Abstract
The aim of this study was to examine the impact of features of dysmetabolism on liver disease severity, evolution, and clinical outcomes in a real‐life cohort of patients treated with direct acting antivirals for chronic hepatitis C virus (HCV) infection. To this end, we considered 7,007 patients treated between 2014 and 2018, 65.3% with advanced fibrosis, of whom 97.7% achieved viral eradication (NAVIGATORE‐Lombardia registry). In a subset (n = 748), liver stiffness measurement (LSM) was available at baseline and follow‐up. Higher body mass index (BMI; odds ratio [OR] 1.06 per kg/m2, 1.03‐1.09) and diabetes (OR 2.01 [1.65‐2.46]) were independently associated with advanced fibrosis at baseline, whereas statin use was protective (OR 0.46 [0.35‐0.60]; P < 0.0001 for all). The impact of BMI was greater in those without diabetes (P = 0.003). Diabetes was independently associated with less pronounced LSM improvement after viral eradication (P = 0.001) and in patients with advanced fibrosis was an independent predictor of the most frequent clinical events, namely de novo hepatocellular carcinoma (HCC; hazard ratio [HR] 2.09 [1.20‐3.63]; P = 0.009) and cardiovascular events (HR 2.73 [1.16‐6.43]; P = 0.021). Metformin showed a protective association against HCC (HR 0.32 [0.11‐0.96]; P = 0.043), which was confirmed after adjustment for propensity score (P = 0.038). Diabetes diagnosis further refined HCC prediction in patients with compensated advanced chronic liver disease at high baseline risk (P = 0.024). Conclusion: Metabolic comorbidities were associated with advanced liver fibrosis at baseline, whereas statins were protective. In patients with advanced fibrosis, diabetes increased the risk of de novo HCC and of cardiovascular events. Optimization of metabolic comorbidities treatment by a multi‐disciplinary management approach may improve cardiovascular and possibly liver‐related outcomes.
Abbreviations
- ASA
acetylsalicylic acid
- BMI
body mass index
- cACLD
compensated advanced chronic liver disease
- CHC
chronic hepatitis C
- CI
confidence interval
- CVE
cardiovascular events
- DAA
direct acting antiviral
- F
female
- HCC
hepatocellular carcinoma
- HIV
human immunodeficiency virus
- HR
hazard ratio
- LSM
liver stiffness measurement
- NS
not significant
- OR
odds ratio
- SVR
sustained virological response
- US
ultrasonography
Chronic hepatitis C (CHC) remains a leading causes of liver disease worldwide. However, direct acting antivirals (DAAs) have revolutionized hepatitis C virus (HCV) treatment with simple, tolerable, pan‐genotypic combinations that currently achieve cure rates exceeding 95% in a few weeks.( 1 ) This progress has allowed us to treat most patients in follow‐up in developed countries, reducing the burden of CHC‐related end‐stage liver disease.( 2 ) Sustained virological response (SVR) to DAA treatment corresponds to the cure of HCV infection, and in patients without severe portal hypertension this results in a reduction of hepatic fibrosis and a decrease in the risk of liver‐related and all clinical events at follow‐up.( 3 , 4 , 5 ) However, in individuals who had already developed advanced fibrosis at the time of cure, some complications such as hepatocellular carcinoma (HCC) development remain a major threat.( 6 , 7 )
Among the drivers of liver injury that can persist after HCV eradication, fatty liver plays a prominent role,( 7 ) through the induction of lipotoxicity and steatohepatitis.( 8 ) However, uncertainties remain on the burden of metabolic comorbidities, in particular of diabetes and obesity, in patients with advanced liver fibrosis who cleared HCV.( 7 ) Furthermore, the impact of dysmetabolism and of the treatments for metabolic comorbidities on the relative risk of hepatic and cardiovascular events (CVEs) has never been reported in large real‐life cohorts.
Within this context, the aim of this study was to examine the impact of dysmetabolism features (diabetes, overweight, and the presence of any of these features or fatty liver) and of pharmacological therapy on liver disease severity and evolution, and on follow‐up liver‐related and CVEs, in a real‐life cohort of patients treated with DAAs representative of an entire European region (NAVIGATORE‐Lombardia Network registry).
Experimental Procedures
Study Cohort
Data of all patients with CHC treated with DAAs in the Lombardy region in Northern Italy, starting from December 2014 to December 2018 in 48 different clinical centers, were collected through the NAVIGATORE Lombardia Network web‐based platform, which is based on REDCap (Research Electronic Data Capture; http://projectredcap.org).( 9 ) Recruitment procedures and disease staging are reported in the Supporting Methods.( 10 , 11 ) Briefly, liver fibrosis was staged in all patients before DAA treatment, either by liver biopsy (METAVIR stage) or noninvasively by transient elastography. Liver stiffness measurement (LSM) thresholds are reported in the Supporting Methods. Patients with decompensated cirrhosis were allocated to fibrosis stage F4.( 10 ) After data retrieval and revision of the database by visual data inspection, we selected 7,007 (74.0%) of 9,470 for whom age, sex, anthropometric features, fibrosis staging, metabolic comorbidities, and pharmacological treatment were available (main study cohort). In 748 (10.7%), systematic LSM re‐evaluation at 24 weeks following treatment was performed. Among 4,578 patients with advanced fibrosis, follow‐up data were available for 2,946 individuals at 24 weeks and for 1,905 at 24 months following treatment (64.4% and 41.6% respectively; Prospective cohort). CVEs were defined as stroke, myocardial infarction, hospitalization due to ischemic heart disease or heart failure, and sudden death.
In a second phase, we collected data relative to the presence of ultrasonography (US) bright liver in a subset of 2,723 of 3,507 patients with cirrhosis (77.6%), from 21 participating centers. In this subset (US cohort), presence of fatty liver was defined by US after confirmation of SVR.( 11 ) To further test the clinical relevance of the findings, we tested the ability of dysmetabolic features to refine risk stratification of de novo HCC in patients who cleared HCV infection with compensated advanced chronic liver disease (cACLD), where risk stratification was assessed based on follow‐up LSM and albumin values (reported in detail in the Supporting Methods).( 12 )
The clinical features of patients included in the main study cohort are presented in Table 1. The study flowchart is presented in Fig. 1. Informed consent was obtained from each patient, and the registry was approved by the ethical committees and review boards of the participating centers and conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The study analysis plan was approved by the Ethics Committee of the University of Milan (on October 23, 2018).
TABLE 1.
Clinical Features of 7,007 Patients From the NAVIGATORE‐Lombardia Cohort (Main Study Cohort) With Complete Baseline Clinical Data Stratified by Fibrosis Severity
| Advanced Fibrosis | P Value | ||
|---|---|---|---|
| No (Stage F0‐F2) | Yes (Stage F3‐F4) | ||
| n | 2,429 (34.7) | 4,578 (65.3) | |
| Age, years | 60.1 ± 13.4 | 61.9 ± 12.2 | <0.0001 |
| Sex, F | 1,187 (48.9) | 1,784 (39.0) | <0.0001 |
| BMI, kg/m2 | 24.2 ± 3.9 | 25.3 ± 4.2 | <0.0001 |
| Alcohol use, yes | 402 (16.6) | 816 (17.8) | 0.18 |
| HCV genotype | <0.0001 | ||
| G1 | 1,355 (55.8) | 2,724 (59.5) | |
| G2 | 604 (24.9) | 688 (15.0) | |
| G3 | 240 (9.9) | 661 (14.4) | |
| G4 | 210 (8.6) | 488 (10.7) | |
| Other | 3 (0.1) | 13 (0.1) | |
| NA | 17 (0.7) | 6 (0.3) | |
| Fibrosis, stage | 0: 455 (18.7) | 3: 1,071 (23.4) | <0.0001 |
| 1: 1,126 (46.4) | 4: 3,507 (76.6) | ||
| 2: 848 (34.9) | |||
| FIB‐4 score | 1.9 ± 2.1 (n = 2,204) | 5.0 ± 4.4 (n = 4,085) | <0.0001 |
| LSM, kPa | 6.3 ± 1.9 (n = 2,272) | 19.9 ± 11.1 (n = 3,849) | <0.0001 |
| HBV, HBcAg or HBsAg+ | 622 (27.7) | 1,243 (30.7) | 0.011 |
| HIV+ | 245 (10.0) | 790 (17.1) | <0.0001 |
| Body weight | <0.0001 | ||
| Underweight (<20 kg/m2) | 270 (11.1) | 314 (6.7) | |
| Normal weight (20‐25 kg/m2) | 1,308 (53.8) | 2,148 (46.9) | |
| Overweight (25.1‐29.9 kg/m2) | 653 (26.9) | 1,658 (34.2) | |
| Obese (≥30 kg/m2) | 198 (8.1) | 549 (12.0) | |
| Treated diabetes | 147 (6.0) | 571 (12.5) | <0.0001 |
| Treated hypertension | 751 (30.9) | 1,581 (34.5) | 0.002 |
| Statin therapy | 133 (5.3) | 136 (3.0) | <0.0001 |
| Treated hypertriglyceridemia | 23 (1.0) | 32 (0.7) | 0.26 |
Data are shown as mean ± SD or n (%) values, as appropriate.
Abbreviations: F, female; FIB‐4, Fibrosis‐4 index; HBcAg, hepatitis B core antigen; HBsAg, hepatitis B surface antigen.
FIG. 1.
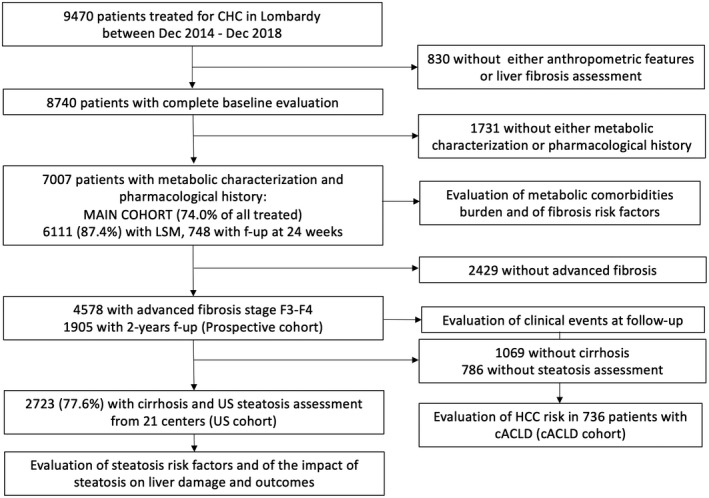
Study flow chart.
Statistical Analysis
For descriptive statistics, categorical variables are shown as number and proportion, while continuous variables are shown as mean and SD or median and interquartile range (IQR), as appropriate.
Observational associations were performed by fitting data to generalized linear models. Logistic regression models were fit to examine binary traits, and the association between metabolic risk factors and liver disease stage/clinical outcomes was adjusted for the covariates specified in the text. The product terms of significant predictors were tested in multivariable generalized linear models to survey for interactions in determining the risk of advanced fibrosis. The impact of dysmetabolism on survival was assessed by log‐rank test, whereas the independent predictors of clinical events were assessed by multivariable Cox regression proportional hazard models. As the main study aim was to examine the impact of metabolic comorbidities and their treatments on the clinical outcomes, we included as covariates in multivariable models demographic features (age, sex), body mass index (BMI), presence of diabetes, use of drugs potentially affecting outcomes (statins and metformin, when significant at univariate analysis), the main clinical risk factors associated with the specific outcomes at univariate analysis (detailed in the Results section and tables), and variables significantly associated with the outcome at univariate analysis. The association between metformin exposure and de novo HCC development was adjusted for propensity score, as reported in the Supporting Methods.
Statistical analysis was carried out using the JMP 16.0 Pro Statistical Analysis Software (SAS Institute, Cary, NC) and R statistical analysis software version 3.5.2 (http://www.R‐project.org/). P values < 0.05 (two‐tailed) were considered significant.
Results
Independent Determinants of Advanced Fibrosis and of Fibrosis Regression
The clinical features of patients in the main study cohort stratified by the presence of advanced fibrosis are reported in Table 1, Supporting Results, and Supporting Fig. S1. The prevalence of obesity according to fibrosis stage and diabetes status is shown in Fig. 2; obesity was more frequently detected in patients with diabetes than in those without (P < 0.0001), and its prevalence increased progressively with fibrosis stage both in the overall cohort and in patients without diabetes (P < 0.0001). The variables independently associated with advanced fibrosis are reported in Table 2. Age was the variable most strongly associated with advanced fibrosis, followed by HCV genotype (G3>G1>G2), BMI, human immunodeficiency virus (HIV) co‐infection, and diabetes (P < 0.0001 for all). A significant interaction between BMI and diabetes (but not between other independent predictors) on the risk of advanced fibrosis was observed, in that the protective impact of lower BMI was attenuated in those with diabetes (interaction term reported in Table 1; P = 0.003).
FIG. 2.
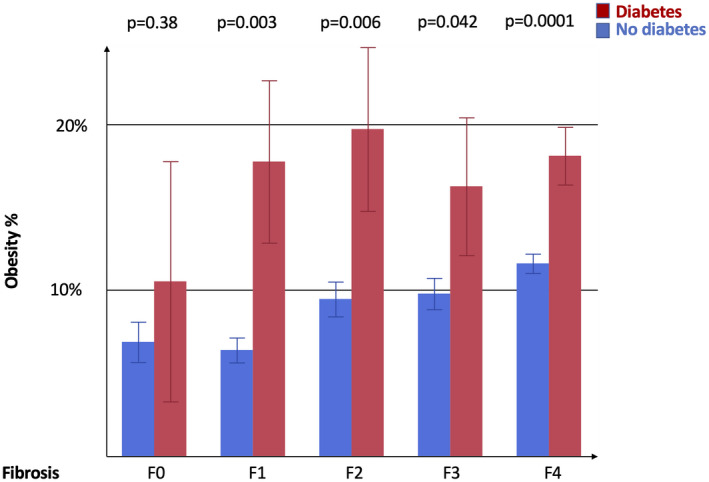
Prevalence of obesity in patients stratified by fibrosis stage and diabetes diagnosis. Data are shown as % and SEM. P < 0.0001 for increased prevalence of obesity with fibrosis stage severity in the overall cohort and patients without diabetes (age and sex adjusted); P = NS in patients with diabetes; and P < 0.05 for the higher prevalence of obesity in diabetes versus without diabetes across fibrosis stages F0‐F4 (age and sex adjusted).
TABLE 2.
Variables Independently Associated With Advanced Fibrosis (Stage F3‐F4) at Baseline at Multivariable Logistic Regression Analysis (n = 7,007)
| OR | 95% CI | P Value | |
|---|---|---|---|
| Age, 10 years | 1.33 | 1.28‐1.38 | <0.0001 |
| Sex, F | 0.68 | 0.61‐0.77 | <0.0001 |
| HCV genotype, vs. 1 | 2: 0.50 | 0.44‐0.58 | <0.0001 |
| 3: 1.52 | 1.28‐1.81 | ||
| 4: 1.18 | 0.98‐1.41 | ||
| HBV, yes | 0.98 | 0.87‐1.11 | 0.76 |
| HIV, yes | 2.10 | 1.77‐2.48 | <0.0001 |
| BMI*, kg/m2 | 1.07 | 1.06‐1.09 | <0.0001 |
| Hypertension, yes | 1.04 | 0.92‐1.17 | 0.53 |
| Diabetes*, yes | 2.01 | 1.65‐2.46 | <0.0001 |
| Statins, yes | 0.46 | 0.35‐0.60 | <0.0001 |
| Treated hypertriglyceridemia, yes | 0.78 | 0.44‐1.38 | 0.39 |
| Alcohol use, yes | 0.98 | 0.85‐1.14 | 0.34 |
Analyses were conducted a multivariable generalized linear model adjusted for the independent variables shown in the table plus liver transplantation.
Estimate. −0.03 ± 01 (P = 0.003) for the BMI × diabetes interaction term ([BMI‐24.88] × diabetes), meaning that the impact of overweight was significantly larger ithose without a diabetes diagnosis.
Abbreviation: HBV, hepatitis B virus.
Conversely, statin therapy was independently and inversely associated with advanced fibrosis (Table 2; P < 0.0001) and LSM values (11.9 ± 8.6 vs. 14.9 ± 11.1 kPa; P < 0.0001). Sensitivity analyses are reported in the Supporting Results and Tables S1‐S2.
SVR was achieved by 6,849 participants (97.7%). Lack of SVR was independently associated with infection by HCV‐G3, advanced fibrosis, and male sex, but not with metabolic risk factors nor with statin use (Supporting Table S3). The independent determinants of fibrosis progression in patients with available paired LSM evaluation at baseline and 24 weeks following treatment are given in Table 3. After adjustment for baseline values and the main clinical covariates, LSM tended to decrease in patients who achieved SVR (estimate −0.191 ± 0.088; P = 0.029), but less so in those affected by diabetes (estimate +0.047 ± 0.023; P = 0.039).
TABLE 3.
Independent Predictors of LSM Changes at 24‐Week Follow‐up After Therapy in 748 Patients With Coupled Evaluations
| Term | Estimate | SEM | P Value |
|---|---|---|---|
| Sex, F | +0.008 | 0.016 | 0.63 |
| Age, 10 years | +0.010 | 0.012 | 0.44 |
| SVR, yes | −0.191 | 0.088 | 0.029 |
| BMI, kg/m2 | +0.002 | 0.004 | 0.57 |
| Diabetes, yes | +0.047 | 0.023 | 0.039 |
A multivariable generalized linear model adjusted for the variants reported in the table and baseline LSM values.
Impact of Diabetes on Clinical Events in Patients With Advanced Fibrosis
The impact of features of dysmetabolism on clinical events, and variables associated with fatty liver in patients with cirrhosis, are presented in the Supporting Results, Table S5, and Fig. S1. The most frequent clinical event was HCC development, followed by CVEs and ascites (incidence rates 2.2%, 1.9% and 0.3%, respectively).
During follow‐up, 80 of 425 (18.8%) patients with previous HCC had a relapse, whereas 145 of 4,178 (3.5%) of patients with advanced fibrosis developed de novo HCC. The independent determinants of HCC risk during follow‐up in all patients with advanced fibrosis are given in Supporting Table S6. Among patients with previous HCC, 80 (20.9%) recurred after a median of 14 (IQR 1‐24) months. The independent determinants of de novo HCC in patients with advanced fibrosis in the Prospective cohort are provided in Table 4, upper panel. Development of de novo HCC was independently associated with lack of SVR (P < 0.0001), older age (P = 0.0002), male sex (P = 0.020), infection by HCV‐G3 (P = 0.021), and cirrhosis (P = 0.043). HCC risk was higher in patients with diabetes (P = 0.009), whereas it was lower in those taking metformin (P = 0.042). Conversely, we did not detect any impact of BMI (P = not significant [NS]), nor of acetylsalicylic acid (ASA) use (P = NS) or of statin treatment (regardless of statin class; Supporting Table S6, upper panel).
TABLE 4.
Independent Determinants of the Risk of Development of de Novo HCC (n = 145) and CVEs (n = 53) in Patients With CHC With Advanced Fibrosis
| HR | 95% CI | P Value | |
|---|---|---|---|
| De Novo HCC (n = 4,178 at risk) | |||
| Age, years | 1.04 | 1.02‐1.06 | 0.0002 |
| Sex, F | 0.60 | 0.39‐0.92 | 0.020 |
| HCV, G3 vs. other | 1.80 | 1.09‐2.96 | 0.021 |
| SVR, yes | 0.28 | 0.16‐0.49 | <0.0001 |
| Cirrhosis vs. fibrosis F3 | 7.65 | 1.07‐54.84 | 0.043 |
| BMI, kg/m2 | 1.01 | 0.97‐1.06 | 0.59 |
| Diabetes, yes | 2.09 | 1.20‐3.63 | 0.009 |
| Metformin, yes | 0.32 | 0.11‐0.96 | 0.042 |
| CVEs (n = 4,578 at risk) | |||
| Age, years | 1.04 | 1.00‐1.08 | 0.035 |
| Sex, F | 0.55 | 0.24‐1.23 | 0.14 |
| BMI, kg/m2 | 1.06 | 0.98‐1.15 | 0.13 |
| HIV, yes | 2.75 | 1.18‐6.46 | 0.020 |
| Diabetes, yes | 2.73 | 1.16‐6.43 | 0.021 |
| Metformin, yes | 0.50 | 0.13‐1.95 | 0.32 |
| Treated hypertension, yes | 1.78 | 0.89‐3.19 | 0.11 |
| Statin, yes | 4.85 | 1.81‐13.0 | 0.0016 |
| ASA, yes | 2.27 | 0.95‐5.38 | 0.063 |
A multivariable Cox proportional hazard model analysis adjusted for the covariates reported in the table.
The combined impact of diabetes diagnosis and metformin treatment on the incidence of de novo HCC in patients with cirrhosis is shown in Fig. 3. HCC incidence was higher in patients with diabetes not taking metformin than in those without diabetes (P = 0.0015) and in those with diabetes taking metformin (P = 0.018). Exposure to metformin remained associated with protection against development of de novo HCC even after adjustment for propensity score (hazard ratio [HR] 0.24, 95% confidence interval [CI] 0.07‐0.87; P = 0.029; Supporting Table S7). The protective association remained significant even after restricting this analysis to patients with diabetes (HR 0.22, 95% CI 0.06‐0.82; P = 0.024).
FIG. 3.
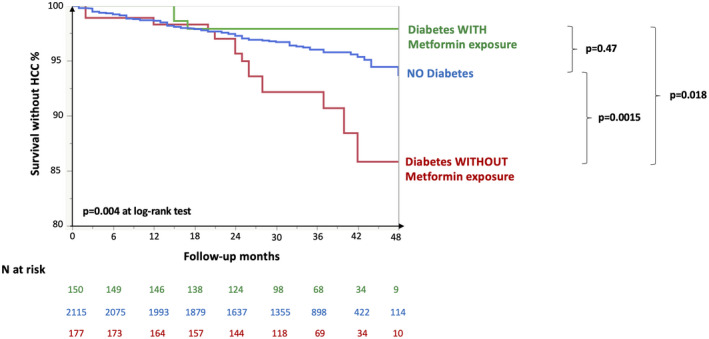
Impact of diabetes and metformin therapy on the incidence of de novo HCC in 2,442 patients with cirrhosis. P values are shown at log‐rank test.
To further evaluate the clinical relevance of these findings, we examined whether diabetes diagnosis could improve HCC risk stratification based on evaluation of LSM and serum albumin levels following SVR in 536 patients with compensated alcohol‐associated liver disease, 305 (56.9%) being classified as at high risk (cACLD cohort; Fig. 4). We first validated the score for prediction of HCC risk (P = 0.009; Fig. 4A). Reclassification of high‐risk patients according to diabetes (Fig. 4B) highlighted a higher HCC risk in those with diabetes (n = 43, 8.0%) as compared to those at low risk (P < 0.0001) and to those at high risk without diabetes (P = 0.024).
FIG. 4.
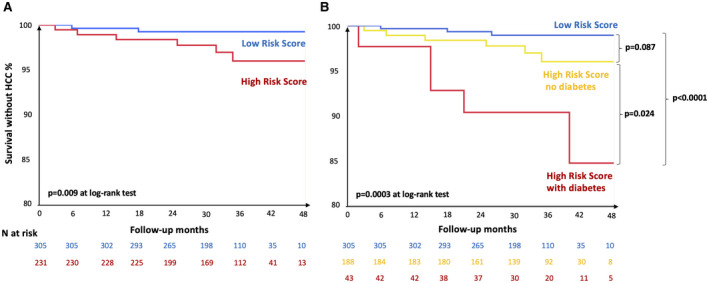
Impact of diabetes on the reclassification of HCC risk in 536 patients with cACLD. We considered patients with LSM > 10 kPa at baseline, who achieved viral eradication, with compensated liver disease, no previous history of HCC, and re‐evaluation of LSM and serum albumin at follow‐up. HCC risk was evaluated according to Pons et al.( 12 ) Patients with LSM ≥ 20 kPa at follow‐up and those with LSM = 10‐20 kPa and albumin levels < 4.4 g/dL at follow‐up were classified as at higher risk. P values are shown at log‐rank test.
The independent determinants of CVEs (n = 53) in the Prospective cohort are provided in Table 4, lower panel. CVEs were associated with being in treatment with statins (P = 0.0016), HIV infection (P = 0.020), diabetes (P = 0.021), and older age (P = 0.035). There was a trend for association between ASA use and higher CVE risk (P = 0.063), whereas sex, smoking status, treated hypertension, and SVR achievement had no significant impact (P = NS). There was no significant difference in the impact on CVE between hydrophilic and lipophilic statins (Supporting Table S6, lower panel; P = 0.79).
Discussion
In this study, we examined the impact of the features and treatments of dysmetabolism on the severity and evolution of liver damage in a real‐life cohort representative of patients with CHC treated with DAAs in a large region of Northern Italy.
First, we confirmed that adiposity and diabetes are robustly associated with the development of advanced fibrosis, which is likely partly mediated by fatty liver.( 13 ) One finding was that the impact of higher BMI was less marked in patients with diabetes. The role of viral factors is discussed in the Supporting Discussion. As most patients with type 2 diabetes have marked insulin resistance regardless of body weight, these data are in line with the notion that insulin resistance leading to hepatic fat accumulation is the main mechanism underlying the association between dysmetabolism and fibrosis. Approximately 1 in 8 patients with advanced fibrosis cured from HCV had diabetes.
Second, after taking into account the impact of pharmacological treatment, we confirmed that diabetes, but not BMI, affects the evolution of liver damage after viral eradication.( 6 , 7 , 14 , 15 , 16 ) In a subset with systematic re‐evaluation at 24 weeks after treatment, diabetes was independently associated with reduced improvement of LSM, which correlated with liver‐related events after SVR.( 17 ) In patients with advanced fibrosis, diabetes was an independent predictor of the two main clinical events at follow‐up, i.e., development of de novo HCC and of CVE. Although previous studies conducted in smaller cohorts of patients with CHC treated with DAAs often failed to detect a significant impact of diabetes on HCC,( 18 , 19 , 20 , 21 ) our data suggest that patients with diabetes should be prioritized for HCC surveillance, as well as for multidisciplinary management to prevent non‐liver‐related events. Supporting the clinical relevance of these findings, the presence of diabetes was able to refine HCC risk prediction in patients with cACLD at high baseline risk, based on noninvasive evaluation by LSM and serum albumin after SVR.( 12 ) In contrast, a more comprehensive definition of dysmetabolism encompassing diabetes, overweight, or the presence of fatty liver was not useful to improve risk stratification of clinical events.
Notably, in the present cohort, patients with advanced fibrosis and diabetes displayed a low use of key drugs prescribed for CVE prevention. The prevalence of statin treatment was approximately 14% in those with diabetes and arterial hypertension. Lack of prescription despite the likely presence of clinical indication did not appear to be justified by the severity of liver damage, as fibrosis stage and liver enzymes were not significantly different between treated and untreated individuals. On the other hand, the impact of HCV replication and of severe liver disease on the reduction of circulating lipid levels may have represented a factor determining low treatment uptake, especially before SVR.( 22 , 23 )
Third, pharmacological treatments for metabolic comorbidities were associated with protection against the main investigated outcomes. Statin use was robustly associated with protection against advanced fibrosis, which persisted even considering only patients without cirrhosis (stage F3 fibrosis as main outcome), suggesting that the association was not accounted for by a prescription bias. Although in the absence of randomized controlled trials definite conclusions cannot be drawn, evidence in fatty liver disease and CHC is consistent with the notion that statins protect from progressive liver disease, with an effect size consistent with the present results.( 24 , 25 , 26 ) The mechanisms underpinning this protective association encompass modulation of lipid accumulation, inflammation, and fibrogenesis,( 27 ) but potentially also inhibition of HCV replication.( 28 ) Of note, recent data support the safety of statin administration in patients with cirrhosis, with potential benefits that extend to the amelioration of portal hypertension,( 29 ) and use of lipophilic statins has been associated with chemoprevention of HCC.( 30 ) Possibly due to the limited power, lack of information about treatment duration and dose, and different metabolisms in patients with severe liver disease, we could not appreciate any significant effect of treatment with either with lipophilic or hydrophilic statins on HCC risk during the follow‐up. On the other hand, although it may be a marker of less‐advanced diabetes and liver dysfunction, exposure to metformin was associated with protection against HCC development independently of cirrhosis and of several clinical confounders in patients with diabetes.( 31 , 32 , 33 ) These data are consistent with previous evidence of a possible chemopreventive activity of metformin on hepatic carcinogenesis.( 31 , 32 , 33 ) Of note, the protective association between metformin exposure and de novo HCC development persisted after adjustment for propensity score.
Although it was previously reported that HCV eradication may also reduce CVEs,( 34 ) no data were yet available on the independent CVE predictors in patients with advanced fibrosis after SVR. Possibly due to the relatively low number of patients who did not achieve SVR, we could not detect a benefit of SVR on CVEs. However, we identified diabetes and HIV coinfection( 35 ) as main CVE risk factors. The risk of CVE after viral eradication was higher in patients taking statins, and a similar trend was observed for ASA. Given the overwhelming evidence from randomized trials that statins reduce CVE, this observation is likely accounted by the low rate of treatment uptake, suggesting that in this subgroup those who were under treatment were at very high CVE risk or already had events and were on secondary prevention. Prescription of inadequate drug dosages due to the fear of hepatic adverse events may also contribute to explain these findings.
Finally, we identified BMI, absence of hepatic decompensation and younger age, HIV co‐infection, and likely diabetes as risk factors for US fatty liver in patients with cirrhosis. Possibly due to the confounding effect of such predictors of clinical events as advanced disease and aging, which favor burnt‐out fatty liver and obscure the identification of fat accumulation by US scan, in the present cohort, fatty liver diagnosis did not significantly improve risk stratification.
This study suffers from common limitations inherent to large registry database related to the detailed characterization of patients. For example, alcohol intake and comorbidities were self‐reported or identified by pharmacological treatment, and although evaluation of the pharmacological history was a strength, the study follow‐up was only relatively long and we could not adjust for competing risk of death; the dosage and duration of treatment were not available, nor were measurements of insulin resistance and determination of genetic risk factors for fatty liver disease. Furthermore, the present findings may not be applicable to different populations and health care settings. In particular, despite an increase in prescriptions during the last years, statin use remains lower in Italy and Southern Europe than in Northern European countries, although data obtained in patients with advanced liver fibrosis are scarce.( 36 )
In conclusion, metabolic comorbidities are associated with liver disease stage and frequently observed in patients with advanced fibrosis who cleared HCV. In this subset, diabetes, but not obesity nor steatosis, was associated with increased risk of de novo HCC and CVE, identifying a subset of patients at higher risk of both hepatic and non‐hepatic clinical events, whereas exposure to metformin was protective. In a clinical setting focused on the prevention of hepatic complications of advanced liver fibrosis, management of metabolic comorbidities was likely suboptimal, and complicated by the presence of severe liver disease. Therefore, optimization of the management of metabolic comorbidities in patients with advanced fibrosis by a multi‐disciplinary management approach may improve cardiovascular and possibly liver‐related outcomes.
Supporting information
Supplementary Material
Acknowledgment
The authors thank Marta Borghi for the data collection and Rossana Carpani for the administrative assistance (Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Milano).
Supported by Gilead Sciences (Gilead Fellowship 2018 Italy, Gilead_IN‐IT‐989‐5790), Ministero della Salute (CV PREVITAL, RF‐2016‐02364358), Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico (PR‐0391 and RC100017A), and European Commission (101016726 and 777377).
Potential conflict of interest: Dr. Valenti received grants from Gilead. Dr. Pasulo advises AbbVie. Dr. D’Ambrosio consults and received grants from Gilead and AbbVie. Dr. Puoti advises, is on the speakers’ bureau, and received grants from Gilead, AbbVie, and Merck. Dr. Rumi consults and received grants from Gilead. She received grants from AbbVie. Dr. Lampertico advises and is on the speakers’ bureau for Bristol‐Myers Squibb, Roche, Gilead, GlaxoSmithKline, MSD, AbbVie, Arrowhead, Alnylam, Eiger, Myr, and Janssen. Dr. Fagiuoli is on the speakers’ bureau for Gilead, MSD, AbbVie, Novartis, Intercept, and Bayer.
References
- 1. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018;69:461‐511. [DOI] [PubMed] [Google Scholar]
- 2. Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090‐1099.e1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Marco V, Calvaruso V, Ferraro D, Bavetta MG, Cabibbo G, Conte E, et al. Effects of eradicating hepatitis C virus infection in patients with cirrhosis differ with stage of portal hypertension. Gastroenterology 2016;151:130‐139.e132. [DOI] [PubMed] [Google Scholar]
- 4. Aghemo A, Prati GM, Rumi MG, Soffredini R, D'Ambrosio R, Orsi E, et al. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology 2012;56:1681‐1687. [DOI] [PubMed] [Google Scholar]
- 5. D'Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 2012;56:532‐543. [DOI] [PubMed] [Google Scholar]
- 6. Degasperi E, Galmozzi E, Pelusi S, D'Ambrosio R, Soffredini R, Borghi M, et al. Hepatic fat—genetic risk score predicts hepatocellular carcinoma in HCV cirrhotic patients treated with DAAs. Hepatology 2020;72:1912‐1923. [DOI] [PubMed] [Google Scholar]
- 7. Negro F. Residual risk of liver disease after hepatitis C virus eradication. J Hepatol 2021;74:952‐963. [DOI] [PubMed] [Google Scholar]
- 8. Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, et al. Non‐invasive stratification of hepatocellular carcinoma risk in non‐alcoholic fatty liver using polygenic risk scores. J Hepatol 2021;74:775‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soria A, Fava M, Bernasconi DP, Lapadula G, Colella E, Valsecchi MG, et al. Comparison of three therapeutic regimens for genotype‐3 hepatitis C virus infection in a large real‐life multicentre cohort. Liver Int 2020;40:769‐777. [DOI] [PubMed] [Google Scholar]
- 10. European Association for Study of L, Asociacion Latinoamericana para el Estudio del H . EASL‐ALEH Clinical Practice Guidelines: non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237‐264. [DOI] [PubMed] [Google Scholar]
- 11. Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol 2007;102:2716‐2717. [DOI] [PubMed] [Google Scholar]
- 12. Pons M, Rodríguez‐Tajes S, Esteban JI, Mariño Z, Vargas V, Lens S, et al. Non‐invasive prediction of liver‐related events in patients with HCV‐associated compensated advanced chronic liver disease after oral antivirals. J Hepatol 2020;72:472‐480. [DOI] [PubMed] [Google Scholar]
- 13. Dyal HK, Aguilar M, Bhuket T, Liu B, Holt EW, Torres S, et al. Concurrent obesity, diabetes, and steatosis increase risk of advanced fibrosis among HCV patients: a systematic review. Dig Dis Sci 2015;60:2813‐2824. [DOI] [PubMed] [Google Scholar]
- 14. Abe K, Wakabayashi H, Nakayama H, Suzuki T, Kuroda M, Yoshida N, et al. Factors associated with hepatocellular carcinoma occurrence after HCV eradication in patients without cirrhosis or with compensated cirrhosis. PLoS One 2020;15:e0243473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ciancio A, Ribaldone DG, Dotta A, Giordanino C, Sacco M, Fagoonee S, et al. Long‐term follow‐up of diabetic and non‐diabetic patients with chronic hepatitis C successfully treated with direct‐acting antiviral agents. Liver Int 2021;41:276‐287. [DOI] [PubMed] [Google Scholar]
- 16. Benhammou JN, Moon AM, Pisegna JR, Su F, Vutien P, Moylan CA, et al. Nonalcoholic fatty liver disease risk factors affect liver‐related outcomes after direct‐acting antiviral treatment for hepatitis C. Dig Dis Sci 2021;66:2394‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alonso López S, Manzano ML, Gea F, Gutiérrez ML, Ahumada AM, Devesa MJ, et al. A model based on noninvasive markers predicts very low hepatocellular carcinoma risk after viral response in hepatitis C virus‐advanced fibrosis. Hepatology 2020;72:1924‐1934. [DOI] [PubMed] [Google Scholar]
- 18. Colussi GianLuca, Donnini D, Brizzi RF, Maier S, Velenti L, Catena C, et al. Sustained virologic response to direct‐acting antiviral agents predicts better outcomes in hepatitis C virus‐infected patients: a retrospective study. World J Gastroenterol 2019;25:6094‐6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of hepatocellular carcinoma in patients with HCV‐associated cirrhosis treated with direct‐acting antiviral agents. Gastroenterology 2018;155:411‐421.e414. [DOI] [PubMed] [Google Scholar]
- 20. Romano A, Angeli P, Piovesan S, Noventa F, Anastassopoulos G, Chemello L, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol 2018;69:345‐352. [DOI] [PubMed] [Google Scholar]
- 21. Piñero F, Mendizabal M, Ridruejo E, Herz Wolff F, Ameigeiras B, Anders M, et al. Treatment with direct‐acting antivirals for HCV decreases but does not eliminate the risk of hepatocellular carcinoma. Liver Int 2019;39:1033‐1043. [DOI] [PubMed] [Google Scholar]
- 22. Prati D, Shiffman ML, Diago M, Gane E, Rajender Reddy K, Pockros P, et al. Viral and metabolic factors influencing alanine aminotransferase activity in patients with chronic hepatitis C. J Hepatol 2006;44:679‐685. [DOI] [PubMed] [Google Scholar]
- 23. Meissner EG, Lee Y‐J, Osinusi A, Sims Z, Qin J, Sturdevant D, et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1‐infected patients. Hepatology 2015;61:790‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, et al. Statin use and nonalcoholic steatohepatitis in at risk individuals. J Hepatol 2015;63:705‐712. [DOI] [PubMed] [Google Scholar]
- 25. Kamal S, Khan MA, Seth A, Cholankeril G, Gupta D, Singh U, et al. Beneficial effects of statins on the rates of hepatic fibrosis, hepatic decompensation, and mortality in chronic liver disease: a systematic review and meta‐analysis. Am J Gastroenterol 2017;112:1495‐1505. [DOI] [PubMed] [Google Scholar]
- 26. Lee JI, Lee HW, Lee KS, Lee HS, Park JY. Effects of statin use on the development and progression of nonalcoholic fatty liver disease: a nationwide nested case‐control study. Am J Gastroenterol 2021;116:116‐124. [DOI] [PubMed] [Google Scholar]
- 27. Musso G, Cassader M, Gambino R. Cholesterol‐lowering therapy for the treatment of nonalcoholic fatty liver disease: an update. Curr Opin Lipidol 2011;22:489‐496. [DOI] [PubMed] [Google Scholar]
- 28. Harrison SA, Rossaro L, Hu K‐Q, Patel K, Tillmann H, Dhaliwal S, et al. Serum cholesterol and statin use predict virological response to peginterferon and ribavirin therapy. Hepatology 2010;52:864‐874. [DOI] [PubMed] [Google Scholar]
- 29. Gu Y, Yang X, Liang H, Li D. Comprehensive evaluation of effects and safety of statin on the progression of liver cirrhosis: a systematic review and meta‐analysis. BMC Gastroenterol 2019;19:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simon TG, Duberg A‐S, Aleman S, Hagstrom H, Nguyen LH, Khalili H, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med 2019;171:318‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int 2018;38:2018‐2027. [DOI] [PubMed] [Google Scholar]
- 32. Zhou Y‐Y, Zhu G‐Q, Liu T, Zheng J‐N, Cheng Z, Zou T‐T, et al. Systematic review with network meta‐analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep 2016;6:33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vilar‐Gomez E, Calzadilla‐Bertot L, Wong VW, Castellanos M, Aller‐de la Fuente R, Eslam M, et al. Type 2 diabetes and metformin use associate with outcomes of patients with nonalcoholic steatohepatitis‐related, child‐pugh A cirrhosis. Clin Gastroenterol Hepatol 2021;19:136‐145.e136. [DOI] [PubMed] [Google Scholar]
- 34. Adinolfi LE, Petta S, Fracanzani AL, Coppola C, Narciso V, Nevola R, et al. Impact of hepatitis C virus clearance by direct‐acting antiviral treatment on the incidence of major cardiovascular events: a prospective multicentre study. Atherosclerosis 2020;296:40‐47. [DOI] [PubMed] [Google Scholar]
- 35. Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta‐analysis. HIV Med 2012;13:453‐468. [DOI] [PubMed] [Google Scholar]
- 36. Vancheri F, Backlund L, Strender LE, Godman B, Wettermark B. Time trends in statin utilisation and coronary mortality in Western European countries. BMJ Open 2016;6:e010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


