Abstract
Acute kidney injury (AKI) and frailty are major drivers of outcomes among patients with cirrhosis. What is unknown is the impact of physical frailty on the development of AKI. We included adults with cirrhosis without hepatocellular carcinoma listed for liver transplantation at nine US centers (n = 1,033). Frailty was assessed using the Liver Frailty Index (LFI); “frail” was defined by LFI ≥ 4.2. Chronic kidney disease as a baseline estimated glomerular filtration rate <60 mL/min/1.73 m2. Our primary outcome, AKI, was defined as an increase in serum creatinine ≥0.3 mg/dL or a serum creatinine ≥1.5‐fold increase. Wait‐list mortality was defined as either a death on the wait list or removal for being too sick. We performed Cox regression analyses to estimate the hazard ratios (HRs) for AKI and wait‐list mortality. Of 1,033 participants, 41% were frail and 23% had CKD. Twenty‐one percent had an episode of AKI during follow‐up. Frail versus nonfrail patients were more likely to develop AKI (25% vs. 19%) and wait‐list mortality (21% vs. 13%) (P < 0.01 for each). In multivariable Cox regression, each of the following groups was associated with a higher risk of AKI as compared with not frail/no CKD: frail/no CKD (adjusted HR [aHR] = 1.87, 95% confidence interval [CI] = 1.29‐2.72); not frail/CKD (aHR = 4.30, CI = 2.88‐6.42); and frail/CKD (aHR = 4.85, CI = 3.33‐7.07). We use a readily available metric, LFI, to identify those patients with cirrhosis most at risk for AKI. We highlight that serum creatinine and creatinine‐based estimations of glomerular filtration rate may not fully capture a patient’s vulnerability to AKI among the frail phenotype. Conclusion: Our work lays the foundation for implementing physical frailty in clinical practice to identify AKI earlier, implement reno‐protective strategies, and expedite liver transplantation.
Abbreviations
- aHR
adjusted hazard ratio
- AKI
acute kidney injury
- BMI
body mass index
- CI
confidence interval
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- FrAILT
Functional Assessment in Liver Transplantation
- GFR
glomerular filtration rate
- HCV
hepatitis C virus
- HR
hazard ratio
- LFI
Liver Frailty Index
- MELD‐Na
Model for End‐Stage Liver Disease–Sodium
- NAFLD
nonalcoholic fatty liver disease
- sCR
serum creatinine
- sHR
subdistribution HR
Kidney dysfunction, specifically acute kidney injury (AKI), is a significant driver of outcomes among patients with cirrhosis. Those with AKI, compared to those with normal kidney function, have more than double the risk of wait‐list mortality, and up to 50% of patients with cirrhosis will die within 30 days of developing acute kidney failure needing renal replacement therapy.( 1 , 2 , 3 ) Although this risk is partially captured by the Model for End‐Stage Liver Disease score including serum sodium (MELD‐Na), the MELD‐Na score does little to inform which cohort of patients will develop AKI.( 4 , 5 ) In fact, few premorbid (i.e., occurring before the onset of the outcome) predictors of AKI have been identified; these have been limited to either estimations of underlying chronic kidney disease (CKD)( 6 , 7 , 8 ) or reflections of more significant portal hypertension (e.g., decreased mean arterial pressure, refractory ascites).( 9 , 10 ) The lack of clinical predictors represents a need to identify premorbid predictors of AKI among patients with cirrhosis.
Recently, validated metrics that capture frailty among patients with cirrhosis, such as the Liver Frailty Index (LFI), have been developed.( 11 , 12 ) These composite metrics capture the degree of malnutrition, muscle wasting, and reduction in functional capacity among patients with cirrhosis, and ultimately serve to identify patients who are most vulnerable to clinical stressors. Whether this vulnerability to physiologic stressors relates to AKI is not known.
Herein, we use a multicenter, prospective cohort—the Functional Assessment in Liver Transplantation (FrAILT) Study—to determine the association and impact of frailty, objectively defined using the LFI, on the development of AKI among patients with cirrhosis. We hypothesized that frailty represents a clinical parameter that may identify patients most vulnerable to AKI.
Patients and Methods
Patients
This study was conducted as part of the multicenter FrAILT study, which includes nine liver transplant centers in the United States: University of California, San Francisco (n = 730); Baylor University Medical Center (n = 42); Columbia University Medical Center (n = 51); Duke University (n = 36); University of Pittsburgh (n = 30); Johns Hopkins Medical Institute (n = 54); Loma Linda University (n = 35); University of Arkansas for Medical Sciences (n = 13); and Northwestern University (n = 42). The FrAILT study enrolled patients with cirrhosis who were listed or eligible for listing for liver transplantation at the FrAILT centers and seen as outpatients. For this analysis, we excluded patients with hepatocellular carcinoma, because the time these patients spend on the wait list is not dependent on their native liver disease (n = 596). Because consent forms were not available in non‐English or non‐Spanish languages, those who did not speak English or Spanish were excluded.
Study Procedures
Frailty
At enrollment, all patients underwent a single baseline objective measurement of frailty:
Grip strength: the average of three trials, measured in the subject’s dominant hand using a hand dynamometer( 13 );
Timed chair stands: measured as the number of seconds it takes to perform five chair stands with the subject’s arms folded across the chest( 14 ); and
Balance testing: measured as the number of seconds that the subject can balance in three positions (feet placed side to side, semitandem, and tandem) for a maximum of 10 seconds each.( 14 )
These three tests were administered by trained study personnel. With these three individual frailty tests, the LFI was calculated using a standardized formula (calculator available at http://liverfrailtyindex.ucsf.edu).( 15 ) The classifications of frailty were determined using previously established cutoffs of the LFI, with physical frailty being defined as an LFI ≥ 4.2. Because most patients were listed for over 90 days (n = 918), this was deemed to be the optimal cutoff based on previous studies.( 15 )
CKD
We treated the serum creatinine recorded at the initial outpatient assessment of physical frailty as the baseline. CKD was defined as having an estimated glomerular filtration rate (eGFR) of ≤ 60 mL/min/1.73 m2 at the initial outpatient enrollment assessment. This is consistent with national guidelines.( 16 , 17 ) We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) creatinine‐based equation.( 18 ) We chose this equation because of the glomerular filtration rate (GFR) calculators that can be used with the data available in the FrAILT study; the CKD‐EPI creatinine‐based equation most closely estimates GFR relative to GFR as measured by iothalamate clearance in patients with cirrhosis.( 19 ) We treated patients who received hemodialysis at the initial assessment to have an eGFR < 15 mL/min/1.73 m2.
Clinical Parameters
We determined clinical parameters based on their records at their initial outpatient assessment. We defined ascites as “absent” if ascites was not present at the physical examination or “present” if ascites was present at the examination and/or the patient was noted to be undergoing large‐volume paracenteses. Hepatic encephalopathy was categorized as “absent” if the patient took <45 seconds to complete the Numbers Connection Test A( 20 ) and “present” if the patient took ≥45 seconds. Data demographics were extracted from the clinic visit note from the initial objective frailty measurement. Patients were considered to have a diagnosis of hypertension, diabetes mellitus, or coronary artery disease if reported in their electronic health record.
Outcomes
Our primary outcome was AKI, defined according to the International Club of Ascites definitions for the diagnosis of AKI among patients with cirrhosis( 21 ):
Stage 1 : increase in serum creatinine (sCR) ≥ 0.3 mg/dL or an increase in sCR ≥ 1.5‐fold to 2‐fold from baseline
Stage 2 : Increase in sCr > 2‐fold to 3‐fold from baseline
Stage 3 : Increase in sCr > 3‐fold from baseline or sCr ≥ 4.0 mg/dL with an acute increase ≥0.3 mg/dL or initiation of renal replacement therapy
Our secondary outcome was wait‐list mortality, defined as either a death on the wait list or removal from the wait list for being too sick for liver transplantation.
Statistical Analysis
Continuous variables were compared between groups by Wilcoxon rank sum or Kruskal‐Wallis. Categorical variables were compared between groups by the chi‐squared test. We used Cox regression analyses to determine the risk of AKI and wait‐list mortality associated with each variable. We chose censored models instead of competing risks, because we hypothesized that there was a biological link between frailty and our outcomes of interest. Patients who underwent living‐donor liver transplantation, patients who underwent liver transplantation, and patients who were removed from the wait list for reasons other than being too sick (i.e., for social reasons) were censored on the day of their removal. If variables were dynamic over the study period (e.g., laboratory values, AKI, hemodialysis), they were treated as time‐dependent variables. Clinical variables, such as CKD, diabetes, hypertension and frailty, were defined at study enrollment. Unadjusted models were used to assess the association of covariates with the outcomes of interest. All covariates with a P < 0.2 in univariate analysis were considered for inclusion in multivariate models. Sequential backward selection was used to eliminate those not reaching the significance of P < 0.05. Survival rates were estimated separately using the Kaplan‐Meier method and compared using the log‐rank test. Postestimation analysis included comparing the estimated hazards between groups.
In a sensitivity analysis, we modeled the cumulative incidence function with Fine and Gray’s competing risk regressions( 22 ) to estimate the risk of AKI and wait‐list mortality associated with each variable. In the analysis of AKI, the competing risks were death and deceased donor liver transplantation; in the analysis of wait‐list mortality, the competing risk was deceased donor liver transplantation. These results are not shown.
Statistical analyses were performed using STATA 15 (StataCorp, College Station, TX). The institutional review boards at all participating sites approved this study.
Results
Population Characteristics
A total of 1,033 patients with cirrhosis were included in this study. The baseline characteristics of the cohort are given in Table 1. The median age of the patients in our cohort was 58 (50‐63) years. Forty‐three percent were female, 61% were non‐Hispanic White, and 19% had nonalcoholic fatty liver disease (NAFLD). Concerning comorbidities, 38% had hypertension, 30% had diabetes mellitus, and 5% had coronary artery disease. The median MELD‐Na was 15 (12‐20), reflecting the outpatient status of the patients in our cohort. Concerning manifestations of decompensation, 35% had ascites, and 68% were treated for hepatic encephalopathy. Twenty‐three percent of patients had CKD, and 7% of patients received any hemodialysis. Thirty percent of the patients in our study were frail. Patients had a median of 3 (2‐4) assessments over a median follow‐up time of 1.1 (0.5‐2.2) years. There were no differences in the number of visits/laboratory draw by frailty status (frail: 3 [2‐4] vs. not frail: 3 [2‐4] visits); MELD‐Na category (MELD‐Na < 30: 3 [2‐4] vs. MELD‐Na ≥ 30: 2.5 [2‐4]) (P > 0.05 for both). Those with CKD had significantly fewer assessments than those without CKD (CKD: 2 [2‐4] vs. no CKD: 3 [2‐4]) (P = 0.001).
TABLE 1.
Baseline Demographics by Frailty Status
| Not Frail (n = 720)* | Frail (n = 313)* | P | |
|---|---|---|---|
| Age, years | 57 (49‐63) | 59 (52‐64) | 0.01 |
| Female sex | 295 (41) | 145 (46) | 0.11 |
| Race | |||
| Caucasian | 626 (87) | 279 (89) | |
| African American | 28 (4) | 18 (6) | 0.18 |
| Other | 66 (9) | 16 (5) | |
| Etiology | |||
| Alcohol | 199 (28) | 61 (20) | |
| HCV | 184 (26) | 97 (31) | |
| NAFLD | 122 (17) | 69 (22) | 0.002 |
| Autoimmune | 120 (17) | 39 (13) | |
| Other | 95 (13) | 47 (15) | |
| BMI (kg/m2) | 28.1 (25.0‐32.4) | 28.8 (25.0‐33.4) | 0.32 |
| MELD‐Na | 15 (13‐18) | 16 (13‐20) | 0.01 |
| Ascites | 214 (30) | 144 (46) | <0.001 |
| Diabetes mellitus | 176 (24) | 133 (43) | <0.001 |
| Hypertension | 258 (36) | 130 (42) | 0.08 |
| Coronary artery disease | 37 (5) | 19 (6) | 0.54 |
| CKD | 137 (19) | 105 (34) | <0.001 |
| Hemodialysis at baseline | 19 (3) | 23 (7) | <0.001 |
| Creatinine at baseline | 0.9 (0.7‐1.2) | 1.0 (0.8‐1.4) | <0.001 |
| Any AKI, n (%) | 135 (19) | 78 (25) | 0.02 |
| Wait‐list outcomes, n (%) | |||
| Waiting | 366 (51) | 146 (47) | |
| Death/too sick | 90 (13) | 67 (21) | 0.008 |
| Transplant | 264 (37) | 110 (35) |
Abbreviations: BMI, body mass index; HCV, hepatitis C virus; and IQR, interquartile range.
Median (IQR) or n (%).
Impact of Frailty on the Development of AKI Among Those With and Without CKD
Of the 1,033 patients, 242 (23%) had CKD. Those with CKD were significantly more likely to be frail (34% vs. 19%, P < 0.001). Excluding patients on hemodialysis at baseline, as they were not at risk for the outcome, there were significant differences in the proportion of patients with AKI based on frailty and CKD status: no CKD/not frail = 13% versus no CKD/frail = 19% versus CKD/not frail = 45% versus CKD/frail = 36% (P < 0.001). Among 213 participants, there were 291 episodes of AKI during the study period. We found an incidence rate of 0.19 episodes of AKI per person‐year (95% confidence interval [CI] = 0.16‐0.21). Among the 291 episodes of AKI, 133 (46%) met both the creatinine and percent change criteria for AKI, 112 (38%) met only the creatinine criteria, and 46 (16%) met only the percent change criteria. There was no difference by frailty status which criteria participants with AKI would meet (P = 0.55). In total, 47 patients experienced a recurrent episode of AKI. There was no significant difference by frailty status on the proportion of participants with AKI who had recurrent AKI (frail = 15% vs. not frail = 26%; P = 0.07).
In a univariable time‐dependent Cox regression analysis, there were significant differences in the rates of AKI based on CKD and frailty status: As compared to those with no CKD/not frail, no CKD/frail: hazard ratio (HR) = 2.24, 95% CI = 1.55‐3.24; CKD/not frail: HR = 6.10, CI = 4.12‐9.00; and CKD/frail: HR = 7.51, CI = 5.20‐10.84 (Table 2). In a multivariable time‐dependent Cox regression analysis, adjusting for MELD‐Na score, there were significant differences in the rates of AKI based on CKD and frailty status: As compared to those with no CKD/not frail, no CKD/frail: adjusted HR (aHR) = 1.87, CI = 1.29‐2.72; CKD/not frail: aHR = 4.30, CI = 2.88‐6.42; and CKD/frail: aHR = 4.85, CI = 3.33‐7.07 (Fig. 1). In the multivariable analysis for AKI, there were no significant differences in the aHR between those who had CKD and were not frail compared with those who had CKD and were frail (multivariable analysis: postestimation coefficient difference = 0.12, CI = −0.28 to 0.52) (Table 2), indicating that CKD and frailty independently contributed to AKI, but having both entities was not additive.
TABLE 2.
Time‐Dependent Cox Regression Analysis for AKI Among All 990 Patients Not on Hemodialysis
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | aHR | 95% CI | P Value | |
| Frailty/CKD status | ||||||
| Not frail/no CKD | — | — | — | — | —— | — |
| Frail/no CKD | 2.24 | 1.55‐3.24 | <0.001 | 1.87 | 1.29‐2.72 | 0.001 |
| Not frail/CKD | 6.1 | 4.13‐9.01 | <0.001 | 4.3 | 2.88‐6.42 | <0.001 |
| Frail/CKD | 7.51 | 5.20‐10.84 | <0.001 | 4.85 | 3.32‐7.07 | <0.001 |
| Age | 1.01 | 0.99‐1.02 | 0.45 | |||
| Female sex | 1.19 | 0.91‐1.56 | 0.2 | |||
| Race | ||||||
| Caucasian | — | — | — | |||
| African American | 1 | 0.49‐2.04 | 0.99 | |||
| Other | 1.24 | 0.73‐2.10 | 0.42 | |||
| Etiology | ||||||
| HCV | — | — | — | |||
| Alcohol | 1.42 | 0.95‐2.10 | 0.08 | |||
| NAFLD | 2.13 | 1.42‐3.20 | <0.001 | |||
| Autoimmune | 1.46 | 0.92‐2.33 | 0.11 | |||
| Other | 1.82 | 0.77‐4.27 | 0.17 | |||
| BMI per 1 kg/m2 | 0.97 | 0.95‐0.99 | 0.03 | |||
| MELD‐Na per point | 1.17 | 1.15‐1.19 | <0.001 | 1.14 | 1.12‐1.16 | <0.001 |
| Albumin per g/dL | 0.69 | 0.58‐0.86 | 0.001 | |||
| Ascites | 2.27 | 1.88‐2.74 | <0.001 | |||
| Diabetes mellitus | 1.71 | 1.30‐2.25 | <0.001 | |||
| Hypertension | 1.32 | 1.01‐1.74 | 0.05 | |||
| Coronary artery disease | 1.45 | 0.87‐2.42 | 0.15 | |||
Abbreviation: HCV, hepatitis C virus.
FIG. 1.
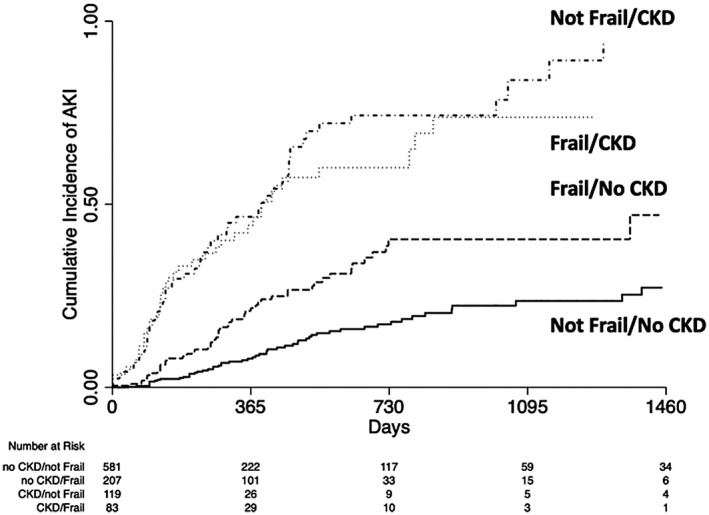
Cumulative incidence of AKI by CKD and frailty status.
Impact of Frailty on the Development of AKI Among Those Without CKD
Among the 788 patients without CKD, 208 (26%) patients were frail. Those who were frail were more likely to experience an episode of AKI (19% vs. 13%, P = 0.02). We next completed a time‐dependent Cox regression analysis to determine the association between frailty and development of the first episode of AKI among the 788 patients without CKD (Supporting Table S1). In univariable analysis, frailty was significantly associated with AKI development (HR = 2.25, CI = 1.55‐3.26). After adjusting for MELD‐Na and ascites, frailty remained significantly associated with AKI development (aHR = 1.55, CI = 1.05‐2.30) (Supporting Table S1).
Association Between Frailty and Hemodialysis Status
A total of 71 (7%) patients received any hemodialysis during the study period. There were 42 (4%) patients on hemodialysis at the initial assessment. Compared to those not on hemodialysis, a greater proportion of patients on hemodialysis at baseline were frail (57% vs. 41%, P = 0.03). There were 29 (3%) of patients started on hemodialysis during follow‐up. There were no significant differences in the proportion of frail patients among those who were and were not started on hemodialysis (28% vs. 30%, P = 0.74).
Impact of Frailty and CKD on Severity of AKI
A total of 57 (6%) patients experienced a severe episode of AKI, defined as stage 2 or greater. Those who had CKD were significantly more likely to have a severe episode of AKI (15% vs. 3%, P < 0.001). Those who were frail did not have a higher proportion of severe AKI (6% vs. 5%, P = 0.83). To evaluate the association between frailty on severe AKI, we completed several time‐dependent cox regression analyses. In univariable, as compared to those who were not frail but with CKD, those not frail and without CKD (subdistribution HR [sHR] = 0.09, CI = 0.04‐0.20), those frail and without CKD (sHR = 0.29, CI 0.13‐0.62), but not those frail with CKD (sHR = 1.48, CI = 0.76‐2.89) had a lower risk of severe AKI (Table 3). Similarly, in the final multivariable model adjusting for confounders, and as compared with those who were not frail but with CKD, those not frail and without CKD (aHR = 0.11, CI = 0.05‐0.27), those frail and without CKD (aHR = 0.36, CI = 0.16‐0.78), but not those frail with CKD (aHR = 1.09, CI = 0.55‐2.16) had a lower risk of severe AKI (Table 3).
TABLE 3.
Cox Regression Analysis for Severe AKI Among All 990 Patients Not on Hemodialysis at Listing Accounting for Liver Transplantation and Wait‐List Mortality
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | aHR | 95% CI | P Value | |
| Frailty/CKD status | ||||||
| Not frail/no CKD | 0.09 | 0.04‐0.20 | <0.001 | 0.11 | 0.05‐0.24 | <0.001 |
| Frail/no CKD | 0.29 | 0.13‐0.62 | 0.001 | 0.32 | 0.15‐0.69 | 0.003 |
| Not frail/CKD | — | — | — | — | — | — |
| Frail/CKD | 1.48 | 0.76‐2.89 | 0.25 | 1.11 | 0.56‐2.21 | 0.76 |
| Age | 1.01 | 0.98‐1.04 | 0.37 | |||
| Female sex | 1.13 | 0.67‐1.91 | 0.64 | |||
| Race | ||||||
| Caucasian | — | — | — | |||
| African American | 1.48 | 0.46‐4.74 | 0.51 | |||
| Other | 0.82 | 0.26‐2.64 | 0.74 | |||
| Etiology | ||||||
| HCV | — | — | — | |||
| Alcohol | 1.13 | 0.49‐2.61 | 0.78 | |||
| NAFLD | 3.3 | 1.57‐6.96 | 0.002 | |||
| Autoimmune | 1.19 | 0.46‐3.07 | 0.72 | |||
| Other | 2.44 | 1.01‐5.89 | 0.05 | |||
| BMI per 1 kg/m2 | 0.97 | 0.93‐1.02 | 0.29 | |||
| MELD‐Na per point | 1.24 | 1.20‐1.28 | <0.001 | |||
| Albumin per g/dL | 0.7 | 0.47‐1.05 | 0.09 | |||
| Ascites | 2.55 | 1.78‐3.64 | <0.001 | 1.9 | 1.29‐2.78 | 0.001 |
| Diabetes mellitus | 3.01 | 1.78‐5.08 | <0.001 | 2.05 | 1.29‐2.78 | 0.009 |
| Hypertension | 2.66 | 1.56‐4.53 | <0.001 | |||
| Coronary artery disease | 2.53 | 1.15‐5.59 | 0.02 | |||
Impact of AKI, CKD, Hemodialysis, and Frailty on Wait‐List Outcomes
There were 155 (15%) wait‐list deaths during the study period. AKI, CKD, hemodialysis status, and frailty all affected wait‐list outcomes: Those who had any AKI (22% vs. 13%, P = 0.001), those with CKD (21% vs. 13%, P = 0.01), those who received any hemodialysis (31% vs. 14%, P < 0.001), and those who were frail (21% vs. 12%, P < 0.001) each experienced significantly higher wait‐list mortality.
In univariable, time‐dependent Cox regression analysis, the following were significantly associated with wait‐list mortality: As compared to those who were not frail and without CKD, those who were frail without CKD (HR = 3.59, CI = 2.43‐5.30), and those who were frail with CKD (HR = 5.28, CI = 3.42‐8.15); AKI (HR = 1.81, CI = 1.23‐2.67); age (HR = 1.03 per year, CI = 1.01‐1.05); MELD‐Na (HR = 1.12 per point, CI = 1.09‐1.15); albumin (HR = 0.49 per 1g/dL, CI = 0.38‐0.62); dialysis (HR = 2.89, CI = 1.82‐4.59); the presence of ascites (HR = 1.88, CI 1.50‐2.36); the presence of diabetes (HR = 1.60, CI = 1.17‐2.21) (Table 4). In the final multivariable model, after adjusting for age, MELD‐Na, and albumin, compared to those who were not frail and without CKD, only those who were frail and had no CKD (aHR = 3.10, CI = 2.07‐4.62) and those who were frail and had CKD (aHR = 3.10, CI = 1.93‐4.97) had higher wait‐list mortality (Supporting Fig. S1).
TABLE 4.
Cox Regression Analysis for Wait‐List Mortality Among All 1,033 Patients
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| sHR | 95% CI | P Value | Adjusted sHR | 95% CI | P Value | |
| Frailty/CKD status | ||||||
| Not frail/no CKD | — | — | — | — | — | — |
| Frail/no CKD | 3.59 | 2.43‐5.30 | <0.001 | 3.1 | 2.07‐4.62 | <0.001 |
| Not frail/CKD | 1.3 | 0.65‐2.56 | 0.45 | 0.92 | 0.45‐1.86 | 0.81 |
| Frail/CKD | 5.28 | 3.42‐8.15 | <0.001 | 3.1 | 1.93‐4.97 | <0.001 |
| AKI | 1.81 | 1.23‐2.67 | 0.003 | |||
| Age | 1.03 | 1.01‐1.05 | 0.002 | 1.03 | 1.01‐1.05 | 0.006 |
| Female sex | 1.32 | 0.96‐1.81 | 0.09 | |||
| Race | ||||||
| Caucasian | — | — | — | |||
| African American | 0.3 | 0.07‐1.20 | 0.09 | |||
| Other | 0.9 | 0.47‐1.71 | 0.75 | |||
| Etiology | ||||||
| HCV | — | — | — | |||
| Alcohol | 0.79 | 0.51‐1.26 | 0.33 | |||
| NAFLD | 1.63 | 1.06‐2.50 | 0.03 | |||
| Autoimmune | 0.91 | 0.54‐1.52 | 0.71 | |||
| Other | 1.19 | 0.69‐2.06 | 0.53 | |||
| BMI per 1 kg/m2 | 0.99 | 0.97‐1.02 | 0.66 | |||
| MELD‐Na per point | 1.12 | 1.09‐1.15 | <0.001 | 1.09 | 1.06‐1.13 | <0.001 |
| Albumin per g/dL | 0.49 | 0.38‐0.62 | <0.001 | 0.62 | 0.48‐0.80 | <0.001 |
| Dialysis | 2.89 | 1.92‐4.59 | <0.001 | |||
| Ascites | 1.88 | 1.50‐2.36 | <0.001 | |||
| Diabetes mellitus | 1.61 | 1.17‐2.21 | 0.003 | |||
| Hypertension | 1.29 | 0.89‐1.69 | 0.2 | |||
| Coronary artery disease | 1.28 | 0.69‐2.27 | 0.43 | |||
Discussion
AKI is a lethal complication of cirrhosis and portal hypertension. However, there are few identified risk factors for the development of AKI in patients with cirrhosis. Using a prospective, multicenter cohort including more than 1,000 outpatients with cirrhosis, we investigated the impact of a risk factor of AKI: frailty. This study demonstrated that frail patients had a 150% higher risk of AKI among patients without CKD. Furthermore, this risk persists even after adjusting for the presence of CKD. These findings highlight that frailty, as captured through the LFI, may identify a cohort of patients with cirrhosis who are most vulnerable to AKI.
These findings are all the more remarkable because frail patients with cirrhosis have less muscle mass and, therefore, may be less able to generate significant increases in sCR as a biomarker of AKI.( 23 , 24 , 25 ) This is supported by our data demonstrating lower rates of severe AKI among frail patients, despite a significantly higher risk of any AKI during the study period. Our study confirms previous work that CKD is one of the strongest predictors of AKI in patients with cirrhosis.( 7 , 26 , 27 ) We build on these findings by investigating the interaction between CKD and frailty on incident AKI. Among those with baseline CKD, there was no difference in the risk of AKI by frailty status. These findings support the possibility that frailty, as captured through the LFI, may be an important tool in clinical practice to help identify a vulnerable subgroup of patients with cirrhosis—a subgroup who are underserved by current creatinine‐based definitions of AKI and CKD.
Another interesting observation from our study is that patients with CKD and frailty were at the greatest risk for wait‐list mortality than all other frailty‐CKD subgroups. This differs from previous work in which CKD, compared with AKI and AKI on CKD, was associated with a lower risk of wait‐list mortality among all patients with cirrhosis (with unknown frailty status).( 3 ) Such data reinforce frailty’s utility as a practical clinical tool to help risk‐stratify patients with cirrhosis—with or without CKD.( 11 , 28 )
We acknowledge the following limitations to this study. First, we defined non‐laboratory‐based variables (e.g., frailty, CKD) at the time of their enrollment in this study. We did not account for subsequent development of frailty, CKD, or other comorbidities in our models. We chose to mimic clinical practice, in which risk stratification and clinical management often must be made upon a single assessment or first clinical encounter. We decided to use laboratory‐based definitions of AKI, and as such, we did not have data regarding the etiology of AKI. Second, definitions of AKI, stages of AKI, and CKD were dependent on sCR measurements and creatinine‐based estimations of GFR. We believe that this strengthens the association between frailty and AKI, as frail patients require a greater degree of kidney injury to meet current clinical criteria. Conversely, there remains the possibility that we underestimated the presence of CKD among those who were frail. However, current clinical practice is dependent on serum creatinine. Although we support the investigation of physical frailty to improve estimations of GFR and the use of more reliable markers of GFR (e.g., cystatin c), we believe our data depict the current clinical landscape. Third, there is the possibility of ascertainment bias: Sicker patients may have more frequent blood work, and therefore more episodes of AKI. This is not what we found: Frail patients and those with higher MELD‐Na scores had similar assessments, whereas those with CKD had fewer assessments, thus providing a similar opportunity for AKI. Finally, this study’s multicenter nature exposes our analyses to heterogeneity related to center‐specific effects that might not have been accounted for in our analyses. That being said, the multicenter nature of our study makes our findings more generalizable. Collectively, our cohort represents the regional and population diversity needed to inform the management of patients with end‐stage liver disease awaiting liver transplantation nationally.
Despite these limitations, this study has important implications for evaluating and managing patients with cirrhosis, specifically those awaiting liver transplantation. We used a readily available metric, LFI, to identify patients with cirrhosis most at risk for AKI, a complication of cirrhosis associated with high mortality rates. This is particularly important because the LFI can identify a subgroup of patients who are likely underserved by using sCR in both the definition of AKI and in the MELD‐Na score. Our work lays the foundation for implementing frailty in clinical practice, to allow for the earlier use of reno‐protective strategies (e.g., augmentation of blood pressure with oral vasopressors or administration of albumin, discontinuation of nonselective beta blockers, decrease in diuretics, avoidance of nephrotoxic substances), and expedition of liver transplantation.
Supporting information
Table S1
Supported by National Institute on Aging (K23AG048337, P30DK026743, and R01AG059183) National Institute of Diabetes and Digestive and Kidney Diseases (F32DK124941, K24DK092291, and K24DK101828).
Potential conflict of interest: Dr. Cullaro received grants from Mallinckrodt. Dr. Duarte‐Rojo advises and received grants from Axcella. Dr. Ganger consults for ACI and received grants from Gilead.
REFERENCES
- 1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749‐1761. [DOI] [PubMed] [Google Scholar]
- 2. Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009;361:1279‐1290. [DOI] [PubMed] [Google Scholar]
- 3. Cullaro G, Verna EC, Lai JC. Association between renal function pattern and mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2019;17:2364‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver‐transplant waiting list. N Engl J Med 2008;10:1018‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamath P, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33:464‐470. [DOI] [PubMed] [Google Scholar]
- 6. Cullaro G, Park M, Lai JC. “Normal” creatinine levels predict persistent kidney injury and waitlist mortality in outpatients with cirrhosis. Hepatology 2018;68:1953‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong F, Reddy KR, O’Leary JG, Tandon P, Biggins SW, Garcia‐Tsao G, et al. Impact of chronic kidney disease on outcomes in cirrhosis. Liver Transpl 2019;25:870‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bassegoda O, Huelin P, Ariza X, Solé C, Juanola A, Gratacós‐Ginès J, et al. Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes. J Hepatol 2020;72:1132‐1139. [DOI] [PubMed] [Google Scholar]
- 9. Wong F, O'Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, et al. New consensus definition of acute kidney injury accurately predicts 30‐day mortality in patients with cirrhosis and infection. Gastroenterology 2013;145:1280‐1288.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut 2013;62:131‐137. [DOI] [PubMed] [Google Scholar]
- 11. Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end‐stage liver disease. Hepatology 2017;66:564‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870‐1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146‐M56. [DOI] [PubMed] [Google Scholar]
- 14. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85‐M94. [DOI] [PubMed] [Google Scholar]
- 15. Kardashian A, Ge J, McCulloch CE, Kappus MR, Dunn MA, Duarte‐Rojo A, et al. Identifying an optimal liver frailty index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology 2021;73:1132‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713‐735. [DOI] [PubMed] [Google Scholar]
- 17. Kidney Disease Improving Global Outcomes (KDIGO) . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. KI Suppl 2013;3. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krones E, Fickert P, Zitta S, Neunherz S, Artinger K, Reibnegger G, et al. The chronic kidney disease epidemiology collaboration equation combining creatinine and cystatin C accurately assesses renal function in patients with cirrhosis. BMC Nephrol 2015;16:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weissenborn K, Rückert N, Hecker H, Manns MP. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol 1998;28:646‐653. [DOI] [PubMed] [Google Scholar]
- 21. Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015;64:1‐8. [DOI] [PubMed] [Google Scholar]
- 22. Fine JP, Gray RJ. Proportional hazards model for the subdistribution of a competing risk a proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;1459:37‐41. [Google Scholar]
- 23. Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in Cirrhosis. Arch Intern Med 1994;154:201‐205. [PubMed] [Google Scholar]
- 24. Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 2008;3:348‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang CW, Feng S, Covinsky KE, Hayssen H, Zhou L‐Q, Yeh BM, et al. A comparison of muscle function, mass, and quality in liver transplant candidates: results from the Functional Assessment in Liver Transplantation (FrAILT) study. Transplantation 2016;100:1692‐1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cullaro G, Verna EC, Lee BP, Lai JC. Chronic kidney disease in liver transplant candidates: a rising burden impacting post‐liver transplant outcomes. Liver Transpl 2020;26:498‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong F, O’Leary JG, Reddy KR, Garcia‐Tsao G, Fallon MB, Biggins SW, et al. Acute kidney injury in cirrhosis: baseline serum creatinine predicts patient outcomes. Am J Gastroenterol 2017;112:1‐8. [DOI] [PubMed] [Google Scholar]
- 28. Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams‐DeMarco M, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology 2019. May 1;156:1675‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


