Abstract
This study focused on the biological evaluation and chemical characterisation of Ficus sur Forssk. (F. sur) (Family: Moraceae). The methanolic and aqueous extracts’ phytochemical profile, antioxidant, and enzyme inhibitory properties were investigated. The aqueous stem bark extract yielded the highest phenolic content (115.51 ± 1.60 mg gallic acid equivalent/g extract), while the methanolic leaves extract possessed the highest flavonoid content (27.47 ± 0.28 mg Rutin equivalent/g extract). In total, 118 compounds were identified in the tested extracts. The methanolic stem bark extract exhibited the most potent radical scavenging potential against 2,2-diphenyl-1 picrylhydrazyl and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (475.79 ± 6.83 and 804.31 ± 4.52 mg Trolox equivalent/g extract, respectively) and the highest reducing Cu2+ capacity (937.86 ± 14.44 mg Trolox equivalent/g extract). The methanolic stem bark extract substantially depressed tyrosinase (69.84 ± 0.35 mg kojic acid equivalent/g extract), α-amylase (0.77 ± 0.01 mmol acarbose equivalent/g extract), acetylcholinesterase and butyrylcholinesterase (2.91 ± 0.07 and 6.56 ± 0.34 mg galantamine equivalent/g extract, respectively) enzymes. F. sur extracts were tested for anticancer properties and antiviral activity towards human herpes virus type 1 (HHV-1). Stem bark infusion and methanolic extract showed antineoplastic activity against cervical adenocarcinoma and colon cancer cell lines, whereas leaf methanolic extract exerted moderate antiviral activity towards HHV-1. This investigation yielded important scientific data on F. sur which might be used to generate innovative phytopharmaceuticals.
Keywords: Ficus, natural products, fig, antioxidant, enzymes, phytochemistry, LC-MS, anticancer, natural antivirals
1. Introduction
For millennia, humans have centred their lives on plants in an effort to maintain good health and treat common ailments. Even though the usage of plants was based simply on people’s intuitive understanding, owing to a lack of suitable techniques to show plants’ therapeutic potential, humans have accepted the use of many medicinal plants and included them in contemporary pharmacotherapy [1]. The Royal Botanic Gardens at Kew’s Bob Allkin recognised around 28,000 plant species as medicinal plants [2]. Since the eureka moment of the discovery of Taxol, the blockbuster anti-cancer medicine produced from the Pacific yew tree, plants have demonstrated their healing ability [3]. Since then, medicinal plants have quickly captured the interest of scientists, resulting in the increased screening of medicinal plants and the use of health-promoting plant extracts in nutrition.
In line with the present worldwide trend, the medicinal plant that this study sought to investigate was Ficus sur Forssk. (F. sur) (Family: Moraceae). The genus Ficus comprises more than 800 species and is generally distributed in tropical and sub-tropical areas [4]. Morphologically, it is a tree that can grow up to 25–30 m tall, with leafy twigs 2–5 mm thick, puberulous, hirtellous, tomentose or hirsute to glabrescent, with the periderm typically not flaking off when dry [5]. F. sur, with the common names Cape fig and broom cluster fig, is used to treat a variety of ailments in many countries. Its leaves and roots are used to cure leukoderma, leprosy, wounds, oedema, respiratory problems, diarrhoea, sexually transmitted illnesses, tuberculosis, anaemia, epilepsy, rickets, dysentery, male infertility, and gonorrhoea in Sudan and Nigeria [6]. According to ethnobotanical research, F. sur is also used to cure swellings [7]. It has long been used in South Africa and other nations to treat renal disorders and as a natural diuretic product [8,9]. In Ethiopia, pulverized fresh F. sur leaves combined with water were administered orally as a traditional medicine for urine retention, effectively alleviating the condition by boosting urine production. There is also a traditional belief that the root of this plant may be utilized to treat bladder diseases [10,11]. Despite the reported beneficial effects of members of the genus Ficus, several side effects (particularly when eating fruits) have been reported. These include stomach problems, obstructions in the intestine and liver damage [8]. It is necessary not to exceed the recommended dose for the fruits.
The findings of Ayele and co-workers are consistent with the traditional usage of F. sur as a diuretic agent. The crude leaf extracts enhanced urine excretion and urinary electrolyte concentrations in a dose-dependent manner [12]. The results of another study indicated that an ethanol extract of F. sur has a substantial anticonvulsant effect, validating the traditional use of the plant in the treatment of epilepsies; processes may entail interaction with GABAergic, glycinergic, serotonergic, and glutaminergic system components [13]. The purpose of another study was to see how feeding a mixture of varying amounts of F. sur fruits and ground maize grain affected intake, digestibility, growth, and blood profile in Yorkshire pigs [14]. The findings demonstrated that the health of the pigs was better when fed with F. sur fruits as creatinine and cholesterol concentrations were lower.
There are reports describing the antiviral potential of different species of Ficus. Ethanolic extracts from F. benjamina leaves inhibited human herpes virus type 1 (HHV-1, HSV-1) and type 2 (HHV-2, HSV-2), and varicella-zoster virus (HHV-3, VZV), while fruit extracts were active only against HHV-3 [15]. F. carica latex inhibited caprine herpes virus-1 (CpHV-1) replication in MDBK cells [16], as well as HHV-1, echovirus type 11 (ECV-11) and adenovirus (ADV) replication in VERO cells [17]. Leaf methanol extract from F. septica impeded dengue virus (DENV) replication in various infected cell types [18], and F. religiosa bark extracts showed activity against human rhinovirus (HRV) and human respiratory syncytial virus (RSV) [19], and HHV-2 [20]. Recently, ethanol extract of Ficus fistulosa leaves was reported to show anti-HIV activity [21].
Our aims with this study were to screen methanolic and aqueous leaves and stem bark extracts of F. sur for antioxidant and anti-enzymatic activities. Additionally, since the absence of detailed characterisation can markedly limit our understanding of their biological activities, the extracts were subjected to detailed phytochemical profiling. The search for new cholinesterase inhibitors (acetyl- and butyryl-cholinesterase) and carbohydrate digesting inhibitors (α-amylase and α-glucosidase) is now underway and our work has evaluated several extracts of F. sur for probable anti-cholinesterase and antidiabetic activities. Since multiple Ficus species were shown to possess antiviral activity, we have undertaken the attempt to evaluate the anti-HHV-1 activity of extracts obtained from Ficus sur. Furthermore, we have evaluated the anticancer potential of this plant species against cervical adenocarcinoma and colon cancer cell lines. We hope that the information offered here will assist in bridging a research gap and, as a result, open up new research opportunities, notably in the production of medicinal bioproducts.
2. Results and Discussion
2.1. Bioactive Compounds
The polarity of the solute of interest was considered in choosing the solvents used to extract bioactive compounds from plants because a solute with equivalent polarity to the solvent will be sufficiently dissolved according to the law of similarity and intermiscibility [22,23]. In this study, polyphenolic compounds, such as phenolic compounds and flavonoids, were quantified from the methanolic and aqueous extracts of F. sur. The results are presented in Table 1.
Table 1.
Total phenolic and flavonoid contents of Ficus sur extracts.
| Parts | Solvents | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|---|
| Leaves | MeOH | 58.46 ± 0.28 c | 27.47 ± 0.28 a |
| Infusion | 51.77 ± 0.77 d | 16.65 ± 0.18 b | |
| Stem barks | MeOH | 109.79 ± 2.19 b | 2.54 ± 0.10 c |
| Infusion | 115.51 ± 1.60 a | 1.13 ± 0.11 d |
Values are reported as mean ± SD of three parallel measurements. GAE: Gallic acid equivalents; RE: Rutin equivalents. Different letters in the same column indicate significant differences in the teste extracts (p < 0.05).
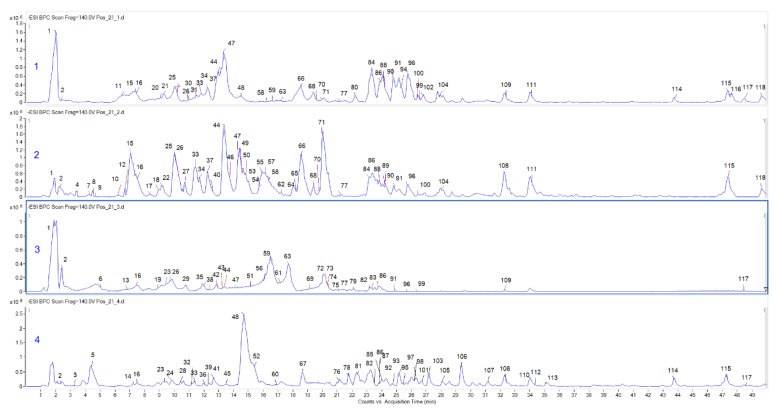
Among the samples studied, the aqueous extract obtained from the stem bark yielded the highest amount of phenolics (115.51 ± 1.60 mg GAE/g), while the methanolic extract prepared from leaves had the highest flavonoid content (27.47 ± 0.28 mg RE/g). LC-ESI-QTOF-MS/MS analysis enabled the chemical characterization of all studied extracts. In total, 118 compounds were described (Table 2, Figure 1). It was observed that the leaf extracts contained more compounds compared to the stem bark extracts. Phenolic acids (such as quinic, citric, dihydroxybenzoic, 3-O-caffeoylquinic, and 5-O-caffeoylquinic acid), quercetin glycosides (quercetin-O-di-rhamnosyl-glucoside/galactoside, quercetin-O-glucoside, quercetin-O-pentoside (arabinoside), quercetin-O-glucuronide), and fatty acids (hydroxy octadecatrienoic acid, palmitic acid derivative) were present in almost all extracts. The methanolic extract from leaves contained mainly phenolic acids and their derivatives, esters of phenolic acids and flavonoids, and flavonoid glycosides (esters of kaempferol and quercetin). Hydroxycoumarin and methyl gallate were present only in this extract. Hydroxycaffeoylquinic, glucogallic, 2-isopropylmalic, tartaric, coumaric, ferulic acids and their esters were characteristic for leaf infusion. The methanolic extract from the stem bark was abundant in tannins, represented by catechins and procyanidins. In the stem bark infusion, apigenin, luteolin, kaempferol, quercetin and their conjugates with one or more sugar moieties dominated. The majority of the detected secondary metabolites were typical for previously studied Ficus species: F. lyrata, F. benghalensis, F. benjamina, F. mysorensis, F. afzelii, F. pyriformis, F. racemose, F. lutea, F. auriculata, F. trigonata, F. spragueana, F. microcarpa var. nitida; F. virens and F. religiosa for which the presence of flavonoids, flavonolignans, anthocyanins and hydroxycinnamic acids derivatives was reported [24,25].
Table 2.
Compounds identified in the studied extracts.
| Comp. No | Tentative Identification | R Time | Molecular Mass | [M − H]− | Fragment Ions (m/z) | Extracts |
|---|---|---|---|---|---|---|
| 1 | Quinic acid | 1.91 | 192.0507 | 191.0507 | 173.0464; 111.0437; 93.0318; 85.0262 | 1,2,3 |
| 2 | Citric acid | 2.33 | 192.0180 | 191.0180 | 111.0035; 87.0052 | 1,2,3,4 |
| 3 | Caffeic acid derivative | 3.40 | 242.0302 | 241.0302 | 179.0273; 153.0497; 135.0406; 123.0407; 109.0230 | 4 |
| 4 | Quinic acid derivative | 3.46 | 534.1621 | 533.1621 | 337.0845; 191.0508 | 2 |
| 5 | Quinic acid derivative | 4.04 | 406.0968 | 405.0968 | 213.0351; 191.0511 | 4 |
| 6 | 4-Hydroxy-2-(hydroxymethyl)benzoic acid | 4.09 | 168.0307 | 167.0307 | 149.0234; 123.0429 | 3 |
| 7 | Dihydroxybenzoic acid glucoside derivative | 4.13 | 532.0929 | 531.0929 | 353.0781; 315.0642; 153.0155; 96.9570 | 2 |
| 8 | Quinic acid derivative | 4.22 | 470.0574 | 469.0574 | 435.1422; 371.0962; 191.0423 | 2 |
| 9 | Hydroxycaffeoyl-quinic acid | 4.76 | 372.0900 | 371.0900 | 353.0840; 197.0360; 191.0533; 179.0312; 173.0431;135.0409 | 2 |
| 10 | Glucogallic acid/Glucosyl gallate | 6.32 | 332.0607 | 331.0607 | 169.0124; 151.0003; 125.0211 | 2 |
| 11 | Dihydroxybenzoic acid glucoside derivative | 6.49 | 436.0075 | 435.0075 | 315.0710; 153.0056 | 1 |
| 12 | Dihydro-caffeoyl-quinic acid | 6.56 | 356.0959 | 355.0959 | 191.0522; 181.0167; 173.0451; 137.0164; 111.0044 | 2 |
| 13 | Hydroxybenzoic acid derivative | 6.77 | 432.1224 | 431.1124 | 137.0243; 93.0383 | 3 |
| 14 | Caffeoyl-hydroxybenzoic acid | 7.23 | 300.0717 | 299.07117 | 239.0562; 179.0356; 137.0228 | 4 |
| 15 | Dihydroxybenzoic acid glucoside isomer 1 | 7.487 | 316.0628 | 315.0628 | 153.0134; 152.0105; 108.0213 | 1,2 |
| 16 | Dihydroxybenzoic acid | 7.59 | 154.0143 | 153.0143 | 109.0302; 108.0225 | 1,2,3,4 |
| 17 | Coumaric acid-hexoside-pentoside | 8.48 | 458.0892 | 457.0892 | 325.0865; 163.0347 | 2 |
| 18 | Dihydroxybenzoic acid glucoside isomer 2 | 8.51 | 316.0660 | 315.0660 | 153.0147; 109.0258 | 2 |
| 19 | 3-Hydroxy-4-methoxymandelate glucoside | 8.86 | 360.0924 | 359.0924 | 197.0449; 182.0215; 153.0557; 138.0321; 123.0129 | 3 |
| 20 | Caffeic acid derivative hexoside | 9.06 | 376.0601 | 375.0601; | 341.1069; 213.0650; 201.0144; 179.0316; 135.0409 | 1 |
| 21 | Quinic acid derivative | 9.08 | 372.0868 | 371.0868 | 251.0544; 191.052; 167.0327 | 1 |
| 22 | 2-Isopropylmalic acid | 9.19 | 176.0571 | 175.0571 | 157.0486; 115.0371; 85.0638 | 2 |
| 23 | Hydroxybenzoic acid | 9.29 | 138.0208 | 137.0208 | 108.0231 | 3, 4 |
| 24 | Unidentified | 9.39 | 376.1236 | 375.1236 | 312.0725; 169.0827; 151.0726 | 4 |
| 25 | Hydroxybenzoic acid 4-O-glucoside | 10.17 | 300.0669 | 299.0669 | 137.0203; 93.0315 | 1,2 |
| 26 | 3-O-Caffeoylquinic acid | 10.36 | 354.0782 | 353.0782 | 191.0578; 179.0370; 161.0243; 135.0461 | 1,2,3 |
| 27 | Dihydroxybenzoic acid glucoside isomer 3 | 10.69 | 316.0660 | 315.0660 | 153.0144; 109.0258 | 2 |
| 28 | Caffeic acid glucoside | 10.76 | 342.0825 | 341.0825 | 179.0357; 161.0246; 135.0457 | 4 |
| 29 | Dihydroxybenzoic acid O-glucoside-pentoside | 10.84 | 448.1382 | 447.1382 | 315.1063; 153.0548; 109.0301; 108.0249 | 3 |
| 30 | Hydroxycoumarin | 10.93 | 340.0715 | 339.0715 | 177.0178 | 1 |
| 31 | Methyl gallate | 11.02 | 184.0234 | 183.0234 | 168.0071; 124.0155; 78.0128 | 1 |
| 32 | Hydroxybenzoic acid | 11.13 | 138.0210 | 137.0210 | 109.0209; 108.0308; 93.0345 | 4 |
| 33 | Glucogallic acid/Glucosyl gallate | 11.50 | 332.0607 | 331.0607 | 169.0113;168.0047;125.0225 | 1,2,4 |
| 34 | 3-O-p-Coumaroylquinic acid | 11.30 | 338.0840 | 337.0840 | 191.0540; 163.0384 | 1,2 |
| 35 | Procyanidin B (dimer of (epi)catechin) | 11.62 | 578.1270 | 577.1270 | 451.1080; 425.0892; 407.0834; 289.0734; 245.0811; 125.0239 | 3 |
| 36 | Caffeic and coumaric acid derivative | 12.20 | 542.1494 | 541.1494 | 523.1466; 475.1519; 361.0900; 235.0490; 215.0901; 179.0358; 163.0398; 137.0249 | 4 |
| 37 | 1-O-Coumaroylquinic acid | 12.28 | 338.0840 | 337.0840 | 191.0509; 173.0417; 163.0356 | 1,2 |
| 38 | Glucogallic acid tartaric ester | 12.33 | 464.1040 | 463.1040 | 331.0608; 169.0147; 168.0037; 149.9937; 125.0212 | 3 |
| 39 | Caffeic acid glucoside | 12.34 | 342.0825 | 341.0825 | 179.0344; 161.0230; 135.0440 | 4 |
| 40 | Gallic acid glucoside derivative | 12.55 | 412.0173 | 411.0173 | 367.355; 331.0632; 240.9965; 169.0091; 125.0157 | 2 |
| 41 | Caffeic acid derivative hexoside | 12.63 | 542.1494 | 541.1494 | 379.0983; 179.0323; 161.0220; 135.0425 | 4 |
| 42 | Methyl gallate hexoside-pentoside | 12.70 | 448.1542 | 447.1542 | 345.1151; 183.0660; 168.0382; 161.0437 | 3 |
| 43 | (Epi)gallocatechin | 13.18 | 306.0612 | 305.0612 | 287.0562; 269.0489; 219.0678; 195.0310; 179.0321; 161.0246; 137.0237; 125. 0235 | 3 |
| 44 | 5-O-Caffeoylquinic acid | 13.27 | 354.0811 | 353.0811 | 191.0521; 161.0214; 135.0406 | 1,2,3 |
| 45 | Dihydroxycoumarin | 13.47 | 178.0152 | 177.0152 | 161.0960; 133.0279; 105.0338 | 4 |
| 46 | 3-O-Feruloylquinic acid | 13.66 | 368.0964 | 367.0964 | 193.0481; 173.0430; 134.0355 | 2 |
| 47 | 4-O-Caffeoylquinic acid | 14.32 | 354.0811 | 353.0811 | 191.0516; 179.0306; 173.0412; 135.0414 | 2 |
| 48 | Caffeic acid | 14.41 | 180.0293 | 179.0293 | 135.0451 | 1,4 |
| 49 | Coumaroyltartaric acid isomer 1 | 14.68 | 296.0404 | 295.0404 | 163.0357; 149.0051; 130.9957; 119.0460 | 2 |
| 50 | Tartaric acid | 14.94 | 150.0055 | 149.0055 | 130.9997; 102.9993; 87.0065 | 2 |
| 51 | 4-O-Methylgallocatechin | 15.25 | 320.0763 | 319.0763 | 287.0497; 243.0251; 197.0451; 161.0242; 125.0233 | 3 |
| 52 | Caffeic and hydroxycinnamic acid derivative | 15.39 | 380.0995 | 379.0995 | 369.0971; 251.0590; 217.0658; 179.0345; 161.0261; 135.0426 | 4 |
| 53 | Coumaric acid hexoside derivative | 15.49 | 442.0969 | 441.0969 | 325.0912; 163.0378; 119.0507 | 2 |
| 54 | Coumaric acid | 15.76 | 164.0361 | 163.0361 | 119.0483 | 2 |
| 55 | Coumaroyltartaric acid isomer 2 | 15.82 | 296.0404 | 295.0404 | 163.0363; 149.0056; 130.9944; 119.0472 | 2 |
| 56 | B-type procyanidin trimer | 16.03 | 866.1873 | 865.1873 | 739.1731; 713.1525; 577.1396; 425.0996; 287.0524; 245.0479; 125.0216 | 3 |
| 57 | Caffeic acid pentoside | 16.15 | 312.0340 | 311.0340 | 179.0036; 135.0380 | 2 |
| 58 | 4-O-p-Coumaroylquinic acid | 16.33 | 338.0871 | 337.0871 | 191.0520; 173.0423; 163.0363 | 1,2 |
| 59 | Procyanidin B (dimer of (epi)catechin) | 16.54 | 578.1226 | 577.1226 | 451.0947; 425.0842; 407.0791; 289.0774; 245.0500 | 1,3 |
| 60 | Caffeic acid and hydroxycoumarin derivative | 16.93 | 458.1282 | 457.1282 | 383.0989; 221.0481; 179.0362; 161.0255; 135.0433; 133.0275; 117.0335 | 4 |
| 61 | (Epi)catechin derivative | 17.11 | 326.0425 | 325.0425 | 289.0667; 245.0790; 205.0449; 125.0275 | 3 |
| 62 | Sinapoyl-ferulate | 17.22 | 400.0876 | 399.0876 | 223.0469; 205.0374; 193.0429; 129.0150; 111.0050; 85.0271 | 2 |
| 63 | (Epi)catechin | 17.26 | 290.0637 | 289.0637 | 245.0785; 205.0476; 187.0356; 179.0295; 125.0258 | 1,3 |
| 64 | Ferulic acid pentoside | 18.03 | 326.0500 | 325.0500 | 193.0474; 178.0238; 134.0347 | 2 |
| 65 | Caffeoylshikimic acid | 18.03 | 336.0713 | 335.0713 | 179.0325; 173. 0429; 161.0208; 135.0420 | 2 |
| 66 | 5-O-p-Coumaroylquinic acid | 18.50 | 338.0871 | 337.0871 | 191.0514; 173.0417; 163.0356 | 1,2 |
| 67 | Unidentified | 18.67 | 578.1268 | 577.1268 | 541.1482; 379.0918; 179.0298; 161.0208; 135.0406 | 4 |
| 68 | Caffeoylshikimic acid isomer | 19.17 | 336.0713 | 335.0713 | 179.0302; 173. 0411; 161.0200; 155.0291; 135.0415 | 1, 2 |
| 69 | B-type procyanidin trimer | 19.20 | 866.1873 | 865.1873 | 739.1553; 713.1344; 577.1240; 425.0756; 287.0531; 245.0383; 125.0183 | 3 |
| 70 | 4-O-Feruloylquinic acid | 19.75 | 368.0972 | 367.0972 | 191.0531; 173.0423 | 1,2 |
| 71 | Caffeoylmalic acid | 20.07 | 296.0416 | 295.0416 | 179.0315; 133.115; 115.0010 | 1,2 |
| 72 | Procyanidin B (dimer of (epi)catechin) derivative | 20.07 | 880.1656 | 879.1656 | 727.1275; 577.1073; 439.0638; 407.0538; 287.0468; 245.0405; 125.0207 | 3 |
| 73 | B-type procyanidin trimer | 20.38 | 866.1873 | 865.1873 | 739.1553; 713.1344; 577.1292; 451.0938; 425.0813; 407.0716; 287.0522; 245.0383; 125.0207 | 3 |
| 74 | (Epi)catechin-(epi)gallocatechin | 20.55 | 594.1023 | 593.1223 | 575.1061; 467.1034; 441.0792; 423.0659; 305.0549; 287.0417; 245.0385; 125.0204 | 3 |
| 75 | Procyanidin B (dimer of (epi)catechin) derivative | 20.76 | 721.6486 | 720.6486 | 644.1217; 577.1295; 289.0645; 245.0399; 125.0202 | 3 |
| 76 | Quercetin-O-di-glucoside | 21.27 | 626.1342 | 625.1342 | 463.0867; 301.0301; 300.0238; 178.9961; 151.0005 | 4 |
| 77 | Quercetin-O-di-rhamnosyl-glucoside/galcactoside | 21.28 | 756.1880 | 755.1880 | 609.1432; 300.0275; 271.0254; 178.9947; 151.0029 | 1,2,3 |
| 78 | Unidentified | 21.74 | 536.1387 | 535.1387 | 491.1498; 323.0715; 281.0600; 179.0314; 161.0215 | 4 |
| 79 | Procyanidin dimer monoglycoside | 22.18 | 740.1601 | 739.1601 | 587.1146; 459.0606; 449.0773; 435.0625; 289.062; 245.0766; 125.0214 | 3 |
| 80 | 5,8-Dihydroxy-7-methoxyflavone-O-glucoside-rhamnoside | 22.22 | 592.1811 | 591.1811; | 445.1129 325.0735; 297.0404; 293.0638; 282.0504 | 1 |
| 81 | Quercetin-3-O-arabino-glucoside | 22.24 | 596.1230 | 595.1230 | 301.0279; 300.0229; 271.0219; 255.0251; 178.9902 | 4 |
| 82 | Rutin | 23.06 | 610.1377 | 609.1377 | 301.0285; 300.0216; 271.0215; 178.9902; 150.9959 | 3,4 |
| 83 | Procyanidin B (dimer of (epi)catechin) | 23.25 | 578.1270 | 577.1270 | 451.0997; 425.0849; 407.0722; 289.0671; 125.0228 | 3 |
| 84 | Rutin-O-(p-coumaroyl) malate | 23.32 | 890.1862 | 889.1862 | 609.1328; 300.0192; 271.0182; 178.9951; 163.0345; 150.9965; 133.0093 | 1, 2 |
| 85 | Quercetin derivative hexoside | 23.85 | 566.1131 | 565.1131 | 403.1489; 301.0262; 300.0204; 271.0208; 255.0250; 178.9927; 150.9985 | 4 |
| 86 | Quercetin-O-glucoside | 23.86 | 464.0778 | 463.0778 | 300.0028; 271.0183; 178.9940; 150.9981 | 1,2,3,4 |
| 87 | Kaempferol-O-pentoside-hexoside | 24.08 | 580.1286 | 579.1286 | 447.0887; 285.0334; 284.0273; 255.0245; 227.0320; 150.9995; 133.0083 | 4 |
| 88 | Kaempferol-3-O-rhamnosyl galactoside | 24.21 | 594.1381 | 593.1381 | 285.0311; 257.0382; 255.0197; 229.0456; 187.0403 | 1, 2 |
| 89 | Ferulic acid | 24.26 | 194.0463 | 193.0463 | 178.0225; 149.0556; 134.0330; 117.0299 | 2 |
| 90 | Quercetin-O-(glucosyl-feruloylmalate) | 24.36 | 774.1473 | 773.1473 | 463.0811; 309.0561; 193.0466; 134.0333 | 1,2 |
| 91 | Quercetin-O-pentoside (arabinoside) | 24.75 | 434.0683 | 433.0686 | 301.0267; 300.0213; 271.0193; 255.0248; 227.0281; 150.9994 | 1,2,3 |
| 92 | Kaempferol-C-di-hexoside-O-hexoside | 24.85 | 772.1694 | 771.1694 | 609.1382; 485.1224; 429.0806; 383.0925; 323.0716; 285.0338; 255.0239; 227.0213; 161.0214; 133.0241 | 4 |
| 93 | Luteolin-O-glucuronide | 25.13 | 462.0664 | 461.0664 | 285.0343; 175.0245; 133.0246; | 4 |
| 94 | Kaempferol-O-glucoside | 25.18 | 448.0832 | 447.0832 | 284.0261; 255.0237; 227.0277; 150.9974 | 1 |
| 95 | Quercetin-O-(caffeoyl-di-glucoside) | 25.44 | 788.1650 | 787.1650 | 625.1372; 461.0677; 387.1494; 301.0286; 300.0232; 179.0375; 161.0161 | 4 |
| 96 | Quercetin-O-glucuronide | 25.71 | 478.0832 | 477.0832 | 301.0285; 271.0556; 178.9914; 150.9982 | 1,2,3 |
| 97 | Quercetin-O-arabinoside-di-glucoside | 25.92 | 758.1530 | 757.1530 | 595.1207; 463.0830; 301.0292; 300.0215; 178.9928; 150.9968 | 4 |
| 98 | Quercetin-O-glucoside-arabinoside-glucuronide | 26.21 | 772.1694 | 771.1694 | 595.1243; 301.0275; 300.0228; 271.0201; 178.9963; 150.9999 | 4 |
| 99 | Kaempferol-O-di-pentoside | 26.40 | 550.1142 | 549.1142 | 417.0841; 285.0453 | 1,3 |
| 100 | Kaempferol-O-pentoside | 26.42 | 418.0743 | 417.0743 | 284.0376; 285.0421 | 1,2 |
| 101 | Di-caffeoyl-dihydroxybenzoic acid | 26.60 | 478.0983 | 477.0983 | 433.1033; 315.0667; 179.0321; 161.0204; 153.0140; 152.0098; 135.0443; 109.0295; 108.0158 | 4 |
| 102 | (Epi)-afzelechin-7-O-glucoside | 26.82 | 436.1199 | 435.1199 | 345.0936; 273.0730; 167.0336 | 1 |
| 103 | Kaempferol-O-(caffeoyl-arabinoside-glucoside | 27.09 | 742.1608 | 741.1608 | 579.1326; 455.1050; 429.0733; 285.0349; 284.0270; 255.0312; 227.0223; 179.0308; 161.0221; 135.0376 | 4 |
| 104 | Kaempferol-O-rhamnoside | 27.88 | 432.0906 | 431.0906 | 285.0431; 284.0317; 255.0287; 227.0334 | 1,2 |
| 105 | Quercetin-O-glucoside-arabinoside-glucuronide | 27.99 | 772.1694 | 771.1694 | 595.1228; 301.0258; 300.0216; 271.0204; 255.0185; 178.9945; 150.9824 | 4 |
| 106 | Kaempferol-O-arabinosisde-glucoside-rhamnoside | 29.28 | 726.1658 | 725.1658 | 579.1227; 285.0346; 284.0272; 255.0222; 227.0298 | 4 |
| 107 | Luteolin | 31.09 | 286.0364 | 285.0364 | 175.0368; 133.0300 | 4 |
| 108 | Trihydroxy-octadecadienoic acid | 32.36 | 328.2116 | 327.2116 | 291.1989; 229.1460; 211.1336; 171.1031 | 2,4 |
| 109 | Unidentified | 32.36 | 396.1960 | 395.1960 | 349.2045; 327.2269; 251.1307; 233.1170; 193.0888; 171.1032 | 1, 3 |
| 110 | Trihydroxy-octadecenoic acid | 33.91 | 330.2292 | 329.2292 | 311.2203; 293.1239; 229.1450; 211.1334; 171.1011 | 4 |
| 111 | 12-oxo-10E-dodecenoic acid | 34.01 | 228.1222 | 227.1222 | 209.1179; 183.1395; 165.1298 | 1,2 |
| 112 | Apigenin | 34.28 | 270.0417 | 269.0417 | 227.0349; 151.0027; 117.0349; 107.0126 | 4 |
| 113 | Trihydroxy-octadecenoic acid derivative | 35.261 | 444.1493 | 443.1493 | 329.2280; 309.1244; 293.1891 | 4 |
| 114 | 12-oxo-10E-dodecenoic acid derivative | 43.89 | 722.3519 | 721.3519 | 675.3594; 397.1348; 227.2159 | 1,4 |
| 115 | Hydroxy octadecatrienoic acid | 47.40 | 294.2048 | 293.2048 | 275.2015; 224.1403; 195.1388 | 1,2,4 |
| 116 | Palmitic acid derivative | 47.50 | 700.3671 | 699.3671 | 653.7889; 397.1369; 255.2329 | 1 |
| 117 | Palmitic acid derivative | 48.36 | 541.3192 | 540.3192 | 480.3059; 255.2310 | 1,3,4 |
| 118 | Hydroxy octadecadienoic acid derivative | 49.62 | 366.2055 | 365.2055 | 317.2080; 295.2254; 277.2110 | 1,2 |
Figure 1.
Base peak chromatograms of studied extracts. 1 Ficus sur leaves-MeOH; 2 Ficus sur leaves-infusion; 3 Ficus sur stem bark -MeOH; 4 Ficus sur stem bark -infusion.
2.2. Antioxidant Effects
The role of oxidative stress in the initiation and progression of human diseases supports the systemic antioxidant assessment of plant extracts under investigation. Antioxidants can perform various functions, including hydrogen atom transfer, single electron transfer, and transition metal chelation [38]. In this study, a battery of antioxidant assays was used to obtain a comprehensive understanding of the antioxidant activities of the prepared extracts of F. sur. The assays were: 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ferric ion reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC), metal-chelating and total antioxidant capacity (phosphomolybdenum). As previously discussed, each test has its own set of advantages and disadvantages [38]. The results are given in Table 3.
Table 3.
Antioxidant properties of Ficus sur extracts.
| Parts | Solvents | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | PBD (mmol TE/g) | MCA (mg EDTAE/g) |
|---|---|---|---|---|---|---|---|
| Leaves | MeOH | 48.66 ± 0.04 c | 81.41 ± 0.05 b | 160.80 ± 1.55 c | 108.50 ± 2.04 c | 1.65 ± 0.11 b | 7.88 ± 0.99 c |
| Infusion | 44.22 ± 0.06 c | 72.32 ± 1.69 b | 147.58 ± 1.59 c | 77.28 ± 0.25 d | 1.65 ± 0.08 b | 22.95 ± 0.20 a | |
| Stem barks | MeOH | 475.79 ± 6.83 a | 804.31 ± 4.52 a | 937.86 ± 14.44 a | 523.17 ± 2.92 b | 5.00 ± 0.30 a | 4.62 ± 0.64 d |
| Infusion | 463.58 ± 1.17 b | 804.91 ± 5.45 a | 910.68 ± 12.14 b | 614.33 ± 2.79 a | 5.05 ± 0.05 a | 13.22 ± 0.18 b |
Values are reported as mean ± SD of three parallel measurements. TE: Trolox equivalents; EDTAE: EDTA equivalents. Different letters in the same column indicate significant differences in the tested extracts (p < 0.05).
Overall, irrespective of the type of extraction solvents used, stem bark extracts demonstrated substantially higher antioxidant activities with DPPH, ABTS, CUPRAC, FRAP, and phosphomolybdenum. For instance, methanolic stem bark extract exhibited the highest DPPH radical scavenging activities (475.79 ± 6.83 mg TE/g). The ABTS assay showed that both methanolic (804.31 ± 4.52mg TE/g) and aqueous stem bark (804.91 ± 5.45 mg TE/g) extracts demonstrated remarkably high activities. ABTS can function with lipophilic and hydrophilic molecules, but DPPH can only be solubilized in organic environments [39]. Our findings confirm the findings of Kim et al. [39]. For example, the DPPH test identified the methanolic extract as the most active, while ABTS identified both methanolic and aqueous extracts as effective ABTS scavengers.
The antioxidant capacity of the extracts was further evaluated in terms of power reduction using the CUPRAC and FRAP tests. Several variables influence antioxidants’ reducing potential, including their ionization potentials, the spin distribution of radical cations, and the bond dissociation energy of the phenolic O-H bond [40]. From Table 3, it can be seen that the methanolic stem bark extract possessed the most potent Cu2+ reducing potential (937.86 ± 14.44 mg TE/g) while the aqueous stem bark extract (614.33 ± 2.79 mg TE/g) was the most robust Fe3+ reducer.
Secondary metabolites are known to have powerful antioxidant properties due to their ability to provide electrons and because they chelate transition metals [41]. Data shown in Table 3 show that the aqueous leaves extract exhibited the highest chelating abilities (22.95 ± 0.20 mg EDTAE/g) while the methanolic stem bark extract displayed the lowest activity (4.62 ± 0.64 mg EDTAE/g). The prepared samples were also tested for their total antioxidant capacity (phosphomolybdenum assay). The latter test is based on antioxidants reducing Mo (VI) to Mo (V), resulting in the formation of a green complex in acidic conditions [42]. The aqueous stem bark extract showed the highest capacity (5.05 ± 0.05 mmol TE/g). It is noteworthy that the stem bark extracts showed stronger antioxidant ability than the leaf extracts for all assays, except the metal-chelating assay. Consequently, it can be said that the antioxidant activity of the active samples could be associated with the presence of bioactive compounds.
2.3. Enzymatic Inhibitory Activities
In the present study, the ability of F. sur extracts to modulate the activity of enzymes related to Alzheimer’s disease [acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)], diabetes type 2 (α-amylase and α-glucosidase), and skin hyperpigmentation (tyrosinase) was investigated. The results are presented in Table 4.
Table 4.
Enzyme inhibitory properties of Ficus sur extracts.
| Parts | Solvents | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| Leaves | MeOH | 2.11 ± 0.24 b | 2.31 ± 0.21 b | 68.12 ± 0.47 b | 0.61 ± 0.01 c | 3.98 ± 0.03 a |
| Infusion | na | na | 0.35 ± 0.08 d | 0.13 ± 0.01 d | 3.90 ± 0.01 b | |
| Stem barks | MeOH | 2.91 ± 0.07 a | 6.56 ± 0.34 a | 69.84 ± 0.35 a | 0.77 ± 0.01 a | na |
| Infusion | 1.88 ± 0.11 b | na | 51.55 ± 0.24 c | 0.74 ± 0.02 b | na |
Values are reported as mean ± SD of three parallel measurements. GALAE: Galantamine equivalents; KAE: Kojic acid equivalents; ACAE: Acarbose equivalents; na: not active: Different letters in the same column indicate significant differences in the tested extracts (p < 0.05).
Because enzymes in the human body contribute to the genesis of disease, inhibiting these enzymes can be advantageous in health care. Cholinesterase inhibitors, for example, are drugs that prevent the breakdown of acetylcholine, a neurotransmitter in the central nervous system that, when present in excessive concentrations, can cause neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease [43]. Our study explored the anti-cholinesterase activity in various extracts of F. sur. High anti-AChE and anti-BChE activities were recorded with the methanolic stem bark extract (2.91 ± 0.07 and 6.56 ± 0.34 mg GALAE/g, respectively). However, the aqueous leaves extract was inactive against AChE and BChE.
Inhibitors of α-amylase and α-glucosidase diminish carbohydrate digestion in the small intestine and, as a result, lower postprandial blood glucose levels, making them an essential therapy option for type II diabetes patients [44]. The methanolic stem bark extract of F. sur was observed to substantially depress α-amylase (0.77 ± 0.01 mmol ACAE/g) but was found to be inactive against α-glucosidase. Instead, the methanolic leaves extract showed high anti-glucosidase activity (3.98 ± 0.03 mmol ACAE/g).
Tyrosinase inhibitors help to protect the skin and prevent hyperpigmentation. They are strongly promoted by the pharmaceutical and cosmetics industries [45]. The methanolic stem bark extract displayed the strongest anti-tyrosinase activity (69.84 ± 0.35 mg KAE/g), while the aqueous leaves extract showed the lowest activity (0.35 ± 0.08 mg KAE/g). It is noteworthy that the methanolic stem bark extract showed the highest activity against four enzymes, namely AChE, BChE, tyrosinase, and α-amylase, although, the extract did not show the highest TFC and TPC.
2.4. Cytotoxicity Evaluation
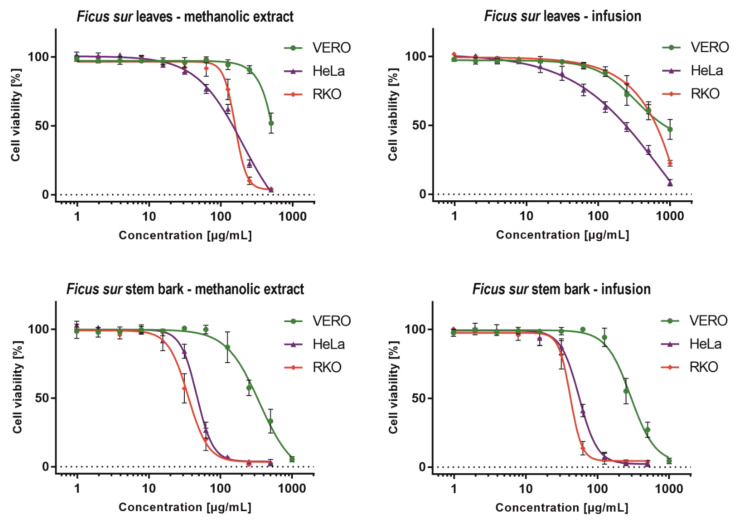
Cytotoxicity evaluation revealed that the infusion and methanolic extract from Ficus sur leaves exerted low toxicity on normal kidney fibroblasts (VERO); the exact CC50 values could not be evaluated because they were above the tested concentration range (Table 5). Stem bark extracts showed a similar effect on VERO cells. Selective toxicity towards HeLa cancer cells was observed for Ficus sur leaves methanolic extract (FLM) and infusion (FLI) with SI of >3.62 and >2.36. In contrast, in the case of RKO, only FLM showed selective toxicity (SI > 3.13). Significant antineoplastic activity towards both cancer cell lines was observed (Figure 2) for Ficus sur stem bark methanolic extract (FSBM) and infusion (FSBI) with CC50 values ranging from 36.8 to 56.12 µg/mL. The anticancer selectivity of FSBM and FSBI towards HeLa cells was 7.1 and 9.24, respectively, whereas against RKO, it was found to be 5.37 and 7.01, respectively. Multiple studies describe the anticancer potential of Ficus spp, ex. Ficus carica [46,47], Ficus salicifolia [46], Ficus religiosa [48], Ficus beecheyana [49], Ficus pandurata H [50] and Ficus exasperata (Vahl) [51], against various cancer cell lines, however, to the best of our knowledge, this is the first report showing Ficus sur stem bark extracts as a possible source of antineoplastic molecules.
Table 5.
Results of cytotoxicity evaluation.
| Ficus sur | Solvent | CC50 ± SD (µg/mL) | |||
|---|---|---|---|---|---|
| VERO | HeLa | RKO | |||
| Leaves | MeOH | FLM | >500 | 138.3 ± 3.78 | 159.97 ± 12.3 |
| Infusion | FLI | >500 | 212.0 ± 19.45 | 594.23 ± 41.0 | |
| Stem bark | MeOH | FSBM | 340.1 ± 22.72 | 47.89 ± 0.27 | 36.8 ± 4.92 |
| Infusion | FSBI | 301.12 ± 30.31 | 56.12 ± 1.89 | 42.96 ± 4.94 | |
Figure 2.
Influence of Ficus sur extracts on normal and cancer cells.
2.5. Antiviral Potential
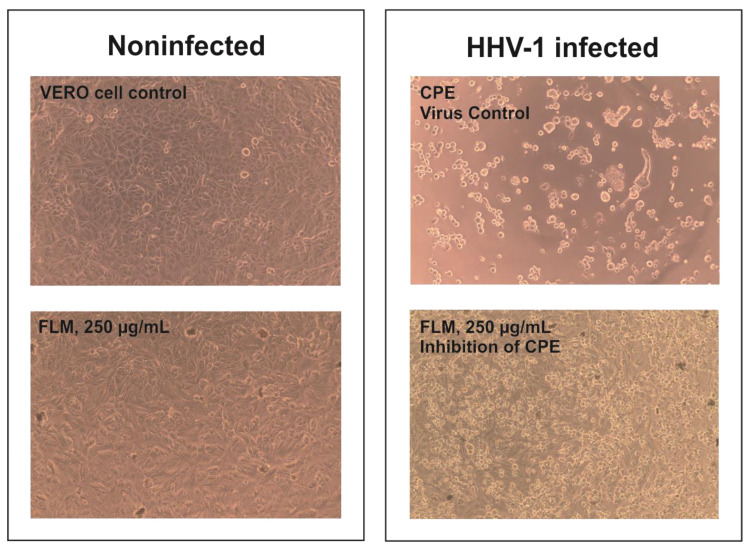
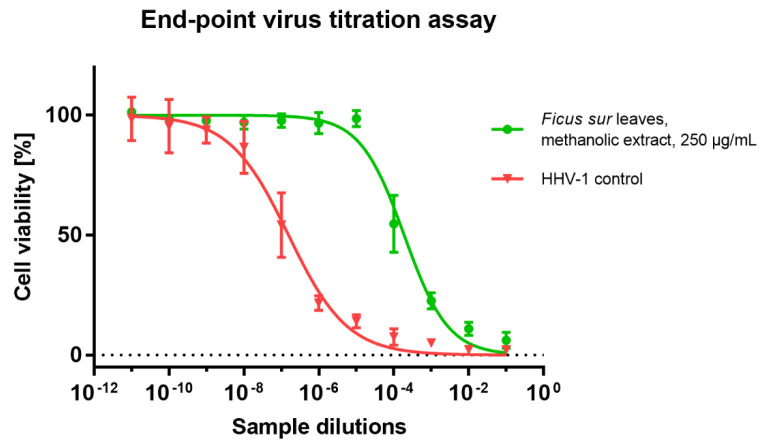
The Ficus sur extracts were incubated with an HHV-1 infected VERO cell line to evaluate the antiviral potential. After CPE was found in the virus control cells, the influence on CPE was observed in extract-treated infected cells. It was found that only one extract, namely FLM at 250 µg/mL, decreased, but did not abolish altogether, CPE formation, as can be seen in Figure 3. The collected samples were further subjected to an end-point dilution assay to evaluate the infectious titer of HHV-1. The data on HHV-1 titer reduction contained in Table 6 confirmed that FLM 250 µg/mL exerted antiviral activity, decreasing the infectious titer by 2.86 log. However, since it is generally agreed that the tested sample should reduce the infectious titer by at least 3 log to show significant antiviral potential, FLM cannot be regarded as such. However, plant extracts are complex mixtures of compounds belonging to various groups of secondary metabolites, and the biological activity of such extracts depends on their composition, and the relative amount of particular substances and possible biological interactions (ex. synergism or antagonism). One of the end-point dilution assays performed for virus-infected cells treated is presented in Figure 4; in this particular experiment, the reduction of HHV-1 titer was 3.1 log. Considering this, the reported results can be regarded as interesting, and the observed antiviral activity will be further evaluated to elucidate the compounds responsible.
Figure 3.
Influence of Ficus sur leaves methanolic extract on HHV-1 generated cytopathic effect.
Table 6.
Abatement of HHV-1 infectious titer in response to Ficus sur treatment.
| Ficus sur | Solvent | Concentration (µg/mL) |
Decrease of HHV-1 Infectious Titer (Δlog *) |
|---|---|---|---|
| Leaves | MeOH | 250 | 2.86 ± 0.17 |
| 125 | 1.03 ± 0.23 | ||
| Infusion | 100 | 0.52 ± 0.19 | |
| 50 | 0.09 ± 0.16 | ||
| Stem bark | MeOH | 125 | 0.12 ± 0.06 |
| 62.5 | 0.07 ± 0.25 | ||
| Infusion | 125 | 0.92 ± 0.52 | |
| 62.5 | 0.35 ± 0.13 |
* Δlog (mean ± SD)–calculated from separate titration assays; Δlog = logCCID50VC–logCCID50E; VC–virus control; E–extract, Δlog ≥ 3 is regarded as significant.
Figure 4.
Titration assay of HHV-1 in the sample treated with Ficus sur leaves methanolic extract.
We have previously reported that Oenanthe aquatica and Oenanthe silaifolia extracts possess significant antiviral activity, and the observed effect may be related to the presence of caffeic acid and its derivatives (caffeic acid glucoside, chlorogenic acid, cryptochlorogenic acid, and neochlorogenic acid) present in those extracts [52]. Interestingly, caffeic acid derivatives were identified in the FLM, which showed the highest anti-HHV-1 activity, and in FSBI, which exerted a noticeable, though much lower, influence on the tested herpes virus, reducing the infectious titer only by 0.92 log. Furthermore, methyl gallate, detected exclusively in the FLM, was proven to be a potent and specific inhibitor of HHV-2 [53]. Additionally, FLM was the only extract showing the presence of 5,8-dihydroxy-7-methoxyflavone-O-glucoside-rhamnoside; there are reports of antiviral activity of some flavone compounds ex. 5,7-dihydroxy-3,4′-dimethoxyflavone (ermanin) and 5,7,4′-trihydroxy-3-methoxyflavone (isokaempferide) against polio [54] or 5,7,4′-trihydroxy-8-methoxyflavone against influenza virus [55], while 5-hydroxy-7-methoxyflavone and 5,7-dimethoxyflavone were found to be protease inhibitors active against HIV-1, HCV, and HCMV (Human cytomegalovirus, HHV-5, CMV) at micromolar concentrations [56]. The kaempferol-O-glucoside present in FLM was also isolated from Securigera securidaca and reported to inhibit HHV-1 attachment to the cell membrane, virus entry and viral polymerase [57], and showed potent anti-HIV-1 reverse transcriptase activity [58]. Flavone glycosides, namely quercetin-3-O-rutinoside, kaempferol-3-O-rutinoside and kaempferol-3-O-robinobioside, were reported by Yarmolinsky et al. [59] as being responsible for the antiviral potential of Ficus benjamina. Interestingly, isolated glycosides exerted significant antiviral activity against HHV-1 and HHV-2, especially when added to infected cells during and after infection, but no activity was found against HHV-3 (varicella-zoster virus, VZV). Flavone aglycones, kaempferol and quercetin, obtained as standards, showed significantly lower activity [59]. Finally, FLM was the only extract that showed the presence of (epi)-afzelechin-7-O-glucoside, and of note, ent-epi-afzelechin-(4-8)-epiafzelechin was reported to inhibit HHV-2 by disrupting virus penetration and interfering with late stages of the viral replication cycle [60].
3. Materials and Methods
3.1. Plant Materials and Preparation of Extracts
Ficus sur samples were collected in the village of Prikro (city of Brobo, Côte d’Ivoire), in January 2020. The species was identified by a plant taxonomist at the National Floristic Center (Universite Felix Houphouet Boigny, Abidjan, Côte d’Ivoire). Voucher specimens were deposited at the herbarium of the above-mentioned center. The leaves and stem barks of the plant samples were dried in shade conditions at room temperature for about one week. Then, the samples were powdered with a mill and stored in dark conditions.
Different solvents (methanol and water) were used to obtain the extracts in this study. Maceration was used as the extraction method for methanol extracts. In addition, the infusion was prepared. For the maceration, the plant materials (10 g) were macerated with 200 mL methanol at room temperature overnight. After that, the mixtures were filtered, and the solvents were evaporated. In preparing the water extracts, the plant materials (10 g) were kept with 200 mL boiled water for 15 min and then filtered. Water extracts were lyophilized, and all extracts were stored at 4 °C until analysis.
3.2. Chromatographic Conditions
The separation was performed on a C18 Gemini® column (3 µm i.d. with TMS end-capping, 110 Å, 100 × 2 mm) supported with a guard column (Phenomenex Inc, Torrance, CA, USA), at a flow rate of 0.2 mL/min under a gradient program operated by Agilent 1200 Infinity HPLC (Agilent Technologies, Santa Clara, CA, USA). Solvent A was water with 0.1% formic acid (v/v), whereas solvent B was 0.1% formic acid in acetonitrile (v/v). Both solvents were mixed according to the following program: 0–60% B for 45 min., next 60–95% B for 1 min., and 95% B for 4 min. The stop time was at 50 min. 10 μL of the sample was injected into a thermostated (20 °C) chromatographic column.
3.3. Detection Conditions
Mass spectra were acquired by the Agilent 6530B QTOF Accurate-Mass QTOF system equipped with Dual Agilent Jet Stream spray source (ESI) (Agilent Technologies, Santa Clara, CA, USA) connected with N2 generator (Parker Hannifin Corporation, Haverhill, MA; generating N2 at purities >99%). Negative ion mode was applied for MS and MS/MS acquisition with drying gas temp: 275 °C, drying gas flow: 10 L/min, sheath gas temp: 325 °C, sheath gas flow: 12 L/min; nebulizer pressure: 35 psig, capillary V (+): 4000 V, skimmer 65 V, fragmentor 140 V. Two spectra per sec were recorded in a range between 100 and 1000 m/z with a collision energy of 10 and 40 eV. The identification of compounds was based on fragmentation patterns and supported by a comparison of obtained mass spectra with those available in databases and the scientific literature.
3.4. Total Phenolic and Flavonoid Content
Total levels of phenolics and flavonoids were assessed based on previously reported methods [61,62]. Total phenolic levels were expressed as mg gallic acid equivalents (GAE)/g dry extract, and mg rutin equivalents (RE)/g dry extract was used to evaluate total flavonoids. All experimental details are given in the Supplementary Materials. The experiments were performed in triplicate, and the results were assessed by ANOVA assays (Tukey’s test).
3.5. Antioxidant and Enzyme Inhibitory Assays
In the current investigation, the antioxidant effects of the tested extracts were detected by different assays [61]. The assays were: [1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline) 6-sulfonic acid (ABTS) radical scavenging, cupric ion reducing antioxidant capacity (CUPRAC), ferric ion reducing antioxidant power (FRAP), metal chelating ability (MCA) and phosphomolybdenum assay (PDA)]. For DPPH, ABTS, CUPRAC and FRAP assays, data were expressed as mg Trolox equivalents (TE)/g extract, whereas in MCA and PDA, mg EDTA equivalents (EDTAE)/g extract and mmol TE/g extract, respectively, were used. The experimental details for acetylcholinesterase, butyrylcholinesterase, tyrosinase, amylase and glucosidase assays were previously provided. Galanthamine was used as a positive control in cholinesterase assays, and data were evaluated as mg galanthamine equivalents (GALAE)/g extract. Kojic acid was used as a standard inhibitor in tyrosinase inhibitory assay, and the results were expressed as mg kojic acid equivalents (KAE)/g extract [61,62]. Acarbose was selected as an inhibitor of both amylase and glucosidase in the antidiabetic assays, and the results are given as mmol acarbose equivalents (ACAE)/g extract. All experimental details are given in the Supplementary Materials. The assays were performed in triplicates, and the differences in the extracts were evaluated by ANOVA (Tukey’s test).
3.6. Cytotoxicity Testing
The evaluation of cytotoxicity was performed against normal kidney fibroblasts (VERO) and cancer cell lines derived from cervical adenocarcinoma (HeLa) and colon cancer (RKO) using microculture tetrazolium assay (MTT) as previously described [52]. Briefly, the cell monolayers were incubated with serial dilutions of the tested extracts for 72 h, and then cellular viability was assessed using the MTT protocol. Details can be found in the Supplementary Materials. The collected data were analyzed using GraphPad Prism to calculate the CC50 values (50% cytotoxic concentration). Additionally, selectivity indexes (SI) were calculated by comparing CC50 values obtained for VERO with those observed for cancer cells (SI = CC50VERO/CC50Cancer, SI > 1 indicates selectivity towards cancer cells).
3.7. Evaluation of Antiviral Potential
The extracts in non-toxic concentrations were tested for their influence on HHV-1 replication in the virus-infected VERO cells after 72 h incubation as previously described [52]. Briefly, the monolayer of VERO cells was treated with HHV-1 (100-fold CCID50, CCID50–50% cell culture infections dose) for 1 h, followed by washing with PBS (phosphate-buffered saline) and further incubated until a cytopathic effect (CPE) was recorded in the virus control (VC). Subsequently, after three cycles of freezing (−72 °C) and thawing, the HHV-1 infectious titer in the collected samples was measured using an end-point titration assay. Finally, the HHV-1 titer (Δlog) difference was calculated (Δlog = logCCID50VC–logCCID50FE, FE-Ficus extract). The difference of ≥3 log is regarded as significant.
4. Conclusions
In conclusion, the F. sur methanolic stem bark extract demonstrated substantial in vitro antioxidant potential with DPPH, ABTS, and CUPRAC assays, but not with FRAP, metal-chelating and phosphomolybdenum assays. The methanolic stem bark extract significantly depressed tyrosinase, α-amylase, AChE and BChE activity. To date, no evidence of enzyme inhibitory actions of Ficus members has been discovered. In this regard, the presented work is the first scientific demonstration of the enzyme inhibitory effects of F. sur extracts, and it may offer a substantial contribution to the scientific platform. Herein, we would like to report that the F. sur leaves methanolic extract exerted noticeable, but limited, antiviral activity against HHV-1, diminishing CPE development and reducing the virus titer by 2.86 log. Furthermore, antineoplastic activity against cervical adenocarcinoma and colon cancer cell lines was observed for stem bark infusion and methanolic extract. However, more study, including in vivo and clinical investigations, is needed to further examine these aforementioned properties to incorporate this traditional herb as a possible therapeutic element.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27061863/s1.
Author Contributions
Conceptualization, E.S., Ł.Ś. and G.Z.; methodology, E.S., Ł.Ś., K.I.S., G.Z., A.B., M.P.-D. and O.K.E.; software, E.S. and Ł.Ś.; validation, K.I.S. and G.Z.; formal analysis, G.Z.; investigation, E.S., K.I.S., G.Z., N.B.S. and M.F.M.; resources, G.Z. and O.K.E.; data curation, E.S., Ł.Ś. and G.Z.; writing—original draft preparation, E.S., Ł.Ś., N.B.S. and M.F.M.; writing—review and editing, G.Z.; visualization, Ł.Ś.; supervision, E.S.; project administration, G.Z.; funding acquisition, E.S. and Ł.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petrovska B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo S., Nam H., Lee D. Phenotype-oriented network analysis for discovering pharmacological effects of natural compounds. Sci. Rep. 2018;8:11667. doi: 10.1038/s41598-018-30138-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stierle A., Strobel G., Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 4.Murugesu S., Selamat J., Perumal V. Phytochemistry, Pharmacological Properties, and Recent Applications of Ficus benghalensis and Ficus religiosa. Plants. 2021;10:2749. doi: 10.3390/plants10122749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [(accessed on 12 December 2021)]. Available online: http://www.worldfloraonline.org/
- 6.Clark A.M. Natural products as a resource for new drugs. Pharm Res. 1996;13:1133–1144. doi: 10.1023/A:1016091631721. [DOI] [PubMed] [Google Scholar]
- 7.Esievo K.B., Anthony S.O., Fatokun O.T., Kunle O.F. Ficus capensis Thumb. (Moraceae): Review of its ethnomedicinal uses, pharmacological activities and phytochemical constituents. Arch. Curr. Res. Int. 2018;12:1–7. doi: 10.9734/ACRI/2018/39495. [DOI] [Google Scholar]
- 8.Lansky E.P., Paavilainen H.M. Figs: The Genus Ficus. CRC Press; Boca Raton, FL, USA: 2010. [Google Scholar]
- 9.Sabiu S., O'Neill F.H., Ashafa A.O.T. The Purview of Phytotherapy in the Management of Kidney Disorders: A Systematic Review on Nigeria and South Africa. Afr J. Tradit. Complement. Altern. Med. 2016;13:38–47. doi: 10.21010/ajtcam.v13i5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyi M. Ethnobotanical investigation of traditional medicinal plants in dugda district, Oromia Regio. SM J. Med. Plant Stud. 2018;2:1007. [Google Scholar]
- 11.Regassa R. Diversity and conservation status of some economically valued indigenous medicinal plants in Hawassa College of Teacher Education Campus, Southern Ethiopia. Int. J. Adv. Res. 2013;1:308–328. [Google Scholar]
- 12.Ayele M., Makonnen E., Ayele A.G., Tolcha Y. Evaluation of the Diuretic Activity of the Aqueous and 80% Methanol Extracts of Ficus sur Forssk (Moraceae) Leaves in Saline-loaded Rats. J. Exp. Pharmacol. 2020;12:619. doi: 10.2147/JEP.S283571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishola I.O., Olayemi S.O., Yemitan O.K., Ekpemandudiri N.K. Mechanisms of anticonvulsant and sedative actions of the ethanolic stem-bark extract of Ficus sur Forssk (Moraceae) in rodents. Pak. J. Biol. Sci. 2013;16:1287–1294. doi: 10.3923/pjbs.2013.1287.1294. [DOI] [PubMed] [Google Scholar]
- 14.Diba D., Mekasha Y., Urge M., Tolera A. Feed intake, digestibility, growth performance, and blood profile of pigs fed mixtures of dried and ground fig (Ficus sur) fruits and graded levels of maize. Trop. Anim. Health Prod. 2015;47:339–346. doi: 10.1007/s11250-014-0725-z. [DOI] [PubMed] [Google Scholar]
- 15.Yarmolinsky L., Zaccai M., Ben-Shabat S., Mills D., Huleihel M. Antiviral activity of ethanol extracts of Ficus binjamina and Lilium candidum in vitro. New Biotechnol. 2009;26:307–313. doi: 10.1016/j.nbt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Camero M., Marinaro M., Lovero A., Elia G., Losurdo M., Buonavoglia C., Tempesta M. In vitro antiviral activity of Ficus carica latex against caprine herpesvirus-1. Nat. Prod. Res. 2014;28:2031–2035. doi: 10.1080/14786419.2014.918120. [DOI] [PubMed] [Google Scholar]
- 17.Lazreg Aref H., Gaaliche B., Fekih A., Mars M., Aouni M., Pierre Chaumon J., Said K. In vitro cytotoxic and antiviral activities of Ficus carica latex extracts. Nat. Prod. Res. 2011;25:310–319. doi: 10.1080/14786419.2010.528758. [DOI] [PubMed] [Google Scholar]
- 18.Huang N.-C., Hung W.-T., Tsai W.-L., Lai F.-Y., Lin Y.-S., Huang M.-S., Chen J.-J., Lin W.-Y., Weng J.-R., Chang T.-H. Ficus septica plant extracts for treating Dengue virus in vitro. PeerJ. 2017;5:e3448. doi: 10.7717/peerj.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cagno V., Civra A., Kumar R., Pradhan S., Donalisio M., Sinha B.N., Ghosh M., Lembo D. Ficus religiosa L. bark extracts inhibit human rhinovirus and respiratory syncytial virus infection in vitro. J. Ethnopharmacol. 2015;176:252–257. doi: 10.1016/j.jep.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh M., Civra A., Rittà M., Cagno V., Mavuduru S.G., Awasthi P., Lembo D., Donalisio M. Ficus religiosa L. bark extracts inhibit infection by herpes simplex virus type 2 in vitro. Arch. Virol. 2016;161:3509–3514. doi: 10.1007/s00705-016-3032-3. [DOI] [PubMed] [Google Scholar]
- 21.Khairunisa S.Q., Indriati D.W., Tumewu L., Widyawaruyanti A., Nasronudin N. Screening of anti-HIV activities in ethanol extract and fractions from Ficus fistulosa leaves. J. Basic Clin. Physiol. Pharmacol. 2021;32:737–742. doi: 10.1515/jbcpp-2020-0413. [DOI] [PubMed] [Google Scholar]
- 22.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q.-W., Lin L.-G., Ye W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elhawary S.S., Younis I.Y., El Bishbishy M.H., Khattab A.R. LC–MS/MS-Based chemometric analysis of phytochemical diversity in 13 Ficus spp. (Moraceae): Correlation to their in vitro antimicrobial and in silico quorum sensing inhibitory activities. Ind. Crops Prod. 2018;126:261–271. doi: 10.1016/j.indcrop.2018.10.017. [DOI] [Google Scholar]
- 25.Farag M.A., Abdelfattah M.S., Badr S.E., Wessjohann L.A. Profiling the chemical content of Ficus lyrata extracts via UPLC-PDA-qTOF-MS and chemometrics. Nat. Prod. Res. 2014;28:1549–1556. doi: 10.1080/14786419.2014.926353. [DOI] [PubMed] [Google Scholar]
- 26.Clifford M.N., Wu W., Kuhnert N. The chlorogenic acids of Hemerocallis. Food Chem. 2006;95:574–578. doi: 10.1016/j.foodchem.2005.01.045. [DOI] [Google Scholar]
- 27.Fotirić Akšić M., Dabić Zagorac D., Sredojević M., Milivojević J., Gašić U., Meland M., Natić M. Chemometric characterization of strawberries and blueberries according to their phenolic profile: Combined effect of cultivar and cultivation system. Molecules. 2019;24:4310. doi: 10.3390/molecules24234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gevrenova R., Zengin G., Sinan K.I., Yıldıztugay E., Zheleva-Dimitrova D., Picot-Allain C., Mahomoodally M.F., Imran M., Dall’Acqua S. UHPLC-MS Characterization and biological insights of different solvent extracts of two Achillea species (A. aleppica and A. santolinoides) from Turkey. Antioxidants. 2021;10:1180. doi: 10.3390/antiox10081180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guimarães R., Barros L., Dueñas M., Carvalho A.M., Queiroz M.J.R., Santos-Buelga C., Ferreira I.C. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013;141:3721–3730. doi: 10.1016/j.foodchem.2013.06.071. [DOI] [PubMed] [Google Scholar]
- 30.Human Metabolome Database (HMDB) 2021. [(accessed on 31 July 2021)]. Available online: https://hmdb.ca/
- 31.MassBank of North America (MoNA) Database. 2021. [(accessed on 31 July 2021)]. Available online: https://mona.fiehnlab.ucdavis.edu/
- 32.Metlin Database. 2021. [(accessed on 31 July 2021)]. Available online: https://metlin.scripps.edu/
- 33.PubChem. [(accessed on 31 July 2021)];2021 Available online: https://pubchem.ncbi.nlm.nih.gov/
- 34.Simirgiotis M.J. Antioxidant capacity and HPLC-DAD-MS profiling of Chilean Peumo (Cryptocarya alba) fruits and comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules. 2013;18:2061–2080. doi: 10.3390/molecules18022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh A., Kumar S., Kumar B. LC-MS Identification of Proanthocyanidins in Bark and Fruit of six Terminalia species. Nat. Prod. Commun. 2018;13:1934578X1801300511. doi: 10.1177/1934578X1801300511. [DOI] [Google Scholar]
- 36.Świątek Ł., Sieniawska E., Sinan K.I., Maciejewska-Turska M., Boguszewska A., Polz-Dacewicz M., Senkardes I., Guler G.O., Bibi Sadeer N., Mahomoodally M.F. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021;22:7621. doi: 10.3390/ijms22147621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zengin G., Mahomoodally M.F., Picot-Allain M.C.N., Sinan K.I., Ak G., Etienne O.K., Sieniawska E., Maciejewska-Turska M., Świątek Ł., Rajtar B. Chemical composition, biological properties and bioinformatics analysis of two Caesalpina species: A new light in the road from nature to pharmacy shelf. J. Pharm. Biomed. Anal. 2021;198:114018. doi: 10.1016/j.jpba.2021.114018. [DOI] [PubMed] [Google Scholar]
- 38.Bibi Sadeer N., Montesano D., Albrizio S., Zengin G., Mahomoodally M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants. 2020;9:709. doi: 10.3390/antiox9080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D.O., Lee K.W., Lee H.J., Lee C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Z., Li Y. Reducing power: The measure of antioxidant activities of reductant compounds? Redox Rep. 2004;9:213–217. doi: 10.1179/135100004225005994. [DOI] [PubMed] [Google Scholar]
- 41.Segura Campos M.R., Ruiz Ruiz J., Chel-Guerrero L., Betancur Ancona D. Coccoloba uvifera (L.) (Polygonaceae) Fruit: Phytochemical Screening and Potential Antioxidant Activity. J. Chem. 2015;2015:534954. doi: 10.1155/2015/534954. [DOI] [Google Scholar]
- 42.Abdel-Hameed E.-S.S., Bazaid S.A., Salman M.S. Characterization of the Phytochemical Constituents of Taif Rose and Its Antioxidant and Anticancer Activities. BioMed Res. Int. 2013;2013:345465. doi: 10.1155/2013/345465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colović M.B., Krstić D.Z., Lazarević-Pašti T.D., Bondžić A.M., Vasić V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tundis R., Loizzo M.R., Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010;10:315–331. doi: 10.2174/138955710791331007. [DOI] [PubMed] [Google Scholar]
- 45.Deri B., Kanteev M., Goldfeder M., Lecina D., Guallar V., Adir N., Fishman A. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016;6:34993. doi: 10.1038/srep34993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AlGhalban F.M., Khan A.A., Khattak M.N.K. Comparative anticancer activities of Ficus carica and Ficus salicifolia latex in MDA-MB-231 cells. Saudi J. Biol. Sci. 2021;28:3225–3234. doi: 10.1016/j.sjbs.2021.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purnamasari R., Winarni D., Permanasari A.A., Agustina E., Hayaza S., Darmanto W. Anticancer activity of methanol extract of Ficus carica leaves and fruits against proliferation, apoptosis, and necrosis in Huh7it cells. Cancer Inform. 2019;18:1176935119842576. doi: 10.1177/1176935119842576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Remadevi V., Mohan Lathika L., Sasikumar Sujatha A., Sreeharshan S. Ficus extract—A promising agent for antimammary tumorigenesis: A review on current status and future possibilities. Phytother. Res. 2019;33:1597–1603. doi: 10.1002/ptr.6348. [DOI] [PubMed] [Google Scholar]
- 49.Yen G.-C., Chen C.-S., Chang W.-T., Wu M.-F., Cheng F.-T., Shiau D.-K., Hsu C.-L. Antioxidant activity and anticancer effect of ethanolic and aqueous extracts of the roots of Ficus beecheyana and their phenolic components. J. Food Drug Anal. 2018;26:182–192. doi: 10.1016/j.jfda.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv H., Hu C., Xie Z., Wang P., Chen X., Wen C. Purification, characterization and anti-tumor activity of a pectic-type polysaccharide isolated from Ficus pandurata H. Int. J. Biol. Macromol. 2020;153:201–206. doi: 10.1016/j.ijbiomac.2020.02.244. [DOI] [PubMed] [Google Scholar]
- 51.Bafor E.E., McKenna J., Rowan E.G., Edrada-Ebel R. Characterisation of the antiproliferative constituents and activity of Ficus exasperata (Vahl) on ovarian cancer cells–a preliminary investigation. Nat. Prod. Res. 2017;31:2164–2168. doi: 10.1080/14786419.2016.1277348. [DOI] [PubMed] [Google Scholar]
- 52.Świątek Ł., Sieniawska E., Mahomoodally M.F., Sadeer N.B., Wojtanowski K.K., Rajtar B., Polz-Dacewicz M., Paksoy M.Y., Zengin G. Phytochemical Profile and Biological Activities of the Extracts from Two Oenanthe Species (O. aquatica and O. silaifolia) Pharmaceuticals. 2022;15:50. doi: 10.3390/ph15010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kane C.J., Menna J.H., Sung C.-C., Yeh Y.-C. Methyl gallate, methyl-3, 4, 5-trihydroxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci. Rep. 1988;8:95–102. doi: 10.1007/BF01128976. [DOI] [PubMed] [Google Scholar]
- 54.Robin V., Boustie J., Amoros M., Girre L. In-vitro antiviral activity of seven psiadia species, Asteraceae: Isolation of two antipoliovirus flavonoids from Psiadia dentata. Pharm. Pharmacol. Commun. 1998;4:61–64. [Google Scholar]
- 55.Nagai T., Suzuki Y., Tomimori T., Yamada H. Antiviral activity of plant flavonoid, 5, 7, 4′-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis against influenza A (H3N2) and B viruses. Biol. Pharm. Bull. 1995;18:295–299. doi: 10.1248/bpb.18.295. [DOI] [PubMed] [Google Scholar]
- 56.Sookkongwaree K., Geitmann M., Roengsumran S., Petsom A., Danielson U.H. Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Die Pharmazie—Int. J. Pharm. Sci. 2006;61:717–721. [PubMed] [Google Scholar]
- 57.Behbahani M., Shanehsazzadeh M., Shokoohinia Y., Soltani M. Evaluation of anti-herpetic activity of methanol seed extract and fractions of Securigera securidaca in vitro. J. Antivir. Antiretrovir. 2013;5:72–76. [Google Scholar]
- 58.Behbahani M., Sayedipour S., Pourazar A., Shanehsazzadeh M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res. Pharm. Sci. 2014;9:463. [PMC free article] [PubMed] [Google Scholar]
- 59.Yarmolinsky L., Huleihel M., Zaccai M., Ben-Shabat S. Potent antiviral flavone glycosides from Ficus benjamina leaves. Fitoterapia. 2012;83:362–367. doi: 10.1016/j.fitote.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Cheng H.-Y., Yang C.-M., Lin T.-C., Shieh D.-E., Lin C.-C. ent-Epiafzelechin-(4α→ 8)-epiafzelechin extracted from Cassia javanica inhibits herpes simplex virus type 2 replication. J. Med. Microbiol. 2006;55:201–206. doi: 10.1099/jmm.0.46110-0. [DOI] [PubMed] [Google Scholar]
- 61.Grochowski D.M., Uysal S., Aktumsek A., Granica S., Zengin G., Ceylan R., Locatelli M., Tomczyk M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017;20:365–372. doi: 10.1016/j.phytol.2017.03.005. [DOI] [Google Scholar]
- 62.Uysal S., Zengin G., Locatelli M., Bahadori M.B., Mocan A., Bellagamba G., De Luca E., Mollica A., Aktumsek A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017;8:290. doi: 10.3389/fphar.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.