Abstract
A survey conducted between 1987 and 1994 at the University Hospital of Besançon, France, demonstrated a dramatic increase (from 0 to 42.5%) in the prevalence of amoxicillin resistance among Salmonella spp. Of the 96 resistant isolates collected during this period (including 77 Typhimurium), 54 were found to produce TEM-1 β-lactamase, 40 produced PSE-1 (equivalent to CARB-2), one produced PSE-1 plus TEM-2, and one produced OXA-1 in isoelectric focusing and DNA hybridization experiments. Plasmids coding for these β-lactamases were further characterized by (i) profile analysis, (ii) restriction fragmentation pattern analysis, (iii) hybridization with an spvCD-orfE virulence probe, and (iv) replicon typing. In addition, isolates of S. typhimurium were genotypically compared by pulsed-field gel electrophoresis of XbaI-macrorestricted chromosomal DNA. Altogether, these methods showed that 40 of the 41 PSE-1 producers were actually the progeny of a single epidemic S. typhimurium strain lysotype DT104. Isolates of that strain were found to harbor RepFIC virulence plasmids with somewhat different restriction profiles, but which all carried the blaPSE-1 gene. Of these virulence/resistance plasmids, 15 were transmissible to Escherichia coli. TEM-1-producing S. typhimurium displayed much greater genotypic and plasmidic diversities, suggesting the acquisition of the blaTEM-1 gene from multiple bacterial sources by individual strains. In agreement with this, 32 of the 35 S. typhimurium plasmids encoding TEM-1 were found to be conjugative. These data show that development of amoxicillin resistance among Salmonella, especially in serovar Typhimurium, results from both gene transfers and strain dissemination.
β-Lactam antibiotics are widely used in the treatment of salmonellosis. Recently, alarming reports have pointed out the rapid development of resistance to these agents, involving Salmonella serovars such as Enteritidis (28, 44), Typhimurium (28, 41, 43), Panama (6), and Typhi (13) in several countries. Clinical strains of Salmonella spp. producing large-spectrum β-lactamases and which are resistant to penicillins (44), or Salmonella spp. producing extended-spectrum β-lactamases and which are resistant to cephalosporins such as cefotaxime, ceftazidime, or ceftriaxone (1, 43), have been isolated from large outbreaks as well as from sporadic cases.
DNA-based typing methods have provided very useful information on the dissemination of resistant Salmonella to epidemiological investigations (39). On some occasions, the relatedness of R plasmids harbored by strains of various origins could be demonstrated by restriction fragmentation pattern analysis (RFP), allowing a better understanding of how resistant strains or R factors may propagate (3, 40, 44). More recently, methods such as random amplified polymorphic DNA fingerprinting analysis (17), IS200 fingerprinting (33), ribotyping, and restriction fragment length polymorphism analysis (24) have been evaluated and found to be valuable tools for tracing large outbreaks due to the circulation of single epidemic clones (45). R plasmid characterization and strain genotyping have, however, rarely been combined to compare resistant isolates over long periods of time or to study the diffusion of resistance determinants among bacterial populations (13, 36).
From 1987 to 1994, we witnessed a tremendous increase in the prevalence of amoxicillin resistance among the Salmonella spp. isolated at the University Hospital of Besançon, France. To establish if such an increase was due to the dissemination of a few resistant clones in the community or whether it resulted from the acquisition of resistance determinants from multiple bacterial sources by Salmonella, we carried out the comparison of the R plasmids harbored by the resistant isolates, as well as the isolates themselves, by applying various typing methods.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
From 1987 to 1994, 489 Salmonella spp. were isolated from blood cultures (3%), stool samples (87%), blood and stool samples (1%), urine samples (3%), urine and stool samples (4%), and biopsies (2%) of patients hospitalized at the University Hospital of Besançon, France. All isolates were biochemically (API 20E strip; BioMérieux) and serotypically characterized (Sanofi Pasteur). Cultures were routinely performed at 37°C on Mueller-Hinton (MH) agar plates (Sanofi Pasteur) or in brain heart (BH) infusion agar (Sanofi Pasteur), supplemented with 300 μg of rifampicin per ml (Sigma) and/or 50 μg of amoxicillin per ml (SmithKline-Beecham), as required. Transferability of amoxicillin resistance was assessed by conjugational matings, with a mutant of Escherichia coli K-12 resistant to rifampicin as a recipient. Conjugations were carried out in BH infusion agar for 4 h at 37°C or, alternatively, on 0.45-μm-pore-size nitrocellulose filters (Millipore) for 18 h at 37°C (22). Transconjugants were selected on MH agar medium containing rifampicin and amoxicillin.
Salmonella plasmids.
Plasmids harbored by Salmonella were extracted by the method of Kieser (15) and were visualized by electrophoresis in a horizontal 0.8% (wt/vol) agarose gel calibrated with reference plasmids from E. coli V517 (18). Total plasmid DNA of Salmonella was purified by the method of Birnboim and Doly (4), cleaved with EcoRI, BamHI, or HindIII (Boehringer Mannheim Biochemicals) according to the manufacturer’s recommendations, and subsequently electrophoresed in a 0.8% (wt/vol) agarose gel.
Hybridization experiments.
DNA probes were prepared from purified plasmids (Quiagen plasmid kit) by digestion with appropriate restriction enzymes or by amplification by PCR with oligonucleotide primers, as specified in Table 1. The DNA fragments were separated by agarose gel electrophoresis, purified with the Bio-Rad Prep-a-gene kit, and labelled by random priming (Random Primed Labelling kit; Boehringer-Mannheim) with [α32P]dCTP. Colony and Southern blot hybridizations were performed under highly stringent conditions. Plasmids R1 (21), RP1 (11), and RPL11 (16) were used as positive controls for the identification of TEM-1 (pI = 5.4), TEM-2 (pI = 5.6), and PSE-1 (pI = 5.7) β-lactamases, respectively. Replicon typing allows the classification of plasmids into replicon groups which, in some cases, match incompatibility groups (7). In this work, we used nine replicon-specific probes, known to be representative of Salmonella plasmids RepFIC, RepFIIA, RepHI1, RepHI2, RepI1, RepA/C, RepP, RepQ, and RepX (14, 37). Plasmids were individually typed by Southern hybridization.
TABLE 1.
DNA probes used in this study
| Probe | Characteristics of probes
|
|||
|---|---|---|---|---|
| Plasmidic origin | Probe size (bp) | Restriction sites or primers | Reference | |
| Replicon typing | ||||
| RepFIC | pULB2440 | 967 | EcoRI-HindIII | 7 |
| RepFIIA | pULB2401 | 543 | PstI | 7 |
| RepHI1 | pULB2436 | 2,250 | EcoRI-HindIII | 7 |
| RepHI2 | pULB2433 | 1,800 | EcoRI | 7 |
| RepI1 | pULB2428 | 1,100 | EcoRI-PstI | 7 |
| RepP | pULB2420 | 750 | HaeII | 7 |
| RepX | pULB2405 | 942 | HindIII | 7 |
| Virulence identification | ||||
| H6 | pIP1350 | 3,500 | HindIII | 12 |
| β-Lactamase determination | ||||
| TEM-1/2a | pBR322 | 489 | 5′GAGTACTCACCAGTCACAGAAAAGC3′ | 35 |
| 5′GACTTCCCGTCGTGTAGATAAC3′ | ||||
| PSE-1 | RPL11 | 586 | 5′AATGGCAATCAGCGCTTCCC3′ | 2, 16 |
| 5′GGGGCTTGATGCTCACTCCA3′ | ||||
Oligonucleotides and probe TEM-1/2 are homologous to TEM-1 and TEM-2 β-lactamases. IEF allowed us to distinguish between both enzymes.
PCR conditions and DNA sequencing.
The PCR mixtures (25 μl) contained 1 μl of bacterial lysate (obtained by heating bacterial colonies to 100°C for 15 min) or 25 to 50 ng of purified DNA, 0.2 U of Taq DNA polymerase (Goldstar; Eurogentec), 1× PCR Goldstar buffer, 0.3 μM each primer (Table 1), and 0.2 mM each deoxynucleoside triphosphate. The amplification step was performed for 30 cycles in a Crocodile II thermal cycler (Appligène). Each amplification cycle consisted of 1 min at 92°C, 2 min at 50°C, and 3 min at 72°C. A final extension was performed at 72°C for 10 min. PCR products obtained after amplification with the PSE primers were purified by using the Wizard PCR Preps kit (Promega) and were sequenced by an ABI 373A automatic sequencer (Perkin-Elmer, Applied Biosystems). Their nucleotide sequences were analyzed with the GeneStream align program (22a).
Macrorestriction analysis.
Preparation of whole cell DNA for pulsed-field gel electrophoresis (PFGE) was as described by Godard et al. (10). DNA-containing agarose plugs were incubated overnight in the presence of 50 U of XbaI (Boehringer-Mannheim) and underwent PFGE as reported previously. The restriction banding patterns of the isolates were compared by means of the Taxotron package (P. Grimont, Pasteur Institute), using SmaI restriction fragments of the Staphylococcus aureus NCTC 8325 genome for intergel calibration. Major restriction patterns were defined as differing by more than three bands and with similarity coefficients less than 85%, according to Struelens et al. (34). The Dice distance coefficient of macrorestriction analysis was calculated to be 1.
Antibiotic susceptibility.
Routine drug susceptibility tests were performed by using the agar diffusion method (disks from Sanofi Pasteur), according to the guidelines of the National Committee for Clinical Laboratory Standards (23). MICs were determined more precisely on MH agar plates containing serial twofold dilutions of the following antibiotics: amoxicillin, amoxicillin-clavulanate (SmithKline-Beecham), piperacillin, piperacillin-tazobactam (Wyeth-Lederlé), cefoperazone (Pfizer), cefuroxime (Glaxo-Wellcome), and cefotaxime (Roussel-Uclaf). An inoculum of 104 bacteria per spot was deposited by a Steers inoculator (30). Isolates were also screened for resistance to chloramphenicol (8 μg/ml), streptomycin (16 μg/ml), spectinomycin (16 μg/ml), tetracycline (8 μg/ml), trimethoprim (2 μg/ml), sulfadiazine (64 μg/ml), and nalidixic acid (16 μg/ml).
IEF of β-lactamases.
Analytical isoelectric focusing (IEF) of β-lactamases (19) produced by Salmonella was performed in precast polyacrylamide gels (Ampholine PAG Plate, pH 4.0 to 6.5 or pH 3.5 to 9.5; Pharmacia Biotech) using an LKB Multiphor 2117 apparatus (Pharmacia), with bacterial suspensions subjected to three cycles of freezing and thawing (5). β-Lactamase activity was revealed in gels by spreading 2 ml of a 0.05% (wt/vol) solution of nitrocefin (Glaxo-Wellcome).
RESULTS
Antimicrobial resistance of Salmonella.
A dramatic increase in the prevalence of amoxicillin resistance was observed among the Salmonella serovars (n = 489) isolated at the University Hospital of Besançon between 1987 (0%) and 1994 (42.5%). Most of the resistant Salmonella isolates belonged to the serovar Typhimurium (n = 77). Other Salmonella serovars were each represented by less than seven resistant isolates: six S. saint-paul, three S. enteritidis, three S. virchow, one S. agona, one S. blockley, one S. brandenburg, one S. heidelberg, one S. kedougou, one S. wien, and one Salmonella sp.
As shown in Table 2, most of the amoxicillin-resistant isolates (74 of 96) were also resistant to piperacillin (MIC at which 90% of the isolates are inhibited [MIC90] = 256 μg/ml). All of these isolates, however, were susceptible to the combination of piperacillin and tazobactam. Similarly, MICs of amoxicillin (MIC90 = > 2,048 μg/ml) were strongly reduced in the presence of the β-lactamase inhibitor clavulanic acid (MIC90 = 16 μg/ml), but seven isolates remained resistant to the combination of both drugs. No resistance to the expanded-spectrum cephalosporin cefotaxime was noted among the selected isolates.
TABLE 2.
Susceptibility levels of PSE-1 and TEM-1 Salmonella isolates to β-lactam antibioticsa
| Antibiotic | MIC (μg/ml)
|
No. resistantb | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| PSE-1 producers (n = 40) | ||||
| Amoxicillin | ≥2,048 | >2,048 | >2,048 | 40 |
| Amoxicillin-clavulanic acidc | 16–32 | 16 | 16 | 4 |
| Piperacillin | 256–>2,048 | 256 | 256 | 40 |
| Piperacillin-tazobactamd | 4–8 | 4 | 4 | 0 |
| Cefuroxime | 4–8 | 8 | 8 | 0 |
| Cefoperazone | 16–512 | 32 | 32 | 1 |
| Cefotaxime | 0.06–0.125 | 0.06 | 0.06 | 0 |
| TEM-1 producers (n = 54) | ||||
| Amoxicillin | 1,024–>2,048 | 2,048 | >2,048 | 54 |
| Amoxicillin-clavulanic acid | 8–32 | 16 | 16 | 1 |
| Piperacillin | 16–>2,048 | 128 | 256 | 32 |
| Piperacillin-tazobactam | 1–8 | 4 | 4 | 0 |
| Cefuroxime | 2–32 | 4 | 8 | 1 |
| Cefoperazone | 1–512 | 4 | 16 | 1 |
| Cefotaxime | 0.03–0.5 | 0.06 | 0.125 | 0 |
The MICs for 2 of 96 strains (the PSE-1/TEM-2- and the OXA-4-producing strains) are not included in this table.
According to the National Committee for Clinical Laboratory Standards breakpoints.
Amoxicillin and clavulanic acid were associated in the ratio of 1/0.5. MIC values refer to the concentrations of amoxicillin.
MIC values refer to the concentrations of piperacillin in the presence of a fixed amount of tazobactam (4 μg/ml).
Characterization of β-lactamases.
As evidenced by IEF and Southern blot hybridizations with specific nucleic acid probes, 94 of 96 isolates were found to produce a single β-lactamase (54 produced TEM-1 and 40 produced PSE-1), and 1 of the 96 isolates produced two enzymes (PSE-1 and TEM-2). All PSE-1 (CARB-2) producers belonged to the serovar Typhimurium. Direct sequencing of the pse-1 PCR products from 13 randomly chosen S. typhimurium isolates revealed that the amplified gene sequences shared 98% (and the derived amino acid sequence, more than 99%) identity with those of the PSE-1 gene carried by plasmid RPL11 in Pseudomonas aeruginosa (16). Finally, 1 of the 96 isolates expressed a β-lactamase of pI 7.4, tentatively identified as an OXA-1 enzyme (33a).
Identification and transferability of R plasmids.
The resistant isolates were found to individually contain one (n = 38), two (n = 28), three (n = 15), four (n = 10), five (n = 3), or six (n = 2) plasmids, with molecular lengths ranging from 1 to 82 kb. Conjugational transfer of the amoxicillin resistance phenotype to a recipient E. coli strain was successful in 67 of 96 (70%) of the Salmonella isolates. Most of the E. coli transconjugants acquired additional resistances to tetracycline, chloramphenicol, trimethoprim-sulfamethoxazole, neomycin, streptomycin, spectinomycin, nalidixic acid, and/or nitrofuranes (data not shown). Hybridization of the transferred plasmids with TEM- and PSE-type probes after Southern blotting demonstrated that the β-lactamase genes were all carried by plasmids larger than 35 kb (43 to 82 kb).
Identification of virulence plasmids.
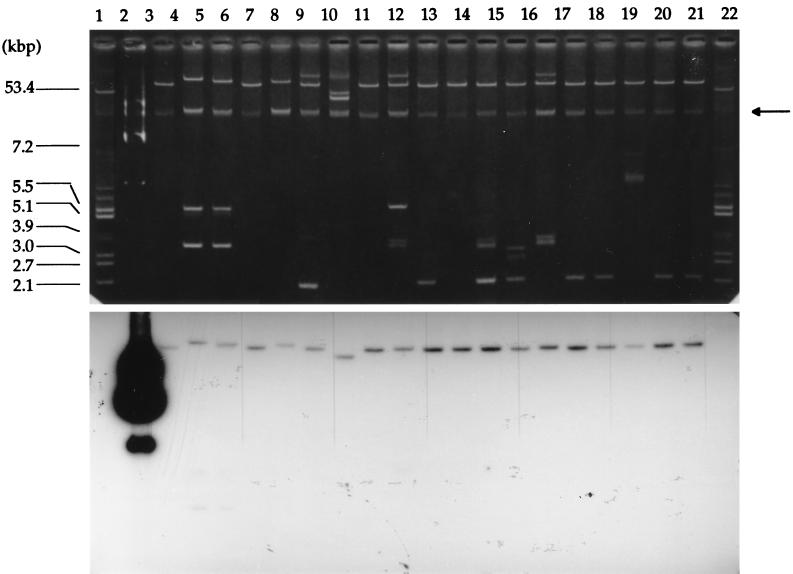
Sixty of the 77 (78%) S. typhimurium isolates and two S. enteritidis isolates harbored large (48- to 82-kb) plasmids that hybridized positively with a spvCD-orfE virulence probe after Southern transfer (Fig. 1). Interestingly, these virulence plasmids were found in 100% of the Salmonella spp. isolated from blood and urine samples, and in 50 and 47% of those obtained from biopsies and stool samples, respectively. Genes homologous to spvCD-orfE were not detected in serovars other than Typhimurium and Enteritidis. It should be stressed here that nearly all of the virulence plasmids (58 of 60) detected in the S. typhimurium isolates also carried genes coding for PSE-1 (n = 41) or TEM-1 (n = 17) β-lactamase. The two virulence plasmids found in S. enteritidis were demonstrated to determine β-lactam resistance as well (TEM-1).
FIG. 1.
Identification of the virulence plasmids in Salmonella typhimurium. (Upper panel) Visualization of Salmonella plasmids by agarose gel electrophoresis after extraction by the method of Kieser (15). Lanes 1 and 22, E. coli V517 plasmids used as molecular size standards (kbp are indicated on the left of the gel); lane 2, plasmid pIP1350 containing the spvCD-orfE virulence determinant was used as a positive control; lanes 3 to 21, selected virulent Salmonella isolates S1, S3, S7, S15, S16, S22, S39, S40, S41, S42, S43, S49, S50, S51, S53, S54, S55, S57, and S58, respectively. Chromosomal DNA bands (arrow) are seen in each lane. (Lower panel) Autoradiogram of the plasmids after Southern transfer and hybridization with the spvCD-orfE probe.
Replicon typing.
Replicon typing was carried out with probes specific to RepFIC, RepFIIA, RepHI1, RepHI2, RepI1, RepA/C, RepP, RepQ, and RepX groups. Eighty-three of the 96 amoxicillin-resistant Salmonella isolates (86.5%) contained one or several plasmids hybridizing with at least one of the selected probes. Plasmids of the RepFIC group or the RepFIC subgroup B (cross-hybridization with RepFIC and RepI1 probes) were predominant among the isolates hybridizing with just one probe (59 of 68), especially in S. typhimurium (n = 34). Other replicon groups were confined in less frequently isolated serovars (e.g., RepP [three Saint-Paul, one Brandenburg, and one Typhimurium], RepHI2 [one Kedougou and one Virchow], and RepQ [one Agona and one Blockley]). Fifteen of 96 strains scored positive with two (n = 9), three (n = 4), or even four (n = 2) different replicon probes, while 16 of 96 contained undetermined replicons. No plasmid hybridized with the RepFIIA, RepHI1, RepA/C, or RepX probes.
Plasmid restriction fragmentation analysis.
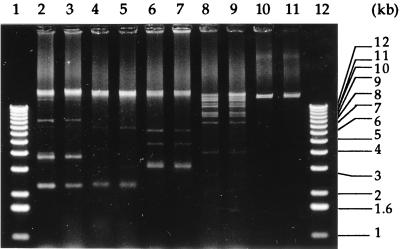
Plasmids of the resistant Salmonella isolates were finally compared on the basis of the restriction banding patterns produced after digestion with endonuclease EcoRI, BamHI, or HindIII. We could thus identify 14 different plasmidic groups showing unique core fragment patterns and differing from strain to strain by less than three bands (Fig. 2). The most frequent restriction profile (RFP I) was present in 25 isolates of S. typhimurium recovered from 1988 to 1994. All isolates of this group harbored a RepFIC virulence plasmid encoding a PSE-1 β-lactamase (Table 3). Thirteen other plasmid patterns (II to XIV) grouped four isolates or less each (Tables 3 and 4).
FIG. 2.
Restriction banding patterns of total plasmid DNA from selected Salmonella isolates. Lanes 1 and 12, DNA marker fragments (sizes in kbp are indicated on the right edge of the gel); lanes 2 to 7, BamHI digestion of DNA from isolates S3 and S5 (RFP II), S78 and S80 (RFP I), and S13 and S14 (RFP III); lanes 8 and 9, EcoRI digestion of S16 and S18 (RFP IV); lanes 10 and 11, HindIII digestion of S27 and S28 (RFP V).
TABLE 3.
Comparison of S. typhimurium isolates by using various phenotypic and genotypic markers
| β-Lactamase | PFGE group | R plasmid characterization
|
Isolates (n) | ||||
|---|---|---|---|---|---|---|---|
| Virulenceab | Replicon type | Transferbc | Length (kbp) | Restriction profile | |||
| PSE-1 | A | + | FIC | +/− | 60–72 | I | 25d |
| FIC | + | 60–72 | VIII | 4 | |||
| FIC | +/− | 60–72 | NRe | 9 | |||
| FIC/Q | − | 72 | NR | 2 | |||
| B | + | FIC | − | 60 | NR | 1 | |
| TEM-1 | C | + | FIC | + | 78–82 | II | 3 |
| D | + | FIC/P/Q | + | 72–82 | NR | 3f | |
| E | − | NDg | + | 43 | V | 3 | |
| − | FIC-Bh | + | 43 | VI | 2 | ||
| − | ND | + | 35 | VII | 2 | ||
| − | ND | + | 43 | NR | 4 | ||
| − | FIC/P/HI2 | + | 75 | NR | 1 | ||
| F | − | ND | + | 43 | XIII | 2 | |
| G | + | FIC | + | 75 | IV | 2 | |
| + | FIC | + | 67–82 | XII | 2 | ||
| + | FIC | + | 67 | NR | 1 | ||
| H | + | FIC | +/− | 82 | XIV | 2 | |
| Ungrouped | +/− | Various | +/− | 45–82 | NR | 8 | |
| OXA-1 | I | − | FIC | − | 78 | NR | 1 |
Hybridization with spvCD-orfE probe.
+, positive; −, negative for the character tested.
Conjugational transfer of amoxicillin resistance.
One of these isolates also produces a TEM-2 β-lactamase.
NR, different by more than three electrophoretic bands.
Two of these isolates harbored two distinct plasmids, one coding for virulence and another coding for β-lactamase; the replicon typing data concern the R plasmid.
ND, not determined.
RepFIC subgroup B.
TABLE 4.
Comparison of the resistant Salmonella serovars other than Typhimurium
| Serovars | β-Lactamase | R plasmid characterization
|
Isolates (n) | ||||
|---|---|---|---|---|---|---|---|
| Virulenceab | Replicon type | Transferbc | Length (kbp) | Restriction profile | |||
| Agona | TEM-1 | − | Q | + | 54 | NRd | 1 |
| Blockley | TEM-1 | − | Q | + | 43 | NR | 1 |
| Brandenburg | TEM-1 | − | P | + | 43 | NR | 1 |
| Enteritidis | TEM-1 | + | FIC | + | 52 | IX | 2 |
| − | FIC-B/P/HI2 | + | 67 | NR | 1 | ||
| Heidelberg | TEM-1 | − | FICA-Bf | + | 75 | NR | 1 |
| Kedougou | TEM-1 | − | HI2 | + | 43 | NR | 1 |
| Saint-Paul | TEM-1 | − | P | +/− | 66 | III | 2 |
| − | P | − | 43 | NR | 1 | ||
| − | P/HI2 | + | 57 | NR | 1 | ||
| − | P/HI2 | + | 43 | X | 2 | ||
| Virchow | TEM-1 | − | NDe | + | 48 | NR | 1 |
| − | HI2 | + | 72 | NR | 1 | ||
| − | P/HI2 | + | 82 | NR | 1 | ||
| Wien | TEM-1 | − | FICA-B | + | 82 | NR | 1 |
| Salmonella sp. | TEM-1 | − | P/HI2 | + | 82 | NR | 1 |
Hybridization with spvCD-orfE probe.
+, positive; −, negative for the character tested.
Conjugational transfer of amoxicillin resistance.
NR, different by more than three electrophoretic bands.
ND, not determined.
RepFIC subgroup A or B.
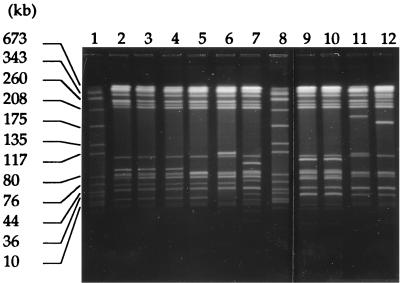
Macrorestriction analysis.
Isolates of the dominating serovar Typhimurium were analyzed by PFGE in order to study their genotypic relatedness. XbaI digestions resulted in approximately 15 fragments in the range of 10 to 675 kbp. A total of 19 different PFGE banding patterns were detected among the 77 isolates (representative patterns are shown in Fig. 3). With one exception, the 41 isolates producing PSE-1 β-lactamase could be grouped into a unique genotype, named A. Altogether, patterns C to H grouped 27 TEM-1-producing isolates, whereas eight isolates showed unique PFGE banding profiles. The isolate that produced OXA-1 β-lactamase showed a particular genotype named I (Table 3).
FIG. 3.
XbaI macrorestriction patterns of selected S. typhimurium isolates. Lanes 1 and 8, DNA marker fragments of S. aureus NCTC 8325 (sizes in kbp are indicated on the left of the gel); lanes 2 to 5, pulsotypes F (isolates S88, S16, S18, and S23); lanes 6, 7, 11, and 12, unrelated pulsotypes (S82, S22, S72, and S5); lanes 9 and 10, pulsotypes C (S41 and S51).
DISCUSSION
The dramatic increase in β-lactam resistance observed during the survey essentially concerned S. typhimurium (77 of 96 isolates), the most prevalent serovar isolated in our hospital (38%). In comparison, S. enteritidis, the second most prevalent serovar (26%), was only rarely resistant to amoxicillin (3 of 96 isolates). Analysis of the resistant S. typhimurium isolates by using various typing methods allowed the identification of two distinct groups of isolates, one producing PSE-1 β-lactamase and one producing TEM-1 enzyme (Table 3).
The PSE-1 group consisted of 40 isolates exhibiting very similar PFGE patterns, showing an identical resistance phenotype (Apr Cmr Spr Smr Sur Tcr), and belonging to the DT104 phage type (as determined from five randomly chosen isolates) (data not presented). These results strongly suggest that all the PSE-1 producers (except one that displayed a unique PFGE profile) actually are the progeny of a single epidemic strain that spread through France beginning in 1988. The observation that all these bacteria shared the rather unusual feature of having RepFIC virulence plasmids carrying the blaPSE-1 gene reinforces this hypothesis. This is also consistent with recent epidemiological data indicating that multiresistant DT104 S. typhimurium is increasing in incidence worldwide mostly because of the transmission of the pathogens from cattle to humans via food (27).
While our isolates appeared to be closely related with respect to the markers cited above, their RepFIC virulence/resistance plasmids showed a somewhat greater diversity. Differences in the size and transferability of the plasmids were indeed noted among the isolates. Furthermore, restriction banding pattern analysis demonstrated major differences between some of the RepFIC virulence/resistance replicons in bacteria harboring single plasmids (as shown by the different plasmidic groups in Table 3). This tends to indicate that variations occurred in the RepFIC virulence/resistance plasmid of the DT104 epidemic strain over time. In support of this speculation, it has been shown that Salmonella plasmids are frequently subjected to molecular rearrangements by homologous or illegitimate recombinations (37). Although the plasmidic profile analysis has been described as a useful epidemiological tool for the differentiation of epidemic from nonepidemic strains of Salmonella in outbreaks (39), this marker appears to be inappropriate to ascertain the epidemiological relatedness of strains isolated over long periods of time.
DT104 isolates resistant to amoxicillin by production of PSE-1 β-lactamase have been reported to be involved in large outbreaks (9, 41). In contrast to the DT104 epidemic strain isolated in our hospital, French, Danish, and British isolates were found to contain the blaPSE-1 gene integrated into the bacterial chromosome (26, 29, 32). In these latter strains, the blaPSE-1 and aadA2 genes (encoding streptomycin and spectinomycin resistance, respectively) were present on two distinct integrons, while the genetic determinants responsible for chloramphenicol and tetracycline resistance were suspected to reside on transposons. As shown by conjugational transfers (in 15 of 41 isolates), the RepFIC virulence/resistance plasmids characterized in this work confered multiresistance to chloramphenicol, spectinomycin, streptomycin, sulfadiazine, and tetracycline, in addition to β-lactam resistance. Therefore, it is tempting to assume that the plasmidic genes that determine these resistances are carried by integrons and/or transposons able to jump from the chromosome to resident plasmids and vice versa (29). There is increasing evidence that integrons are responsible for the acquisition and dissemination of resistance genes among Salmonella serovars through plasmid transfers. According to Tosini et al. (42), RepFI (including RepFIC) plasmids are frequent vehicles of class 1 integrons, which possibly explains their molecular evolution. The fact that the virulence plasmid of S. typhimurium may serve as a carrier for resistance genes is, however, unprecedented in DT104 isolates. The implications of this finding are not clear, but this finding raises the question of what role antibiotics play as selective agents in the possible dissemination of such virulence/resistance plasmids among gram-negative enteric bacteria. Interestingly, the locations of resistance genes on virulence plasmids in S. typhimurium isolate phage type 193 (40) and Shigella dysenteriae type 1 have recently been described (8).
The bacterial reservoir of the blaPSE-1 gene (equivalent to blaCARB-2) also remains unclear, since PSE-1/CARB-2 producers are infrequently encountered among enteric gram-negative bacteria such as E. coli (38) and Shigella sp. (20), in contrast to P. aeruginosa (2). Our sequencing results demonstrate that the β-lactamase gene carried by the RepFIC virulence/resistance plasmids of S. typhimurium is highly homologous to that previously detected in P. aeruginosa plasmid RPL11. Possible transfers of plasmids between Salmonella serovars and P. aeruginosa must be confirmed, since these species do not occur in similar ecological niches (20).
The second group of amoxicillin-resistant S. typhimurium isolates (n = 54) produced TEM-1 enzyme. In striking contrast to the PSE-1-producing isolates, members of the TEM-1 group showed a great genomic diversity when examined by PFGE (Table 3). Furthermore, the blaTEM-1 gene was detected on transferable plasmids (in 32 of 35 isolates) with dissimilar phenotypic and genotypic features. The transferability of R plasmids coding for TEM-1 β-lactamase was also a common feature for amoxicillin-resistant Salmonella other than S. typhimurium (17 of 19) (Table 4). The observation that TEM-1 β-lactamase is met with increasing frequencies in other gram-negative enteric species, such as E. coli (31), suggests that Salmonella may inherit the blaTEM-1 gene from the intestinal flora of humans (25) or animals. Indeed, transfers of amoxicillin resistance between S. enteritidis and E. coli have recently been demonstrated to occur in vivo (3).
Altogether, our results reinforce the notion that Salmonella serovars may efficiently acquire β-lactamase genes from various bacterial sources and that the increasing prevalence of amoxicillin resistance in these bacteria is part of a global trend that involves many other gram-negative species producing TEM-1 enzyme. In contrast, the development of β-lactam resistance due to the production of PSE-1 enzyme results from the dissemination of a few epidemic clones into the population.
ACKNOWLEDGMENTS
We thank D. Sirot (Faculté de Médecine, Clermont-Ferrand, France) for help in the identification of OXA-1 β-lactamase and F. Grimont (Unité des Entérobactéries, Institut Pasteur, Paris, France) for performing phage typing analysis of PSE-1 Salmonella isolates. We also thank T. Köhler and J.-C. Pechere (Centre Médical Universitaire, Geneva, Switzerland) for critical reading of the manuscript. We are grateful to Linda Bouchaour and Sandra Tasik for technical assistance. The DNA sequencing was performed at the Institut d’Etude et de Transfert de Gènes (Besançon, France).
REFERENCES
- 1.Archambaud M, Gerbaud G, Labau E, Marty N, Courvalin P. Possible in-vivo transfer of β-lactamase TEM-3 from Klebsiella pneumoniae to Salmonella kedougou. J Antimicrob Chemother. 1991;27:427–436. doi: 10.1093/jac/27.4.427. [DOI] [PubMed] [Google Scholar]
- 2.Arlet G, Philippon A. Construction by polymerase chain reaction and intragenic DNA probes for three main types of transferable β-lactamases (TEM, SHV, CARB) FEMS Microbiol Lett. 1991;82:19–26. doi: 10.1016/0378-1097(91)90414-6. [DOI] [PubMed] [Google Scholar]
- 3.Balis E, Vatopoulos A C, Kanelopoulou M, Mainas E, Hatzoudis G, Kontogianni V, Malamou-Lada H, Kitsou-Kiriakopoulou S, Kalapothaki V. Indications of in vivo transfer of an epidemic R plasmid from Salmonella enteritidis to Escherichia coli to the normal human gut flora. J Clin Microbiol. 1996;34:977–979. doi: 10.1128/jcm.34.4.977-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush K, Sykes R B. Methodology for the study of β-lactamases. Antimicrob Agents Chemother. 1986;30:6–10. doi: 10.1128/aac.30.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornado A M, Virgilio R. Evolution of drug resistance in Salmonella panama isolates in Chile. Antimicrob Agents Chemother. 1996;40:336–341. doi: 10.1128/aac.40.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couturier M, Bex F, Bergquist P L, Maas W K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988;52:375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta S, Pal A, Basu S, Banerjee P C. Involvement of a 70-kb plasmid of the epidemic Shigella dysenteriae type 1 (Dt66) strain in drug-resistance, lipopolysaccharide synthesis, and virulence. Microb Drug Resist. 1997;3:351–357. doi: 10.1089/mdr.1997.3.351. [DOI] [PubMed] [Google Scholar]
- 9.Glynn M K, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo F J. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 10.Godard C, Plésiat P, Michel-Briand Y. Persistance of Pseudomonas aeruginosa strains in seven cystic fibrosis patients followed over 20 months. Eur J Med. 1993;2:117–120. [PubMed] [Google Scholar]
- 11.Grinsted J, Saunders J R, Ingram L C, Sykes R B, Richmond M H. Properties of an R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972;110:529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulig P A, Danbara H, Guiney D G, Lax A J, Norel F, Rhen M. Molecular analysis of spv virulence genes of the Salmonella virulence plasmids. Mol Microbiol. 1993;7:825–830. doi: 10.1111/j.1365-2958.1993.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 13.Hermans P W M, Saha S K, van Leeuwen W J, Verbrugh H A, van Belkum A, Goessens W H F. Molecular typing of Salmonella typhi strains from Dhaka (Bangladesh) and development of DNA probes identifying plasmid-encoded multidrug-resistant isolates. J Clin Microbiol. 1996;43:1373–1379. doi: 10.1128/jcm.34.6.1373-1379.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones C, Stanley J. Salmonella plasmids of the pre-antibiotic era. J Gen Microbiol. 1992;138:189–197. doi: 10.1099/00221287-138-1-189. [DOI] [PubMed] [Google Scholar]
- 15.Kieser T. Factors affecting the isolation of cccDNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984;12:19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 16.Korfhagen T R, Loper J C. RPL11, an R factor of Pseudomonas aeruginosa determining carbenicillin and gentamicin resistance. Antimicrob Agents Chemother. 1975;7:69–73. doi: 10.1128/aac.7.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin A W, Usera M A, Barrett T J, Goldsby R A. Application of random amplified polymorphic DNA analysis to differentiate strains of Salmonella enteritidis. J Clin Microbiol. 1996;34:870–876. doi: 10.1128/jcm.34.4.870-876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macrina F L, Kopecko D J, Jones K R, Ayers D J, MacCowen S M. A multiple plasmid containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978;1:417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- 19.Matthew M, Harris A M, Mashall M J, Ross G W. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros A A, Hedge R W, Jacoby G A. Spread of a Pseudomonas-specific β-lactamase to plasmids of enterobacteria. J Bacteriol. 1982;149:700–707. doi: 10.1128/jb.149.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meynell E, Datta N. The relation of resistance transfer factors to the F factor (sex factor) of E. coli K12. Genet Res. 1966;7:134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- 22.Michel-Briand Y, Dupont M-J, Chardon-Loriaux I, Jouvenot M. Isolation of an antibiotic multiresistance plasmid from Pseudomonas aeruginosa. J Antimicrob Chemother. 1981;7:371–378. doi: 10.1093/jac/7.4.371. [DOI] [PubMed] [Google Scholar]
- 22a.National Center for Biotechnology. posting date. [Online.] GeneStream align program. 24 November 1997. http://vega.igh.cnrs.fr http://vega.igh.cnrs.fr. [3 April 1998, last date accessed.] . [3 April 1998, last date accessed.] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Zone diameter interpretive standards and equivalent minimum inhibitory concentration. Standard M100-S5, table M2-A5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 24.Navarro F, Llovet T, Echeita M A, Coll P, Aladuena A, Usera M A, Prats G. Molecular typing of Salmonella enterica serovar typhi. J Clin Microbiol. 1996;34:2831–2834. doi: 10.1128/jcm.34.11.2831-2834.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platt D J, Sommerville J S, Gribben J. Sequential acquisition of R-plasmids in vivo by Salmonella typhimurium. J Antimicrob Chemother. 1984;13:65–69. doi: 10.1093/jac/13.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Poirel L, Guibert M, Bellais S, Naas T, Nordmann P. Integron- and carbenicillinase-mediated reduced susceptibility to amoxicillin-clavulanic acid in isolates of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 from French patients. Antimicrob Agents Chemother. 1999;43:1098–1104. doi: 10.1128/aac.43.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poppe C, Smart N, Khakhria R, Johnson W, Spika J, Prescott J. Salmonella typhimurium DT104: a virulent and drug-resistant pathogen. Can Vet J. 1998;39:559–565. [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos J M, Alés J M, Cuenca-Estrella M, Fernandez-Roblas R, Soriano F. Changes in susceptibility of Salmonella enteritidis, Salmonella typhimurium and Salmonella virchow to six antimicrobial agents in a Spanish hospital. Eur J Clin Microbiol Infect Dis. 1996;15:85–88. doi: 10.1007/BF01586193. [DOI] [PubMed] [Google Scholar]
- 29.Ridley A, Threlfall E J. Molecular epidemiology of antibiotic resistance genes in multiresistant epidemic Salmonella typhimurium DT104. Microb Drug Resist. 1998;4:113–118. doi: 10.1089/mdr.1998.4.113. [DOI] [PubMed] [Google Scholar]
- 30.Sahm D F, Washington J A., II . Antibacterial susceptibility tests: dilution methods. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 1105–1116. [Google Scholar]
- 31.Sanders C C, Sanders E., Jr β-Lactam resistance in gram-negative bacteria: global trends and clinical impact. Clin Infect Dis. 1992;15:824–839. doi: 10.1093/clind/15.5.824. [DOI] [PubMed] [Google Scholar]
- 32.Sandvang D, Aarestrup F M, Jensen L B. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica typhimurium DT104. FEMS Microbiol Lett. 1998;160:37–41. doi: 10.1111/j.1574-6968.1998.tb12887.x. [DOI] [PubMed] [Google Scholar]
- 33.Schiaffino A, Beuzon C R, Uzzau S, Leori G, Cappuccinelli P, Casadesus J, Rubino S. Strain typing with IS200 fingerprints in Salmonella abortusovis. Appl Environ Microbiol. 1996;62:2375–2380. doi: 10.1128/aem.62.7.2375-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Sirot, D. Personal communication.
- 34.Struelens M J, Deplano A, Godard C, Maes N, Serruys E. Epidemiologic typing and delineation of genetic relatedness of methicillin-resistant Staphylococcus aureus by macrorestriction analysis of genomic DNA by pulsed-field gel electrophoresis. J Clin Microbiol. 1992;30:2599–2605. doi: 10.1128/jcm.30.10.2599-2605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tassios P T, Markogiannakis A, Vatopoulos A C, Katsanikou E, Velonakis E N, Kourea-Kremastinou J, Legakis N J. Molecular epidemiology of antibiotic resistance of Salmonella enteritidis during a 7-year period in Greece. J Clin Microbiol. 1997;35:1316–1321. doi: 10.1128/jcm.35.6.1316-1321.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor D E, Chumpitaz J C, Goldstein F. Variability of IncHI1 plasmids from Salmonella typhi with special reference to Peruvian plasmids encoding resistance to trimethoprim and other antibiotics. Antimicrob Agents Chemother. 1985;28:452–455. doi: 10.1128/aac.28.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson K S, Weber D A, Sanders C C, Sanders W E., Jr β-Lactamase production in members of the family Enterobacteriaceae and resistance to β-lactam-enzyme inhibitor combinations. Antimicrob Agents Chemother. 1990;34:622–627. doi: 10.1128/aac.34.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Threlfall E J, Frost J A. The identification, typing and fingerprinting of Salmonella: laboratory aspects and epidemiological applications. J Appl Bacteriol. 1990;68:5–16. doi: 10.1111/j.1365-2672.1990.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 40.Threlfall E J, Hampton M D, Chart H, Rowe B. Identification of a conjugative plasmid carrying antibiotic resistance and Salmonella plasmid virulence (spv) genes in epidemic strains of Salmonella typhimurium phage type 193. Lett Appl Microbiol. 1994;18:82–85. doi: 10.1111/j.1472-765X.1994.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 41.Threlfall E J, Ward L R, Skinner J A, Rowe B. Increase in multiple antibiotic resistance in nontyphoidal Salmonellas from humans in England and Wales: a comparison of data for 1994 and 1996. Microb Drug Resist. 1997;3:263–266. doi: 10.1089/mdr.1997.3.263. [DOI] [PubMed] [Google Scholar]
- 42.Tosini F, Visca P, Luzzi I, Dionisi A-M, Pezzella C, Petrucca A, Carattoli A. Class 1 integron-borne multiple-antibiotic resistance carried by Inc/FI and Inc/L/M plasmids in Salmonella enterica serotype Typhimurium. Antimicrob Agents Chemother. 1998;42:3053–3058. doi: 10.1128/aac.42.12.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vahaboglu H, Hall L M C, Mulazimoglu L, Dodanli S, Yildirim I, Livermore D M. Resistance to extended-spectrum cephalosporins, caused by PER-1 β-lactamase, in Salmonella typhimurium from Istanbul, Turkey. J Med Microbiol. 1995;43:294–299. doi: 10.1099/00222615-43-4-294. [DOI] [PubMed] [Google Scholar]
- 44.Vatopoulos A C, Mainas E, Balis E, Threlfall E J, Kanelopoulou M, Kalapothaki V, Malamou-Lada H, Rowe N J. Molecular epidemiology of ampicillin-resistant clinical isolates of Salmonella enteritidis. J Clin Microbiol. 1994;32:1322–1325. doi: 10.1128/jcm.32.5.1322-1325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wegener H C, Baggesen D L. Investigation of an outbreak of human salmonellosis caused by Salmonella enterica ssp. enterica serovar Infantis by use of pulsed field gel electrophoresis. Int J Food Microbiol. 1996;32:125–131. doi: 10.1016/0168-1605(96)01114-2. [DOI] [PubMed] [Google Scholar]