Abstract
Background
A large proportion of individuals who have recovered from an acute COVID-19 infection continue to experience symptoms months later. Post-acute COVID-19 (long-haul COVID-19) can range from serious complications to quality of life symptoms such as fatigue or insomnia. The purpose of this study was to evaluate the potential for inhalation of essential oils to improve energy levels among otherwise healthy female survivors of acute COVID-19 who experience a lack of energy more than five months after recovery. This study was conducted in the United States in late 2021.
Method
This was a randomized double blind, placebo controlled trial to evaluate the potential for inhalation of Longevity™, a proprietary essential oil blend manufactured by Young Living Essential Oils (Lehi, Utah, USA), on energy levels among female survivors of COVID-19 who continue to experience fatigue more than 5 months recovery from the acute infection. Forty women were randomized to two groups: intervention and placebo. Both groups inhaled the assigned product twice daily for fourteen consecutive days. Fatigue scores were measured using the Multidimensional Fatigue Symptom Inventory (MFSI). Secondary outcomes included scores on each of the MFSI’s ten subscales.
Results
Individuals who inhaled the essential oil blend for 2 weeks had significantly lower fatigue scores after controlling for baseline scores, employment status, BMI, olfactory function, and time since diagnosis, with a large effect size (F (1,39) = 6.15, p = .020, partial eta squared = 0.198). Subscale analysis identified subscales of vigor, as well as global, behavioral, general, and mental fatigue as benefiting from the intervention. This study provides evidence that a proprietary aromatherapy blend can significantly improve energy levels among women who are experiencing fatigue after recovering from COVID-19.
1. Introduction
1.1. Background
Since the beginning of the COVID-19 pandemic in late 2019, over 250 million documented cases have been reported worldwide, with nearly 1 in 5 of those occurring in the United States.28 The SARs-CoV-2 virus is widely understood to produce an acute respiratory infection with potentially fatal complications. However, its long-term effects have received less attention. Estimates of prevalence of long-haul COVID-19 range from approximately 23% to as high as 73%, depending on epidemiological methodology and sampling population.8, 18
Over 50 symptoms have been identified among survivors of COVID-19.3, 13 These range from mild symptoms, such as fatigue, cognitive impairment, and headaches, to serious symptoms such as difficulty breathing, post-traumatic stress disorder (PTSD), hypertension, and chest pain.25 Regardless of severity, these symptoms dramatically reduce quality of life among survivors of COVID-19, reducing productivity, adversely affecting employment status, and impacting mental wellbeing.16 Furthermore, severity of the initial infection does not appear to predict the long haul extent to which these symptoms impair survivors.10
Fatigue is the most common post-COVID-19 symptom, affecting approximately 58% of those with long-haul symptoms.13 This is not unusual as fatigue is common among patients who have recovered from respiratory illnesses.9 Fatigue differs from sleepiness in that it encompasses a feeling of exhaustion and tiredness regardless of sleep quantity and quality. It is a nonspecific symptom that leads to a reduced overall capacity to accomplish routine tasks. This may involve the inability to initiate activity, inability to continue routine activities, or difficulty focusing and concentrating, with symptoms worsening after physical or mental exertion.23
This lack of energy affects physical, mental, and emotional states, altering overall quality of life.20 COVID-19 has also been shown to produce chronic health conditions such as diabetes, kidney impairment, or pulmonary fibrosis.14, 21, 22 Addressing these newly diagnosed conditions may require invasive treatments or long-term medications, so interventions to address mild concerns should be non-invasive to reduce potential for interaction with advanced treatments.
Aromatherapy is the practice of inhalation essential oils. Essential oils are made up of lipid soluble volatile compounds found in various parts of plant matter, including flower petals, leaves, stems, and roots Thomas.26 They enter the bloodstream through the respiratory system. The molecules in the oils activate the body’s limbic system, which affects emotions and behaviors. The exact mechanism of action for many essential oils is unknown but its recognized effects are often attributed to these actions on the limbic system.
Sensory-based interventions, such as aromatherapy, music therapy, and massage have been found to boost energy levels in many populations.12, 17, 27 There is evidence that aromatherapy inhalation can increase energy among cardiac patients, hypothyroid patients, and patients undergoing hemodialysis, using essential oils such as peppermint, clove, and orange. Mahdavikian et al.,1, 15, 7 Many commercial products, including this blend, are marketed for their invigorating or energizing effects. However, to our knowledge, aromatherapy has not been evaluated as a tool to boost energy levels among patients who have recovered from acute COVID-19.
1.2. Objectives
The purpose of this study was to evaluate the potential for inhalation of essential oils to improve energy levels among otherwise healthy female survivors of acute COVID-19 who experience a lack of energy more than five months after recovery. This study is reported using the CONSORT checklist for reporting clinical trials with the Herbal Medicinal Interventions extension.29, 30
2. Methods
This study was a randomized double blind, placebo controlled trial to evaluate the effects of an aromatherapy blend on energy levels among female survivors of COVID-19 who are experiencing low energy levels after recovery.
2.1. Participants
Participants were eligible if they had recovered from COVID-19 five or more months before the start of the intervention, if they reported experiencing fatigue at a level that was not present prior to COVID-19, and if they were otherwise healthy. Additional inclusion criteria include: aged 19–49, living in the United States, and self-identification as a woman. To capture a population within 6–9 months of their diagnosis, participants were required to have a COVID-19 diagnosis between December 1, 2020, and March 31, 2021.
Participants were not eligible if they had a positive COVID-19 test at any time before December 1, 2020 or after March 31, 2021. Exclusion criteria also included an allergy to any of the intervention ingredients or symptoms of serious long-haul-COVID-19 including recurring headaches, chest pain, abnormal pulmonary function, or uncontrolled hypertension. Those who were going to receive a COVID-19 vaccine during the study period or who had received one within the week prior to the study period were excluded. Additionally, those with a diagnosis of chronic fatigue syndrome, those who experienced fatigue prior to the COVID-19 diagnosis, those with hypothyroidism, and those who were pregnant, trying to conceive, or breastfeeding were excluded.
2.2. Intervention
Those randomized to the intervention group were given one 15 ml bottle containing a blend of essential oils extracted from the following plants: thyme (Thymus vulgaris), orange peel (Citrus sinensis), clove bud (Eugenia caryophyllus), and frankincense (Boswellia carterii). Essential oils from thyme and clove bud were prepared via steam distillation. Frankincense essential oil was prepared via hydrodistillation of the resin. Orange essential oil was expressed via cold pressing from the peels of the fruit. The product was provided by Young Living Essential Oils (Lehi, Utah, USA). This blend is marketed in the United States as an aromatherapy product and is sold worldwide under the product name Longevity™. The placebo product contained an inert, odorless fractionated coconut oil and was also provided by Young Living Essential Oils.
The bottles were sealed with an orifice reducer to ensure that it was dispensed one drop at a time. The intervention protocol was developed by a certified professional aromatherapist to be administered by the participant in their own home. This protocol enables the findings to reflect real-world settings where aromatherapy interventions are self-administered in the home. Participants were monitored during the study for protocol adherence by completing mid-point reports and routine compliance audits conducted via phone and/or email, depending on participant preference.
Each participant was instructed to dispense 4 total drops of the provided bottle of oil onto a small paper scent tester strip, which was also provided to the participants. To avoid bias from other sensory experiences, they were instructed to sit in a calm, quiet location for the intervention. To inhale the oil, they took deep breaths, holding the tester strip within 2 in. of their nose. They were instructed to continue breathing the scent for a total of 15 min, each morning and evening. Each participant did this twice a day for a total of 14 consecutive days (2 weeks).
2.3. Outcomes
The primary outcome for this study was the total score on the Multidimensional Fatigue Symptom Inventory, Short Form developed by Stein et al.,24 after controlling for baseline scores. The MFSI is a validated measurement instrument which measures multiple patterns of fatigue in patients with chronic illness.6 This instrument has been used significantly in research on fatigue and Cronbach's alphas for subscales range from 0.87 to 0.96.24 Secondary outcomes included scores on each of the ten subdomains of the Multidimensional Fatigue Symptom Inventory (MFSI), after controlling for baseline scores.
Endpoint scores on the Patient Health Questionnaire-9 (PSQ-9) were also evaluated as a quality of life exploratory outcome. This instrument measures symptoms traditionally associated with depression such as changes to sleeping habits, eating habits, difficulty concentrating, and satisfaction with life. It is scored on a Likert scale, with higher scores indicating higher levels of depression.
Socioeconomic status control variables included age, race, height, weight, BMI, household income, educational attainment, marital status, and employment status. Health status control variables included the number of days between COVID-19 diagnosis and the start of the intervention, duration of COVID-19 infection, and post-COVID-19 olfactory function.
2.4. Sample size
Sample size was determined by conducting a power analysis using G*Power 3.1.9.6. To achieve 80% power with an alpha of.05, a total of 36 participants were required to detect a large effect on total fatigue scores with an ANCOVA analysis. A large effect size (f=0.4) was chosen as the minimum effect for this study to ensure that the intervention produced clinically meaningful results for patients with post-COVID-19 fatigue. This target effect size is consistent with effect sizes found in previous work measuring the effects of aromatherapy inhalation on energy levels for other populations.7 To account for participant loss due to dropouts, the minimum required sample size was increased by 10% to 40.
2.5. Randomization and blinding
Participants were adaptively randomized using the method developed by Kang et al.11 This ensures even distribution between groups on the known factor of age. Participants and participant-facing study staff were blinded to allocation of participants. Each participant received an unbranded bottle of the study oil, labeled with their participant ID. All study oil bottles were identical in color, shape, and size.
During the informed consent and orientation process, participants were not told which oils were being studied, nor were they conditioned regarding what to expect from the inhalation exercise. To confirm the success of blinding, participants were asked open-ended questions regarding their group assignment after the intervention concluded. Similar numbers of participants in each group believed they had an active intervention and similar numbers of participants in each group reported that they felt the intervention improved their energy levels.
2.6. Statistical analyses
Continuous demographics and randomization success were assessed using two-sample t-tests. Categorical variables were assessed using chi-square analysis. Between-groups comparisons of total fatigue scores on day 14 were evaluated using ANCOVA with baseline scores as the covariate. Secondary outcomes included each of the ten subdomains of the MSFI and were analyzed using the same approach. All data were analyzed using STATA v17.
3. Results
3.1. Participant flow and recruitment
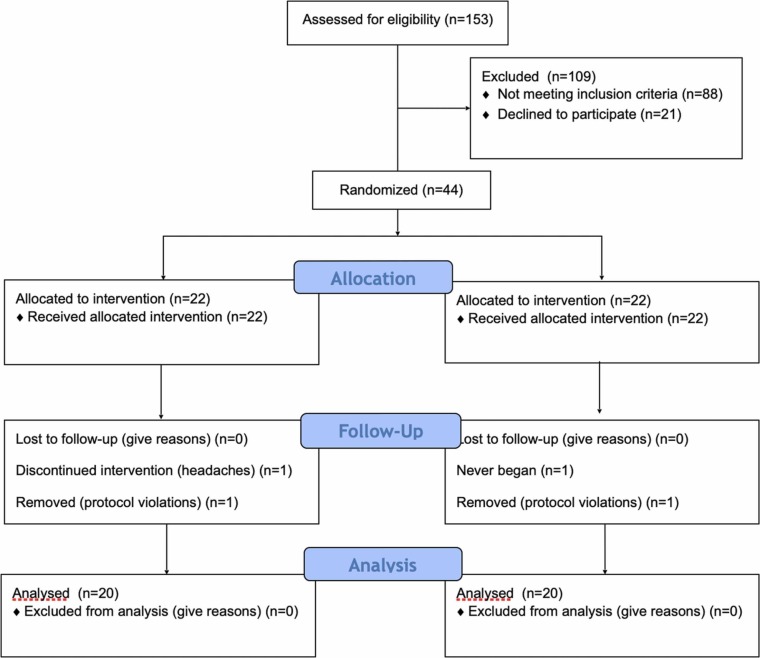
Participants were recruited from the research center database via email between early August and early October, 2021. Prior to recruiting participants, the study protocol was reviewed and approved by a central IRB and registered at ClinicalTrials.gov, NCT04980573. A total of 230 individuals requested information about the study and 153 were screened for inclusion. Forty-four individuals qualified for the study and provided informed consent. Of those participants, one failed to begin the study, two were removed for protocol violations, and one withdrew due to side effects. This produced a total sample size of 40 individuals (n = 40) for a modified intent to treat analysis. See Fig. 1: Flow Chart.
Fig. 1.
CONSORT flow diagram.
3.2. Baseline data
The total number of participants was 40, with 20 in each group. Baseline descriptive statistics were evaluated, and t-tests were performed to ensure balance between each group. No differences between the two groups were identified. Participants were analyzed using a modified intent-to-treat analysis. Participants were mostly white (80%) and married (67.5%). Educational attainment varied, with 25% completing a 4-year degree, and 40% completing an advanced degree. The majority of participants (67.5%) were employed full time. See Table 1.
Table 1.
Demographic data (n = 40).
| Demographics | Intervention (n = 20) | Placebo (n = 20) |
p |
|---|---|---|---|
| Race | .247 | ||
| White | 14 | 18 | |
| Black | 2 | 0 | |
| Asian | 1 | 0 | |
| Hawaiian or Pacific Islander | 1 | 0 | |
| Hispanic | 1 | 1 | |
| Other | 1 | 1 | |
| Marital Status | .462 | ||
| Married | 14 | 13 | |
| Single/Never Married | 3 | 6 | |
| Divorced | 3 | 1 | |
| Educational Attainment | .470 | ||
| Some High School | 1 | 0 | |
| Some College | 3 | 1 | |
| Associate’s Degree | 3 | 6 | |
| Four-Year Degree | 5 | 5 | |
| Advanced Degree | 8 | 8 | |
| Employment Status | .573 | ||
| Full Time | 13 | 14 | |
| Part Time | 3 | 3 | |
| Student | 2 | 1 | |
| Stay at Home Parent | 0 | 1 | |
| Unemployed | 1 | 1 | |
| Retired | 1 | 0 |
Health history was also similar between groups. Average length of COVID-19 infection was 2.3 weeks (SD=1.26 weeks), with 72.5% of participants recovering from acute COVID-19 within 2 weeks. Most participants (67.6%) lost both their sense of taste and smell during their illness. Some lost only their sense of taste (2.5%) or smell (7.5%), and 22.5% lost neither. Taste and smell had returned to the majority of participants (65%), though 32.5% had only regained some sensory function and 2.5% had not regained any function. There were no differences between groups on any of the baseline control variables.
3.3. Outcomes
The assumptions of normality, linearity, homogeneity of variances, and homogeneity of regression slopes were checked and met. To comprehensively evaluate the relationship between inhalation of this essential oil blend and fatigue scores, a fully saturated model was developed, including all potential main effects, then a parsimonious model was developed using the statistically and theoretically significant variables. The fully saturated model explained a large portion of the variance (adjusted R² =0.74). The majority of the contribution came from group, baseline scores, employment status, BMI, olfactory function, and time since COVID-19 infection.
The parsimonious model including relevant variables resulted in a good overall fit (adjusted R² =0.64) This produced adjusted mean scores of 37.13 (SE: 3.10) for the placebo group and 25.42 (SE: 3.10) for the intervention group. Individuals who inhaled the essential oil blend for 2 weeks had significantly lower fatigue scores after controlling for baseline scores, employment status, BMI, olfactory function, and time since diagnosis, with a large effect size (F (1,39) = 6.15, p = .020, partial eta squared = 0.198). See Table 2.
Table 2.
MFSI Scores on day 14 with baseline as covariate.
| Source | df | SS | MS | F | p | η 2 |
|---|---|---|---|---|---|---|
| Baseline Score | 1 | 5178.514 | 5178.514 | 32.14 | < 0.001 | .562 |
| Treatment Group | 1 | 991.317 | 991.317 | 6.15 | .020 | .198 |
| Employment Status | 5 | 1713.147 | 342.629 | 2.13 | .095 | .298 |
| BMI | 1 | 440.279 | 440.279 | 2.73 | .111 | .098 |
| Olfactory Function | 3 | 911.897 | 303.966 | 1.89 | .681 | .185 |
| Time Since Diagnosis | 1 | 325.602 | 235.602 | 1.46 | .238 | .055 |
| Error | 27 | 4027.836 | 161.113 | |||
| Total | 39 | |||||
| Adjusted R Squared = 0.623 |
3.4. Secondary outcomes
Responses for the complete MFSI were used to produce ten subscale scores for each participant to identify which types of fatigue are most affected by the essential oil blend. Baseline scores were used as the covariate. Demographic and health history control variables were used. For each outcome, preliminary checks were conducted to ensure that there were no violations of assumptions of normality, linearity, homogeneity of variances, or homogeneity of regression slopes. Multicollinearity was ruled out using a correlation matrix. For each outcome, a fully saturated model was developed using all control variables to identify which factors contributed to the outcome. From those results, a parsimonious model was developed using all of the variables deemed critical to the model.
Significant results were found on the subscales of global, behavioral, general, and mental fatigue, as well as vigor. For the outcome of global fatigue, adjusted mean scores for the placebo group were 2.53 (SE=0.124) compared to 2.03 (SE=0.124) in the intervention group after controlling for baseline scores, employment status, BMI, and olfactory function. The effect size for treatment group differences was substantial (F (1.39) = 7.57, p = .010, partial eta squared = 0.213).
Behavioral fatigue scores were mildly affected by group assignment. After controlling for baseline scores, age, BMI, length of COVID-19 infection, and days since COVID-19 infection, adjusted mean scores were 2.06 (SE=0.075) for the placebo group and 1.82 (SE=0.75) for the intervention group. This subscale achieved statistical significance with a large effect size (F (1.39) = 4.46, p = .044, partial eta squared = 0.146).
For the outcome of general fatigue, adjusted mean scores were 17.75 (SE=1.02) in the placebo group and 12.95 (SE=1.02) in the intervention group. Analysis of covariance found a significant difference between groups (F (1.39) = 5.17, p = .030, partial eta squared = 0.147), after controlling for baseline scores, BMI, and olfactory function.
Mental fatigue scores were also affected by group assignment. After controlling for baseline scores, employment status, BMI, and duration of COVID-19 infection, a significant difference was found between groups (F (1.39) = 10.45, p = .003, partial eta squared = 0.240). Adjusted mean scores were 13.01 (SE= 0.57) in the placebo group and 11.14 (SE=0.57) in the intervention group.
Vigor scores were also found to improve with the intervention. After controlling for baseline scores, marital status, and olfactory function, a significant difference was found between groups (F (1.39) = 5.79, p = .022, partial eta squared = 0.149). Adjusted mean scores for the placebo group were 13.84 (SE=0.80) and 16.66 (SE=0.80) in the intervention group.
For each of the ten subgroup outcomes, baseline scores were significantly associated with final outcome scores. Additionally, BMI and race were significant for the outcome of somatic fatigue, employment status and duration of COVID-19 were significantly related to the outcome of affective fatigue, and employment status and BMI significantly related to cognitive fatigue. Emotional fatigue was related to race, employment status, and duration of COVID-19 infection. Baseline scores were the only measured factor related to post-intervention physical fatigue scores.
3.5. Exploratory outcomes
For the prespecified exploratory outcome of PSQ-9 scores, a one-way between groups analysis of covariance was performed using socioeconomic and health control variables. The fully saturated model produced an adjusted R² of.63. The majority of the contribution came from baseline scores, employment status, BMI, olfactory function, and duration of time since COVID-19 infection.
The parsimonious model including relevant variables resulted in a good overall fit (adjusted R² =0.63) This produced adjusted mean scores of 16.98 (SE: 0.70) for the placebo group and 13.22 (SE: 0.70) for the intervention group. Individuals who inhaled the essential oil blend for 2 weeks had significantly lower symptom scores after controlling for baseline scores, employment status, BMI, olfactory function, and time since diagnosis, with a substantial effect size (F (1.39) = 12.65, p = .002, partial eta squared = 0.336). See Table 3.
Table 3.
PSQ-9 scores on day 14 with baseline as covariate.
| Source | df | SS | MS | F | p | η 2 |
|---|---|---|---|---|---|---|
| Baseline Score | 1 | 233.077 | 233.077 | 27.16 | < 0.001 | .521 |
| Treatment Group | 1 | 108.585 | 108.585 | 12.65 | .002 | .336 |
| Employment Status | 5 | 139.910 | 27.982 | 3.26 | .021 | .395 |
| BMI | 1 | 24.066 | 24.066 | 2.80 | .107 | .101 |
| Olfactory Function | 3 | 46.665 | 15.555 | 0.99 | .387 | .177 |
| Time Since Diagnosis | 1 | 10.674 | 10.674 | 1.24 | .275 | .047 |
| Error | 27 | |||||
| Total | 39 | |||||
| Adjusted R Squared = 0.635 |
3.6. Adverse events
Participants were monitored for adverse events and for the development of any exclusion criteria during the intervention. One participant in the intervention group reported experiencing headaches and withdrew on day 13. This participant also reported experiencing recurring headaches prior to the start of the study, which is common among individuals with post-COVID-19 symptoms.5 No other adverse effects were identified.
4. Discussion
4.1. Interpretation
This study provides evidence that the inhalation of Longevity™ essential oil blend twice daily for 14 days provides substantial improvements to energy levels. These improvements are specifically useful for energy levels related to global fatigue, behavioral fatigue, general fatigue, and mental fatigue. Vigor was also improved in this population, and exploratory analysis identified a substantial improvement to quality of life after the use of the essential oil blend, as measured by the PHQ-9.
Energy levels associated with somatic fatigue, affective fatigue, emotional fatigue, and cognitive fatigue improved during the intervention but did not achieve statistical significance. This study was powered to identify areas with large effects. Therefore, the lack of significance may indicate that aromatherapy does not increase energy on these domains or it may be explained by a smaller effect size for those subdomains.
Secondary outcomes of specific subdomains may also be affected by multiplicity, increasing the risk of type 1 errors. Subdomain outcomes with higher p-values should be interpreted with caution. Using a Bonferroni correction, the majority of the energy improvement from aromatherapy would come from the mental fatigue domain. Using a less stringent correction, global and mental fatigue, as well as vigor, would account for the largest effects. This is also consistent with the effect sizes for each of these subdomains.
These findings are consistent with previous work on aromatherapy for other health conditions associated with fatigue.1, 15, 7 This study extends the knowledge of aromatherapy for fatigue to include those who have recovered from COVID-19 and are still experiencing fatigue more than 5 months later. The secondary findings indicating that the greatest effect was found in the mental fatigue domain are also consistent with the existing literature on post-COVID-19 fatigue. Cognitive impairment or “brain fog” has been closely associated with fatigue among those with long-COVID-19.2, 19
4.2. Limitations
Participants in this study were women living in the United States. Women are known to experience fatigue at higher levels than men, across multiple subtypes of fatigue.4 Therefore, these findings may not be generalizable to men. Future studies should examine the role of aromatherapy in populations less likely to experience fatigue.
Additionally, because COVID-19 is a novel illness, the expected duration of post-COVID-19 challenges to overall health status and quality of life is not yet known. It is not yet clear whether interventions that boost energy after surviving COVID-19 are also applicable to lingering symptoms after other respiratory conditions or in populations who experience low levels of energy from other causes.
4.3. Overall evidence
The use of aromatherapy with Longevity™ essential oil blend to boost energy levels in women who have recovered from COVID-19 provides a novel, non-invasive approach to improving quality of life in this population. This intervention is particularly beneficial for global and mental fatigue, as well as vigor. Other subdomains may experience improvements to energy levels with a smaller effect size; future studies should be conducted to explore this potential.
Funding
This trial was funded by Young Living Essential Oils.
CRediT authorship contribution statement
Jessie Hawkins: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing. Christy Hires: Investigation, Methodology, Supervision, Writing – original draft. Elizabeth Dunne: Investigation, Methodology, Validation, Writing – original draft. Lindsey Keenan: Data curation, Investigation, Project administration, Writing – original draft.
Disclosures
All authors of this study confirm there are no conflicts of interest to declare. This was an investigator initiated study and the design, management, and analysis of this trial was completely independent of the research finder. Additionally, the funder has no influence in the interpretation, reporting, or dissemination of the trial results.
References
- 1.Ahmady S., Rezaei M., Khatony A. Comparing effects of aromatherapy with lavender essential oil and orange essential oil on fatigue of hemodialysis patients: a randomized trial. Complement Ther Clin Pract. 2019;36:64–68. doi: 10.1016/j.ctcp.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Ceban F., Ling S., Lui L.M., et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2021 doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.101019. Available at SSRN 3820561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engberg I., Segerstedt J., Waller G., Wennberg P., Eliasson M. Fatigue in the general population-associations to age, sex, socioeconomic status, physical activity, sitting time and self-rated health: the northern Sweden MONICA study 2014. BMC Public Health. 2017;17(1):1–9. doi: 10.1186/s12889-017-4623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández‐de‐las‐Peñas C., Navarro‐Santana M., Gómez‐Mayordomo V., et al. Headache as an acute and post‐COVID‐19 symptom in COVID‐19 survivors: a meta‐analysis of the current literature. Eur J Neurol. 2021 doi: 10.1111/ene.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentile S., Delaroziere J.C., Favre F., et al. Validation of the French ‘multidimensional fatigue inventory’(MFI 20) Eur J Cancer Care. 2003;12:58–64. doi: 10.1046/j.1365-2354.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins J., Hires C.Y., Dunne E.W., Keenan L.A. Aromatherapy reduces fatigue among women with hypothyroidism: a randomized placebo-controlled clinical trial. J Complement Integr Med. 2020;17:1. doi: 10.1515/jcim-2018-0229. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Pinto M.D., Borelli J.L., et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: looking for clarity in the haze of the pandemic. medRxiv. 2021 doi: 10.1177/10547738221125632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam M.F., Cotler J., Jason L.A. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue Biomed Health Behav. 2020;8(2):61–69. [Google Scholar]
- 10.Jacobson K.B., Rao M., Bonilla H., et al. Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang M., Ragan B.G., Park J.H. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43(2):215–221. doi: 10.4085/1062-6050-43.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarus E.R., Amirtharaj A.D., Jacob D., Chandrababu R., Isac C. The effects of an olive-oil massage on hemodialysis patients suffering from fatigue at a hemodialysis unit in southern India–a randomized controlled trial. J Complement Integr Med. 2021;18(2):397–403. doi: 10.1515/jcim-2019-0338. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021 doi: 10.1038/s41598-021-95565-8. Available at SSRN 3769978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maestre-Muñiz M.M., Arias Á., Mata-Vázquez E., et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. 2021;10(13):2945. doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahdavikian S., Fallahi M., Khatony A. Comparing the effect of aromatherapy with peppermint and lavender essential oils on fatigue of cardiac patients: a randomized controlled trial. Evid-Based Complement Altern Med. 2021 doi: 10.1155/2021/9925945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik P., Patel K., Pinto C., et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—a systematic review and meta-analysis. J Med Virol. 2021 doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miladinia M., Voss J.G., Molavynejad S., et al. Slow-stroke back massage compared with music therapy for leukemia-related pain and fatigue: a randomized controlled trial. JCO Oncol Pract. 2021 doi: 10.1200/OP.21.00156. OP-21. [DOI] [PubMed] [Google Scholar]
- 18.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.11417. e2111417-e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortelli P., Ferrazzoli D., Sebastianelli L., et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J Neurol Sci. 2021;420 doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooney S., Webster A., Paul L. Systematic review of changes and recovery in physical function and fitness after severe acute respiratory syndrome-related coronavirus infection: implications for COVID-19 rehabilitation. Phys Ther. 2020;100(10):1717–1729. doi: 10.1093/ptj/pzaa129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubino F., Amiel S.A., Zimmet P., et al. New-onset diabetes in Covid-19. N Engl J Med. 2020;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathish T., Kapoor N., Cao Y., Tapp R.J., Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. 2021 doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P., Bharti S., Garg I. Post covid fatigue: can we really ignore it? Indian J Tuberc. 2021 doi: 10.1016/j.ijtb.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein K.D., Martin S.C., Hann D.M., Jacobsen P.B. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6 doi: 10.1046/j.1523-5394.1998.006003143.x. 143-52. [DOI] [PubMed] [Google Scholar]
- 25.Taquet M., Dercon Q., Luciano S., et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas D.V. Aromatherapy: mythical, magical, or medicinal? Holist Nurs Pract. 2002;16(5):8–16. doi: 10.1097/00004650-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wang T., Zhai J., Liu X.L., Yao L.Q., Tan J.Y.B. Massage therapy for fatigue management in breast cancer survivors: a systematic review and descriptive analysis of randomized controlled trials. Evid-Based Complement Altern Med. 2021 doi: 10.1155/2021/9967574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Coronavirus Disease (COVID-19): Weekly Epidemiological, Update 1; 2021.
- 29.Gagnier J.J., Boon H., Rochon P., Moher D., Barnes J., Bombardier C. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144(5):364–367. doi: 10.7326/0003-4819-144-5-200603070-00013. [DOI] [PubMed] [Google Scholar]
- 30.Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P.J., Altman D.G. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International journal of surgery. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]