Abstract
Introduction
Hemodialysis (HD) patients have increased risk for short-term adverse outcomes of COVID-19. However, complications and survival at the post–COVID-19 period have not been published extensively.
Methods
We conducted a national, multicenter observational study that included adult maintenance HD patients recovered from confirmed COVID-19. A control HD group without COVID-19 was selected from patients in the same center. We investigated the characteristics and outcomes in the follow-up of HD patients and compare them with the non–COVID-19 group.
Results
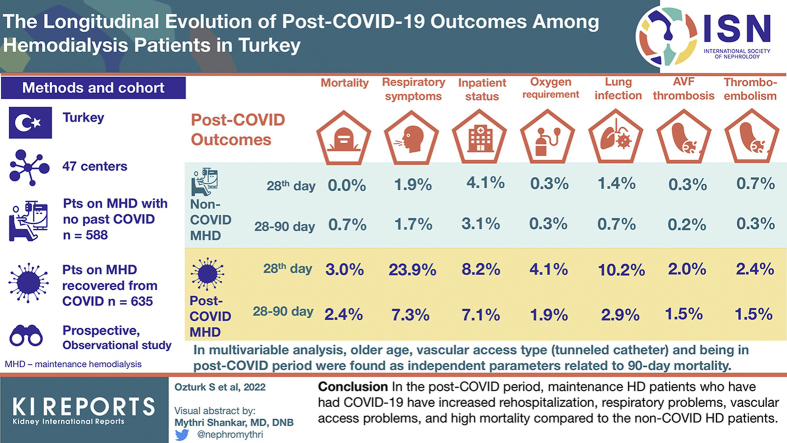
A total of 1223 patients (635 patients in COVID-19 group, 588 patients in non–COVID-19 group) from 47 centers were included in the study. The patients' baseline and HD characteristics were almost similar. The 28th-day mortality and mortality between 28th day and 90th day were higher in the COVID-19 group than non–COVID-19 group (19 [3.0%] patients vs. none [0%]; 15 [2.4%] patients vs. 4 [0.7%] patients, respectively). The presence of respiratory symptoms, rehospitalization, need for home oxygen therapy, lower respiratory tract infection, and arteriovenous (AV) fistula thrombosis was significantly higher in the COVID-19 group in both the first 28 days and between 28 and 90 days. In the multivariable analysis, age (odds ratio [OR] [95% CI]: 1.029 [1.004–1.056]), group (COVID-19 group vs. non–COVID-19 group) (OR [95% CI]: 7.258 [2.538–20.751]), and vascular access type (tunneled catheter/AV fistula) (OR [95% CI]: 2.512 [1.249–5.051]) were found as independent parameters related to 90-day mortality.
Conclusion
In the post–COVID-19 period, maintenance HD patients who have had COVID-19 have increased rehospitalization, respiratory problems, vascular access problems, and high mortality compared with the non–COVID-19 HD patients.
Keywords: COVID-19, hemodialysis, nationwide study, outcome
Graphical abstract
Hemodialysis (HD) patients have an increased risk for short-term adverse outcomes, such as hospitalization, need for intensive care support, and mortality, of COVID-19.1, 2, 3 The European Renal Association COVID-19 database, including 768 dialysis patients (72%), showed that the 28-day probability of death was 25.0% (95% CI: 20.2%–30.0%) in dialysis patients.4 The data from New York, United States, showed 28% in-hospital mortality among HD patients.5 Our group showed that undergoing maintenance HD was an independent risk factor for intensive care unit (ICU) admission and in-hospital mortality in Turkey.3,6
COVID-19–related symptoms, such as persistent shortness of breath, elevated biomarkers, and abnormalities in chest radiograph results, can persist into the late post–COVID-19 periods.7, 8, 9 In a study from Wuhan, China, describing the main clinical sequelae of 538 COVID-19 survivors, they found that general symptoms (49.6%), respiratory symptoms (39%), cardiovascular-related symptoms (13%), psychosocial symptoms (22.7%), and alopecia (28.6%) might persist for >3 months.10 In a general population cohort, lung scan abnormalities have been reported in 63% and dyspnea in 16% of patients 4 months after discharge.9 Post–COVID-19 complications and survival of the patients have not been published extensively among HD patients. We aimed to investigate the outcomes data, including symptoms, rehospitalization, and mortality, in the follow-up of HD patients in the post–COVID-19 period and compare them with the non–COVID-19 group.
Methods
This retrospective study cohort followed the report Strengthening the Reporting of Observational Studies in Epidemiology.11 Ethics Committee of Health Sciences University Haseki Training and Research Hospital approved this study (number: 2020-255).
Population and Setting
We conducted a national, multicenter observational study that included maintenance HD patients aged 18 years or older with confirmed COVID-19. In addition, a control HD group was established from the patients who did not have COVID-19 but followed in the same HD center. We did not use historical controls, both to avoid possible bias in selection and to exclude possible changes in the quality of care and treatment that might cause differences in the outcomes in all HD patients that may occur during the COVID-19 pandemic. Instead, control participants were chosen as the patient who had started dialysis closest in time to the patient with COVID-19 at each center. A web-based database was specifically designed to collect the data. This study has included the data recorded in this database from April 21, 2021, to June 11, 2021.
The main database included only patients whose SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) test result became negative and were not receiving any antiviral treatment for COVID-19 and had data from at least 28 days after diagnosis of COVID-19. We included patients who had confirmed SARS-CoV-2 infection based on positive RT-PCR testing result of a nasopharyngeal swab. We did not include patients in the acute period of COVID-19 (SARS-CoV-2 RT-PCR still positive and/or still receiving antiviral treatment for COVID-19). The peritoneal dialysis patients, kidney transplant recipients, or patients undergoing temporary HD due to acute kidney injury were not included. Patients with COVID-19 with SARS-CoV-2 RT-PCR negative results and patients without outcome data were also excluded.
Measurements and Definitions
We recorded demographic data, comorbidities and medications, primary kidney diseases that cause end-stage renal disease, duration of HD, vascular access type, residual urine amount, dialysis membrane surface area, and data regarding HD sessions (predialysis weight, systolic and diastolic blood pressures, mean erythropoietin-stimulating agent dosage, Kt/V, urea reduction rate, mean ultrafiltration volume per session, venous pressures, and speed of HD pump). We also collected data regarding routine laboratory tests (hemogram, serum creatinine, electrolytes, alanine aminotransferase, albumin, C-reactive protein [CRP], ferritin) at the last monthly routine check before the development of COVID-19. The same laboratory tests were also obtained in the same monthly check for the non–COVID-19 group. In addition, we obtained data for presenting symptoms, the COVID-19 treatment, presence of pneumonia at chest computed tomography and ICU admission, mechanical ventilation, and main treatments in the ICU for the COVID-19 group.
We have classified patients with COVID-19 according to the clinical severity of the disease at presentation according to the Ministry of Health guideline12: patients with no symptoms and/or detected at screening were classified as having an asymptomatic disease; if there is fever, cough, and so on, but no dyspnea—(there may be abnormal finding on computed tomography) were called mild disease; if there is dyspnea requiring oxygen administration—(maybe other symptoms together) were called moderate-severe disease; and blood arterial oxygen saturation < 90% despite oxygen support at admission or hemodynamic disorders requiring ICU follow-up were classified as a serious life-threatening disease.
Follow-up and Outcome
The primary end points in the study were death within 28 days and between 28 and 90 days. The secondary composite end points included rehospitalization, the persistence of respiratory symptoms associated with COVID-19, and the development of lower respiratory system infection or vascular access problems (thrombosis or catheter placement) within the 90 days after diagnosis of COVID-19. For the non–COVID-19 group patients, primary and secondary end points were also questioned during the same period (28 and 90 days) and compared with the COVID-19 group.
Statistical Analyses
IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY) was used for statistical analyses. The normality of variables was decided using visual methods (histograms and probability plots) and Kolmogorov-Smirnov tests. We presented numbers and percentages for categorical variables and median and interquartile ranges (IQRs) (25%–75%) for numeric variables in descriptive statistics. The χ2 test was used for 2 or multiple group comparisons of categorical variables. We used the independent t test or Mann-Whitney U test as appropriate in the comparison of numerical variables. In the multiple group comparisons of numerical variables, we used the variance (analysis of variance) test for numerical variables with normal distribution and the Kruskal-Wallis test for numerical variables that were not normally distributed. To determine the independent parameters related to the 90th-day mortality, a multivariable binary logistic regression model with “enter” method was used, including the different parameters between nonsurvivors and survivors. The missing data were considered as pairwise missing in the analyses and were not imputed in the study. P < 0.05 was accepted as the level of significance.
Results
Demographic and Baseline Characteristics
The main database had 1362 patients from 47 centers in Turkey. We excluded patients with a negative RT-PCR test result (n = 85 patients), patients not have a non-COVID group from that center (8 patients), patients with duplicated records (8 patients), patients with missing primary outcome data (3 patients), and active patients with COVID-19 (35 patients). The remaining 1223 patients (635 COVID-19 group, 588 non–COVID-19 group) were included in the study.
The median age of the COVID-19 group was 58.9 + 14.8 years (minimum–maximum: 19–93), and the median age of the non–COVID-19 group: 57.1 + 15.4 years (minimum–maximum: 18–94). The number of women was 292 (45.9%) in the COVID-19 group and 239 (40.7%) in the non–COVID-19 group. Table 1 and Supplementary Table S1 represent the patients’ baseline demographics, comorbidities, HD-related data, baseline, and first- and third-month laboratory test results. The most common cause of end-stage renal disease was diabetic kidney disease in the COVID-19 group (36.2%) and hypertensive nephrosclerosis in the non–COVID-19 group (36.9%). The presence of comorbidities, medicines, and smoking status of the groups was not statistically different. The rates of medication use were consistent with the rate of comorbidities.
Table 1.
The baseline demographics, comorbidities, and baseline laboratory test results of the patients
| Characteristic | Sub-group | COVID-19 group (n = 636) | Non–COVID-19 group (n = 587) | Total (N = 1223) |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr), median (IQR) | 61 (49–70) | 60 (47–69) | 60 (48–69) | |
| Gender, n (%) | Women | 292 (46.0) | 239 (40.6) | 531 (43.4) |
| Men | 343 (54.0) | 349 (59.4) | 692 (56.6) | |
| Primary kidney disease, n (%) | Diabetic kidney disease | 230 (36.2) | 160 (27.2) | 390 (31.9)a |
| Primary Glomerulonephritis | 39 (6.1) | 43 (7.3) | 82 (6.7) | |
| Hypertensive nephrosclerosis | 209 (32.9) | 217 (36.9) | 426 (34.8) | |
| ADPCKD | 28 (4.4) | 31 (5.3) | 59 (4.8) | |
| Other | 129 (20.3) | 137 (23.3) | 266 (21.7) | |
| HD duration (mo), median (IQR) | 48 (24–96) | 53 (24–96) | 48 (24–96) | |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 276/629 (43.9) | 196/339 (33.5) | 472/1214 (38.9)a | |
| Hypertension | 501/632 (79.3) | 443/586 (75.6) | 944/1218 (77.5) | |
| COPD | 78/621 (12.6) | 58/583 (9.9) | 136/1204 (11.3) | |
| Cardiac disease | 253/624 (40.5) | 214/582 (36.8) | 467/1206 (38.6) | |
| Cerebrovascular disease | 30/612 (4.9) | 24/581 (4.1) | 54/1193 (4.5) | |
| Malignancy | 21/610 (3.4) | 18/580 (3.1) | 39/1190 (3.3) | |
| Chronic liver disease | 17/614 (2.8) | 21/581 (3.6) | 38/1195 (3.2) | |
| Autoimmune/autoinflammatory diseases | 16/611 (2.6) | 24/578 (4.2) | 40/1189 (3.4) | |
| History of fistula thrombosis | 76/599 (12.7) | 59/581 (10.2) | 135/1180 (11.4) | |
| History of nonfistula thromboembolic disease | 15/593 (2.5) | 13/582 (2.2) | 28/1175 (2.4) | |
| Medicines, n/N (%) | ||||
| ACE inhibitor | 110/602 (18.3) | 119/580 (20.5) | 229/1182 (19.4) | |
| ARB | 60/605 (9.9) | 47/576 (8.2) | 107/1181 (9.1) | |
| Calcium channel blockers | 277/611 (45.3) | 236/574 (41.1) | 513/1185 (43.3) | |
| β-blocker | 278/607 (45.8) | 238/577 (41.2) | 516/1184 (43.6) | |
| Other antihypertensives | 122/600 (20.3) | 136/575 (23.7) | 258/1175 (22.0) | |
| Insulin | 198/615 (32.2) | 139/581 (23.9) | 337/1196 (28.2)a | |
| Oral antidiabetics | 46/609 (7.6) | 33/579 (5.7) | 79/1188 (6.6) | |
| Statin | 119/602 (19.8) | 99/579 (17.1) | 218/1181 (18.5) | |
| Antiaggregant | 315/596 (52.9) | 311/579 (53.7) | 626/1175 (53.3) | |
| Anticoagulants | 142/598 (23.7) | 116/583 (19.9) | 258/1181 (21.8) | |
| ESA | 459/601 (76.4) | 429/583 (73.6) | 888/1184 (75.0) | |
| i.v. iron | 378/601 (62.9) | 375/581 (64.5) | 753/1182 (63.7) | |
| Vitamin D or analogs | 325/602 (54.0) | 339/585 (57.9) | 664/1187 (55.9) | |
| Phosphorus binders containing calcium | 429/602 (71.3) | 397/584 (68.0) | 826/1186 (69.6) | |
| Lanthanum | 33/578 (5.7) | 41/580 (7.1) | 74/1158 (6.4) | |
| Cinacalcet | 84/578 (14.5) | 77/580 (13.3) | 161/1158 (13.9) | |
| Smoking, n (%) | Never smoked | 352 (58.4) | 315 (56.4) | 667 (57.4) |
| Still smoking | 70 (11.6) | 80 (14.3) | 150 (12.9) | |
| Quite smoking | 181 (30.0) | 164 (29.3) | 345 (29.7) | |
| Baseline laboratory data (before COVID-19), median (IQR) | ||||
| Potassium (mmol/l) | 5 (5–6) | 5 (5–6) | 5 (5–6) | |
| Calcium (mg/dl) | 8.6 (8–9) | 8.6 (8–9) | 8.6 (8–9) | |
| Phosphorus (mg/dl) | 5 (4–6) | 4.9 (4–6) | 5 (4–6) | |
| Parathormone (pg/ml) | 362 (220–617) | 352 (193–687) | 358 (206–646) | |
| ALT (U/l) | 11 (8–17) | 11 (7–16) | 11 (8–16) | |
| Albumin (g/dl) | 3.8 (4–4) | 3.8 (4–4) | 3.8 (4–4) | |
| Ferritin (ng/ml) | 533 (295–868) | 492 (278–764) | 513 (289–813)a | |
| CRP (mg/l) | 8 (3–20) | 4 (2–11) | 5.9 (2–15)a | |
| Hemoglobin (g/dl) | 11 (10–12) | 11 (10–12) | 11 (10–12) | |
| Leukocyte(/mm3) | 6480 (5050–8050) | 6230 (4800–7900) | 6355 (4910–7985)a | |
| Number of neutrophils (/mm3) | 3850 (2600–5400) | 3900 (2670–5120) | 3885 (2600–5240) |
ACE, angiotensin-converting enzyme; ADPCKD, autosomal-dominant polycystic kidney disease; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; ESA, erythropoietin-stimulating agents; HD, hemodialysis; IQR, interquartile range.
All the data were obtained at the month before the development of COVID-19 in the COVID-19 group and the same month in the non–COVID-19 group with the patient with COVID-19.
P < 0.05.
HD-Related Data of the Groups
The median HD duration of the COVID-19 group was 48 (IQR: 24–96) months and 53 (IQR: 24–96) months in the non–COVID-19 group (Supplementary Table S1). The most common vascular access type was AV fistula in both groups. However, this rate was significantly higher in the non–COVID-19 group than the COVID-19 group (82.8% vs. 76.9%, respectively). Tunneled HD catheter rate was significantly higher in the COVID-19 group than the non–COVID-19 group (20.3% vs. 15.1%, respectively). Predialysis weights, residual urine volumes, blood pressures, duration of the sessions, ultrafiltration volumes, blood pump rate, venous pressures, rate of erythropoietin-stimulating agent use, and used anticoagulant types were not different between the groups. Kt/V, urea reduction rate, and dialyzer surface areas were significantly lower in the COVID-19 group than the non–COVID-19 group (1.56 [IQR: 1.40–1.75] vs. 1.60 [IQR: 1.40–1.80]; 72% [IQR: 68–77] vs. 74% [IQR: 68–78] and 1.7 [1.6–1.8] vs. 1.8 [IQR: 1.7–1.8], respectively).
Predialysis serum creatinine in the COVID-19 group was significantly lower. CRP, ferritin, and leukocyte counts were significantly higher than the non–COVID-19 group (Table 1). All other laboratory test results were not different between the groups.
The Data Regarding COVID-19
Table 2 represents the data of COVID-19 in the patients with COVID-19 during the active period of the disease. Of the patients, 12.3% were asymptomatic. Fever (67.2%) and cough (63.4%) were the most common presenting symptoms. The total HD session number during the active phase of COVID-19 was 6 (IQR: 5–8). Furthermore, 451 patients (71.0%) in the COVID-19 group were hospitalized owing to COVID-19, and 56 (12.4%) of them were admitted to the ICU. Almost half of the ICU-admitted patients (n = 27, 48.2%) needed mechanical ventilation. The total length of hospital stay was 12 (IQR: 8–18) days.
Table 2.
The data of COVID-19 in the patients with COVID-19 during the active period of the disease
| Characteristic | Sub-group | n (%) |
|---|---|---|
| Symptoms | ||
| Fever | 414/616 (67.2) | |
| Dyspnea | 267/617 (43.3) | |
| Cough | 391/617 (63.4) | |
| Diarrhea | 88/604 (14.6) | |
| Loss of smell | 96/600 (16.0) | |
| Loss of taste | 111/597 (18.6) | |
| Presence of pneumonia at CT | 430/563 (76.4) | |
| Clinical severity at the time of diagnosis | Asymptomatic disease | 78 (12.3) |
| Mild disease | 336 (52.9) | |
| Moderate-to-severe disease | 201 (31.7) | |
| Serious life-threatening disease | 20 (3.1) | |
| Outpatient treatment | 185 (29.1) | |
| Inpatient treatment | 450 (70.9) | |
| Treatments for COVID-19 | ||
| Hydroxychloroquine | 134/586 (22.9) | |
| Oseltamivir | 33/585 (5.7) | |
| Macrolides | 121/580 (20.9) | |
| Favipiravir | 559/613 (91.2) | |
| Glucocorticoids | 218/586 (37.2) | |
| Tocilizumab | 8/582 (1.4) | |
| Anakinra | 5/585 (0.9) | |
| Convalescent plasma | 25/583 (4.3) | |
| HD place during active period of COVID-19 | In another center | 85 (13.7) |
| In attending center | 536 (86.3) | |
| Changes in HD session during active COVID-19 | No changes | 20 (3.2) |
| Isolated room | 351 (56.9) | |
| Specific COVID-19 session | 223 (36.1) | |
| Other | 23 (3.7) | |
| Total HD session number during acute phase of COVID-19a | 6 (5–8) | |
| Treatment method | Outpatient | 185 (29.1) |
| Inpatient | 451 (71.0) | |
| ICU data | ||
| ICU admission | 56/451 (12.4) | |
| Noninvasive mechanical ventilation | 37/53 (69.8) | |
| Mechanical ventilation | 27/56 (48.2) | |
| ECMO administration | 6/54 (11.1) | |
| Total length of hospital stay, (d), median (IQR)b | 12 (8–18) |
CT, computed tomography; ECMO, extracorporeal membrane oxygenation; HD, hemodialysis; ICU, intensive care unit; IQR, interquartile range.
Median (IQR).
Includes ward (+ ICU if applicable).
Outcomes at 28th Day and Between 28th Day and 90th Day
Some characteristics of the patients on the 28th day of the diagnosis of COVID-19 showed significant differences between the groups. The median venous pressure during HD, serum creatinine, potassium, calcium, albumin, and hemoglobin levels were significantly lower in the COVID-19 group (Supplementary Table S2). However, median erythropoietin-stimulating agent doses, alanine aminotransferase, ferritin, and CRP levels were significantly higher in the COVID-19 group than the non–COVID-19 group. There were no significant differences in other HD characteristics between the groups. However, serum CRP, ferritin levels, and leukocyte count were significantly higher in the COVID-19 group.
There were significant differences between the groups regarding the outcomes in this period (Table 3 and Figure 1). A total of 19 patients (3.0%) from the COVID-19 group died within the 28th day of the diagnosis of COVID-19. However, none of the patients from the non–COVID-19 group died within the same period. In addition, 28-day mortality was significantly higher in patients with persistent respiratory symptoms than the patients without respiratory symptoms (6 of 163 [3.7%] vs. 13 of 1060 [1.2%], respectively, P = 0.018).
Table 3.
Comparative outcome data of the patients at 28th day and 28th to 90th days
| Characteristic | COVID-19 group (n = 635) | Non–COVID-19 group (n = 588) | Total (N = 1223) |
|---|---|---|---|
| 28th-day laboratory data, median (IQR) | |||
| Creatinine (mg/dl) | 7.1 (5.6–8.7) | 7.9 (6.3–9.6) | 7.5 (5.9–9.1)a |
| Potassium (mmol/l) | 4.9 (4.4–5.4) | 5.1 (4.6–5.6) | 5.0 (4.5–5.5)a |
| Calcium (mg/dl) | 8.5 (7.9–9.0) | 8.6 (8.2–9.1) | 8.6 (8.0–9.1)a |
| Phosphorus (mg/dl) | 4.8 (3.9–5.7) | 4.83 (3.9–5.8) | 4.8 (3.9–5.8) |
| Parathormone (pg/ml) | 342 (198–600) | 365 (207–659) | 352 (200–613) |
| ALT (U/l) | 12 (8–18) | 11 (8–15) | 12 (8–17)a |
| Albumin (g/dl) | 3.6 (3.3–3.9) | 3.8 (3.6–4.0) | 3.73 (3.4–4.0)a |
| Ferritin (ng/ml) | 651 (357–1026) | 505 (307–750) | 566 (328.10–876)a |
| CRP (mg/l) | 10.35 (3.70–25.50) | 3.4 (1.90–11.60) | 6.82 (2.10–18.0)a |
| Hemoglobin (g/dl) | 10.1 (9.00–11.30) | 11 (10.00–12.00) | 10.6 (9.47–11.60)a |
| Leukocyte(/mm3) | 6390 (4960–7950) | 6200 (4600–7800) | 6300 (4815–7850)a |
| Number of neutrophils (/mm3) | 3810 (2612–5348) | 3910 (2770–5040) | 3880 (2700–5130) |
| Outcomes, n (%) | |||
| Nonsurvivor | 19 (3.0) | 0 (0) | 19 (1.6)a |
| Any respiratory symptom | 152 (23.9) | 11 (1.9) | 163 (13.3)a |
| Rehospitalization for any reason | 52 (8.2) | 24 (4.1) | 76 (6.2)a |
| Oxygen support at home | 26 (4.1) | 2 (0.3) | 28 (2.3)a |
| Lower respiratory infection | 65 (10.2) | 8 (1.4) | 73 (6)a |
| Urinary tract infection | 3 (0.5) | 6 (1) | 9 (0.7) |
| AV fistula thrombosis | 13 (2.0) | 2 (0.3) | 15 (1.2)a |
| Any other thromboembolic event | 15 (2.4) | 4 (0.7) | 19 (1.6)a |
| Need of HD catheter placement | 21 (3.3) | 9 (1.5) | 30 (2.5)a |
| n = 616b | n = 588 | N = 1204 | |
|---|---|---|---|
| 28th–90th-day laboratory data, median (IQR) | |||
| Creatinine (mg/dl) | 7.12 (5.7–8.8) | 7.9 (6.3–9.5) | 7.5 (5.9–9.1) |
| Potassium (mmol/l) | 5.0 (4.5–5.5) | 5.0 (4.6–5.6) | 5 (4.6–5.5) |
| Calcium (mg/dl) | 8.7 (8.1–9.2) | 8.7 (8.1–9.1) | 8.7 (8.1-9.1) |
| Phosphorus (mg/dl) | 5 (4.0–5.9) | 4.97 (4.0–5.90) | 5 (4.0–5.90) |
| Parathormone (pg/ml) | 324 (177–564) | 388 (215–651) | 354 (196–600)a |
| ALT (U/l) | 11 (8–17) | 10 (7–15) | 11(8-16) |
| Albumin (g/dl) | 3.8 (3.5–4.0) | 3.8 (3.6–4.0) | 3.8 (3.6–4.0)a |
| Ferritin (ng/ml) | 525 (280–888) | 476 (292–762) | 500 (288–804)a |
| CRP (mg/l) | 7 (3.0–16.0) | 4 (1.8–11.9) | 5.2 (2.0–13.7)a |
| Hemoglobin (g/dl) | 11.0 (10.00–11.90) | 11.2 (10.20–12.00) | 11.1 (10.10–12.00) |
| Leukocyte(/mm3) | 6415 (5080–8030) | 6335 (4840–7715) | 6400 (4990–7890)a |
| Number of neutrophils (/mm3) | 3820 (2740–5140) | 3900 (2790–4950) | 3845 (2769–5040) |
| Outcomes, n (%) | |||
| Nonsurvivor | 15 (2.4) | 4 (0.7) | 19 (1.6)a |
| Any respiratory symptom | 45 (7.3) | 10 (1.7) | 55 (4.6)a |
| Rehospitalization for any reason | 44 (7.1) | 18 (3.1) | 62 (5.1)a |
| Oxygen support at home | 12 (1.9) | 2 (0.3) | 14 (1.2)a |
| Lower respiratory infection | 18 (2.9) | 4 (0.7) | 22 (1.8)a |
| Urinary tract infection | 7 (1.1) | 7 (1. 2) | 14 (1.2) |
| AV fistula thrombosis | 9 (1.5) | 1 (0.2) | 10 (0.8)a |
| Any other thromboembolic event | 9 (1.5) | 2 (0.3) | 11 (0.9)a |
| Need of HD catheter placement | 13 (2.1) | 10 (1.7) | 23 (1.9) |
ALT, alanine aminotransferase; AV, arteriovenous; CRP, C-reactive protein; HD, hemodialysis; IQR, interquartile range.
P < 0.05
Did not include 19 patients who died before the 28th day.
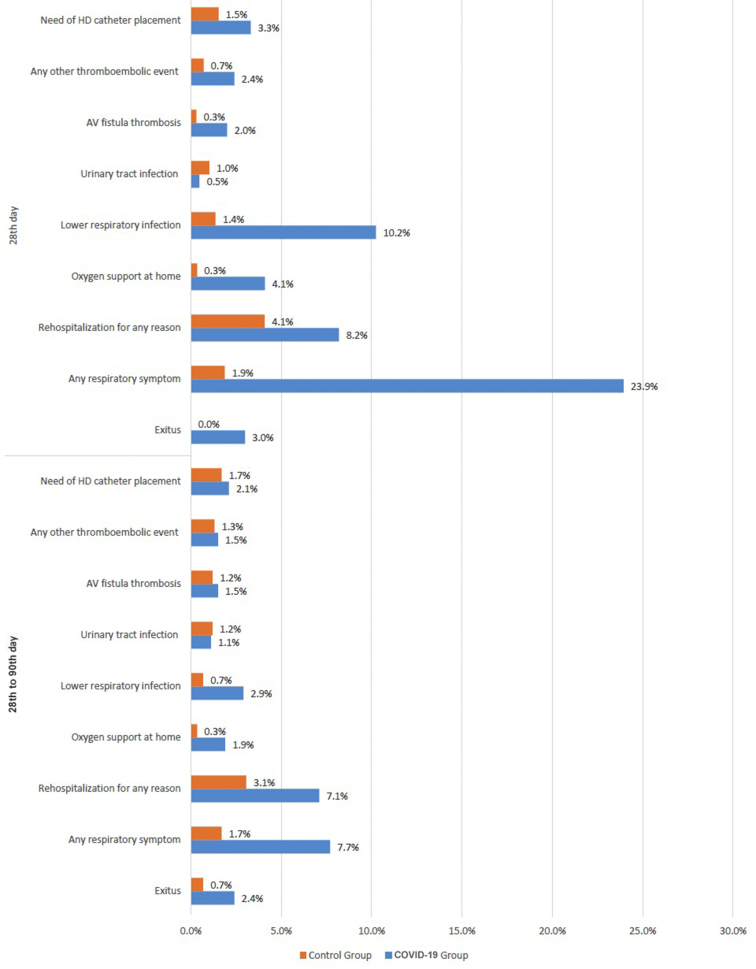
Figure 1.
The 28th-day and 28th to 90th-day outcomes of the groups. AV, arteriovenous; HD, hemodialysis.
A total of 15 patients (2.4%) from the COVID-19 group died between the 28th and 90th days of the diagnosis of COVID-19. However, 4 patients (0.7%) from the non–COVID-19 group died within the same period. Correspondingly, both in the first 28 days of the disease and between 28 and 90 days, presence of respiratory symptoms, readmission to hospital for any reason, need for home oxygen therapy, development of lower respiratory tract infection, development of AV fistula thrombosis, and development of other venous or arterial thromboembolic events were significantly higher in the COVID-19 group. Although the need for HD catheter insertion was significantly higher in the COVID-19 group within the first 28 days, it was not significantly different between the 28th and 90th days.
When the characteristics of patients who did not survive were compared with those who survived (Table 4 and Supplementary Table S2), being in the COVID-19 group, age, current smokers, the patients with tunneled HD catheters, presence of dyspnea or coughing at presentation, serious life-threatening disease at presentation, the patients who were given corticosteroids, tocilizumab, or anakinra, ICU admission, mechanical ventilation, and using continuous dialysis therapies in the ICU had significantly higher mortality. Moreover, the patients with ongoing respiratory symptoms, rehospitalized patients, the patients needing oxygen support at home, the patients developing lower respiratory infection within 28 days, and the patients with ongoing respiratory symptoms developing lower respiratory infection between 28 and 90 days had significantly higher mortality. Mortality was also related to age, serum albumin, and CRP levels in the first month and serum albumin, ferritin, and hemoglobin levels in the third month.
Table 4.
Characteristics of survivor and nonsurvivor patients on 90th day and differences between the groups
| Characteristic | Sub-group | Nonsurvivor (n = 38) | Survivor (n = 1185) |
|---|---|---|---|
| Demographics | |||
| aPatient group, n (%) | Non–COVID-19 group | 4 (0.7) | 584 (99.3) |
| COVID-19 group | 34 (5.4) | 601 (94.6) | |
| aAge (yr), median (IQR) | 68.5 (59–74) | 60 (48–69) | |
| HD duration (mo), median (IQR) | 48 (24–72) | 48 (24–96) | |
| Gender, n (%) | Women | 18 (47.4) | 513 (43.3) |
| Men | 20 (52.6) | 672 (56.7) | |
| Primary kidney disease, n (%) | Diabetic nephropathy | 16 (42.1) | 374 (31.6) |
| Glomerulonephritis | 1 (2.6) | 81 (6.8) | |
| Hypertensive nephrosclerosis | 11 (28.9) | 415 (35.0) | |
| ADPCKD | 2 (5.3) | 57 (4.8) | |
| Others | 8 (21.1) | 258 (21.8) | |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 19 (50.0) | 453 (38.5) | |
| Hypertension | 30 (78.9) | 914 (77.5) | |
| COPD | 5 (13.2) | 131 (11.2) | |
| Cardiac disease | 17 (44.7) | 450 (38.5) | |
| Cerebrovascular disease | 2 (5.3) | 52 (4.5) | |
| Malignancy | 2 (5.3) | 37 (3.2) | |
| Chronic liver disease | 1 (2.6) | 37 (3.2) | |
| Autoimmune/autoinflammatory disease | 1 (2.7) | 39 (3.4) | |
| AV fistula thrombosis history | 5 (13.5) | 130 (11.4) | |
| HD information | |||
| Vascular access, n (%) | aAV fistula | 22 (57.9) | 953 (80.4) |
| AV graft | 1 (2.6) | 19 (1.6) | |
| Catheter (transient) | 0 (0) | 10 (0.8) | |
| aCatheter (tunneled-permanent) | 15 (39.5) | 203 (17.1) | |
| Duration of current vascular access (mo), median (IQR) | 24.5 (18–48) | 36 (16–72) | |
| Number of sessions during active COVID-19, median (IQR) | 6 (4–10) | 6 (5–8) | |
| ICU data, n (%) | |||
| aAdmission to ICU | 15/32 (46.9) | 41/420 (9.8) | |
| Noninvasive mechanical ventilation | 13/15 (86.7) | 24/38 (63.2) | |
| aNeed of mechanical ventilation | 16/17 (94.1) | 11/41 (26.8) | |
| aECMO application | 0/14 (0) | 6/40 (15) | |
| aSlow continuous treatments (HF/HDF) | 6/15 (40) | 3/40 (7.5) | |
| aTotal length of hospital stay (including service + ICU), days, median (IQR) | 13 (8–20) | 12 (9–17) | |
| Length of stay at ICU, median (IQR) | 10 (4–17) | 8 (6–14) | |
| 28th-day outcomes, n (%) | |||
| aAny respiratory symptoms | 16/38 (42.1) | 147/1185 (12.4) | |
| aRehospitalization | 7/38 (18.4) | 69/1185 (5.8) | |
| aNeed of oxygen treatment at home | 5/38 (13.2) | 23/1185 (1.9) | |
| aLower respiratory infection | 8/38 (21.1) | 65/1185 (5.5) | |
| Urinary tract infection | 0/38 (0) | 9/1185 (0.8) | |
| Any venous or arterial thromboembolic events | 1/38 (2.6) | 18/1185 (1.5) | |
| AV fistula thrombosis | 1/38 (2.6) | 14/1185 (1.2) | |
| Need of HD catheter | 1/38 (2.6) | 29/1185 (2.4) | |
| 90th-day outcomes, n (%) | |||
| aAny respiratory symptoms | 7/38 (18.4) | 52/1185 (4.4) | |
| Rehospitalization | 6/38 (15.8) | 59/1185 (5) | |
| Need of oxygen treatment at home | 2/38 (5.3) | 13/1185 (1.1) | |
| aLower respiratory infection | 3/38 (7.9) | 21/1185 (1.8) | |
| Urinary tract infection | 0/38 (0) | 14/1185 (1.2) | |
| Any venous or arterial thromboembolic events | 1/38 (2.6) | 10/1185 (0.8) | |
| AV fistula thrombosis | 0/38 (0) | 10/1185 (0.8) | |
| Need of HD catheter insertion | 0/38 (0) | 23/1185 (1.9) |
ADPCKD, autosomal-dominant polycystic kidney disease; AV, arteriovenous; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; HD, hemodialysis; HDF, hemodiafiltration; HF, hemofiltration; ICU, intensive care unit; IQR, interquartile range.
P < 0.05.
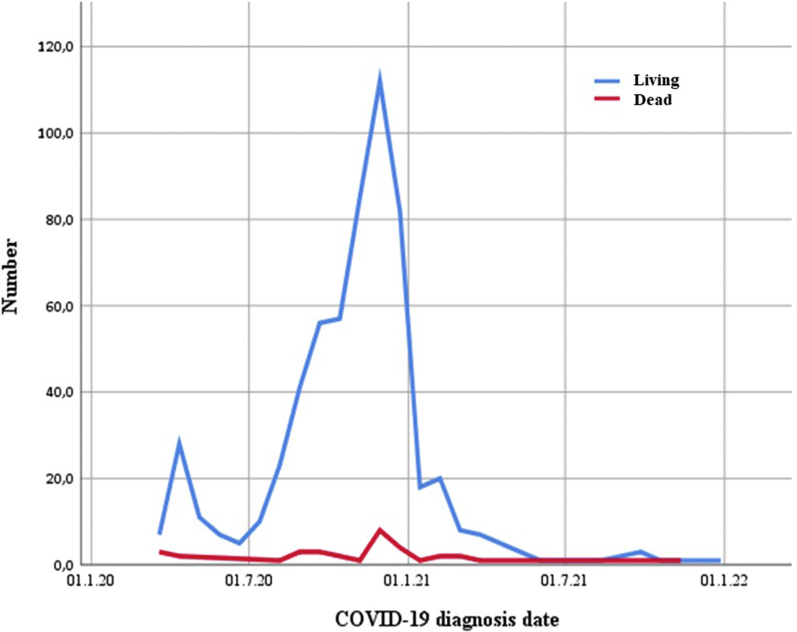
In the analysis made according to the date of diagnosis of COVID-19 (Figure 2), we saw that most of the cases occurred in the second half of 2020, with the most deaths in this period. Moreover, we found that the centers showed a similar distribution to this graph in terms of case diagnosis date (Supplementary Figure S1).
Figure 2.
Graph showing case survivals by date of diagnosis of COVID-19.
We divided the COVID-19 group into outpatient/hospitalized and compared it with the non–COVID-19 group (Supplementary Table S3). Hospitalized patients were older, had more comorbidities, had worse basic laboratory tests, had more COVID-19 symptoms, and had a more severe clinical presentation. All deaths on day 28 (all 19 patients) and most deaths between 28 and 90 days (32 of 38 patients) were from the hospitalized group. Almost all studied clinical problems (any respiratory symptom, rehospitalization for any reason, need of oxygen support at home, lower respiratory infection, AV fistula thrombosis, any other thromboembolic event) on the 28th day and any respiratory symptom and lower respiratory infection on the 90th day were significantly higher than the other groups.
In the multivariable analysis, age (OR [95% CI]: 1.029 [1.004–1.056]), group (COVID-19 vs. non–COVID-19) (OR [95% CI]: 7.258 [2.538–20.751]), and vascular access type (tunneled catheter vs. AV fistula) (OR [95% CI]: 2.512 [1.249–5.051]) were found as independent parameters related to 90-day mortality (Table 5). When this analysis is repeated according to the results of the univariable analysis by dividing the COVID-19 group into 2 groups according to hospitalization, group (hospitalized COVID-19 vs. non–COVID-19 group) (OR [95% CI]: 7.854 [1.032–59.757]) and vascular access type (tunneled catheter vs. AV fistula) (OR [95% CI]: 3.522 [1.496–8.292]) were found as independent parameters related to 90th-day mortality (Supplementary Table S4).
Table 5.
Binary logistic regression analysis of the baseline parameters related to the 90th-day mortality
| Parameters | OR | 95% CI for OR |
P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (yr) | 1.029 | 1.004 | 1.056 | 0.026 |
| Gender (male/female) | 1.009 | 0.514 | 1.983 | 0.979 |
| Diabetes mellitus | 1.116 | 0.567 | 2.196 | 0.751 |
| Group (COVID-19 group/non–COVID-19 group) | 7.258 | 2.538 | 20.751 | <0.001 |
| Vascular access (tunneled catheter/AV fistula) | 2.512 | 1.249 | 5.051 | 0.010 |
| Constant | 0.004 | <0.001 | ||
AV, arteriovenous; OR, odds ratio.
The model included the parameters that were found different between survivor and nonsurvivor in Table 4. We also added gender and diabetes mellitus, which might be effective in survival based on the literature.
Discussion
In this multicenter retrospective study involving maintenance HD patients recovering from COVID-19 and a non–COVID-19 group, we presented a detailed comparative follow-up data, including demographics, symptoms, laboratory tests, treatments, and outcomes of the groups. One of the most striking findings was significantly higher 28th-day and 90th-day mortality in the COVID-19 HD group compared with the non–COVID-19 group. Although the short-term mortality of HD patients with COVID-19 has been shown to be higher than the patients with COVID-19 from the general population,3,13 as far as we know, no study has shown the increased mortality after the acute phase of COVID-19 (post–COVID-19 period) than a non–COVID-19 HD cohort. We showed that all deaths on the 28th day were from the COVID-19 group (3.0% of patients), and most deaths (15 of 19) on the 90th day were also from the COVID-19 group, especially among hospital discharged patients. In multivariable analyses, we also found that age, being in the COVID-19 group, and using a tunneled catheter for vascular access were independent parameters related to 90th-day mortality. These data clearly show the ongoing high mortality risk after COVID-19 in HD patients compared with the non–COVID-19 HD patients.
When we compared nonsurvivors with survivors, being in the COVID-19 group, age, current smokers, the patients with tunneled HD catheters, presence of dyspnea or coughing at presentation, serious life-threatening disease at presentation, corticosteroids, tocilizumab, or anakinra use, ICU admission, mechanical ventilation, and using continuous dialysis therapies in the ICU were related with significantly higher mortality. Serum albumin, CRP, ferritin, and hemoglobin levels were worse in nonsurvivors. Especially ICU admission and mechanical ventilation were significant predictors of 90th-day mortality, as 26.8% of ICU-admitted patients and 59.3% of mechanical ventilated patients died during 90 days. Almost all papers in the literature regarding the outcome of HD patients with COVID-19 included only the acute phase of the disease. As far as we know, this is the first study researching the outcomes of HD patients at the post–COVID-19 period and comparing them with a non–COVID-19 group. Although there are no studies with similar designs and outcomes, some indirect results of studies involving the acute phase of patients with COVID-19 may support our outcomes. In a multicenter study of our group including 567 maintenance HD patients with active COVID-19, in-hospital mortality was 16.3%.14 In that study, age (hazard ratio: 1.022 [95% CI: 1.003–1.041], P = 0.025), severe-critical disease clinical presentation at the time of diagnosis (hazard ratio: 6.223 [95% CI: 2.168–17.863], P < 0.001), presence of congestive heart failure (hazard ratio: 2.247 [95% CI: 1.228–4.111], P = 0.009), and ferritin levels on admission (hazard ratio: 1.057 [95% CI: 1.006–1.111], P = 0.028) were among the risk factors for mortality. In another study conducted by the European Renal Association COVID-19 Database, including 1423 end-stage renal disease patients with COVID-19 (HD = 1017 patients/kidney transplant recipients = 406 patients), the higher age, prior smoking history, higher clinical frailty score, and self-reported shortness of breath at first presentation were identified as predictors of mortality in those discharged at initial triage.15 They also showed that among nonhospitalized patients, 10% (n = 36) were readmitted to the hospital; these patients had worsening respiratory symptoms, a fall in oxygen saturation (97% vs. 90%), and high CRP between attendances (26 vs. 73 mg/l).
Our study gave important data regarding the nonfatal outcomes after the COVID-19. The presence of respiratory symptoms, readmission to hospital for any reason, need for home oxygen therapy, development of lower respiratory tract infection, development of AV fistula thrombosis, and development of other venous or arterial thromboembolic events were significantly higher in the COVID-19 group at both first 28 days of the disease and between 28 and 90 days. We found that dyspnea was reported at a rate of 23.9% on the 28th day and 7.3% on the 90th day of the diagnosis of COVID-19. A similar high prevalence of shortness of breath was reported in the general population after the COVID-19 period. Halpin et al.16 showed that breathlessness was a significantly higher symptom among patients discharged from ICU than the ward group (65.6% in ICU group and 42.6% inward group), which was assessed 4 to 8 weeks after hospital discharge. Although our study included not only hospitalized but also outpatient COVID-19 cases, it seems to be a very significant late persisting symptom. Moreover, among COVID-19 group, 4.1% of the patients on the 28th day and 2.9% on the 90th day required oxygen support at home, which was significantly higher than the non–COVID-19 group patients. Moreover, in our study, serum CRP, ferritin levels, and leukocyte counts were significantly higher in the COVID-19 group during follow-ups. The findings of persisting respiratory symptoms, need for oxygen support at home, and development of lower respiratory infections in the COVID-19 group were significantly higher than the non–COVID-19 group. All these might show the ongoing pulmonary inflammation together with the lung sequela of patients with COVID-19.
The rehospitalization rates within 28 days and between the 28th day and 90th day in the COVID-19 group were significantly higher than that of the non–COVID-19 group (8.2% vs. 4.1% and 7.1% vs. 3.1%, respectively). To the best of our knowledge, the rehospitalization rate of HD patients in the post–COVID-19 period has not been published yet. Some studies among nonuremic populations represented comparable data. A retrospective study including the general population cohort aimed to determine 30-day posthospitalization outcomes after COVID-19 from New York City showed that among 1344 patients, 16.5% returned to an emergency department, 9.8% were rehospitalized, and 2.4% died.17 In a study from Wuhan, China, aiming to describe the long-term health consequences of patients with COVID-19 who have been discharged from hospital, 25 of 1733 patients (1.5%) were readmitted to the hospital.18
We showed increased AV fistula thrombosis and other arterial or venous thromboses among COVID-19 group patients than the non–COVID-19 group within 28 days and between the 28th and 90th days. During COVID-19, there may be a hypercoagulation condition in which different and complex mechanisms play a role. Microvascular and macrovascular thrombosis with associated inflammation (thrombo-inflammation) ensues commonly in ICU admitted patients with COVID-19.19, 20, 21 It has been shown that catheter-related thrombosis is significantly more frequent in patients with COVID-19,22 and having an AV fistula as vascular access among HD patients might contribute to higher survival of HD patients with COVID-19.23 However, there is no study showing increased vascular access thrombosis among COVID-19 survivors. A study from France that collected fistula thrombosis cases among active COVID-19 HD patients in 7 dialysis units included only 17 patients.24 10 patients (59%) were men, and 10 patients (59%) had diabetes. The mortality rate in these patients was 47%. All thrombosis was successfully treated with declotting procedures but with an early relapse rate of 36%. Shabaka et al.25 showed increased late thrombotic events in COVID-19 survivors compared with the noninfected cohort after a median follow-up of 7 months (18.5% vs. 1.9%, P = 0.002) among 185 prevalent HD patients. In that study, 6 of 158 patients (3.8%) in the non–COVID-19 group with a previous history of vascular access dysfunction had vascular access thrombosis 6 months after COVID-19 but none of the COVID-19 group (27 patients) developed vascular access thrombosis.
Another interesting finding of our study is that the predialysis serum Kt/V and urea reduction rate values of the COVID-19 group before COVID-19 were significantly lower and CRP, ferritin, and leukocyte counts were higher than the non–COVID-19 group. This may indicate that HD patients with low muscle mass and high inflammation, which may be a component of the malnutrition-inflammation-atherosclerosis complex, may therefore be more susceptible to COVID-19.26
In our study, use of corticosteroids, tocilizumab, or anakinra was related to high mortality. All these treatments were given to severe patients with COVID-19 according to our national guideline; hence, increased mortality in these patients seems to be associated with the severity of the disease. Moreover, to determine the independent parameters associated with the late survival of the patients, we included data that showed significant differences between the patients' baseline demographic data in the multivariable analysis. Age, patient group, vascular access, and gender and diabetes mellitus, which are promising parameters in the literature, were included in this analysis. In the multivariable analysis, age (OR [95% CI]: 1.029 [1.004–1.056]), group (COVID-19 group vs. non-COVID group) (OR [95% CI]: 7.258 [2.538–20.751]), and vascular access type (tunneled catheter/AV fistula) (OR [95% CI]: 2.512 [1.249–5.051]) were found as independent parameters related to 90-day mortality.
In our study, it was seen that there were no patients with serious life-threatening disease in the clinical presentation in the outpatient COVID-19 group, and the rate of moderate-to-severe disease was very low (3.3%) among them. This may be related to the health structure in our country. All HD treatments and COVID-19 treatments (hospitalizations, ICU admissions—even in private hospitals) in Turkey are covered by the government. All hospitals are allocated to serving pandemic patients as well. In addition, from the onset of the pandemic, there was no problem of finding beds in hospitals or ICUs. Computed tomography was readily and easily available and widely used for diagnosis in all patient groups. This study had some limitations. First, it was retrospective and the groups were not fully randomized. However, the recording of data from different regions in a structured database and the inclusion of a non–COVID-19 group from each center in the study can be considered as close to those encountered in real life. In addition, the fact that our study was designed with simple randomization made the comparisons of the results more valuable. Second, causes of mortality and rehospitalization were not studied in detail, which may provide important data on the causation of COVID-19 complications. However, because COVID-19 is a systemic disease, any cause of death may be associated with COVID-19. Therefore, all-cause mortality may be the best way to evaluate COVID-19–related deaths. In our study, especially the death rate in the non–COVID-19 group seems to be lower than in many other studies. There may be many reasons for this, but the annual mortality rate of chronic HD patients in the Turkish cohort is known to be significantly (up to 50% after adjustments) lower than that published in the US Renal Data System Annual Data Report.27 In contrast, the annual mortality rates of patients in the Turkish cohort in this study were close to the rates in the Chinese patient cohort published on similar dates.28 This suggests that there may be regional and racial differences.
As a result, in the post–COVID-19 period, maintenance HD patients who have had COVID-19 have increased rehospitalization, respiratory problems, vascular access problems, and mortality compared with the non–COVID-19 HD patients. These adverse outcomes are particularly evident in the post–COVID-19 period of hospitalized COVID-19 HD patients. Thus, longer follow-up of post–COVID-19 in maintenance HD patients may provide further insight into the burden of this infection.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank the Turkish Society of Nephrology for the organization of the study and the OMEGA Contract Research Organization in Turkey for data processing and statistical analysis. We also appreciate Prof. Dr. Halim Issever, from Istanbul University, Istanbul Faculty of Medicine, Department of Public Health, for his invaluable contributions.
Footnotes
Table S1. The HD-related data and laboratory analyses at first and third months after diagnosing COVID-19 data. All data in the non–COVID-19 group were obtained at the same month with the COVID-19 patient in the non–COVID-19 group.
Table S2. Characteristics of survivor and nonsurvivor patients on the 90th day and differences between the groups.
Table S3. The comparative presentation demographics, comorbidities, and laboratory tests of the patients stratified according to hospitalization.
Table S4. Binary logistic regression analysis of the baseline parameters related to the 90th-day mortality including COVID-19 group divided into two groups according to hospitalization.
Figure S1. Chart showing case distributions and case survivals of centers by COVID-19 diagnosis date.
STROBE Statement.
Supplementary Material
Table S1. The HD-related data and laboratory analyses at first and third months after diagnosing COVID-19 data. All data in the non–COVID-19 group were obtained at the same month with the COVID-19 patient in the non–COVID-19 group.
Table S2. Characteristics of survivor and nonsurvivor patients on the 90th day and differences between the groups.
Table S3. The comparative presentation demographics, comorbidities, and laboratory tests of the patients stratified according to hospitalization.
Table S4. Binary logistic regression analysis of the baseline parameters related to the 90th-day mortality including COVID-19 group divided into 2 groups according to hospitalization.
Figure S1. Chart showing case distributions and case survivals of centers by COVID-19 diagnosis date.
STROBE Statement.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. Published online March 31, 2020. https://doi.org/10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed]
- 3.Ozturk S., Turgutalp K., Arici M., et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35:2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilbrands L.B., Duivenvoorden R., Vart P., et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher M., Yunes M., Mokrzycki M.H., et al. Chronic hemodialysis patients hospitalized with COVID-19: short-term outcomes in the Bronx, New York. Kidney360. 2020;1:755–762. doi: 10.34067/KID.0003672020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozturk S., Turgutalp K., Arici M., et al. Characteristics and outcomes of hospitalised older patients with chronic kidney disease and COVID-19: a multicenter nationwide controlled study. Int J Clin Pract. 2021;75:e14428. doi: 10.1111/ijcp.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal S., Barnett J., Brill S.E., et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalization for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Writing Committee for the COMEBAC Study Group. Morin L., Savale L., et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19 [published correction appears in JAMA. 2021;326:1874] JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong Q., Xu M., Li J., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenbroucke J.P., von Elm E., Altman D.G., et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Republic of Turkey Ministry of Health. Guidance to COVID-19 (SARS Cov2 Infection) (Scientific Board Study). Republic of Turkey Ministry of Health. Published April 14, 2021. Accessed April 18, 2021. https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID-19_Rehberi20200414_eng_v4_002_14.05.2020.pdf
- 13.Savino M., Casula A., Santhakumaran S., et al. Sociodemographic features and mortality of individuals on haemodialysis treatment who test positive for SARS-CoV-2: a UK Renal Registry data analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turgutalp K., Ozturk S., Arici M., et al. Determinants of mortality in a large group of hemodialysis patients hospitalized for COVID-19. BMC Nephrol. 2021;22:29. doi: 10.1186/s12882-021-02233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra S., Jayanti A., Vart P., et al. Clinical triage of patients on kidney replacement therapy presenting with COVID-19: an ERACODA registry analysis. Nephrol Dial Transplant. 2021;36:2308–2320. doi: 10.1093/ndt/gfab196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpin S.J., McIvor C., Whyatt G., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 17.Kingery J.R., Bf Martin P., Baer B.R., et al. Thirty-day post-discharge outcomes following COVID-19 infection. J Gen Intern Med. 2021;36:2378–2385. doi: 10.1007/s11606-021-06924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill J.B., Garcia D., Crowther M., et al. Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Blood Adv. 2020;4:5373–5377. doi: 10.1182/bloodadvances.2020003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menter T., Haslbauer J.D., Nienhold R., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gidaro A., Vailati D., Gemma M., et al. Retrospective survey from vascular access team Lombardy net in COVID-19 era. J Vasc Access. 2021 doi: 10.1177/1129729821997252. [DOI] [PubMed] [Google Scholar]
- 23.Murt A, Yadigar S, Yalin SF, et al. Arteriovenous fistula as the vascular access contributes to better survival of hemodialysis patients with COVID-19 infection. J Vasc Access. Published February 23, 2021. https://doi.org/10.1177/1129729821997252 [DOI] [PMC free article] [PubMed]
- 24.Desbuissons G, Michon A, Attias P, et al. Arteriovenous fistulas thrombosis in hemodialysis patients with COVID-19. J Vasc Access. Published online February 24, 2021. https://doi.org/10.1177/1129729821996091 [DOI] [PubMed]
- 25.Shabaka A., Gruss E., Landaluce-Triska E., et al. Late thrombotic complications after SARS-CoV-2 infection in hemodialysis patients. Hemodial Int Int Symp Home Hemodial. 2021;25:507–514. doi: 10.1111/hdi.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betjes M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 27.Asci G., Marcelli D., Celtik A., et al. Comparison of Turkish and US haemodialysis patient mortality rates: an observational cohort study. Clin Kidney J. 2016;9:476–480. doi: 10.1093/ckj/sfw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng X., Nayyar S., Wang M., et al. Mortality rates among prevalent hemodialysis patients in Beijing: a comparison with USRDS data. Nephrol Dial Transplant. 2013;28:724–732. doi: 10.1093/ndt/gfs326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.