Abstract
The multidrug transporter NorA contributes to the resistance of Staphylococcus aureus to fluoroquinolone antibiotics by promoting their active extrusion from the cell. Previous studies with the alkaloid reserpine, the first identified inhibitor of NorA, indicate that the combination of a chemical NorA inhibitor with a fluoroquinolone could improve the efficacy of this class of antibiotics. Since reserpine is toxic to humans at the concentrations required to inhibit NorA, we sought to identify new inhibitors of NorA that may be used in a clinical setting. Screening of a chemical library yielded a number of structurally diverse inhibitors of NorA that were more potent than reserpine. The new inhibitors act in a synergistic manner with the most widely used fluoroquinolone, ciprofloxacin, by substantially increasing its activity against both NorA-overexpressing and wild-type S. aureus isolates. Furthermore, the inhibitors dramatically suppress the emergence of ciprofloxacin-resistant S. aureus upon in vitro selection with this drug. Some of these new inhibitors, or their derivatives, may prove useful for augmentation of the antibacterial activities of fluoroquinolones in the clinical setting.
Fluoroquinolone antibiotics are an important class of antibiotics that exhibit a broad spectrum of potent antibacterial activity. The most widely used fluoroquinolone, ciprofloxacin, was the fifth most prescribed antibiotic in 1998 (24). Although highly active against most gram-negative microorganisms (MIC at which 90% of isolates are inhibited [MIC90], about 0.1 μg/ml), ciprofloxacin is less effective against gram-positive bacteria, particularly aerobic gram-positive cocci (MIC90 for Staphylococcus aureus, about 0.25 to 2 μg/ml; MIC90 for Streptococcus pneumoniae, 1 to 4 μg/ml; and MIC90 for Enterococcus faecalis, 0.5 to 4 μg/ml) (6). One of the reasons for the reduced susceptibility of gram-positive cocci to fluoroquinolones is the expression by these bacteria of multidrug efflux transporters, membrane proteins that actively extrude fluoroquinolones and other multiple drugs from the cell (4, 14, 27).
The efflux of fluoroquinolones and the expression of multidrug transporters have been demonstrated for enterococci and pneumococci (4, 16, 28) but have been best documented for one of the most important human pathogens, S. aureus, in which the major role in fluoroquinolone efflux is played by the membrane transporter NorA (13, 25). NorA, which is a close homolog of the Bmr multidrug transporter of Bacillus subtilis (18), promotes the active efflux of a wide variety of organic compounds, including ethidium bromide, rhodamine, acridines, tetraphenylphosphonium, puromycin, benzalkonium, centrimide, and pentamidine, with fluoroquinolone antibiotics being one of the best transporter substrates (10, 19).
We have previously shown that drug efflux mediated by NorA can be inhibited by the plant alkaloid reserpine (19), which reduces the MIC of norfloxacin for wild-type S. aureus by at least fourfold (17) and which has an effect similar to that of the genetic disruption of the NorA gene (10, 26). In addition to being involved in the reduced susceptibility of gram-positive bacteria to fluoroquinolones, multidrug transporters contribute to the acquired resistance, which is selected upon exposure to these antibiotics. Although this resistance is usually attributed to mutations in the target proteins of fluoroquinolones, DNA gyrase and topoisomerase IV (8, 21), many strains of S. aureus selected for fluoroquinolone resistance both in vitro (11, 23) and in vivo (12, 13, 20, 25) also overexpress NorA or at least exhibit reserpine-sensitive resistance mechanisms. A recent study demonstrates that the ciprofloxacin resistance of 48 of 102 clinical isolates of S. aureus could be reversed at least fourfold by reserpine, suggesting a contribution of NorA and/or other reserpine-sensitive transporters to fluoroquinolone resistance in almost half of such isolates (20).
Recently, it was demonstrated that chemical inhibition of NorA increased the bactericidal activity and postantibiotic effect of ciprofloxacin on S. aureus (1). Additionally, we have shown in in vitro selection experiments that the addition of reserpine to the selection medium reduces the rate of emergence of norfloxacin-resistant variants of S. aureus by almost two orders of magnitude (17). It appears, therefore, that the clinical use of fluoroquinolones in combination with an inhibitor of multidrug transporters could dramatically improve the efficacies of these antibiotics by both reducing their effective concentration severalfold (shifting it below their practically achievable levels in tissue) and preventing the emergence of drug-resistant variants.
Unfortunately, reserpine cannot be used to potentiate the activities of fluoroquinolones because of its neurotoxicity at the concentrations required for NorA inhibition. Therefore, in this study we sought to identify additional inhibitors of NorA that may be used in combination with fluoroquinolones to augment the effective therapeutic action of this class of antibiotics against S. aureus.
MATERIALS AND METHODS
Chemicals.
The DiverSet chemical library, which consists of 9,600 structurally diverse drug-like compounds, was purchased from ChemBridge Corp., Mountainview, Calif. Ethidium bromide and reserpine were purchased from Sigma (St. Louis, Mo.).
Bacterial strains and media.
All strains were cultivated in Luria-Bertani (LB) medium (Difco). Strain ΔΔ is a B. subtilis strain, BD170/bmr::cat blt::erm, in which the genes that encode the multidrug transporters Bmr and Blt are genetically inactivated (3). Its derivative strain, ΔΔNA, expresses a functional NorA transporter from the plasmid expression vector pBEV (19). S. aureus SA1199B (11–13), which overexpresses the chromosomal norA gene and which harbors a mutation in grlA, and SA1199 (12, 13), its wild-type counterpart, were gifts from G. Kaatz, Wayne State University, Detroit, Mich. On the day of the experiment strains ΔΔNA and SA1199B were grown from a frozen stock in medium containing 10 μg of ethidium bromide per ml.
Library screening.
The chemical library was screened for compounds effective, at concentrations of 20 μg/ml or less, in reversing the resistance of strain ΔΔNA to the NorA substrate ethidium bromide. ΔΔNA cells at the logarithmic phase of growth were inoculated into the wells of a 96-well plate to a final optical density at 600 nm (OD600) of 0.001. Each compound was added to a final concentration of 20 μg/ml, and ethidium bromide was added to a final concentration of 10 μg/ml (fourfold less than the MIC for this strain). Plates were incubated for 18 h at 37°C and were examined visually for growth. Compounds that inhibited growth were subsequently tested at different concentrations in the presence or absence of ethidium bromide to determine toxicity and effectivity. Ethidium bromide was chosen as a diagnostic NorA substrate since transporter-mediated efflux is the only mechanism known to confer resistance to this compound.
Monitoring of ethidium bromide efflux.
Efflux of ethidium bromide from cells was performed essentially as described previously (15). Strain ΔΔNA at the logarithmic stage of growth was loaded with ethidium bromide at a concentration of 10 μg/ml for 20 min at 37°C in the presence of reserpine (20 μg/ml). Subsequently, the cells were centrifuged and the cell pellet was resuspended to an OD600 of 0.2 in a minimal growth medium (GM1) (5) alone or in the presence of a NorA inhibitor. Fluorescence of ethidium bromide was monitored in a Shimadzu fluorimeter at an excitation λ of 530 nm and an emission λ of 600 nm.
Synergy testing.
Synergy testing was performed with strains ΔΔNA and SA1199B by checkerboard titration in microtiter plates with twofold serial broth microdilutions (7). Each candidate inhibitor was tested at 11 concentrations (50 ng/ml to 50 μg/ml). Ethidium bromide was tested at 11 concentrations ranging from 40 ng/ml to 40 μg/ml (the MIC for strain ΔΔNA) and ciprofloxacin was tested at 11 concentrations ranging from 4 ng/ml to 4 μg/ml for ΔΔNA (two times the MIC) and 16 ng/ml to 16 μg/ml for SA1199B (two times the MIC). Wells were assessed visually for growth after an 18-h incubation period at 37°C. The fractional inhibitory concentration (FIC) was calculated for each inhibitor and ethidium bromide or ciprofloxacin combination. The following formulas were used to calculate the FIC index: FIC of drug A = MIC of drug A in combination/MIC of drug A alone, FIC of drug B = MIC of drug B in combination/MIC of drug B alone, and FIC index = FIC of drug A + FIC of drug B. Synergy was defined as an FIC index of <0.5.
Selection of ciprofloxacin-resistant mutants.
Spontaneous mutants were obtained 48 h after plating SA1199 cells on LB agar plates containing ciprofloxacin at a concentration of 1 μg/ml (two times the MIC). The frequency of mutant selection was determined to be 2 × 10−8 by comparing the number of colonies that grew on plates containing the drug with the number of colonies obtained upon plating appropriate dilutions in the absence of drug. For chemical mutagenesis, cells were treated with ethylmethane sulfonate as described previously (17). Briefly, 100 ml of cells in the logarithmic phase of growth was centrifuged and washed with 1 M Tris-HCl (pH 7.4). After centrifugation, the cells were resuspended in 500 μl of 1 M Tris-HCl (pH 7.4) and 5 μl of ethylmethane sulfonate was added while the cells in the suspension were swirled. Cells were shaken vigorously for 5 min, washed once with LB medium, and resuspended in 200 ml of LB medium. After incubation at 37°C for 3 h, the cells were centrifuged and plated on selection plates. Appropriate dilutions of cells were plated to determine the number of cells selected.
Determination of IC50.
The effect of inhibitors on the 50% inhibitory concentration (IC50) of ciprofloxacin for the wild-type strain S. aureus SA1199 was determined as described previously (17). Cells at the logarithmic phase of growth and at an OD600 of 0.01 were inoculated into 2 ml of LB medium containing ciprofloxacin at 1.5-fold dilutions ranging from 0.45 to 0.0178 μg/ml. The OD600 was determined after 3 h of incubation with shaking at 37°C.
RESULTS
Screening for NorA inhibitors in B. subtilis ΔΔNA.
The DiverSet chemical library, which consists of 9,600 structurally diverse compounds (molecular weights, 200 to 700) was screened for inhibitors of NorA. The screening was performed in a model system in which compounds were tested for the ability to inhibit the NorA-mediated resistance of the specially constructed strain B. subtilis ΔΔNA to the NorA substrate ethidium bromide. The use of this strain, which is devoid of the two most important endogenous B. subtilis multidrug transporters, Bmr and Blt, but which expresses a plasmid-borne norA gene ensured that NorA represented the major transporter that causes resistance. Ethidium bromide was chosen as a tester drug since, unlike the situation with fluoroquinolones, active efflux represents the only known mechanism of bacterial resistance to this drug. Compared to the original strain, ΔΔ, strain ΔΔNA exhibits a 20-fold increase in resistance to ethidium bromide (MICs, of 2 versus 40 μg/ml, respectively).
Compounds from the library were tested at a concentration of 20 μg/ml and those which reversed the resistance to ethidium bromide by at least fourfold, while being nontoxic to the bacteria themselves, were identified. Surprisingly, as many as 399 compounds (4% of the library) were found to be positive by this screening test. Twenty-eight of these compounds were at least as potent as reserpine, being active at 5 μg/ml, and 11 of these were found to be more potent than reserpine, being effective at concentrations of 2.5 μg/ml or less.
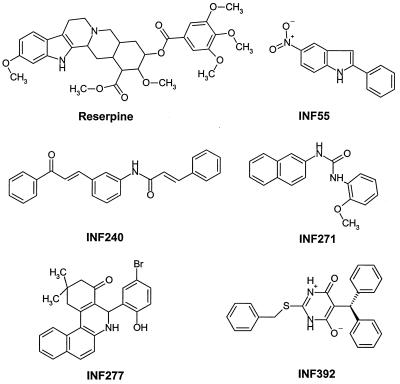
Most of the active compounds could be divided into several broad chemical groups. Considering that reserpine contains an indole moiety, it was not surprising to find a number of active indole derivatives. Thirty of 370 indole derivatives present in the library were active, and 7 of these were nitroindoles. Another large group (10 of the 28 most active compounds) was best characterized as having a trichloromethylaminal functional group. These compounds were not characterized further, however, since this chemical group is likely to make them toxic to humans. Additionally, of 32 biphenyl urea derivatives in the library, 11 were found to be active. Other compounds, including INF 392, INF 277, and INF 240 (Fig. 1), did not show any obvious structural similarity to other active compounds. The five INF compounds shown in Fig. 1, which were active at concentrations of 5 μg/ml or less, were selected for further evaluation. These compounds included the most potent compound (INF 392), the most potent indole (INF 55), and the most potent biphenyl urea derivative (INF 271).
FIG. 1.
Chemical structures of the selected NorA inhibitors.
Testing of the combination of each of the five inhibitors with ethidium bromide and ciprofloxacin for synergy was performed with test strain ΔΔNA by checkerboard titration. The calculated FIC indices are shown in Table 1. The most potent inhibitor, INF 392, reversed ethidium bromide and ciprofloxacin resistance by eightfold at a concentration of 0.4 μg/ml, a concentration 32 fold less than the MIC of INF 392 for strain ΔΔNA. The FIC indices for all five of the candidate inhibitors were ≪0.5, indicating that these compounds are strongly synergistic in promoting the antibacterial effects of both ethidium bromide and ciprofloxacin, which is what one should expect to observe for an inhibitor of the resistance mechanism, NorA, in this particular case.
TABLE 1.
Synergy results for NorA inhibitors with ethidium bromide or ciprofloxacin
| Inhibitor | FIC indexa
|
||
|---|---|---|---|
| ΔΔNA
|
SA1199B | ||
| EtBr | Cip | Cip | |
| INF 55 | 0.08 | 0.25 | 0.25 |
| INF 240 | 0.16 | 0.12 | 0.28 |
| INF 271 | 0.07 | 0.12 | 0.18 |
| INF 277 | 0.09 | 0.15 | 0.28 |
| INF 392 | 0.14 | 0.28 | 0.15 |
FIC indices were for the combination of either ethidium bromide (EtBr) or ciprofloxacin (Cip) with NorA inhibitors by using strain ΔΔNA or SA1199B. FIC indices were calculated as described in Materials and Methods. An FIC index of <0.5 was considered to be an indicator of synergistic activity.
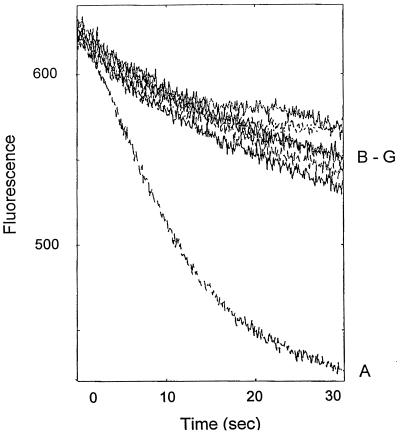
The use of ethidium as a test drug allowed us to demonstrate directly that the five INF compounds evaluated inhibit the drug resistance of the ΔΔNA cells by suppressing drug efflux. The cells were loaded with ethidium bromide in the presence of reserpine (20 μg/ml) and were placed in a fluorimeter cuvette with fresh medium. Since ethidium bromide fluoresces only when it is located inside the cell and is bound to nucleic acids, cells exhibited a rapid decrease in fluorescence due to NorA-mediated ethidium bromide efflux. As shown in Fig. 2, this decrease in fluorescence was inhibited when cells were allowed to extrude ethidium bromide in the presence of reserpine or each of the five INF compounds tested.
FIG. 2.
Effect of NorA inhibitors on ethidium efflux from ΔΔNA cells. ΔΔNA cells were loaded with ethidium bromide in the presence of reserpine and efflux was allowed to occur in the absence of inhibitor (A) or in the presence of reserpine at 20 μg/ml (B), INF 55 at 10 μg/ml (C), INF 240 at 5 μg/ml (D), INF 271 at 5 μg/ml (E), INF 277 at 5 μg/ml (F), or INF 392 at 1 μg/ml (G).
Effects of identified NorA inhibitors on ciprofloxacin resistance of S. aureus.
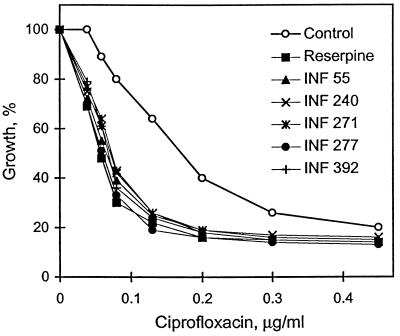
The five representative NorA inhibitors exhibited strong potentiating activity on the bacteriotoxic action of ciprofloxacin against S. aureus. As shown in Fig. 3, they decreased the IC50 of ciprofloxacin by two- to threefold at concentrations 8- to 30-fold less than the concentration of reserpine. The decrease in OD600 in these experiments was confirmed to represent a decrease in cell number by determining, in parallel, cell viability at the end of the experiment. For example, in the presence of 0.13 μg of ciprofloxacin per ml alone (one-fourth the MIC), cell viability was determined to be 3 × 107 CFU/ml, whereas in the presence of either reserpine or INF 271, cell viability was reduced at least 100-fold (5 × 104 and 1.2 × 105 CFU/ml, respectively). Furthermore, as Table 1 demonstrates, the inhibitors acted synergistically (FIC index, <0.5) with ciprofloxacin against the fluoroquinolone-resistant strain of S. aureus, SA1199B, in which resistance involves both the overexpression of NorA and a mutation in the DNA topoisomerase gene, grlA (11). The most potent inhibitor, INF 392, reversed ciprofloxacin resistance fourfold (2 versus 8 μg/ml) at a concentration of 0.2 μg/ml (1/64 the MIC for SA1199B). The other inhibitors, INF 55, INF 240, INF 271, and INF 277, required 1.5, 0.4, 1.5, and 0.8 μg/ml, respectively, to reverse ciprofloxacin resistance to the same extent.
FIG. 3.
Effect of lead inhibitors on the susceptibility of wild-type S. aureus SA1199 to ciprofloxacin. Cells were diluted to an OD600 of 0.01 into tubes with LB medium containing different concentrations of ciprofloxacin (1.5 fold dilutions) and either no inhibitor, 20 μg of reserpine per ml, 2.5 μg of INF 55 per ml, 2.5 μg of INF 240 per ml, 2.5 μg of INF 271 per ml, 0.6 μg of INF 277 per ml, or 0.6 μg of INF 392 per ml. ODs were determined after 3 h of incubation.
Probably the most important from the standpoint of their potential use in clinics was the effects of these inhibitors on the emergence of ciprofloxacin-resistant mutants of S. aureus. Similar to our previous studies with norfloxacin (17), reserpine dramatically inhibited the emergence of ciprofloxacin resistance by more than one order of magnitude. Importantly, as shown in Table 2, each of the tested inhibitors also decreased the frequency of spontaneous emergence of ciprofloxacin resistance by 50-fold or more. This dramatic effect could not be attributed to a toxic effect of the inhibitor since the same concentration of inhibitor, which was at least 10-fold less than its MIC for SA1199, affected neither the colony-forming ability nor the colony size of SA1199 cells plated in the absence of ciprofloxacin.
TABLE 2.
Emergence of resistance to ciprofloxacin in the presence of NorA inhibitors
| Inhibitor (concn [μg/ml]) | Frequency of emergence of resistancea
|
|
|---|---|---|
| Spontaneous | Mutagenized | |
| None | 2 × 10−8 | 1 × 10−7 |
| Reserpine (20) | <1 × 10−10 | <2.5 × 10−10 |
| INF 55 (5) | <1 × 10−10 | <2.5 × 10−10 |
| INF 240 (5) | 2 × 10−10 | 2.5 × 10−10 |
| INF 271 (5) | <1 × 10−10 | <2.5 × 10−10 |
| INF 277 (5) | <1 × 10−10 | <2.5 × 10−10 |
| INF 392 (1) | 2 × 10−10 | 1 × 10−9 |
Frequency of emergence of in vitro-selected variants of SA1199 resistant to 1 μg of ciprofloxacin per ml (two times the MIC for strain SA1199) in either the absence or the presence of NorA inhibitors. The results of one of three experiments is presented.
Ciprofloxacin resistance in first-step, in vitro-selected mutants of S. aureus is predominantly due to specific point mutations in the targets of this drug, topoisomerase IV and gyrase (8, 21). In our hands, chemical mutagenesis of S. aureus by ethylmethane sulfonate increases the rate of emergence of ciprofloxacin-resistant variants by approximately fivefold (Table 2). However, even with mutagenized cells, the NorA inhibitors strongly suppressed the appearance of drug-resistant colonies, demonstrating that the identified lead inhibitors, like reserpine, inhibited the emergence of fluoroquinolone resistance in S. aureus.
DISCUSSION
Since the mechanism underlying the ability of NorA, or any other multidrug transporter, to recognize multiple structurally dissimilar drugs is unknown, there is no structure-based approach to designing an inhibitor of this transporter. In fact, reserpine was discovered serendipitously to reverse Bmr and subsequently NorA-mediated drug resistance (19). In this study we have identified a number of structurally different compounds as inhibitors of NorA by screening a chemical library. The explanation for the surprisingly large number of identified inhibitors and their broad structural diversity apparently lies in the low substrate specificity of this multidrug transporter, since a similar structural diversity has been observed for inhibitors of the mammalian multidrug transporter P-glycoprotein (22). The multiple inhibitors identified, many of which belong to the same chemical classes, have provided a basis for the structure-activity analysis of the lead compounds that is in progress. The goal of the analysis is the development of improved inhibitors.
The newly identified inhibitors are more potent than reserpine in promoting the bacteriotoxicity of ciprofloxacin. These inhibitors not only increased the intrinsic sensitivity of S. aureus to ciprofloxacin but also reversed the ciprofloxacin resistance of a fluoroquinolone-resistant strain and, perhaps most importantly, dramatically reduced the rate of emergence of ciprofloxacin-resistant mutants of S. aureus. Although the toxicities of the identified inhibitors are unknown, their large number and broad chemical diversity raises the hope that further studies will allow the identification of a nontoxic potent lead compound that can be developed into a clinically useful adjuvant for fluoroquinolone therapy of staphylococcal infections, particularly for those caused by methicillin-resistant S. aureus isolates, among which the rate of fluoroquinolone resistance is considerably higher than that among methicillin-susceptible S. aureus strains (9). Determination of the prevalence of efflux-mediated resistance mechanisms in such clinical isolates, studies we have planned for the near future, should be valuable in predicting the potential usefulness of NorA inhibitors. Such inhibitors are also likely to be active against multidrug transporters of other gram-positive bacteria, since the five inhibitors evaluated here also inhibit the B. subtilis Bmr multidrug transporter and INF 271 and INF 55 inhibit efflux-mediated fluoroquinolone resistance in S. pneumoniae (16a).
The mechanism of inhibition of NorA by the inhibitors is unknown. There is strong evidence that reserpine exerts its inhibitory action directly, by binding to the transporters that mediate this efflux. Several variants of the B. subtilis Bmr transporter that exhibit markedly reduced sensitivity to the inhibitory effects of reserpine have been obtained (2, 15). Since some of these mutations have been shown to inhibit reserpine binding to the transporter, these residues are likely to be involved in the formation of the reserpine-binding site within the transporter molecule. Considering the high level of sequence homology between Bmr and NorA, there is little doubt that the same is true for the interaction of reserpine with NorA. Similar studies will be performed with the new inhibitors to prove their direct interaction with NorA.
Although ciprofloxacin is the most frequently used fluoroquinolone and is being approved for a growing number of therapeutic indications, a new group of these antibiotics with increased activity against gram-positive bacteria (sparfloxacin and trovafloxacin) has recently been introduced into clinical practice. Several other new fluoroquinolones are at various stages of development by major pharmaceutical companies. Although some of these new quinolones are subject to multidrug efflux mechanisms of resistance, both sparfloxacin and trovafloxacin are poor substrates of the NorA multidrug transporter (17a), which may be one of the reasons for their superior effectiveness compared to that of ciprofloxacin. In general, combining high efficacy with resistance to efflux is a formidable challenge. Thus, we believe that the future development of fluoroquinolone antibiotics should not be limited to the rare compounds which are poorly recognized by multidrug transporters. As we demonstrate here, the development of a transporter inhibitor as a supplement to a particularly attractive drug appears to be a feasible alternative.
ACKNOWLEDGMENT
This work was supported by NIH grant R44 GM55449 (to P.N.M.).
REFERENCES
- 1.Aeschlimann J R, Dresser L D, Kaatz G W, Rybak M J. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:335–340. doi: 10.1128/aac.43.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed M, Borsch C M, Neyfakh A A, Schuldiner S. Mutants of the Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensive alkaloid reserpine. J Biol Chem. 1993;268:11086–11089. [PubMed] [Google Scholar]
- 3.Ahmed M, Lyass L, Markham P N, Taylor S S, Vazquez-Laslop N, Neyfakh A A. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J Bacteriol. 1995;177:3904–3910. doi: 10.1128/jb.177.14.3904-3910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranova N, Neyfakh A A. Apparent involvement of a multidrug transporter in the fluoroquinolone resistance of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1396–1398. doi: 10.1128/aac.41.6.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bron S. Plasmids. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons; 1990. pp. 75–174. [Google Scholar]
- 6.Eliopoulus G M. In vitro activity of fluoroquinolones against gram positive bacteria. Drugs. 1995;49(Suppl. 2):48–57. doi: 10.2165/00003495-199500492-00009. [DOI] [PubMed] [Google Scholar]
- 7.Eliopoulus G M, Moellering R C., Jr . Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 330–396. [Google Scholar]
- 8.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein F W, Acar J F. Epidemiology of quinolone resistance: Europe and North and South America. Drugs. 1995;49(Suppl. 2):36–42. doi: 10.2165/00003495-199500492-00007. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh P C, Siegel S A, Rogers B, Davis D, Lewis K. Bacteria lacking a multidrug pump: a sensitive tool for drug discovery. Proc Natl Acad Sci USA. 1998;95:6602–6606. doi: 10.1073/pnas.95.12.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaatz G, Seo S M. Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2733–2737. doi: 10.1128/aac.41.12.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaatz G W, Seo S M, Ruble C A. Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J Infect Dis. 1990;163:1080–1086. doi: 10.1093/infdis/163.5.1080. [DOI] [PubMed] [Google Scholar]
- 13.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaatz G W, Seo S. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:2650–2655. doi: 10.1128/aac.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klyachko K, Schuldiner S, Neyfakh A A. Mutations affecting substrate specificity of the Bacillus subtilis multidrug transporter Bmr. J Bacteriol. 1997;179:2189–2193. doi: 10.1128/jb.179.7.2189-2193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch C, Courvalin P, Nikaido H. Active efflux of antimicrobial agents in wild-type strains of enterococci. Antimicrob Agents Chemother. 1997;41:869–871. doi: 10.1128/aac.41.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Markham, P. N. Unpublished data.
- 17.Markham P N, Neyfakh A A. Inhibition of the multidrug transporter NorA prevents emergence of norfloxacin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:2673–2674. doi: 10.1128/aac.40.11.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Markham, P. N., and A. A. Neyfakh. Unpublished data.
- 18.Neyfakh A A. The multidrug efflux transporter of Bacillus subtilis is a structural and functional homolog of the Staphylococcus NorA protein. Antimicrob Agents Chemother. 1992;36:484–485. doi: 10.1128/aac.36.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neyfakh A A, Borsch C M, Kaatz G W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob Agents Chemother. 1993;37:128–129. doi: 10.1128/aac.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz F-J, Fluit A C, Luckefahr M, Engler B, Hofmann B, Verhoef J, Heinz H-P, Hadding U, Jones M E. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of S. aureus. J Antimicrob Chemother. 1998;42:807–810. doi: 10.1093/jac/42.6.807. [DOI] [PubMed] [Google Scholar]
- 21.Sreedharan S, Oram M, Jensen B, Peterson L R, Fisher L M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990;172:7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein W D. Kinetics of the multidrug transporter (P-glycoprotein) and its reversal. Physiol Rev. 1997;77:545–589. doi: 10.1152/physrev.1997.77.2.545. [DOI] [PubMed] [Google Scholar]
- 23.Sulavik M C, Barg N L. Examination of methicillin-resistant and methicillin-susceptible Staphylococcus aureus mutants with low-level fluoroquinolone resistance. Antimicrob Agents Chemother. 1998;42:3317–3319. doi: 10.1128/aac.42.12.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Top 200 Prescriptions. 1999. 1998 U.S. prescriptions. [Online.] RxList.Com, Inc. http://www.rxlist.com/top200a.htm. [27 August, 1999, last date accessed.]
- 25.Trucksis M, Wolfson J S, Hooper D C. A novel locus conferring fluoroquinolone resistance in Staphylococcus aureus. J Bacteriol. 1991;173:5854–5860. doi: 10.1128/jb.173.18.5854-5860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada H, Kurose-Hamada S, Fukuda Y, Mitsuyama J, Takahata M, Minami S, Watanabe Y, Narita H. Quinolone susceptibility of norA-disrupted Staphylococcus aureus. Antimicrob Agents and Chemother. 1997;41:2308–2309. doi: 10.1128/aac.41.10.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeller V, Janoir C, Kitzis M, Gutmann L, Moreau N J. Active efflux as a mechanism of resistance to ciprofloxacin in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1973–1978. doi: 10.1128/aac.41.9.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]