom | 1217 | Local protocol | Post-stroke pneumonia in the first 14 days after stroke | Functional outcome (mRS) at 90 days, mortality, adverse events | Standard care |
| Westen-dorp et al., 2015 8 | Ischemic and hemorrhagic stroke patients | The Nether-lands | 2538 | Ceftriaxone | Functional outcome at 3 months on the mRS | Infections, pneumonia, mortality, adverse events | Standard care |
| Harms et al., 2008 4 | Patients with ischemic stroke (NIHSS>11) in middle cerebral artery territory | Germany | 79 | Moxifloxacin | Infection within 11 days after stroke | Neurological outcome (mRS), survival, immune-depression, induction of bacterial resistance | Placebo |
| Chamorro et al., 2005 3 | Ischemic and hemorrhagic stroke patients | Spain | 136 | Levo-floxacin | Infection within 7 days after stroke | Neurological outcome (mRS) and mortality at 90 days | Placebo |
| Chang et al., 2017 23 | Hemorrhagic stroke patients | USA | 20 | Minocycline | Adverse events | Change in serial NIHSS score, hematoma volume, MMP-9 measurements, 3-month functional outcome (mRS) and mortality | Placebo |
| Fouda et al., 2017 24 | Hemorrhagic stroke patients | USA | 16 | Minocycline | Serum concentrations of minocycline | ICH volume, inflammatory parameters | Standard care |
| Amiri-Nikpour et al., 2015 25 | Ischemic stroke patients | Iran | 53 | Minocycline | NIHSS at 3 months | NIHSS at 30, 60 days | Standard care |
| Kohler et al., 2013 26 | Ischemic and hemorrhagic stroke patients | Australia | 92 | Minocycline | Survival free of handicap (mRS ≤2) at day 90 | NIHSS at day 7, Barthel index at 90 days | Standard care |
| Blacker et al., 2013 27 | Ischemic stroke patients that received thrombolysis | Australia | 46 | Minocycline | Hemorrhagic transformation on CT-scan | — | Standard care |
The baseline characteristics were similar in antibiotic and control or placebo groups within the nine studies (Table 2). 2100 patients (50.0%) received preventive antibiotic treatment and 2097 (50.0%) standard care or placebo. Ischemic stroke was diagnosed in 3580 of 4196 patients (85%), hemorrhagic stroke in 467 patients (11%), TIA in 94 patients (2%), and another diagnosis was made in 55 patients (1%).
Table 2.
Baseline characteristics of all patients.
| Characteristic | Preventive antibiotic treatment (n = 2100) | Standard care/placebo (n = 2097) |
|---|---|---|
| Age (years) | 75 (65–82) | 75 (65–83) |
| Male sex | 52 (1095/2097) | 52 (1091/2096) |
| Medical history | ||
| Obstructive pulmonary disease | 9 (176/2055) | 7 (149/2047) |
| Diabetes mellitus | 20 (418/2098) | 20 (420/2095) |
| Atrial fibrillation | 22 (436/1995) | 23 (463/1990) |
| Pre-stroke disability (mRS) | 0 (0–1) | 0 (0–1) |
| Stroke severity (NIHSS) | 8 (4–15) | 7 (4–15) |
| Stroke type | ||
| Ischemic | 85 (1781/2100) | 86 (1799/2096) |
| Hemorrhagic | 12 (252/2100) | 10 (215/2096) |
| TIA | 2 (44/2100) | 2 (50/2096) |
| Other diagnosis | 1 (23/2100) | 2 (32/2096) |
| Intravenous thrombolysis | 32 (659/2061) | 31 (634/2057) |
| Dysphagia | 51 (922/1793) | 51 (918/1795) |
| Use of urinary cathether | 20 (262/1314) | 22 (286/1315) |
Data in % (n/N), median with interquartile range or mean with standard deviation.
mRS: modified Rankin scale; NIHSS: National Institute of Stroke Severity Scale; TIA: transient ischemic attack.
Preventive antibiotic therapy was not associated with a shift in functional outcome on the mRS at 3 months (OR 1.13, 95%CI 0.98–1.31, p = 0.0896, moderate heterogeneity, I2 47%, Supplementary figure 2 and 3, table 3). Preventive antibiotics was also not associated with favorable outcome in the analysis using dichotomization of the mRS (Table 3). However, in type 2 trials, preventive minocycline was associated with worse functional outcome on the total range of the mRS at 3 months (OR 1.46, 95%CI 1.02–2.09, p = 0.04, Supplementary figure 2).
Table 3.
Study outcomes.
| Antibiotics (n = 2100) % (n/N) | Standard care/placebo (n = 2097) % (n/N) | Odds ratio (95%CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Functional worsening on mRS | - | - | 1.13 (0.98–1.31) | 0.09 |
| Type 1 | 1.08 (0.94–1.25) | 0.27 | ||
| Type 2 | 1.46 (1.02–2.09) | 0.04 | ||
| Unfavorable functional outcome a | 52.0 (1057/2032) | 52.2 (1060/2029) | 0.85 (0.60–1.19) | 0.348 |
| Type 1 | 53.0 (1033/1949) | 53.1 (1032/1942) | 0.85 (0.60–1.19) | 0.348 |
| Type 2 | 28.9 (24/83) | 32.2 (28/87) | 0.25 (0.04–1.44) | 0.120 |
| Secondary outcomes | ||||
| Death | 16.7 (344/2066) | 15.3 (316/2067) | 1.13 (0.95–1.36) | 0.165 |
| Type 1 | 17.3 (339/1957) | 16.0 (313/1952) | 1.13 (0.94–1.35) | 0.199 |
| Type 2 | 4.6 (5/109) | 2.6 (3/115) | 1.82 (0.36–9.12) | 0.468 |
| Any infection | 13.4 (276/2066) | 20.3 (417/2059) | 0.60 (0.51–0.71) | 0.001 |
| Pneumonia | 9.2 (191/2066) | 9.9 (205/2061) | 0.92 (0.75–1.14) | 0.450 |
| UTI | 3.6 (74/2066) | 9.7 (201/2062) | 0.34 (0.24–0.48) | <0.001 |
| Other infection | 1.7 (35/2066) | 1.9 (39/2062) | 0.90 (0.56–1.46) | 0.639 |
All analyses are adjusted for age and stroke severity (NIHSS).
mRS: modified Rankin Scale; UTI: urinary tract infection.
aUnfavorable functional outcome: mRS 3–6 or Barthel Index <60 or deceased.
Preventive antibiotic therapy was associated with a decrease of any infections (13.4% in the preventive antibiotic group vs 20.3% in the control group, OR 0.60, 95% CI 0.51–0.71, p < 0.001). This was mainly driven by a decrease in UTI (74 of 2066 [3.6%] in the preventive antibiotic group vs. 201 of 2062 [9.7%] in the control group, OR 0.34 95%CI 0.26–0.45, p < 0.001), while the proportion of patients with pneumonia and other infections were similar between groups (191 of 2066 [9.2%] in the preventive antibiotic group vs. 205 of 2061 [9.9%] in the control group, OR 0.92 (0.75–1.14), p = 0.450 and 1.7 35/2066 [1.7%] vs 39/2062 [1.9%], OR 0.90 (0.56–1.46), p = 0.639). Any infection (OR 2.65, 95% CI 2.08–3.37, p < 0.001) and pneumonia (OR 6.76 95% CI 4.34–10.54, p < 0.001) were associated with unfavorable functional outcome in analyses adjusted for age and stroke severity.
Sex, stroke severity, type of stroke, treatment with thrombolysis, subgroups based on risk scores ISAN and A2DS2 score, dysphagia, time to treatment, antibiotic class, placebo-controlled versus open-label study, and whether treatment was administered according to protocol or not did not significantly influence treatment response of preventive antibiotic therapy (Supplementary table 12–14).
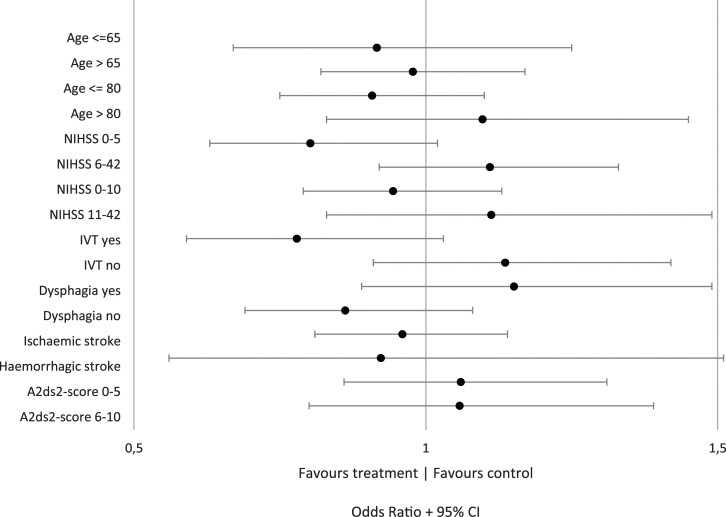
The analysis of preventive antibiotics in patients with lower stroke severity (NIHSS ≤5) suggested a favorable effect (p-value interaction 0.028) in type 1 trials (Figure 2). However, this analysis was merely a comparison between trials as 90% of patients with NIHSS ≤5 were derived from 1 trial and 1 other trial only included patients with NIHSS >5 (Supplementary table 19).
Figure 2.
Subgroup analysis for unfavorable outcome (mRS 3–6) for all trials. This figure shows the odds ratios for unfavorable outcome in patients randomized to antibiotic therapy versus patients randomized to standard care, for each subgroup of patients (y-axis). An odds ratio larger than one favors control, smaller than one favors antibiotic therapy.
The analyses of longer time to treatment suggested possible harm (p-value interaction 0.02, Supplementary table 15). The analysis for those treated with iv-thrombolysis showed a trend toward benefit (301 of 639 [47%] with unfavorable outcome in the preventive antibiotic group vs. 327 of 607 [54%] in the control group, p-value interaction 0.08, Supplementary table 15). Overall, the number of adverse events was comparable in both treatment arms (Supplementary table 18). A post-hoc sample size analysis for primary outcome showed sufficient power to detect a clinical meaningful effect even in smaller subgroups of patients (Supplementary appendix page 28).
Discussion
This individual patient meta-analysis did not show benefit of preventive antibiotic therapy in stroke patients. Preventive antibiotic therapy did reduce the occurrence of any infection but this was largely driven by a decrease in the proportion of patients with UTI but not a decrease in the incidence of pneumonia which was independently associated with unfavorable functional outcome. Extensive exploration of pre-specified subgroups did not show robust evidence of benefit in any particular subgroup. The suggested benefit for those treated with iv-thrombolysis is likely to have occurred by chance and the apparent harm in patients with longer time to treatment is likely to be due to confounding. The effect of preventive antibiotic therapy found in patients with lower stroke severity (NIHSS < 5) was merely a comparison between trials as 90% of patients with NIHSS < 5 were derived from 1 trial.
Preventive antibiotics did not reduce occurrence of pneumonia. In this meta-analysis, approximately 1 in 10 patients suffered from pneumonia, which is in line with previous evidence. 2 Pneumonia is one of the most common complications after stroke and contributes strongly to unfavorable outcome. 17 In a cohort study of 8251 stroke patients, the occurrence of pneumonia was associated with less favorable outcome at discharge (OR 0.2, 95% CI 0.14–0.29, p-value 0.001) and increased 1-year mortality (OR 3.0, 95%CI 2.5–3.7, p-value <0.0001). 18 The lack of effect of antibiotic therapy on the incidence of pneumonia raises the question whether the type and timing of antibiotic therapy may have been important. The antibiotic therapies used in included trials cover most of the pathogens associated with pneumonia after stroke, 19 although anaerobes might not have been covered in the PASS trial as ceftriaxone did not cover anaerobic pathogens. The preferred antibiotic regimen used in 70% of patients in the STROKE-INF trial did cover anaerobic pathogens but in this trial pneumonia frequency was also not reduced. Next, timing of start of antibiotic therapy might have been too late. In the two largest trials (PASS and STROKE-INF), patients had to start therapy within 24 and 48 h, respectively. This might have been too late as 75% of infections are diagnosed within 3 days after admission. 20 The time to treatment subgroup analysis showed that delay in treatment was associated with unfavorable outcome in the analysis of all trials and type 2 trials, but not in the separate analysis of the type 1 trials that were specifically aimed at preventing infections. In addition, it is likely that confounding exists in this subgroup analysis as patients who present early to the hospital benefit more from more rapid specialist stroke unit care and we could not correct for this possible confounding. Another potential explanation for the lack of effect of preventive antibiotics on outcome is that pneumonia after stroke could also incorporate a non-infective respiratory syndrome which antibiotics cannot prevent. 21 This respiratory syndrome may relate to chemical injury caused by inhalation of sterile gastric contents rather than a true infection by pathogenic bacteria, and therefore may not be preventable with antibiotic therapy. 21 Because antibiotic therapy did not reduce pneumonia frequency, it remains unclear whether pneumonia has a causal relationship with unfavorable outcome or is merely an epi-phenomenon of severe stroke.
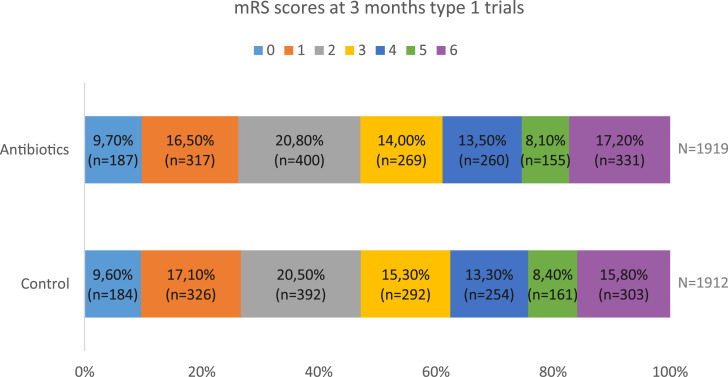
In this analysis, we aimed to include all the available randomized trial evidence but some data remained unavailable despite our best efforts. For type 1 trials, it is unlikely that the one missing trial would change the results of this analysis, as the number of included patients in this analysis is high (3970) and the missing study only included 60 patients. 6 In contrast, for type 2 trials focused on the neuroprotective effect of minocycline two missing trials could have impacted our results, in particular because the number of patients in these missing trials is similar to the number of patients included in the current analysis of type 2 trials.15,16 Indeed, in a recent study-level meta-analysis of minocycline trials that included these trials, a trend toward a favorable effect (mRS 0–2) of minocycline on functional outcome was seen (RR = 1.31; 95% CI 0.98–1.74, p = 0.06). 22 In addition, risk of bias was moderate in some of the studies on minocycline. Therefore, the evidence on the effect of minocycline on outcome in stroke patients is inconclusive. As trials for minocycline were small and probably underpowered, a subsequent larger trial could give more reliable estimates on the efficacy of minocycline (Figure 3).
Figure 3.
Modified Rankin Scale score at 3 months for patients included in type 1 trials.
In the current analysis, we did not adjust for multiple comparisons. As we did not find a robust effect in one of the subgroups, this is unlikely to have influenced results.
We found no benefit of preventive antibiotics aimed at reducing infection in prespecified subgroups of patients. One ongoing trial might significantly change the abovementioned results: the PRECIOUS-trial (ISRCTN 82217627). In the PRECIOUS-trial, not only preventive antibiotic therapy to prevent infections (ceftriaxone) is investigated, but also two other pharmacological interventions for post-stroke complications: metoclopramide for aspiration and paracetamol for fever. As the sample size of the study is 3800 patients, this study has the potential to change the abovementioned results for patients aged 66 or older.
In conclusion, preventive antibiotic therapy in patients with acute stroke decreases any infections but does not reduce pneumonia or unfavorable functional outcome. There were no significant treatment effects in any of the pre-specified subgroups. Preventive antibiotics did not benefit all or any subgroup of patients with acute stroke and can currently not be recommended.
Supplementary Material
Acknowledgments
None.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: WFW, JDV, CJS, AK, JH, LK, AM, AC, JJC, YR, MRAN, FAF, JAS, MGWD, PJN, DvdB declare no competing interests. DB is clinical advisor and stockholder in Argenica Therapeutics, a biotech company developing a neuroprotective drug.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: J.H. is partially supported by National Institute of Health Research NF-SI-0617–10120 and Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the UK NHS, NIHR, or the Department of Health and Social Care. Otherwise, this research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval: Ethical approval was not sought for the present study because of the nature of the study (systematic review and meta-analysis). This study was completed in accordance with the Helsinki Declaration as revised in 2013.
Informed consent: Informed consent was not sought separately for the present study because of the nature of the study (systematic review and meta-analysis).
Trial registration: Not applicable
Guarantor: WFW
Contributorship: WFW, JDV, PJN, DvdB researched literature and conceived the study. WFW, JDV, PJN, DvdB, JH, LK, AM, AC, JJC, YR, MRAN, FAF, JAS, DB were involved in patient recruitment of the original studies and providing data for the current study. WFW, JDV, CJS, AK, MGW, PJN, DvdB were involved in the development of the study. WFW wrote the first draft of the manuscript. Statistical analysis was performed by WFW and MGW. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Supplemental material: Supplemental material for this article is available online.
ORCID iD
Willeke F Westendorp https://orcid.org/0000-0003-3847-6605
References
- 1.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017; 120(3): 439–448. [DOI] [PubMed] [Google Scholar]
- 2.Westendorp WF, Nederkoorn PJ, Vermeij J-D, et al. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol 2011; 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamorro A, Horcajada JP, Obach V, et al. The early systemic prophylaxis of infection after stroke study: a randomized clinical trial. Stroke 2005; 36(7): 1495–1500. [DOI] [PubMed] [Google Scholar]
- 4.Harms H, Prass K, Meisel C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS One 2008; 3(5): e2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra L, Irshad S, Hodsoll J, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015; 386(10006): 1835–1844. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz S, Al-Shajlawi F, Sick C, et al. Effects of prophylactic antibiotic therapy with mezlocillin plus sulbactam on the incidence and height of fever after severe acute ischemic stroke: the Mannheim infection in stroke study (MISS). Stroke 2008; 39(4): 1220–1227. [DOI] [PubMed] [Google Scholar]
- 7.van de Beek D, Wijdicks EF, Vermeij FH, et al. Preventive antibiotics for infections in acute stroke: a systematic review and meta-analysis. Arch Neurol 2009; 66(9): 1076–1081. [DOI] [PubMed] [Google Scholar]
- 8.Westendorp WF, Vermeij J-D, Zock E, et al. The preventive antibiotics in stroke study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015; 385(9977): 1519–1526. [DOI] [PubMed] [Google Scholar]
- 9.Vermeij JD, Westendorp WF, Dippel DW, et al. Antibiotic therapy for preventing infections in people with acute stroke. Cochrane Database Syst Rev 2018; 1: Cd008530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CJ, Bray BD, Hoffman A, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc 2015; 4(1): e001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann S, Malzahn U, Harms H, et al. Development of a clinical score (A 2 DS 2 ) to predict pneumonia in acute ischemic stroke. Stroke 2012; 43(10): 2617–2623. [DOI] [PubMed] [Google Scholar]
- 12.Churilov L, Arnup S, Johns H, et al. An improved method for simple, assumption-free ordinal analysis of the modified Rankin Scale using generalized odds ratios. Int J Stroke 2014; 9(8): 999–1005. [DOI] [PubMed] [Google Scholar]
- 13.Cumming TB, Churilov L, Sena ES. The missing medians: exclusion of ordinal data from meta-analyses. PloS One 2015; 10(12): e0145580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340: c221. [DOI] [PubMed] [Google Scholar]
- 15.Padma Srivastava M, Bhasin A, Bhatia R, et al. Efficacy of minocycline in acute ischemic stroke: a single-blinded, placebo-controlled trial. Neurol India 2012; 60(1): 23–28. [DOI] [PubMed] [Google Scholar]
- 16.Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 2007; 69(14): 1404–1410. [DOI] [PubMed] [Google Scholar]
- 17.Davenport RJ, Dennis MS, Wellwood I, et al. Complications after acute stroke. Stroke 1996; 27(3): 415–420. [DOI] [PubMed] [Google Scholar]
- 18.Finlayson O, Kapral M, Hall R, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 2011; 77(14): 1338–1345. [DOI] [PubMed] [Google Scholar]
- 19.Kishore AK, Vail A, Jeans AR, et al. Microbiological etiologies of pneumonia complicating stroke: a systematic review. Stroke 2018; 49(7): 1602–1609. [DOI] [PubMed] [Google Scholar]
- 20.Vermeij FH, Scholte op Reimer WJM, de Man P, et al. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis 2009; 27(5): 465–471. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med 2001; 344(9): 665–671. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra K, Chang JJ, Khunger A, et al. Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J Neurol 2018; 265(8): 1871–1879. [DOI] [PubMed] [Google Scholar]
- 23.Chang JJ, Kim-Tenser M, Emanuel BA, et al. Minocycline and matrix metalloproteinase inhibition in acute intracerebral hemorrhage: a pilot study. Eur J Neurol 2017; 24(11): 1384–1391. [DOI] [PubMed] [Google Scholar]
- 24.Fouda AY, Newsome AS, Spellicy S, et al. Minocycline in acute cerebral hemorrhage: an early phase randomized trial. Stroke 2017; 48(10): 2885–2887. [DOI] [PubMed] [Google Scholar]
- 25.Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, et al. An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent effect. Acta Neurol Scand 2015; 131(1): 45–50. [DOI] [PubMed] [Google Scholar]
- 26.Kohler E, Prentice DA, Bates TR, et al. Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta-analysis. Stroke 2013; 44(9): 2493–2499. [DOI] [PubMed] [Google Scholar]
- 27.Blacker DJ, Prentice D, Alvaro A, et al. Reducing haemorrhagic transformation after thrombolysis for stroke: a strategy utilising minocycline. Stroke Res Treat 2013; 2013: 362961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.