Abstract
Introduction
Limited treatment options exist for COVID-19 infections; thus, attempts from complementary and alternative systems (CAM) of medicine are being explored as possible therapeutic options. Ayurcov is a formulation made of ingredients mentioned in Ayurveda. These constituents have proven antiviral, detoxifying, immune-modulating, and bio-enhancing properties. The present study was carried out to evaluate the therapeutic effect and safety of Ayurcov in patients with various severity states of COVID-19 infections.
Methods
A randomized, single blinded, controlled trial was carried out in adults diagnosed with mild-to-moderate, and severe COVID-19 infections confirmed by real time reverse transcriptase polymerase chain reaction (rRTPCR) test. The interventional group received three doses of ‘Ayurcov’. It is constituted of Haridra Churna (Curcuma longa), Go ark (Bos Indicus Distilled Urine), Sphatika (Alum), Sita (Rock Candy), Godugdham (Bos Indicus Milk) milk, Goghritam (Bos Indicus ghee) on Day 1, as an adjuvant to the standard of care, and the control group received only the standard of care. Key outcomes included: proportion of patients and time taken for symptom resolution, reduction in the rRT-PCR Ct values, safety, and functional status until 42 days after discharge.
Results
Ninety patients with mild-to-moderate and 30 patients with severe COVID-19 disease were recruited. It was observed that significantly more proportions of patients receiving Ayurcov had symptom relief much earlier than control group. Additionally, the interventional group showed significantly lower rRT-PCR Ct values. However, a shorter time of resolution of symptoms was observed with the interventional group in the mild to moderate category but not with those having severe symptoms. Similarly, a significantly better functional status was observed with interventional group on days 7 and 28 after discharge. Ayurcov was not observed with higher risks of any adverse/serious adverse events.
Conclusions
Ayurcov as adjuvant with standard of care was associated with significantly earlier resolution of COVID-19 related symptoms than standard of care alone.
Keywords: COVID-19, Ayurveda, CAM, Complementary and alternative medicine, Ayurcov
1. Introduction
The unprecedented COVID-19 pandemic shook the world with a mortality of 3.42 million out of the total infected cases of 165 million as of May 20, 2021. Considering the limited therapeutic options, investigators from across the globe are also exploring the benefits of various complementary and alternative medicines for the prevention and treatment of COVID-19 infections. Traditional Chinese Medicine (TCM) has been used in China to contain COVID-19.1 Ayurveda is a holistic system of medicine with roots from India and is one of the oldest indigenous systems of medicine with unbroken clinical practice dating back to thousands of years.2
The Ayurvedic basis of any disease is an imbalance between three doshas (vata, pitta and kapha), one, two or three simultaneously. According to Acharya Charak, Nija hetu (Endogenous causes) directly leads to dosha imbalance but Agantuj hetu (Exogenous causes), ultimately after a while leads to dosha imbalance as Sannipatik Doshadushti.3 The Covid 19 is caused by an exogenous cause as virus ultimately affects All three doshas imbalance inside.4 Doshas represent the functional classification adopted in Ayurveda to understand the complex human system and manage health and diseases.5, 6
Ayurcov is constituted of Haridra Churna (Curcuma longa),7 Go ark (Bos Indicus Distilled urine),8, 9 Sphatika (Pottasium Alum),10 Sita (Rock Candy),10 Godugdham (Bos Indicus Milk),11 Goghritam (Bos Indicus ghee).11, 12
Although antiviral medicines are required for treating covid-19, as per Rasayan chikitsa (a specialty of Ayurveda, stating about rejuvenation therapy) host immunity plays a vital role for prevention of cytokine storm and quick tissue regeneration and retard aging.13 Bos Indicus ghee (BIG) has been shown to possess anti-toxic, improves digestion, antipyretic, endurance enhancement, and immune boosting properties.14 Bos Indicus Milk (BIM), promotes tissue repair and boosts immunity.15
Bos Indicus distilled urine (BIDU) possesses anti-toxic properties, ameliorates cough, taste, and enhances the endurance.16 BIDU was found to enhance B-lymphocyte and T-lymphocyte blastogenesis as well as IgG, IgA, and IgM antibody titers.17 US Patents (No. 6896907 and 6410059) for BIDU have also mentioned that it possesses bioenhancer, antibiotic, antifungal, and anticancer properties.18 Pottasium Alum (PA) is a wound healer and possesses a local pungent action on the throat with anti-microbial property.19 PA is known to induce an innate immune response through macrophage and mast cell sensors.20 It is widely tested as the safest and most efficacious adjuvant component, inducing high levels of neutralizing antibodies, which are increasingly recognized as the cornerstone of the protection afforded by COVID-19 vaccines.21 It is known to act as an antiseptic, as an antimicrobial and as a cleanser - in the throat and respiratory tract. Rock Candy (RC) reduces throat dryness and targets in treating all the three doshas.22
Given these properties, it is imperative that the combination of above constituents is likely to improve the symptoms and signs associated with COVID-19 infections. Hence, we envisaged the present study to investigate the efficacy and safety of ‘Ayurcov’ in patients with varied severity of COVID-19 illnesses.
2. Materials and methods
2.1. Study design and ethics
This study was carried out after obtaining approval from the Institutional Ethics Committee that is approved by Ministry of Health, Government of India (EC/NEW/INST/2019/245). The study was carried out between June and Nov 2020. This study was a single center, assessor blinded, randomized, controlled trial. Written informed consent was obtained from the study participants. We adhered to the Declaration of Helsinki guidelines, New Drug Clinical Trials - 2019 guidelines (India) and Indian Council of Medical Research - 2017 guidelines. This trial was registered with Clinical Trial Registry of India (CTRI/2020/06/026262).
2.2. Study participants
Adults (>18 years) (n = 120) with symptoms of COVID-19 disease with real-time reverse transcription polymerase chain reaction (rRTPCR) confirmed infection were recruited after their consent. Pregnant or breastfeeding women, patients on ventilator support, patients with known allergies or any hypersensitivity to study drugs were excluded. Mild, moderate, and severe COVID-19 disease was defined based on 'Clinical management protocol COVID-19' version 3, dated 13 June 2020, by the Ministry of Health and Family Welfare, Directorate General of Health Services of the Government of India.22 Patients with uncomplicated upper respiratory tract infection, with mild symptoms such as fever, cough, sore throat, nasal congestion, malaise, and headache were classified as ‘mild COVID-19 disease’. Symptoms suggestive of pneumonia without any sign of severe disease were considered ‘moderate’ and those experiencing any of these symptoms - respiratory frequency ≥ 30/min, blood oxygen saturation (SpO2) ≤ 90%, PaO2/FiO2 ratio < 300, or severe pneumonia were categorized as ‘severe COVID-19′ disease.
2.3. Study procedure
During the initial (baseline) visit, patients presenting with symptoms/signs suggestive of COVID-19 disease were confirmed with rRTPCR tests. Participants were admitted in the hospital and were evaluated on days 1, 3, from day 4 until discharge, and days 7, 28, and 42 after discharge from the hospital. The details of the assessments are listed in Table 1. rRTPCR tests for each study participant were carried out on days 1 and 3. The eligible participants were randomized to either standard of care arm (control) or adjuvant intervention (Ayurcov) with standard of care.
Table 1.
Study procedures carried out amongst the study participants.
| S. No. | Procedure | D-1 | D-3 | D-4 to Discharge | Discharge | Days-7, 28, and 42 follow up |
|---|---|---|---|---|---|---|
| 1 | Signed Informed a Consent Form | X | ||||
| 2 | Medical History | X | ||||
| 3 | Clinical Examination | X | X | X | X | |
| 4 | Review of eligibility criteriaa | X | ||||
| 5 | Vital signsb | X | ||||
| 6 | Randomization | X | ||||
| 7 | Study medication administrationc | X | ||||
| 8 | rRTPCR COVID-19 testd | X | X | |||
| 9 | AE/SAE recordinge | X | X | X | X | X |
| 10 | Comorbid conditions | X | ||||
| 11 | Concomitant medications | X | ||||
| 12 | Safety follow-upf | X |
X indicates procedure required on that visit day.
Abbreviations: AE=Adverse Event, SAE=Serious Adverse Event, rRTPCR=real time reverse transcription polymerase chain reaction.
3Demographic characteristics captured included age, gender, and date of admission. Other clinical features capture include fever, cough, cold, breathlessness, body ache, diarrhea, tastelessness, loss of smell, concomitant diseases (diabetes, hypertension, ischemic heart disease, and renal disease), rRT-PCR values, and outcomes (discharge/death).
Eligibility criteria were evaluated on Day-1 after informed consent procedure. For the study purpose first day is defined as randomization visit day, on which rRTPCR test was carried out.
Vital signs included blood pressure, pulse rate, and oxygen saturation.
Ayurcov medication was a single day regimen, with 10 ml dose constituted medicine, three times a day.
Specimen samples included swabs from the nose/throat/both nose and throat/nasopharyngeal sites. Specimens collected were kept in 2–8 °C ice bags and immediately transported to the laboratory. Nucleic acid extraction of SARS-CoV-2 was manually carried out in a biosafety cabinet or by automatic nucleic acid extraction system.
Adverse events (AE) were defined as emergence of any new symptom/s or worsening of pre-existing symptoms and were followed until complete resolution of symptoms.
Post discharge follow-up was done at day 7, 28, and 42 using validated functional assessment scale.
The standard of care consisted of the following:
-
(i)
Mild COVID-19 disease: Symptomatic treatment such as antipyretic (Paracetamol 500 mg orally every 6 h) for fever and pain, and adequate nutrition and rehydration. Hydroxychloroquine (HCQ) at 400 mg twice daily on the first day and 200 mg twice daily for four more days orally was administered for patients with mild disease with one or more of the following risk factors for severe disease: age > 60 years, systemic hypertension, diabetes mellitus, chronic lung / kidney / liver disease, cerebrovascular disease, and obesity. Electrocardiogram (ECG) was assessed before deciding to administer HCQ.
-
(ii)
Moderate COVID-19 disease: Oxygen was administered through nasal prongs/masks/ masks with breathing/non-rebreathing reservoir bag depending on the degree of requirement of oxygen therapy. HCQ 400 mg twice daily orally on the first day and 200 mg twice daily for four more days was provided after ECG assessment. Methylprednisolone intravenously at 0.5–1 mg/kg for 3 days was administered in case of increasing requirement of oxygen/elevated markers of inflammation (erythrocyte sedimentation rate and C-reactive protein). Unfractionated heparin/low molecular weight Heparin was preferred for the purpose of anticoagulation. In addition, symptomatic treatment with adequate nutrition/hydration was ensured.
-
(iii)
Severe COVID-19 disease: Oxygen therapy was initiated at 5 L/min and then titrated to reach a target SpO2 (≥ 90%) in non-pregnant adults, and SpO2 (≥ 92%) in pregnant patients. A diagnosis of acute respiratory distress syndrome (ARDS) was made in case of hypoxemic respiratory failure and was failing with the standard oxygen therapy and was put on mechanical ventilation. Additionally, HCQ, methylprednisolone, and anticoagulants at the above-mentioned doses and other symptomatic management were carried out.
The interventional arm consisted of the following:
-
(i)
Ayurcov medication is a single day regimen, consisting of oral medication three times a day and two times gargles as detailed below.
First gargle one hour prior to lunch around 12.00 noon and the second gargle around 9.00 PM to be done with 500 mg of Haridra (Curcuma longa) mixed in 10 ml BIDU diluted in 200 ml warm water, each time.
PA (150 mg) with RC (450 mg) in 10 ml BIDU (diluted in 100 ml of warm water) was administered orally. The first dose of this combination was started one hour post lunch, the second dose was administered two hours post first dose, and the third dose after two hours following the second dose. One glass (200 ml) of pure BIM with two teaspoons of BIG was administered post one hour of the third oral dose.
-
(ii)
The above-mentioned intervention was administered along with standard of care to all the patients.
2.4. Details on the processing and standardization of the Ayurcov ingredients
2.4.1. Haridra churna
Good manufacturing practices were adhered to as specified in the Indian Food and Drug Administration Act. Haridra was checked for adulteration or contamination, following which it was grinded to a fine powder. Then, it is sieved under 60 mesh nylon cloth for uniformity and the powder was collected and checked for the basic characteristics as color, taste, and pH.
2.4.2. BIDU
We collected (BIDU) from non-lactating Indian cows (Bos Indicus) early in the morning (Brahmamuhurta). It was then filtered and transported to the distillation plant. The urine was inspected for altered color or presence of suspended particles, and distillation was carried out. A final check was carried out for pH, clarity, and for suspended particles.
2.4.3. PA
A check for adulteration or contamination was carried out initially. Then, a small part of the alum was dissolved in water for checking suspended particles and pH. It was then grinded into small crystals and subsequently it was heated until fine opaque white powder was obtained. It was then sieved with 60 mesh nylon cloth for uniformity, and a final check on the basic characteristics as color, taste, and pH were carried out.
2.4.4. RC
An initial inspection was carried out for adulteration and contamination. Then, the suspended particles were grinded to a fine powder. After sieving the powder with 60 mesh nylon cloth for uniformity, basic characteristics such as color, taste, and pH were checked.
2.5. Endpoints
2.5.1. Primary endpoint
Proportion of patients with clinical recovery from COVID-19 related presenting symptoms.
2.5.2. Secondary endpoints
-
1.
Mild to Moderate COVID-19 disease:
-
(i)
rRTPCR values on days 1 and 3, and proportion of patients with rRTPCR negative status on day 3.
-
(ii)
Time of resolution of presenting symptoms.
-
(iii)
Proportion of patients requiring intensive care unit (ICU) admission.
-
(iv)
Duration of hospitalization.
-
(v)
Functional status at the time of discharge, at days 7, 28, and 42.
-
(vi)
Incidence of adverse events.
-
2.
Severe COVID-19 disease:
-
(i)
Proportion of patients requiring mechanical ventilator support.
-
(ii)
Proportion of patients requiring oxygen on Days 1 and 3.
-
(iii)
Time of resolution of presenting symptoms.
-
(iv)
Duration of hospitalization.
-
(v)
Functional status at the time of discharge, at days 7, 28, and 42.
-
(vi)
Incidence of adverse events. Serious adverse events (SAE) were defined as per the New Drug Clinical Trials - 2019 guidelines (India) and ICMR 2017 guidelines.
The study participants were discharged following complete recovery. In addition, the quality of life in terms of a validated functional status scale on days 7, 28, and 42 after discharge was evaluated.
2.6. Statistical analysis
2.6.1. Randomization and blinding
Simple unrestricted randomization technique was used, and the randomization list was generated using computer-generated (SAS) program. Treatment allocation was concealed using sequentially numbered, opaque, sealed envelopes. The assessors of the clinical response (and adverse events) as well as the investigators involved in statistical analyses were blinded to the treatment arms.
2.6.2. Sample size estimation
The pilot study with the same hypothesis revealed a difference in the proportions of the primary endpoint to be around 9%. At a precision of 5%, with a significance level of 5%, using the formula, , and 5% drop-out rate, the sample size for each arm was estimated to be 120.
2.6.3. Statistical tests
Intention to-treat (ITT) analysis was carried out for all the statistical tests. Time to clinical improvement (days) for cough, breathlessness, fever, and tastelessness were assessed for all patients on day-3 and till symptoms resolution. Statistical analysis was done at three time points, namely days 3, 5 and at the time of symptom resolution. The numerical data were summarized using descriptive statistics [mean and standard deviation (SD)]. Normal distribution was tested using Kolmogorov-Smirnov test and accordingly either parametric or non-parametric tests were used. For Day-1 and Day-3, all subjects (belonging to both the Intervention and Control Arms) were sorted in the descending order of Ct values to measure the quantity Ct-based precision@k. The Ct-based precision@k was defined as the percentage of patients in the intervention arm that were ranked (in the top k) above the control arm participants in this sorted list. Several clinical trials have evaluated a strong relationship between rRTPCR Ct values and a proportionate disease outcome; the more the rRTPCR Ct values, the better is the outcome. The statistical tests were carried out at 5% level of significance and a power of 90% using ‘R version 4.0.2′, Python 3, and ‘SPSS version 20′. The study was carried out in compliance with consolidated standards of reporting trials (CONSORT).23
3. Results
3.1. Demographics of the study participants
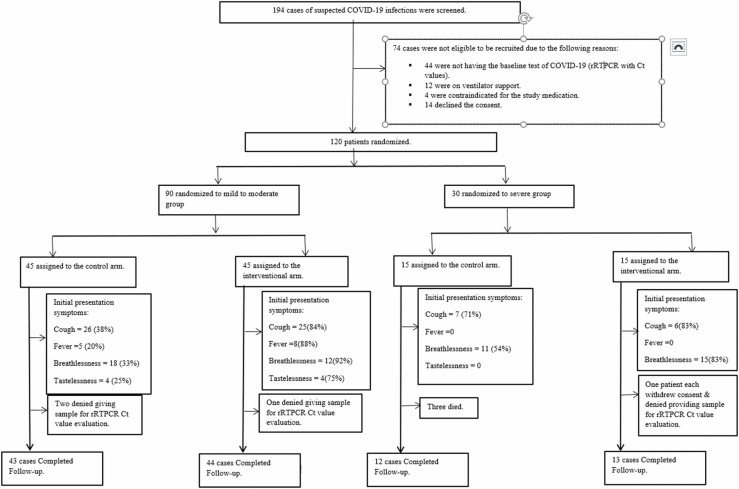
One-hundred and ninety-four patients suspected with COVID-19 infections were screened of which 74 were found ineligible (44 – negative rRTPCR; 12 – required mechanical ventilator support; 4 – had one of the contraindications for receiving the study medication; and 14 denied consent for participation), remaining 120 participants were enrolled. Ninety participants were in the 'mild to moderate group' (45 each in the control and interventional arms) and 30 (15 each in the control and interventional arms) in the 'severe group'. Fig. 1 depicts the study flow diagram. In the mild to moderate group of patients, mean (SD) age was 50.92 (14.99) years and 51.29 (15.27) years in the control and intervention arms, respectively; in the severe group, 55.93 (11.48) and 56.75 (9.71) years, respectively. The other demographic characteristics are listed in Table 2.
Fig. 1.
Study flow diagram.
Table 2.
Demographic characteristics of the study participants (N = 120).
| Parameters | Mild to moderate |
Severe |
||
|---|---|---|---|---|
| Control (n = 45) | Interventional (n = 45) | Control (n = 15) | Interventional (n = 15) | |
| Age (years)α | 50.92 (14.99) | 51.29 (15.27) | 55.93 (11.58) | 56.75 (9.71) |
| Maleβ | 31 (68.89) | 34 (75.56) | 13 (86.66) | 11 (73.33) |
| Co-existing diseases | ||||
| Hypertensionβ | 22 (48.88) | 9 (22) | 2 (13) | 3 (20) |
| Ischemic heart diseaseβ | 5 (11.11) | 3 (6.66) | 3 (20) | 1 (6.66) |
| Diabetesβ | 13 (28.88) | 10 (22.22) | 4 (26.66) | 4 (26.66) |
| Presenting symptoms | ||||
| Coughβ | 26(57.78) | 24 (54.55) | 6 (50) | 5 (35.71) |
| Breathlessnessβ | 18 (40) | 12(27.27) | 7 (58.33) | 11 (78.57) |
| Tastelessnessβ | 4 (8.89) | 4 (9.1) | 0 | 0 |
| Feverβ | 5 (11.11) | 7 (15.9) | 1 (8.33) | 0 |
rRTPCR – real time reverse transcriptase polymerase chain reaction; α – represented in mean (SD); β – represented in n (%).
In the mild to moderate group, 43/45 cases and 44/45 were considered for ITT analysis in the control and interventional arms respectively, because two patients in the control and one patient in the intervention arms, respectively denied the follow-up rRTPCR tests. In the severe group, 12/15 and 13/15 cases were considered for ITT analysis in the control and interventional arms, respectively. In the severe group, three patients died in the control arm and hence could not be considered for rRTPCR analysis. In the severe group (in the intervention arm), one patient withdrew consent while another patient refused the rRTPCR test and so were excluded from the analysis.
3.2. Proportion of patients with clinical improvement
Numbers of patients presenting with each of the COVID-19 symptoms at baseline, and on days 3, 5, and at the time of discharge are listed in Table 3. Significantly more proportions of patients with mild to moderate disease showed symptom resolution on days 3 and 5 compared with interventional arm compared to control group. No significant differences were observed in patients with severe disease.
Table 3.
Comparison of number of patients with each of the presenting symptoms in both the groups.
| Symptoms | Days | Mild-to-moderate |
Odds ratios for symptom resolution [95% CI] | Severe |
Odds ratios for symptom resolution [95% CI] | ||
|---|---|---|---|---|---|---|---|
| Control arm | Interventional arm | Control arm | Interventional arm | ||||
| Cough | D-1 (Baseline) | 26(57.78) | 24 (54.55) | NA | 6 (50) | 5 (35.71) | NA |
| D-3 | 16 (35.56) | 4 (9.09) | 5.7 [1.7, 18.7] * | 2 (16.67) | 1 (7.14) | 2.6 [0.2, 32.9] | |
| D-5 | 8 (17.78) | 1 (2.27) | 9.5 [1.1, 79.6] * | 2 (16.67) | 0 | 6.9 [0.3, 159.3] | |
| Discharge | 1 (2.2) | 0 | 3.1 [0.1, 77.3] | 0 | 0 | 1.2 [0.02, 62.9] | |
| Breathlessness | D-1 (Baseline) | 18 (40) | 12(27.27) | NA | 7 (58.33) | 11 (78.57) | NA |
| D-3 | 13 (28.89) | 2 (4.55) | 8.7 [1.8, 41.5] * | 3 (25) | 3 (21.43) | 1.2 [0.2, 7.6] | |
| D-5 | 4 (8.89) | 1 (2.27) | 5.5 [0.6, 49.1] | 1 (8.33) | 1 (7.14) | 1.2 [0.07, 21.2] | |
| Discharge | 2 4.44) | 0 | 5.2 [0.2, 112] | 0 | 0 | 1.2 [0.02, 62.9] | |
| Tastelessness | D-1 (Baseline) | 4 (8.89) | 4 (9.1) | NA | 0 | 0 | NA |
| D-3 | 3 (6.67) | 1 (2.3) | 3.1 [0.3, 31.4] | 0 | 0 | 1.2 [0.02, 62.9] | |
| D-5 | 1 (2.2) | 0 | 3.1 [0.1, 77.3] | 0 | 0 | 1.2 [0.02, 62.9] | |
| Discharge | 0 | 0 | 1 [0.01, 51] | 0 | 0 | 1.2 [0.02, 62.9] | |
| Fever | D-1 (Baseline) | 5 (11.11) | 7 (15.9) | NA | 1 (8.33) | 0 | NA |
| D-3 | 4 (8.88) | 2 (4.5) | 2.1 [0.3 12] | 0 | 0 | 1.2 [0.02, 62.9] | |
| D-5 | 1 (2.22) | 0 | 3.1 [0.1, 77] | 0 | 0 | 1.2 [0.02, 62.9] | |
| Discharge | 0 | 0 | 1 [0.02, 51.5] | 0 | 0 | 1.2 [0.02, 62.9] | |
NA-Not applicable; * - Statistically significant.
3.3. Proportion of patients requiring life-support for severe COVID-19 disease group
Three (20%) in the control group required mechanical ventilation but none in the interventional group (p = 0.1).
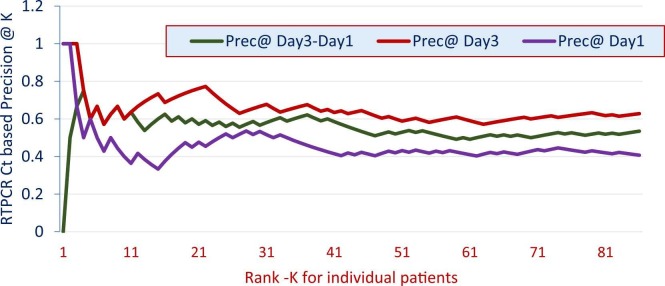
3.4. Real time reverse transcription polymerase chain reaction results
Ct-based precision@k values for both the groups on days 1 and 3 are depicted in Fig. 2. Their mean average precision (MAP) values were 0.46 and 0.53, respectively. MAP of Ct-based precision@k difference in Ct values between 'Day-1′ and 'Day-3′ (red) was 0.59. It is concluded, both from Fig. 2 (for all values of k) as well as the MAP values that on 'Day-3′ (relative to 'Day-1′).
Fig. 2.
Ct-based precision@k values.
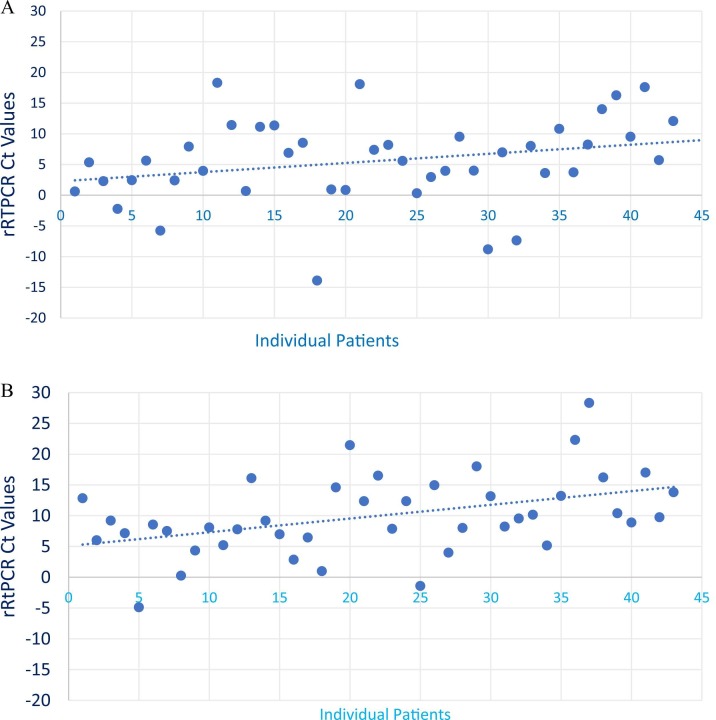
There is a relatively much larger increase in the rRTPCR Ct values [mean (SD)] was observed [9.98 (6.39), 5.55 (6.91); p < 0.001] with the interventional arm compared to the control group in the mild to moderate group ( Fig. 3A and B).
Fig. 3.
Comparison of the differences in rRTPCR Ct values. A: Dots represents difference of RTPCR Ct values at Day-1 and Day-3 in control group. B: Dots represents difference of RTPCR Ct values at Day-1 and Day-3 in interventional group.
3.5. Time of resolution of symptoms
Significantly shorter time of resolution [mean (SD)] were observed for cough [6.71 (2.96), 10.13 (2.1) days; p < 0.0000002], breathlessness [5.94 (2.62), 9.30 (2.85) days; p = 0.00000001], tastelessness [5.48 (2.66), 7.80 (2.40) days; p < 0.00006923], and fever [5.38 (2.62), 7.90 (2.66) days; p < 0.00003249] with the interventional arm compared to the control group in the mild to moderate group. However, no significant differences were observed for those with severe symptoms {cough [8.5 (2.25), 8.71 (2.25) days; p = 0.8], breathlessness [7 (2.25), 6.68 (2.25) days; p = 0.1], tastelessness [9.66(1.86), 8.83 (1.94) days; p = 0.4], and fever [7.33(3.82), 7.66 (3.82) days; p < 0.7405]}.
3.6. Proportion of patients requiring ICU admission
In the severe category, we did not observe any significant difference between the number of patients requiring admission in ICU with the interventional compared to control group [1 (2.2%), 3 (6.7%); p = 0.6].
3.7. Duration of hospitalization
Median (range) of duration of stay in the hospital was not significantly different between the interventional and control arms in both mild and moderate group [9 (19), 10 (19) days; p = 0.4] and in the severe category [10 (20), 12 (26) days; p = 0.2].
3.8. Functional status scale
Median (range) of functional status scores were not significantly different between the interventional and control groups on the day 7 [1(2), 1(2); p = 1], day 28 [1(1), 2(6); p = 0.9], and day 42 [1(4), 1(3); p = 0.9] in the mild to moderate category. However, for those with severe symptoms, the scores were significantly lower in the interventional group on day 7 [1 (1), 5 (6); p = 0.0001], day 28 [1 (1), 2.5 (3); p = 0.0009] but not on day 42 [1(1), 1(2); p = 0.08].
3.9. Adverse event and serious adverse events
Table 4 shows the comparison of adverse events between the control and the intervention arms and no significant differences were observed between the groups. No serious adverse events other than three deaths in the control arm were observed.
Table 4.
Adverse events amongst the study participants.
| Variables | Hospital Stay |
Post Discharge at Day-7 follow-up |
Post Discharge at Day-28 follow-up |
Post Discharge at Day-42 follow-up |
||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Mild to moderate category | ||||||||
| Nausea | 2 | 4 | 0 | 2 | 0 | 0 | 0 | 0 |
| Flatulence | 1 | 2 | 1 | 2 | 1 | 1 | 0 | 1 |
| Loss of appetite | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 |
| Disturbed sleep | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 1 |
| Loose Motion | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 0 |
| Headache | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 1 |
| Epigastric Pain | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Severe category | ||||||||
| Nausea | 2 | 4 | 0 | 3 | 0 | 0 | 0 | 0 |
| Disturbed sleep | 1 | 4 | 0 | 3 | 0 | 2 | 0 | 2 |
| Loose Motion | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 0 |
| Burning micturition | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 2 | 3 | 0 | 2 | 0 | 1 | 0 | 1 |
| Epigastric Pain | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
4. Discussion
Ayurveda interventions for prophylaxis, prevention and treatment of mild-moderate and severe cases of COVID-19 were pragmatically proposed as early as in April 2020 and was much appreciated by the global community working in the area of COVID-19 (COVID-19 pandemic.24
Further to this, number of case reports have been published endorsing the strength of Ayurvedic interventions in COVID −19 cases of various severity (Ayurveda co-interventions have supported complete recovery in severe COVID-19 infection with a chest severity score 18/25).25, 26
We carried out the present RCT to evaluate the therapeutic effect of ‘Ayurcov’ formulation in patients presenting with COVID-19 symptoms. We observed that significantly more proportions of patients receiving Ayurcov had symptom relief much earlier than control group. Additionally, the interventional group showed significantly lower rRTPCR Ct values. However, a shorter time of resolution of symptoms was observed with the interventional group in the mild to moderate category but not with those having severe symptoms. Similarly, a significantly better functional status was observed with interventional group on days 7 and 28 after discharge. Ayurcov was not observed with greater risks of any adverse/serious adverse events. Similar encouraging results were observed in few of the studies published on clinical trials conducted on Ayurcov.27, 28, 29
Despite the approval of remdesivir and potential claims of other anti-viral drugs in addressing the issues of hyper-inflammation of lungs, lymphocytopenia, and cytokine storms, high-quality evidence is lacking.30, 31 Given this state, evidence backing up the therapeutic efficacy and safety for drugs belonging to CAM is the need of the hour. We could demonstrate one of such CAM formulation, Ayurcov, in the present study. In addition to clinical improvement in terms of symptom resolution, we also observed that rRTPCR Ct values significantly lowered with the Ayurcov. A strong relationship between rRTPCR Ct values and disease severity was observed in other studies.31, 32 We did not observe any difference in the rRTPCR Ct values between day 3 and baseline amongst those with severe disease. One possible explanation could be the same dose of Ayurcov medicine used in all our study participants irrespective of their disease severity.
Ayurcov has multiple ingredients and the documented properties of each of the constituents are summarized in Table 5 [ For few ingredients literature reference is provided and remaining ingredients are identified by High resolution liquid chromatography, mass spectrometer (HRLCMS) and liquid state NMR spectroscopy performed on Study Medication at Indian Institute of Technology(IIT-B) Bombay laboratory]. As can be ascertained, the combination provides a spectrum of activity critical for controlling the disease phenomenon in COVID-19 such as specific antiviral effects, immune-stimulation, anti-oxidation, rheological modification, and anti-inflammatory properties. Evidence states there is a high possibility that COVID-19 has distinct pathophysiologic events than other respiratory disorders.33 Hyperinflammation is a characteristic of severe COVID-19 disease as observed by hyperferritinemia, and elevated interleukin-6 with cytokine storm.34 based on our results, it is speculated that the immunomodulatory roles of the ingredients in Ayurcov are probably controlling the progression of the disease although firm conclusions can be reached only with estimation of inflammatory markers from future pre-clinical and clinical studies. The ingredients used in Ayurcov are easily available in almost any region of the world. Although we did not estimate the cost-effectiveness, it is imperative that in resource-limited countries that are severely constrained for affording remdesivir, the only approved treatment for COVID-19, Ayurcov shall be a possible alternative. We did not observe any increased incidence of adverse events with Ayurcov even in patients with severe disease, although three deaths were observed with standard of care arm. Considering the encouraging results in this study, clinicians along with conventional treatment can consider using this Ayurvedic formulation as adjuvant, under strict supervision from registered Ayurvedic medicine practitioners.
Table 5.
Documented properties of the Ayurcov constituents.
| Antiviral effects | Antimicrobial effects | Immunity Booster | Anti-oxidation | Rheological modifier | Anti-inflammatory/ Anti-Allergic | |
|---|---|---|---|---|---|---|
| Alum | S-Methyl-L-cysteine | |||||
| Bos Indicus milk | MDGI12 | |||||
| Bos Indicus Urine | Phenyl phenol8 | Urea, Uric Acid, Creatinine, gold hydroxide, Undecanoic acid, Sulfadimidine | Iron and Erythropoietin for RBC, gold hydroxide, Squalene | D-Saccharic Acid | Urokinase, Limaprost | |
| Bos Indicus Ghee | Butyric acid | Malyngamide, Myristoleic Acid methyl ester | 17-trifluoromethylphenyl trinor PGF2α ethyl amide | Phospholipids, α-tocopherol, vitamin A, amino acids, proteins with free sulfuryl groups | 2-O-methyl PAF, C-18, Butenoyl PAF, Gallopamil, N-arachidonoyl taurine | Iridodial glucoside, Butyric acid, Hecogenin acetate, Malyngamide |
| Rock Candy | Enoxolone | Sulfamethazine, Myristoleic Acid methyl ester, Enoxolone | Anisodamine, Tafluprost | Trimeprazine |
MDGI – Mammary-derived growth inhibitor.
Jwara is Rasapradoshaj Vyadhi,35 as per the ayurvedic literature Covid 19 mimics Vata Kaphaj Pitta hina Sannipat jwara. In this Sannipat jwara as Covid-19 there is low Pitta (Mandagni) and high Vata, Kapha Dosha.36 Physiologically food is converted into Rasadhatu within a day.37
And so medicines also reaches the Rasadhatu within a day. As the spread of toxins in Covid 19 is Ashukari (Quick), we need medicine which has potential to target these toxins at the earliest preferably immediate. According to Vagbhata Sharirsthan Prabhavi Dravya (Wonder drugs) reaches to all the dhatus immediately.38
The spread of doshas if targeted at early stage by Doshapachan with Ashukari qualities, has immense potential to halt the progress of the disease leading to prevention of Dhatupaak (Tissue involvement). In view of above mechanism of action and rational, study medicine Ayurcov acts on Kapha Dosha first in first kaal (Kaphakala) i.e 1 h of meal, acts on Pitta Dosha in Pitta kala with 3–4 hrs of meal and Vata Dosha in Vaata Kaala with respect to food (Bhojankala). Ingredients like BIDU and PA acts as Prabhavi Vishaghna dravyas to counteract the low digestive fire by Agnivardhan and digests the Doshas (Doshapachan).
We observed the reversal of normal taste in participants in intervention group within few hours of administration of Ayurcoro-3, there by resuming appetite for food, by Agnivardhan and Doshapachan. Also Laghuta (lightness), Swedajanan (perspiration) and Balavardhan (energizing body) due to detoxification and improved food intake was observed in this population. These signs implies halt in disease progression and thus states the significance and rational of one day dose. So effect of various modalities of treatment explained in Ayurveda as external application, internal consumption of medicine and dietary modification synergized the optimal effect locally and systematically.
The strength of the present study is the RCT design with assessors blinded, thus the observed results are reasonably robust. However, the study is limited in not evaluating the levels of various inflammatory and anti-inflammatory markers such as interleukins, and ferritin.
5. Conclusion
Significantly shorter time of resolution [mean (SD)] was observed for cough [6.71 (2.96), 10.13 (2.1) days], breathlessness [5.94 (2.62), 9.30 (2.85) days], tastelessness [5.48 (2.66), 7.80 (2.40) days], and fever [5.38 (2.62), 7.90 (2.66) days] with the interventional arm compared to the control group in the mild to moderate group.
There is a relatively much larger increase in the Ct values [mean (SD)] was observed [9.98 (6.39), 5.55 (6.91); p < 0.001] with the interventional arm compared to the control group in the mild to moderate group, indicating rapid reduction of virulence in the interventional group in just 2 days of evaluation. However no significant differences were observed in patients with severe disease, another clinical trial with increased frequency of study medicine doses is required to be carried out to further explore its efficacy in severe disease. Ayurcov was not observed with greater risks of any adverse/serious adverse events.
As we did not observe any increased risk of adverse events, albeit superior benefits, Ayurcov as an adjuvant to standard of care can be considered in clinical practice.
Funding
Study is funded by Sarveshwar Seva Sahkar Sanstha. However, it has no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Author contributions
Conception and design: Dr. Ajay Sankhe, Dr. Nanasaheb Memane, Dr. Vijaykumar Gawali. Administrative Support: Dr. Ajay Sankhe. Collection and assembly of data: Dr. Sonal Memane, Mr. Mayur Bagul, Dr. Vijaykumar Gawali. Analysis and interpretation: Dr. Ganesh Ramakrishnan, Dr. Ashutosh Kumar, Prof. Tapanendu Kundu, Mr. Vikram Bansal, Dr. Rashmi Tiwari, Dr. Ajay Sankhe, Dr. Nanasaheb Memane, Dr. Vijaykumar Gawali. Manuscript writing: Dr. Ajay Sankhe, Dr. Nanasaheb Memane, Dr. Sonal Memane, Dr. Vijaykumar Gawali, Dr. Ganesh Ramakrishnan, Mr. Vikram Bansal, Mr.Mayur Bagul.
Conflict of interest
Authors do not have any conflict of interest.
Acknowledgments
Mr. Umakant Gaikwad (Director of Sarveshwar Seva Sahkar Sanstha), Dr. Sivaprasad Gourabathini, Dr. Ajay Godse, Mr. Praveen Muley, Mr. Kabir Das, Dr Rishikesh Karpe,Dr. Sanjay Parab, Dr. Sharad Waje, Ms. Sayli Jadhav, Mr. Yogesh Singh, Dr. Bala, Dr. Kinjal Patel, Mrs. Saakesh Shahid, Dr. Sakash Shah, Mr Ayush Maheshwari.
References
- 1.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal Y.S., Williams L.L. A glimpse of ayurveda – the forgotten history and principles of Indian traditional medicine. J Tradit Complement Med. 2016;7(1):50–53. doi: 10.1016/j.jtcme.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tripathi Brahmanand (Ed.). Charaka Samhita of Agnivesa, Nidansthan, Choukhamba Prakashan, Varanasi, 1st Chapter, Verse-30; 2001.
- 4.Pandkar Prasad Dilip, Sachdeva Vinay. Pathophysiology of Covid-19 and host centric approaches in ayurveda. J Ayurveda Integr Med. 2022;13(1) doi: 10.1016/j.jaim.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devi D., Srivastava R., Dwivedi B.K. A critical review of concept of aging in ayurveda. AYU. 2010;31(4):516–519. doi: 10.4103/0974-8520.82030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee P.K., Harwansh R.K., Bahadur S., et al. Development of ayurveda – tradition to trend. J Ethnopharmacol. 2017;197:10–24. doi: 10.1016/j.jep.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Agnivesha, Charak Samhita with Ayurveda Deepika commentary by Chakrapani Datta Edited by Yadav ji Trikamji Acharya, Chaukhambha Prakashan Edition, Sutrasthana-1/121; 2007: p. 22.
- 8.Charak Samhita, Sutra Sthana, 1/70, Satyanarayan Shastri, Chaukhamba Bharti Academy, Varanasi, 1st ed.; 1992.
- 9.Randhawa G.K. Cow urine distillate as bioenhancer. J Ayurveda Integr Med. 2010;1(4):240. doi: 10.4103/0975-9476.74089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garde GK, Vagbhat Sanhita, Saartha Vagbhat, Chapter 1, Verse 126; 2007.
- 11.Charak Sanhita, Sutrasthan, 27/217-218, DR Brahmanand Tripathi, Chaukhamba Prakashan, Varanasi; 2001.
- 12.Kaushik R., Jain J., Rai P. Therapeutic potentials of cow derived products-a review. Int J Pharm Sci Res. 2016;7(4):1383–1390. [Google Scholar]
- 13.Tillu G., Chaturvedi S., Chopra A., Patwardhan B. Public health approach of ayurveda and yoga for COVID-19 prophylaxis. J Altern Complement Med. 2020;26(5):360–364. doi: 10.1089/acm.2020.0129. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A., Naik S.N. Ghee: Its properties, importance and health benefits. Lipid Universe. 2018;6:1–9. [Google Scholar]
- 15.Bonfoh B., Zinsstag J., Farah Z., et al. Raw milk composition of Malian Zebu cows (Bos indicus) raised under traditional system. J Food Compos Anal. 2005;18:29–38. [Google Scholar]
- 16.Minochecherhomji F.P. Bio-enhancing properties of cow urine – a review. Int J Innov Res Sci Eng Technol. 2016;5:16283–16287. [Google Scholar]
- 17.Randhawa G.K., Sharma R. Chemotherapeutic potential of cow urine: a review. J Intercult Ethnopharmacol. 2015;4(2):180. doi: 10.5455/jice.2015022210032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajinkya C., Yogesh G., Varsha G., Reshma P., Avinash B. Physicochemical study of gomutra and formulation of gomutra ARK. J Pharm Biol Sci. 2019;14(04):60–64. [Google Scholar]
- 19.Zeenat F. An appraisal of medicinal properties of Shibb-e-Yamani (Alum): a review. UniMed-Kulliyat. 2018;10:78–87. [Google Scholar]
- 20.McKee A.S., Munks M.W., MacLeod M.K., et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183(7):4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotez P.J., Corry D.B., Strych U., Bottazzi M.E. COVID-19 vaccines: neutralizing antibodies and the alum advantage. Nat Rev Immunol. 2020:1–2. doi: 10.1038/s41577-020-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical Management Protocol: COVID-19. Government of India. Available at: 〈https://www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID19dated03072020.pdf〉. Accessed June 2020.
- 23.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rastogi S., Pandey D.N., Singh R.H. COVID-19 pandemic: a pragmatic plan for ayurveda intervention. J Ayurveda Integr Med. 2022;13(1) doi: 10.1016/j.jaim.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rastogi S. Ayurveda co-interventions have supported complete recovery in severe COVID-19 infection with a chest severity score 18/25: a case report. J Ayurveda Integr Med. 2022;13(2) doi: 10.1016/j.jaim.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rastogi S. COVID-19-affected family treated at home through integrative approach: upbringing the concept of ayurvedic family physician for COVID cluster management. J Ayurveda Case Rep. 2021;4(3):84. [Google Scholar]
- 27.Sankhe A.P., Memane N.S., Gawali V.P., et al. A prospective, multi center, single blind, randomized controlled study evaluating “AyurCoro3″ as an adjuvant in the treatment of mild to moderate COVID-19 patients. J Ayurveda Integr Med Sci. 2021;6(4):31–40. [Google Scholar]
- 28.Sankhe A.P., Memane N.S., Gawali V.P., et al. Use of “AyurCoro-3″ as a prophylactic drug in frontline healthcare workers involved in treating COVID-19 patients: a pilot study. Int J. 2021;4(4):821. [Google Scholar]
- 29.Sankhe A.P., Memane N.S., Gawali V.P., et al. Retrospective evaluation of the efficacy, safety and satisfaction of AyurCoro3: a patient-reported outcomes study. J Ayurveda Integr Med Sci. 2021;6(4):08–12. [Google Scholar]
- 30.Liu Y.Y., Yang Y., Zhang C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao X., Ye F., Zhang M., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y.W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58(6):e00512–e00520. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021. S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed]
- 34.Ruscitti P., Berardicurti O., Di Benedetto P., et al. Severe COVID-19, another piece in the puzzle of the hyperferritinemic syndrome. An immunomodulatory perspective to alleviate the storm. Front Immunol. 2020;11:1130. doi: 10.3389/fimmu.2020.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charak Sanhita, Nidansthan, 1/20, DR Brahmanand Tripathi, Chaukhamba Prakashan, Varanasi; 2001.
- 36.Charak Samhita, Nidan Sthana, 1/20, Satyanarayan Shastri, Chaukhamba Bharti Academy, Varanasi, 1st ed.; 1992.
- 37.Sushrut Sanhita, Purvardha 14/15, Ambika Dutta Shastri, Chaukhamba Samskrut Sanstahan, Varanasi; 2006.
- 38.Vagbhat Sanhita Shariri Sthan 3/67, Parashuram Vaidya, Yadneshwar Dikshit, 1st ed.; 1915.