Abstract
Sporothrix schenckii is one of the etiological agents of sporotrichosis. In this review, we discuss the virulence factors that have been proven to participate in the S. schenckii-host interaction. Among these known factors, we can find cell wall glycoproteins, adhesins, melanin, extracellular vesicles, and dimorphism. Furthermore, the morphological transition of S. schenckii in response to environmental conditions such as pH and temperature represents a means by which the fungus is able to establish mycosis in mammals. One of the key features in the development of sporotrichosis is the adhesion of the fungus to the host extracellular matrix. This event represents the first step to developing the mycosis, which involves adhesins such as the glycoproteins Gp70, Hsp60, and Pap1, which play a key role during the infection. The production of melanin helps the fungus to survive longer in the tissues and to neutralize or diminish many of the host’s attacks, which is why it is also considered a key factor in pathogenesis. Today, the study of human fungal pathogens’ virulence factors is a thriving area of research. Although we know some of the virulence factors in S. schenckii, much remains to be understood about the complex process of sporotrichosis development and the factors involved during the infection.
Keywords: Sporothrix schenckii, virulence factors, extracellular vesicles, pathogenesis
1. Introduction
Sporotrichosis is a worldwide distributed mycosis, mainly found in tropical and subtropical areas, caused by the species of the Sporothrix pathogenic clade, including Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa [1,2]; S. schenckii and S. brasiliensis are considered to be sister species, and their clade is considered to be closely related to that of S. globosa, which suggests that they share a more recent common ancestor than the species of the environmental clade [3]. These organisms are thermodimorphic fungi found in soil, plant organic matter, and decomposing organic matter in nature, as the mycelial or saprophytic phase, while they are found in the host as yeasts, also known as the parasitic phase [4]. The infection transmission is through traumatic inoculation into the skin with contaminated material or through zoonosis, with cats as the main vector, and while the lesions are usually restricted to the skin, several clinical forms can be developed, which range from the most commonly found lymphocutaneous and fixed cutaneous forms, to the extracutaneous and disseminated forms, present mainly in immunocompromised patients [5]. In addition, the infection can also be acquired through spore inhalation, affecting mainly the lungs [6].
Although the first report of sporotrichosis dates back to 1898 by Benjamin R. Schenck [7], and even though the genome sequencing of the three main Sporothrix pathogenic species is already available [8,9] and the transcriptomic data from both S. schenckii morphologies have been reported (http://sporothrixgenomedatabase.unime.it/, accessed on 13 February 2022), limited information about this fungus virulence factors is known [10].
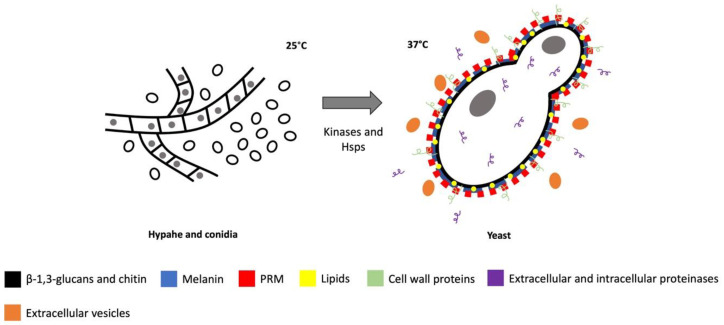
A virulence factor is defined as an element of the pathogen that contributes to damaging the host, whose absence causes reduction of fungal virulence without affecting the organism’s growth or fitness [11]. Based on this definition, only a few virulence factors (Figure 1) of S. schenckii, the most studied species of the clinical clade, are reported, such as cell wall proteins, involved in the fungal adherence to the host; kinases and heat shock proteins, which participate in thermotolerance and dimorphism; extracellular and intracellular proteinases, which help the fungus to colonize the host tissues; melanin, which protects the fungus against environmental stresses; extracellular vesicles, which transport elements needed for virulence outside the cell; lipids, found on the cell wall; and biofilm, a structure that participates in the resistance against antifungals; all of which will be described in this review.
Figure 1.
S. schenckii virulence factors.
The known host immune mechanism against the main S. schenckii virulence factors have been thoroughly reviewed elsewhere, for which they will be not discussed herein. Garcia-Carnero et al. (2018) [1] and Ruiz-Baca et al. (2021) [12] offer a broad and in-depth summary of the immune response induced by S. schenckii.
2. Cell Wall Proteins
The fungal cell wall plays an essential role during the host-pathogen interaction, besides participating in the cell wall integrity and remodeling [13]. Several pathogen-associated molecular patterns (PAMPs) can be found in the cell surface, which might be recognized through pattern recognition receptors (PRRs) of the host immune system, or which function as virulence factors, participating during the infection with a role in the organism pathogenicity [1,14,15].
These proteins are highly glycosylated and can be classified as typical and atypical proteins [16], depending on their secretion mechanisms [17,18,19].
In pathogenic fungi, many glycoproteins participate during the infection, having a role in how the pathogen invades the host, promotes its survival, and evades the host immune response [14,15]. Therefore, protein secretion is important for the pathogenic processes, and can take place by three main mechanisms: (i) co-translational translocation, through the endoplasmic reticulum and Golgi apparatus, which requires the presence of a signal sequence (signal peptide) in the N-terminus of the protein; (ii) post-translational translocation, which is SRP (signal recognition particle)-independent; and (iii) as cargo of extracellular vesicles (EVs) [17,18,19].
Typical cell wall proteins are those with a signal peptide in their N-terminus, which get covalently linked to the cell wall by different bonds to fulfill their biological function [19,20]. Most of these proteins have a glycosyl phosphatidyl inositol-anchor (GPI) that can be either linked to the cell wall or attached to the plasma membrane [19,21,22]. These proteins have a common structure: the peptide signal, domains for ligand binding, an optional central Ser/Thr rich glycosylated region, and a hydrophobic domain at the C-terminal that gets cleaved off and replaced with the GPI-anchor [20,22].
On the other hand, atypical proteins have also been found in the cell wall of many organisms, which neither present the signal peptide needed for the classical secretion pathway nor the GPI-anchor, but instead, they are secreted by non-classical mechanisms, such as post-translational translocation or EVs, and remain non covalently bound to the cell wall [19]. These proteins are known as moonlight, and they can be highly glycosylated and are usually intracellular proteins with enzymatic activities that have different functions on the cell wall, many of which have been found to play important roles in the virulence of pathogenic fungi [23].
The proteins from the cell wall of Sporothrix have not been well characterized, but there are several reports about some adhesins that bind to the host extracellular matrix (ECM) proteins, such as fibronectin, laminin, and type II collagen (Table 1) [24,25,26]. Adhesion of the pathogen to the host cells is essential for its correct colonization and further dissemination [27].
Table 1.
Known S. schenckii virulence factors.
| S. schenckii Virulence Factors | Function | References | |
|---|---|---|---|
| Cell wall proteins | Gp70 | Adhesin that binds to fibronectin, laminin, and type II collagen Immunodominant antigen |
[27,30,31,33,88] |
| Hsp60 | Adhesin that binds to laminin, elastin, fibrinogen, and fibronectin | [40] | |
| Pap1 | Adhesin that binds to laminin, elastin, fibrinogen, fibronectin, and type I and II collagen | [40] | |
| Kinases and heat shock proteins in dimorphism and thermotolerance | SSCMK1 | Morphological switching and thermotolerance | [49,51] |
| Hsp90 | Response to heat shock and proteotoxic stress Thermotolerance | [49] | |
| DRK1 | Morphological switching and thermotolerance | [57,59] | |
| Extracellular and intracellular proteinases | Degradation of skin constituents and cleaving of antibodies | [60,61,62] | |
| Melanin | Protection against environmental stresses and phagocytosis Neutralization of reactive oxygen species and nitric oxide Resistance to antifungals |
[67,69,70,72,73] | |
| Extracellular vesicles | Transportation of molecules involved in pathogenesis | [77,78] | |
| Lipids | Protection against the immune response and phagocytosis | [30] | |
| Biofilm | Resistance to antifungals | [79,85,87,89] | |
Some S. schenckii cell wall proteins that bind to fibronectin have been identified, although only a few have been well characterized. These proteins were reported to be of different molecular masses of 152, 92, 70, 67, 55, 50, 44, and 37 kDa, varying among isolates [27].
The most characterized adhesin of Sporothrix is the Gp70, found on the three clinically most important species, S. brasiliensis, S. schenckii, and S. globosa [28]. Gp70 is a highly glycosylated typical cell wall protein [29] that can present a molecular weight in a range of 60–70 kDa, probably due to changes in the glycosylation pattern, and with a 3-carboxymuconate cyclase domain that provides the enzymatic activity to degrade aromatic derivates from the environment when Sporothrix is growing as a saprophyte on the vegetal matter and debris [29]. This protein has been found attached to the cell wall, but also as secreted and as part of EVs [28,30]. The expression of this protein has been related to the virulence of the species and strains; in highly virulent S. brasiliensis isolates, the presence of Gp70 was poorly observed, while in low virulent S. brasiliensis and S. schenckii isolates, a higher density of the protein in the cell wall was found [30]. The importance of this protein for Sporothrix pathogenesis has been widely studied, suggesting that it has a dual role: adhesion and immunogenicity [31]. Due to its lower expression in most pathogenic isolates, and the fact that is the major antigenic protein in the cell wall [30,32,33], it is thought to contribute to the immune response against the fungus, as it was observed by the protective effect of passive immunization with monoclonal antibodies against Gp70 during infection, causing a decreased number in the CFUs [31], and by the fact that sera from all infected patients recognize the protein [34]. On the other hand, its adhesion properties were attributed since the purified protein is capable of binding extracellular matrix proteins, like fibronectin, laminin, and type II collagen [27,31], and treatment of endothelial EMC with antibodies against this protein decreased the attachment of Sporothrix yeasts [31]. In addition, it was found that Gp70 contributes to the fungal adherence to mouse tail, since anti-Gp70 antibodies reduced adhesion to the host tissue [34].
Another element of the S. schenckii cell wall is the peptidorhamnomannan (PRM), a glycoconjugate composed of mannose (57%), rhamnose (33.5%), proteins (14.2%), and galactose (1%) [35]. Although the PRM has been identified as one of the main antigenic components of the cell wall and, despite its carbohydrate moiety, has been widely studied [35,36,37,38,39], the proteins that compose it were only recently identified [40]. By mass spectrometry, it was found that this complex is composed of 325 proteins, most of which lack signal peptide and, therefore, could be moonlight proteins transported to the cell surface by non-canonical secretory pathways [40,41]. Two of these PRM proteins, Hsp60 and Pap1, were identified as adhesins that bind to different ECM proteins in the host cells [40]. Hsp60 is a chaperone highly conserved in all living organisms and upregulated in response to stress conditions, playing an important role in the cell housekeeping functions [42]. This protein has also been found on the cell wall of other pathogenic fungi, such as Histoplasma capsulatum [43], Paracoccidioides brasiliensis, and Paracoccidioides lutzii [44,45], as immunodominant antigens and participating as virulence factors by giving the fungus adhesion properties [43,45,46].
In S. schenckii, it was found that Hsp60 binds to laminin, elastin, fibrinogen, and fibronectin (Table 1). Correspondingly, this protein participates in the fungus virulence, as observed in Galleria mellonella assays. When S. schenckii yeasts were opsonized with antibodies against the recombinant Hsp60 (rHsp60) and then used to cause infection in the larvae, the fungus lost its ability to kill the host, probably due in part to an increase in the elimination of the pathogen, since the CFUs were significantly reduced. Additionally, this led to an increased humoral immune response, since hemocytes levels, phenoloxidase activity, and melanin production were also increased in this model, which was also probably due to a loss of the adhesion properties of the fungus. In addition, when the rHsp60 was inoculated in the larvae prior to a lethal challenge of S. schenckii, in concentrations equal or higher than 40 μg, an increase of the host survival was observed, thus representing a prophylaxis method for the protection against infection by S. schenckii [40]. Similar to the Hsp60, the Peptidorhamnomannan-associated protein 1 (Pap1), an uncharacterized protein present in S. brasiliensis but not in S. globosa, was found to lack a signal peptide and to function as an adhesin. Pap1 binds to laminin, elastin, fibrinogen, fibronectin, and type I and type II collagen and, like Hsp60, participates in the S. schenckii virulence. G. mellonella assays demonstrated that when yeast cells are opsonized with antibodies against the recombinant Pap1 (rPap1) and that when the rPap1 is used as prophylaxis, the fungus is incapable of killing the host [40].
It is also noteworthy to mention that the genes that code for both proteins were found to be upregulated in the yeast parasitic morphology and when the yeast interacts with HeLa cells, confirming their role during the interaction with the host [40].
3. Kinases and Heat Shock Proteins
As in other pathogenic fungi, dimorphism is an important virulence factor for S. schenckii (Figure 1) [10], since the fungus needs to change from its saprophytic phase to its parasitic phase, the yeast morphology, in order to grow and disseminate in the host [47]. It has been demonstrated that calcium is highly related to S. schenckii dimorphism, stimulating the fungus morphology transition to adapt to environmental changes [48,49]. By binding to calmodulin (CaM), calcium activates Ca2+/calmodulin-dependent protein kinases (CaMKs), which are serine/threonine proteins with two major domains, a highly conserved N-terminal catalytic domain and a C-terminal regulatory domain [50,51].
The S. schenckii sscmk1 gene codes for a CaMK, named SSCMK1, of 407 amino acids with a molecular weight of 45.6 kDa and with the 12 conserved subdomains needed for its function [51,52]. Furthermore, a pak-box/p21-rho-binding domain was found in the protein, which is involved in the regulation of the MAPK pathway that participates in the control of morphogenetic and proliferative processes. In addition, this protein is homologous to members of the CaMK family in other fungi, with a high degree of conservation [51]. Inhibition assays in S. schenckii using inhibitors of the CaMK activity, such as W-7, KN-62, and lavendustin C, demonstrated that the yeast is incapable of reentering into the budding cycle. These compounds have different action mechanisms with different degrees of specificity towards the enzyme: W-7 is a calmodulin inhibitor [53]; KN-62 is the most specific inhibitor, affecting the CaM binding domain [54], and lavendustin C inhibits CaMKs as well as tyrosine kinases [55]; yet, with all of the inhibitors used, an increase of the yeast to mycelium transition and an inhibition of the re-entry into the yeast cell cycle was observed, which suggest that the CaM kinase activity and the participation of calcium are needed for the G1/S transition during the budding cycle and are important in the regulation of S. schenckii dimorphism [51].
The sscmk1 gene was later silenced by RNAi and the results obtained by the inhibitors were confirmed with the mutants obtained. These mutants were incapable of growing efficiently in the yeast morphology at 35 °C, growing mainly as mycelium, which is suggested to be due to the decreased expression of the SSCMK1 [49]. In addition, a two-hybrid analysis of proteins that interact with the SSCMK1 identified an Hsp90 homolog that interacts with the kinase [49]. Hsp90 is a protein with essential housekeeping functions for the cell, including the promotion of folding and refolding of proteins during heat shock conditions and proteotoxic stress. This protein has been reported to bind to many client proteins, including kinases/phosphatases related to calcium, in other fungi like Candida albicans and Aspergillus fumigatus [56]. It was observed that SSCMK1 binds to the C-terminal domain of the S. schenckii Hsp90, which might cause an alteration of the Hsp90 activity that induces the release of effector proteins that normally bind to the Hsp90 N-terminal domain, such as calcineurin and other kinases that function as effectors for dimorphism. Therefore, regulation of the Hsp90 by SSCMK1 is suggested, and thus, decreasing the SSCMK1 levels is thought to affect the activity of Hsp90, making the cell more vulnerable to temperature changes [49]. To confirm this, the inhibitor geldanamycin was used to inhibit the Hsp90 activity, which caused an abnormal mycelial morphology, similar to that observed in the sscmk1 silencing mutants. Altogether, these results support the fact that SSCMK1 is necessary for the correct functioning of Hsp90 and, therefore, for S. schenckii thermotolerance and dimorphism [49].
Another histidine kinase, DRK1, has also been suggested to participate in S. schenckii dimorphism. The cDNA sequence of the DRK1, named SsDRK1, was found in the fungus, with an open reading frame of 4071 bp that codes for a protein of 1356 amino acids of 147.3 kDa [57]. This protein is predicted to be a soluble histidine kinase without transmembrane segments and with three domains: a sensor domain, a linker domain, and a functional domain. This hybrid histidine kinase is an important regulator of dimorphism in other pathogenic fungi such as Blastomyces dermatitidis and H. capsulatum [58], and since the SsDRK1 has a 65% identity match to that of B. dermatitidis and is overexpressed in the yeast morphology, it was suggested to be involved in the S. schenckii dimorphism [57]. This was later confirmed by the silencing of SsDRK1. The silencing mutants presented retarded growth in both mycelial and yeast morphologies, demonstrating a delay in asexual development. Likewise, yeast cells had abnormalities in their ultrastructure, with the presence of short and abundant plasma membrane invaginations, while conidia and hyphae had irregular morphologies, with increased and variable cell wall thickness, lack of the electron-dense zone on the cell wall surface, and plasma membrane invaginations [59]. In addition, the mutants had an increased sensitivity to congo red and zymolyase, and defects in colony pigmentation were observed since the mutants failed to produce as much melanin as the wild type strain. All of these results suggest that a decrease in SsDRK1 levels is associated with decreased polysaccharide levels in the S. schenckii cell wall [59].
Further, the silencing mutants had decreased virulence in a murine model of cutaneous sporotrichosis, since mice infected with these strains had reduced inflammatory cell infiltration, such as neutrophils and mononuclear cells, in the lesions, which might be explained by the deficiencies in asexual development and dimorphism, cell wall composition and integrity, and melanin synthesis [59].
4. Extracellular and Intracellular Proteinases
For pathogenic fungi, proteinase production for enzymatic digestion of the host tissues is essential for cell growth and infection development, and several of these enzymes have been suggested in S. schenckii. It was observed that when the yeast morphology of the fungus grows in media with albumin or collagen as nitrogen sources, it produces extracellular proteinases. Two of these were isolated and characterized, Proteinase I and Proteinase II. Proteinase I is a serine proteinase, with a molecular weight of 36.5 kDa, and an optimal working activity at pH of 6.0, with chymotrypsin like characteristics, while Proteinase II is an aspartic proteinase, with a molecular weight of 39 kDa, and an optimal working activity at pH of 3.5, with cathepsin D-like characteristics. Hemoglobin was the best substrate for both enzymes, but these were also capable of hydrolyzing stratum corneum, type I collagen, and elastin [60,61]. In vivo expression of these proteins was proved with a murine model of intracutaneous infection, where high titers of antibodies against both proteinases were found during the whole infection period [61], and treatment of the lesions with the proteinases’ inhibitors, chymostatin and pepstatin, reduced nodule development and caused faster regression [62]. These results suggest that Proteinase I and II help S. schenckii to degrade skin constituents, multiply in the dermis, and invade the host [60,61,62].
In addition, the secretion products of an S. schenckii pure yeast preparation were analyzed, demonstrating the presence of highly immunogenic proteins with molecular weights between 40 and 70 kDa, some of them with proteolytic activity. Although Proteinase I and II are thought to be present in this extract, proteases that were capable of cleaving different subclasses of human IgG were also found, which confirms the presence of different proteases activities performed by S. schenckii [63].
On the other hand, the presence of intracellular proteolytic activity in the yeast morphology was also reported. At pH of 5.0, proteolytic activity was observed and associated with proteins between 200 and 116 kDa, while at pH 7.0, this activity was even higher and associated with proteins between 200 and 70 kDa. It was demonstrated that these proteinases induce actin cytoskeleton alteration in epithelial cells, since cells treated with these enzymes showed a decrease of cytoplasmic actin stress fibers, producing spherical-shaped epithelial cells, membrane blebbing, and cytoplasmic actin filament alteration. This proteolytic activity was then inhibited with cysteine or serine protease inhibitors, which blocked the disruption of the actin stress fibers, confirming the proteinases substrates. These results suggest the presence of intracellular proteinases that alter the morphology and structure of the host cells, which also promotes the host tissue colonization by S. schenckii [64].
5. Melanins
Melanins are a class of pigments generated by oxidative polymerization of phenolic compounds, and although those pigments are not essential for the growth of the fungus, they play a fundamental role in the organism survival, conferring stability against certain environmental stressors, such as UV radiation and host defenses [65,66]. Although different biosynthetic routes are used for melanin production, in the case of fungi, the DOPA and DHN routes are the ones that predominate, and it has been shown that S. schenckii uses both routes. The first pathway requires the presence of exogenous substrates, such as 3,4 dihydroxyphenylalanin, and the melanin produced is known as eumelanin, while the second route can use precursors produced via acetyl-CoA from the glycolysis. Furthermore, there is a third pathway employed by S. schenckii called pyomelanin that uses L-tyrosine precursors for the melanin synthesis employed by S. schenckii [67,68,69,70]. Melanins confer great advantages on the organisms that possess them, and a feature that we will highlight is their role as a virulence factor in the pathogenic fungus S. schenckii. For instance, nonmelanized cells of S. schenckii are more susceptible to being engulfed by human monocytes and murine macrophages [67]. Melanin produced by the DHN route is of greater quantity and faster production in S. brasiliensis compared to S. schenckii, which correlates with the greater virulence that S. brasiliensis presents in the murine model, demonstrating the impact that the pigment has on the virulence of Sporothrix [71]. Furthermore, melanin protects fungal cells against reactive oxygen species (ROS) and nitric oxide (NO), chemical compounds released by host cells as a defense mechanism. Moreover, this pigment has been proposed as a scavenger that protect the fungus against UV radiation [67]. The mutant strain of S. schenckii lacking melanin causes less progression of sporotrichosis in the murine model compared to the wild type strain; therefore, it has been proposed that the presence of melanin in S. schenckii may be an important factor in the development of the mycosis [72]. In the human pathogenic fungi H. capsulatum, Cryptococcus neoformans, and P. brasiliensis, the melanin acts as a fungal component that provides resistance to the antifungal drugs amphotericin B and caspofungin [73]. The same role has been demonstrated for S. brasiliensis [70]. In addition, melanized cells of S. schenckii and S. brasiliensis have shown resistance to the antifungal drug terbinafine, another of the drugs of choice to treat sporotrichosis [69]. As an antifungal resistance factor, melanin and its synthesis pathways could be a target for the design of new antifungal drugs for sporotrichosis therapies. A recent study has shown that melanin is a key factor to prevent phagocytosis of S. globosa by THP-1 macrophages, given that this cell wall component neutralizes the respiratory burst and suppresses the ROS and NO production; besides, the presence of melanin in S. globosa decreases the expression of TLR2 and TLR4, receptors involved in the recognition of the fungus [74,75,76]. Therefore, the presence of a said component in the cell wall of the pathogen reduces the possibility of the host to fight the fungus. Finally, the pro-inflammatory response is also decreased, especially TNFα and IL-6, in the presence of S. schenckii wild-type fungus compared to the mutant lacking melanin [74].
6. Extracellular Vesicles
Extracellular vesicles, lipid bilayer structures released by cells, have been described in a variety of human pathogenic fungi such as C. albicans, Candida parapsilosis and H. capsulatum; in the last one, the vesicular content has molecules involved in the cell wall architecture, but also includes phospholipids and proteins related with virulence, pathogenesis, and stress response [77]. The formation of said structures has been described in S. schenckii [77], and presumably, the macromolecules in this vesicular transport contain products that are associated with the pathogenesis of the fungus. In a recent proteomic analysis of S. schenckii EVs, 40 different proteins were found, of which 35% were not characterized; in the case of S. brasiliensis, the EVs harbor a variety of 63 proteins, 27% of which are not characterized. However, in both species, most of the characterized proteins are related to metabolic process, transport, oxidation-reduction reactions, stress response, DNA metabolic process, and cellular component organization [78]. The EVs of S. brasiliensis activate the production of the cytokines TNFα, as well as INFγ and the interleukins IL-12p40, and IL-1β, in BALB/c mice [78]. This suggests that EVs participate as virulence factors in the establishment of infection.
7. Lipids and Biofilm
Lipids associated with the cell wall of S. schenckii are involved in pathogenesis since these components inhibit the phagocytic process by macrophages of Swiss mice. However, lipids can also trigger a high release of NO and TNFα, which help to eliminate the pathogen and to stimulate the immune response [79].
Biofilm formation has been considered a virulence factor in different human pathogenic fungi such as Candida, Aspergillus and Cryptococcus [80,81,82], and recent studies have shown that S. schenckii is capable of generating this complex structure, whose main components are glycoproteins, polysaccharides, nucleic acids, and lipids [83,84,85]. This structure complicates antifungal treatment since, apart from being a barrier that prevents the access of certain molecules to the interior of the biofilm, it is a key participant in the acquisition of resistance to the antifungals of choice, such as itraconazole, fluconazole, voriconazole, posaconazole, amphotericin B, flucytosine, and caspofungin [84,85,86,87]. The little access that antifungals have to reach their target cells, either by impediment, adsorption, inactivation, neutralization, or expulsion caused by the biofilm, causes the mycosis to become recalcitrant, for which biofilm development has become an important factor for the development and persistence of sporotrichosis.
8. Conclusions
Some human pathogenic fungi possess virulence factors that give them the ability to cause disease in the host. Despite the immune response that is triggered to combat these pathogens, they survive and persist in the host thanks to virulence factors that help them fight and damage host tissue. The study of S. schenckii virulence factors is important to continue building knowledge about sporotrichosis, and to continue with the development of new drugs to combat mycosis, as well as the possibility of generating vaccines that allow the prevention of the infection.
Acknowledgments
We would like to thank both working teams: The Fungi Physiology Laboratory and The Glycobiology of Pathogenic Fungi Laboratory of the Department of Biology of the Universidad de Guanajuato. A special thanks to CONACYT for the postdoctoral fellowship received.
Author Contributions
Both authors, L.C.G.-C. and J.A.M.-Á. contributed equally in Conceptualization; Writing; Review and Editing to the completion of this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.García-Carnero L.C., Pérez-García L.A., Martínez-Álvarez J.A., Reyes-Martínez J.E., Mora-Montes H.M. Current Trends to Control Fungal Pathogens: Exploiting Our Knowledge in the Host-Pathogen Interaction. Infect. Drug Resist. 2018;11:903–913. doi: 10.2147/IDR.S170337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes-Bezerra L.M., Walker L.A., Niño-Vega G., Mora-aMontes H.M., Neves G.W.P., Villalobos-Duno H., Barreto L., Garcia K., Franco B., Martínez-Álvarez J.A., et al. Cell Walls of the Dimorphic Fungal Pathogens Sporothrix schenckii and Sporothrix brasiliensis Exhibit Bilaminate Structures and Sloughing of Extensive and Intact Layers. PLoS Negl. Trop. Dis. 2018;12:e0006169. doi: 10.1371/journal.pntd.0006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Carvalho J.A., Beale M.A., Hagen F., Fisher M.C., Kano R., Bonifaz A., Toriello C., Negroni R., Rego R.S.d.M., Gremião I.D.F., et al. Trends in the Molecular Epidemiology and Population Genetics of Emerging Sporothrix Species. Stud. Mycol. 2021;100:100129. doi: 10.1016/j.simyco.2021.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes-Bezerra L.M., Schubach A., Costa R.O. Sporothrix schenckii and Sporotrichosis. An. Acad. Bras. Cienc. 2006;78:293–308. doi: 10.1590/S0001-37652006000200009. [DOI] [PubMed] [Google Scholar]
- 5.Barros M.B.D.L., de Almeida Paes R., Schubach A.O. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011;24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Romero E., Reyes-Montes M., Pérez-Torres A., Ruiz-Baca E., Villagómez-Castro J.C., Mora-Montes H.M., Flores-Carreón A., Toriello C. Sporothrix schenckii Complex and Sporotrichosis, an Emerging Health Problem. Future Microbiol. 2011;6:85–102. doi: 10.2217/fmb.10.157. [DOI] [PubMed] [Google Scholar]
- 7.Hektoen L., Perkins C.F. Refractory Subcutaneous Abscesses Caused by Sporothrix schenckii. A New Pathogenic Fungus. J. Exp. Med. 1900;5:77–89. doi: 10.1084/jem.5.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira M.M.M., de Almeida L.G., Kubitschek-Barreira P., Alves F.L., Kioshima É.S., Abadio A.K., Fernandes L., Derengowski L.S., Ferreira K.S., Souza R.C., et al. Comparative Genomics of the Major Fungal Agents of Human and Animal Sporotrichosis: Sporothrix schenckii and Sporothrix brasiliensis. BMC Genom. 2014;15:943. doi: 10.1186/1471-2164-15-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L., Gao W., Giosa D., Criseo G., Zhang J., He T., Huang X., Sun J., Sun Y., Huang J., et al. Whole-Genome Sequencing and In Silico Analysis of Two Strains of Sporothrix globosa. Genome Biol. Evol. 2016;8:3292–3296. doi: 10.1093/gbe/evw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamez-Castrellón A.K., Romeo O., García-Carnero L.C., Lozoya-Pérez N.E., Mora-Montes H.M. Virulence Factors in Sporothrix schenckii, One of the Causative Agents of Sporotrichosis. Curr. Protein Pept. Sci. 2019;21:295–312. doi: 10.2174/1389203720666191007103004. [DOI] [PubMed] [Google Scholar]
- 11.Rementeria A., López-Molina N., Ludwig A., Vivanco A.B., Bikandi J., Pontón J., Garaizar J. Genes and Molecules Involved in Aspergillus fumigatus Virulence. Rev. Iberoam. Micol. 2005;22:1–23. doi: 10.1016/S1130-1406(05)70001-2. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Baca E., Pérez-Torres A., Romo-Lozano Y., Cervantes-García D., Alba-Fierro C.A., Ventura-Juárez J., Torriello C. The Role of Macrophages in the Host’s Defense against Sporothrix schenckii. Pathogens. 2021;10:905. doi: 10.3390/pathogens10070905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora-Montes H.M., Ponce-Noyola P., Villagómez-Castro J.C., Gow N.A.R., Flores-Carreón A., López-Romero E. Protein Glycosylation in Candida. Future Microbiol. 2009;4:1167–1183. doi: 10.2217/fmb.09.88. [DOI] [PubMed] [Google Scholar]
- 14.Lin B., Qing X., Liao J., Zhuo K. Role of Protein Glycosylation in Host-Pathogen Interaction. Cells. 2020;9:1022. doi: 10.3390/cells9041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Retanal C., Ball B., Geddes-Mcalister J. Post-Translational Modifications Drive Success and Failure of Fungal–Host Interactions. J. Fungi. 2021;7:124. doi: 10.3390/jof7020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tronchin G., Pihet M., Lopes-Bezerra L., Bouchara J.P. Adherence Mechanisms in Human Pathogenic Fungi. Med. Mycol. 2008;46:749–772. doi: 10.1080/13693780802206435. [DOI] [PubMed] [Google Scholar]
- 17.Delic M., Valli M., Graf A.B., Pfeffer M., Mattanovich D., Gasser B. The Secretory Pathway: Exploring Yeast Diversity. FEMS Microbiol. Rev. 2013;37:872–914. doi: 10.1111/1574-6976.12020. [DOI] [PubMed] [Google Scholar]
- 18.Hernández-Chávez M.J., González-Hernández R.J., Trujillo-Esquivel J.E., Hernández-Cervantes A., Mora-Montes H.M. The Secretory Pathway in the Filamentous Fungus Trichoderma. Elsevier; Amsterdam, The Netherlands: 2014. [DOI] [Google Scholar]
- 19.Satala D., Karkowska-Kuleta J., Zelazna A., Rapala-Kozik M., Kozik A. Moonlighting Proteins at the Candidal Cell Surface. Microorganisms. 2020;8:1046. doi: 10.3390/microorganisms8071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latgé J.-P. Tasting the Fungal Cell Wall. Cell. Microbiol. 2010;12:863–872. doi: 10.1111/j.1462-5822.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 21.Ielasi F.S., Decanniere K., Willaert R.G. The Epithelial Adhesin 1 (Epa1p) from the Human-Pathogenic Yeast Candida glabrata: Structural and Functional Study of the Carbohydrate-Binding Domain. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012;68:210–217. doi: 10.1107/S0907444911054898. [DOI] [PubMed] [Google Scholar]
- 22.de Groot P.W.J., Bader O., de Boer A.D., Weig M., Chauhan N. Adhesins in Human Fungal Pathogens: Glue with Plenty of Stick. Eukaryot. Cell. 2013;12:470–481. doi: 10.1128/EC.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffery C.J. Why Study Moonlighting Proteins? Front. Genet. 2015;6:211. doi: 10.3389/fgene.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima O.C., Figueiredo C.C., Pereira B.A.S., Coelho M.C.P., Morandi V., Lopes-Bezerra L.M. Adhesion of the Human Pathogen Sporothrix schenckii to Several Extracellular Matrix Proteins. Braz. J. Med. Biol. Res. 1999;32:651–657. doi: 10.1590/S0100-879X1999000500020. [DOI] [PubMed] [Google Scholar]
- 25.Previato O., Mendonc L., Lima O.C., Figueiredo C.C., De Biologia D. Involvement of Fungal Cell Wall Components in Adhesion of Sporothrix schenckii to Human Fibronectin. Infect Immun. 2001;69:6874–6880. doi: 10.1128/IAI.69.11.6874-6880.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima O.C., Bouchara J.P., Renier G., Marot-Leblond A., Chabasse D., Lopes-Bezerra L.M. Immunofluorescence and Flow Cytometry Analysis of Fibronectin and Laminin Binding to Sporothrix schenckii Yeast Cells and Conidia. Microb. Pathog. 2004;37:131–140. doi: 10.1016/j.micpath.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Teixeira P.A.C., de Castro R.A., Nascimento R.C., Tronchin G., Torres A.P., Lazéra M., de Almeida S.R., Bouchara J.-P., Loureiro y Penha C.V., Lopes-Bezerra L.M. Cell Surface Expression of Adhesins for Fibronectin Correlates with Virulence in Sporothrix schenckii. Microbiology. 2009;155:3730–3738. doi: 10.1099/mic.0.029439-0. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues A.M., Kubitschek-Barreira P.H., Fernandes G.F., de Almeida S.R., Lopes-Bezerra L.M., de Camargo Z.P. Immunoproteomic Analysis Reveals a Convergent Humoral Response Signature in the Sporothrix schenckii Complex. J. Proteom. 2015;115:8–22. doi: 10.1016/j.jprot.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Álvarez J.A., García-Carnero L.C., Kubitschek-Barreira P.H., Lozoya-Pérez N.E., Belmonte-Vázquez J.L., De Almeida J.R.F., De Gómez-Infante A.J., Curty N., Villagómez-Castro J.C., Peña-Cabrera E., et al. Analysis of Some Immunogenic Properties of the Recombinant Sporothrix schenckii Gp70 Expressed in Escherichia coli. Future Microbiol. 2019;14:397–410. doi: 10.2217/fmb-2018-0295. [DOI] [PubMed] [Google Scholar]
- 30.Castro R.A., Kubitschek-Barreira P.H., Teixeira P.A.C., Sanches G.F., Teixeira M.M., Quintella L.P., Almeida S.R., Costa R.O., Camargo Z.P., Felipe M.S.S., et al. Differences in Cell Morphometry, Cell Wall Topography and Gp70 Expression Correlate with the Virulence of Sporothrix brasiliensis Clinical Isolates. PLoS ONE. 2013;8:e75656. doi: 10.1371/journal.pone.0075656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nascimento R.C., Espíndola N.M., Castro R.A., Teixeira P.A.C., Penha C.V.L., Lopes-Bezerra L.M., Almeida S.R. Passive Immunization with Monoclonal Antibody against a 70-KDa Putative Adhesin of Sporothrix schenckii Induces Protection in Murine Sporotrichosis. Eur. J. Immunol. 2008;38:3080–3089. doi: 10.1002/eji.200838513. [DOI] [PubMed] [Google Scholar]
- 32.Nascimento R.C., Almeida S.R. Humoral Immune Response against Soluble and Fractionate Antigens in Experimental Sporotrichosis. FEMS Immunol. Med. Microbiol. 2005;43:241–247. doi: 10.1016/j.femsim.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Baca E., Mora-Montes H.M., López-Romero E., Toriello C., Mojica-Marín V., Urtiz-Estrada N. 2D-Immunoblotting Analysis of Sporothrix schenckii Cell Wall. Mem. Inst. Oswaldo Cruz. 2011;106:248–250. doi: 10.1590/S0074-02762011000200021. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Baca E., Toriello C., Pérez-Torres A., Sabanero-Lopez M., Villagómez-Castro J.C., López-Romero E. Isolation and Some Properties of a Glycoprotein of 70 KDa (Gp70) from the Cell Wall of Sporothrix schenckii Involved in Fungal Adherence to Dermal Extracellular Matrix. Med. Mycol. 2009;47:185–196. doi: 10.1080/13693780802165789. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd K.O., Bitoon M.A. Isolation and Purification of a Peptido-Rhamnomannan from the Yeast Form of Sporothrix schenckii. Structural and Immunochemical Studies. J. Immunol. 1971;107:663–671. [PubMed] [Google Scholar]
- 36.Lopes-Alves L.M., Mendonça-Previato L., Fournet B., Degand P., Previato J.O. O-Glycosidically Linked Oligosaccharides from Peptidorhamnomannans of Sporothrix schenckii. Glycoconj. J. 1992;9:75–81. doi: 10.1007/BF00731702. [DOI] [PubMed] [Google Scholar]
- 37.Alves L.L., Travassos L.R., Previato J.O., Mendonça-previato L. Novel Antigenic Determinants from Peptidorhamnomannans of Sporothrix schenckii. Glycobiology. 1994;4:281–288. doi: 10.1093/glycob/4.3.281. [DOI] [PubMed] [Google Scholar]
- 38.Lima O.C., Lopes Bezerra L.M. Identification of a Concanavalin A-Binding Antigen of the Cell Surface of Sporothrix schenckii. J. Med. Vet. Mycol. 1997;35:167–172. doi: 10.1080/02681219780001101. [DOI] [PubMed] [Google Scholar]
- 39.Lopes-Bezerra L.M. Sporothrix schenckii Cell Wall Peptidorhamnomannans. Front. Microbiol. 2011;2:243. doi: 10.3389/fmicb.2011.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Carnero L.C., Salinas-Marín R., Lozoya-Pérez N.E., Wrobel K., Wrobel K., Martínez-Duncker I., Niño-Vega G.A., Mora-Montes H.M. The Heat Shock Protein 60 and Pap1 Participate in the Sporothrix schenckii-Host Interaction. J. Fungi. 2021;7:960. doi: 10.3390/jof7110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen M.J., Chirico W.J., Lipke P.N. Through the Back Door: Unconventional Protein Secretion. Cell Surf. 2020;6:100045. doi: 10.1016/j.tcsw.2020.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiwari S., Thakur R., Shankar J. Role of Heat-Shock Proteins in Cellular Function and in the Biology of Fungi. Biotechnol. Res. Int. 2015;2015:132635. doi: 10.1155/2015/132635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez F.J., Allendoerfer R., Deepe G.S. Vaccination with Recombinant Heat Shock Protein 60 from Histoplasma Capsulatum Protects Mice against Pulmonary Histoplasmosis. Infect. Immun. 1995;63:2587–2595. doi: 10.1128/iai.63.7.2587-2595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izacc S.M.S., Gomez F.J., Jesuino R.S.A., Fonseca C.A., Felipe M.S.S., Deepe G.S., Soares C.M.A. Molecular Cloning, Characterization and Expression of the Heat Shock Protein 60 Gene from the Human Pathogenic Fungus Paracoccidioides brasiliensis. Med. Mycol. 2001;39:445–455. doi: 10.1080/mmy.39.5.445.455. [DOI] [PubMed] [Google Scholar]
- 45.Thomaz L., Nosanchuk J.D., Rossi D.C.P., Travassos L.R., Taborda C.P. Monoclonal Antibodies to Heat Shock Protein 60 Induce a Protective Immune Response against Experimental Paracoccidioides lutzii. Microbes Infect. 2014;16:788–795. doi: 10.1016/j.micinf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Long K.H., Gomez F.J., Morris R.E., Newman S.L. Identification of Heat Shock Protein 60 as the Ligand on Histoplasma Capsulatum That Mediates Binding to CD18 Receptors on Human Macrophages. J. Immunol. 2003;170:487–494. doi: 10.4049/jimmunol.170.1.487. [DOI] [PubMed] [Google Scholar]
- 47.Silva-Bailão M.G., de SousaLima P., Oliveira M.M.E., Oliveira L.C., Almeida-Paes R., Borges C.L., Bailão A.M., Coelho A.S.G., de AlmeidaSoares C.M., Zancopé-Oliveira R.M. Comparative Proteomics in the Three Major Human Pathogenic Species of the Genus Sporothrix. Microbes Infect. 2021;23:4762. doi: 10.1016/j.micinf.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Serrano S., Del Valle N.R. Calcium Uptake and Efflux during the Yeast to Mycelium Transition in Sporothrix schenckii. Mycopathologia. 1990;112:1–9. doi: 10.1007/BF01795170. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Caban J., Gonzalez-Velazquez W., Perez-Sanchez L., Gonzalez-Mendez R., Del Valle N.R. Calcium/Calmodulin Kinase1 and Its Relation to Thermotolerance and HSP90 in Sporothrix schenckii: An RNAi and Yeast Two-Hybrid Study. BMC Microbiol. 2011;11:162. doi: 10.1186/1471-2180-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braun A.P., Schulman H. KINASE: From Form to Function. Annu. Rev. Physiol. 1995;4:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 51.Valle-Aviles L., Valentin-Berrios S., Gonzalez-Mendez R.R., Rodriguez-Del Valle N. Functional, Genetic and Bioinformatic Characterization of a Calcium/Calmodulin Kinase Gene in Sporothrix schenckii. BMC Microbiol. 2007;7:107. doi: 10.1186/1471-2180-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanks S.K., Hunter T. The Eukaryotic Protein Kinase Superfamily: Kinase (Catalytic) Domain Structure and Classification 1. FASEB J. 1995;9:576–596. doi: 10.1096/fasebj.9.8.7768349. [DOI] [PubMed] [Google Scholar]
- 53.Périanin A., Pedruzzi E., Hakim J. W-7, a Calmodulin Antagonist, Primes the Stimulation of Human Neutrophil Respiratory Burst by Formyl Peptides and Platelet-Activating Factor. FEBS Lett. 1994;342:135–138. doi: 10.1016/0014-5793(94)80487-7. [DOI] [PubMed] [Google Scholar]
- 54.Hidaka H., Yokokura H. Molecular and Cellular Pharmacology of a Calcium/Calmodulin-Dependent Protein Kinase II (CaM Kinase II) Inhibitor, KN-62, and Proposal of CaM Kinase Phosphorylation Cascades. Adv. Pharmacol. 1996;36:193–219. doi: 10.1016/S1054-3589(08)60583-9. [DOI] [PubMed] [Google Scholar]
- 55.Schneider J.C., El Kebir D., Chéreau C., Lanone S., Huang X.L., De Buys Roessingh A.S., Mercier J.C., Dall’Ava-Santucci J., Dinh-Xuan A.T. Involvement of Ca2+/Calmodulin-Dependent Protein Kinase II in Endothelial NO Production and Endothelium-Dependent Relaxation. Am. J. Physiol.-Heart Circ. Physiol. 2003;284:2311–2319. doi: 10.1152/ajpheart.00932.2001. [DOI] [PubMed] [Google Scholar]
- 56.Leach M.D., Klipp E., Cowen L.E., Brown A.J.P. Fungal Hsp90: A Biological Transistor That Tunes Cellular Outputs to Thermal Inputs. Nat. Rev. Microbiol. 2012;10:693–704. doi: 10.1038/nrmicro2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou B., Zhang Z., Zheng F., Liu X. Molecular Cloning, Characterization and Differential Expression of DRK1 in Sporothrix schenckii. Int. J. Mol. Med. 2013;31:99–104. doi: 10.3892/ijmm.2012.1193. [DOI] [PubMed] [Google Scholar]
- 58.Nemecek J.C., Wüthrich M., Klein B.S. Global Control of Dimorphism and Virulence in Fungi. Science. 2006;312:583–588. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z., Hou B., Wu Y.Z., Wang Y., Liu X., Han S. Two-Component Histidine Kinase DRK1 Is Required for Pathogenesis in Sporothrix schenckii. Mol. Med. Rep. 2018;17:721–728. doi: 10.3892/mmr.2017.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanada T. Isolation and Characterization of Extracellular Proteinases from Sporothrix schenckii. Nippon Hifuka Gakkai Zasshi. Jpn. J. Dermatol. 1987;97:1223–1230. doi: 10.14924/dermatol.97.1223. [DOI] [PubMed] [Google Scholar]
- 61.Yoshiike T., Lei P.C., Komatsuzaki H., Ogawa H. Antibody Raised against Extracellular Proteinases of Sporothrix schenckii in S. schenkii Inoculated Hairless Mice. Mycopathologia. 1993;123:69–73. doi: 10.1007/BF01365082. [DOI] [PubMed] [Google Scholar]
- 62.Lei P.C., Yoshiike T., Ogawa H. Effects of Proteinase Inhibitors on the Cutaneous Lesion of Sporothrix schenckii Inoculated Hairless Mice. Mycopathologia. 1993;123:81–85. doi: 10.1007/BF01365084. [DOI] [PubMed] [Google Scholar]
- 63.Rosa D.D.A., Gezuele E., Calegari L., Goñi F. Excretion-Secretion Products and Proteases from Live Sporothrix schenckii Yeast Phase. Immunological Detection and Cleavage of Human IgG. Rev. Inst. Med. Trop. Sao Paulo. 2009;51:1–7. doi: 10.1590/S0036-46652009000100001. [DOI] [PubMed] [Google Scholar]
- 64.Sabanero López M., Flores Villavicencio L.L., Soto Arredondo K., Barbosa Sabanero G., Villagómez-Castro J.C., Cruz Jiménez G., Sandoval Bernal G., Torres Guerrero H. Proteases of Sporothrix schenckii: Cytopathological Effects on a Host-Cell Model. Rev. Iberoam. Micol. 2018;35:32–38. doi: 10.1016/j.riam.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Nosanchuk J.D., Casadevall A. The Contribution of Melanin to Microbial Pathogenesis. Cell. Microbiol. 2003;5:203–223. doi: 10.1046/j.1462-5814.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 66.Casadevall A., Cordero R.J.B., Bryan R., Nosanchuk J., Dadachova E. The Fungal Kingdom. ASM Press; Washington, DC, USA: 2017. Melanin, Radiation, and Energy Transduction in Fungi; pp. 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romero-Martinez R., Wheeler M., Guerrero-Plata A., Rico G., Torres-Guerrero H. Biosynthesis and Functions of Melanin in Sporothrix schenckii. Infect. Immun. 2000;68:3696–3703. doi: 10.1128/IAI.68.6.3696-3703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris-Jones R., Youngchim S., Gomez B.L., Aisen P., Hay R.J., Nosanchuk J.D., Casadevall A., Hamilton A.J. Synthesis of Melanin-like Pigments by Sporothrix schenckii in Vitro and during Mammalian Infection. Infect. Immun. 2003;71:4026–4033. doi: 10.1128/IAI.71.7.4026-4033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almeida-Paes R., Figueiredo-Carvalho M.H.G., Brito-Santos F., Almeida-Silva F., Oliveira M.M.E., Zancopé-Oliveira R.M. Melanins Protect Sporothrix brasiliensis and Sporothrix schenckii from the Antifungal Effects of Terbinafine. PLoS ONE. 2016;11:e0152796. doi: 10.1371/journal.pone.0152796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almeida-Paes R., Frases S., Araújo G.D.S., de Oliveira M.M.E., Gerfen G.J., Nosanchuk J.D., Zancopé-Oliveira R.M. Biosynthesis and Functions of a Melanoid Pigment Produced by Species of the Sporothrix Complex in the Presence of L-Tyrosine. Appl. Environ. Microbiol. 2012;78:8623–8630. doi: 10.1128/AEM.02414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Almeida-Paes R., De Oliveira L.C., Oliveira M.M.E., Gutierrez-Galhardo M.C., Nosanchuk J.D., Zancopé-Oliveira R.M. Phenotypic Characteristics Associated with Virulence of Clinical Isolates from the Sporothrix Complex. BioMed Res. Int. 2015;2015:212308. doi: 10.1155/2015/212308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madrid I.M., Xavier M.O., Mattei A.S., Fernandes C.G., Guim T.N., Santin R., Schuch L.F.D., Nobre M.D.O., Araújo Meireles M.C. Role of Melanin in the Pathogenesis of Cutaneous Sporotrichosis. Microbes Infect. 2010;12:162–165. doi: 10.1016/j.micinf.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 73.Taborda C.P., Da Silva M.B., Nosanchuk J.D., Travassos L.R. Melanin as a Virulence Factor of Paracoccidioides brasiliensis and Other Dimorphic Pathogenic Fungi: A Minireview. Mycopathologia. 2008;165:331–339. doi: 10.1007/s11046-007-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guan M.-Q., Yao L., Zhen Y., Song Y., Cui Y., Li S. shan. Melanin of Sporothrix globosa Affects the Function of THP-1 Macrophages and Modulates the Expression of TLR2 and TLR4. Microb. Pathog. 2021;159:105158. doi: 10.1016/j.micpath.2021.105158. [DOI] [PubMed] [Google Scholar]
- 75.Negrini T.D.C., Ferreira L.S., Arthur R.A., Alegranci P., Placeres M.C.P., Spolidorio L.C., Carlos I.Z. Influence of TLR-2 in the Immune Response in the Infection Induced by Fungus Sporothrix schenckii. Immunol. Investig. 2014;43:370–390. doi: 10.3109/08820139.2013.879174. [DOI] [PubMed] [Google Scholar]
- 76.Sassá M.F., Ferreira L.S., de Abreu Ribeiro L.C., Carlos I.Z. Immune Response against Sporothrix schenckii in TLR-4-Deficient Mice. Mycopathologia. 2012;174:21–30. doi: 10.1007/s11046-012-9523-1. [DOI] [PubMed] [Google Scholar]
- 77.Albuquerque P.C., Nakayasu E.S., Rodrigues M.L., Frases S., Casadevall A., Zancope-Oliveira R.M., Almeida I.C., Nosanchuk J.D. Vesicular Transport in Histoplasma Capsulatum: An Effective Mechanism for Trans-Cell Wall Transfer of Proteins and Lipids in Ascomycetes. Cell. Microbiol. 2008;10:1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ikeda M.A.K., De Almeida J.R.F., Jannuzzi G.P., Cronemberger-Andrade A., Torrecilhas A.C.T., Moretti N.S., Da Cunha J.P.C., De Almeida S.R., Ferreira K.S. Extracellular Vesicles from Sporothrix brasiliensis are an Important Virulence Factor That Induce an Increase in Fungal Burden in Experimental Sporotrichosis. Front. Microbiol. 2018;9:2286. doi: 10.3389/fmicb.2018.02286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carlos I.Z., Sgarbi D.B.G., Santos G.C., Placeres M.C.P. Sporothrix schenckii Lipid Inhibits Macrophage Phagocytosis: Involvement of Nitric Oxide and Tumour Necrosis Factor-α. Scand. J. Immunol. 2003;57:214–220. doi: 10.1046/j.1365-3083.2003.01175.x. [DOI] [PubMed] [Google Scholar]
- 80.Ajesh K., Sreejith K. Cryptococcus Laurentii Biofilms: Structure, Development and Antifungal Drug Resistance. Mycopathologia. 2012;174:409–419. doi: 10.1007/s11046-012-9575-2. [DOI] [PubMed] [Google Scholar]
- 81.Alim D., Sircaik S., Panwar S.L. The Significance of Lipids to Biofilm Formation in Candida albicans: An Emerging Perspective. J. Fungi. 2018;4:140. doi: 10.3390/jof4040140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shopova I., Bruns S., Thywissen A., Kniemeyer O., Brakhage A.A., Hillmann F. Extrinsic Extracellular DNA Leads to Biofilm Formation and Colocalizes with Matrix Polysaccharides in the Human Pathogenic Fungus Aspergillus fumigatus. Front. Microbiol. 2013;4:141. doi: 10.3389/fmicb.2013.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sánchez-Herrera R., Flores-Villavicencio L.L., Pichardo-Molina J.L., Castruita-Domínguez J.P., Aparicio-Fernández X., López M.S., Villagómez-Castro J.C. Analysis of Biofilm Formation by Sporothrix schenckii. Med. Mycol. 2021;59:31–40. doi: 10.1093/mmy/myaa027. [DOI] [PubMed] [Google Scholar]
- 84.Brilhante R.S.N., Da Silva M.L.Q., Pereira V.S., De Oliveira J.S., Maciel J.M., Da Silva I.N.G., Garcia L.G.S., Guedes G.M.D.M., Cordeiro R.D.A., Pereira-Neto W.D.A., et al. Potassium Iodide and Miltefosine Inhibit Biofilms of Sporothrix schenckii Species Complex in Yeast and Filamentous Forms. Med. Mycol. 2019;57:764–772. doi: 10.1093/mmy/myy119. [DOI] [PubMed] [Google Scholar]
- 85.Brilhante R.S.N., De Aguiar F.R.M., Da Silva M.L.Q., De Oliveira J.S., De Camargo Z.P., Rodrigues A.M., Pereira V.S., Serpa R., De Souza Collares Maia Castelo-Branco D., Correia E.E.M., et al. Antifungal Susceptibility of Sporothrix schenckii Complex Biofilms. Med. Mycol. 2018;56:297–306. doi: 10.1093/mmy/myx043. [DOI] [PubMed] [Google Scholar]
- 86.Rodrigues A.M., De Hoog G., Zhang Y., De Camargo Z.P. Emerging Sporotrichosis Is Driven by Clonal and Recombinant Sporothrix Species. Emerg. Microbes Infect. 2014;3:1–10. doi: 10.1038/emi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X., Huang H., Feng P., Zhang J., Zhong Y., Xue R., Xie Z., Li M., Xi L. In Vitro Activity of Itraconazole in Combination with Terbinafine against Clinical Strains of Itraconazole-Insensitive Sporothrix schenckii. Eur. J. Dermatol. 2011;21:573–576. doi: 10.1684/ejd.2011.1400. [DOI] [PubMed] [Google Scholar]
- 88.Rodrigues A.M., Fernandes G.F., Araujo L.M., Della Terra P.P., dos Santos P.O., Pereira S.A., Schubach T.M.P., Burger E., Lopes-Bezerra L.M., de Camargo Z.P. Proteomics-Based Characterization of the Humoral Immune Response in Sporotrichosis: Toward Discovery of Potential Diagnostic and Vaccine Antigens. PLoS Negl. Trop. Dis. 2015;9:e0004016. doi: 10.1371/journal.pntd.0004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodrigues A.M., de Hoog G.S., de Cássia Pires D., Brihante R.S.N., da Costa Sidrim J.J., Gadelha M.F., Colombo A.L., De Camargo Z.P. Genetic Diversity and Antifungal Susceptibility Profiles in Causative Agents of Sporotrichosis. BMC Infect. Dis. 2014;14:219. doi: 10.1186/1471-2334-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.