Abstract
The genes encoding the DNA gyrase A and B subunits of Bacteroides fragilis were cloned and sequenced. The gyrA and gyrB genes code for proteins of 845 and 653 amino acids, respectively. These proteins were expressed in Escherichia coli, and the combination of GyrA and GyrB exhibited ATP-dependent supercoiling activity. To analyze the role of DNA gyrase in quinolone resistance of B. fragilis, we isolated mutant strains by stepwise selection for resistance to increasing concentrations of levofloxacin. We analyzed the resistant mutants and showed that Ser-82 of GyrA, equivalent to resistance hot spot Ser-83 of GyrA in E. coli, was in each case replaced with Phe. These results suggest that DNA gyrase is an important target for quinolones in B. fragilis.
Bacteroides fragilis is an obligate anaerobic bacterium composing intestinal flora and is the major pathogen in intra-abdominal infection following a perforated appendix or surgery on the gastrointestinal tract (11). B. fragilis often presents a serious problem in therapy, as it is intrinsically resistant to many antibiotics, including most of the penicillins, cephalosporins, and quinolones (9). β-Lactam resistance is usually explained by the combination of low permeability of the outer membrane (34) and the presence of highly active β-lactamases of the Bush 2e and 3 classes (4). However, the molecular basis of quinolone resistance remains poorly defined (20, 27).
Studies with Escherichia coli have shown that quinolones act by inhibiting the activity of DNA gyrase, which catalyzes ATP-dependent DNA supercoiling (3, 7, 8, 21). Moreover, it was revealed that mutations in the GyrA quinolone resistance-determining region (QRDR), located between amino acid residues 67 and 106 (5, 10, 23, 31), were related to quinolone resistance. Recently, the type II enzyme topoisomerase IV, essential for chromosome segregation, was shown to be another target of quinolones (14). In gram-negative bacteria, such as E. coli and Neisseria gonorrhoeae, strains with low-level resistance contained gyrA mutations whereas those with higher levels of resistance had mutations in both gyrA and parC (1, 13, 15, 16). On the other hand, in gram-positive bacteria such as Staphylococcus aureus and Streptococcus pneumoniae, mutations in parC (grlA) conferred low-level resistance and preceded those in gyrA (6, 24, 25). Moreover, mutations in the B subunits of DNA gyrase and topoisomerase IV (2, 30, 33), and the appearance of efflux pumps, were shown to be related to quinolone resistance (17, 18, 22, 26, 32).
As a first step, we report here the cloning and characterization of gyrA and gyrB of B. fragilis and examine the role of DNA gyrase in the stepwise acquisition of levofloxacin resistance in vitro. This study complements the genetic characterization of the type II DNA topoisomerases of B. fragilis and reveals the molecular basis of quinolone resistance.
MATERIALS AND METHODS
Antibacterial agents.
All quinolones used in this study were synthesized at Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan.
Bacterial strains, plasmids, and DNA manipulations.
B. fragilis ATCC 25285 was grown in general anaerobic GAM broth (Nissui, Tokyo, Japan) at 37°C in an anaerobic box. To construct a genomic library, chromosomal DNA was extracted from B. fragilis ATCC 25285. The E. coli strains used for plasmid transformation were MC1061 and DH5α (19). Plasmid pUC18 was used to construct libraries and to subclone DNA inserts. Plasmid pMAL-c2 (New England Biolabs) was used to construct plasmids for overexpression of the GyrA and GyrB proteins of B. fragilis in E. coli. Manipulations of DNA, including plasmid extraction, electrophoresis, Southern hybridization, and colony hybridization, were carried out by standard methods (19). For Southern and colony hybridization, DNA was radiolabeled with 50 μCi of [α-32P]dCTP (300 Ci/mmol), using the Multiprime DNA labeling kit (Pharmacia-Amersham).
Determination of MICs.
The MICs were determined by a standard agar dilution method with GAM agar (Eiken Chemical Co., Ltd., Tokyo, Japan). Drug-containing agar plates were inoculated with one loopful (5 μl) of an inoculum corresponding to about 104 CFU per spot and were incubated for 18 h at 37°C. The MIC was defined as the lowest drug concentration that prevented visible growth of bacteria.
DNA sequence analysis.
DNA fragments were subcloned into plasmid pUC18 and sequenced by the chain termination method with a fluorescence sequencer (Pharmacia-Amersham). Amplification of the QRDR of the gyrA and gyrB genes from B. fragilis ATCC 25285 and its levofloxacin-resistant mutants was carried out by PCR with genomic DNA as a template. For the QRDR of gyrA, the forward primer was Pr-BFGA03, 5′-ATGCTTGAACAAGACAGAATTATAAAG-3′ (gyrA positions 1 to 27) and the reverse primer was Pr-BFGA02, 5′-GACTGTCGCCGTCTACAGAACCG-3′ (324 to 346). The primers for the QRDR of the gyrB gene were Pr-BFGB03, 5′-GACCCGCAGAAGTGTGAGTTATTCC-3′ (gyrB positions 1279 to 1303) and Pr-BFGB04, 5′-TTTCAAGCGCTTTGTGATACATGGC-3′ (1405 to 1429). The PCR conditions were 25 cycles of 94°C for 0.5 min, 60°C for 0.5 min, and 72°C for 1 min. The 346-bp gyrA and 151-bp gyrB PCR products were cloned into pCRII (Invitrogen) for DNA sequence analysis.
Protein expression.
GyrA and GyrB of DNA gyrase were expressed separately as fusion proteins with maltose-binding protein (MBP) by using the pMAL-c2 expression vector. Each gene was amplified by PCR and inserted into the expression vector. In the reverse primers, a HindIII or PstI site was introduced for cloning purposes. For gyrA, the forward primer was Pr-BFGA03, 5′-ATGCTTGAACAAGACAGAATTATAAAG-3′ (gyrA positions 1 to 27), and the reverse primer was Pr-BFGA04, 5′-AGTTGTTAAGCTTTTGCGAAGTCAGG-3′ (2777 to 2802; HindIII). The primers for the gyrB gene were Pr-BFGB01, 5′-ATGAGCGAAGAACAGAATCCCACC-3′ (gyrB positions 1 to 24), and Pr-BFGB02, 5′-ATTTTCCTGCAGCGCCGGCGCTTC-3′ (2001 to 2024; PstI). PCR was carried out on genomic DNA from strain ATCC 25285 as follows: 20 cycles of 94°C for 0.5 min, 65°C for 0.5 min, and 72°C for 2 min. The PCR products were digested with restriction enzymes, ligated into expression vectors, and transformed into E. coli MC1061. Protein production was induced with isopropyl-β-d-thiogalactopyranoside (IPTG), and each protein was purified as described previously (29).
DNA gyrase assay.
The supercoiling activity of DNA gyrase, the conversion of relaxed pBR322 DNA to the supercoiled form, was detected by the method described previously (28).
Nucleotide sequence accession numbers.
The DNA sequences corresponding to the gyrA and gyrB genes have been assigned GenBank accession no. AB017712 and AB017713, respectively.
RESULTS
Cloning and sequencing the gyrA and gyrB genes of B. fragilis.
Southern blot hybridization analysis of genomic DNA from B. fragilis ATCC 25285 revealed that a 1.5-kb EcoRI fragment and a 4-kb SphI fragment hybridized to the E. coli gyrA and gyrB probes, respectively (data not shown). These fragments were isolated by colony hybridization of a size-selected B. fragilis ATCC 25285 EcoRI fragment library and an SphI fragment library. DNA sequence analysis of both clones indicated that the sequences showed high homology with gyrA and gyrB of E. coli. To obtain full-length gyrA and gyrB genes, a partially Sau3AI-digested genomic library was screened with the two genes as probes. Fragments of 1.8 and 3 kb were screened by the gyrA probe, and a 0.6-kb fragment was screened by the gyrB probe. Analysis of the nucleotide sequences revealed two open reading frames for GyrA and GyrB. The gyrA and gyrB genes encoded 845- and 653-residue proteins with predicted molecular masses of 95.7 and 70.9 kDa (Fig. 1 and 2). The deduced products of gyrA and gyrB exhibited 48 and 52% identity, respectively, to GyrA and GyrB of E. coli. The homology of the GyrA QRDR between B. fragilis and E. coli was particularly high (70%), suggesting that this region of B. fragilis is also related to quinolone resistance (Fig. 3).
FIG. 1.
Nucleotide and deduced amino acid sequence of a 3,124-bp fragment which contains the gyrA gene of B. fragilis ATCC 25285. The methionine initiation codon is underlined. An asterisk indicates the stop codon.
FIG. 2.
Nucleotide and deduced amino acid sequence of a 2,215-bp fragment which contains the gyrB gene of B. fragilis ATCC 25285. The symbols are defined in the legend to Fig. 1.
FIG. 3.
Alignment of B. fragilis GyrA (A) and GyrB (B) protein sequence with their counterparts in E. coli and S. aureus. An asterisk indicates identity among all three proteins. The numbers indicate amino acid residues. Residue Ser-82 (S) in B. fragilis GyrA and the position of the catalytic tyrosine (Y) residue involved in DNA breakage reunion (12) are in boldface and underlined.
Purification of GyrA and GyrB in E. coli.
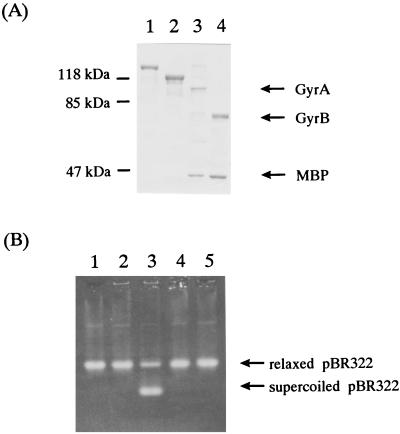
To identify the proteins encoded by gyrA and gyrB, we overexpressed the proteins and examined their enzymatic properties. The putative GyrA and GyrB proteins were expressed as MBP fusion proteins and purified separately. The bands for each protein on a sodium dodecyl sulfate-polyacrylamide gel stained with Coomassie brilliant blue were about 95 and 70 kDa for GyrA and GyrB, respectively (Fig. 4). Neither protein alone had supercoiling activity, but the reconstituted proteins showed ATP-dependent enzymatic activity (Fig. 4). These results demonstrate that the 95- and 70-kDa proteins of B. fragilis are GyrA and GyrB, respectively.
FIG. 4.
Purification of B. fragilis GyrA and GyrB proteins. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of purified B. fragilis GyrA and GyrB proteins. The proteins were electrophoresed in a 10% polyacrylamide gel and stained with Coomassie brilliant blue. The masses of the protein markers are indicated in kilodaltons on the left. Lane 1, MBP-GyrA fusion protein; lane 2, MBP-GyrB fusion protein; lane 3, MBP-GyrA fusion protein after factor Xa cleavage; lane 4, MBP-GyrB fusion protein after factor Xa cleavage. (B) Supercoiling activity of purified GyrA and GyrB proteins. Lane 1, purified GyrA (1 U); lane 2, purified GyrB (1 U); lane 3, purified GyrA (1 U) and GyrB (1 U); lane 4, purified GyrA (1 U) and GyrB (1 U) without ATP; lane 5, no addition. The source of DNA is pBR322.
Sequence analysis of stepwise-selected levofloxacin-resistant mutants of B. fragilis.
In order to examine the role of DNA gyrase in quinolone-resistant B. fragilis, we developed mutants of susceptible strain ATCC 25285 by stepwise exposure to levofloxacin. In the first round of selection, isolate ATCC 25285 (approximately 108 CFU) was plated on GAM agar plates containing increasing concentrations of levofloxacin in multiples of the MIC. More than 100 colonies (first-step mutants) grew on the plate containing 0.78 μg of levofloxacin/ml, and no growth was seen at higher drug concentrations. Two first-step mutants (L1-1 and L1-2) were selected for gyrA sequence analysis. Mutant L1-1 was exposed to increased drug levels on plates. At a concentration of 3.13 μg/ml, more than 100 colonies (second-step mutants) were able to grow. Third- and fourth-step mutants, which grew in the presence of 12.5 and 25 μg of levofloxacin per ml, respectively, were generated similarly. Mutant strains were also cross-resistant to other quinolones: sitafloxacin, ciprofloxacin, and sparfloxacin (Table 1).
TABLE 1.
Properties of mutants of B. fragilis ATCC 25285 selected for resistance by stepwise exposure in vitro to levofloxacin
| Straina | MIC (μg/ml)b
|
Mutation
|
||||
|---|---|---|---|---|---|---|
| LVFX | STFX | CPFX | SPFX | gyrA | gyrB | |
| ATCC 25285 | 0.78 | 0.025 | 1.56 | 0.78 | ||
| L1 | 3.13 | 0.10 | 12.5 | 1.56 | None | None |
| L2 | 12.5 | 0.78 | 25 | 6.25 | Ser-82 (TCT)→Phe (TTT) | None |
| L3 | 50 | 1.56 | 50 | 25 | Ser-82 (TCT)→Phe (TTT) | None |
| L4 | 50 | 1.56 | 50 | 50 | Ser-82 (TCT)→Phe (TTT) | None |
Two clones were analyzed for each strain.
The MIC is the lowest drug concentration at which no bacterial growth on GAM agar plates was observed after anaerobic incubation overnight at 37°C. LVFX, levofloxacin; STFX, sitafloxacin; CPFX, ciprofloxacin; SPFX, sparfloxacin.
A 346-bp gyrA fragment spanning codons 1 to 115 was amplified by PCR from levofloxacin-resistant mutants. This region encompasses sequence equivalent to the quinolone resistance-determining region of E. coli GyrA (residues 67 to 106). PCR products were ligated into plasmid pCRII, and the inserts were sequenced. The nucleotide sequences of the PCR products from L1-1 and L1-2 were identical to that of ATCC 25285. However, PCR products from second-step mutants carried a single-nucleotide change compared to the wild type. A TCT-to-TTT alteration was found at codon 82, which would result in a Ser-to-Phe substitution in GyrA. Sequence analysis of PCR products from third- and fourth-step mutants (in each case, two mutants were examined) did not reveal further mutations in this region of the gyrA gene. The QRDR of gyrB (residues 436 to 467) of levofloxacin-resistant mutants was also amplified and analyzed. The nucleotide sequence of this region of all mutants was identical to that of ATCC 25285.
DISCUSSION
We have cloned and characterized the gyrA and gyrB genes of B. fragilis. Assignment was based on close sequence homology to E. coli DNA gyrase subunits and the demonstration that when expressed in E. coli, the reconstituted GyrA and GyrB proteins showed ATP-dependent supercoiling activity, which is characteristic of DNA gyrase. The QRDR is highly conserved among B. fragilis, E. coli, and S. aureus. Ser-82 and Tyr-121, which are reported to be sites important in quinolone resistance and DNA breakage reunion (12, 21), respectively, were conserved among the three strains.
We isolated a series of B. fragilis ATCC 25285 mutants resistant to levofloxacin by stepwise selection on plates containing increasing drug concentrations (Table 1). These strains also exhibited cross-resistance to other quinolones. By examining gyrA genes in the quinolone-resistant ATCC 25285 mutants, we found mutation of Ser-82 to Phe in GyrA. As this residue is equivalent to the resistance hot spot Ser-83 of GyrA in E. coli (5, 10, 23, 31), the mechanisms of quinolone resistance for the two species are likely identical, and the mutation is related to quinolone resistance. Mutations in gyrB are also related to quinolone resistance (33), but no mutation was detected in our strains. Although gyrB mutations were not involved in quinolone resistance in this study, mutations in gyrB may, in general, be related to quinolone resistance in B. fragilis. Since no other mutation was detected in the GyrA and GyrB QRDRs of the highly quinolone-resistant strains L3 and L4, mutations in other regions may occur. Mutations in parC or parE are possible explanations. No mutation was detected in the first-step mutants (L1). As the level of resistance is modest, it is conceivable that an efflux pump or outer membrane permeability is related to quinolone resistance in first-step mutants (20). In this study, no mutation besides Ser-82 was observed in the QRDR of gyrA in the quinolone-resistant mutants, but alteration of Phe-86, which is equivalent to Asp-87 of GyrA in E. coli (5, 10, 23), or other alterations of GyrA may also confer quinolone resistance in B. fragilis.
In the gram-negative species E. coli and N. gonorrhoeae, quinolone resistance arises initially from a mutation in gyrA, and additional mutation of parC leads to highly resistant isolates (1, 13, 15). Thus, DNA gyrase appears to be the primary target in these bacteria, with topoisomerase IV acting as a secondary target. Although the parC gene of B. fragilis is not yet cloned and analyzed, the observation of GyrA mutations in quinolone-resistant mutants indicates that DNA gyrase is an important target for quinolones in B. fragilis.
For further study of quinolone resistance in B. fragilis, analysis of the topoisomerase IV gene and efflux pumps is needed. Additional characterization of the B. fragilis gyrA and gyrB genes reported here should facilitate further understanding of this important anaerobic pathogen.
REFERENCES
- 1.Belland R J, Morrison S G, Ison C, Huang W H. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol Microbiol. 1994;14:371–380. doi: 10.1111/j.1365-2958.1994.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 2.Breins D M, Ouadbesselam S, Ng E Y, Tankovic J, Shah S, Soussy C J, Hooper D C. Quinolone resistance locus nfxD of Escherichia coli is a mutant allele of the parE gene encoding a subunit of topoisomerase IV. Antimicrob Agents Chemother. 1997;41:175–179. doi: 10.1128/aac.41.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown P O, Cozzarelli N R. A sign inversion mechanism for enzymatic supercoiling of DNA. Science. 1980;206:1081–1083. doi: 10.1126/science.227059. [DOI] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen M E, Wyke A W, Kuroda R, Fisher L M. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989;33:886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrero L, Cameron B, Manse B, Lagneux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target for fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 7.Gallert M, Mizuuchi K, O’Dea M H, Itoh T, Tomizawa J I. Nalidixic acid resistance: a second character involved in DNA gyrase activity. Proc Natl Acad Sci USA. 1977;74:4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gellert M, Mizuuchi K, O’Dea M H, Nash H A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci USA. 1977;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecht D W, Wexler H M. In vitro susceptibility of anaerobes to quinolones in the United States. Clin Infect Dis. 1996;23:S2–S8. doi: 10.1093/clinids/23.supplement_1.s2. [DOI] [PubMed] [Google Scholar]
- 10.Heisig P, Schedletzky H, Falkenstein-Paul H. Mutations in the gyrA gene of a highly fluoroquinolone-resistant clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1993;37:696–701. doi: 10.1128/aac.37.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill G B. Introduction to the anaerobic bacteria: non-spore-forming anaerobes. In: Joklik W K, Willett H P, Amos D B, Willett C M, editors. Zinsser microbiology. 20th ed. Norwalk, Conn: Appleton & Lange; 1992. pp. 621–635. [Google Scholar]
- 12.Horowitz D S, Wang J C. Mapping the activity site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987;269:5339–5344. [PubMed] [Google Scholar]
- 13.Hoshino K, Akasaka A, Morrissey I, Sato K, Kato J, Ikeda H. Comparison of inhibition of Escherichia coli topoisomerase IV by quinolones with DNA gyrase inhibition. Antimicrob Agents Chemother. 1994;38:2623–2627. doi: 10.1128/aac.38.11.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato J, Nishimura Y, Imamura R, Niki H, Higara S, Suzuki H. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 15.Khodursky A B, Zechiedrich E L, Cozzarelli N R. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11801–11805. doi: 10.1073/pnas.92.25.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai Y, Kato J, Hoshino K, Akasaka T, Sato K, Ikeda H. Quinolone-resistant mutants of Escherichia coli DNA topoisomerase IV parC gene. Antimicrob Agents Chemother. 1996;40:710–714. doi: 10.1128/aac.40.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis K. Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem Sci. 1994;19:119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 18.Li X-Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch R F, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Miyamae S, Nikaido H, Tanaka Y, Yoshimura F. Active efflux of norfloxacin by Bacteroides fragilis. Antimicrob Agents Chemother. 1998;42:2119–2121. doi: 10.1128/aac.42.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuuchi K, Fisher L M, O’Dea M H, Gallert M. DNA gyrase action involves the introduction of transient double-strand DNA breaks into DNA. Proc Natl Acad Sci USA. 1980;77:1847–1851. doi: 10.1073/pnas.77.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oram M, Fisher L M. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob Agents Chemother. 1991;35:387–389. doi: 10.1128/aac.35.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan X S, Fisher L M. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J Bacteriol. 1996;178:4060–4069. doi: 10.1128/jb.178.14.4060-4069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan X S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piddock L J V. Mechanism of resistance to fluoroquinolones: state-of-the-art 1992–1994. Drugs. 1995;49:29–35. doi: 10.2165/00003495-199500492-00006. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen B, Bush K, Tally F P. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997;24:S110–S120. doi: 10.1093/clinids/24.supplement_1.s110. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Sato K, Kimura Y, Hayakawa I, Osada Y, Nishino T. Inhibition by quinolones of DNA gyrase from Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:1489–1491. doi: 10.1128/aac.35.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Onodera Y, Uchida Y, Sato K, Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2362–2366. doi: 10.1128/aac.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka M, Onodera Y, Uchida Y, Sato K. Quinolone resistance mutation in the GrlB protein of Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:3044–3046. doi: 10.1128/aac.42.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Kanno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida H, Bogaki M, Nakamura M, Yamanaka L M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991;35:1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yotsuji A, Mituyama J, Hori R, Yasuda T, Saikawa I, Inoue M, Mitsuhashi S. Outer membrane permeation of B. fragilis by cephalosporins. Antimicrob Agents Chemother. 1988;32:1097–1099. doi: 10.1128/aac.32.7.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]