Abstract
In adults, embryonal rhabdomyosarcoma (ERMS) is rare and has a poor prognosis. Giant perianal ERMS with severe multiple bone metastases at initial diagnosis has not been reported and lacks effective treatment options. This current case report describes a 31-year-old female patient that presented with a large lump on the right side of the anus. ERMS was diagnosed, accompanied by multiple bone metastases throughout the body and severe thrombocytopenia. She had an extremely low platelet count at initial diagnosis, making systemic chemotherapy inappropriate. Genetic testing did not help identify effective targeted drugs. A multi-target tyrosine kinase inhibitor, anlotinib, was selected to control the tumours combined with local radiotherapy to relieve pain. The lump became smaller and this reduction was maintained for 5 months. At 7 months after the diagnosis, the patient died of thrombocytopenia. This current case may provide supportive evidence for a potential treatment for patients with advanced ERMS, especially those not suitable for chemotherapy or surgery.
Keywords: Embryonal rhabdomyosarcoma, adult, multiple bone metastases, targeted therapy, case report
Introduction
Rhabdomyosarcoma (RMS) is a malignant tumour that originates from mesenchymal cells. The embryonal histological variation was discovered by Bernard in 1894 1 and it mainly occurs in the head, neck and genitourinary regions.2,3 Embryonal RMS (ERMS) is frequently seen in children under 10 years of age, accounting for approximately 70% of all cases of RMS in children. 4 Adult RMS accounts for only 1% of all solid malignancies and ERMS is a rare histological type. 5
Due to the increased prevalence of adult patients with advanced RMS and a greater chance of recurrence and metastasis, 5 the clinical outcome remains poor.6,7 In 2016, a case of perineal ERMS in an adult male with lymph node metastasis was reported and the patient died after 6 months of follow-up with only supportive care. 8 This current case report describes an adult female patient with a poor prognosis. She presented with a huge perineal ERMS in addition to multiple bone metastases and severe thrombocytopenia. The patient received multi-target tyrosine kinase inhibitor therapy and achieved a giant mass reduction for 5 months.
Case report
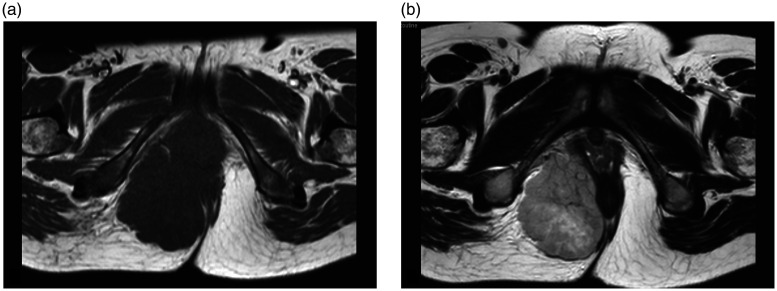
In November 2018, a 31-year-old female patient found a hard mass on the right side of her anus 2 months after delivery of a baby. She felt pain in the waist accompanied by a progressively increasing anal mass. This prompted her to visit the Department of Oncology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu Province, China for a consultation. First, a digital rectal examination was conducted, which identified a hard mass near the wall of the rectum, 8 cm in diameter, with poor mobility and a clear boundary. The patient experienced slight pain when the lump was touched. Subsequent magnetic resonance imaging of the pelvis revealed a mass (78 × 75 ×61 mm) with long T1 and T2 signals (Figures 1a and 1b). After contrast-enhanced imaging, the lesions showed a septal enhancement signal. Multiple small lymph node shadows were observed in bilateral groins.
Figure 1.
Pelvic magnetic resonance imaging scan showing a perianal mass in a 31-year-old female patient that presented with a hard mass on the right side of her anus. The lesion had a long T1 (a) and long T2 (b) signal. The mass had a size of 78 × 75 × 61 mm and heterogeneous signal. The surrounding tissue was compressed and changed.
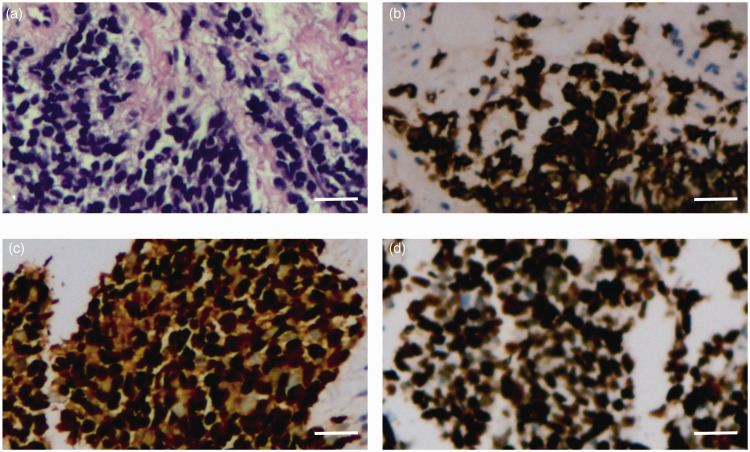
A needle biopsy of the mass was undertaken and the pathology was reviewed by two experienced pathologists from different hospitals. Small round tumour cells in the fibrous tissue were observed under light microscopy, with a cord-like and nested arrangement. The nuclei were deeply stained and atypical. Necrosis was also observed in the tumour tissue (Figure 2a). Tumour cells were positive for cluster of differentiation (CD)56, desmin (Figure 2b), myogenin (Figure 2c) and myoblast determination protein 1; and negative for solute carrier family 4 member 1/solute carrier family 4 member 3 (AE1/AE3), synaptophysin, chromogranin-A, cytokeratin 20, leukocyte common antigen, CD3, CD20, CD99, melanosome, S-100 and thyroid transcription factor-1 as determined using immunohistochemical staining. The Ki-67 proliferation index was 90% (Figure 2d). ERMS was diagnosed on the basis of the histomorphological and immunohistochemical results. This woman was in good health, without a history of surgery, allergies, hypertension, diabetes mellitus, heart disease or any related family history.
Figure 2.
Haematoxylin and eosin (H&E) and immunohistochemical staining observations of a needle biopsy sample from the perianal mass of a 31-year-old female patient that presented with a hard mass on the right side of her anus. (a) H&E staining revealed small round tumour cells, with a cord-like and nested arrangement. Nuclei were deeply stained and evidently atypical (scale bar 50 µm). Immunohistochemical staining showed (b) positive desmin staining in tumour cells (scale bar 50 µm), (c) positive myogenin staining in tumour cells (scale bar 50 µm) and (d) positive Ki-67 staining in tumour cells (scale bar 50 µm. The colour version of this figure is available at: http://imr.sagepub.com.
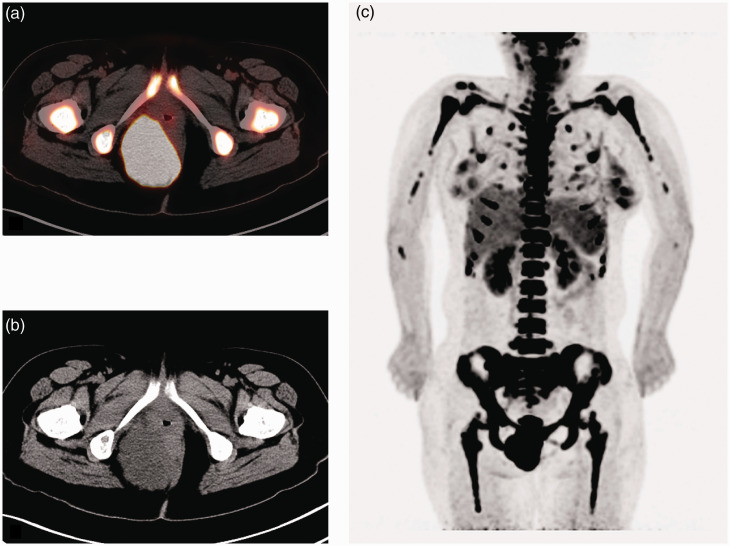
Positron emission tomography-computed tomography (PET-CT) was performed to further evaluate the distant metastases. The mass in the perineum (Figures 3a and 3b) had a standardized uptake value (SUV) of 9.9; a pelvic lymph node 1.8 cm in diameter had an SUV of 4.0; and multiple bone metastases in the whole body (Figure 3c) had an SUV of 10.4. Meanwhile, this patient had small bilateral pleural effusions. According to the workup described above, this current patient was categorized as T2N1M1 and identified as stage IV.
Figure 3.
Positron emission tomography-computed tomography (PET-CT) scans of the perianal mass and multiple bone metastases in the whole body of a 31-year-old female patient that presented with a hard mass on the right side of her anus. (a) PET scan of the lesion near the right side of the anus. (b) CT scan of the lesion near the right side of the anus. (c) Severe multiple bone metastases in the whole body, including limb bone, spinal, rib and pelvic involvement. The colour version of this figure is available at:http://imr.sagepub.com.
More than 500 genes for solid tumours were investigated using next-generation sequencing technology to obtain more accurate information before treatment. The gene detection results showed two gene mutations, including the PIK3C2G gene in exon 23 and RTEL1 in exon 10, with an abundance of 48.65% and 2.17%, respectively. The tumour mutation burden (TMB) value was 1.4 mutations/Mb, which was lower than the reference value (4.5 mutations/Mb) from The Cancer Genome Atlas, along with microsatellite stabilization and negative expression of programmed cell death receptor ligand 1 (PD-L1). As part of the diagnostic workup, blood tests revealed a low platelet count (14 × 109/l), haemoglobin (86 g/l) and high lactate dehydrogenase (2035 U/l).
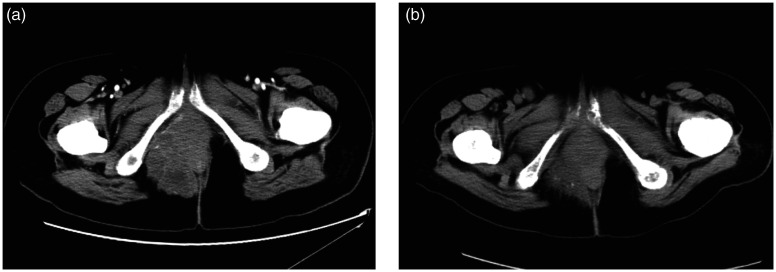
The patient received the drug (12 mg anlotinib oral, once a day, continued for 14 days, every 3 weeks for a cycle) from two attending oncologists after obtaining informed consent. After 4 weeks, the platelet count increased gradually and the mass reduced in size on CT re-examination (after 1.5 cycles of anlotinib) (Figure 4a). Four months later (following five cycles of anlotinib), the mass continued to decrease in size from the results of the two previous CT scans (Figure 4b). The toxicity of anlotinib was manageable and acceptable in the first five cycles. However, the patient did not take anlotinib regularly in the sixth cycle due to side-effects, such as nausea, vomiting and weakness. Despite these adverse reactions, the mass continued to shrink on physical examination six cycles later even with an incomplete sixth cycle of medication.
Figure 4.
Pelvic computed tomography imaging of a 31-year-old female patient that presented with a hard mass on the right side of her anus showing the perianal mass at different times after treatment. (a) At 4 weeks after taking multi-targeted therapy. (b) At 4 months after taking multi-targeted therapy.
The patient received palliative radiotherapy (radiotherapy dose: 30 Gy/3 Gy/10 fractionation) on both shoulders to relieve pain. The pain in her back gradually worsened and she could not even lie down when she could not adhere to the medication regimen. She then underwent vertebroplasty at the T9, T10 and L3 centrum to prevent vertebral fractures. Radiotherapy was used as the only treatment for lumbar metastasis (radiotherapy dose: 30 Gy/3 Gy/10 fractionation) to relieve pain. At 2 weeks after radiotherapy, the perineal mass was larger on physical examination and the platelet count had irreversibly decreased. At 7 months after the diagnosis, the patient died of thrombocytopenia. The reduction in the size of the mass was maintained for 5 months.
This case report was approved by the Ethics Committee of Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu Province, China (no. BR2015021) and conformed to the CARE guidelines. 9 Written informed consent for publication was obtained from the patient’s relatives.
Discussion
Embryonal rhabdomyosarcoma is a type of RMS derived from immature cells and myogenic satellite cells. 10 ERMS can manifest as an asymptomatic mass that can cause pain due to compression of adjacent nervous structures. 11 To the best of our knowledge, this is the first report of perianal ERMS with severe bone metastases. To date, there have been no reports of related cases.
Among distant metastases, bone metastases are the third most common site of metastases (34%) in RMS,4,12 which may cause severe pain. A previous report compared the application of PET-CT with conventional imaging examinations, including CT and bone scan, in childhood RMS staging. 13 PET-CT identified nodules, bones and bone marrow involvement better than the other modalities. 14 Bone metastasis is often accompanied by other distant metastases, with there being only a 3% chance of metastasis to the bone without lung involvement. 12 It was also reported that bone metastasis strongly correlated with bone marrow involvement (P < 0.001). 13 Patients with either bone or bone marrow involvement have a poorer prognosis. 14 However, in this current case, it is not clear whether there was bone marrow infiltration due to the absence of a bone marrow biopsy.
Adult metastatic ERMS is relatively rare and there is currently no consensus on treatment. Most clinical studies for metastatic ERMS were conducted in children and adolescents, including the IRS Group Studies, 15 SIOP-MMT Group Studies 16 and European Intergroup MMT89-91 Studies. 17 Compared with the prognosis of localized tumours in childhood, survival in patients with metastatic tumours has not significantly improved for more than 10 years.15–17 The patients in these studies received conventional multi-drug chemotherapy based on alkylating drugs (cyclophosphamide or ifosfamide), vincristine and actinomycin.15–17 Despite the development of more intensive therapies, including high-dose chemotherapy stem cell support and local treatment (surgical resection and/or radiotherapy) after tumour remission, the 5-year survival rate is still 20–30%.15–17 A comprehensive analysis found that not all patients with metastatic RMS had a poor prognosis. 4 Age <1 year or >10 years, primary tumours in unfavourable locations (e.g. extremities, bladder, prostate), bone or bone marrow involvement and three or more metastatic sites were all unfavourable prognostic factors. 4 The 3-year event-free survival (EFS) of patients without the above four adverse factors was 50%, while the EFS with one, two, three and four adverse factors was 42%, 18%, 12% and 5%, respectively (P < 0.0001). 4
Unfortunately, the current patient had an extremely low platelet count. Therefore, choosing a chemotherapy regimen with cyclophosphamide, vincristine and actinomycin or other drugs would have been dangerous. Patients with ERMS have relatively more genetic aberrations, which may lead to opportunities to choose targeted treatments. 18 This current patient had mutations in the PIK3C2G and RTEL1 genes. PIK3C2G codes for the type II catalytic subunit of PI3-kinase, 19 which is a lipid kinase that participates as an auxiliary messenger in key signalling pathways in the cell cycle, movement, differentiation and transformation. 20 PIK3C2G is mutated in many cancers. 21 RTEL1 (telomere length regulator 1) encodes DNA helicase, which is involved in the regulation of telomere length, DNA repair and maintenance of genome stability. 22 It is essential for maintaining the integrity of chromosome ends. 22 However, the corresponding targeted therapies for these mutations are currently absent. The use of PD-1/PD-L1 immune checkpoint inhibitors to actively immunize patients and achieve clinical benefits has become the standard treatment plan for patients with cancer that have failed traditional treatments. 23 Increasing evidence shows that TMB is related to treatment effects and a high TMB can predict treatment benefit. 24 PD-L1 expression was negative, with a low TMB value in this current patient. Therefore, it was not possible to identify potential treatment options for specific targeted therapy and immunotherapy based on gene detection. Consequently, there is a clear need for novel treatment strategies in similar cases.
It is known that anlotinib acts as a multi-target tyrosine kinase inhibitor involving a variety of signalling pathways, such as vascular endothelial growth factor (VEGF) receptor, platelet-derived growth factor receptor α/β, mast cell/stem cell growth factor receptor, rearranged during transfection (Ret), aurora kinase B and colony stimulating factor 1 receptor, inhibiting tumour proliferation, vasculature generation and changing the tumour microenvironment.25,26 A multicentre phase II study from China in 2018 enrolled several soft tissue sarcomas that progressed after anthracycline-based chemotherapy, demonstrating that anlotinib had antitumor activity. 27 The ALTER0203 study confirmed that anlotinib was effective in a variety of soft tissue sarcomas. 28 VEGF expression was detected in RMS cell lines and the inhibition of VEGF signalling delayed RMS proliferation. 29 Chemotherapy is the most extensive treatment option for ERMS. 30 In the current era, when genetic testing is more convenient, it is also a good option to look for gene mutations and find possible effective drug targets. Meanwhile, immunotherapy has attracted increasing attention in tumour treatment and has gradually been found to improve the prognosis of patients with tumours. 31 In addition, when there is no suitable treatment option, a multi-target inhibitor with supportive care may also be a good option for patients with EMRS.
In conclusion, to the best of our knowledge, this is the first report of a giant perianal ERMS with severe multiple bone metastases. The current patient was classified as having a high-risk status and intolerant of surgery or chemotherapy because of thrombocytopenia. Fortunately, the tumour showed significant atrophy after multi-target tyrosine kinase inhibitor therapy. A multi-target tyrosine kinase inhibitor may be a potential treatment option for patients with metastatic ERMS. In general, this current case highlights a treatment option for patients that are not suitable for chemotherapy. Further relevant studies are required and it is hoped that there will be an exchange of treatment options for this disease.
Footnotes
Author contributions: J.J.L., M.B.C., X.J.G., Y.Z., Y.Y.L., Y.Y. and P.L. contributed equally to this study. J.J.L., M.B.C., X.J.G. and P.L. wrote the manuscript, collected data, reviewed the pathology and organized the imaging data. P.L. supervised the entire process and contributed to the final draft. P.L. also acts as a guarantor for the work described here. Y.Z., Y.Y.L. and Y.Y. commented on previous versions of the manuscript and revised it critically for important intellectual content. All authors have read and approved the final manuscript.
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by the Suzhou Science and Technology Plan Project (no. KJXW2019064).
ORCID iD: Jing-Jing Lu https://orcid.org/0000-0001-9938-2560
Reference
- 1.Geiger S, Czernobilsky B, Marshak G, et al. Embryonal rhabdomyosarcoma: Immunohistochemical characterization. Oral Surg Oral Med Oral Pathol 1985; 60: 517–523. DOI: 10.1016/0030-4220(85)90241-5. [DOI] [PubMed] [Google Scholar]
- 2.Daya H, Chan HSL, Sirkin W, et al. Pediatric rhabdomyosarcoma of the head and neck: Is there a place for surgical management? Arch Otolaryngol Head Neck Surg 2000; 126: 468–472. DOI: 10.1001/archotol.126.4.468. [DOI] [PubMed] [Google Scholar]
- 3.Hays DM, Lawrence W, Crist WM, et al. Partial cystectomy in the management of rhabdomyosarcoma of the bladder: a report from the Intergroup Rhabdomyosarcoma Study. J Pediatr Surg 1990; 25: 719–723. DOI: 10.1016/s0022-3468(05)80004-1. [DOI] [PubMed] [Google Scholar]
- 4.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol 2008; 26: 2384–2389. DOI: 10.1200/jco.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little DJ, Ballo MT, Zagars GK, et al. Adult rhabdomyosarcoma: outcome following multimodality treatment. Cancer 2002; 95: 377–388. DOI: 10.1002/cncr.10669. [DOI] [PubMed] [Google Scholar]
- 6.Dumont SN, Araujo DM, Munsell MF, et al. Management and outcome of 239 adolescent and adult rhabdomyosarcoma patients. Cancer Med 2013; 2: 553–563. DOI: 10.1002/cam4.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Gaal JC, De Bont ES, Kaal SE, et al. Building the bridge between rhabdomyosarcoma in children, adolescents and young adults: the road ahead. Crit Rev Oncol Hematol 2012; 82: 259–279. DOI: 10.1016/j.critrevonc.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Ka S, Gnangnon F, Dieng MM, et al. Embryonal rhabdomyosarcoma of the perineum in an adult: a case report. J Med Case Rep 2016; 10: 353. DOI: 10.1186/s13256-016-1166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013; 2: 38–43. DOI: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd RV, Hajdu SI, Knapper WH. Embryonal rhabdomyosarcoma in adults. Cancer 1983; 51: 557–565. DOI: 10.1002/1097-0142(19830201)51:3<557::aid-cncr2820510333>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Egas-Bejar D, Huh WW. Rhabdomyosarcoma in adolescent and young adult patients: current perspectives. Adolesc Health Med Ther 2014; 5: 115–125. DOI: 10.2147/ahmt.s44582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss AR, Lyden ER, Anderson JR, et al. Histologic and clinical characteristics can guide staging evaluations for children and adolescents with rhabdomyosarcoma: a report from the Children's Oncology Group Soft Tissue Sarcoma Committee. J Clin Oncol 2013; 31: 3226–3232. DOI: 10.1200/jco.2012.44.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benz MR, Tchekmedyian N, Eilber FC, et al. Utilization of positron emission tomography in the management of patients with sarcoma. Curr Opin Oncol 2009; 21: 345–351. DOI: 10.1097/CCO.0b013e32832c95e2. [DOI] [PubMed] [Google Scholar]
- 14.Weigel BJ, Lyden E, Anderson JR, et al. Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children's Oncology Group. J Clin Oncol 2016; 34: 117–122. DOI: 10.1200/JCO.2015.63.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lager JJ, Lyden ER, Anderson JR, et al. Pooled analysis of phase II window studies in children with contemporary high-risk metastatic rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol 2006; 24: 3415–3422. DOI: 10.1200/jco.2005.01.9497. [DOI] [PubMed] [Google Scholar]
- 16.McDowell HP, Foot AB, Ellershaw C, et al. Outcomes in paediatric metastatic rhabdomyosarcoma: results of The International Society of Paediatric Oncology (SIOP) study MMT-98. Eur J Cancer 2010; 46: 1588–1595. DOI: 10.1016/j.ejca.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Carli M, Colombatti R, Oberlin O, et al. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. J Clin Oncol 2004; 22: 4787–4794. DOI: 10.1200/jco.2004.04.083. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins DS, Chi YY, Anderson JR, et al. Addition of Vincristine and Irinotecan to Vincristine, Dactinomycin, and Cyclophosphamide Does Not Improve Outcome for Intermediate-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J Clin Oncol 2018; 36: 2770–2777. DOI: 10.1200/JCO.2018.77.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li A, Chen H, Lin M, et al. PIK3C2G copy number is associated with clinical outcomes of colorectal cancer patients treated with oxaliplatin. Int J Clin Exp Med 2015; 8: 1137–1143. [PMC free article] [PubMed] [Google Scholar]
- 20.Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol 1999; 11: 219–225. DOI: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 21.Falasca M, Maffucci T. Regulation and cellular functions of class II phosphoinositide 3-kinases. Biochem J 2012; 443: 587–601. DOI: 10.1042/bj20120008. [DOI] [PubMed] [Google Scholar]
- 22.Vannier JB, Sandhu S, Petalcorin MI, et al. RTEL1 is a replisome-associated helicase that promotes telomere and genome-wide replication. Science (New York, NY) 2013; 342: 239–242. DOI: 10.1126/science.1241779. [DOI] [PubMed] [Google Scholar]
- 23.Ri MH, Ma J, Jin X. Development of natural products for anti-PD-1/PD-L1 immunotherapy against cancer. J Ethnopharmacol 2021; 281: 114370. DOI: 10.1016/j.jep.2021.114370. [DOI] [PubMed] [Google Scholar]
- 24.Hsiehchen D, Espinoza M, Valero C, et al. Impact of tumor mutational burden on checkpoint inhibitor drug eligibility and outcomes across racial groups. J Immunother Cancer 2021; 9: e003683. DOI: 10.1136/jitc-2021-003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong CC, Chen F, Yang JL, et al. Pharmacokinetics and disposition of anlotinib, an oral tyrosine kinase inhibitor, in experimental animal species. Acta Pharmacol Sin 2018; 39: 1048–1063. DOI: 10.1038/aps.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016; 9: 105. DOI: 10.1186/s13045-016-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi Y, Fang Z, Hong X, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients With Refractory Metastatic Soft Tissue Sarcoma. Clin Cancer Res 2018; 24: 5233–5238. DOI: 10.1158/1078-0432.CCR-17-3766. [DOI] [PubMed] [Google Scholar]
- 28.Zhang RS, Liu J, Deng YT, et al. The real-world clinical outcomes and treatment patterns of patients with unresectable locally advanced or metastatic soft tissue sarcoma treated with anlotinib in the post-ALTER0203 trial era. Cancer Med 2022. DOI: 10.1002/cam4.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onisto M, Slongo ML, Gregnanin L, et al. Expression and activity of vascular endothelial growth factor and metalloproteinases in alveolar and embryonal rhabdomyosarcoma cell lines. Int J Oncol 2005; 27: 791–798. [PubMed] [Google Scholar]
- 30.Leaphart C, Rodeberg D. Pediatric surgical oncology: management of rhabdomyosarcoma. Surg Oncol 2007; 16: 173–185. DOI: 10.1016/j.suronc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Gu S, Yin M, et al. Analysis of infantile fibrosarcoma reveals extensive T-cell responses within tumors: Implications for immunotherapy. Pediatr Blood Cancer 2018; 65. DOI: 10.1002/pbc.26813. [DOI] [PubMed] [Google Scholar]