Abstract
The effect of antibiotics on the acute lung injury induced by virulent Pseudomonas aeruginosa PA103 was quantitatively analyzed in a rat model. Lung injury was induced by the instillation of PA103 directly into the right lower lobes of the lungs of anesthetized rats. The alveolar epithelial injury, extravascular lung water, and total plasma equivalents were measured as separate, independent parameters of acute lung injury. Four hours after the instillation of PA103, all the parameters were increased linearly depending on the dose of P. aeruginosa. Next, we examined the effects of intravenously administered antibiotics on the parameters of acute lung injury in d-galactosamine-sensitized rats. One hour after the rats received 107 CFU of PA103, an intravenous bolus injection of aztreonam (60 mg/kg) or imipenem-cilastatin (30 mg/kg) was administered. Despite an MIC indicating resistance, imipenem-cilastatin improved all the measurements of lung injury; in contrast, aztreonam, which had an MIC indicating sensitivity, did not improve any of the lung injury parameters. The antibiotics did not generate different quantities of plasma endotoxin; therefore, endotoxin did not appear to explain the differences in lung injury. This in vivo model is useful to quantitatively compare the efficacies of parenteral antibiotic administration on Pseudomonas airspace infections.
Pseudomonas aeruginosa is a major cause of lung infections, particularly nosocomial pneumonia (9, 13, 23, 28). Mortality rates of patients with P. aeruginosa pneumonia are higher than the mortality rates of patients with pneumonia caused by other pathogens (14) because P. aeruginosa pneumonia frequently disseminates to cause bacteremia and sepsis (6, 22, 27). This rapid dissemination is associated with the generation of acute lung epithelial injury by some strains of P. aeruginosa (29, 30). To protect patients who have P. aeruginosa pneumonia from bacteremia, sepsis, and death, the effective utilization of antibiotics is important. However, most of the strains of P. aeruginosa are inherently resistant to many antibiotics (10).
The hypothesis tested by this study was that acute lung injury may be decreased by a single bolus of antibiotics if the antibiotics decrease the number of bacteria instilled in the lungs of rats. We also sought to determine whether the amount of lung injury was related to the change in endotoxin concentration over the experimental period. In this investigation, we quantitated the acute lung injury caused by the airspace instillation of a virulent strain of P. aeruginosa, PA103, in anesthetized rats. The effects of different inoculums of the same strain, PA103, on multiple, independent parameters of lung injury were determined. We then investigated the effect of antibiotic treatment on these parameters of bacterially induced lung injury. We chose two antibiotics, aztreonam and imipenem-cilastatin, and compared their effects on the parameters of acute lung injury in the rat model. Both aztreonam (1, 5, 11) and imipenem (3, 4, 26) are utilized clinically to treat infections caused by this organism.
The MIC in vitro bacterial sensitivity test is utilized routinely to choose an antibiotic that can effectively kill a bacterial pathogen. However, the concentration of antibiotics at the site of infection, which is affected by various pharmacokinetic factors, including the rate of penetration into the specific tissues and the speed of metabolism of the drugs, may be more important in the efficacy of antibiotics in vivo. Therefore, we utilized this animal model to compare two antibiotics that had different MICs to determine which of the antibiotics was more efficacious in improving the parameters of bacterially induced lung injury.
MATERIALS AND METHODS
Surgical preparation and ventilation.
Male Sprague-Dawley rats (300 to 350 g; Simonsen, Gilroy, Calif.), certified pathogen free, were anesthetized with 30 mg of pentobarbital given intraperitoneally; anesthesia was maintained with 12 mg of intraperitoneally administered pentobarbital every 2 h. All rats remained anesthetized, intubated, and ventilated throughout the entire experiment. The right carotid artery was cannulated with a polyethylene tube (PE50; Clay Adams, Parsippany, N.J.), for monitoring arterial blood pressure and for blood sampling. The right jugular vein was cannulated with a PE50 tube, and the drugs were administered intravenously. Pancuronium bromide (0.3 mg/kg of body weight) was given intravenously every 2 h for neuromuscular blockade. An endotracheal tube (PE240; Clay Adams) was inserted into the trachea via a tracheostomy. The rats were ventilated with a constant-volume animal respirator (Harvard Apparatus, South Natick, Mass.) with an O2 fraction of 1.0 and 3-cm positive end-expiratory pressure. The respiratory rate was adjusted to maintain an arterial PCO2 between 35 and 45 mm Hg. All animal experiments were conducted in compliance with the guidelines of the Animal Care Committee of the University of California at San Francisco.
Culture conditions, bacterial strains, and instillate preparation.
P. aeruginosa PA103 was instilled to induce lung injury, as it has been shown to significantly increase the measurements of lung injury (20). The strain was stored as a bacterial stock at −70°C in a 10% sterile skim milk solution. The bacteria were streaked onto a Vogel-Bonner minimal medium plate for 36 h at 37°C, and then the bacteria were cultured in tryptic soy broth for 13 h at 32°C in a shaking incubator.
The instillate consisted of a 1-ml iso-osmolar albumin solution prepared from Ringer’s lactate, 2 mg of Evans blue dye, and 3 μCi of 125I-labeled albumin. P. aeruginosa at a concentration of 106, 107, or 108 CFU, determined spectrophotometrically, was added to the instillate just prior to airspace instillation. A sample of the instillate was saved for measurement of radioactivity, protein concentration, and quantitative bacterial cultures to assure accurate inoculations.
Treatments.
The MICs of the antibiotics were determined by the standard microdilution technique, according to the methods of the National Committee for Clinical Laboratory Standards (21). Aztreonam (Squibb Inc., Princeton, N.J.) at 60 mg/kg or imipenem-cilastatin (Merck, Inc., Rahway, N.J.) at 30 mg/kg was administered as an intravenous bolus dose 1 h after the airspace bacterial instillation. Control rats received an equal volume of normal saline (Baxter, Roundlake, Ill.) as a bolus. The investigator was blinded to whether a drug was administered. Due to the length of surgery, only two rats were studied in 1 day to maintain reproducibility of the model; a control rat was always one of the experimental animals.
Endotoxin measurements.
Serum samples for measurements of endotoxin were obtained just prior to and 4 h after the bacterial instillation. The samples were allowed to clot for 20 min and then were centrifuged and immediately frozen at −70°C until they were analyzed. The serum samples were measured for free endotoxin with the Limulus amebocyte lysate assay (Chromogenic-Limulus Amebocyte; Wittaker M. A. Bioproducts, Walkersville, Md.).
General experimental protocol.
In all experiments, after the surgical preparation, 3 μCi of 131I-albumin (Merck-Frosst, Kirkland, Quebec, Canada) was injected intravenously. The rats were then placed in a right lateral decubitus position. With the use of 1-ml syringes and PE50 tubes, the instillates were delivered to the right lungs (primarily the right lower lobes) over a 30-min interval. The rats were injected with 500 mg of d-galactosamine/kg intraperitoneally during the bacterial instillation in order to sensitize them to the effects of endotoxin (2, 15, 16). Systemic arterial and airway pressures were measured at 1-h intervals. Blood samples were obtained for 131I-albumin and 125I-albumin measurement every hour after the instillation for 6 h.
After 6 h, the rats were deeply anesthetized, their abdomens were opened, and they were exsanguinated. Samples of blood, urine, right pleural fluid, and liver were obtained for bacterial cultures and radioactivity counts. The lungs were removed, and any remaining instillate was aspirated with a 3-ml syringe and a PE50 tube passed into the right lower lobes. The total protein and radioactivity of the alveolar samples were measured. The right and left lungs were homogenized separately for water-to-dry-weight ratio measurements and for culture. Measurements of the total protein concentrations of plasma, pleural fluid, and initial and final instillate samples were performed by the Biruet method.
Measurements of lung injury.
Lung injury was quantified in four ways, as previously described (29, 30). The first method quantified the movement of the alveolar protein tracer, 125I-albumin, from the lung into the blood by measuring the amount of residual 125I-albumin in the lung as well as the accumulation of the tracer in the plasma over the 6-h course of the experiment. Total 125I-albumin instilled into the lung was determined by measuring duplicate samples of the instillate for total radioactivity (counts per minute per gram) and multiplying this amount by the total volume instilled into the lung. To calculate the residual 125I-albumin in the lung after 6 h, the average of two 0.5-g samples obtained from the lung homogenate was multiplied by the total volume of the lung homogenate. The radioactivities in the lung homogenates were added to the recovered radioactive counts in the aspirated fluid from the lungs. Circulating plasma 125I-albumin was measured from a sample of plasma obtained at the end of 6 h. The plasma fraction was accounted for by multiplying the counts per milliliter by the plasma volume [body weight × 0.07 (1 hematocrit)].
The second method required the measurement of 131I-albumin (the vascular protein tracer) in the airspace compartment of the lung at the end of the experiment. This was accomplished by measuring the 131I-albumin counts in the final airspace sample. Plasma 131I-albumin counts were averaged over the course of the experiment, and the 131I-albumin counts in the airspaces were expressed as a ratio to the plasma counts. This ratio provides a good index of equilibration of the vascular protein tracer into the alveolar compartment, as had been shown previously (30).
Third, extravascular lung water was determined for each separate lung by calculating the water-to-dry-weight ratio. The excess water in the experimental lung was calculated with the same equation described previously (30). Briefly, the volume of excess lung water (ELW; in ml) of the serum-instilled experimental lung was calculated as follows: ELW = [We/(De − P) − Wc/Dc](De − P), where W and D are extravascular lung water and blood-free dry weights, respectively, of the experimental lung (e) and the control lung (c) and P is the blood-free dry weight of the initial alveolar fluid multiplied by the fraction of 125I-albumin remaining in the lung.
Finally, the accumulation of the vascular protein tracer into the extravascular space of the lung was calculated by determining the total extravascular count of 131I-albumin in the lung divided by the average counts in the plasma over the 6-h study period and was expressed as total plasma equivalents in milliliters.
Statistics.
All data presented are means ± standard errors. The data were analyzed by one-way analysis of variance followed by Dunnet’s multiple-comparison test. A P value of <0.05 was accepted as statistically significant.
RESULTS
Acute lung injury induced by the airspace instillation of P. aeruginosa.
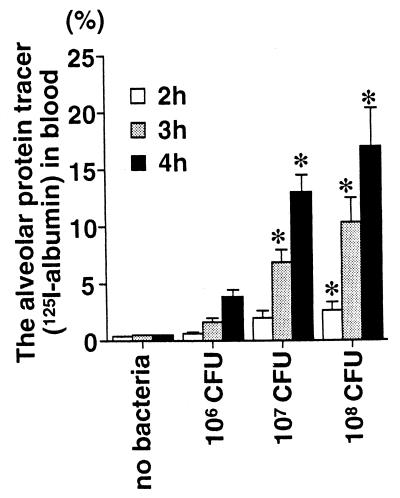
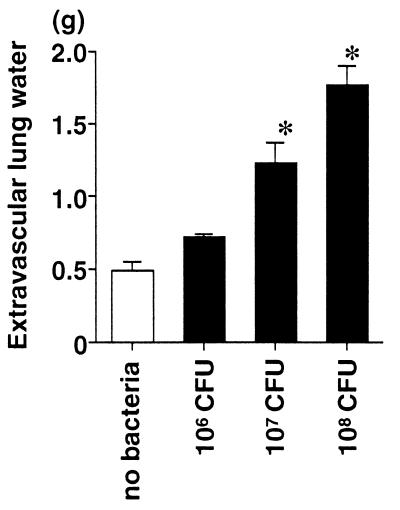
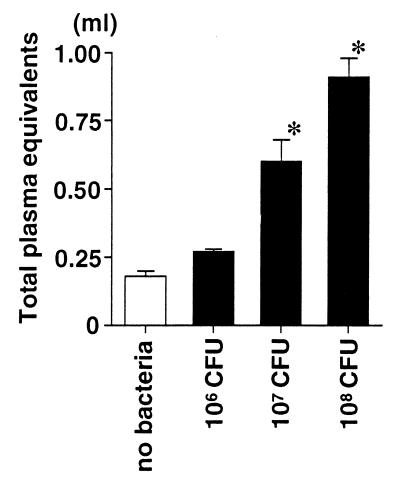
First, we quantified the acute lung injury in rats receiving the airspace instillation of P. aeruginosa. Rats receiving three different doses (106, 107, and 108 CFU/rat) of P. aeruginosa were compared with the control rats that received vehicle without bacteria. The measurements of alveolar epithelial injury (the alveolar protein tracer in blood) and two measurements of lung edema (extravascular lung water and total plasma equivalents) were calculated in the 4-h experiments. The alveolar epithelial injury quantified by the movement of the radiolabeled alveolar protein tracer was increased linearly with increasing doses of P. aeruginosa or by increasing the duration of bacterial exposure in the lung from 2 to 4 h (Fig. 1). Both parameters of lung edema, the extravascular lung water (Fig. 2) and the total plasma equivalents (Fig. 3), were also increased linearly with increasing doses of P. aeruginosa. Therefore, in this rat model, increasing quantities of acute lung injury can be consistently produced by modifying the dose of bacteria and/or the interval of bacterial exposure.
FIG. 1.
Alveolar epithelial injury in rats instilled with three different doses of P. aeruginosa. The rats received an instillate that included the alveolar protein tracer 125I-albumin without bacteria (control) or with P. aeruginosa PA103 (106, 107, or 108 CFU) in their lungs. The percentage of the alveolar protein tracer in the blood at 2, 3, or 4 h after the instillation was calculated. The data are means + standard errors. ∗, P < 0.05. P values are by comparison with the results for the control group that did not receive bacteria (analysis of variance followed by Dunnet’s test). The control (no-bacteria) and 106-, 107-, and 108-CFU groups had seven, six, nine, and five rats, respectively.
FIG. 2.
Extravascular lung water measurements in rats instilled with three different doses of P. aeruginosa. The rats received an instillate without bacteria (control) or with P. aeruginosa PA103 (106, 107, or 108 CFU) in their lungs. The amount of extravascular lung water was calculated 4 h after the instillation. The data are means + standard errors. ∗, P < 0.05. P values are by comparison with the results for the control group that did not receive bacteria. The control (no-bacteria) and 106-, 107-, and 108-CFU groups had seven, six, nine, and five rats, respectively.
FIG. 3.
Total plasma equivalents in rats instilled with three different doses of P. aeruginosa. The rats received an instillate without bacteria (control) or with P. aeruginosa PA103 (106, 107, or 108 CFU) in their lungs. The total plasma equivalent was calculated 4 h after the instillation. The data are means + standard errors. ∗, P < 0.05. P values are by comparison with the results for the control group that did not receive bacteria. The control (no-bacteria) and 106-, 107-, and 108-CFU groups had seven, six, nine, and five rats, respectively.
Effects of antibiotics on acute lung injury caused by P. aeruginosa.
Based on the results described above, we evaluated the effects of two antibiotics, aztreonam and imipenem-cilastatin, on acute lung injury. A dose (107 CFU) of P. aeruginosa was administered to each rat to produce a moderate quantity of lung injury that might be modified by the administration of antibiotics. We observed the animals for 6 h in this series to evaluate the effect of antibiotics. In addition to the parameters of acute lung injury, we measured the number of bacteria in the instilled lung 6 h after the tracheal instillation of P. aeruginosa.
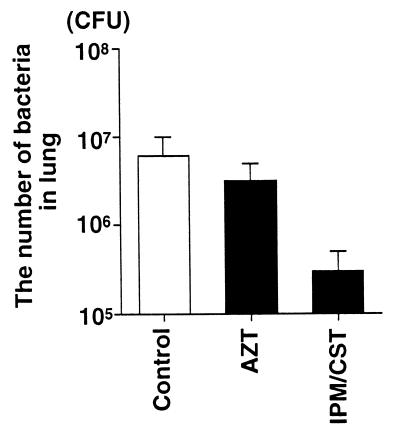
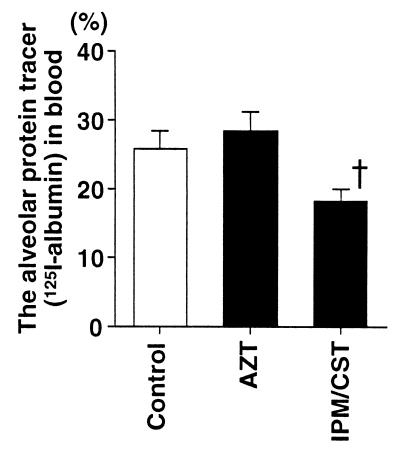
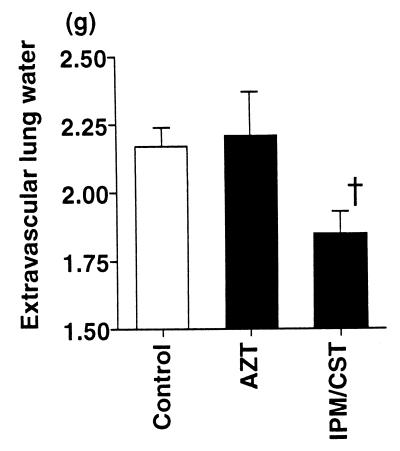
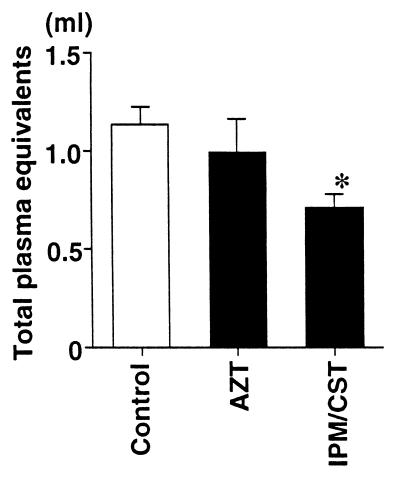
The MIC of imipenem-cilastatin was 16 μg/ml, considered to be resistant by the National Committee for Clinical Laboratory Standards (20). In contrast, the MIC of aztreonam was 0.5 μg/ml, considered sensitive by the same standards. Nonetheless, among the rats that received imipenem-cilastatin after the instillation of bacteria there was a trend toward fewer bacteria remaining in the lungs (3.0 × 105 ± 2.0 × 105 CFU/ml) than among the rats that had received aztreonam (3.2 × 106 ± 1.7 × 106 CFU/ml) or the rats that had not received antibiotics (6.0 × 106 ± 4.0 × 106 CFU/ml) (Fig. 4). The blood culture results from all rats at the end of the experimental period were negative. The culture results from a liver sample taken at the end of the experiment were positive for all rats. Imipenem-cilastatin significantly improved all the parameters of lung injury measured compared to the control, untreated rats or to the aztreonam-treated rats. The administration of imipenem-cilastatin resulted in significantly less alveolar epithelial injury (Fig. 5), significantly less extravascular lung edema (Fig. 6), and significantly fewer total plasma equivalents (Fig. 7). The baseline blood pressure measurements of the groups were not different (P > 0.05). Treatment with aztreonam resulted in greater reduction in systolic and diastolic blood pressure 5 h after instillation compared to treatment with imipenem-cilastatin and the control (Table 1). At 6 h, systolic blood pressure was significantly reduced in the aztreonam-treated group (P = 0.026), and there was a trend toward statistical significance for reduction in diastolic blood pressure in the aztreonam-treated group (P = 0.052) at this time point. The reduction in lung injury and hypotension in the imipenem-cilastatin-treated rats was not explained by the serum endotoxin levels 4 h after the tracheal instillation of P. aeruginosa; the levels were not different in the antibiotic-treated rats and the control animals (Table 2).
FIG. 4.
Effect of administration of aztreonam or imipenem-cilastatin on the number of bacteria in the right lung 6 h after the instillation of P. aeruginosa PA103. One hour after the airspace instillation of PA103 (107 CFU), an intravenous bolus dose of aztreonam (AZT; 60 mg/kg) or imipenem-cilastatin (IPM/CST; 30 mg/kg) was administered. Control rats (Control) received an equal volume of normal saline intravenously 1 h after the airspace instillation of PA103 (107 CFU). The data are means + standard errors (there were no statistically significant differences among groups by one-way analysis of variance). The control, AZT, and IPM/CST groups had seven, four, and four rats, respectively.
FIG. 5.
Alveolar epithelial injury in rats instilled with P. aeruginosa and then treated with aztreonam or imipenem-cilastatin. One hour after the airspace instillation of PA103 (107 CFU) with the alveolar protein tracer 125I-albumin, an intravenous bolus dose of aztreonam (AZT; 60 mg/kg) or imipenem-cilastatin (IPM/CST; 30 mg/kg) was administered. The percentage of the alveolar protein tracer in the blood 6 h after the instillation was calculated. The data are means + standard errors. †, P < 0.05. P values are by comparison with the results for the AZT group (one-way analysis of variance followed by Dunnet’s test). The control, AZT, and IPM/CST groups had seven, four, and four rats, respectively.
FIG. 6.
Extravascular lung water measurements in rats instilled with P. aeruginosa and then treated with aztreonam or imipenem-cilastatin. One hour after the airspace instillation of PA103 (107 CFU) with the alveolar protein tracer 125I-albumin, an intravenous bolus dose of aztreonam (AZT; 60 mg/kg) or imipenem-cilastatin (IPM/CST; 30 mg/kg) was administered. The extravascular lung water 6 h after the instillation was calculated. Data are means + standard errors. †, P < 0.05. P values are by comparison with the results for the AZT group (one-way analysis of variance followed by Dunnet’s test). The control, AZT, and IPM/CST groups had seven, four, and four rats, respectively.
FIG. 7.
Total plasma equivalents in rats instilled with P. aeruginosa and then treated with aztreonam or imipenem-cilastatin. One hour after an airspace instillation of PA103 (107 CFU), an intravenous bolus dose of aztreonam (AZT; 60 mg/kg) or imipenem-cilastatin (IPM/CST; 30 mg/kg) was administered. The total plasma equivalents 6 h after the instillation of antibiotic were calculated. Data are means + standard errors. ∗, P < 0.05. P values are by comparison with the results for the control group (one-way analysis of variance followed by Dunnet’s test). The control, AZT, and IPM/CST groups had seven, four, and four rats, respectively.
TABLE 1.
Arterial blood pressure before instillation of bacteria and at 5 and 6 h
| Group | n | Mean blood pressure (mm Hg)a
|
|||||
|---|---|---|---|---|---|---|---|
| Systolic
|
Diastolic
|
||||||
| Before instillation | 5 h after instillation | 6 h after instillation | Before instillation | 5 h after instillation | 6 h after instillation | ||
| Imipenem-cilastatin | 4 | 174 ± 20 | 113 ± 4b | 110 ± 8c | 130 ± 11 | 90 ± 5d | 89 ± 7e |
| Aztreonam | 4 | 169 ± 5 | 83 ± 9 | 75 ± 6 | 123 ± 11 | 49 ± 10 | 55 ± 9 |
| Control | 7 | 145 ± 7 | 93 ± 5 | 93 ± 6 | 116 ± 8 | 59 ± 7 | 65 ± 8 |
Values are mean ± standard error.
Different from aztreonam (P < 0.05).
Different from aztreonam (P < 0.05).
Different from aztreonam and control (P < 0.05).
Trend toward statistically significant difference between treatments (P = 0.052).
TABLE 2.
Serum endotoxin concentration before instillation of bacteria and at 4 h
| Group | n | Serum endotoxin concn (pg/ml)a
|
|
|---|---|---|---|
| Before instillation | 4 h after instillation | ||
| Imipenem-cilastatin | 3 | 120 ± 19 | 39 ± 11 |
| Aztreonam | 3 | 60 ± 21 | 46 ± 4 |
| Control | 6 | 105 ± 30 | 52 ± 38 |
Values are means ± standard errors. There were no statistical differences among the groups.
DISCUSSION
A model that produces a consistent quantity of bacterially-induced lung injury is a useful tool for comparing the effects of various antibiotic treatments on markers of lung injury. In the development of such a model we have shown that the administration of small doses (106 CFU) of P. aeruginosa for longer intervals (4 h) consistently led to moderate quantities of lung injury. The administration of larger doses (108 CFU) of P. aeruginosa for longer intervals produced larger quantities of lung injury with very small standard deviations in the measurements. Therefore, the airspace administration of P. aeruginosa can be utilized to produce a consistent quantity of lung injury that can be increased or decreased by modifying the dose or the interval. These results allowed us to choose a dose of P. aeruginosa, 107 CFU, and an interval, 6 h, to produce a moderate amount of lung injury. While this model has some limitations, such as the short evaluation period of 6 h, it also has advantages, such as reproducible, quantifiable lung injury measurements. Because of these advantages, we elected to use this model to test the hypothesis that acute lung injury may be decreased by a single bolus of antibiotics if the antibiotics decrease the number of bacteria instilled in the lungs of rats. We also sought to determine whether the amount of lung injury was related to the change in serum endotoxin concentration over the experimental period. In fact, we were able to document that one bolus of imipenem-cilastatin, but not aztreonam, significantly improved all parameters of the bacterially induced lung injury as evidenced by the extravascular lung water and total plasma equivalents. The extravascular lung water is an overall measurement of lung edema. Total plasma equivalents is also a measure of lung edema but is more specific for the movement of plasma into the lung. We were not able to correlate these parameters of lung injury with plasma endotoxin concentration.
Imipenem-cilastatin, but not aztreonam, was able to significantly improve lung injury. P. aeruginosa PA103 is sensitive to aztreonam, as the MIC for it was 0.5 μg/ml; therefore the lack of improved killing compared to untreated controls was not due to antibiotic resistance. In contrast, there was a trend for increased killing by imipenem-cilastatin despite a MIC of imipenem (16 μg/ml) higher than the MIC of aztreonam (0.5 μg/ml). This result suggests that measurement of the in vitro killing of bacteria does not correlate with antibiotic-induced killing in an airspace infection in vivo. The pharmacokinetics of aztreonam and imipenem-cilastatin have been described previously in rats (17, 19). The penetrations of these antibiotics into the lung tissue are comparable, measuring approximately 50% of that in the serum. The protein binding of aztreonam is nearly 50 to 60%, whereas that of imipenem-cilastatin is only 10 to 20% (7, 8). In order to account for this difference in protein binding in the model, we elected to give a higher dose of aztreonam (60 mg/kg) than of imipenem-cilastatin (30 mg/kg). Aztreonam displays an effect of inoculum on the MIC at 107 to 108 CFU/ml, whereas impenem does not show an inoculum effect on the MIC until 108 CFU/ml (7, 8). It is possible that the inoculum selected for study, 107 CFU/ml, favored the activity of imipenem-cilastatin. However, the MIC of aztreonam was five times lower that that of imipenem-cilastatin, which would favor the activity of aztreonam.
This model of lung injury also resulted in bacteremia, as evidenced by the positive liver cultures. Administration of imipenem-cilastatin, but not aztreonam, resulted in a reduction of systemic hypotension over the experimental period, which may have contributed to the increased lung injury observed in the aztreonam-treated rats.
It has been demonstrated that antibiotics which target PBP-2, such as imipenem, liberate less endotoxin than antibiotics which bind PBP-3, such as aztreonam (12, 18, 25). Aztreonam may cause endotoxin release due to filamentation, which appears to be secondary to the properties of the penicillin binding protein. In fact, we previously documented that the airspace instillation of aztreonam along with P. aeruginosa caused endotoxin release and increased acute lung injury (24). However, in this model, there were no differences in the serum levels of endotoxin between the groups. Therefore, antibiotic-induced release of serum endotoxin did not appear to explain the differences in the lung injury measurements between the aztreonam-treated rats and the imipenem-cilastatin-treated rats. It is possible that lung endotoxin levels were different in the treatment groups; however, we were unable to measure lung endotoxin levels using this model.
In conclusion, a virulent strain of P. aeruginosa, PA103, can be utilized to create a consistent quantity of lung injury for comparisons of antibiotic effectiveness in airspace infections. Imipenem-cilastatin administration led to significant improvement in all the measured parameters of lung injury. In contrast, aztreonam, which was effective in killing this bacterium in vitro, did not improve any of the parameters of lung injury compared to nontreated animals. These results suggest that the evaluation of antibiotic effects in vivo may be important in airspace infections. Our animal model for Pseudomonas pneumonia is useful for evaluation of the comparative effects of antibiotics.
ACKNOWLEDGMENTS
We thank Richard Shanks for his technical support.
This work was supported by National Heart and Lung Institute grants HL49810 and HL59239 (J.P.W.-K.).
REFERENCES
- 1.Allen K D, Green H T. Aztreonam in infections due to aerobic gram-negative bacteria. J Antimicrob Chemother. 1989;23:290–291. doi: 10.1093/jac/23.2.290. [DOI] [PubMed] [Google Scholar]
- 2.Bahrami S, Redl H, Buurman W A, Schlag G. Influence of the xanthine derivative HWA 138 on endotoxin-related coagulation disturbances: effects in non-sensitized vs. D-galactosamine sensitized rats. Thromb Haemost. 1992;68:418–423. [PubMed] [Google Scholar]
- 3.Barza M. Imipenem: first of a new class of beta-lactam antibiotics. Ann Intern Med. 1985;103:552–560. doi: 10.7326/0003-4819-103-4-552. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum J F, Kahan M, Kropp H, MacDonald J S. Carbapenems, a new class of beta-lactam antibiotics. Discovery and development of imipenem/cilastatin. Am J Med. 1987;78:3–21. doi: 10.1016/0002-9343(85)90097-x. [DOI] [PubMed] [Google Scholar]
- 5.Boccazzi A, Langer M, Mandeli M, Ranzi A M, Urso R. The pharmacokinetics of aztreonam and penetration into the bronchial secretions of critically ill patients. J Antimicrob Chemother. 1989;23:401–407. doi: 10.1093/jac/23.3.401. [DOI] [PubMed] [Google Scholar]
- 6.Brewer S C, Wunderink R G, Jones C B, Leeper K V., Jr Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 7.Brogden R N, Heel R C. Aztreonam, a review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1986;31:96–130. doi: 10.2165/00003495-198631020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Buckley M M, Brogden R N, Barradell L B, Goa K L. Imipenem/cilastatin, a reappraisal of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1992;44:408–444. doi: 10.2165/00003495-199244030-00008. [DOI] [PubMed] [Google Scholar]
- 9.Craven D E, Steger K. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect. 1996;11:32–53. [PubMed] [Google Scholar]
- 10.Cullmann W, Buscher K H, Dick W. Selection and properties of Pseudomonas aeruginosa variants resistant to beta-lactam antibiotics. Eur J Clin Microbiol. 1987;6:467–473. doi: 10.1007/BF02013112. [DOI] [PubMed] [Google Scholar]
- 11.Demaria A, Treadwell T L, Saunders C A, Porat R, McCabe W R. Randomized clinical trial of aztreonam and aminoglycoside antibiotics in the treatment of serious infections caused by gram-negative bacilli. Antimicrob Agents Chemother. 1989;33:1137–1143. doi: 10.1128/aac.33.8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dofferhoff A S M, Nijland J H, de Vries-Hispers H G, Mulder P O M, Veits J, Bom V J. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: an in-vitro and in-vivo study. Scand J Infect Dis. 1991;23:745–754. doi: 10.3109/00365549109024303. [DOI] [PubMed] [Google Scholar]
- 13.Fabregas N, Torres A, El-Ebiary M, Ramfrez J, Hernandez C, Gonzalez J, de la Bellacasa J P, de Anta J, Rodoriguez-Roisin R. Histopathologic and microbiologic aspects of ventilator-associated pneumonia. Anesthesiology. 1996;84:760–771. doi: 10.1097/00000542-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Fagon J Y, Chastre J, Hance A J, Montravers P, Novara A, Gilbert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 15.Galanos C, Freudenberg M A. Mechanisms of endotoxin shock and endotoxin hypersensitivity. Immunobiology. 1993;187:346–356. doi: 10.1016/S0171-2985(11)80349-9. [DOI] [PubMed] [Google Scholar]
- 16.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto S, Wolfe E, Guglielmo B, Shanks R, Sundelof J, Pittet J F, Thomas E, Wiener-Kronish J. Aerosolization of imipenem/cilastatin prevents pseudomonas-induced acute lung injury. J Antimicrob Chemother. 1996;38:809–818. doi: 10.1093/jac/38.5.809. [DOI] [PubMed] [Google Scholar]
- 18.Jackson J J, Kropp H. β-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP-3-specific ceftazidime. J Infect Dis. 1992;165:1033–1041. doi: 10.1093/infdis/165.6.1033. [DOI] [PubMed] [Google Scholar]
- 19.Kripilani K J, Singhvi S M, Weinstein S H, Everett D W, Bathala M S, Dean A V, Ita C E, Lawrence L, Meeker F S, Jr, Shaw J M, Walker B D, Migdalof B H. Disposition of [14C]aztreonam in rats, dogs, and monkeys. Antimicrob Agents Chemother. 1984;26:119–126. doi: 10.1128/aac.26.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudoh I, Wiener-Kronish J P, Hashimoto S, Pittet J-F, Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar injury. Am J Physiol. 1994;267:L511–L516. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for determining dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 22.Parrillo J E, Parker M M, Nathanson C, Suffredini A F, Danner R L, Cunnion R E, Ognibene F P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction and therapy. Ann Intern Med. 1990;113:227–237. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 23.Rouby J J. Nosocomial infection in the critically ill. The lung as a target organ. Anesthesiology. 1996;84:757–758. doi: 10.1097/00000542-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Sawa T, Kurahashi K, Ohara M, Gropper M A, Doshi V, Larrick J W, Wiener-Kronish J P. Evaluation of antimicrobial and lipopolysaccharide-neutralizing effects of a synthetic CAP18 fragment against Pseudomonas aeruginosa in a mouse model. Antimicrob Agents Chemother. 1998;42:3269–3275. doi: 10.1128/aac.42.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shenep J L, Barton R P, Mogan K A. Role of antibiotic class in the rate of liberation of endotoxin during therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1985;151:1012–1018. doi: 10.1093/infdis/151.6.1012. [DOI] [PubMed] [Google Scholar]
- 26.Stradvik G, Malmborg A S, Bergan T, Michalsen H, Storrosten O T, Wretlind B. Imipenem/cilastatin, an alternative treatment of Pseudomonas infection in cystic fibrosis. J Antimicrob Chemother. 1988;21:471–480. doi: 10.1093/jac/21.4.471. [DOI] [PubMed] [Google Scholar]
- 27.Taylor G D, Buchanan-Chell M, Kirkland T, McKenzie M, Wiens R. Bacteremic nosocomial pneumonia. A 7-year experience in one institution. Chest. 1994;108:786–788. doi: 10.1378/chest.108.3.786. [DOI] [PubMed] [Google Scholar]
- 28.Torres A, Aznar R, Gatell J M, Jimenez P, Gonzalez J, Ferrer A, Celis R, Rodriguez-Roisin R. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–528. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- 29.Wiener-Kronish J P, Albertine K H, Matthay M A. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Investig. 1991;88:864–875. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiener-Kronish J P, Sakuma T, Kudoh I, Pittet J-F, Frank D, Dobbs L, Vasil M L, Matthay M. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol. 1993;75:1661–1669. doi: 10.1152/jappl.1993.75.4.1661. [DOI] [PubMed] [Google Scholar]