Summary
Background
In moderate-to-severe COVID-19 pneumonia, dexamethasone (DEX) and tocilizumab (TCZ) reduce the occurrence of death and ventilatory support. We investigated the efficacy and safety of DEX+TCZ in an open randomized clinical trial.
Methods
From July 24, 2020, through May 18, 2021, patients with moderate-to-severe COVID-19 pneumonia requiring oxygen (>3 L/min) were randomly assigned to receive DEX (10 mg/d 5 days tapering up to 10 days) alone or combined with TCZ (8 mg/kg IV) at day 1, possibly repeated with a fixed dose of 400 mg i.v. at day 3. The primary outcome was time from randomization to mechanical ventilation support or death up to day 14, analysed on an intent-to-treat basis using a Bayesian approach. ClinicalTrials.gov number, NCT04476979.
Findings
A total of 453 patients were randomized, 3 withdrew consent, 450 were analysed, of whom 226 and 224 patients were assigned to receive DEX or TCZ+DEX, respectively. At day 14, mechanical ventilation or death occurred in 32/226 (14%) and 27/224 (12%) in the DEX and TCZ+DEX arms, respectively (hazard ratio [HR] 0·85, 90% credible interval [CrI] 0·55 to 1·31). At day 14, the World health Organization (WHO) clinical progression scale (CPS) was significantly improved in the TCZ+DEX arm (OR 0·69, 95% CrI, 0·49 to 0.97). At day 28, the cumulative incidence of oxygen supply independency was 82% in the TCZ+DEX arms and 72% in the DEX arm (HR 1·36, 95% CI 1·11 to 1·67). On day 90, 24 deaths (11%) were observed in the DEX arm and 18 (8%) in the TCZ+DEX arm (HR 0·77, 95% CI 0·42–1·41). Serious adverse events were observed in 25% and 21% in DEX and TCZ+DEX arms, respectively.
Interpretation
Mechanical ventilation need and mortality were not improved with TCZ+DEX compared with DEX alone. The safety of both treatments was similar. However, given the wide confidence intervals for the estimate of effect, definitive interpretation cannot be drawn.
Funding
Programme Hospitalier de Recherche Clinique [PHRC COVID-19–20–0151, PHRC COVID-19–20–0029], Fondation de l'Assistance Publique – Hôpitaux de Paris (Alliance Tous Unis Contre le Virus) and from Fédération pour la Recherche Médicale” (FRM). Tocilizumab was provided by Roche.
Keywords: COVID-19, Tocilizumab+Dexamethasone, Randomized clinical trial
Research in context.
Evidence before this study
Dexamethasone (DXM) and Tocilizumab (TCZ), an anti-IL-6 receptor, are now considered individually as the standard of care for moderate, severe or critical COVID-19 pneumonia. We searched PubMed from inception to July 30, 2021, for clinical trials published in English evaluating the effect of tocilizumab, a monoclonal anti-IL-6 receptor antibody, and dexamethasone in patients with laboratory-confirmed COVID-19 using the search terms (“COVID-19″[All Fields] OR “2019-nCoV”[All Fields]) OR “SARS-CoV-2″[All Fields]) AND (“Tociliizumab” [All Fields] (filters: Clinical Trial, Randomized Controlled Trial). We identified one retrospective study, three post hoc analysis of randomized studies evaluating Dexamethasone and Tocilizumab, and no randomized clinical trial that compared Tocilizumab and Dexamethasone with Dexamethasone in patients with COVID-19.
Added value of this study
We designed the first randomized clinical trial to assess whether the combination of Tocilizumab and Dexamethasone could improve the outcome of patients hospitalised with moderate-to-severe COVID-19 pneumonia. The study was arrested after the data safety monitoring board advised stopping the inclusions despite the absence of crossing the planned futility boundaries because of the dramatic decrease of inclusions occurring at the end of the third wave in France when the infection rate had become very low. We found no statistical difference between the two arms in terms of mechanical ventilation need and mortality up to day 90 in the DEX and TCZ+DEX arms, respectively. However, WHO CPS significantly improved in the TCZ+DEX arm. On day 28, the cumulative incidence of oxygen supply independency was 82% in the TCZ+DEX arms, and 72% in the DEX arm and the cumulative incidence of discharge was 83% in the TCZ+DEX arm and 73% in the DEX arm. Safety was similar in both arms.
Implications of all the available evidence
The primary endpoint of our study was not reached. However, the fact that Hazard Ratio (HR 0.77, 95% CI 0.42–1.41) is consistent with the WHO PMA post hoc analysis and that pre-specified secondary analysis (WHO CPS, cumulative incidence of oxygen supply independency and of hospital discharge) are in favour of the TCZ+DEX arm supports the need for further studies for assessing the efficacy of TCZ in patients who failed to improve upon DEX in patients with COVID-19 and moderate-to-severe pneumonia.
Alt-text: Unlabelled box
Introduction
COVID-19 is a respiratory disease due to a novel coronavirus (SARS CoV-2) causing substantial morbidity and mortality.1, 2, 3, 4 Approximately 10 to 15% of patients develop a moderate or severe disease that requires hospitalization and oxygen support, including non-invasive (NIV) and mechanical ventilation (MV) for cases complicated by acute respiratory distress syndrome (ARDS) and multi-organ failure. It is now well established that hyperinflammation with the production of high levels of cytokines, particularly IL-6, is responsible for oedema, cell infiltration, and thrombosis in the lungs of patients with Sars-Cov-2 infection resulting in tissue lesions and non-effective host adaptive immune responses. To circumvent this deleterious hyperinflammation, strategies using anti-inflammatory drugs, particularly corticosteroids, antibodies directed against cytokines, and JAK inhibitors, have been tested by several cooperative groups and pharmaceutical companies throughout the world during the successive epidemic waves. The CORIMUNO-19 platform was set up in France in March 2020, with the overall objective to determine the best anti-inflammatory strategy using already marketed immune modulator drugs in adult patients hospitalized with moderate/severe COVID-19 or critically ill. At the end of the first epidemic wave, the RECOVERY study demonstrated that dexamethasone (DEX), a steroid with high anti-inflammatory activity, reduces death by one-third in patients receiving MV and one-fifth in patients receiving oxygen without MV.5 Since then (July 2020), DEX has become the new standard of care (SOC) in these groups of patients and is highly recommended in most countries. At the same time, different studies,6, 7, 8, 9 including the CORIMUNO-TOCI-1 trial,10,11 suggested that Tocilizumab (TCZ), an anti-human IL-6 receptor monoclonal antibody approved for the treatment of rheumatoid arthritis and cytokine release syndrome associated with CAR-T cell therapy, reduces the occurrence of death or ventilation significantly in patients with moderate-to-severe pneumonia without an increase in serious adverse events including infections. Nevertheless, no randomized clinical trials (RCT) evaluated the association of DEX + TCZ versus DEX. Therefore, we set up an open randomized trial to assess the efficacy and safety of the combination of DEX and TCZ in comparison to DEX in COVID-19 patients with moderate-to-severe pneumonia.
Methods
Trial design and study oversight
Since the beginning of the SARS CoV-2 epidemy (March 2020), we enroled COVID-19 patients to perform a series of randomised controlled trials testing different therapeutic regimens in a short period of time (CORIMUNO-19 cohort). Patients with moderate-to-severe pneumonia and patients with critical pneumonia were included in independent open-label RCTs. The CORIMUNO Cohort and all embedded trials (i.e. trials using data collected in the CORIMUNO cohort) were approved by an ethics committee (CPP Île-de-France VI) and relevant authorities. This article reports on CORIMUNO-TOCIDEX, a CORIMUNO, multicentric, open-label RCT in patients with moderate-to-severe COVID-19 pneumonia (NCT04476979). CORIMUNO-TOCI-DEX trial was conducted at the beginning of the second wave in France, which started in French Guyana, from July 24, 2020, through May 18, 2021 (the date of enrolment of the last patient) in 25 French university and general hospitals. At that time, vaccines were not available (https://www.santepubliquefrance.fr/recherche/#search=COVID%2019%20%20% 20point%20epidemiologique&publications=donn% C3%A9es®ions=National&sort=date). Written informed consent was obtained from all patients or from the patient's legal representative for entering the CORIMUNO Cohort, and longitudinal data (including clinical status, biological data and outcomes) were recorded as part of their participation in the cohort.
In this consent, patients were made aware that they were randomly selected to be offered Tocilizumab and Dexamethasone or Dexamethasone and agreed to receive this treatment. The full trial protocol the statistical analysis plan (SAP) can be accessed in the supplementary materials. This trial was reported according to CONSORT guidelines.
Randomisation and masking
Eligible patients were randomly assigned in a 1:1 ratio to receive either DEX (10 mg/d 5 days and tapering doses up to 10 days (5 mg/d for 5 days and 2·5 mg/d for 5 days) or combined with TCZ (8 mg/kg intravenously) at day 1. Administration of an additional fixed dose of the assigned drug (TCZ 400 mg i.v.) was recommended if no response, i.e. no decrease of oxygen requirement of more than 50%, was observed at day 3. In slight modification from the recovery trials, we decided to increase and tapered the initial dose of DEX to have a higher anti-inflammatory effect and to prevent any rebound and eventually reduced the total dose of DEX received by the patients. In both groups, supportive care, including supplemental oxygen, NIV and MV, antibiotics and antiviral agents, vasopressor support, prophylactic anticoagulants, renal-replacement therapy, and extracorporeal membrane oxygenation (ECMO), was provided at the discretion of the clinicians. An executive coordination committee was responsible for the design, conduct, and reporting of the trial (see Appendix 1). An independent data and safety monitoring board oversaw all CORIMUNO-19 trials every 60 patients (see Appendix 1). Legal issues and trial procedures are presented in detail in Appendix 2.
Study population
Patients with confirmed SARS CoV-2 infection (positive PCR and/or typical chest CT-scan) with moderate and severe pneumopathy requiring oxygen (>3 L/min) but without ventilation support (NIV), high flow or MV, WHO class 5 according to the WHO 10 points-Clinical Progression Scale (CPS)12 for COVID-19 pneumopathy were eligible for CORIMUNO-TOCI-DEX. Exclusion criteria included known hypersensitivity to TCZ, pregnancy, current documented bacterial infection, patients with absolute neutrophil count (ANC) less 1·0 × 109/L or less, or platelets (PLT) less 50 G /L, ALAT more 5 N (for further details, see Appendix 2)
Study endpoints
The primary endpoint was survival without the need for invasive ventilation at day 14.
Secondary endpoints were clinical status as assessed with the WHO progression scale at 7 and 14 days, overall survival up to day 14, 28, 60, and 90, survival without needs of ventilator utilization (including NIV and high-flow oxygen) at day 14, and the rate of oxygen supply independency and of hospital discharged at day 14 and 28. Safety outcomes included adverse events during treatment and follow up serious adverse events and premature treatment discontinuation.
Data quality monitoring included remote and on-site monitoring performed by dedicated staff independent of the site investigators, with source data verification performed for all patients recruited at every site for all critical data points.
Statistical analysis
Bayesian analyses were used for the primary outcome. Using the results of previous CORIMUNO trials, we hypothesized that the proportion of patients with MV or death at day 14 would be 25%. A frequentist sample size calculation suggested that a total sample size of 634 patients was necessary to demonstrate an HR of 0·65 with power 80%, using a one-sided 5% type I error rate. Keeping the analysis strategy every 60 patients of CORIMUNO trials, an indicative maximum sample size would be 660 (330 per arm). The previous Bayesian design (see Statistical Analysis Plan and previous publications10,13) was then extended to analyse the trial data every 60 patients reaching at least 7 follow-up days. At each analysis, the three planned posterior probabilities were computed for the primary outcome: the posterior probability of any benefit P1 = P(HR < 1 | data), the posterior probability of at least a fair benefit P2 = P(HR < 0·8 | data), and the posterior probability of inefficacy or harm P3 = P(HR > 1 | data). The following actions were then planned according to the thresholds given below, adapted from the Statistical Design and Analysis Plan for Sequential Parallel-Group RCF for COVID-19 (Harrell & Lindsell, 2020. http://hbiostat.org/proj/covid19/bayesplan.html): Stop with evidence for efficacy if P1 > 0·95 (P1 > 0·99 at the first analysis); Stop for futility if P2〈 0·10 or P3〉 0·80; Stop with evidence for efficacy if P2 > 0·80 (only actionable when at least 180 patients had been randomized). Those decision rules were non-binding, and the DSMB was free to recommend stopping or continuing the trial at each interim analysis. The treatment effect was expressed in terms of hazard ratio (HR) in a Bayesian Cox model adjusted for age and centre as a random effect. Posterior medians and credible intervals were derived from the posterior distribution of parameters obtained using Monte Carlo Markov Chains. Details on the Bayesian analyses are presented in the Statistical Analysis Plan.
Secondary outcomes were analysed with frequentist methods. Overall survival up to the predefined timepoints was analysed using Cox models. Time to discharge and oxygen supply independency were analysed using Fine-gray models, considering death as a competing event. WHO—CPS scores were analysed as an ordinal outcome using a proportional odds model. All those models were adjusted for age and centre, as for the primary outcome. Since no correction for multiplicity was used, those analyses should be considered exploratory. For safety analyses, the proportion of participants with at least one adverse event and at least one serious adverse event were compared using Fisher's exact tests, and the total numbers of adverse events and serious adverse events were compared using Poisson models.
All analyses were performed on an intention-to-treat basis and included all patients who had undergone randomization, analysed in the arm they were allocated to, unless consent was withdrawn. Statistical analyses were conducted with R version 4.0.5 (The R Foundation for Statistical Computing, Vienna, Austria).
Role of the funding source
Assistance Publique Hôpitaux de Paris was involved at every stage of the study, participating in the design and conduct of the study (including the development of the study protocol and statistical analysis plan); collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Roche provided tocilizumab in an unrestricted grant. Raphael Porcher, Tabassome Simon, Matthieu Resche-Rigon, Philippe Ravaud had access to the raw data and verified the data and analyses and the fidelity of this report to the study protocol and data analysis plan. Olivier Hermine, Xavier Mariette, Pierre Louis Tharaux, Matthieu Resche-Rigon, Tabassome Madjlessi Simon, Raphael Porcher, and Philippe Ravaud had full access to all of the data and the final responsibility to submit for publication.
Results
Patients
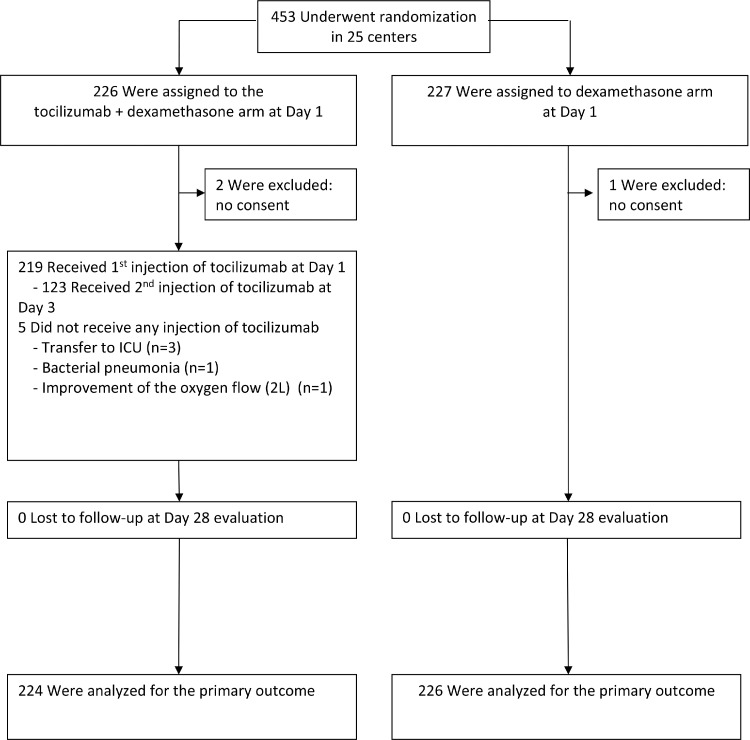
Between July 24th 2020 and May 18 2021, 453 patients (TCZ+DEX (n = 226); DEX (n = 227) were enroled at 25 sites. Inclusions were stopped per DSMB recommendation on May 18, 2021, after an interim analysis and in the light of the sample size needed to reach a reasonable power given the observed event rate and effect size and the dramatic decrease in the pace of inclusions at that time. Three patients (two in TCZ+DEX and one in DEX) were excluded because of consent withdrawal or declined participation before any study drug administration. Accordingly, 450 patients were analysed, (Figure 1). The median age was 63 years (interquartile range [IQR], 52·6 to 73·3 years), and 68% of participants were men (Table 1). Baseline CRP level was high (median 95 mg/ml, [IQR], 53 to 147). At enrolment, there were no between-group differences in demographic characteristics, comorbidities, or biological (e.g., CRP, Ferritin, d-Dimers, LDH levels or lymphocytes count). Amongst those treated by TCZ, 123/224 (59%) received a second injection of 400 mg at Day 3 (Supplementary Table 1). Antiviral drugs were barely used, and prophylactic anticoagulants were administered in 83% of cases in the two arms (Supplementary Table 2). Few patients received additional immuno-modulators, one in the TCZ+DEX group (convalescent plasma, n = 1) and 5 in the DEX group (TCZ, n = 5).
Figure 1.
Trial flow chart.
Table 1.
Demographic, clinical, biological characteristics at baseline.
| TCZ+DEX (n = 224) | DEX (n = 226) | |
|---|---|---|
| Age (years) | 63·6 [52·6–73·3] | 63·2 [53·6–73·3] |
| Male, n/N (%) | 146/224 (65%) | 159/226 (70%) |
| Weight (kg) | 82·0 [70·0–93·5] | 81·5 [73·5–90·5] (n = 224) |
| BMI (kg/m²) | 27·4 [24·8–31·2] (n = 178) | 28·2 [24·7–31·9] (n = 191) |
| Obesity (BMI ≥30 kg/m²), n/N (%) | 62/220 (28%) | 63/224 (28%) |
| WHO score (0–10) | 5 [5–5] | 5 [5–5] |
| WHO score (0–10) = 5, n/N (%) | 224/224 (100%) | 226/226 (100%) |
| Temperature ( °C) | 37·1 [36·7–38·0] | 37·1 [36·7–38·0] |
| Respiratory rate (breaths / min) | 24·0 [21·0–30·0] (n = 203) | 25·0 [22·0–30·0] (n = 202) |
| Flow (L/min) | 5·0 [3·0–6·0] | 5·0 [3·0–6·0] |
| SpO2 (%) | 94·0 [92·0–96·0] | 94·0 [92·0–95·0] |
| Time from symptoms onset to randomization (days) | 9 [7–11] (n = 218) | 9 [7–11] (n = 222) |
| Co-existing conditions, n/N (%) | ||
| Hypertension | 80/221 (36%) | 84/221 (38%) |
| Chronic cardiac disease | 32/221 (14%) | 38/222 (17%) |
| Diabetes | 56/220 (25%) | 49/221 (22%) |
| Chronic kidney disease (stage 1 to 3) or dialysis | 20/221 (9%) | 12/221 (5%) |
| Asthma | 24/220 (11%) | 13/220 (6%) |
| Chronic pulmonary disease (not asthma) | 14/221 (6%) | 18/222 (8%) |
| Active malignant neoplasm | 8/221 (4%) | 11/222 (5%) |
| Smoking | ||
| No | 184/220 (84%) | 177/219 (80%) |
| Current | 18/220 (8%) | 10/219 (5%) |
| Former | 18/220 (8%) | 32/219 (15%) |
| Laboratory values | ||
| C-reactive protein (CRP) (mg/L) | 98 [58–147] (n = 215) | 94 [54–150] (n = 220) |
| D-Dimer (µg/L) | 807 [596–1344] (n = 204) | 922 [562–1347] (n = 201) |
| Lymphocyte count (G/L), | 0·7 [0·5–1·0] (n = 218) | 0·7 [0·6–1·1] (n = 220) |
| Ferritin (mg/L) | 988 [525–1746] (n = 147) | 1026 [484–1693] (n = 145) |
| LDH (IU/L) | 429 [335–537] (n = 169) | 398 [314–509] (n = 169) |
BMI denotes body mass index, LDH Lactate dehydrogenase. Values are median [interquartile range] unless stated.
Primary outcome
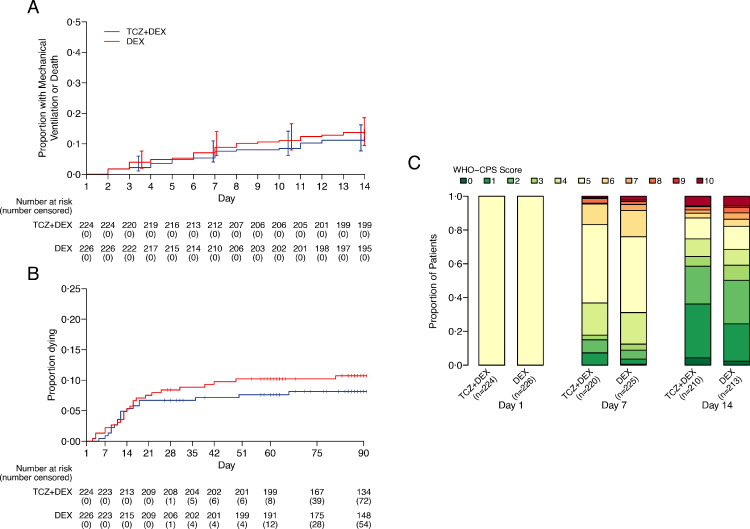
At day 14, invasive ventilation or death occurred in 27/224 (12%) and 32/226 (14%) patients in the TCZ+DEX and DEX arms, respectively (median posterior HR 0·85, 90% credible interval [CrI] 0·55 to 1·31, posterior probability of any benefit 72·8%) (Figure 2A, Tables 2, Supplementary 3). Sensitivity analyses using different prior distributions or a frequentist analysis yielded very similar results (Supplementary Table 4 and Supplementary Fig. 1). The posterior probability that TCZ+DEX would increase the hazard of MV or death compared to DEX by more than 10% was 16·5% (posterior probability of a HR > 1·10).
Figure 2.
Proportion of patients with the occurrence of A the primary event (mechanical ventilation and survival) and B overall survival. C Distribution of the 10-point WHO—CPS scores during follow-up (See also Appendix 3).
Table 2.
Primary and secondary efficacy outcomes.
| TCZ+DEX (n = 224) | DEX (n = 226) | Treatment effect (95% CI) | |
|---|---|---|---|
| Primary outcomes | |||
| Mechanical ventilation or death up to day 14 | 27 (12%) | 32 (14%) | 0·85 (90% CrI 0·55 to 1·31)* |
| Posterior probability of any benefit | 0·728 | ||
| Posterior probability of at least a fair benefit | 0·405 | ||
| Secondary outcomes | |||
| Overall survival | |||
| Mortality at day 14 | 12 (5%) | 12 (5%) | 1·03 (0·46 to 2·29)† |
| Mortality at day 28 | 15 (7%) | 19 (8%) | 0·82 (0·41 to 1·61)† |
| Mortality at day 60 | 17 (8%) | 23 (10%) | 0·76 (0·41 to 1·42)† |
| Mortality at day 90 | 18 (8%)** | 24 (11%) | 0·77 (0·42 to 1·41)† |
| WHO—CPS score (10 pt-scale) | |||
| Day 7, median [IQR] | 5 [4–5] ¶ | 5 [4–5]†† | 0·70 (0·50 to 0·98)‡ |
| Day 14, median [IQR] | 2 [1–5]‡‡ | 2 [2–5]¶¶ | 0·68 (0·49 to 0·96)‡ |
| Time to discharge | |||
| Discharged at day 14 | 162 (72%) | 144 (64%) | 1·23 (0·98 to 1·54)† |
| Discharged at day 28 | 186 (83%) | 169 (75%) | 1·24 (1·01 to 1·53)† |
| Time to oxygen supply independency | |||
| Independent from oxygen at day 14 | 158 (71%) | 138 (62%) | 1·34 (1·07 to 1·68)† |
| Independent from oxygen at day 28 | 182 (82%) | 161 (72%) | 1·36 (1·11 to 1·67)† |
CrI: credible interval (Bayesian analysis); CI: confidence interval (frequentist analysis); NIV: non-invasive ventilation (including high-flow oxygen); MV: mechanical ventilation; WHO—CPS: World Health Organization Clinical Progression Scale.
Probability of any benefit was defined as P(HR < 1), and probability of at least a fair benefit as P(HR < 0·8).
* Median posterior hazard ratio adjusted for age and centre with 90% CrI.
** One patient died on day 94, and is not counted amongst the 18.
† Hazard ratio adjusted for age and centre with 95% CI.
‡ Odds ratio in a proportional odds model adjusted for age and centre with 95% CI.
¶ n = 220 with available data.
†† n = 225 with available data.
‡‡ n = 210 with available data.
¶¶ n = 213 with available data.
Secondary outcomes
Secondary outcomes are summarized in Table 2. In the TCZ+DEX arm, 62 (28%) patients needed any ventilation support (MV and NIV) or died by day 14, compared to 75 (33%) in the DEX arms (HR 0·81, 95%CI 0·58 to 1·13) (Supplementary Figure 2, Supplementary Table 5). On the 10 points WHO CPS scale, an improvement was observed more frequently in the TCZ+DEX arm than in the DEX arm at day 7 and day 14 (OR 0·70, 95% CI 0·50 to 0·98 and OR 0·69, 95% CI 0·49 to 0·97, respectively) (Figure 2C, and Supplementary Table 6).
During follow-up until day 90 (17,468 and 17,629 person-days in TCZ+DEX and DEX arms, respectively), 42 patients died, 18/224 (8%) in the TCZ+DEX arm and 24/226 (11%) died in the DEX arm (HR 0·77, 95% CI 0·42 to 1·41) (Figure 2B). Causes of death are shown in Table 3 and are mainly due to ARDS progression
Table 3.
Safety analysis. Adverse events, serious adverse events and causes of deaths.
| TCZ+DEX (n = 224) | DEX (n = 226) | P | |
|---|---|---|---|
| Adverse events | |||
| Patients with at least one AE | 147 (66%) | 139 (62%) | 0·38* |
| Patients with multiple AEs | 94 (42%) | 89 (39%) | |
| Number of AEs | 424 | 470 | 0·16** |
| Serious adverse events | |||
| Patients with at least one SAE | 48 (21%) | 56 (25%) | 0·43* |
| Patients with multiple SAEs | 13 (6%) | 24 (11%) | |
| Number of SAEs | 73 | 106 | 0·017** |
| Acute respiratory distress syndrome | 17 | 21 | |
| Multivisceral failure | 3 | 1 | |
| Sudden death | 1 | 0 | |
| Bacterial sepsis | 28 | 50 | |
| Fungal sepsis | 2 | 1 | |
| Viral hepatitis | 1 | 0 | |
| Acute renal failure | 2 | 5 | |
| Pulmonary embolism | 2 | 8 | |
| Other ischaemic events | 6 | 4 | |
| Haemorrhagic events | 2 | 3 | |
| Cardiac failure | 1 | 3 | |
| Cardiac rhythm abnormalities | 2 | 1 | |
| Worsening of pre-existing cancer | 0 | 4 | |
| Cytopenia | 2 | 1 | |
| Hepatic cytolysis | 2 | 2 | |
| Abdominal occlusion | 2 | 0 | |
| Diabetes | 0 | 1 | |
| Dyskinesia | 0 | 1 | |
| Deaths | 19 (8%) | 24 (11%) | |
| Cause of death | |||
| Acute respiratory distress syndrome | 13 | 17 | |
| Multiple organ failure | 1 | 1 | |
| Cardiac arrest | 1 | 0 | |
| Sudden death | 1 | 0 | |
| Bacterial sepsis | 3 | 6 |
Values are n (%). AE: adverse event; SAE: Serious adverse events.
* Fisher's exact test; ** Poisson model.
At day 28, the cumulative incidence of oxygen supply independency was 82% in the TCZ+DEX arms, and 72% in the DEX arm (HR 1·36, 95% CI 1·11 to 1·67) (Tables 2 and Supplementary 7), and the cumulative incidence of discharge was 83% in the TCZ+DEX arm and 73% in the DEX arm (HR 1·24, 95% CI 1·01 to 1·53) (Tables 2 and Supplementary 8).
Post hoc analysis did not find any interaction between CRP and the primary outcome (P = 0·74), with a HR slightly lower in patients with CRP > 100 mg/L (HR 0·74, 95% CI 0·40 to 1·37) vs HR 0·88, 95% CI 0·47 to 1·64) (Supplementary Figure 3).
Safety
A total of 147/224 patients (66%) in the TCZ+DEX arm and 139/226 (62%) in the DEX group reported adverse events between randomization and day 28 (Table 3). Serious adverse events (SAE) occurred in 48/224 patients (21%) in the TCZ+DEX group and 56/226 patients (25%) in the DEX group. The number of SAE was higher in the DEX group (106 vs 73, P = 0·017) with an increased number of secondary bacterial infections (50 vs 28). Up to day 90, death occurred in 19 patients from the TCZ+DEX arm (mainly from 13 ARDS and 3 bacterial sepsis) and in 24 patients from the DEX arm (mainly from 17 ARDS and 6 bacterial sepsis).
Discussion
In hospitalized adults with COVID-19 with moderate-to-severe pneumonia, TCZ plus DEX had a numerically lower number of events. Still, it was not superior for reducing the risk of death and disease progression at 14 days. This randomised controlled trial addresses an important question since TCZ and DEX are now considered, individually, as standard of care. Furthermore, post hoc analyses of previous randomised control trials (RCT), recently reported by the WHO metanalysis, have suggested that the use of TCZ might amplify the beneficial effect of DEX.6 The present trial failed in demonstrating the beneficial effect of combining the two drugs; however, the wide confidence interval does not allow a definite conclusion. There was no significant reduction in death or mechanical ventilation (or any ventilation support) at day 14, and the comparison of the number of deaths at day 90 observed with TOCI+DEX versus DEX did not reach a statistical significance. Nevertheless, the Bayesian analysis shows that the TCZ+DEX combination has a 72·8% chance of being superior to DEX alone. The DSMB advised stopping the inclusions despite the absence of crossing the planned futility boundaries, in particular, because of the dramatic decrease of inclusions occurring at the end of the third wave in France when the infection rate had become very low. Moreover, the DSMB was aware on mid-May 2021 of the results of the recently published WHO prospective meta-analysis on the effect of anti-IL-6 receptor antibodies that showed a significant reduction of mortality at day 286. Of note, in this meta-analysis, the odds ratio of day 28 survival in patients treated with the combination of TCZ and corticosteroids versus standard of care or placebo of TCZ was the same that in the TOCIDEX trial: 0·77 (95% CI0·68 to 0.87) and 0·77 (0·42 to 1·41), respectively.
In secondary pre-specified endpoints, TCZ+DEX improved the WHO score on the 10 points WHO CPS scale. The potential improvement of the clinical status appreciated by the WHO CPS scale in the TCZ+DEX arm may be relevant to reduce the pressure on hospitalizations in general and particularly in intensive care units. Likewise, other secondary endpoints such as time to oxygen independency and time to discharge potentially appeared better in the combination arm versus the DEX arm.
The incidence of severe adverse events was similar in both arms; especially, the risk of severe infection was not increased by combining TCZ and DEX, which is consistent with all previous studies on TCZ in COVID-19, suggesting that the control of the hyperinflammation may help to restore antibacterial and antiviral responses.
The main limitation of this study is its insufficient power. A conditional power analysis showed that, with the observed event rate, which was markedly lower than that observed in earlier trials, the trial would have had less than 20% chance of reaching the efficacy boundary even if it had included the planned 660 patients and accrual of at least another 1000 patients would have been necessary to get 80% power (Supplementary Figure 4). Their recruitment was no longer possible while, fortunately, the third wave was ending in France. Because of the improvement of the standard of care, including anticoagulation therapy, antibiotics, and more recently, corticosteroids, larger studies or meta-analyses are now warranted to demonstrate the efficacy of the new regimen in this target population of COVID-19 patients with moderate-to-severe pneumonia (WHO class 5 patients). In addition, as observed in most reported trials including those of CORIMUNO (TOCI-110 and ANA-111 and SARI-1 (in preparation)), patients included in TOCIDEX may be less severe with, for example, a baseline CRP of 100 mg/L vs 150 mg/L in the other trials. The second limitation is the absence of blinding. However, we considered objective outcomes (death or MV), and it is unlikely that the lack of a placebo may have influenced them. Finally, the use of forbidden drugs may occur in any RCT, especially in patients with severe disease in whom the clinician want to save his/her patient. In this study, only 5 out the 226 patients (2.2%) randomised in the DXM arm received TCZ. Of course, for the quality of the trial, this patient may disfavour the TCZ+DEX arms, but this very low number does not affect the result of the overall trial. Obviously, like in every RCT, in an intention to treat analysis these patients who have been randomized in the DXM arm have to be analysed in the DXM arm and cannot be excluded. Since the beginning of our trial, JAK inhibitors like Baricitinib14 (JAK1 and JAK2) and tofacitinib15 (JAK1 and JAK3) have also shown some beneficial effects with no significant safety issues. However, although the best effect was observed in WHO CPS class 6 for baricitinib, it is currently impossible to define which COVID-19 patients would benefit the most from JAK inhibitors or TCZ+DEX. In conclusion, In patients with SARS CoV-2 infection with moderate and severe pneumopathy requiring oxygen but without ventilation support, the combination of TCZ and DEX did not have a significant benefit over DEX alone, with regard to the risk of mortality or reduction of disease progression at day 14, but improve significantly WHO CPS, length of hospitalisation and oxygen requirement. However, given the wide confidence intervals for the estimate of effect, the findings do not allow for a conclusive interpretation. Further studies are warranted to identify patients who do not respond to DEX to develop personalized therapeutic strategies that may include the addition of TCZ.
Contributors
A steering committee [Philippe Ravaud (Chair), Olivier Hermine, Xavier Mariette, Pierre-Louis Tharaux, Maxime Dougados, Raphael Porcher, Matthieu Resche-Rigon, Serge Bureau, Annick Tibi, and Tabassone Simon] was responsible for the design, conduct, and reporting of the trial, and an independent data and safety monitoring board oversees all CORIMUNO-19 trials (appendix 1). The manuscript was initially drafted by the writing committee (OH, XM, P-LT, MR-R, RP, TS and PhR), who contributed equally, and was then approved by all members of the trial steering committee and co-authors. TS organised collection of the data. RP and PhR did the statistical analysis. All authors from the writing committee contributed to design of the trial and of study protocol, data interpretation, and critical review and revision of the manuscript. RP and MR-R had access to the raw data and verified the data and analyses and the fidelity of this report to the study protocol and data analysis plan. All members of the writing committee had full access to all of the data and the final responsibility to submit for publication.
Declaration of interests
The authors have the following interests to declare:
Laurent Savale reports personal fees from Janssen and Janssen, andMSD, grants and personal fees from GSK, non-financial support from Acceleron, outside the submitted work.
Gilles Pialoux has received consulting fees from Gilead, Abbvie, ViiVHealthcare, MSD and research grants from Gilead.
Karine Lacombe Advisory boards in the Covid19 field of MSD, Gilead, GSK, Educational activities in the Covid19 field Chiesi, Sobi, MSD, Gilead, MSD.
Xavier Lescure is COVID-19 advisor and has received grant from French ministry of health
Jade Ghosn reports grants and personal fees from Gilead, personal fees from MSD, personal fees from ViiV Healthcare, personal fees from Janssen, personal fees from Astra Zeneca, outside the submitted work.
Jean Marie Michot reports investigator fees from Amgen, BMS-Celgene, Roche, Sanofi, Xencor, Astex, outside the submitted work.
François Raffi personal fees from Gilead Sciences, Janssen, MSD, Pfizer, Roche, ViiV Healthcare, outside the submitted work.
Muriel Fartoukh Advisory board (community acquired pneumonia) of Pfizer, educational activities (community acquired pneumonia) of BioMérieux, (ARDS) of Fisher&Paykel.
Marc Humbert personal fees from Acceleron, AstraZeneca, Bayer, Merck, Novartis, Roche, Sanofi, GSK, outside the submitted work.
Frédéric Pene reports grants from ALEXION, personal fees from GILEAD, outside the submitted work.
Gabriel Steg reports grants from Amarin, Bayer, Sanofi, Servier, consulting fees from Amarin, Amgen, AstraZeneca, Bayer, BMS, Idorsia, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Servier, honoraria for lectures from Mylan, support for travels from Astra Zeneca, Co inventor on Patent on alirocumab to reduce cardiovascular risk with all royalties to Sanofi, Participation on a Data Safety Monitoring Board or Advisory Board of Sanofi, New Amsterdam Pharma, Co founder of Bioquantis, all outside the submitted work.
Philippe Ravaud has received grant from French ministry of health.
All other authors have nothing to declare.
Acknowledgments
Funding
Programme Hospitalier de Recherche Clinique [PHRC COVID-19–20–0151, PHRC COVID-19–20–0029], Fondation de l'Assistance Publique – Hôpitaux de Paris (Alliance Tous Unis Contre le Virus) and from Fédération pour la Recherche Médicale” (FRM). Tocilizumab was provided by Roche.
Data sharing statement
Assistance Publique – Hôpitaux de Paris is committed to responsible and transparent sharing of clinical trial data with patients, healthcare practitioners, and independent researchers to improve scientific and medical knowledge and foster innovative treatment approaches. Researchers interested in obtaining access to documents and/or data can make their requests on the APHP portal at the following address: raphael.porcher@aphp.fr. Request will be reviewed by the CORIMUNO scientific committee. The steering committee will need to be satisfied that any proposed publication is of high quality, honours the commitments made to the study participants in the consent documentation and ethical approvals, and is compliant with relevant legal and regulatory requirements (eg, relating to data protection and privacy). To gain access, data requesters will need to sign a data access agreement and to confirm that data will only be used for the agreed purpose for which access was granted. The steering committee will have the right to review and comment on any draft manuscripts before publication.
Acknowledgements
We are grateful to all patients who participated in the CORIMUNO study and their families. The authors also thank the investigators who collaborated in this study (Supplementary Appendix 1). This trial was publicly funded by the Ministry of Health, Programme Hospitalier de Recherche Clinique (PHRC COVID-19–20–0151, PHRC COVID-19–20–0029), and Assistance Publique – Hôpitaux de Paris Foundation and Foundation for Medical Research. We are grateful to all patients who participated in the CORIMUNO-19 study, and their families. The authors also thank Prof Maxime Dougados who was in charge of the logistics, as well as the investigators who collaborated in this study and Universities of Paris, Paris-Saclay, Paris-Sorbonne, Paris-Nord Sorbonne, Paris-Est Créteil, Versailles-Saint Quentin and Strasbourg (medical students support), INSERM, and Reacting. Roche donated Tocilizumab as an unrestricted grant and had no role in the study design, no role in the collection, analysis, or interpretation of the data, and no role in the writing of the report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101362.
Supplementary materials
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a Report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group R.C., Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group WHOREAfC-TW, Shankar-Hari M., Vale C.L., et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021 doi: 10.1001/jama.2021.11330. Jul 6. pii: 2781880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S., Wang W., Hayek S.S., et al. Association between early treatment with tocilizumab and mortality among critically Ill patients with COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermine O., Mariette X., Tharaux P.L., et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariette X., Hermine O., Tharaux P.L., et al. Effectiveness of tocilizumab in patients hospitalized with COVID-19: a follow-up of the CORIMUNO-TOCI-1 randomized clinical trial. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192. doi: 10.1016/S1473-3099(20)30483-7. -e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group C.C. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalil A.C., Patterson T.F., Mehta A.K., ACTT-2 Study Group Members Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guimarães P., Quirk D., Furtado R., STOP-COVID Trial Investigators Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385(5):406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.