Abstract
Thrombin-induced platelet microbicidal protein 1 (tPMP-1) is a small, cationic peptide released from rabbit platelets following thrombin stimulation. In vitro resistance to this peptide among strains of Staphylococcus aureus correlates with the survival advantage of such strains at sites of endothelial damage in humans as well as in experimental endovascular infections. The mechanisms involved in the phenotypic resistance of S. aureus to tPMP-1 are not fully delineated. The plasmid-encoded staphylococcal gene qacA mediates multidrug resistance to multiple organic cations via a proton motive force-dependent efflux pump. We studied whether the qacA gene might also confer resistance to cationic tPMP-1. Staphylococcal plasmids encoding qacA were found to confer resistance to tPMP-1 in an otherwise susceptible parental strain. Deletions which removed the region containing the qacA gene in the S. aureus multiresistance plasmid pSK1 abolished tPMP-1 resistance. Resistance to tPMP-1 in the qacA-bearing strains was inoculum independent but peptide concentration dependent, with the level of resistance decreasing at higher peptide concentrations for a given inoculum. There was no apparent cross-resistance in qacA-bearing strains to other endogenous cationic antimicrobial peptides which are structurally distinct from tPMP-1, including human neutrophil defensin 1, protamine, or the staphylococcal lantibiotics pep5 and nisin. These data demonstrate that the staphylococcal multidrug resistance gene qacA also mediates in vitro resistance to cationic tPMP-1.
Thrombin-induced platelet microbicidal protein 1 (tPMP-1) is a small, cationic peptide released from rabbit platelets by agonists associated with endovascular infection (e.g., thrombin) (31). This peptide has potent activity against organisms which commonly invade the bloodstream, including Staphylococcus aureus (1, 30). S. aureus strains that exhibit a tPMP-1-resistant phenotype in vitro appear to have a survival advantage in vivo at sites of endovascular damage with respect to induction, progression, and treatment outcomes of experimental and human endovascular infections, such as infective endocarditis (IE) (1, 5–7, 30). However, the mechanisms of such phenotypic tPMP-1 resistance have not been fully elucidated.
The naturally occurring S. aureus plasmid pSK1 carries genes that confer resistance to a number of antimicrobial agents, including aminoglycosides (aacA-aphD) and trimethoprim (dfrA) (23). Additionally, pSK1 contains the antiseptic and disinfectant resistance determinant qacA, which encodes a proton motive force-dependent, multidrug export protein belonging to the major facilitator superfamily of transport proteins (17, 22). This gene mediates resistance to a broad range of antimicrobial organic cations, including quaternary ammonium compounds, intercalating dyes, diamidines, and biguanidines (14, 21). On pSK1, the qacA locus consists of the qacA gene itself and a divergently transcribed repressor, qacR, which regulates the transcription of qacA (9, 24). Epidemic S. aureus strains isolated in Australia and the United Kingdom since 1980 commonly carry pSK1-like plasmids, and other qacA-encoding plasmids have been identified in clinical isolates of S. aureus and coagulase-negative staphylococci (13, 23).
The aim of the current study was to investigate whether S. aureus strains possessing the qacA gene might also be resistant to cationic tPMP-1 and whether this resistance is specific for tPMP-1 or if there is cross-resistance to other antimicrobial cationic peptides which are structurally unrelated to tPMP-1.
(This study was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998.)
MATERIALS AND METHODS
Bacterial strains and plasmids.
The S. aureus strains used in this study are described in Table 1. The plasmid-free strains NCTC8325 (19) and SK982 (15), the latter of which is a rifampin- and novobiocin-resistant variant of the restrictionless strain SA113 (10), were used as plasmid recipients. Plasmids, which are represented diagrammatically in Fig. 1 (see below), were transferred into NCTC8325 or SK982 by mixed-culture transfer (15). Briefly, donor and recipient cells recovered from cultures grown for 6 h at 37°C in brain heart infusion medium (Difco Laboratories, Detroit, Mich.) were resuspended in nutrient broth (Difco) to approximately 107 CFU/ml. One milliliter each of donor and recipient were mixed with 0.1 ml of 0.2 M CaCl2 and incubated at 37°C for 18 h with agitation. Transferred plasmids were selected by plating on brain heart infusion agar plates containing rifampin (20 μg/ml) and novobiocin (2 μg/ml).
TABLE 1.
S. aureus strains
| Strain | Background | Plasmida | Reference or source |
|---|---|---|---|
| SK982 | 15 | ||
| SK2355 | SK982 | pSK1 | 14 |
| SK4787 | SK982 | pSK105 | 13 |
| SK2413 | SK982 | pSK107 | 13 |
| SK4027 | SK982 | pSK638 | 13 |
| SK5127 | SK982 | pSK30 | 16 |
| SK2269 | SK982 | pSK78 | 28 |
| NCTC8325 | 19 | ||
| SK2237 | NCTC8325 | pSK1 | This study |
See Fig. 1 for relevant genes encoded by these plasmids.
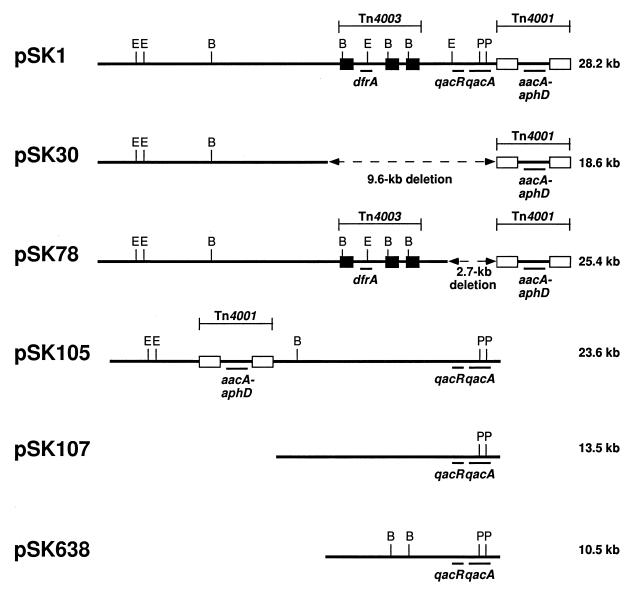
FIG. 1.
Maps of the S. aureus multiresistance plasmid pSK1 (23), its deletion derivatives pSK30 and pSK78, and the related S. epidermidis plasmid pSK105 (13) are aligned with respect to the qacA locus; in comparison to pSK1, pSK105 lacks Tn4003, and Tn4001 is located in a different position. The qacA-carrying S. epidermidis plasmids pSK107 and pSK638 are unrelated to the pSK1 family plasmids (13). Plasmid sizes are indicated on the right. The positions of the transposons are indicated above each map. White and black solid boxes denote copies of IS256 and IS257, respectively. The DNA segments deleted from pSK30 and pSK78 are denoted by broken lines, and the size of the deletion is shown below the line. Loci encoding the indicated functions are shown below the maps: aacA-aphD, resistance to the aminoglycosides, gentamicin, tobramycin, and kanamycin; dfrA, resistance to trimethoprim; qacA, multidrug resistance to antiseptics and disinfectants; and qacR, transcriptional regulation of qacA (the presence of qacR in pSK105, pSK107, and pSK638 is assumed but not proven at this time). Restriction sites: B, BglII; E, EcoRI; P, PvuII.
Nucleotide sequence determination.
DNA segments to be sequenced were amplified by PCR with Taq DNA polymerase (Sigma Chemical Co., St. Louis, Mo.) according to the manufacturer’s recommendations and purified with Microcon 100 microconcentrators (Millipore, Bedford, Mass.). Primers were made with an Oligo 1000 synthesizer (Beckman, Fullerton, Calif.), and nucleotide sequencing was performed at the Australian Genome Research Facility. Sequences were stored and assembled with the program SEQUENCHER (Gene Codes, Ann Arbor, Mich.).
Preparation of tPMP-1.
tPMP-1 was prepared as previously described (33, 34). In brief, platelets were isolated from freshly collected and anticoagulated rabbit blood. After platelets were washed twice in Tyrode’s salts solution and resuspended in Eagle’s minimal essential medium (MEM) (Irvine Scientific, Santa Ana, Calif), tPMP-1-rich preparations were produced by stimulation with bovine thrombin (400 μl; 1,000 U/ml) in the presence of CaCl2 and platelet-poor plasma. The tPMP-1 preparations were stored at −70°C until use. This process has been shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and acid-urea gel electrophoresis, as well as by reversed-phase, high-performance liquid chromatography, to yield preparations containing predominantly tPMP-1 with respect to cationic peptide microbicidal activity (34).
Bioactivity of tPMP-1.
The bioactivity of the tPMP-1 preparations was assessed by a microbiologic assay as previously described with Bacillus subtilis ATCC 6633 as the tPMP-1-hypersusceptible marker organism (33). After B. subtilis was washed twice, bacterial cells were added at a final inoculum of 103 CFU/ml to microtiter wells containing a range of tPMP-1 dilutions from 1:1 to 1:1,024. After 30 min of incubation, a 15-μl aliquot was removed from each well and quantitatively cultured on agar plates. tPMP-1 bioactivity (in units per milliliter) was quantified and defined as the reciprocal of the highest tPMP-1 dilution which retained ≥95% lethality for B. subtilis. Specific tPMP-1 bioactivity was then estimated as units per milligram of protein, and the value was converted to the tPMP-1 concentration as previously described (100 U/ml is 2 μg of tPMP-1 per ml) (33).
In vitro tPMP-1 susceptibility of S. aureus isolates.
Overnight cultures of the study S. aureus isolates were washed twice and resuspended in phosphate-buffered saline. Dilutions of tPMP-1 and S. aureus isolates were added to polypropylene microculture tubes to achieve a final tPMP-1 concentration of 2, 3, 4, 6, or 12 μg/ml and a final bacterial inoculum of 103 CFU/ml (the standard inoculum used for in vitro tests of this peptide against S. aureus strains) (30, 33). In parallel, we tested all S. aureus isolates against the same tPMP-1 concentration range at a final inoculum of 104 CFU/ml to determine the presence of an in vitro inoculum effect. All assays reported in this paper were done with logarithmic-phase bacterial cells. Pilot studies showed no substantial differences in the extent of tPMP-1-mediated killing of logarithmic-phase versus stationary-phase S. aureus cells at the above peptide concentrations. One tube contained only bacteria and MEM alone as a positive growth control. After 2 h of incubation at 37°C, suspensions were vortexed, and a 15-μl aliquot was removed from each tube and quantitatively cultured on blood agar plates. The proportion of S. aureus cells of each isolate surviving a 2-h exposure to tPMP-1 was expressed as a percentage of the CFU of the positive growth control. All assays were performed in triplicate, and the mean percentage of survival (mean ± standard deviation) was determined. S. aureus resistance was defined as ≥40% survival of the initial inoculum after a 2-h exposure to 2 μg of tPMP-1 per ml. This arbitrary in vitro resistance breakpoint correlates with an enhanced capacity of resistant strains to induce human or experimental IE in vivo (1, 5, 7, 30).
Assays of bactericidal activity of other cationic antimicrobial peptides.
To evaluate if qacA-bearing strains exhibit cross-resistance to well-characterized, cationic antimicrobial peptides structurally unrelated to tPMP-1, the in vitro susceptibility profiles of S. aureus were determined for the following peptides (12, 32): protamine, a basic polypeptide found in salmon sperm (Sigma); the coagulase-negative staphylococcal lantibiotics nisin (Sigma) and pep5 (kindly provided by H.-G. Sahl, Bonn, Germany); and the human neutrophil defensin 1 (hNP-1) (Sigma). For these cationic peptides, bactericidal assays were independently performed in triplicate.
Lantibiotics and protamine were diluted in Trypticase soy broth (TSB) (Difco) to their respective final concentrations. For bactericidal assays of protamine and lantibiotics, the broth microdilution technique was performed with 96-well microtiter plates and a final inoculum of 106 CFU/ml to establish the minimum bactericidal concentration. The range of concentrations tested encompassed serial twofold dilutions between 0.5 and 10 μg/ml for protamine, 12.5 and 300 μg/ml for nisin, and 0.04 and 90 μg/ml for pep5. These peptide concentration ranges and the in vitro inoculum are based on data from previous studies in our laboratory (3, 12). Control wells contained S. aureus in the presence of TSB or TSB alone. After 12 h of incubation at 37°C, a 25-μl sample was removed from each well and cultured on a TSB plate. The number of surviving colonies was assessed after an additional 16 h of incubation at 37°C. The MBC was defined as the lowest peptide concentration yielding ≥99.5% killing of the initial inoculum.
Like tPMP-1, hNP-1 is inactivated in the presence of most nutrient growth media. Therefore, bactericidal assays of hNP-1 are performed with MEM, and the data are expressed as the percentage of survival of S. aureus after a 2-h exposure. For the hNP-1 bactericidal assay, dilutions of hNP-1 and each S. aureus isolate were added to polypropylene microculture tubes to achieve a final hNP-1 concentration of 30, 40, or 50 μg/ml and a final bacterial inoculum of 103 CFU/ml. These hNP-1 concentrations represent the range which exerts in vitro action against S. aureus (32). S. aureus cells were added to one tube with MEM alone as a positive growth control. After 2 h of incubation at 37°C, a 15-μl aliquot was removed from each tube and quantitatively cultured on agar plates. Survival was calculated as a percentage of the CFU of the positive growth control.
RESULTS AND DISCUSSION
To examine the possibility that the qacA multidrug resistance gene mediates resistance to tPMP-1, we compared the in vitro susceptibility of plasmid-free S. aureus SK982 with that of a derivative harboring the qacA-encoding plasmid pSK1 (Fig. 1). When tested at a standard inoculum (103 CFU/ml) and a standard peptide concentration (2 μg/ml), SK982 cells were found to be tPMP-1 susceptible (Table 2). In contrast, SK982 cells containing pSK1 were tPMP resistant under these conditions (Table 2). Equivalent results were obtained when S. aureus NCTC8325 (background strain) was used instead of SK982.
TABLE 2.
Survival of S. aureus strains (inoculum of 103 CFU/ml) after exposure to tPMP-1 in vitro
| tPMP-1 (μg/ml) | Mean ± SD % survival ofa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SK982 | SK2355(pSK1) | SK4787(pSK105) | SK2413(pSK107) | SK4027(pSK638) | SK5127(pSK30) | SK2269(pSK78) | NCTC8325 | SK2237(pSK1) | |
| 12 | 3 ± 1 | 4 ± 1 | 8 ± 1 | 7 ± 2 | 9 ± 3 | 3 ± 1 | 4 ± 2 | 2 ± 0.3 | 7 ± 1 |
| 6 | 8 ± 1 | 14 ± 2 | 13 ± 2 | 11 ± 2 | 13 ± 1 | 6 ± 0.4 | 10 ± 2 | 5 ± 0.2 | 6 ± 3 |
| 4 | 19 ± 4 | 25 ± 4 | 24 ± 3 | 24 ± 3 | 26 ± 3 | 7 ± 2 | 16 ± 3 | 7 ± 2 | 33 ± 4 |
| 3 | 20 ± 3 | 47 ± 3 | 44 ± 3 | 45 ± 3 | 44 ± 4 | 20 ± 3 | 31 ± 4 | 23 ± 4 | 32 ± 4 |
| 2 | 22 ± 3 | 59 ± 5 | 70 ± 5 | 72 ± 3 | 82 ± 3 | 26 ± 3 | 35 ± 4 | 29 ± 8 | 61 ± 4 |
After 2 h of in vitro exposure under the given conditions. Results represent the mean of three independent experiments.
Since the tPMP-1 resistance conferred by pSK1 might be attributable to genes, other than qacA encoded by this multiresistance plasmid, we determined the ability of other qacA-encoding plasmids to confer this phenotype. Plasmids pSK105, pSK107, and pSK638 were each originally isolated from S. epidermidis, confer the antiseptic and disinfectant resistance phenotype associated with qacA, and hybridize with a qacA-specific probe (13). Whereas pSK105 is structurally related to pSK1, pSK107 and pSK638 are thought to be otherwise unrelated, apart from the qacA locus (Fig. 1) (13). As shown in Table 2, the three plasmids from coagulase-negative staphylococci conferred levels of tPMP-1 resistance in SK982 comparable to that mediated by pSK1. Of importance, preliminary experiments have indicated that the unrelated staphylococcal multidrug resistance gene smr (carried by plasmid pSK89), which encodes a cation efflux pump distinct from QacA (13, 14, 22), does not mediate resistance to tPMP-1.
Further support for the involvement of qacA in the observed tPMP-1 resistance was obtained with two independently isolated pSK1 deletion derivatives, pSK30 and pSK78, which were recovered after bacteriophage 80α transduction (16) and mixed-culture transfer (28), respectively. The deletion junctions on pSK30 and pSK78 were amplified and sequenced, and the sequences were compared to the complete nucleotide sequence of pSK1 (8). This comparison demonstrated that the deletions in pSK30 and pSK78 had removed 9,611 and 2,721 nucleotides, respectively, and that both deletions extended precisely from the terminus of the IS256 copy of Tn4001 to the left of the aacA-aphD aminoglycoside resistance gene, as shown in Fig. 1. The deletion in pSK30 encompassed a DNA segment encoding loci that mediate resistance to trimethoprim (dfrA) and to antiseptics and disinfectants (qacA), whereas the deletion in pSK78 specifically removed the qacA locus, namely, the open reading frame of qacA and that of its transcriptional regulator, qacR (9, 24). In contrast to pSK1, from which they were derived, neither pSK30 (i.e., strain SK5127) nor pSK78 (i.e., strain SK2269) was found to confer resistance to tPMP-1 (Table 2).
We observed a peptide concentration-dependent bactericidal effect for all strains tested against tPMP-1 at both inocula used (103 and 104 CFU/ml) (Tables 2 and 3). Thus, a progressively higher proportion of bacterial cells was killed as the tPMP-1 concentration was increased from 2 to 12 μg/ml. Of note, all strains exhibiting in vitro tPMP-1 susceptibility at 103 CFU/ml remained tPMP-1 susceptible at the higher inoculum (104 CFU/ml), indicating a lack of appreciable inoculum effect over this inoculum range (Table 3).
TABLE 3.
Survival of S. aureus strains (inoculum of 104 CFU/ml) after exposure to tPMP-1 in vitro
| tPMP-1 (μg/ml) | Mean ± SD % survival ofa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SK982 | SK2355(pSK1) | SK4787(pSK105) | SK2413(pSK107) | SK4027(pSK638) | SK5127(pSK30) | SK2269(pSK78) | NCTC8325 | SK2237(pSK1) | |
| 12 | 2 ± 1 | 7 ± 1 | 8 ± 3 | 6 ± 2 | 11 ± 2 | 2 ± 1 | 5 ± 2 | 2 ± 0.3 | 15 ± 2 |
| 6 | 10 ± 4 | 19 ± 4 | 14 ± 2 | 13 ± 2 | 15 ± 1 | 11 ± 5 | 14 ± 3 | 4 ± 1 | 36 ± 4 |
| 4 | 12 ± 3 | 37 ± 4 | 30 ± 4 | 28 ± 3 | 33 ± 2 | 15 ± 3 | 19 ± 2 | 17 ± 2 | 43 ± 5 |
| 3 | 15 ± 2 | 83 ± 5 | 61 ± 4 | 64 ± 3 | 62 ± 5 | 19 ± 4 | 28 ± 3 | 15 ± 4 | 69 ± 4 |
| 2 | 15 ± 2 | 91 ± 4 | 76 ± 5 | 85 ± 5 | 89 ± 6 | 21 ± 3 | 31 ± 4 | 20 ± 3 | 83 ± 4 |
After 2 h of in vitro exposure under the given conditions. Results represent the mean of three independent experiments.
The apparent association of the presence of qacA-containing plasmids and a tPMP-1-resistant phenotype in S. aureus posed the question as to whether the presence of this gene might render S. aureus strains less susceptible to other endogenous antimicrobial peptides structurally unrelated to tPMP-1. To investigate this notion, susceptibilities to the peptides nisin, pep5, protamine, and hNP-1 were determined. Of note, similar in vitro susceptibility profiles were observed for these latter peptides in comparisons of the plasmid-free, qacA-bearing, and qacA deletion derivative-bearing strains. Thus, the minimum bactericidal concentrations of nisin, pep5, and protamine were found to be 100 to 110 μg/ml, 11.25 to 11.5 μg/ml, and 2 mg/ml, respectively, and a mean survival of 47 to 52% was determined for an hNP-1 concentration of 50 μg/ml, with no differences in survival at 30 and 40 μg/ml. Additionally, it was recently shown that the presence of the qacA-bearing plasmid pSK1 in S. aureus strains did not influence their in vitro susceptibilities to protegrin 1 (25), the cysteine-rich cationic peptide from porcine leukocytes (11), or to CG 117-136 (25), the 20-mer amphipathic, cationic peptide derived from human lysosomal cathepsin G (26). Collectively, these data suggest a degree of specificity for qacA-mediated tPMP-1 resistance.
We have previously shown that S. aureus strains which acquire resistance to the microbicidal action of tPMP-1 in vitro have a survival advantage in vivo with respect to experimental endovascular infections compared to their genetically related tPMP-1-susceptible counterpart strains (5–7). For example, a tPMP-1-resistant strain, ISP479R, derived via transposon (Tn551) insertion into the chromosome of the tPMP-1-susceptible strain ISP479, exhibits a significant virulence advantage over the parental strain in experimental IE in terms of (i) intravegetation proliferation, (ii) hematogenous dissemination and proliferation within the kidneys and spleen, and (iii) reduced rates of intravegetation clearance during antibiotic therapy (6, 7). Similar survival advantages have been observed by Dankert et al. studying experimental IE with platelet peptide-resistant viridans group streptococci (4). Moreover, studies of human bloodstream S. aureus have indicated that strains causing endocarditis and other endovascular infections are substantially more resistant to tPMP-1 in vitro than bacteremic strains emanating from soft tissue abscesses (1). Thus, 52 and 83% of bacteremic S. aureus strains isolated from patients with vascular catheter sepsis and IE, respectively, were tPMP-1 resistant in vitro, compared to only 33% of strains from patients with soft tissue abscesses (1). Of note, the degrees of in vitro tPMP-1 resistance seen in these clinical bacteremic S. aureus strains closely parallel those observed for the qacA-bearing strains in the current study.
Studies with genetically related tPMP-1-susceptible and -resistant S. aureus strain pairs have implicated the bacterial cytoplasmic membrane as a principal target for the microbicidal action of tPMP-1 (2, 3, 12, 32). These investigations demonstrated that the cytoplasmic membranes of tPMP-1-resistant variants differ from those of their tPMP-1-susceptible parental strains in multiple aspects, including (i) diminished ability to generate a normal transmembrane electrical potential; (ii) increased resistance to tPMP-1-induced damage and lysis; (iii) increased fluidity properties; and (iv) increased O2 consumption and ATP generation, despite a reduced transmembrane electrical potential (2, 3, 12, 32). Collectively, these studies suggested the possible existence of an energy-dependent, cytoplasmic membrane-based pathway linked to phenotypic resistance to cationic tPMP-1 (e.g., a cation antiporter system). Indeed, two recent reports have described plasmid-mediated microbial resistance to endogenous cationic peptides via energy-dependent efflux systems. In one study, gonococci were able to cause protegrin 1 efflux via a proton motive force-dependent transporter (27). In a second investigation, an S. epidermidis strain was able to cause extrusion of the lantibiotics gallidermin and epidermin from its cytoplasmic membrane via an ATP-dependent transporter system (20).
In summary, the results described here demonstrate an association between the qacA locus, which encodes a proton motive force-dependent, membrane-bound cation exporter (18, 22, 24, 29), and the observed in vitro tPMP-1 resistance. Although it is tempting to speculate that qacA-mediated tPMP-1 resistance is due to active efflux of the peptide, it is also conceivable that there are more global alterations in the target cytoplasmic membranes of strains expressing qacA. Investigations to evaluate these issues are in progress.
ACKNOWLEDGMENTS
We thank William Shafer (Birmingham, Ala.) for providing unpublished data on the bactericidal assays with protegrin 1 and CG 117-136 and Brendon O’Rourke for excellent technical assistance.
L.I.K. was supported by a grant from the Deutsche Forschungsgemeinschaft (KU 1155/1-1). This study was also supported in part by research grants from the National Institutes of Health to A.S.B. (AI39108) and to M.R.Y. (AI39001 and AI39108). Work in the laboratory of R.A.S. was supported by a project grant from the National Health & Medical Research Council (Australia).
REFERENCES
- 1.Bayer A S, Cheng D, Yeaman M R, Corey G R, McClelland R S, Harrell L E, Fowler V G. In vitro resistance to thrombin-induced platelet microbicidal protein (tPMP) among Staphylococcus aureus bacteremic isolates correlates with endovascular infection source. Antimicrob Agents Chemother. 1998;42:3169–3172. doi: 10.1128/aac.42.12.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer A S, Yeaman M R, Koo S P, Prasad R. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Resistance to staphylococcal effects of thrombin-induced platelet microbicidal protein is associated with alterations in membrane fluidity and lipid content, abstr. A-107; p. 19. [Google Scholar]
- 3.Bayer A S, Yeaman M R, Sahl H G, Brar D, Proctor R A. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Relationship of phenotypic resistance to thrombin-induced platelet microbicidal protein (tPMP) and cytoplasmic membrane bioenergetics in Staphylococcus aureus (SA), abstr. A-106; p. 19. [Google Scholar]
- 4.Dankert J, van der Werff J, Zaat S A, Joldersma W, Klein D, Hess J. Involvement of bacterial factors from thrombin-stimulated platelets in clearance of adherent viridans group streptococci in experimental infective endocarditis. Infect Immun. 1995;63:663–671. doi: 10.1128/iai.63.2.663-671.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhawan V K, Bayer A S, Yeaman M R. In vitro resistance to thrombin-induced platelet microbicidal protein is associated with enhanced progression and hematogenous dissemination in experimental Staphylococcus aureus infective endocarditis. Infect Immun. 1998;66:3476–3479. doi: 10.1128/iai.66.7.3476-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhawan, V. K., M. R. Yeaman, and A. S. Bayer. Thrombin-induced platelet microbicidal protein susceptibility phenotype influences the treatment outcome in experimental Staphylococcus aureus endocarditis. J. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 7.Dhawan V K, Yeaman M R, Cheung A L, Kim E, Sullam P M, Bayer A S. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect Immun. 1997;65:3293–3299. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firth, N., S. Apisiridej, and R. A. Skurray. Unpublished data.
- 9.Grkovic S, Brown M H, Roberts N J, Paulsen I T, Skurray R A. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J Biol Chem. 1998;273:18665–18673. doi: 10.1074/jbc.273.29.18665. [DOI] [PubMed] [Google Scholar]
- 10.Iordanescu S, Surdeanu M. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J Gen Microbiol. 1976;96:277–281. doi: 10.1099/00221287-96-2-277. [DOI] [PubMed] [Google Scholar]
- 11.Kokryakow V N, Harwig S S, Panyutich E A, Shevchenko A A, Aleshina G M, Shamova O V, Korneva H A, Lehrer R I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 12.Koo S P, Bayer A S, Sahl H-G, Proctor R A, Yeaman M R. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect Immun. 1996;64:1070–1074. doi: 10.1128/iai.64.3.1070-1074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leelaporn A, Paulsen I T, Tennent J M, Littlejohn T G, Skurray R A. Multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. J Med Microbiol. 1994;40:214–220. doi: 10.1099/00222615-40-3-214. [DOI] [PubMed] [Google Scholar]
- 14.Littlejohn T G, Paulsen I T, Gillespie M T, Tennent J M, Midgley M, Jones I G, Purewal A S, Skurray R A. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol Lett. 1992;95:259–266. doi: 10.1016/0378-1097(92)90439-u. [DOI] [PubMed] [Google Scholar]
- 15.Lyon B R, May J W, Skurray R A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193:554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- 16.Lyon, B. R., and R. A. Skurray. Unpublished data.
- 17.Marger M D, Saier M H., Jr A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell B A, Brown M H, Skurray R A. QacA multidrug efflux pump from Staphylococcus aureus: comparative analysis of resistance to diamidines, biguanidines, and guanylhydrazones. Antimicrob Agents Chemother. 1998;42:475–477. doi: 10.1128/aac.42.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novick R P. The Staphylococcus as a molecular genetic system. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 1–37. [Google Scholar]
- 20.Otto M, Peschel A, Götz F. Producer self-protection against the lantibiotic epidermin by the ABC transporter EpiFEG of Staphylococcus epidermidis Tu3298. FEMS Microbiol Lett. 1998;166:203–211. doi: 10.1111/j.1574-6968.1998.tb13891.x. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen I T, Brown M H, Littlejohn T G, Mitchell B A, Skurray R A. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc Natl Acad Sci USA. 1996;93:3630–3635. doi: 10.1073/pnas.93.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulsen I T, Firth N, Skurray R A. Resistance to antimicrobial agents other than β-lactams. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 175–212. [Google Scholar]
- 24.Rouch D A, Cram D S, DiBerarino D, Littlejohn T G, Skurray R A. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline- and sugar-transport proteins. Mol Microbiol. 1990;4:2051–2062. doi: 10.1111/j.1365-2958.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 25.Shafer, W. M. Personal communication.
- 26.Shafer W M, Hubalek F, Huang M, Pohl J. Bactericidal activity of a synthetic peptide (CG 117-136) of human lysosomal cathepsin G is dependent on arginine content. Infect Immun. 1996;64:4842–4845. doi: 10.1128/iai.64.11.4842-4845.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafer W M, Qu X-D, Waring A J, Lehrer R I. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA. 1998;95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tennent J M, Lyon B R, Gillespie M T, May J W, Skurray R A. Cloning and expression of Staphylococcus aureus plasmid-mediated quaternary ammonium resistance in Escherichia coli. Antimicrob Agents Chemother. 1985;27:79–83. doi: 10.1128/aac.27.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tennent J M, Lyon B R, Midgley M, Jones I G, Purewal A S, Skurray R A. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J Gen Microbiol. 1989;135:1–10. doi: 10.1099/00221287-135-1-1. [DOI] [PubMed] [Google Scholar]
- 30.Wu T, Yeaman M R, Bayer A S. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among staphylococcal isolates. Antimicrob Agents Chemother. 1994;38:729–732. doi: 10.1128/aac.38.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeaman M R. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 32.Yeaman M R, Bayer A S, Koo S P, Foss W, Sullam P M. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Investig. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeaman M R, Norman D C, Bayer A S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeaman M R, Tang Y-Q, Shen A-J, Bayer A S, Selsted M E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]