Key Points
Questions
Is SARS-CoV-2 vaccination during pregnancy associated with adverse pregnancy outcomes?
Findings
In this population-based retrospective cohort study that included 157 521 deliveries in Sweden and Norway, SARS-CoV-2 vaccination during pregnancy, compared with no SARS-CoV-2 vaccination during pregnancy, was not significantly associated with risk of preterm birth (adjusted hazard ratio [aHR], 0.98), stillbirth (aHR, 0.86), small for gestational age (adjusted odds ratio [aOR], 0.97), low Apgar score (aOR, 0.97), or neonatal care admission (aOR, 0.97).
Meaning
In this population-based study conducted in Sweden and Norway, vaccination against SARS-CoV-2 during pregnancy was not associated with an increased risk of adverse pregnancy outcomes.
Abstract
Importance
Data about the safety of vaccines against SARS-CoV-2 during pregnancy are limited.
Objective
To examine the risk of adverse pregnancy outcomes after vaccination against SARS-CoV-2 during pregnancy.
Design, Setting, and Participants
This registry-based retrospective cohort study included 157 521 singleton pregnancies ending after 22 gestational weeks from January 1, 2021, until January 12, 2022 (Sweden), or January 15, 2022 (Norway). The Pregnancy Register in Sweden and the Medical Birth Registry of Norway were linked to vaccination and other registries for identification of exposure and background characteristics.
Exposures
Data on mRNA vaccines—BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna)—and 1 viral vector vaccine—AZD1222 (AstraZeneca)—were collected from national vaccination registries.
Main Outcomes and Measures
The risk of preterm birth and stillbirth was evaluated using Cox regression models, with gestational day as the time metric and vaccination as a time-dependent exposure variable. The risk of small for gestational age, low Apgar score, and neonatal care admission was evaluated using logistic regression. Random-effects meta-analysis was used to combine results between countries.
Results
Among the 157 521 singleton births included in the study (103 409 in Sweden and 54 112 in Norway), the mean maternal age at the time of delivery was 31 years, and 28 506 (18%) were vaccinated against SARS-CoV-2 (12.9% with BNT162b2, 4.8% with mRNA-1273, and 0.3% with AZD1222) while pregnant. A total of 0.7%, 8.3%, and 9.1% of individuals delivering were vaccinated during the first, second, and third trimester, respectively. Vaccination against SARS-CoV-2 was not significantly associated with increased risk of preterm birth (6.2 vs 4.9 per 10 000 pregnancy days; adjusted hazard ratio [aHR], 0.98 [95% CI, 0.91 to 1.05]; I2 = 0%; P for heterogeneity = .60), stillbirth (2.1 vs 2.4 per 100 000 pregnancy days; aHR, 0.86 [95% CI, 0.63 to 1.17]), small for gestational age (7.8% vs 8.5%; difference, –0.6% [95% CI, –1.3% to 0.2%]; adjusted OR [aOR], 0.97 [95% CI, 0.90 to 1.04]), low Apgar score (1.5% vs 1.6%; difference, –0.05% [95% CI, –0.3% to 0.1%]; aOR, 0.97 [95% CI, 0.87 to 1.08]), or neonatal care admission (8.5% vs 8.5%; difference, 0.003% [95% CI, –0.9% to 0.9%]; aOR, 0.97 [95% CI, 0.86 to 1.10]).
Conclusions and Relevance
In this population-based study conducted in Sweden and Norway, vaccination against SARS-CoV-2 during pregnancy, compared with no SARS-CoV-2 vaccination during pregnancy, was not significantly associated with an increased risk of adverse pregnancy outcomes. The majority of the vaccinations were with mRNA vaccines during the second and third trimesters of pregnancy, which should be considered in interpreting the findings.
This cohort study examines the risk of adverse pregnancy outcomes after vaccination against SARS-CoV-2 during pregnancy based on registry data from Sweden and Norway.
Introduction
A systematic review of 27 studies published between December 2019 and December 2020 confirmed that pregnant individuals are at a higher risk of severe COVID-19.1 Several vaccines against SARS-CoV-2 have been proven highly effective both in the pregnant and nonpregnant populations.2,3 Both the US Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, in addition to several European health authorities, have therefore recommended that pregnant individuals get vaccinated against SARS-CoV-2.4,5,6 Still, individuals may be hesitant to get vaccinated while pregnant due to concerns about the safety of vaccination during pregnancy.
Pregnant individuals were not included in the phase 3 trials of the vaccines approved in the EU and the US. However, no major adverse events signals have been detected in the Centers for Disease Control and Prevention’s surveillance systems7,8 or from the unplanned pregnancies in vaccination trials.9 A few studies have investigated pregnancy outcomes in vaccinated individuals and did not find evidence of adverse maternal or neonatal outcomes.10,11,12,13,14 However, these studies were based on a limited number of participants and were mostly hospital-based or they were conducted during periods when vaccines were prioritized to high-risk groups and given to a restricted number of individuals. Vaccination against SARS-CoV-2 was not associated with risk of miscarriage in a study with Norwegian registry data.15
This study used national birth registries in Sweden and Norway with information on births linked to mandatory registrations on SARS-CoV-2 vaccination to investigate the risk of adverse pregnancy outcomes after vaccination during pregnancy.
Methods
This study was approved by the Swedish Ethical Review Authority (approval numbers: 2020-01499, 2020-02468, 2021-00274) and the Regional Committee for Medical and Health Research Ethics of South/East Norway (No. 141135). Each committee provided a waiver of consent for participants, due to the use of registry data.
Study Population
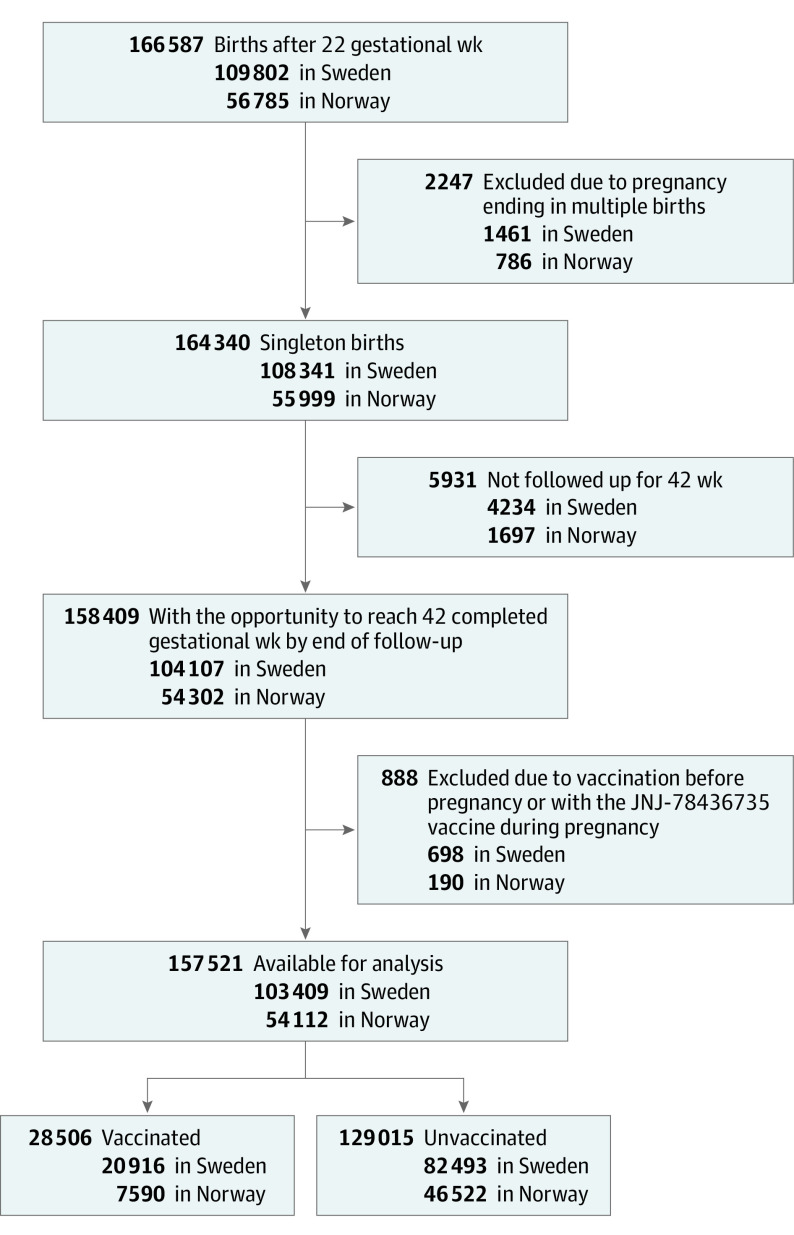
This study included all singleton pregnancies ending after 22 completed gestational weeks registered in the Pregnancy Register in Sweden and the Medical Birth Registry of Norway from January 1, 2021, until January 12, 2022 (Sweden), or January 15, 2022 (Norway). The universal access to free prenatal care is similar in the 2 countries. Unique national personal identification numbers were used to link individual information on births to national vaccination registries, positive laboratory test results for SARS-CoV-2, and population registers. For Norway, education and income information was obtained from Statistics Norway. Neonatal care admission in Sweden was retrieved from the Neonatal Quality Register. The different registers providing information on characteristics and other health conditions are described in more detail in the eAppendix in the Supplement. Pregnancies ending in multiple births were excluded. To avoid oversampling short pregnancies, we only included pregnancies that had the opportunity to reach 42 completed gestational weeks by the end of the follow-up in the registries. Individuals vaccinated prior to pregnancy were excluded, as were individuals who received the Johnson & Johnson vaccine (JNJ-78436735), because this vaccine was not part of the vaccination program in the 2 countries. The selection of study participants is shown in Figure 1.
Figure 1. Development of Cohorts in a Study of Pregnancy Outcomes With and Without SARS-CoV-2 Vaccination.
SARS-CoV-2 Vaccination
In Sweden, the 2 mRNA vaccines from Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) and 1 viral vector vaccine from AstraZeneca (AZD1222) were part of the national vaccination program throughout the study period, although the AZD1222 vaccine was not recommended to individuals younger than 65 years from March 16, 2021. In Norway, the AZD1222 vaccine was excluded from the vaccination program on May 12, 2021, and after this only the 2 mRNA vaccines were part of the program. General recommendations on vaccination for pregnant individuals in the second or third trimester were in May 2021 in Sweden and in August 2021 in Norway. Prior to this, only pregnant individuals considered to be at high risk of severe COVID-19, or high risk of acquiring COVID-19, were recommended vaccination. From the initiation of the vaccination program, vaccination during the first trimester was still not recommended in Sweden and Norway except for individuals with particularly high risk of severe COVID-19. Vaccinations during pregnancy were defined as vaccinations occurring between the start of pregnancy (delivery date minus the gestational age in days) and date of delivery.
Pregnancy Outcomes
We studied the following pregnancy outcomes: preterm birth (<37 completed gestational weeks), very preterm birth (<32 completed gestational weeks), stillbirth, small for gestational age (birth weight <10th percentile standardized for gestational age and sex), low Apgar score (Apgar score at 5 minutes below 7), and neonatal care admission. We further examined spontaneous and induced preterm birth separately, where induced preterm birth was defined by onset of labor initiated by either elective cesarean delivery or induction of labor, excluding individuals with preterm premature rupture of membranes. Gestational age at birth was estimated at the routine ultrasound assessment at approximately 18 gestational weeks for more than 95% of births in both countries or by using date of last menstrual period if ultrasound estimates were missing.
Covariates
We adjusted for the following potential confounders: maternal age at the start of pregnancy (continuous), parity (0, 1, and ≥2), educational level (≤9 years, 10-12 years, and >12 years), whether the individual was living with a partner (yes or no), household income (Norway only; categorized in tertiles), country of birth categorized into 4 regions (Scandinavia, other European countries, Middle East/Africa, Other [ie, North America, South America, Latin America, Asia, Australia, and New Zealand]), whether the individual had tested positive for SARS-CoV-2 (no, tested positive before pregnancy, and tested positive during pregnancy), and underlying chronic conditions (yes or no). Underlying chronic conditions included any registrations in the antenatal or birth records of hypertension, chronic kidney disease, asthma, cardiovascular disease, thrombosis, and diabetes. These are all conditions associated with both the likelihood of vaccination and risk of adverse pregnancy complications. Secondary analyses further adjusted for smoking during pregnancy (yes or no) and prepregnancy body mass index for pregnancies with this information available. Separate categories were included for unknown or missing for all other variables than smoking and body mass index. We did not impute missing information on covariates because individuals with missing information, particularly for education and income, are more likely to be immigrants and we had limited information in the registries to impute the missing values for this group.
Statistical Analysis
Hazard ratios (HRs) for preterm birth, very preterm birth, and stillbirth after vaccination were obtained using Cox regression where gestational age in days was the time-metric. Vaccination status was included as a time-varying exposure. Pregnant individuals could contribute to both unexposed and exposed follow-up time while pregnant. Pregnancies were followed up from 22 completed gestational weeks until 37 completed gestational weeks (for preterm birth), until 32 completed gestational weeks (for very preterm birth), or the end of pregnancy (for stillbirth). The proportional hazards assumption was evaluated by visually inspecting the Schoenfeld residuals, and there were no substantial deviations from the assumption. We used logistic regression to obtain odds ratios (ORs) for small for gestational age, low Apgar score, and neonatal care admission according to whether the individual was vaccinated during pregnancy.
The main analysis evaluated any vaccination during pregnancy, while secondary analyses examined differences according to pregnancy trimester of vaccination, number of vaccine doses, and different vaccine subtypes. Vaccinations in different trimesters were defined as during the first (<84 gestational days), second (84-195 gestational days), or third (>195 gestational days) trimester. There was a small number of individuals vaccinated with AZD1222 or vaccinated in the first trimester. Because vaccination was not recommended for pregnant individuals during the first trimester, this subgroup was likely to be highly selected, and we do not present estimates for these 2 specific exposure groups.
The main multivariable analyses adjusted for all covariates except for smoking during pregnancy and prepregnancy body mass index, while secondary analyses (including 92% of the total sample) adjusted for these 2 additional covariates. In the multivariable analyses of low Apgar score and neonatal care admission, we additionally adjusted for gestational age. We also conducted a sensitivity analysis restricting to term births for small for gestational age, low Apgar score, and neonatal care admission. Because any potential effect of vaccination on fetal growth would require some time to have an effect, we added a sensitivity analysis for small for gestational age in which those exposed to vaccination during the last 2 weeks of pregnancy were set to be unexposed.
All analyses were conducted separately for the 2 countries according to a common protocol and subsequently meta-analyzed using the DerSimonian and Laird random effects model, which takes into account any heterogeneity in the estimates between the 2 countries. Differences in the magnitude of the associations between the 2 countries were tested using the I2 statistic. We conducted a meta-analysis in which the data from the 2 countries were analyzed separately and subsequently combined the effect estimates, and not a pooled analysis in which individual-level data from both countries would be analyzed together, because the data were located at 2 different institutions. We used a 5% threshold to define statistical significance and all tests conducted were 2-sided. Because of the potential for type I error due to multiple comparisons, findings for the analyses should be interpreted as exploratory. Analyses were conducted in Stata version 15 (StataCorp) and SAS version 9.4 (SAS Institute).
Results
A total of 158 409 eligible births occurred during the study period in the 2 countries. Of these, 888 individuals were excluded because they were vaccinated prior to pregnancy or because they received the JNJ-78436735 vaccine (Figure 1). Of the 157 521 singleton births included in the study (103 409 in Sweden and 54 112 in Norway), 28 506 (18%) of the births were among individuals vaccinated against SARS-CoV-2 during pregnancy. In total, 4.4% of the participants had only 1 vaccine dose, whereas 13.7% had 2 doses while pregnant. The median gestational day of first vaccination was 184 days in Sweden (IQR, 154-221) and 209 days in Norway (IQR, 175-238). Among those vaccinated during pregnancy, most individuals (50.4% of those vaccinated) were vaccinated in the third trimester, while 45.6% were vaccinated in the second trimester, and only 3.9% during the first trimester. The gestational age distribution of vaccination in both countries is shown in the eFigure in the Supplement. There was a statistically significant difference in the gestational age at vaccination between the 2 countries (P < .001). A total of 4.8% of births were exposed to mRNA-1273, 12.9% were exposed to BNT162b2, while only 0.3% were exposed to AZD1222.
In both countries, individuals who were vaccinated during pregnancy were generally older, had a higher parity, were more highly educated, had a higher income, and were more likely to be born in Scandinavia. They were also more likely to have an underlying chronic disease and less likely to have a positive test result for SARS-CoV-2 during pregnancy (Table). Of those who remained unvaccinated throughout pregnancy, 8.5% tested positive for SARS-CoV-2 during pregnancy, while 3.4% of those vaccinated during pregnancy tested positive for SARS-CoV-2 during pregnancy after being vaccinated.
Table. Maternal Characteristics According to Vaccination Status During Pregnancy.
| Background characteristic | No. (%) | |||
|---|---|---|---|---|
| Sweden | Norway | |||
| Vaccinated (n = 20 916) | Unvaccinated (n = 82 493) | Vaccinated (n = 7 590) | Unvaccinated (n = 46 522) | |
| Age at start of pregnancy, mean (SD), y | 32.2 (4.6) | 30.5 (4.8) | 31.4 (4.5) | 30.7 (4.7) |
| Parity, y | ||||
| 0 | 9050 (43.3) | 34 864 (42.3) | 3400 (44.8) | 19 442 (41.8) |
| 1 | 8282 (39.6) | 30 477 (36.9) | 2834 (37.3) | 17 573 (37.8) |
| ≥2 | 3584 (17.1) | 17 152 (20.8) | 1356 (17.9) | 9507 (20.4) |
| Educational level, y | 17 202 | 67 384 | 7185 | 41 751 |
| ≤9 | 563 (3.3) | 5403 (8.0) | 593 (8.3) | 6573 (15.7) |
| 10-12 | 4530 (26.3) | 25 046 (37.2) | 1197 (16.7) | 9033 (21.6) |
| >12 | 12 109 (70.4) | 36 935 (54.8) | 5395 (75.1) | 26 145 (62.6) |
| Household income, tertilea | NA | NA | 7315 | 44 032 |
| First | NA | NA | 2194 (30.0) | 14 922 (33.9) |
| Second | NA | NA | 2313 (31.6) | 14 803 (33.6) |
| Third | NA | NA | 2808 (38.4) | 14 307 (32.5) |
| Living with partner | 20 472 | 80 553 | 7464 | 45 743 |
| Yes | 19 287 (94.2) | 74 475 (92.5) | 7237 (97.0) | 43 877 (95.9) |
| Region of origin | 18 566 | 74 410 | 7583 | 46 446 |
| Scandinavia | 15 646 (84.3) | 52 341 (70.3) | 6492 (85.6) | 33 936 (73.1) |
| Middle East/Africa | 1196 (6.4) | 13 048 (17.5) | 138 (1.8) | 3552 (7.7) |
| Other European countries | 735 (4.0) | 5445 (7.3) | 481 (6.3) | 5476 (11.8) |
| Otherb | 989 (5.3) | 3576 (4.8) | 472 (6.2) | 3482 (7.5) |
| Condition associated with risk of complication of pregnancyc | 2775 (13.3) | 8702 (10.5) | 805 (10.6) | 4094 (8.8) |
| Asthma | 1855 (8.9) | 5795 (7.0) | 601 (7.9) | 3031 (6.5) |
| Cardiovascular disease | 380 (1.8) | 1274 (1.5) | 53 (0.7) | 289 (0.6) |
| Diabetes | 325 (1.6) | 900 (1.1) | 92 (1.2) | 301 (0.7) |
| Prior thrombosis | 204 (1.0) | 679 (0.8) | 15 (0.2) | 96 (0.2) |
| Chronic hypertension | 133 (0.6) | 405 (0.5) | 52 (0.7) | 280 (0.6) |
| Chronic kidney disease | 92 (0.4) | 344 (0.4) | 37 (0.5) | 283 (0.6) |
| Positive SARS-CoV-2 test resultd | ||||
| Only positive prior to start of pregnancy | 1138 (5.4) | 1944 (2.4) | 73 (1.0) | 273 (0.6) |
| Positive during pregnancy | 1390 (6.6) | 9733 (11.8) | 123 (1.1) | 1255(2.3) |
| Smoking in pregnancy | 20 208 | 79 695 | 6831 | 41 587 |
| Yes | 377 (1.9) | 2938 (3.7) | 280 (4.1) | 2330 (5.6) |
| Prepregnancy body mass index, median (IQR)e | 25 (22-29) | 24 (22-28) | 24 (22-27) | 24 (21-27) |
| SARS-CoV-2 vaccinef | ||||
| BNT162b2 | 16 726 (80.0) | 3698 (48.7) | ||
| mRNA-1273 | 3926 (18.8) | 3681 (48.5) | ||
| AZD1222 | 264 (1.3) | 211 (2.8) | ||
| SARS-CoV-2 vaccination status | ||||
| 1 dose | 4541 (21.7) | 2436 (32.1) | ||
| 2 doses | 16 375 (78.3) | 5154 (67.9) | ||
| Pregnancy trimester of SARS-CoV-2 vaccinationg | ||||
| First trimester | 654 (3.1) | 471 (6.2) | ||
| Second trimester | 10 519 (50.3) | 2493 (32.9) | ||
| Third trimester | 9743 (46.6) | 4625 (60.9) | ||
Abbreviation: NA, not available.
Household income was not available as part of the Swedish registry linkage.
The other category includes North America, South America, Latin America, Asia, Australia, and New Zealand.
These 6 conditions (asthma, cardiovascular disease, diabetes, prior thrombosis, hypertension, and kidney disease) are associated both with the likelihood of receiving vaccination and with the risk of an adverse pregnancy complication.
These are mutually exclusive categories.
Calculated as weight in kilograms divided by height in meters squared.
A total of 1753 women received 2 different vaccines during pregnancy. The frequencies shown in the table indicate the last type of vaccine the individual was given during pregnancy.
The trimester of vaccination against SARS-CoV-2 refers to the trimester when the individual received their first dose of the vaccine.
Of all births, 4.2% were preterm (2.6% spontaneous and 1.6% medically indicated) and 0.5% were very preterm. The cumulative incidence curves for preterm birth in the 2 exposure groups are shown in Figure 2. We observed no statistically significant increased risk of preterm birth among individuals who were vaccinated during pregnancy (6.2 vs 4.9 per 10 000 pregnancy days at risk; adjusted HR [aHR], 0.98 [95% CI, 0.91 to 1.05]; I2 = 0%; P for heterogeneity = .60) (Figure 2), with similar results for spontaneous or medically indicated preterm deliveries (eTable 1 in the Supplement) and for very preterm birth (eTable 2 in the Supplement). Adjustment for smoking or body mass index did not change our findings (eTable 3 in the Supplement). The results were similar for vaccination during the second or third trimester (eTable 4 in the Supplement), for vaccination with 1 or 2 doses (eTable 5 in the Supplement), and for the 2 mRNA vaccines (eTable 6 in the Supplement).
Figure 2. Cumulative Incidence Curves of the Risk of Preterm Birth, Very Preterm Birth, and Stillbirth According to SARS-CoV-2 Vaccination.

All pregnancies were observed from 22 completed gestational weeks until event or 32 completed gestational weeks (very preterm birth), 37 completed gestational weeks (preterm birth), or end of pregnancy (stillbirth).
Analyses were adjusted for age at start of pregnancy, parity, education level, living with a partner, household income (Norway only), whether the individual had tested positive for SARS-CoV-2, and underlying chronic condition.
The I2 heterogeneity statistic for the difference in the estimates between Sweden and Norway was 0% (P = .60) for preterm birth, 59% (P = .12) for very preterm birth, and 0% (P = .71) for stillbirth.
Combining the estimates for Sweden and Norway in a random-effects meta-analysis, the adjusted hazard ratio was 0.98 (95% CI, 0.91 to 1.05) for preterm birth, 0.91 (95% CI, 0.63 to 1.31) for very preterm birth, and 0.86 (95% CI, 0.63 to 1.17) for stillbirth.
Only 0.2% of births ended in stillbirth during the study period. The cumulative incidence curves for stillbirth in the 2 exposure groups is shown in Figure 2. Adjusted analyses indicated no statistically significant increased risk of stillbirth after vaccination during pregnancy (2.1 vs 2.4 per 100 000 pregnancy days at risk; aHR, 0.86 [95% CI, 0.63 to 1.17]; I2 = 0%; P for heterogeneity = .71) (Figure 2; eTable 7 in the Supplement). We did not explore risk of stillbirth according to vaccination by pregnancy trimester, number of doses, or vaccine type due to the small number of stillbirths in our study.
Of all births, 8.4% were small for gestational age, 1.6% had a low Apgar score, and 8.5% were admitted for neonatal care. There was no statistically significant increased risk among infants who were exposed to maternal vaccinations during pregnancy for small for gestational age (7.8% vs 8.5%; difference, –0.6% [95% CI, –1.3% to 0.2%]; adjusted OR [aOR], 0.97 [95% CI, 0.90 to 1.04]; I2 = 39%; P for heterogeneity = .20), low Apgar score (1.5% vs 1.6%; difference, –0.05% [95% CI, –0.3% to 0.1%]; aOR, 0.97 [95% CI, 0.87 to 1.08]; I2 = 0%; P for heterogeneity = .93), or neonatal care admission (8.5% vs 8.5%; difference, 0.003% [95% CI, –0.9% to 0.9%]; aOR, 0.97 [95% CI, 0.86 to 1.10]; I2 = 80%; P for heterogeneity = .03) (Figure 3). Adjustment for smoking and body mass index did not change the results (eTable 8 in the Supplement). The results were also similar for vaccination during the second and third trimesters (eTable 9 in the Supplement), for 1 or 2 doses (eTable 10 in the Supplement), and for the 2 mRNA vaccines (eTable 11 in the Supplement). The sensitivity analysis restricted to term births (n = 150 905 deliveries) yielded similar associations for small for gestational age (7.5% vs 8.2%; difference, –0.6% [95% CI, –1.4% to 0.2%]; aOR, 0.97 [95% CI, 0.90 to 1.03]; I2 = 35%; P for heterogeneity = .22), low Apgar score (1.3% vs 1.3%; difference, 0.01% [95% CI, –0.1% to 0.2%]; aOR, 1.01 [95% CI, 0.90 to 1.14]; I2 = 0%; P for heterogeneity = .61), and neonatal care admission (6.6% vs 6.4%; difference, 0.01% [95% CI, –1.0% to 1.2%]; aOR, 1.01 [95% CI, 0.87 to 1.17]; I2 = 84%; P for heterogeneity = .01). The sensitivity analysis of small for gestational age defining those vaccinated during the last 2 weeks of pregnancy as unexposed also did not indicate any increased risk as observed in the main analysis (7.8% vs 8.5%; difference, –0.7% [95% CI, –1.4% to 0.3%]; aOR, 0.97 [95% CI, 0.88 to 1.06]; I2 = 63%; P for heterogeneity = .10).
Figure 3. Odds Ratios of Low Apgar Score at 5 Minutes, Small for Gestational Age, and Neonatal Care Admission According to Vaccination Against SARS-CoV-2 During Pregnancy.

Adjusted for age at start of pregnancy, parity, education level, living with a partner, household income (Norway only), whether the individual had tested positive for SARS-CoV-2, and underlying chronic condition.
The I2 heterogeneity statistic for difference in the estimates between Sweden and Norway was 0% (P = .93) for low Apgar score, 39% (P = .20) for small for gestational age, and 80% (P = .03) for neonatal care admission. The vertical dashed lines indicate the values for the meta-analyzed effect estimates.
Discussion
In this large registry-based study of births in Sweden and Norway, there was no statistically significant increased risk of adverse pregnancy outcomes among individuals vaccinated against SARS-CoV-2 during pregnancy. The results were similar for vaccinations during the second or third trimester, with 1 or 2 doses of vaccines, and with the different mRNA vaccine types. These findings support the continued recommendation of vaccination of pregnant individuals against SARS-CoV-2. Vaccination during the third trimester was associated with a modest decreased risk of neonatal care admission and low Apgar score, and vaccination with the mRNA-1273 vaccine was also associated with a modest decreased risk of neonatal care admission.
These findings should be addressed in other populations, although higher health awareness in vaccinated individuals may explain these associations.16 It is also possible that the reduced risk of these adverse pregnancy outcomes could be explained by a lower risk of SARS-CoV-2 among these individuals.17,18
Vaccination campaigns are still ongoing worldwide, and many individuals vaccinated while pregnant have not yet given birth. The evidence available on SARS-CoV-2 vaccination and pregnancy outcomes is still emerging. Previous studies have been limited in size in terms of the number of vaccinated pregnant individuals,10,11,12,14 inclusion of 1 type of vaccine,12,13 or reliance on participant-reported surveillance systems,7 which have led to difficulties in drawing conclusions. While previous results are in line with the findings of this study, the current study substantially expands on available data that support no evidence of an increased risk of adverse pregnancy outcomes among individuals vaccinated against SARS-CoV-2 during pregnancy.
Strengths of this study included the inclusion of nearly all births in Sweden and Norway in the time period from when vaccinations became available in both countries (January 2021) until the end of the study period (January 2022). The study was based on national mandatory registrations on births, vaccinations, characteristics, and underlying health conditions, limiting potential bias from selection of participants. Detailed registry information enabled adjustment for a range of potential confounding factors and to evaluate differences with 1 or 2 doses, vaccination in different trimesters, and vaccine type.
The findings of this study support no adverse events following SARS-CoV-2 vaccination during pregnancy. It is particularly important to increase vaccination rates in pregnant individuals because they are at higher risk of severe COVID-19 and complications. Recent population-based data from pregnant individuals in Scotland showed that approximately 91% of hospital admissions and 98% of critical care admissions associated with SARS-CoV-2 occurred in unvaccinated pregnant individuals.19
COVID-19 in pregnancy is also associated with adverse pregnancy outcomes affecting the child,17,18 and vaccination reduces the risk of COVID-19 during pregnancy.2 An added benefit of vaccination of individuals against SARS-CoV-2 during pregnancy is that antibodies are transferred to the fetus,20,21,22 which offers infants protection against the virus during the first months of life. This is particularly beneficial because none of the existing vaccines are currently approved for use in children younger than 2 years.
Limitations
This study had several limitations. First, potential misclassification of registrations of pregnancy outcomes, vaccinations, and background characteristics cannot be excluded. The registries in Norway and Sweden have been shown to be complete and accurate,23 but misclassification may occur for underlying chronic conditions and lifestyle factors. If misclassification was random and not related to vaccination or pregnancy outcome, it would most likely have resulted in an underestimation of the association.
Second, although the analyses adjusted for a broad range of potential confounding factors, there was likely residual confounding given the observational design of the study.
Third, there was not a sufficient number of pregnancies exposed to vaccination with AZD1222 to provide any evidence regarding adverse events associated with this vaccine during pregnancy.
Fourth, there were few individuals who were vaccinated in the first trimester among individuals who completed pregnancies in the study period. Because Norway and Sweden do not recommend vaccination during the first trimester except for particular high-risk groups, those exposed to vaccination during the first trimester are likely to constitute a highly selected group. Thus, it was not possible to provide any evidence regarding adverse events associated with vaccination during the first trimester. Most individuals in the cohort were vaccinated during the third trimester, which may have limited the assessment of fetal growth restriction.
Fifth, the overall vaccination rate among pregnant individuals included in this study was relatively low and vaccination was most often in the third trimester, likely reflecting a delayed vaccination uptake among pregnant individuals. This may limit the generalizability of the findings.
Conclusions
In this population-based study conducted in Sweden and Norway, vaccination against SARS-CoV-2 during pregnancy, compared with no SARS-CoV-2 vaccination during pregnancy, was not significantly associated with an increased risk of adverse pregnancy outcomes. The majority of the vaccinations were with mRNA vaccines during the second and third trimesters of pregnancy, which should be considered in interpreting the findings.
eAppendix. Data Sources and Linkages in Sweden and Norway
eTable 1. Hazard Ratios for Spontaneous and Medically Indicated Preterm Birth According to SARS-CoV-2 Vaccination During Pregnancy
eTable 2. Hazard Ratios of Very Preterm Birth According to SARS-CoV-2 Vaccination During Pregnancy
eTable 3. Hazard Ratios for Preterm Birth, According to Vaccination Against SARS-CoV-2 During Pregnancy Exploring Additional Adjustment for Smoking and Body-Mass Index
eTable 4. Hazard Ratios for Preterm Birth According to SARS-CoV-2 Vaccination by Pregnancy Trimester
eTable 5. Hazard Ratios for Preterm Birth According to One or Two Doses of Vaccine Against SARS-CoV-2 During Pregnancy
eTable 6. Hazard Ratios for Preterm Birth According to SARS-CoV-2 Vaccination by Vaccine Subtypes
eTable 7. Hazard Ratios for Stillbirth According to SARS-CoV-2 Vaccination During Pregnancy
eTable 8. Odds Ratios for Apgar Score, Small-for-Gestational-Age and Neonatal Care Admission According to SARS-CoV-2 Vaccination During Pregnancy With Additional Adjustment for Smoking and Body To Mass Index
eTable 9. Odds Ratios for Apgar Score, Small-for-Gestational-Age and Neonatal Care Admission According to SARS-CoV-2 Vaccination During Pregnancy by Trimester
eTable 10. Odds Ratios for Apgar Score, Small-for-Gestational-Age and Neonatal Care Admission According to One or Two Doses of Vaccine Against SARS-CoV-2 During Pregnancy
eTable 11. Odds Ratios for Apgar Score, Small-for-Gestational-Age and Neonatal Care Admission According to Different Types of SARS-CoV-2 Vaccines During Pregnancy
eFigure. Gestational Age Distribution of SARS-CoV-2 Vaccination
References
- 1.Giesbers S, Goh E, Kew T, et al. ; PregCOV-19 Group . Treatment of COVID-19 in pregnant women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;267:120-128. doi: 10.1016/j.ejogrb.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693-1695. doi: 10.1038/s41591-021-01490-8 [DOI] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412-1423. doi: 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists . COVID-19 vaccination considerations for obstetric–gynecologic care. Accessed January 18, 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- 5.Centers for Disease Control and Prevention . Information about COVID-19 vaccines for people who are pregnant or breastfeeding. Accessed May 11, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- 6.Martins I, Louwen F, Ayres-de-Campos D, Mahmood T. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimabukuro TT, Kim SY, Myers TR, et al. ; CDC v-safe COVID-19 Pregnancy Registry Team . Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273-2282. doi: 10.1056/NEJMoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zauche LH, Wallace B, Smoots AN, et al. ; CDC v-safe Covid-19 Pregnancy Registry Team . Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385(16):1533-1535. doi: 10.1056/NEJMc2113891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen SA, Kelley CF, Horton JP, Jamieson DJ. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1-236.e14. doi: 10.1016/j.ajog.2021.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. doi: 10.1016/j.ajogmf.2021.100467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bookstein Peretz S, Regev N, Novick L, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021;58(3):450-456. doi: 10.1002/uog.23729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. COVID-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129(2):248-255. doi: 10.1111/1471-0528.16941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037-6040. doi: 10.1016/j.vaccine.2021.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. COVID-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385(21):2008-2010. doi: 10.1056/NEJMc2114466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remschmidt C, Wichmann O, Harder T. Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC Infect Dis. 2015;15:429. doi: 10.1186/s12879-015-1154-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allotey J, Stallings E, Bonet M, et al. ; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman M, Navér L, Söderling J, et al. Association of maternal SARS-CoV-2 infection in pregnancy with neonatal outcomes. JAMA. 2021;325(20):2076-2086. doi: 10.1001/jama.2021.5775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. Published online January 13, 2022. doi: 10.1038/s41591-021-01666-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill L, Jones CW. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in neonatal cord blood after vaccination in pregnancy. Obstet Gynecol. 2021;137(5):894-896. doi: 10.1097/AOG.0000000000004367 [DOI] [PubMed] [Google Scholar]
- 21.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1-303.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul G, Chad R. Newborn antibodies to SARS-CoV-2 detected in cord blood after maternal vaccination: a case report. BMC Pediatr. 2021;21(1):138. doi: 10.1186/s12887-021-02618-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephansson O, Petersson K, Björk C, Conner P, Wikström AK. The Swedish Pregnancy Register: for quality of care improvement and research. Acta Obstet Gynecol Scand. 2018;97(4):466-476. doi: 10.1111/aogs.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Data Sources and Linkages in Sweden and Norway
eTable 1. Hazard Ratios for Spontaneous and Medically Indicated Preterm Birth According to SARS-CoV-2 Vaccination During Pregnancy
eTable 2. Hazard Ratios of Very Preterm Birth According to SARS-CoV-2 Vaccination During Pregnancy
eTable 3. Hazard Ratios for Preterm Birth, According to Vaccination Against SARS-CoV-2 During Pregnancy Exploring Additional Adjustment for Smoking and Body-Mass Index
eTable 4. Hazard Ratios for Preterm Birth According to SARS-CoV-2 Vaccination by Pregnancy Trimester
eTable 5. Hazard Ratios for Preterm Birth According to One or Two Doses of Vaccine Against SARS-CoV-2 During Pregnancy
eTable 6. Hazard Ratios for Preterm Birth According to SARS-CoV-2 Vaccination by Vaccine Subtypes
eTable 7. Hazard Ratios for Stillbirth According to SARS-CoV-2 Vaccination During Pregnancy
eTable 8. Odds Ratios for Apgar Score, Small-for-Gestational-Age and Neonatal Care Admission According to SARS-CoV-2 Vaccination During Pregnancy With Additional Adjustment for Smoking and Body To Mass Index
eTable 9. Odds Ratios for Apgar Score, Small-for-Gestational-Age and Neonatal Care Admission According to SARS-CoV-2 Vaccination During Pregnancy by Trimester
eTable 10. Odds Ratios for Apgar Score, Small-for-Gestational-Age and Neonatal Care Admission According to One or Two Doses of Vaccine Against SARS-CoV-2 During Pregnancy
eTable 11. Odds Ratios for Apgar Score, Small-for-Gestational-Age and Neonatal Care Admission According to Different Types of SARS-CoV-2 Vaccines During Pregnancy
eFigure. Gestational Age Distribution of SARS-CoV-2 Vaccination