Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, and its prevalence continues to rise. Fibrosis‐4 index (FIB‐4) has been shown to be a prognostic marker of liver‐related outcomes in patients with NAFLD. We analyzed data from TriNetX global federated research network, combining data on 30 million patients. Patients were categorized into three diagnostic groups: NAFLD, nonalcoholic steatohepatitis (NASH), and at risk of NASH. Primary outcome was all‐cause mortality, and secondary outcomes included progression to NASH, development of cirrhosis, end‐stage liver disease, hepatocellular carcinoma (HCC), and liver transplantation. A total of 442,277 subjects (1.5% of the cohort) were assessed, and 81,108 were retained for analysis. Median follow‐up was 34.8 months (interquartile range 12.2). FIB‐4 was < 1.3 in 52.3% patients and ≥ 2.67 in 11.4% patients. In multivariate analysis, FIB‐4 ≥ 2.67 was significantly and independently associated with all‐cause mortality (hazard ratio [HR] 2.49, 95% confidence interval [CI] 2.20‐2.82, P < 0.001) as well as with progression to NASH (HR 5.78, 95% CI 4.72‐7.07, P < 0.001), cirrhosis (HR 2.04, 95% CI 1.86‐2.24, P < 0.001), end‐stage liver disease (HR 1.86, 95% CI 1.68‐2.05, P < 0.001), HCC (HR 3.66, 95% CI 2.71‐4.94, P < 0.001), and liver transplantation (HR 7.98, 95% CI 4.62‐13.79, P < 0.001). Conclusion: In a real‐world nationwide database, FIB‐4 ≥ 2.67 was a strong predictor of both all‐cause mortality and liver‐related adverse outcomes independently of the baseline diagnostic group and common risk factors. Our findings indicate that FIB‐4 could play a role as a risk‐stratification tool for a population health approach. Significant underdiagnosis of both NAFLD/NASH and NASH cirrhosis in electronic medical records was observed.

Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- BMI
body mass index
- CI
confidence interval
- EMR
electronic medical records
- FIB‐4
Fibrosis‐4 Index
- HCC
hepatocellular carcinoma
- HDL‐C
high‐density lipoprotein–cholesterol
- HR
hazard ratio
- ICD
International Classification of Diseases
- IQR
interquartile range
- LDL‐C
low‐density lipoprotein–cholesterol
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- T2DM
type 2 diabetes mellitus
- U.S.
United States
Nonalcoholic fatty liver disease (NAFLD) is a major public health problem affecting 25% of the worldwide population, and its global burden is expected to rise in the next decades.( 1 , 2 ) In the United States (U.S.), it is estimated that NAFLD currently affects approximately 80 million people, with a projection of more than 100 million affected individuals by 2030.( 3 )
NAFLD, defined by the accumulation of fat (steatosis) in more than 5% of hepatocytes, in the absence of excessive alcohol consumption, is a heterogenous disease that encompasses simple steatosis without fibrosis, steatohepatitis (NASH) with different stages of fibrosis, and, ultimately, cirrhosis with its life‐threatening complications. Within the spectrum of NAFLD, simple steatosis has a low risk of liver‐related complications, whereas NASH has a potentially progressive course that can lead to liver fibrosis, cirrhosis, end‐stage liver disease, hepatocellular carcinoma (HCC), liver transplantation, and/or extra‐hepatic complications.( 4 , 5 , 6 )
Over the last decades, NAFLD has become one of the leading causes of cirrhosis, end‐stage liver disease, HCC, and liver transplantation worldwide.( 7 , 8 ) NASH is the second most common indication—and also the fastest increasing indication—for liver transplantation in 2019, the leading indication for liver transplantation among women without HCC,( 9 ) and the most rapidly growing cause of HCC among US patients listed for liver transplantation.( 10 )
NAFLD is often underdiagnosed in clinical practice,( 11 ) contributing to higher rates of morbidity and mortality, as most patients are diagnosed when therapeutic interventions are limited. The prevalence of NAFLD is highly variable in the literature, depending on the clinical setting and diagnostic methods. The use of International Classification Diseases (ICD) coding to diagnose NAFLD/NASH is consistently associated with an underestimation of the “true” prevalence of the disease( 3 , 12 ) compared with studies using imaging (ultrasound, computed tomography scan, and magnetic resonance imaging/spectroscopy), suggesting a significant underdiagnosis in real‐world clinical practice. Indeed, the prevalence of NAFLD in recent claims‐database studies showed a low NAFLD prevalence, ranging from less than 1% to almost 6%,( 11 , 13 ) compared with 21% in other studies.( 3 )
The development and progression of liver fibrosis are the best predictors of liver disease progression and long‐term outcomes.( 14 , 15 , 16 , 17 , 18 ) Hence, the early identification of patients with fibrosis is vital to recognize high‐risk patients who will need to be referred to liver specialists for monitoring and treatment of liver complications, potential coming treatments, and, in the case of end‐stage liver disease, assessment for liver transplantation. Although liver biopsy is still the gold standard to assess liver fibrosis, its invasive nature, high cost, sampling variability, and interobserver and intra‐observer variability makes it unsuitable for screening and disease monitoring in clinical practice.( 19 ) Therefore, the identification of accurate noninvasive methods to identify those patients with advanced fibrosis is of high clinical interest. Transient elastography is a very well validated noninvasive method to assess liver stiffness and estimating stage of liver fibrosis; however, it is not widely accessible, and from a primary care perspective, would not be appropriate as a first‐line approach.
Noninvasive scoring systems based on clinical and biochemical variables have been widely used in recent years, and numerous studies have validated their diagnostic accuracy for the assessment of liver fibrosis in the population with NAFLD.( 20 , 21 ) The Fibrosis‐4 index (FIB‐4) is a clinical score based on common clinical parameters (age, aspartate aminotransferase [AST], alanine aminotransferase [ALT], and platelets) and has been shown to have the best diagnostic accuracy for advanced fibrosis when compared with other noninvasive clinical scores.( 22 ) Recent evidence suggests that, in addition to its diagnostic accuracy, FIB‐4 also has prognostic value and can predict adverse outcomes among patients with NAFLD.( 23 , 24 , 25 , 26 , 27 ) However, current studies are based in small cohorts, from tertiary hospitals and specialized centers, and their findings may be hard to extrapolate in the general population.
Therefore, the aim of this study is to evaluate the long‐term prognostic value of FIB‐4 in a population with NAFLD using a large U.S. database of 30 million patients and to estimate the extent of the underdiagnosis of NAFLD/NASH across different clinical settings.
Patients and Methods
Study Population and Design
This study was a retrospective, observational analysis of longitudinal medical data from the TriNetX global federated research network. The TriNetX network is a large electronic medical records (EMR) database that at the time of data obtainment contained EMR for 30 million patients in care in one of 25 health care organizations. The database represents a combination of hospital, primary care, and specialty care organizations geographically dispersed across the U.S., providing inpatient and outpatient care. The database includes deidentified enrollee information (sex, age, race/ethnicity) and longitudinal clinical information (including laboratory, diagnosis, procedures, medications) with historical data of 5 or more years on average from each organization. This study was approved by the Western Review Board as an institutional review board exemption under 45 CFR 46.101 (b).( 4 )
Inclusion criteria for the study were (1) ≥18 years old at the beginning of the observation time (July 2015) and less than 90 years by the end of the observation window (June 2019); (2) all patients with an ICD‐based diagnosis of NAFLD, NASH, and subjects with a risk profile of NAFLD/NASH (risk algorithm and ICD codes detailed in Table 1); (3) having sufficient demographic, clinical, and laboratory data to calculate FIB‐4 or to estimate the risk profile; and (4) at least 1 year of medical history and 2 years of follow‐up available. Our exclusion criteria were (1) >90 years during the time of observation; (2) a concurrent diagnosis or history of other chronic liver disease, including viral, alcoholic, cholestatic, genetics, or autoimmune liver disease; and (3) human immunodeficiency virus infection.
TABLE 1.
Definition of the Diagnostic Groups According to ICD Coding
| Diagnostic Group | ICD Code |
|---|---|
| Risk of NASH | Age > 50 years, ALT > 30 U/L, and (BMI > 30 kg/m2 or T2DM) |
| NAFLD | ICD‐10 K76, ICD‐9 571.8, and ICD‐9 571.9 |
| NASH | ICD‐10 K75.81 |
Definition of Diagnostic Groups: NAFLD, NASH, and RISK
In our study, we defined three different diagnostic groups: (1) NAFLD, (2) NASH, and (3) RISK. The first two groups included all patients with a formal ICD diagnosis of NAFLD or NASH, respectively. The ICD codes are detailed in Table 1. Given the underdiagnosis of NAFLD and NASH,( 11 ) we created a third group (RISK), to identify patients with NAFLD/NASH who did not carry ICD code. RISK‐group patients were identified via an algorithm designed by an expert panel based on established risk factors for NAFLD/NASH( 28 , 29 , 30 , 31 ) that reflect daily practice and could be implemented by primary care providers and diabetics clinics. The risk criteria used were age >50 years, ALT > 30 U/L, and either body mass index (BMI) > 30 kg/m2 or presence of type 2 diabetes mellitus (T2DM).
For this study, the index date was the first calculable FIB‐4 between July 2015 and June 2017 with more than 1‐year history and more than 2 years follow‐up or to death.
Clinical Characteristics and Outcomes of Interest
Diagnoses were considered if they occurred before or on the index date. Baseline characteristics were within 12 months before to 3 months following the index date, with reported measures being closest to the index date, and preference given to measures before the index date. Comorbidities associated with NAFLD, such as T2DM, dyslipidemia and hypertension, were identified using ICD codes. Metabolic syndrome was indicated when three or more of the following criteria were met: (1) plasma glucose ≥ 1.1 g/L; (2) high‐density lipoprotein–cholesterol (HDL‐C)–cholesterol < 40 mg/dL for males and <50 mg/dL for females; (3) triglycerides ≥ 1.5 g/L; and (4) hypertension defined as ≥130 mmHg systolic or ≥90 mmHg diastolic blood pression. Patients were classified according to their BMI as follows: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5‐24.9 kg/m2), overweight (BMI 25‐29.9 kg/m2), and obese (BMI ≥ 30 kg/m2). Major adverse cardiac events included any of the following: (1) myocardial infarction; (2) hospitalization for unstable angina; (3) hospitalization for heart failure; (4) coronary revascularization; and (5) stroke, according to ICD codes. The Charlson Comorbidity Index was assessed at the index date based on previous comorbidities of patients, and was calculated using ICD codes as described in a previous study. FIB‐4 was calculated using the following formula: age × AST/(platelets × sqrt [ALT]). According to FIB‐4 values, patients were classified as (1) low risk, FIB‐4 < 1.3; (2) intermediate risk, FIB4 1.3‐2.66; and (3) high risk, FIB‐4 ≥ 2.67.( 32 )
The primary outcome was all‐cause mortality. The prespecified secondary outcomes included (1) progression to NASH; (2) development of cirrhosis; (3) development of end‐stage liver disease; (4) HCC; and (5) liver transplantation. By study design, all subjects in the cohort had a minimum follow‐up until death or 24 months. Progression to NASH, development of cirrhosis, HCC, and liver transplantation were assessed using ICD codes that occurred during follow‐up and at least 90 days past index. End‐stage liver disease was defined by the presence of a Model for End‐Stage Liver Disease score > 14 and/or the presence of any of the following: (1) portal hypertension, (2) ascites, (3) encephalopathy, and (4) liver failure.
Statistical Analysis
Continuous variables were summarized as median (interquartile range [IQR]) and categorial variables as frequency (percentage). Comparisons of baseline characteristics between cohorts were performed using Wilcoxon test (continuous) and exact test (categorical). Cumulative incidence curves were generated by the Kaplan‐Meier method plotting cumulative incidence of study outcomes by years of follow‐up and compared using a log‐rank test. Time to all‐cause mortality and progression to advanced liver disease analyses were performed using Cox proportional models with strata for age group and BMI group status at index. Each outcome was analyzed using complete‐case analysis by predictors included in multiple regression model. All models included diagnosis group and FIB‐4 status as predictors, and all demographic and clinical covariates with P < 0.200 for individual models were selected to adjust results in multiple regression models. Univariate and multivariate Cox regression results were reported as hazard ratio (HR) and 95% confidence interval (95% CI). The final models were adjusted for diagnostic group, FIB‐4 category, gender, race/ethnicity and T2DM, hypertension, major adverse cardiac events, hyperglycemia, high low‐density lipoprotein–cholesterol (LDL‐C), low HDL‐C, and high triglycerides. All analyses were performed using SPSS statistical software version 25.0 (IBM, Armonk, NY) and R statistical software, version 3.6.1 (R Foundation for Statistical Computing). A two‐tailed P value < 0.05 was considered significant.
Results
Baseline Characteristics of the Cohort
After reviewing the EMR database, 442,277 patients were identified as having NAFLD, NASH, or met criteria for RISK group, which represents 1.5% of the entire cohort (442,277 of 30 million). Of those, 81,108 individuals met the remaining study inclusion and exclusion criteria for follow‐up and calculable FIB‐4 (Supporting Fig. S1). Of those, 62,612 (77.2%) patients were included in the RISK group, 17,582 (21.7%) in the NAFLD group, and 914 (1.1%) in the NASH group. Baseline characteristics are summarized in Table 2.
TABLE 2.
Characteristics of the Cohort at Baseline
| Overall, n (%) | RISK*, 62,612 (77.2%) | NAFLD, 17,582 (21.7%) | NASH, 914 (1.1%) | Total, 81,108 (100%) | P Value of RISK vs. NAFLD | P Value of RISK vs. NASH | P Value of NAFLD vs. NASH |
|---|---|---|---|---|---|---|---|
| Age at enrollment (years), median (IQR) | 63 (14) | 57 (20) | 58 (20) | 62 (14) | <0.001 | <0.001 | 0.318 |
| Male sex, n (%) | 33,010 (52.7) | 6,852 (39.0) | 332 (36.3) | 40,194 (49.6) | <0.001 | <0.001 | 0.110 |
| Race/ethnicity, n (%) | <0.001 | <0.001 | <0.001 | ||||
| White | 50,604 (80.8) | 13,752 (78.2) | 765 (83.7) | 65,121 (80.3) | |||
| Black or African American | 8,421 (13.4) | 2,455 (14.0) | 58 (6.3) | 10,934 (13.5) | |||
| Other † | 1,096 (1.8) | 455 (2.6) | 29 (3.2) | 1,580 (1.9) | |||
| Unknown | 2,491 (4.0) | 920 (5.2) | 62 (6.8) | 3,473 (4.3) | |||
| BMI (kg/m † ), median (IQR) | 32.2 (5.3) | 31.0 (8.3) | 32.1 (7.8) | 32 (5.9) | <0.001 | 0.128 | <0.001 |
| Patients by BMI groups, n (%) | <0.001 | <0.001 | <0.001 | ||||
| Underweight (<18.5 kg/m † ) | 217 (0.5) | 209 (1.6) | 1 (0.2) | 427 (0.7) | |||
| Normal weight (18.5‐24.9 kg/m † ) | 2,424 (5.1) | 1,834 (14.4) | 59 (8.9) | 4,317 (7.1) | |||
| Overweight (25‐29.9 kg/m † ) | 8,440 (17.8) | 3,329 (26.2) | 179 (27) | 11,948 (19.6) | |||
| Obese (≥30 kg/m † ) | 36,435 (76.7) | 7,332 (57.7) | 423 (63.9) | 44,190 (72.6) | |||
| Tobacco, n (%) | 95 (0.2) | 55 (0.3) | 0 (0.0) | 150 (0.2) | <0.001 | <0.001 | 0.012 |
| Comorbidities, n (%) | |||||||
| Metabolic syndrome | 9,397 (15) | 2,691 (15.3) | 121 (13.2) | 12,209 (15.1) | <0.001 | <0.001 | <0.001 |
| T2DM | 22,312 (35.6) | 5,048 (28.7) | 422 (46.2) | 27,782 (34.3) | <0.001 | 0.002 | <0.001 |
| Hyperlipidemia | 41,481 (66.3) | 10,195 (58) | 500 (54.7) | 52,176 (64.3) | <0.001 | <0.001 | <0.001 |
| Hypertension | 36,168 (57.8) | 9,274 (52.7) | 537 (58.8) | 45,979 (56.7) | <0.001 | 0.219 | 0.751 |
| Cardiovascular disease | 39,109 (62.5) | 10,624 (60.4) | 622 (68.1) | 50,355 (62.1) | <0.001 | <0.001 | <0.001 |
| MACE ‡ | 11,097 (17.7) | 2,620 (14.9) | 118 (12.9) | 13,835 (17.1) | 0.095 | <0.001 | <0.001 |
| Charlson Comorbidity Index, median (IQR) | 2 (1) | 3 (2) | 3 (2) | 2 (1) | <0.001 | <0.001 | 0.176 |
| Laboratory data, n (%) | |||||||
| HbA1c > 5.6% | 30,820 (88.5) | 7,471 (82.3) | 393 (77.8) | 38,683 (87.1) | <0.001 | <0.001 | <0.001 |
| Hyperglycemia ≥ 1.1 g/L | 28,714 (46.8) | 7,414 (43.1) | 461 (52.2) | 36,589 (46.1) | <0.001 | 0.174 | 0.891 |
| LDL‐C ≥ 100 mg/dL | 17,853 (42.5) | 4,851 (47.3) | 213 (43.1) | 22,917 (43.5) | <0.001 | 0.938 | 0.446 |
| HDL‐C < 40 mg/dL/m), <50 mg/dL (f) | 20,872 (33.3) | 6,077 (34.6) | 290 (31.7) | 27,239 (33.6) | 0.002 | 0.322 | 0.080 |
| Triglycerides > 150 mg/dL | 19,136 (43.9) | 5,112 (47.4) | 235 (47) | 24,483 (44.6) | 0.177 | 0.012 | 0.034 |
| Platelets < 150 K/mcl | 6,038 (9.7) | 1,998 (11.4) | 275 (30.2) | 8,311 (10.3) | 0.032 | 0.004 | 0.020 |
| FIB‐4 category, n (%) | <0.001 | <0.001 | <0.001 | ||||
| <1.3 | 32,092 (51.3) | 10,002 (56.9) | 349 (38.2) | 42,443 (52.3) | |||
| 1.3‐2.66 | 23,700 (37.9) | 5,473 (31.1) | 282 (30.9) | 29,455 (36.3) | |||
| ≥2.67 | 6,820 (10.9) | 2,107 (12.0) | 283 (31.0) | 9,210 (11.4) | <0.001 | <0.001 | <0.001 |
| Liver‐related outcomes at baseline, n (%) | |||||||
| Cirrhosis § | <0.001 | <0.001 | <0.001 | ||||
| Compensated Cirrhosis | 130 (14.2) | 348 (2.0) | 89 (0.1) | 567 (0.7) | |||
| End‐stage liver disease | 11,856 (18.9) | 4,829 (27.5) | 351 (38.4) | 17,036 (21.0) | |||
| MELD ≥ 15 | 5,269 (8.4) | 1,313 (7.5) | 113 (12.4) | 6,695 (8.3) | <0.001 | <0.001 | <0.001 |
| Portal hypertension | 10 (0.0) | 46 (0.3) | 23 (2.5) | 79 (0.1) | <0.001 | <0.001 | <0.001 |
| Ascites | 3,595 (5.7) | 2,123 (12.1) | 178 (19.5) | 5,896 (7.3) | <0.001 | <0.001 | <0.001 |
| Encephalopathy | 1,633 (2.6) | 6,661 (3.8) | 104 (11.4) | 2,398 (3.0) | <0.001 | <0.001 | <0.001 |
| Varices | 273 (0.4) | 275 (1.6) | 63 (6.9) | 611 (0.8) | <0.001 | <0.001 | <0.001 |
| Liver failure | 127 (0.2) | 208 (1.2) | 91 (10.0) | 426 (0.5) | <0.001 | <0.001 | <0.001 |
| HCC | 91 (0.1) | 163 (0.9) | 21 (2.3) | 275 (0.3) | <0.001 | 0.019 | <0.001 |
| Liver transplantation | 152 (0.2) | 124 (0.7) | 47 (5.1) | 323 (0.4) | <0.001 | 0.114 | 0.653 |
Statistical analyses for continuous variables were performed using Fisher’s exact test for categorical variables and the Wilcoxon rank‐sum test for continuous variables.
RISK includes patients with risk profile of NASH using with the following formula: age > 50 years and ALT > 30 U/L and (BMI > 30 kg/m2 or T2DM).
Other includes American Indian or Alaska, Asian, Native Hawaiian, or other Pacific Island.
Major adverse cardiac events included myocardial infarction, hospitalization for unstable angina or heart failure, coronary revascularization and stroke.
Does not consider FIB‐4.
Abbreviations: HbA1c, hemoglobin A1c; and MACE, major adverse cardiac events.
Median age in the overall population was 62.0 (IQR 14) years with no gender predominance (50.4% female), and 80.3% of patients were of White race. NAFLD and NASH patients were younger (median age 57 and 58 years, respectively) and predominantly of female sex (61% and 63.7%, respectively) compared to patients in the RISK group (median age 63 years, female sex 47.3%) (P < 0.001 for all). Median BMI was 32 kg/m2 (IQR 5.9), with 19.6% of patients being overweight and 72.6% obese. Overall, 15.1% percent of patients had metabolic syndrome, 34.3% T2DM, 64.3% dyslipidemia, and 56.7% hypertension.
With regard to FIB‐4 values, 52.3% presented a FIB‐4 < 1.3, 36.3% a FIB‐4 1.3‐2.66, and 11.4% a FIB‐4 ≥ 2.67. The proportion of patients with FIB‐4 ≥ 2.67 was significantly higher for NASH (31.0%, P < 0.001) compared to all patients with NAFLD (12.0%, P < 0.001), which was also significantly higher compared to the RISK group (10.9%, P < 0.001). Among patients with cirrhosis, 41.6% (236 of 567) had a FIB‐4 ≥ 2.67, whereas among patients without cirrhosis, only 8.3% (5,288 of 63,505) had a FIB‐4 ≥ 2.67.
Regarding liver‐related comorbidities at baseline, 21.7% of patients had established cirrhosis (compensated in 0.7% of patients and end‐stage liver disease in 21% of patients), 0.3% had HCC and 0.4% of patients had undergone a liver transplantation. The proportion of patients with cirrhosis was significantly higher in patients with NASH compared to RISK and patients with NAFLD (38.5% vs. 33.1% vs. 29.5%, P < 0.001). Similarly, patients with NASH presented a significantly higher number of HCC than patients with NAFLD (2.3% vs. 0.9%, P < 0.001), whereas patients with NAFLD presented a significantly higher proportion of HCC than patients in the RISK group (0.9% vs. 0.1%, P < 0.001).
Univariate and Multivariate Associations With All‐Cause Mortality and Secondary Outcomes
During median follow‐up of 34.8 months (IQR 12.2), out of 81,108 patients, 8,652 (10.7%) died, 1,875 (2.3%) developed NASH, 12,838 (15.8%) developed cirrhosis, 13,162 (16.2%) developed end‐stage liver disease, 425 (0.5%) developed an HCC, and 207 (0.3%) received a liver transplant.
The incident rate (cases per 1,000 person‐years) for all‐cause mortality and secondary outcomes, stratified by FIB‐4 category, is described in Fig. 1. We observed a higher incidence rate for all‐cause mortality and secondary outcomes in patients with FIB‐4 ≥ 2.67 compared to patients with FIB‐4 < 2.67 (P < 0.001).
FIG. 1.
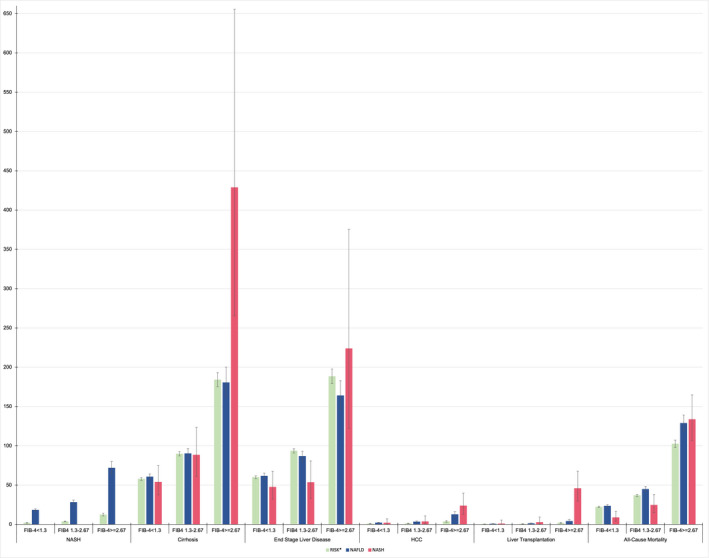
Incidence rates of liver‐related outcomes and all‐cause mortality. Incidence rate (number of cases/1,000 person‐years) of liver‐related outcomes (NASH, cirrhosis, end‐stage liver disease, HCC, liver transplantation) and all‐cause mortality according to FIB‐4 and diagnostic group (RISK, NAFLD, and NASH). RISK includes patients with a risk profile of NASH using with the following formula: age > 50 years and ALT > 30 U/L and (BMI > 30 kg/m2 or T2DM).
Significant predictors of all‐cause mortality on univariate analysis (Table 3) include NAFLD, FIB‐4 1.3‐2.67, FIB‐4 ≥ 2.67, male sex, T2DM, major adverse cardiac events, hyperglycemia, high LDL, low HDL, and high triglycerides. In cumulative incidence curves, patients with FIB‐4 ≥ 2.67 experienced a substantial increase in all‐cause mortality (Fig. 2) and liver‐related outcomes (Supporting Figs. S2‐S6) compared to those with FIB‐4 < 2.67 (P < 0.001). This finding was consistent in all diagnostic groups (P < 0.001 for NALFD, NASH, and RISK groups).
TABLE 3.
Univariate and Multivariate Analyses of Patient Clinical and Demographic Characteristics of FIB‐4 With All‐Cause Mortality
| All‐Cause Mortality | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Diagnostic group | ||||
| RISK | 1 (reference) | 1 (reference) | ||
| NAFLD | 1.15 (1.02‐1.28) | 0.018 | 1.15 (1.02‐1.29) | 0.018 |
| NASH | 1.33 (0.88‐2.01) | 0.175 | 1.14 (0.75‐1.72) | 0.540 |
| Sex | ||||
| Female | 1 (reference) | 1 (reference) | ||
| Male | 1.15 (1.05‐1.25) | 0.002 | 0.99 (0.91‐1.08) | 0.820 |
| Race/ethnicity | ||||
| White | 1 (reference) | 1 (reference) | ||
| Black/African American | 0.93 (0.8‐ 1.06) | 0.256 | 0.78 (0.68‐0.89) | <0.001 |
| Hispanic | 0.99 (0.77‐1.27) | 0.919 | 1.01 (0.79‐1.30) | 0.920 |
| Other | 0.91 (0.7‐1.18) | 0.480 | 0.95 (0.73‐1.24) | 0.711 |
| Clinical characteristics | ||||
| T2DM | 1.17 (1.07‐1.28) | <0.001 | 0.97 (0.87‐1.07) | 0.534 |
| Hypertension | 0.92 (0.77‐1.11) | 0.403 | ‐ | ‐ |
| MACE* | 2.93 (2.68‐3.2) | <0.001 | 2.53 (2.31‐2.78) | <0.001 |
| Hyperglycemia | 1.37 (1.26‐1.5) | <0.001 | 1.25 (1.14‐1.37) | <0.001 |
| High LDL‐C | 0.7 (0.63‐0.77) | <0.001 | 0.85 (.077‐0.94) | 0.002 |
| Low HDL‐C | 1.19 (1.09‐1.29) | <0.001 | 1.07 (0.98‐1.17) | 0.148 |
| High triglycerides | 0.85 (0.77‐0.93) | <0.001 | 0.83 (0.75‐0.91) | <0.001 |
| FIB‐4 | ||||
| <1.3 | 1 (reference) | 1 (reference) | ||
| 1.3‐2.66 | 1.24 (1.12‐1.38) | <0.001 | 1.13 (1.02‐1.26) | 0.020 |
| ≥2.67 | 3.19 (2.83‐3.60) | <0.001 | 2.49 (2.20‐2.82) | <0.001 |
Statistical analyses were performed using Cox regression and results were reported as HR and 95% CI.
MACE included myocardial infarction, unstable angina, heart failure, and coronary revascularization, including coronary artery bypass graft and stroke.
Abbreviation: aHR, adjusted HR.
FIG. 2.
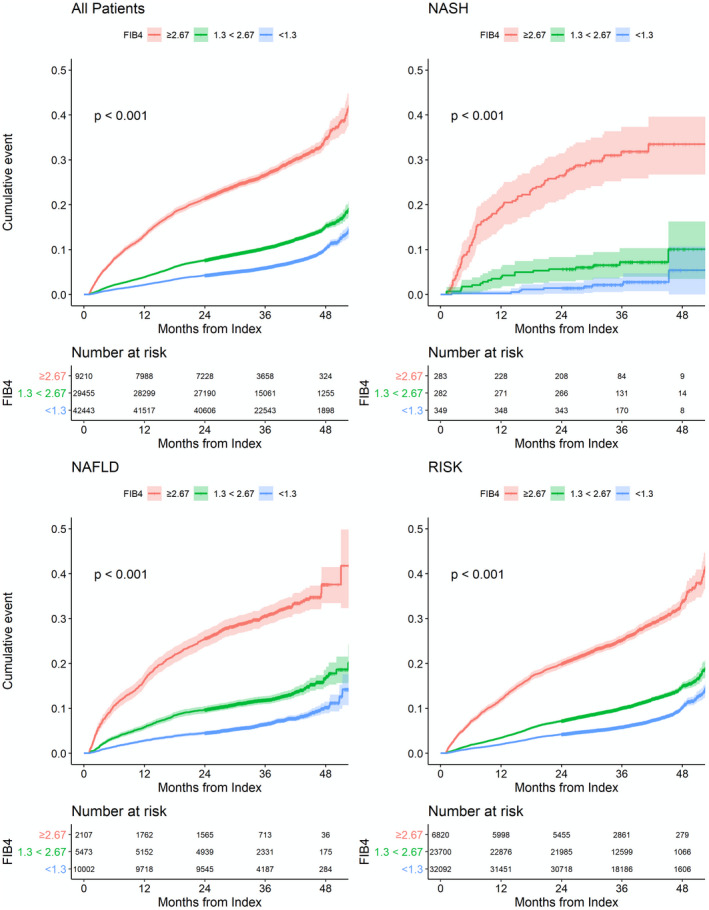
Cumulative incidence curves for all‐cause mortality. Cumulative incidence curves for all‐cause mortality according to FIB‐4 and diagnostic group (all patients, RISK, NAFLD, and NASH). Cumulative incidence curves were generated by the Kaplan‐Meier method plotting cumulative incidence of study outcomes by years of follow‐up and compared using a log‐rank test. RISK includes patients with risk profile of NASH using with the following formula: age > 50 years and ALT > 30 U/L and (BMI > 30 kg/m2 or T2DM).
These variables were further analyzed on multivariate analysis, adjusting for diagnostic group (RISK, NAFLD, NASH), FIB‐4 category (<1.3, 1.3‐2.66, ≥ 2.67), gender, race/ethnicity and T2DM, major adverse cardiac events, hyperglycemia, high LDL, low HDL, and high triglycerides, and results are presented in Table 3. Variables that maintained a significant association with all‐cause mortality after adjustment for confounders included NAFLD, FIB‐4 1.3‐2.67, FIB‐4 ≥ 2.67, hyperglycemia, high LDL, major cardiac adverse events, and high triglycerides. FIB‐4 ≥ 2.67 conferred a 2.5‐fold higher risk of all‐cause mortality. After excluding patients with cirrhosis at baseline from the analysis, FIB‐4 ≥ 2.67 was the strongest predictor of mortality (HR 2.13, 95% CI 1.88‐2.42, P < 0.001).
The univariate associations of FIB‐4 ≥ 2.67 with the secondary outcomes are described in Table 4. FIB‐4 ≥ 2.67 was the strongest or the second‐strongest predictor across all of the secondary outcomes (Table 4). In multivariate analysis (Table 4), FIB‐4 ≥ 2.67 remained a strong predictor of progression to NASH, cirrhosis, end‐stage liver disease, HCC, and liver transplantation.
TABLE 4.
Univariate and Multivariate Analyses of FIB‐4 With Progression to NASH, Cirrhosis, End‐Stage Liver Disease, HCC, and Liver Transplantation
| Unadjusted HR | Progression to NASH | Progression to Cirrhosis | End‐Stage Liver Disease | HCC | Liver Transplantation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| FIB‐4 | ||||||||||
| <1.3 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| 1.3‐2.66 | 2.13 (1.8 – 2.53) | <0.001 | 1.28 (1.2‐1.46) | <0.001 | 1.21 (1.14‐1.29) | <0.001 | 1.2 (0.9‐1.6) | 0.206 | 1.35 (0.77‐2.37) | 0.291 |
| ≥2.67 | 6.71 (5.49‐8.2) | <0.001 | 2.36 (2.15‐2.58) | <0.001 | 2.11 (1.92‐2.33) | <0.001 | 3.97 (2.96‐5.33) | <0.001 | 10.03 (5.92‐17) | <0.001 |
| aHR* | Progression to NASH | Progression to Cirrhosis | End‐Stage Liver Disease | HCC | Liver Transplantation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| FIB‐4 | ||||||||||
| <1.3 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| 1.3‐2.66 | 2.08 (1.75‐2.47) | <0.001 | 1.2 (1.13‐1.28) | <0.001 | 1.14 (1.07‐1.22) | <0.001 | 1.18 (0.89‐1.58) | 0.246 | 1.23 (0.7‐2.16) | 0.482 |
| ≥2.67 | 5.78 (4.72‐7.07) | <0.001 | 2.04 (1.86‐2.24) | <0.001 | 1.86 (1.68‐2.05) | <0.001 | 3.66 (2.71‐4.94) | <0.001 | 7.98 (4.62‐13.79) | <0.001 |
Statistical analyses were performed using Cox regression and results were reported as HR and 95% CI.
HR adjusted for risk group (NALFD, NASH, and RISK), sex, race/ethnicity, and clinical characteristics (T2DM, hypertension, MACE, hyperglycemia, high LDL‐C, low HDL‐C, and high triglycerides).
Discussion
In this retrospective multicenter population‐wide study, using a database of 30 million patients representing nearly 10% of the U.S. population, and combining data from hospital, primary and specialty care, we identified 18,496 patients who carried a diagnosis of NAFLD and NASH and 62,612 patients who met our definition of at risk of NASH. Our main findings were (1) NAFLD/NASH and NASH cirrhosis are underdiagnosed in real‐world clinical practice; (2) high‐risk FIB‐4 score (≥2.67) was a significant independent predictor of all‐cause mortality, after controlling for the diagnostic group (RISK, NAFLD, and NASH) and established clinical risk factors; (3) high risk FIB‐4 score (≥2.67) was one of the strongest independent predictors of liver‐related adverse outcomes including progression to NASH, cirrhosis, end‐stage liver disease, HCC and liver transplantation, incremental to the diagnostic group (RISK, NAFLD, and NASH) and risk factors.
Underdiagnosis of NAFLD, NASH, and Cirrhosis
In this large database, we identified 442,277 patients with a diagnosis of NAFLD, NASH, or at RISK of NASH—representing 1.5% of our population, which is a significantly lower prevalence compared with the 24% previously described in the literature,( 12 ) indicating a significant underdiagnosis of NAFLD and NASH in real‐world clinical practice. This finding is consistent with results obtained from other claims database studies.( 11 , 13 ) In an attempt to address the underdiagnosis bias, we included in our study a RISK group of patients with common clinical criteria and recognized risk factors—T2DM and obesity—who likely have NAFLD or NASH but do not carry a formal ICD‐diagnosis. Several NAFLD clinical screening tools have been validated but can be challenging to implement in clinical daily practice due to unavailability of liver imaging tests in primary care clinics,( 33 ) the lack of necessary clinical parameters,( 34 ) or the absence of validation in the U.S. population.( 35 ) Hence, based on consensus of an expert panel, we opted for a straightforward widely available algorithm, based on established risk factors for NAFLD, that would reflect daily practice and could be readily implemented by primary care providers and diabetic clinics.
We also observed a significantly higher percentage of patients with end‐stage liver disease compared to patients with compensated cirrhosis (21% vs. 0.7%). We believe that this discrepancy may be due to an undercoding of compensated cirrhosis. In a study by Loomba et al.,( 11 ) describing data from a Medicare cohort, more than 90% of patients with cirrhosis were first diagnosed with decompensated cirrhosis. By definition, patients with compensated cirrhosis are, in most cases, asymptomatic, which contributes to both undercoding and underdiagnosis by primary care providers. Moreover, identifying patients with NASH cirrhosis in EMR is challenging, as there is no ICD‐specific diagnostic code. However, a recent systematic analysis( 36 ) showed that from 1990 to 2017 the number of prevalent cases of decompensated cirrhosis due to NASH more than tripled, and predictions estimate that incidence of NASH‐related decompensated cirrhosis will increase 165% by 2030.( 37 ) Early recognition of NASH cirrhosis by primary care providers is crucial for timely referrals to liver specialists and prevent adverse liver‐related outcomes.
Furthermore, in our cohort, nearly 50% had a FIB‐4 ≥ 1.3 (corresponding to an intermediate‐to‐high risk of advanced liver fibrosis), from whom 11% had a FIB‐4 ≥ 2.67 (corresponding to a high risk of advanced liver fibrosis). Previous data validating FIB‐4 as a predictor of fibrosis in patients with NAFLD showed a substantially lower number of patients classified as high risk. Indeed, in the study published by Srivastava et al.,( 38 ) with 1,452 patients with NAFLD, only 29% had a FIB‐4 ≥ 1.3. In another study published by Davyduke et al.,( 39 ) with 560 patients with suspected NALFD (criteria: 16‐65 years old, elevated ALT and/or steatosis on imaging, absence of previous liver diagnosis), only 13% of patients had a FIB‐4 ≥ 1.3. In contrast to these studies, almost 80% of our sample consisted of a high‐risk population with established risk factors for NASH (age, obesity, T2DM), which can explain a bias toward those with more severe disease.
Gender and Racial Disparities
In our study, men were significantly less likely to have a formal diagnosis of NAFLD/NASH than women (NAFLD: 39% vs. 61.0%, NASH: 36.3% vs. 63.7%, P < 0.001). Recent data have showed that NAFLD is more prevalent in men than in premenopausal women but occurs at an even higher rate in postmenopausal women.( 40 ) In our study, the mean age of our population was over 50 years, which may explain the lesser rate of NAFLD/NASH in men.
It is noteworthy that in our study Black patients were associated with a higher overall mortality and adverse liver‐related outcomes than White and Hispanic patients. A previous study by Younossi et al.( 41 ) showed a higher risk of overall mortality in Black individuals, although a recent meta‐analysis was inconclusive.( 42 ) A poorer access to health care system, leading to a later a diagnosis ‐ when the disease has already progressed to an advanced stage ‐ may play a role in the worse prognosis observed in these patients.
Association Between FIB‐4 and Overall Mortality
In this large‐scale multicenter cohort combining data from inpatient and outpatient care, FIB‐4 score was a strong predictor of all‐cause mortality, after adjustment for demographic and the most relevant clinical variables, and this finding was independent of the diagnostic group. In patients with FIB‐4 ≥ 2.67, we observed a high rate of cumulative incidence of all‐cause mortality, which may be explained by an advanced age (median age: 69 years), a high burden of cardiovascular risk factors, and a high probability of advanced hepatic disease in this population. In comparison, a previous study of 14,967 patients with cirrhosis showed a mortality rate superior to 50% in patients with a Charlson Comorbidity Index > 1, with diabetes being the second most frequent comorbidity.( 43 ) Of note, when patients with cirrhosis were excluded from analysis, FIB‐4 ≥ 2.67 was the strongest predictor of mortality.
Although the association between FIB‐4 and all‐cause mortality has been described in prior studies, the populations were predominantly selected from specialist clinics. Indeed, in previous studies of patients with biopsy‐proven NAFLD, a high FIB‐4 score (≥2.67) was associated with overall mortality.( 23 , 24 , 25 ) However, the studied population was smaller (<700 patients) and biased toward those with more severe disease (patients with biopsy‐proven NAFLD from tertiary centers). Moreover, in comparison to our study, variables were not consistently adjusted to key confounders such as cardiovascular disease, race or dyslipidemia, which may influence their association to the outcome. Another study in 11,154 individuals, from which 34% had a NAFLD diagnosis by liver ultrasound, also showed the association between FIB‐4 and overall mortality.( 26 ) However, the diagnosis of NAFLD by liver ultrasound may miss mild steatosis, is operator‐dependent, and accuracy may be influenced by BMI.
Association Between FIB‐4 and Adverse Liver‐Related Outcomes
We further observed a strong association between a high FIB‐4 score (≥2.67) and all the major liver‐related adverse outcomes, including cirrhosis, end‐stage liver disease, HCC, and liver transplantation. Again, these findings were independent of the diagnostic group.
In a study published by Angulo et al.,( 25 ) with 320 patients with biopsy‐proven NAFLD, a high FIB‐4 score was strongly associated with liver‐related events, defined as the development of ascites, gastroesophageal varices/bleeding, portosystemic encephalopathy, hepatopulmonary syndrome, HCC, spontaneous bacterial peritonitis, and hepatorenal syndrome. Onnerhag et al.( 24 ) reported similar findings in 144 patients with biopsy‐proven NAFLD, confirmed these findings, and Kim et al.( 27 ) recently showed that FIB‐4 was a strong predictor of the development of HCC in a population of patients with NAFLD.
Both of these findings ‐ FIB‐4 as a predictor of overall mortality and adverse liver‐related events ‐ obtained in a “real‐world population” representing almost 10% of the U.S. population, may have a significant impact in clinical practice. Being a readily accessible noninvasive tool that can be used in all clinical settings (primary care, diabetics clinics, and specialized care), FIB‐4 can be applied not only to identify patients at risk of developing more advanced disease but also to stratify those at higher risk of overall mortality and adverse liver‐related outcomes, who could benefit from closer surveillance, intensive lifestyle measures, and inclusion in clinical trials.
Strengths and Limitations
To our knowledge, this is the largest U.S. study analyzing the prognostic value of FIB‐4 and combining data from all health care settings (hospital, primary care, and specialty care). Our study represents a “real‐world population” with the inclusion of a RISK group, with established risk factors for NASH, including obesity and T2DM.
Our study has several limitations. First, diagnoses were based on ICD coding, which may lead to an underestimation of NAFLD due to misclassification bias related to coding errors. In addition, ICD codes for NAFLD/NASH lack accuracy, particularly discriminating patients with biopsy‐proven diagnosis or NASH cirrhosis, and depend on the individual physician practices to code. All ‐ these aspects may impact the quality of data collection and contribute to coding errors. Nonetheless, this study includes not only patients with a formal diagnosis of NAFLD and NASH, but also patients at risk of NASH ‐ defined by the presence of common and established risk factors ‐ who have a high likelihood of having NAFLD or NASH minimizing the underdiagnosis bias. We did, however, observe significant differences between NAFLD and NASH with regard to clinical characteristics, FIB‐4, and liver outcomes. Second, NASH diagnosis was based on ICD coding, and we do not have information if it was truly based on histological analysis. Moreover, the progress to NASH outcomes should be interpreted carefully, as this may not represent a real progression to NASH but only a formal ICD coding by the physician. With regard to the statical analysis, we did not use imputation methods for missing data. However, we do not believe that the main takeaways from the results would have changed substantially given the sample size. Furthermore, we were unable to assess the independent predictive value of AST, ALT, and platelet count, as we used the integrated FIB‐4 score in our multivariate model. However, all Cox models were appropriately stratified by age. Finally, and as discussed previously, we observed an unexpectedly high number of patients with end‐stage liver disease, which may indicate a selection bias in the studied population. As a calculable FIB‐4 was necessary for enrollment, it is possible that our population was biased toward those with more severe hepatic disease. Patients followed by hepatologists may be more likely to have an ICD code for cirrhosis and its complications, compared to those followed by primary care providers. Increasing awareness about underdiagnosis, undercoding and underrecognition of NASH in primary care was one of our study objectives.
In conclusion, in a nationwide database assessing the association between FIB‐4 and NAFLD outcomes in a real‐world setting, a high FIB‐4 score (≥2.67) was strongly associated with both all‐cause mortality and liver‐related adverse outcomes, independently of traditional risk prognosticators, in patients at risk of NASH and/or a NAFLD and NASH diagnosis. These findings underscore that fibrosis stage, and not NASH per se, is the strongest predictor of all‐cause mortality and liver‐related adverse outcomes. The FIB‐4 clinical score can accurately identify patients at risk of severe liver disease and adverse outcomes and should be used as a part of a population health approach to screen high‐risk populations, such as those with T2DM and patients fulfilling “at RISK” criteria described in our study. Furthermore, this real‐world database highlights the significant underdiagnosis of both NAFLD/NASH and NASH cirrhosis in the U.S., leading to a late diagnosis of liver complications and consequently a higher rate of morbidity and mortality.
Supporting information
Fig S1‐6
Supported by University Hospital of Lausanne (Centre Hospitalier Universitaire Vaudois), Gottfried und Julia Bangerter‐Rhyner‐Stiftung, and Novartis Foundation for Medical‐Biological Research (to J.V.B.).
Potential conflict of interest: Dr. Younossi consults for Intercept, Gilead, Novo Nordisk, Siemens, and Terns. Dr. Milligan, A. Frick and J. Broestl are employed by Trio Health Analytics and have received research support from AbbVie, Gilead, GSK, Johnson & Johnson, Merck, Sanofi, Takeda, and UCB. Dr. Afdhal consults for Echosens and Sonic Incytes.
References
Author names in bold designate shared co‐first authorship.
- 1. Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564‐568. [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019;69:2672‐2682. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 4. Cotter TG, Rinella M. NAFLD 2020: the state of the disease. Gastroenterology 2020;158:1851‐1864. [DOI] [PubMed] [Google Scholar]
- 5. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA 2020;323:1175‐1183. [DOI] [PubMed] [Google Scholar]
- 6. Vieira Barbosa J, Lai M. Nonalcoholic fatty liver disease screening in type 2 diabetes mellitus patients in the primary care setting. Hepatol Commun 2021;5:158‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330‐344. [DOI] [PubMed] [Google Scholar]
- 8. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 9. Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2021;19:580‐589.e5. [DOI] [PubMed] [Google Scholar]
- 10. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748‐755.e743. [DOI] [PubMed] [Google Scholar]
- 11. Loomba R, Wong R, Fraysse J, Shreay S, Li S, Harrison S, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther 2020;51:1149‐1159. [DOI] [PubMed] [Google Scholar]
- 12. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 13. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real‐world data from a large U.S. claims database. Hepatology 2018;68:2230‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 16. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology 2020;158:1611‐1625. [DOI] [PubMed] [Google Scholar]
- 17. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J Hepatol 2017;67:1265‐1273. [DOI] [PubMed] [Google Scholar]
- 18. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris R, Harman DJ, Card TR, Aithal GP, Guha IN. Prevalence of clinically significant liver disease within the general population, as defined by non‐invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol 2017;2:288‐297. [DOI] [PubMed] [Google Scholar]
- 20. Vilar‐Gomez E, Chalasani N. Non‐invasive assessment of non‐alcoholic fatty liver disease: clinical prediction rules and blood‐based biomarkers. J Hepatol 2018;68:305‐315. [DOI] [PubMed] [Google Scholar]
- 21. Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1264‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut 2010;59:1265‐1269. [DOI] [PubMed] [Google Scholar]
- 23. Hagstrom H, Nasr P, Ekstedt M, Stal P, Hultcrantz R, Kechagias S. Accuracy of noninvasive scoring systems in assessing risk of death and liver‐related endpoints in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:1148‐1156. [DOI] [PubMed] [Google Scholar]
- 24. Onnerhag K, Hartman H, Nilsson PM, Lindgren S. Non‐invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non‐alcoholic fatty liver disease (NAFLD). Scand J Gastroenterol 2019;54:328‐334. [DOI] [PubMed] [Google Scholar]
- 25. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013;145:782‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non‐alcoholic fatty liver disease and cancer incidence rate. J Hepatol 2017. Nov 2. 10.1016/j.jhep.2017.09.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28. Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1224‐1229. [DOI] [PubMed] [Google Scholar]
- 29. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol 2019;71:793‐801. [DOI] [PubMed] [Google Scholar]
- 30. Younossi ZM, Corey KE, Alkhouri N, Noureddin M, Jacobson I, Lam B, et al. Clinical assessment for high‐risk patients with non‐alcoholic fatty liver disease in primary care and diabetology practices. Aliment Pharmacol Ther 2020;52:513‐526. [DOI] [PubMed] [Google Scholar]
- 31. Chang Y, Jung H‐S, Cho J, Zhang Y, Yun KE, Lazo M, et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol 2016;111:1133‐1140. [DOI] [PubMed] [Google Scholar]
- 32. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. NASH Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McHenry S, Park Y, Browning JD, Sayuk G, Davidson NO. Dallas Steatosis Index identifies patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2020;18:2073‐2080.e2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee J‐H, Kim D, Kim HJ, Lee C‐H, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503‐508. [DOI] [PubMed] [Google Scholar]
- 36. GBD 2017 Cirrhosis Collaborators . The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:24‐ 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, et al. Prospective evaluation of a primary care referral pathway for patients with non‐alcoholic fatty liver disease. J Hepatol 2019;71:371‐378. [DOI] [PubMed] [Google Scholar]
- 39. Davyduke T, Tandon P, Al‐Karaghouli M, Abraldes JG, Ma MM. Impact of implementing a “FIB‐4 First” strategy on a pathway for patients with NAFLD referred from primary care. Hepatol Commun 2019;3:1322‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lonardo A, Nascimbeni F, Ballestri S, Fairweather DeLisa, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology 2019;70:1457‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Younossi ZM, Otgonsuren M, Venkatesan C, Mishra A. In patients with non‐alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism 2013;62:352‐360. [DOI] [PubMed] [Google Scholar]
- 42. Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2018;16:198‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sorensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population‐based cohort study. Hepatology 2008;48:214‐220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐6


