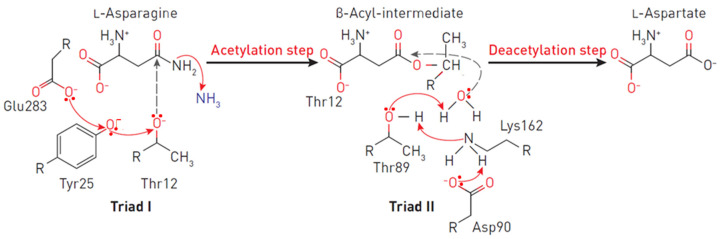
Figure 2.
Reaction mechanism at the EcA II catalytic triads. The triad I acylates the substrate (L-asparagine) to form a β-aspartyl enzymatic intermediate. The triad II deacylates the intermediate in the presence of a water molecule to release L-aspartic acid and ammonia as products. In the first reaction, the electron density migrates from Glu283 to the oxygen of the Tyr25, and consequently, to the oxygen Thr12. A nucleophilic attack occurs, leading to the release of ammonia and the formation of ether. In the second reaction, due to the presence of a charge on Asp90, an ionic bond is formed with the amino group Lys162, leading to the removal of the proton from Thr89, followed by the nucleophilic attack of the water molecule on the carbon of the ester. Thus, a deacetylation reaction occurs.