Abstract
Antimicrobial-resistant bacteria in food animals pose a major public health threat worldwide. In this study, we aimed to assess the antimicrobial resistance profiles and resistance trends of commensal Escherichia coli isolated from the feces of healthy cattle, pigs, and chickens in South Korea during 2010 and 2020. A total of 7237 E. coli isolates (2733 cattle, 2542 pig, and 1962 chicken isolates) were tested for susceptibility towards 12 antimicrobials. About 48%, 90%, and 97% of cattle, pig, and chicken isolates, respectively, were resistant to one or more antimicrobial agents. Cattle isolates presented low resistance (<15%) to most of the tested antimicrobials. In contrast, chicken and pig isolates demonstrated a relatively high (>45%) resistance rate to ampicillin, chloramphenicol, streptomycin, and tetracycline. We observed high ciprofloxacin and nalidixic acid resistance rates in chicken (76.1% and 88.6%, respectively), isolates in pig (12.7% and 26.7%, respectively) and cattle (2.7% and 8.2%, respectively) isolates. Notably, a very small proportion of isolates (<5%) from cattle, chickens, and pigs demonstrated resistance to amoxicillin/clavulanic acid, cefoxitin, and colistin. We identified ceftiofur resistance in a small proportion of chicken (8.8%), pig (3.7%), and cattle (0.7%) isolates. We noted an increasing but fluctuating trend of ampicillin, amoxicillin/clavulanic acid, ceftiofur, cefoxitin, chloramphenicol, ciprofloxacin, and streptomycin resistance in pig isolates. Similarly, the ampicillin, ceftiofur, and chloramphenicol resistance rates were increased but fluctuated through time in chicken isolates. Overall, 56% of the isolates showed multidrug-resistant (MDR). The proportion of MDR isolates was low in cattle (17.1%); however, this proportion was high in chickens (87.1%) and pigs (73.7%). Most of the resistance patterns included streptomycin and tetracycline in pigs and cattle, and ciprofloxacin and nalidixic acid in chickens. In conclusion, this study showed high resistance of commensal E. coli isolated from major food animals in Korea to commonly used antimicrobials including critically important antimicrobials. These bacteria could not only be a resistance reservoir but also could have potential to spread this resistance through gene transfer to pathogenic bacteria. Thus, the high prevalence of antimicrobial resistance in food animals highlights the urgent need for measures to restrict and ensure the prudent use of antimicrobials in Korea.
Keywords: antimicrobial resistance, E. coli, food animals
1. Introduction
Escherichia coli is a commensal bacterium colonizing the gastrointestinal tract of humans and animals. Most strains are harmless and seldom cause disease. However, pathogenic strains, especially enterotoxigenic E. coli. have been associated with food poisoning outbreaks in humans [1]. Enterotoxigenic E. coli was responsible for about 51,000 human deaths globally in 2016, with 24,666 in Asia, 25,075 in Africa, 796 in Latin America, and 237 in the EU [2]. In the Republic of Korea (Korea), E. coli was associated with 2200 annual illnesses between 2010 and 2018 [3].
E. coli strains are potential reservoirs of antimicrobial resistance genes and are considered to be excellent indicators to monitor the general level of resistance [4]. Antimicrobial resistance in commensal bacteria such as E. coli may serve as an early warning for the development of resistance in pathogenic bacteria [5]. A recent study in Europe has shown that more than half of the E. coli isolates were resistant to at least one class of antimicrobials, including those considered critically important for humans [6]. Frequent and uncontrolled use of antimicrobials in animals and humans raises the potential risk for the selection of antimicrobial resistance in commensal bacteria such as E. coli [7]. E. coli isolates with antimicrobial resistance potential can transfer from food animals to humans, either through direct contact or indirectly through the food chain [8].
Monitoring antimicrobial resistance in commensal bacteria such as E. coli from food animals is vital to determine the emergence of antimicrobial resistance and its associated risk to humans. Our recent study demonstrated that more than 90% of E. coli isolated from broiler chickens in Korea exhibited resistance to several antimicrobials, including quinolones and extended-spectrum cephalosporins [9]. Several other studies have been conducted in Korea to assess the extent of antimicrobial resistance in E. coli isolated from food animals [10,11,12,13,14]. However, most of these studies were on a relatively small number of isolates or isolates collected over a short duration. In addition, only a few studies have looked at the antimicrobial resistance trend. The main goal of this study is to determine the antimicrobial susceptibility and the resistance trend of E. coli isolated from healthy cattle, chickens, and pigs in South Korea from 2010 to 2020.
2. Materials and Methods
2.1. Isolation and Identification of E. coli
A total of 7237 E. coli isolates (2733 isolates from cattle, 2542 from pigs, and 1962 from chickens) were obtained from 16 laboratories/centers participating in the Korean Veterinary Antimicrobial Resistance Monitoring System (Table 1).
Table 1.
E. coli isolates obtained from feces of healthy cattle, pigs, and chickens during 2010–2020 in Korea.
| Cattle | Pigs | Chickens | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | No. of Slaughter-Houses |
No. of Farms |
No. of Isolates |
No. of Slaughter-Houses |
No. of Farms |
No. of Isolates |
No. of Slaughter- Houses |
No. of Farms |
No. of Isolates |
| 2010 | 27 | 211 | 231 | 27 | 160 | 221 | 15 | 151 | 155 |
| 2011 | 29 | 322 | 347 | 26 | 195 | 231 | 14 | 135 | 141 |
| 2012 | 25 | 265 | 282 | 28 | 243 | 277 | 12 | 181 | 200 |
| 2013 | 22 | 207 | 209 | 28 | 186 | 199 | 16 | 183 | 187 |
| 2014 | 23 | 287 | 299 | 26 | 251 | 294 | 11 | 190 | 192 |
| 2015 | 23 | 204 | 206 | 24 | 204 | 218 | 13 | 177 | 189 |
| 2016 | 27 | 365 | 401 | 26 | 296 | 347 | 15 | 281 | 303 |
| 2017 | 26 | 260 | 263 | 28 | 244 | 262 | 13 | 133 | 137 |
| 2018 | 24 | 177 | 178 | 25 | 171 | 189 | 21 | 162 | 163 |
| 2019 | 27 | 152 | 152 | 21 | 136 | 139 | 22 | 138 | 143 |
| 2020 | 21 | 162 | 165 | 19 | 163 | 165 | 19 | 146 | 152 |
| Total | 83 | 2478 | 2733 | 85 | 2039 | 2542 | 60 | 1606 | 1962 |
E. coli was isolated from fecal samples collected from 228 slaughterhouses during 2010–2020. The animals were delivered to the slaughterhouses from 6123 farms (≤5 samples per farm). The authors do not have information about the history of antimicrobial use in the farms, the number of animals, or the number of samples considered for this study. E. coli strains were isolated and identified as previously described [15]. Isolates were then confirmed by matrix-assisted laser desorption and ionization-time-of-flight mass spectrometry (MALDI-TOF, Biomerieux, Marcy L’Etoile, France). Only a single isolate per sample was considered for antimicrobial susceptibility testing.
2.2. Antimicrobial Susceptibility
Antimicrobial susceptibility was carried out by the broth microdilution method [1] using the commercially available Sensititre plates KRVP5F (Thermo Trek Diagnostics, Waltham, MA, USA). The isolates were tested for susceptibility toward 12 antimicrobials: amoxicillin/clavulanic acid, ampicillin, cefoxitin, ceftiofur, chloramphenicol, ciprofloxacin, colistin, gentamicin, nalidixic acid, streptomycin, tetracycline, and trimethoprim/sulfamethoxazole. E. coli ATCC 25922 and E. coli ATCC 35218 were used as quality control strains. The resulting minimum inhibitory concentration (MICs) values were interpreted according to the CLSI [16], the National Antimicrobial Resistance Monitoring System [17], and the European Committee on Antimicrobial Susceptibility Testing [18] guidelines. The MIC50 and MIC90 were calculated as the MIC that inhibited 50% and 90% of the isolates, respectively. Multi-drug resistance (MDR) was defined as resistance to three or more antimicrobial subclasses.
2.3. Statistical Analysis
The analysis of the antimicrobial resistance rates and Pearson correlation were conducted using Excel (Microsoft-Excel, 2016, Microsoft Corporation, Redmond, WA, USA) and Rex software (Version 3.0.3, RexSoft Inc., Seoul, Korea). p values less than 0.05 were considered significant.
3. Results
3.1. Antimicrobial Resistance Rate
In general, the antimicrobial resistance rate of E. coli isolated from chickens and pigs was significantly (p ≤ 0.0001) higher than that of cattle (Table 2). More than 60% of the pig and chicken isolates were resistant to ampicillin (64.1% and 72.7%), streptomycin (68.6% and 63.0%), and tetracycline (74.0% and 73.9 %, respectively). Cattle isolates presented low resistance (0.3–11.7%) to the tested antimicrobials except for streptomycin (39.2%) and tetracycline (41.4%). Antimicrobial resistance varied significantly (p ≤ 0.0001) among animal species. We observed, higher ciprofloxacin and nalidixic acid resistance rates in isolates from chicken (76.1% and 88.6%, respectively) than isolates from pigs (12.7% and 26.7%, respectively) and cattle (2.7% and 8.2%, respectively). In addition, significantly (p ≤ 0.0001) high resistance rate to chloramphenicol was observed in isolates from pigs (67.3%) than in cattle (10.2%) and chickens (45.6%). Moreover, resistance pattern screening among antimicrobials showed that a very small proportion of isolates (<5%) from cattle, chicken, and pigs demonstrated resistance against amoxicillin/clavulanic acid, cefoxitin, and colistin, while, isolates from pigs (38.6%) and chickens (42.2%) were more resistant against trimethoprim/sulfamethoxazole as compared to cattle (6.4%). Of note, we have identified 8.8%, 3.7%, and 0.7% of ceftiofur resistance in isolates from chickens, pigs, and cattle, respectively. The MIC50 and MIC90 values of the tested antimicrobials are summarized in Tables S1–S3.
Table 2.
Antimicrobial resistance of E. coli isolated from healthy cattle, pigs, and chickens during 2010–2020 in Korea (n = 7237).
| % (No. of Resistant Isolates) | p-Value | ||||
|---|---|---|---|---|---|
| Antimicrobials | Cattle (n = 2733) |
Pigs (n = 2542) |
Chickens (n = 1962) |
Total (n = 7237) |
|
| Amoxicillin/clavulanic acid | 0.6 (16) | 1.2 (30) | 3.3 (65) | 1.5 (111) | ≤0.0001 |
| Ampicillin | 11.7 (320) | 64.1 (1630) | 72.7 (1427) | 46.7 (3377) | ≤0.0001 |
| Cefoxitin | 0.6 (17) | 1.5 (37) | 3.7 (73) | 1.8 (127) | ≤0.0001 |
| Ceftiofur | 0.7 (19) | 3.7 (93) | 8.8 (172) | 3.9 (284) | ≤0.0001 |
| Chloramphenicol | 10.2 (279) | 67.3 (1712) | 45.6 (895) | 39.9 (2886) | ≤0.0001 |
| Ciprofloxacin | 2.7 (75) | 12.7 (322) | 76.1 (1493) | 26.1 (1890) | ≤0.0001 |
| Colistin | 0.3 (9) | 0.8 (20) | 1.1 (21) | 0.7 (50) | 0.0021 |
| Gentamicin | 2.4 (65) | 16.0 (407) | 16.4 (321) | 11.0 (793) | ≤0.0001 |
| Nalidixic acid | 8.2 (225) | 26.7 (678) | 88.6 (1738) | 36.5 (2641) | ≤0.0001 |
| Streptomycin | 39.2 (1070) | 68.6 (1743) | 63.0 (1236) | 55.9 (4049) | ≤0.0001 |
| Tetracycline | 41.4 (1131) | 74.0 (1881) | 73.9 (1450) | 61.7 (4462) | ≤0.0001 |
| Trimethoprim/sulfamethoxazole | 6.4 (175) | 38.6 (980) | 42.2 (828) | 27.4 (1983) | ≤0.0001 |
| MDR | 17.1 (466) | 73.7 (1874) | 87.1 (1709) | 55.9 (4049) | ≤0.0001 |
p < 0.05 was considered significant change in antibiotic resistance trend. MDR, multi-drug resistant (resistant to at least three antimicrobial subclasses).
3.2. Antimicrobial Resistance Trends
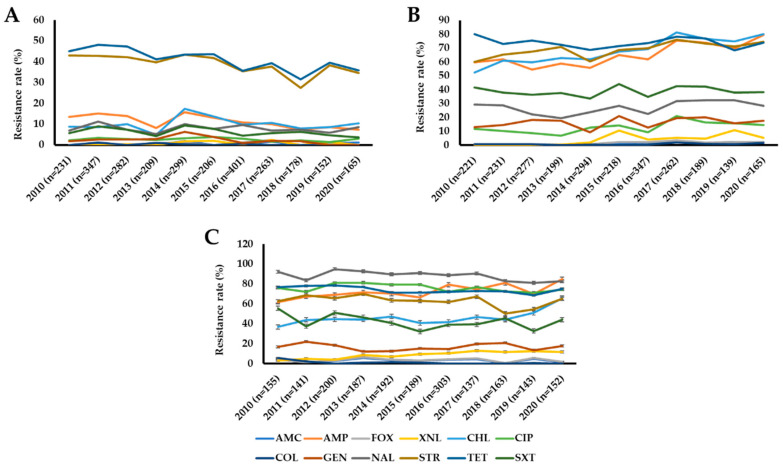
The antimicrobial resistance trend varied significantly (p < 0.05) among isolates recovered from cattle, pigs, and chickens (Figure 1, Tables S1–S3).
Figure 1.
Antimicrobial resistance trends of E. coli isolates recovered from cattle (A), pigs (B), and chickens (C) in Korea from 2010 to 2020. Abbreviations: AMC, amoxicillin/clavulanic acid; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; COL, colistin; FOX, cefoxitin; GEN, gentamicin; NAL, nalidixic acid; STR, streptomycin; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline; XNL, ceftiofur.
The antimicrobial resistance rate screening throughout the study period showed that the isolates from cattle maintained their resistance rate below 20% against most of the tested antimicrobials except for streptomycin (27.5–43.5%) and tetracycline (31.5–48.1%) (Figure 1A, Table S1). However, we have observed a decreasing but fluctuating trend of ampicillin, streptomycin, and tetracycline resistance in cattle isolates. Pig isolates demonstrated an increasing but fluctuating resistance trend of several antimicrobials: ampicillin, amoxicillin/clavulanic acid, cefoxitin, ceftiofur, ciprofloxacin, and streptomycin (Figure 1B, Table S2). The ampicillin, tetracycline, chloramphenicol, and streptomycin resistance trend among pig isolates ranged from between 50% and 80% throughout the study period, while a moderate or low resistance rate was observed against the remaining antimicrobials. We also observed an increasing but fluctuating trend of ampicillin and ceftiofur resistance in chicken isolates, whereas colistin, nalidixic acid, and tetracycline resistance rates were decreased through time (Figure 1C, Table S3). Notably, resistance to chloramphenicol was dramatically increased in pig and chicken isolates from 2010 (52.5% and 36.8%, respectively) to 2020 (80.0% and 65.8%).
3.3. MDR and Antimicrobial Resistance Patterns
In the present study, 47.8% (1307/2733) of cattle isolates, 89.7% (2281/2542) of pig isolates, and 96.8% (1899/1962) of chicken isolates exhibited resistance to at least one antimicrobial agent (Table 3, Table 4 and Table 5). Overall, 56% of the isolates were multidrug-resistant. The isolates recovered from cattle showed lower MDR with 17.1% proportion as compared to isolates from chickens and pigs with a relatively higher proportion of 87.1% and 73.7%, respectively. A total of 108, 216 and 221 MDR combination patterns were observed in the cattle, pig, and chicken isolates, respectively (Tables S4–S6). Resistance to streptomycin and tetracycline was the most frequent (20.3%, 556/2733) pattern among cattle isolates (Table 3).
Table 3.
Frequent resistance patterns in E. coli isolated from healthy cattle between 2010 and 2020 in Korea (n = 2733).
| No. of Antimicrobials |
Total No. of Isolates (%) |
Most Common Pattern (No. of Isolates) |
|---|---|---|
| 0 | 1426 (52.2) | - |
| 1 | 233 (8.5) | TET (n = 122) |
| 2 | 603 (22.1) | STR TET (n = 556) |
| 3 | 219 (8.0) | NAL STR TET (n = 72) |
| 4 | 110 (4.0) | AMP CHL STR TET (n = 52) |
| 5 | 59 (2.2) | AMP CHL STR TET SXT (n = 22) |
| 6 | 40 (1.5) | AMP CHL GEN STR TET SXT (n = 10) |
| AMP CHL NAL STR TET SXT (n = 10) | ||
| 7 | 25 (0.9) | AMP CHL CIP NAL STR TET SXT (n = 15) |
| 8 | 13 (0.5) | AMP CHL CIP GEN NAL STR TET SXT (n = 11) |
| 9 | 2 (0.1) | AMP XNL CHL CIP GEN NAL STR TET SXT (n = 2) |
| 10 | 2 (0.1) | AMP FOX XNL CHL CIP GEN NAL STR TET SXT (n = 2) |
| 11 | 1 (0.04) | AMC AMP FOX XNL CHL CIP GEN NAL STR TET SXT (n = 1) |
Abbreviations: AMC, amoxicillin/clavulanic acid; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; COL, colistin; FOX, cefoxitin; GEN, gentamicin; NAL, nalidixic acid; STR, streptomycin; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline; XNL, ceftiofur).
Table 4.
Frequent resistance patterns in E. coli isolated from healthy pigs between 2010 and 2020 in Korea (n = 2542).
| No. of Antimicrobials |
Total No. of Isolates (%) |
Most Common Pattern (No. of Isolates) |
|---|---|---|
| 0 | 261 (10.3) | |
| 1 | 160 (6.3) | TET (n = 73) |
| 2 | 232 (9.1) | STR TET (n = 94) |
| 3 | 377 (14.8) | CHL STR TET (n = 92) |
| 4 | 579 (22.7) | AMP CHL STR TET (n = 305) |
| 5 | 480 (18.9) | AMP CHL STR TET SXT (n = 244) |
| 6 | 236 (9.3) | AMP CHL NAL STR TET SXT (n = 63) |
| 7 | 126 (5.0) | AMP CHL CIP NAL STR TET SXT (n = 56) |
| 8 | 64 (2.5) | AMP CHL CIP GEN NAL STR TET SXT (n = 45) |
| 9 | 20 (0.8) | AMP XNL CHL CIP GEN NAL STR TET SXT (n = 9) |
| 10 | 5 (0.2) | AMC AMP FOX CHL CIP GEN NAL STR TET SXT (n = 2) |
| AMP FOX XNL CHL CIP GEN NAL STR TET SXT (n = 2) | ||
| 11 | 2 (0.1) | AMC AMP FOX XNL CHL CIP GEN NAL STR TET SXT (n = 2) |
Abbreviations: AMC, amoxicillin/clavulanic acid; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; COL, colistin; FOX, cefoxitin; GEN, gentamicin; NAL, nalidixic acid; STR, streptomycin; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline; XNL, ceftiofur).
Table 5.
Frequent resistance patterns in E. coli isolated from healthy chickens between 2010 and 2020 in Korea (n = 1962).
| No. of Antimicrobials |
Total No. of Isolates (%) |
Most Common Pattern (No. of Isolates) |
|---|---|---|
| 0 | 63 (3.2) | |
| 1 | 63 (3.2) | NAL (n = 29) |
| 2 | 123 (6.3) | CIP NAL (n = 55) |
| 3 | 227 (11.6) | AMP CIP NAL (n = 47) |
| 4 | 272 (13.9) | AMP CIP NAL TET (n = 55) |
| 5 | 366 (18.7) | AMP CIP NAL STR TET (n = 73) |
| 6 | 359 (18.3) | AMP CIP NAL STR TET SXT (n = 104) |
| 7 | 309 (15.8) | AMP CHL CIP NAL STR TET SXT (n = 198) |
| 8 | 142 (7.2) | AMP CHL CIP GEN NAL STR TET SXT (n = 107) |
| 9 | 24 (1.2) | AMC AMP FOX XNL CHL CIP NAL STR TET (n = 7) |
| 10 | 12 (0.6) | AMC AMP FOX XNL CHL CIP NAL STR TET SXT (n = 8) |
| 11 | 2 (0.1) | AMC AMP FOX XNL CHL CIP GEN NAL STR TET SXT (n = 2) |
Abbreviations: AMC, amoxicillin/clavulanic acid; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; COL, colistin; FOX, cefoxitin; GEN, gentamicin; NAL, nalidixic acid; STR, streptomycin; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline; XNL, ceftiofur).
Resistance to ampicillin, chloramphenicol, streptomycin, and tetracycline was predominant (12.0%, 305/2542) in pig isolates (Table 4).
In addition, the major MDR pattern in chicken isolates was resistance to seven antimicrobials (10.1%, 198/1962), including ciprofloxacin and trimethoprim/sulfamethoxazole (Table 5).
4. Discussion
In food animals, E. coli strains are considered as a potential reservoir for antimicrobial resistance, and they are frequently used as a sentinel for antimicrobial resistance [19]. In this report, we studied antimicrobial resistance patterns of E. coli strains isolated from food animals. The isolates were collected using uniform methods of sampling and isolation. In addition, MICs of selected antimicrobials considered important in human and/or veterinary medicine were performed in a single central laboratory at the Animal and Plant Quarantine Agency, Korea. Resistance bacteria isolated from food animals may transfer to pathogenic bacteria and subsequently reduce the effectiveness of antimicrobials in humans [20]. Therefore, it is essential to know both the prevalence and trends of antimicrobial resistance in bacteria isolated from food sources. The findings of this study could be used to design and implement appropriate prevention and control strategies. [21].
Consistent with other reports in Korea [11,12,13,14] and other countries [20,22,23,24,25,26,27], we noted high levels of resistance to some specific antimicrobials in chicken and pig isolates throughout the study period. This was the case for ampicillin, streptomycin, and tetracycline, for which the occurrence of resistant strains remained continuously high (>60%). During the study period, tetracyclines, penicillin, phenicol (florfenicol), and aminoglycosides were the antibiotics most sold for use in food animals, especially pigs and chickens in Korea (APQA, 2020).
It was noteworthy that E. coli isolated from cattle were less frequently resistant to the tested antimicrobials compared with those isolated from chickens and pigs, a finding that has also been reported in other studies [21,28,29]. One possible reason may be that antimicrobial use is lower in cattle than in other animals. In addition, the differences in antimicrobial treatment regimens in cattle, chickens, and pigs (group vs. individual and oral (feed) vs. parenteral treatment) could contribute to the differences in antimicrobial resistance rates [30]. The slaughtering of broiler chickens at early ages (5–6 weeks), when they harbor more resistant strains than older animals [30], and continuous antimicrobial treatment until a few days before slaughter might also contribute to the occurrence of high resistant chicken isolates [21].
Overall, there was no significant difference in antimicrobial resistance in all animal species during the study period except for chloramphenicol. In 2020, in isolates from pigs and chicken, resistance to chloramphenicol increased drastically by 1.3–1.8 times when compared to 2010. Although chloramphenicol has been prohibited for use in veterinary medicine, the use of other phenicol (florfenicol) or the co-selection with unrelated antimicrobial(s) could be associated with the emergence of chloramphenicol-resistant E. coli [20]. In general, the high resistance to these older antimicrobials is not hard to explain because they are frequently used in food animals, especially pigs and chickens in Korea [14]. Therefore, the high-level resistance observed in chicken and pig isolates could reflect the use of these antimicrobials in poultry and pig farms.
Cephalosporins are among the critically important antimicrobial agents indicated for the treatment of MDR bacterial infections in humans [31]. In this study, the overall cefoxitin and ceftiofur resistance rates in cattle, chicken, and pig isolates remained low (<10%). Of note, we observed a trend of increasing resistance to ceftiofur or cefoxitin in chicken and pig isolates. The ceftiofur resistance rates in cattle, chicken, and pig isolates were consistent with previous reports in Korea [11], Japan [32], some European countries [24,33,34,35], and North America [33,36]. In contrast, Zhang et al. (2017) have reported a relatively high ceftiofur resistance in E. coli isolates from swine (16%) and broiler chickens (47%) in China [22]. Furthermore, consistent with previous studies in Korea and other countries [28,37,38,39], we found relatively high cefoxitin and ceftiofur resistance in chicken isolates than in pig and cattle isolates. Recently, we have identified extended-spectrum β-lactamase-producing (mainly CTX-M type) Enterobacteriaceae including E. coli from food animals in Korea [9,40,41]. Cefoxitin and ceftiofur-resistant strains might contribute to the emergence of resistance to other critically important cephalosporins. This could reduce the availability of these antimicrobials that can be used for critical infections [42].
Quinolones are among the priority antimicrobials used in human antimicrobial therapy [31]. Consistent with our previous study [37], we identified very high nalidixic acid and ciprofloxacin resistance in chicken isolates (88.6% and 76.1%, respectively) compared with those isolated from pigs (26.7% and 12.7%, respectively) and cattle (8.2% and 2.7%. respectively). Similarly, despite fluctuations in the levels of resistance, previous studies in Ghana [23], China [28,43], Qatar [44], and Poland [38] reported a high incidence in ciprofloxacin and/or nalidixic acid resistance more often in broiler isolates than in the pig and cattle isolates. Notably, the ciprofloxacin resistance rate in this study was higher than those reported in European countries [20] (2.0%, 0.2%, and 5.0% in cattle, pig, and chicken isolates, respectively). Ciprofloxacin is not approved for use in food animals in Korea. However, the use of other quinolones, especially enrofloxacin, in chickens might lead to an increased ciprofloxacin resistance rate. The high occurrence of ciprofloxacin resistance in healthy chicken isolates could pose a serious threat to public health.
The emergence of E. coli strains resistant to critically important antimicrobials such as colistin is a worldwide problem. Consistent with this study, previous reports in Korea [37] and other countries [20,21,23,28,38,45] identified a small proportion of colistin- and/or amoxicillin/clavulanic acid-resistant isolates from cattle, chickens, and pigs. In contrast, Kyung-Hyo et al. (2020) and Zhang et al. (2017) reported a relatively high occurrence of colistin (11–26%) and amoxicillin/clavulanic acid (29%) resistance in isolates from broiler chickens and/or swine in Korea and China, respectively [12,22]. Recently, the plasmid-borne mcr-1 and mcr-3 genes which are associated with the emergence of colistin resistance in Enterobacteriaceae including E. coli have been detected in food animals in Korea [46,47]. The World Health Organization (WHO) has classified the third and higher generation of cephalosporins, quinolones and colistin as priorities among critically important antimicrobials for treating serious infections caused by multidrug-resistant bacteria [48]. Therefore, the emergence of resistance to these antimicrobials in isolates from food animals warrants special concern and requires close monitoring.
In this study, we found high MDR rates in chicken (87.1%) and pig (73.7%) isolates compared with cattle isolates (17.1%). The prevalence of MDR remained above 60% and 80% during the whole study period for pig and chicken isolates, respectively. Similarly, several studies have reported high MDR rates in chicken (60–89%) and pig isolates (45–95%) than in cattle isolates (11–35%) [11,12,21,22,28,30]. These results suggest that stronger selective pressures for antibiotic resistance are present in the chicken and pig isolates than cattle isolates. In addition, MDR in Enterobacteriaceae is also complicated by the presence of mobile genetic elements, such as plasmids and transposons [49,50,51,52,53]. Our recent study demonstrated that E. coli isolated from healthy chickens had diverse plasmids containing mobile genetic elements and antibiotic resistance genes. Thus, the resistance noted could be owing to plasmid transfer [9].
In this study, we observed diverse MDR patterns, especially in chicken and pig isolates. The most frequent MDR patterns in cattle, chicken, and pig isolates commonly include resistance to streptomycin and tetracycline. Ciprofloxacin resistance was noted as a component of the most frequent MDR pattern in chicken isolates and should be considered highly important. Indeed, ciprofloxacin, ceftiofur, and/or colistin resistances were also noted in the less frequent MDR pattern (>7 antimicrobial agents) in chicken and pig isolates. MDR E. coli may spread to humans through direct contact with infected or colonized animals or their carcasses or the food chain, and poses a high risk to humans [8].
Overall, caution must be exercised when comparing and contrasting antimicrobial resistance rates and MDR profiles among studies because of the differences in the health status of animals, their history of antimicrobial use, farm management systems, and methodologies used, particularly with the determination of resistance breakpoints.
In conclusion, our study has shown a high occurrence of resistance and increasing resistance trends to commonly used antimicrobials including those considered critical for humans. Regular surveillance of antimicrobial resistance in food animals, farmworkers, and veterinarians as well as the implementation of administrative guidelines and regulations for the rational use of antimicrobials is essential to mitigate the antimicrobial resistance burden in food animals in Korea.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10030524/s1, Table S1: The MIC50 and MIC90 of the tested antimicrobials against E. coli isolated from healthy cattle between 2010 and 2020 in Korea (n = 2733); Table S2. The MIC50 and MIC90 of the tested antimicrobials against E. coli isolated from healthy pigs between 2010 and 2020 in Korea (n = 2542); Table S3. The MIC50 and MIC90 of the tested antimicrobials against E. coli isolated from healthy chickens between 2010 and 2020 in Korea (n = 1962); Table S4. Antimicrobial resistance patterns of E. coli isolated from healthy cattle between 2010 and 2020 in Korea (n = 2733); Table S5. Antimicrobial resistance patterns of E. coli isolated from healthy pigs between 2010 and 2020 in Korea (n = 2542); Table S6. Antimicrobial resistance patterns of E. coli isolated from healthy chickens between 2010 and 2020 in Korea (n = 1962).
Author Contributions
Conceptualization, S.-K.L. and H.-J.S.; Methodology, D.C.M., N.B., J.-H.C., A.F.M. and S.-J.K.; Software, H.-J.S., D.C.M. and N.B.; Validation, N.B. and J.-H.C.; Formal analysis, H.Y.K., S.-S.Y. and H.-J.S.; Investigation, D.C.M., H.Y.K. and H.-J.S.; Data Curation, S.-J.K. and S.-S.Y.; Writing—Original Draft Preparation, A.F.M. and N.B.; Writing-Review and Editing, S.-S.Y. and S.-K.L.; Supervision, S.-S.Y. and S.-K.L.; Project Administration, S.-S.Y. and S.-K.L.; Funding Acquisition; S.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food, and Rural Affairs, Korea (Grant number: N-1543081-2017-24-01).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Basak Unver K., Ahmet K. Vero-Toxigenic Escherichia coli (VTEC): To sum up all we know. J. Gastroenterol. Res. 2017;1:14–23. doi: 10.36959/621/583. [DOI] [Google Scholar]
- 2.Khalil I.A., Troeger C., Blacker B.F., Rao P.C., Brown A., Atherly D.E., Brewer T.G., Engmann C.M., Houpt E.R., Kang G. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018;18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H., Yoon Y. Etiological agents implicated in foodborne illness worldwide. Food Sci. Anim. Resour. 2021;41:1. doi: 10.5851/kosfa.2020.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolejska M., Bierošová B., Kohoutova L., Literak I., Čížek A. Antibiotic-resistant Salmonella and Escherichia coli isolates with integrons and extended-spectrum beta-lactamases in surface water and sympatric black-headed gulls. J. Appl. Microbiol. 2009;106:1941–1950. doi: 10.1111/j.1365-2672.2009.04155.x. [DOI] [PubMed] [Google Scholar]
- 5.Abbas G., Khan I., Mohsin M. High rates of CTX-M group-1 extended-spectrum β-lactamases producing Escherichia coli from pets and their owners in Faisalabad, Pakistan. Infect. Drug Resist. 2019;12:571. doi: 10.2147/IDR.S189884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EFSA The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15:e05077. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill J. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste. Volume 40 The Review on Antimicrobial Resistance; London, UK: 2015. [Google Scholar]
- 8.Geenen P., Graat E., Haenen A., Hengeveld P., Van Hoek A., Huijsdens X., Kappert C., Lammers G., Van Duijkeren E., Van De Giessen A., et al. Prevalence of livestock-associated MRSA on Dutch broiler farms and in people living and/or working on these farms. J. Epidemiol. 2013;141:1099–1108. doi: 10.1017/S0950268812001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H.-J., Moon D.C., Mechesso A.F., Kang H.Y., Kim M.H., Choi J.-H., Kim S.-J., Yoon S.-S., Lim S.-K. Resistance profiling and molecular characterization of extended-spectrum/plasmid-mediated Ampc β-lactamase-producing Escherichia coli isolated from healthy broiler chickens in South Korea. Microorganisms. 2020;8:1434. doi: 10.3390/microorganisms8091434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tark D.-S., Moon D.C., Kang H.Y., Kim S.-R., Nam H.-M., Lee H.-S., Jung S.-C., Lim S.-K. Antimicrobial susceptibility and characterization of extended-spectrum β-lactamases in Escherichia coli isolated from bovine mastitic milk in South Korea from 2012 to 2015. J. Dairy Sci. 2017;100:3463–3469. doi: 10.3168/jds.2016-12276. [DOI] [PubMed] [Google Scholar]
- 11.Lim S.-K., Nam H.-M., Moon D.-C., Jang G.-C., Jung S.-C. Antimicrobial resistance of Escherichia coli isolated from healthy animals during 2010–2012. Korean J. Vet. Res. 2014;54:131–137. doi: 10.14405/kjvr.2014.54.3.131. [DOI] [Google Scholar]
- 12.Kyung-Hyo D., Jae-Won B., Wan-Kyu L. Antimicrobial Resistance Profiles of Escherichia coli from Diarrheic Weaned Piglets after the Ban on Antibiotic Growth Promoters in Feed. Antibiotics. 2020;9:755. doi: 10.3390/antibiotics9110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Do K.-H., Byun J.-W., Lee W.-K. Serogroups, Virulence Genes and Antimicrobial Resistance of F4+ and F18+ Escherichia coli Isolated from Weaned Piglets. Pak. Vet. J. 2019;39:266–270. doi: 10.29261/pakvetj/2019.021. [DOI] [Google Scholar]
- 14.Cho J., Ha J., Kim K. Antimicrobial drug resistance of Escherichia coli isolated from cattle, swine and chicken. Korean J. Vet. Public Health. 2006;30:9–18. [Google Scholar]
- 15.Tamang M.D., Nam H.-M., Kim S.-R., Chae M.H., Jang G.-C., Jung S.-C., Lim S.-K. Prevalence and molecular characterization of CTX-M β-lactamase–producing Escherichia coli isolated from healthy swine and cattle. Foodborne Pathog. Dis. 2013;10:13–20. doi: 10.1089/fpd.2012.1245. [DOI] [PubMed] [Google Scholar]
- 16.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Clinical and Laboratory Standard Institute; Wayne, PA, USA: 2018. CLSI Supplement M100. [Google Scholar]
- 17.FDA . NARMS Integrated Report: 2014—The National Antimicrobial Resistance Monitoring System: Enteric Bacteria. FDA; Silver Spring, MD, USA: 2016. [Google Scholar]
- 18.European Committee on Antimicrobial Susceptibility Testing . Breakpoint Tables for Interpretation of MICs and Zone Diameters. European Society of Clinical Microbiology and Infectious Diseases; Basel, Switzerland: 2018. [Google Scholar]
- 19.Astorga F., Navarrete-Talloni M.J., Miró M.P., Bravo V., Toro M., Blondel C.J., Hervé-Claude L.P. Antimicrobial resistance in E. coli isolated from dairy calves and bedding material. Heliyon. 2019;5:e02773. doi: 10.1016/j.heliyon.2019.e02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Jong A., Thomas V., Simjee S., Godinho K., Schiessl B., Klein U., Butty P., Vallé M., Marion H., Shryock T.R. Pan-European monitoring of susceptibility to human-use antimicrobial agents in enteric bacteria isolated from healthy food-producing animals. J. Antimicrob. Chemother. 2012;67:638–651. doi: 10.1093/jac/dkr539. [DOI] [PubMed] [Google Scholar]
- 21.Wasyl D., Hoszowski A., Szulowski K., Zając M. Antimicrobial resistance in commensal Escherichia coli isolated from animals at slaughter. Front. Microbiol. 2013;4:221. doi: 10.3389/fmicb.2013.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P., Shen Z., Zhang C., Song L., Wang B., Shang J., Yue X., Qu Z., Li X., Wu L. Surveillance of antimicrobial resistance among Escherichia coli from chicken and swine, China, 2008–2015. Vet. Microbiol. 2017;203:49–55. doi: 10.1016/j.vetmic.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Ohene Larbi R., Ofori L.A., Sylverken A.A., Ayim-Akonor M., Obiri-Danso K. Antimicrobial Resistance of Escherichia coli from Broilers, Pigs, and Cattle in the Greater Kumasi Metropolis, Ghana. Int. J. Microbiol. 2021;2021:1–7. doi: 10.1155/2021/5158185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EFSA/ECDC . The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2012. Volume 12. EFSA; Parma, Italy: 2014. p. 3590. [Google Scholar]
- 25.Deckert A., Gow S., Rosengren L., Leger D., Avery B., Daignault D., Dutil L., Reid-Smith R., Irwin R. Canadian integrated program for antimicrobial resistance surveillance (CIPARS) farm program: Results from finisher pig surveillance. Zoonoses Public Health. 2010;57:71–84. doi: 10.1111/j.1863-2378.2010.01356.x. [DOI] [PubMed] [Google Scholar]
- 26.Borck Høg B., Korsgaard H.B., Wolff Sönksen U., Bager F., Bortolaia V., Ellis-Iversen J., Hendriksen R.S., Borck Høg B., Jensen L.B., Korsgaard H.B. Danish Integrated Antimicrobial Resistance Monitoring (DANMAP) 2016—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, food, and Humans in Denmark. Technical University of Denmark National Food Institute; Kongens Lyngby, Denmark: 2016. [Google Scholar]
- 27.Hayer S.S., Lim S., Hong S., Elnekave E., Johnson T., Rovira A., Vannucci F., Clayton J.B., Perez A., Alvarez J. Genetic determinants of resistance to extended-spectrum cephalosporin and fluoroquinolone in Escherichia coli isolated from diseased pigs in the United States. mSphere. 2020;5:e00990-20. doi: 10.1128/mSphere.00990-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yassin A.K., Gong J., Kelly P., Lu G., Guardabassi L., Wei L., Han X., Qiu H., Price S., Cheng D. Antimicrobial resistance in clinical Escherichia coli isolates from poultry and livestock, China. PLoS ONE. 2017;12:e0185326. doi: 10.1371/journal.pone.0185326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J., Huang X.-Y., Xia Y.-B., Guo Z.-W., Ma Z.-B., Yi M.-Y., Lv L.-C., Lu P.-L., Yan J.-C., Huang J.-W. Clonal spread of Escherichia coli ST93 carrying mcr-1-harboring IncN1-IncHI2/ST3 plasmid among companion animals, China. Front. Microbiol. 2018;9:2989. doi: 10.3389/fmicb.2018.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanon J.-B., Jaspers S., Butaye P., Wattiau P., Méroc E., Aerts M., Imberechts H., Vermeersch K., Van der Stede Y. A trend analysis of antimicrobial resistance in commensal Escherichia coli from several livestock species in Belgium (2011–2014) Prev. Vet. Med. 2015;122:443–452. doi: 10.1016/j.prevetmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Wendlandt S., Shen J., Kadlec K., Wang Y., Li B., Zhang W.-J., Feßler A.T., Wu C., Schwarz S. Multidrug resistance genes in staphylococci from animals that confer resistance to critically and highly important antimicrobial agents in human medicine. Trends Microbiol. 2015;23:44–54. doi: 10.1016/j.tim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Ohnishi M., Okatani A., Esaki H., Harada K., Sawada T., Murakami M., Marumo K., Kato Y., Sato R., Shimura K. Herd prevalence of E nterobacteriaceae producing CTX-M-type and CMY-2 β-lactamases among Japanese dairy farms. J. Appl. Microbiol. 2013;115:282–289. doi: 10.1111/jam.12211. [DOI] [PubMed] [Google Scholar]
- 33.FDA . Guidance for Industry. Clinical Pharmacogenomics: Premarket Evaluation in Early-Phase Clinical Studies and Recommendations for Labeling. FDA; Silver Spring, MD, USA: 2013. [Google Scholar]
- 34.Dahmen S., Haenni M., Madec J.-Y. IncI1/ST3 plasmids contribute to the dissemination of the bla CTX-M-1 gene in Escherichia coli from several animal species in France. J. Antimicrob. Chemother. 2012;67:3011–3012. doi: 10.1093/jac/dks308. [DOI] [PubMed] [Google Scholar]
- 35.Thomas V., de Jong A., Moyaert H., Simjee S., El Garch F., Morrissey I., Marion H., Vallé M. Antimicrobial susceptibility monitoring of mastitis pathogens isolated from acute cases of clinical mastitis in dairy cows across Europe: VetPath results. Int. J. Antimicrob. Agents. 2015;46:13–20. doi: 10.1016/j.ijantimicag.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Saini V., McClure J., Léger D., Keefe G., Scholl D., Morck D., Barkema H. Antimicrobial resistance profiles of common mastitis pathogens on Canadian dairy farms. J. Dairy Sci. 2012;95:4319–4332. doi: 10.3168/jds.2012-5373. [DOI] [PubMed] [Google Scholar]
- 37.Boireau C., Morignat É., Cazeau G., Jarrige N., Jouy É., Haenni M., Madec J.Y., Leblond A., Gay É. Antimicrobial resistance trends in Escherichia coli isolated from diseased food-producing animals in France: A 14-year period time-series study. Zoonoses Public Health. 2018;65:e86–e94. doi: 10.1111/zph.12412. [DOI] [PubMed] [Google Scholar]
- 38.Wasyl D., Hasman H., Cavaco L.M., Aarestrup F.M. Prevalence and characterization of cephalosporin resistance in nonpathogenic Escherichia coli from food-producing animals slaughtered in Poland. Microb. Drug Resist. 2012;18:79–82. doi: 10.1089/mdr.2011.0033. [DOI] [PubMed] [Google Scholar]
- 39.Lim J.-S., Choi D.-S., Kim Y.-J., Chon J.-W., Kim H.-S., Park H.-J., Moon J.-S., Wee S.-H., Seo K.-H. Characterization of Escherichia coli–producing extended-spectrum β-lactamase (ESBL) Isolated from chicken slaughterhouses in South Korea. Foodborne Pathog. Dis. 2015;12:741–748. doi: 10.1089/fpd.2014.1921. [DOI] [PubMed] [Google Scholar]
- 40.Na S.H., Moon D.C., Kang H.Y., Song H.-J., Kim S.-J., Choi J.-H., Yoon J.W., Yoon S.-S., Lim S.-K. Molecular characteristics of extended-spectrum β-lactamase/AmpC-producing Salmonella enterica serovar Virchow isolated from food-producing animals during 2010–2017 in South Korea. Int. J. Food Microbiol. 2020;322:108572. doi: 10.1016/j.ijfoodmicro.2020.108572. [DOI] [PubMed] [Google Scholar]
- 41.Na S.H., Moon D.C., Choi M.-J., Oh S.-J., Jung D.-Y., Sung E.J., Kang H.Y., Hyun B.-H., Lim S.-K. Antimicrobial resistance and molecular characterization of extended-spectrum β-lactamase-producing Escherichia coli isolated from ducks in South Korea. Foodborne Pathog. Dis. 2019;16:799–806. doi: 10.1089/fpd.2019.2644. [DOI] [PubMed] [Google Scholar]
- 42.Collignon P., Aarestrup F.M., Irwin R., McEwen S. Human deaths and third-generation cephalosporin use in poultry, Europe. Emerg. Infect. Dis. 2013;19:1339–1340. doi: 10.3201/eid1908.120681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei T., Tian W., He L., Huang X.-H., Sun Y.-X., Deng Y.-T., Sun Y., Lv D.-H., Wu C.-M., Huang L.-Z. Antimicrobial resistance in Escherichia coli isolates from food animals, animal food products and companion animals in China. J. Vet. Microbiol. 2010;146:85–89. doi: 10.1016/j.vetmic.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Eltai N.O., Abdfarag E.A., Al-Romaihi H., Wehedy E., Mahmoud M.H., Alawad O.K., Al-Hajri M.M., Al Thani A.A., Yassine H.M. Antibiotic resistance profile of commensal Escherichia coli isolated from broiler chickens in Qatar. J. Food Prot. 2017;81:302–307. doi: 10.4315/0362-028X.JFP-17-191. [DOI] [PubMed] [Google Scholar]
- 45.Manishimwe R., Moncada P.M., Bugarel M., Scott H.M., Loneragan G.H. Antibiotic resistance among Escherichia coli and Salmonella isolated from dairy cattle feces in Texas. PLoS ONE. 2021;16:e0242390. doi: 10.1371/journal.pone.0242390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alhababi D.A., Eltai N.O., Nasrallah G.K., Farg E.A., Al Thani A.A., Yassine H.M. Antimicrobial resistance of commensal Escherichia coli isolated from food animals in Qatar. Microb. Drug Resist. 2020;26:420–427. doi: 10.1089/mdr.2019.0402. [DOI] [PubMed] [Google Scholar]
- 47.Lim S.-K., Kang H.Y., Lee K., Moon D.-C., Lee H.-S., Jung S.-C. First Detection 45 the mcr-1 Gene in Escherichia coli Isolated from Livestock between 2013 and 2015 in South Korea. Antimicrob. Agents Chemother. 2016;60:6991–6993. doi: 10.1128/AAC.01472-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belaynehe K.M., Shin S.W., Park K.Y., Jang J.Y., Won H.G., Yoon I.J., Yoo H.S. Emergence of mcr-1 and mcr-3 variants coding for plasmid-mediated colistin resistance in Escherichia coli isolates from food-producing animals in South Korea. Int. J. Infect. Dis. 2018;72:22–24. doi: 10.1016/j.ijid.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization . Critically Important Antimicrobials for Human Medicine: Ranking of Antimicrobial Agents for Risk Management of Antimicrobial Resistance due to Non-Human Use. WHO; Geneva, Switzerland: 2018. [Google Scholar]
- 50.Wyrsch E.R., Reid C.J., DeMaere M.Z., Liu M.Y., Chapman T.A., Roy Chowdhury P., Djordjevic S.P. Complete sequences of multiple-drug resistant IncHI2 ST3 plasmids in Escherichia coli of porcine origin in Australia. Front. Sustain. Food Syst. 2019;3:18. doi: 10.3389/fsufs.2019.00018. [DOI] [Google Scholar]
- 51.Mbelle N.M., Feldman C., Sekyere J.O., Maningi N.E., Modipane L., Essack S.Y. The resistome, mobilome, virulome and phylogenomics of multidrug-resistant Escherichia coli clinical isolates from Pretoria, South Africa. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-52859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Exner M., Bhattacharya S., Christiansen B., Gebel J., Goroncy-Bermes P., Hartemann P., Heeg P., Ilschner C., Kramer A., Larson E. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria. GMS Hyg. Infect. Control. 2017;12:20170410. doi: 10.3205/dgkh000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chalmers G., Cormier A.C., Nadeau M., Côté G., Reid-Smith R.J., Boerlin P. Determinants of virulence and of resistance to ceftiofur, gentamicin, and spectinomycin in clinical Escherichia coli from broiler chickens in Québec, Canada. Vet. Microbiol. 2017;203:149–157. doi: 10.1016/j.vetmic.2017.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.