Abstract
Dysfunction in the olfactory system of a person can have adverse effects on their health and quality of life. It can even increase mortality among individuals. Olfactory dysfunction is related to many factors, including post-viral upper respiratory infection, head trauma, and neurodegenerative disorders. Although some clinical therapies such as steroids and olfactory training are already available, their effectiveness is limited and controversial. Recent research in the field of therapeutic nanoparticles and stem cells has shown the regeneration of dysfunctional olfactory systems. Thus, we are motivated to highlight these regenerative approaches. For this, we first introduce the anatomical characteristics of the olfactory pathway, then detail various pathological factors related to olfactory dysfunctions and current treatments, and then finally discuss the recent regenerative endeavors, with particular focus on nanoparticle-based drug delivery systems and stem cells. This review offers insights into the development of future therapeutic approaches to restore and regenerate dysfunctional olfactory systems.
Keywords: Olfactory dysfunction, stem cells, nanoparticles, regeneration
Introduction
Olfactory dysfunction, estimated to affect 3%–20% of the population, significantly affects the health and quality of life of the afflicted individual,1,2 and increases the likelihood of mortality among individuals up to four times. 3 This is mainly related to the body’s weakened defense (immune) system and the inability to sense dangerous signals (e.g. fires, hazardous chemical vapors, gas leaks, and decayed food).4–6 The principal causes of olfactory dysfunction are sinonasal diseases, viral infections, head injuries, and neurodegenerative diseases.
The olfactory system has several unique characteristics. One notable feature is that the cells (mainly olfactory sensory neurons (OSNs)) are always exposed to various harmful substances while in direct contact with external air that enters the nasal cavity 7 ; as such, the OSNs are easily damaged. More importantly, OSNs can also regenerate throughout the lifetime of a person 8 ; this fact allows the possibility of regenerative approaches to treat olfactory dysfunction. While treatment varies depending on the etiology of the olfactory dysfunction, medications such as oral/intranasal steroids, surgery, and olfactory training are clinically used. However, as the success of these various treatment modalities is not guaranteed, it is currently challenging to treat patients with olfactory dysfunctions. Recent efforts in the field have yielded promising outcomes in the treatment of anosmia using stem cell therapies.
Thus, we are motivated to highlight recent studies that endeavored to regenerate the dysfunctional olfactory system. For this, we first introduce the anatomical characteristics of the olfactory pathway, then detail the various pathological factors related to olfactory dysfunction and current clinical options, and then finally discuss the recent emerging therapeutic approaches, particularly with respect to nanoparticle-based delivery systems and stem cells. We also added the availability of the combinatory approach of nanoparticles with stem cells to potentiate regenerative functions. This review offers insights into the development of future therapeutic approaches to restore and regenerate the dysfunctional olfactory system.
Anatomy of the olfactory system and the dysfunctions: A brief overview
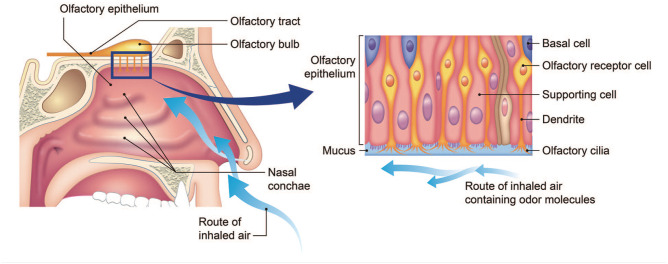
Figure 1 shows the anatomical structure of the olfactory pathway. The olfactory nerve is the first of the 12 cranial nerves and specifically carries olfactory sensory information (sense of smell). The olfactory epithelium (OE), a mucosal membrane that lines the roof and sides of the nasal cavity, contains the olfactory receptor cells. When an odorant passes through the nasal vestibule and contacts the OE, the perception of smell begins. The OE consists of the pseudostratified columellar neuroepithelium and basal cells which reside in the deep layers of the OE, and function as stem cells with multipotency. 9 These basal cells can give rise to new olfactory sensory neurons. 10
Figure 1.
Anatomy of the olfactory system, and the tissue structure and cells comprising of.
Olfactory neurons reside superficially to basal cells. Each olfactory neuron expresses a single olfactory receptor. One odor is capable of activating multiple receptor types to varying degrees. The binding of an odorant to olfactory receptors results in signal transmission via the olfactory nerves to the olfactory bulb. Efferent neurons of the olfactory bulb form the olfactory tract. The axons from the olfactory bulb cells project information to the thalamus, hypothalamus, and dorsolateral frontal cortex, which ultimately results in the sense of smell. 11
Clinically, olfactory dysfunction results from many underlying diseases such as sinonasal diseases, post-infectious disorders, and post-traumatic disorders.12–14 Other etiologies such as congenital, idiopathic, toxic, or neurodegenerative disease-associated problems are related, but less prevalent; however, they must not be ruled out. Depending on the site of olfactory nerve injury, olfactory dysfunction is categorized as follows: (i) direct damage to the olfactory nerve epithelium or subepithelial tissue, and (ii) degeneration and damage to the olfactory nerve axons or olfactory bulb. 15
Table 1 summarizes the common etiology of olfactory dysfunction. Most etiologies are attributed to common conductive or traumatic processes (such as, sinonasal disease and head trauma) and common sensorineural processes (such as, upper respiratory infection and age-related loss), and congenital disorders. 16 Regardless of the specific etiology, neurogenic exhaustion is likely a common feature of many acquired anosmia in which the normal replacement of damaged or senescent OSNs from progenitor basal cells is overwhelmed. 17
Table 1.
Etiologies of olfactory dysfunctions.
| Sinonasal conditions |
| Upper respiratory infection (especially viral), allergic rhinitis, chronic rhinosinusitis, nasal polyps |
| Head trauma |
| Damage to cribriform plate, shearing forces, intracranial damage, facial trauma |
| Neurodegenerative disorders |
| Parkinson disease, parkinsonism, Alzheimer disease, mild cognitive impairment, multiple sclerosis |
| Medications |
| Chemotherapy, angiotension-converting enzyme inhibitors, angiotensin receptor blockers, dihydropyridine calcium channel blockers, diuretics, intranasal zinc, antimicrobials (macrolides, terbinafine, fluoroquinolones, protease inhibitors, griseofulvin, penicillins, tetracyclines, nitromidazoles, antiarrhythmics, antithyroid agents, antidepressants, anticonvulsants, lipid-lowering agents) |
| Toxins or intoxicants |
| Alcohol, cocaine, ammonia, hairdressing chemicals, gasoline, formaldehyde, paint solvents, welding agents, benzene, sulfuric acids, cadmium, acrylates, iron, lead, chromium |
| Chronic medical conditions |
| Renal of hepatic failure, diabetes mellitus, cancer, human immunodeficiency virus |
| Structural or mechanical conditions |
| Ischemic stroke, subarachnoid or intracranial hemorrhage, brain or sinonasal tumor |
| Nutritional deficiencies |
| Malnutrition, pernicious anemia, deficiencies in vitamin A/B6/B12, niacin, zinc or copper |
| Postsurgical state |
| Nasal surgery (septal or sinus), total laryngectomy, pharyngectomy, tonsillectomy |
| Post-radiation (especially to head and neck area) |
| Congenital conditions |
| Kallmann syndrome |
| Phychiatric conditions |
| Anorexia nervosa, major depressive disorder, bipolar disorder, schiznophrenia |
| Endocrine conditions |
| Pregnancy, hypothyroidism, Addison disease, Cushing syndrome |
| Autoimmune/inflammatory conditions |
| Sjögren syndrome, systemic lupus erythematosus, sarcoidosis, herpes encephalitis |
Current clinical treatments and therapeutic molecules and their limitations
Clinical treatment options
For patients with chronic rhinosinusitis, functional endoscopic sinus surgery was shown to improve some of the olfactory functions, ameliorating ventilation, and decreasing inflammation in the olfactory cleft area 18 ; a spontaneous recovery in 32%–66% of the patients was observed in post-URI anosmia. 19 On the other hand, post-traumatic olfactory disorders showed a much lower recovery rate, due to scarring in the cribriform plate area, accompanied by shearing injuries and intracranial lesions. 20 Olfactory training is also recommended to gain some olfactory functions in post-traumatic anosmia/hyposmia 21 and in chronic rhinosinusitis.22,23 Exposure to certain odors may modulate the regenerative capacity of olfactory receptor neurons.24,25 In this study, patients with post infection anosmia showed brain remodeling during functional magnetic resonance imaging following 12 weeks of olfactory training. 26 Patients with hyposmia, who have neurodegenerative disorders like Parkinson’s disease, may also benefit from olfactory training. 27
Clinical therapies based on oral or systemic steroids have often been proven to be effective for sinonasal disorders, although the duration and dose of steroids remain to be optimized.28,29 Topical steroids have also been shown to be effective in allergic rhinitis combined with antihistamines.30,31 In a double-blind, randomized, placebo-controlled study, the effect of fluticasone nasal spray on patients with olfactory dysfunction was evaluated. Eighty-three percent of the patients had improved smell after systemic treatment, with no difference observed in the topical versus placebo groups. 32 Although the administration of steroids was proven to be effective in many animal models,33,34 studies in humans showed variable outcomes and often had limited efficacy.35,36 In patients with post-traumatic olfactory dysfunction, the treatment effect of steroids was known to occur only in 12%–16% of the patients. 37 More importantly, the side effects of steroids such as osteonecrosis and iatrogenic Cushing syndrome (adrenal insufficiency) have been raised as significant considerations.38,39 Other side effects of steroids include cataracts, gastritis, hyperglycemia, hypertension, delayed wound healing, and bacterial/fungal/viral infection.
Other medications such as statins and vitamins have been used in clinical settings, in addition to steroids. A recent study has revealed that vitamin A plays a role in the regeneration of olfactory receptor neurons. 40 In post-traumatic and post-infectious anosmic patients, topical treatment with vitamin A increased the olfactory function in 37% of anosmic patients, while 23% improvement was shown in the control group. 40
Candidates of therapeutic molecules studied in vivo
Some of the candidate small molecules and proteins, albeit not clinically proven, have been tested in dysfunctional olfactory in vivo models.
Statin, a β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitor with putative neuroprotective properties, has shown effects on the nervous system. 41 Kim et al. 42 showed that the statin treatment group had an increased expression of olfactory marker protein (OMP) and thickness of OE, compared to the control group. Furthermore, a significantly higher pass rate in a food-finding test was shown in the treatment group compared to that in the control group. 42
Valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, has shown some neuro-regenerative properties in rodents with spinal cord injury. 43 Ogawa et al. 44 investigated the effects of VPA on olfactory sensory neuron regeneration. In a mice model of OE degeneration induced by methimazole injection, daily administration of VPA increased epithelial thickness, the proliferation of OMP positive cells, and the expression of growth-associated protein-43 (GAP43), which is a nervous tissue-specific cytoplasmic protein in the OE, suggesting that VPA stimulates proliferation and differentiation of olfactory precursor cells, which in turn promotes regeneration of the olfactory system. 44
Treatment with growth factors is also a potential tool to improve olfactory dysfunction by restoring homeostasis and normal neurogenesis, as growth factors stimulate cellular growth, proliferation, and regeneration. 45 In young and aged mice, intranasal administration of basic fibroblast growth factor (bFGF) significantly increased the proliferation of GAP43-positive cells, although there was no significant change in the number of OMP positive cells and mature olfactory receptor neurons. 46 Nota et al. 47 examined the effect of bFGF on the injured OE of mice. In a murine anosmia model, intranasal treatment with bFGF and hydrogel increased the thickness of the OE and the number of mature OSNs expressing OMP. 47 With hydrogel, sustained release of bFGF could be achieved.
Platelet-rich plasma (PRP) is a small amount of blood from a subject that is separated into its components via centrifugation. It is a biocompatible physiological material and contains many growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and numerous neurotrophic factors such as the neurotrophin-3, angiopoietin-1 (Ang1), and glial cell line-derived neurotrophic factor (GDNF).48–52 A recent study demonstrated the effectiveness of PRP on anosmia in a mouse model, showing that PRP treatment induced significant functional and histological improvements. 53 However, this study had some limitations; the authors only investigated the histopathological findings with hematoxylin and eosin staining.
These studies in in vivo anosmia models revealed some of the important roles that small molecules and proteins play in various biological pathways in restoring the functions of OE, highlighting their potential use as candidate molecules for the treatment of olfactory dysfunction in the future. Despite their therapeutic effectiveness, the potential dose-dependent human toxicity and side effects of small molecules and proteins, particularly when administered systematically, must be resolved, which might be alleviated by utilizing adequate delivery systems.
Nanoparticle-based intranasal drug delivery system
Nanoparticles have been extensively studied as drug delivery systems in diverse fields, including for the treatment of tumors, neurodegenerative disorders, and cardiovascular diseases.54,55 Nanoparticles have recently been studied for applications in olfactory disorders. Like in the other areas, the treatment of olfactory system disorders requires the nanoparticles to satisfy several prerequisites: biocompatibility and the capacity to load large amounts of cargo molecules and subsequently release them in a controllable manner. Furthermore, the nanoparticles are tailored to exert specific functions; for example, surface modification is required to target cells or cellular components, which depends on the administration route (oral, intravenous, or intranasal delivery), and the composition needs to be properly chosen (polymers, inorganics, or composites) to allow controlled drug release profiles (diffusion, swelling, erosion, or degradation). Sometimes, the nanoparticles are equipped with more advanced properties such as stimuli-responsiveness and imaging/diagnostic capacity. Authors are guided to refer to some of the key reviews in this nanoparticle development area.56–58 Among other delivery routes, intranasal delivery has been the most widely studied route for the use of nanoparticles in the treatment of olfactory system disorders.
Overcoming mucus clearance and the therapeutic efficacy in vitro and in vivo
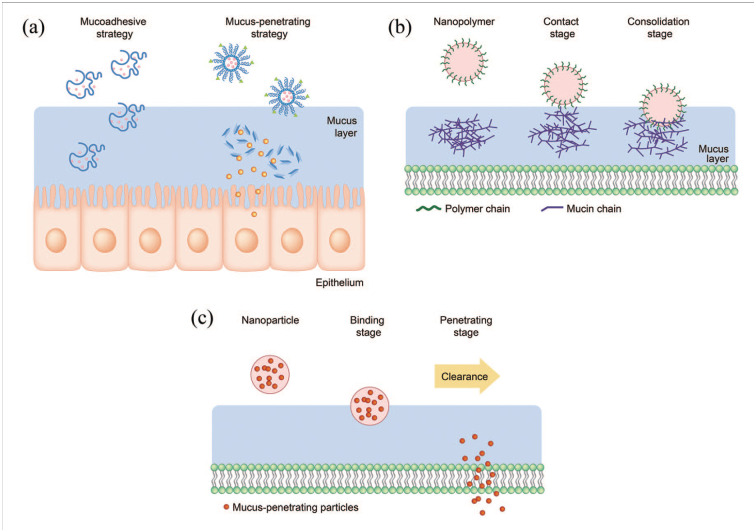
Above all, the mucus layer is considered a unique characteristic of the olfactory system; thus, “mucociliary clearance” has remained a major physiological barrier that the nanoparticles with drugs need to overcome in the olfactory pathway. 59 This protection mechanism of the respiratory system operates efficiently and rapidly eliminates noxious substances (particles and microorganisms) trapped in the 10–15 μm thick mucus layer. As such, this system greatly limits the residence time of therapeutic substances administered into the nasal cavity.
Two strategies made with nanoparticles are used to address the mucociliary clearance issue: (i) mucoadhesive and (ii) mucus-penetrating, as depicted schematically in Figure 2. The mucus layer has different physicochemical properties depending on the organ. The nasal mucus is slightly acidic (pH 5.5–6.5) and is negatively charged because of the presence of high amount of mucins.60,61 Therefore, mucoadhesive nanoparticles are often developed to have a positively-charged surface to maximize nanoparticle adhesion to the nasal mucus based on their electrostatic attraction with mucins. Moreover, the surface can be tailored to be hydrophobic to enable hydrophobic interaction with mucin hydrophobic domains. 62 For this reason, chitosan has been widely studied as a mucoadhesive nanoparticle for intranasal drug delivery. It is not only biocompatible, biodegradable, mucoadhesive, and positively charged in the slightly acidic pH of nasal mucus, but is also an efficient permeation enhancer that can transiently open the tight junctions between epithelial cells in mucosal tissues.63–65
Figure 2.
(a) Schematic illustrating the mucoadhesive and mucus-penetrating strategy with developed nanoparticles. Mucoadhesive nanoparticles (b) are good at catching the surface of mucous membrane whereas the mucus-penetrating nanoparticles (c) transport more effectively through the mucus layer.
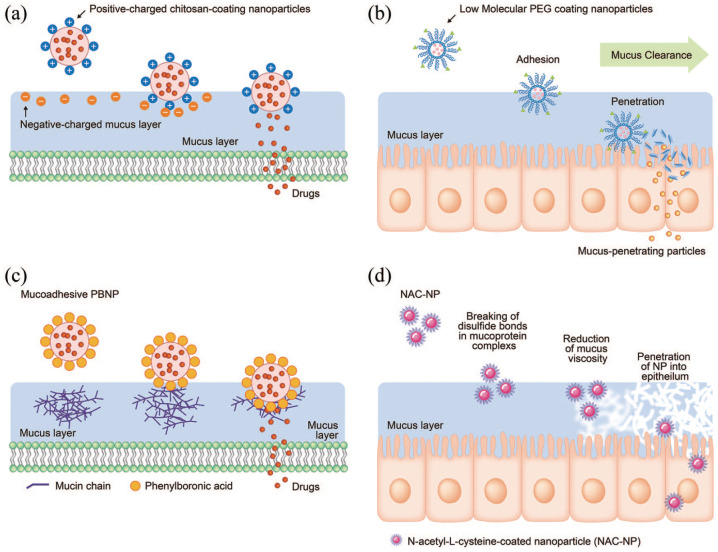
Several studies have reported mucoadhesive nanocarriers based on chitosan derivatives (Figure 3(a)). Trimethyl chitosan (TMC), for example, is a water-soluble, permanently positively charged chitosan derivative that has been used to encapsulate the analgesic neurotransmitter leucine-enkephalin (Leu-Enk). Trimethyl chitosan nanoparticles could increase the permeability of the peptide across porcine nasal mucosa 35 times, leading to a significant increase in the antinociceptive effect. 66 Liposomes coated with a chitosan derivative have also been proposed for nasal delivery. Liposomes loaded with ghrelin were prepared by the lipid film-rehydration-extrusion technique, followed by coating with N-([2-hydroxy-3-trimethylammonium] propyl) chitosan chloride (HTCC). The chitosan-coated liposomes bound mucin more efficiently than the uncoated anionic liposomes and improved permeation. 67 Clementino et al.68,69 developed hybrid chitosan–lipid nanocapsules for drug (statin) delivery. The nanocapsules with sizes of 200 nm and that were positively charged, were administered intranasally to rats and showed a higher intake rate than free-standing drugs. Another study developed a mucin-controlled drug release system from mucoadhesive phenylboronic acid-rich nanoparticles (PBNPs) that specifically adhered to mucin (Figure 3(b)). 70
Figure 3.
Exemplar studies on the development of mucoadhesive or mucus-penetrating nanoparticles. (a) Mucoadhesive nanoparticles based on chitosan-coating for binding to negative-charged mucus layer. (b) Mucoadhesive nanoparticles based on phenylboronic acid-rich nanoparticles (PBNP) for adhesion to mucin and mucin-controlled drug release. (c) Mucus-penetrating nanoparticles by low molecular weight PEG coating. (d) Mucus-penetrating nanoparticles based on NAC-coating by reducing mucus viscosity.
On the other hand, the mucus-penetrating nanoparticles had their surface modified to reduce mucoadhesion (Figure 3(c)). Mucus is a dense molecular network with a mesh spacing (20–500 nm) that prevents the diffusion of larger particles through it.71,72 Thus, the use of sufficiently small nanoparticles coated with proper polymers that minimize interactions with mucins, may increase their penetration through the mucus layer. Polyethylene glycol (PEG) is often used to coat the surfaces of polymeric nanoparticles. For example, the presence of PEG on the surface of PLA nanoparticles at high density was shown to enhance nanoparticle transportation within the nasal mucosa when administered intranasally to rats. 73 Furthermore, nanoparticles of 100 and 200 nm coated with low MW PEG were also shown to penetrate the mucus of individuals suffering from chronic rhinosinusitis effectively. 74 The unique characteristics of nanoparticles, such as, the small size (<200 nm) and negatively charged surface (ζ potential between −15 and −30 mV), makes them favorable for drug delivery across the nasal mucosa (and even to brain transport), and superior to naked drug delivery. When poly (lactic-co-glycolic acid) (PLGA)/PEG nanostructured particles loaded with resveratrol (RSV) were used to treat a mouse nasal polyp model, polyp formation was inhibited, and epithelial integrity was increased. 75 The lipid was also combined with PEG to coat the PLGA nanoparticles as an effective mucus-penetrating nanocarrier of the drug. Although the work was actually aimed at nose-to-brain delivery, the highlighted point was that the dose fraction accumulated in the liver and spleen was significantly reduced, confirming a higher safety of the nasal treatment via a mucus-penetrating approach. 76
Another approach for designing mucus-penetrating nanoparticles is in conjugation with a mucolytic agent to disrupt the mucus barrier (Figure 3(d)). N-acetyl-L-cysteine (NAC), a potent mucolytic agent, remarkably enhanced nasal absorption of large molecular weight compounds, in combination with nonionic surfactants. 77 NAC can disrupt the mucus structure by substituting the free thiol (sulfhydryl) group for the disulfide bonds connecting with mucin proteins, resulting in its clinical use in bronchopulmonary diseases to reduce both the viscosity and tenacity of mucus, as well as to facilitate its removal.77,78
Discussion and outlook on nanoparticle-based therapies
Along with the design of nanoparticles to be mucoadhesive or mucus-penetrating, other properties might be helpful in future developments. Some of the intrinsic properties of the newly developed nanoparticles, such as enzymatic activity, require special attention to treat olfactory injuries. The enzymatic activity involves catalase-, superoxide dismutase-, oxidase, and peroxidase-like properties; thus, the nanoparticles developed to have these properties can play roles similar to those of the body’s natural antioxidant enzymes.79,80 For example, nanoparticles such as cerium oxide (CeO2), copper oxide, and polyoxometalate (POM), have been shown to exert some of those properties in vitro and in vivo and were thus highly effective in scavenging reactive oxygen species (ROS) under oxidative stress conditions (e.g. inflamed tissues such as osteoarthritis, skin infection, and spinal cord injury).81–84 Acute injuries and infections in the olfactory system mostly entail severe inflammation with excessively generated ROS; thus, using such enzymatic nanoparticles would help attenuate local inflammation, possibly contributing to olfactory tissue recovery; this, however, requires further investigation. Furthermore, when the enzymatic nanoparticles are modified to be mucoadhesive or mucus-penetrating and to deliver drugs, their therapeutic functions in inflamed olfactory tissues could be synergized and potentiated, which constitutes a potential future research area.
While we focused on nanoparticle delivery to the site of olfactory injuries, nasal delivery often reaches the brain region, and thus, olfactory dysfunction is closely linked to brain diseases. Recently, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been shown to significantly influence the respiratory and central nervous systems, leading to anosmia and several neurological diseases.85,86 Olfactory dysfunctions caused by sensorineural or traumatic etiologies are difficult to treat clinically. Although researchers and medical companies are focusing on developing drugs for anosmia and SARS-CoV-2-induced anosmia, there are few reports on the use of nanoparticles to treat anosmia. As a key mechanism of sensorineural/traumatic anosmia, the disruption of the olfactory neurons is considered 87 ; therefore, strategies for drug delivery from intranasal to brain are thought to be a promising treatment option.69,88,89 As listed in Table 2, some of the nanocarrier systems developed for nose-to-brain delivery are also based on mucoadhesive or mucus-penetrating polymeric nanoparticles, with compositions such as lipids, fatty acids, PEG, PEG-PLGA, and Pluronic F127.69,90,91 The olfactory sensory neurons directly cross-talk with the brain and central nervous system, so nanoparticle-based therapeutics through the route of nose-to-brain would be a promising therapeutic strategy to address olfactory dysfunction.
Table 2.
Nanocarriers with mucoadhesive or mucus-penetrating property developed for the intranasal drug delivery.
| Drug | Application | Size (nm) | ζ-potential (mV) | References | |
|---|---|---|---|---|---|
| Mucoadhesive nanocarriers | |||||
| Chitosan | Chitosan | Olfactory dysfunction | — | — | Li et al. 92 |
| Chitosan NPs | Estradiol | Alzheimer’s disease | 269.3 ± 31.6 | +25.4 ± 0.7 | Wang et al. 93 |
| Rivastigmine | Alzheimer’s disease | 185.4 ± 8.4 | +38.4 ± 2.8 | Fazil et al. 94 | |
| Thymoquinone | Alzheimer’s disease | 172.4 ± 7.4 | +30.3 ± 2.2 | Alam et al. 95 | |
| Bromocriptine | Parkinsons’ disease | 161.3 ± 4.7 | +40.3 ± 2.7 | Md et al. 96 | |
| Pramipexole | Parkinsons’ disease | 292.5 ± 8.8 | +14.0 ± 2.9 | Raj et al. 97 | |
| Ropinirole | Parkinsons’ disease | 173.7 ± 2.3 | +32.7 ± 1.5 | Jafarieh et al. 98 | |
| Tapentadol | Chronic pain | 201.2 ± 1.5 | +49.3 ± 1.2 | Javia and Thakkar 99 | |
| Thiolated chitosan NPs | Cyclobenzaprine | Chronic pain | 272.1 ± 11.5 | +20.9 ± 1.7 | Patel et al. 100 |
| Selegiline | Depression | 215.0 ± 34.7 | +17.1 | Singh et al. 101 | |
| Chitosan-PLGA NPs | Chlorpromazine | Schizophrenia | 463.9 ± 12.0 | +21 ± 2 | Chalikwar et al. 102 |
| Chitosan-coated liposomes | Ghrelin | Cachexia | 194.0 ± 6.1 | +6.0 ± 0.4 | Salade et al. 67 |
| Mucus-penetrating/penetration-enhancing nanocarriers | |||||
| Pluronic® F127 PLGA NPs | Diazepam | Epilepsy | 183.2 | < −15 | Sharma et al. 103 |
| Midazolam | Epilepsy | 164.0 ± 4.5 | −16.6 ± 2.5 | Sharma et al. 104 | |
| Pluronics-coated PLGA | — | Chronic rhinosinusitis | 188 ± 7 | −7 ± 1 | Lai et al. 74 |
| PEGylated Zinc | Zinc | Olfactory enhancement | 1.4 ± 0.4 | −27.5 ± 2.5 | Singletary et al. 105 |
| Lipid/PEG-PLGA NPs | FTA | Glioblastoma | 164.3 ± 10.3 | −12.0 ± 1.3 | Sekerdag et al. 76 |
| TPSG micelles | Zolmitriptan | Migraine | 24.2 ± 0.7 | — | Jain et al. 106 |
| Sumatriptan | Migraine | 23.1 ± 0.4 | — | Jain et al. 107 | |
| Polysorbate 80 SLN | Rosmarinic acid | Huntington’s Disease | 149.2 ± 18.2 | −38.27 | Bhatt et al. 108 |
FTA: farnesylthiosalicylic acid; SLN: solid lipid nanoparticles.
Stem cell-based transplantation therapies
Stem cells have self-renewal potential and multi-lineage differentiation properties and have therefore been used for therapeutic purposes in regenerative medicine. In particular, multipotent adult stem cells including hematopoietic stem cells (HSCs), mesenchymal stem/stromal cells (MSCs), and fetal tissue-derived stem cells, can be specialized more toward tissue- and lineage-specific cell types; thus, a wide range of clinical trials have been performed for over 60 years. 109 Although these stem cells have beneficial effects in regenerative medicine in vitro, several pathophysiological conditions such as hypoxia, restricted nutrient supply, oxidative stress, and inflammation, suppress the therapeutic efficacy in vivo. Thus, to enhance stem cell functions against pathophysiological conditions, recent studies on stem cell-based therapy have adopted new technologies such as virus-mediated transduction of stem cells, gene-editing tools, optogenetics, chemogenetics, extracellular vesicles (EVs), and application of nanoparticles.109–111
Therapeutic evidences of stem cell transplantation to olfactory dysfunctions
Various cell transplantation treatments have recently been studied for the restoration of dysfunctional olfactory system (Table 3). Transplanted stem cells are more likely to secrete beneficial substances (proteins such as growth factors or exosomes) that help the survival and regeneration of target cells, rather than directly differentiating to provide new cell sources.112,113 Among other secretome molecules, neurotrophic factors enhance neuronal survival, regulate progenitor cell proliferation, and promote neurogenesis.114–116 For example, nerve growth factor (NGF) is known to transport to OE, and its presence in OE can modulate neuronal turnover.117,118 Additionally, brain-derived neurotrophic factor (BDNF) has been implicated in generating and differentiating new olfactory receptor neurons. 119 The upregulation of neurotrophic factors by the stem cells transplanted to the olfactory injury sites would thus be a promising approach for increasing the healing capacity of dysfunctional olfactory systems.
Table 3.
Stem cell therapies used to treat olfactory dysfunctions.
| Material | Application | Measured parameters | Strengths & Defects | Reference |
|---|---|---|---|---|
| BM-MSCs | TX-100 induced anosmic rat | Behavioral test (food finding test), histologic changes of olfactory epithelium, mRNA level of NGF and BDNF | - Proposed BM-MSC as new potential therapeutic modality for anosmia - Lacks mechanism study - Clinical application can be limited due to invasive direct injection method. |
Jo et al. 120 |
| TX-100 induced anosmic rat | Histological changes of olfactory epithelium, western blot of NGF | - Proposed BM-MSC as new potential therapeutic modality for anosmia - Lacks mechanism study, no behavioral test was performed - Clinical application can be limited due to invasive direct injection method. |
Kwon et al. 121 | |
| Ad-MSCs | Dichlobenil induced anosmic mice | Histologic changes of olfactory epithelium, EOG on mice olfactory mucosa | - Suggest the possibility of a future central role in regenerative medicine for ADSCs - Lacks mechanism study - Clinical application can be limited due to invasive direct injection method. - No behavioral test was performed. |
Franceschini et al. 122 |
| HSC | Dichlobenil induced anosmia mice | Histologic changes of olfactory epithelium. | - First evidence that transplanted HSCs migrating to the olfactory epithelium and contribute to epithelial restoration. - Lacks mechanism study - Clinical application can be limited due to invasive direct injection method. - No behavioral test was performed. |
Franceschini et al. 123 |
| NSCs | 3-MI induced anosmic mice | Behavioral test (food finding test), histologic analyses on olfactory epithelium, western blot of olfactory epithelium (OMP, α-tubulin) | - Evaluated both functional and histologic recovery of anosmic mice - Non-invasive (intranasal) injection of NSCs - Lacks mechanism study. |
Lee et al. 124 |
| OSCs | Ift88 gene deleted hyposmia mice | Histologic changes of olfactory epithelium, EOG on mice olfactory mucosa | - Proposed OSCs as new potential therapeutic modality for hyposmia - Non-invasive (intranasal) injection of OSCs - Recover of sensory losses in olfactory tissues - Study on human-derived OSCs need to be investigated. |
Kurtenbach et al. 125 |
Ad-MSC: adipose-derived mesenchymal stem cell; BDNF: brain derived neurotrophic factor; BM-MSC: bone marrow derived mesenchymal stem cell; EOG: electroolfactogram; HSC: human cord blood-selected CD133+ stem cell; NGF: nerve growth factor; NSC: neural stem cell; OMP: olfactory marker protein; OSC: olfactory stem cell; TX-100: triton X-100.
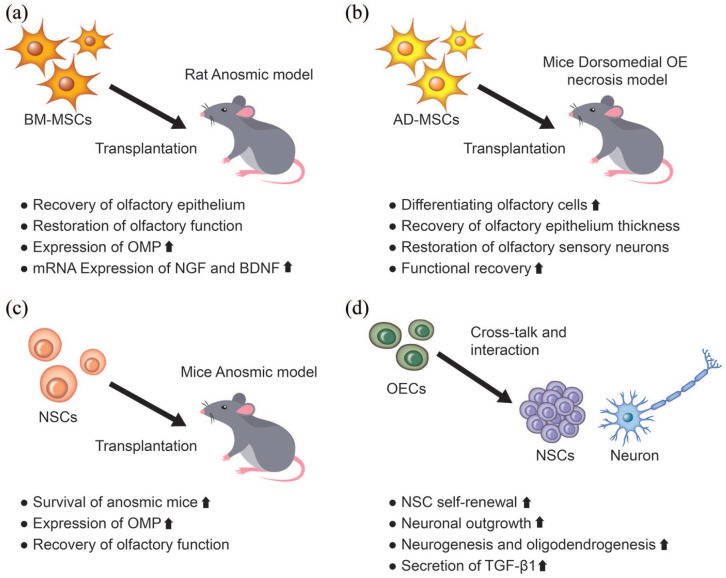
Tissue healing, including those in the olfactory system such as repairing endothelial cells and sensory neurons, entails neo-blood vessel formation and angiogenesis. MSCs are known to induce and secrete angiogenic cytokines. 126 Therefore, MSCs are an effective therapeutic cell source for the treatment of anosmia. In a recent representative study, bone marrow-derived MSCs (BM-MSCs), adipose tissue-derived MSCs (Ad-MSCs), and HSCs were transplanted into an OE degeneration rat model (Figure 4(a)). 120 The intranasal injection of BM-MSCs could augment the thickness and cellular composition of the OE to the normal level. In addition, the restoration rate of olfactory functions due to the BM-MSC injection was significantly enhanced compared to that of the control group. 120 This study also found a close relationship between the restoration of olfactory functions and the activated neurotrophic factors, including NGF and BDNF. In another study, in a rat anosmia model, the transplantation of BM-MSCs promoted the morphological restoration of the olfactory mucosa when compared to the contralateral control side. 121 Franceschini et al. 122 transplanted Ad-MSCs into immunodeficient mice with permanent damage in the dorsomedial olfactory region induced by dichlobenil inoculation (Figure 4(b)). The transplanted cells integrated into the lesioned OE and clusters of differentiated cells were observed in the epithelium. 122 In particular, there was a marked increase in the thickness of the OE and the expression of OMP in the Ad-MSC transplanted group compared to the control group.
Figure 4.
Application of stem cell transplantation in olfactory dysfunction and the regenerative effects of cell-based therapeutics.
AD-MSCs: adipose-derived mesenchymal stem cells; BDNF: brain-derived neurotrophic factor; BM-MSCs: bone marrow-derived mesenchymal stem cells; NGF: nerve growth factor; NSCs: neural stem cells; OE: olfactory epithelium; OECs: olfactory ensheathing cells; OMP: olfactory marker protein; TGF-β1: transforming growth factor beta 1.
Other types of stem cells have also shown therapeutic efficacy in restoring olfactory dysfunction. One study has shown that HSCs transplanted into injured OE of NOD-SCID mice resulted in improved neuronal recovery, with an increased expression of GAP43. 123 Lee et al. 124 investigated neural stem cells (NSCs) as a potential treatment for olfactory dysfunction (Figure 4(c)). The olfactory bulb-derived NSCs recovered olfactory function and the expression of OMP in a murine OE-injured model. Compared to the control, NSC-treated mice showed a better recovery of olfactory function in terms of the food-finding test and the expression of OMP. 124 Olfactory ensheathing cells (OECs) are a unique glial cell type that ensheathe olfactory axons into large bundles as they traverse from the lamina propria to the nerve fiber layer of the olfactory bulb. They express guidance cues and extracellular matrix molecules to assist in the growth and provide directional and tropic support to the primary neurons to reach the olfactory bulb (Figure 4(d)). 127 A recent study revealed that OECs secrete transforming growth factor β1 (TGF-β1), which can increase their phagocytic activity by regulating integrin/milk fat globule-epidermal growth factor (EGF) factor 8 (MFG-E8) protein signaling pathway. 128
Discussion and outlook of stem cell-based therapies
As noted, stem cell transplantation to the dysfunctional olfactory system is effective with convincing evidence. However, a couple of issues remain that need to be addressed. First, the cells considered as the stem cells of the olfactory nerve epithelium have not been accurately identified in the molecular biology context, which makes it difficult to determine the differentiated fate of transplanted stem cells and their possible specific roles. Second, it is also unclear what kinds of biochemical factors are essentially involved in the differentiation of stem cells into olfactory-specific cells, complicating the identification of the decisive role of secreted molecules from the transplanted cells. In addition, the effects of injection route, injection dose, number of administrations, and source of stem cells used for olfactory dysfunction, have not been well examined, requiring more systematic studies in the area. Given that these issues are addressed in the future, we may identify the fate of stem cells transplanted (whether they are differentiated or not, and if so, what fraction and which lineage cells would be) and the mechanisms underlying the differentiation into specific cell types that are needed for the recovery of dysfunctional olfactory tissues.
As the surrounding matrix influences stem cell properties, such as survival and differentiation capacity, it is highly recommended to use biomaterials and scaffolds for stem cell delivery. Many hydrogels and 3D scaffold systems for the delivery of stem cells have been developed with tunable physico-mechano-chemical properties such as stiffness, dynamic mechanical properties (e.g. stress relaxation), ligand type, and density.129–132 Depending on these extracellular matrix properties, the biological fate of stem cells can be modulated. For example, when MSCs were cultured in stress-relaxing hydrogels, their differentiation into osteogenic or chondrogenic lineage was significantly enhanced compared to those in non-stress-relaxing hydrogels,133,134 implying the importance of dynamic mechanical properties of hydrogels in cell fate determination. In addition, the ligand type of 3D scaffolds primarily determined the lineage differentiation of NSCs,135–137 demonstrating the difference in initial cellular perception of the extracellular matrix ligand governing the intracellular signaling that leads to altered cell fate. Therefore, future studies on stem cell delivery to the olfactory system are needed that use the hydrogels and 3D scaffolds that are designed to specifically stimulate the cells to induce secretome and/or differentiate to the cells helpful for the recovery of dysfunctional olfactory tissues.
Although here we focused on the delivery of stem cells, their secretome such as extracellular vesicles (EVs), are considered to be of utmost importance as an alternative to stem cells because of their merits over living cells, including delivery to target specific cell types, less immune responses, stabilization in the body, and the ability to contain drugs.111,138 Some of the applications of EVs to dysfunctional tissues such as kidney diseases, cardiovascular diseases, bone defects, fibrosis, stroke, and spinal cord injuries, have shown the therapeutic efficacy of EVs to levels comparable to those treated with conventional stem cell delivery approaches.111,138–140 The applications of stem cell-based therapies for the dysfunctional olfactory system are still in infancy; thus, future strategies need to harness the new technologies that progress in scaffold development and EV biogenesis, in order to potentiate the cellular capacity for regenerating the dysfunctional olfactory tissues.
Footnotes
Authorship: Shin Hyuk Yoo and Hae-Won Kim contributed to the concept of the work, data acquisition, and drafting of the manuscript and revised the article critically for important intellectual content. Jun Hee Lee designed the study, drafted the manuscript, procured funding, and supervised the study.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present research was supported by the research fund of Dankook University in 2020.
ORCID iDs: Hae-Won Kim  https://orcid.org/0000-0001-6400-6100
https://orcid.org/0000-0001-6400-6100
Jun Hee Lee  https://orcid.org/0000-0002-2026-1406
https://orcid.org/0000-0002-2026-1406
References
- 1. Hoffman HJ, Rawal S, Li CM, et al. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord 2016; 17(2): 221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kern DW, Wroblewski KE, Schumm LP, et al. Olfactory function in wave 2 of the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci 2014; 69(Suppl 2): S134–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinto JM, Wroblewski KE, Kern DW, et al. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One 2014; 9: e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santos DV, Reiter ER, DiNardo LJ, et al. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg 2004; 130: 317–319. [DOI] [PubMed] [Google Scholar]
- 5. Bonfils P, Faulcon P, Tavernier L, et al. Home accidents associated with anosmia. Presse Med 2008; 37: 742–745. [DOI] [PubMed] [Google Scholar]
- 6. Miwa T, Furukawa M, Tsukatani T, et al. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg 2001; 127: 497–503. [DOI] [PubMed] [Google Scholar]
- 7. Min YG, Kim JW, Hong SC, et al. Pathogenetic mechanism of olfactory cell injury after exposure to sulfur dioxide in mice. Laryngoscope 2003; 113: 2157–2162. [DOI] [PubMed] [Google Scholar]
- 8. Graziadei PP, Monti Graziadei AG. Regeneration in the olfactory system of vertebrates. Am J Otolaryngol 1983; 4: 228–233. [DOI] [PubMed] [Google Scholar]
- 9. Holbrook EH, Leopold DA. An updated review of clinical olfaction. Curr Opin Otolaryngol Head Neck Surg 2006; 14: 23–28. [DOI] [PubMed] [Google Scholar]
- 10. Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol 2004; 469(4): 457–474. [DOI] [PubMed] [Google Scholar]
- 11. Isaacson JS. Odor representations in mammalian cortical circuits. Curr Opin Neurobiol 2010; 20(3): 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damm M, Temmel A, Welge-Lüssen A, et al. Olfactory dysfunctions. Epidemiology and therapy in Germany, Austria and Switzerland. HNO 2004; 52: 112–120. [DOI] [PubMed] [Google Scholar]
- 13. Nordin S, Brämerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol 2008; 8: 10–15. [DOI] [PubMed] [Google Scholar]
- 14. Wrobel BB, Leopold DA. Clinical assessment of patients with smell and taste disorders. Otolaryngol Clin North Am 2004; 37: 1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beites CL, Kawauchi S, Crocker CE, et al. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res 2005; 306: 309–316. [DOI] [PubMed] [Google Scholar]
- 16. Daramola OO, Becker SS. An algorithmic approach to the evaluation and treatment of olfactory disorders. Curr Opin Otolaryngol Head Neck Surg 2015; 23: 8–14. [DOI] [PubMed] [Google Scholar]
- 17. Holbrook EH, Leopold DA, Schwob JE. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope 2005; 115: 2144–2154. [DOI] [PubMed] [Google Scholar]
- 18. Yee KK, Pribitkin EA, Cowart BJ, et al. Neuropathology of the olfactory mucosa in chronic rhinosinusitis. Am J Rhinol Allergy 2010; 24: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seiden AM, Duncan HJ, Smith DV. Office management of taste and smell disorders. Otolaryngol Clin North Am 1992; 25: 817–835. [PubMed] [Google Scholar]
- 20. Lotsch J, Reither N, Bogdanov V, et al. A brain-lesion pattern based algorithm for the diagnosis of posttraumatic olfactory loss. Rhinol J 2015; 53: 365–370. [DOI] [PubMed] [Google Scholar]
- 21. Konstantinidis I, Tsakiropoulou E, Bekiaridou P, et al. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope 2013; 123: E85–E90. [DOI] [PubMed] [Google Scholar]
- 22. Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum Allergy Rhinol 2016; 6: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope 2014; 124: 826–831. [DOI] [PubMed] [Google Scholar]
- 24. Hudson R, Distel H. Induced peripheral sensitivity in the developing vertebrate olfactory system. Ann N Y Acad Sci 1998; 855: 109–115. [DOI] [PubMed] [Google Scholar]
- 25. Hummel T, Rissom K, Reden J, et al. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009; 119: 496–499. [DOI] [PubMed] [Google Scholar]
- 26. Kollndorfer K, Kowalczyk K, Hoche E, et al. Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast 2014; 2014: 140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haehner A, Tosch C, Wolz M, et al. Olfactory training in patients with Parkinson’s disease. PLoS One 2013; 8: e61680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jafek BW, Moran DT, Eller PM, et al. Steroid-dependent anosmia. Arch Otolaryngol Head Neck Surg 1987; 113: 547–549. [DOI] [PubMed] [Google Scholar]
- 29. Banglawala SM, Oyer SL, Lohia S, et al. Olfactory outcomes in chronic rhinosinusitis with nasal polyposis after medical treatments: a systematic review and meta-analysis. Int Forum Allergy Rhinol 2014; 4: 986–994. [DOI] [PubMed] [Google Scholar]
- 30. Stuck BA, Blum A, Hagner AE, et al. Mometasone furoate nasal spray improves olfactory performance in seasonal allergic rhinitis. Allergy 2003; 58: 1195. [DOI] [PubMed] [Google Scholar]
- 31. Klimek L, Poletti SC, Sperl A, et al. Olfaction in patients with allergic rhinitis: an indicator of successful MP-AzeFlu therapy. Int Forum Allergy Rhinol 2017; 7: 287–292. [DOI] [PubMed] [Google Scholar]
- 32. Blomqvist EH, Lundblad L, Bergstedt H, et al. Placebo-controlled, randomized, double-blind study evaluating the efficacy of fluticasone propionate nasal spray for the treatment of patients with hyposmia/anosmia. Acta Otolaryngol 2003; 123: 862–868. [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi M, Costanzo RM. Olfactory nerve recovery following mild and severe injury and the efficacy of dexamethasone treatment. Chem Senses 2009; 34: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim BY, Park JY, Kim E. Differences in mechanisms of steroid therapy and olfactory training for olfactory loss in mice. Am J Rhinol Allergy 2020; 34: 810–821. [DOI] [PubMed] [Google Scholar]
- 35. Jiang RS, Wu SH, Liang KL, et al. Steroid treatment of posttraumatic anosmia. Eur Arch Otorhinolaryngol 2010; 267: 1563–1567. [DOI] [PubMed] [Google Scholar]
- 36. Fujii M, Fukazawa K, Takayasu S, et al. Olfactory dysfunction in patients with head trauma. Auris Nasus Larynx 2002; 29: 35–40. [DOI] [PubMed] [Google Scholar]
- 37. Ikeda K, Sakurada T, Takasaka T, et al. Anosmia following head trauma: preliminary study of steroid treatment. Tohoku J Exp Med 1995; 177: 343–351. [DOI] [PubMed] [Google Scholar]
- 38. Dilisio MF. Osteonecrosis following short-term, low-dose oral corticosteroids: a population-based study of 24 million patients. Orthopedics 2014; 37: e631–e636. [DOI] [PubMed] [Google Scholar]
- 39. Flynn MD, Beasley P, Tooke JE. Adrenal suppression with intranasal betamethasone drops. J Laryngol Otol 1992; 106: 827–828. [DOI] [PubMed] [Google Scholar]
- 40. Hummel T, Whitcroft KL, Rueter G, et al. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur Arch Otorhinolaryngol 2017; 274: 2819–2825. [DOI] [PubMed] [Google Scholar]
- 41. Douma TN, Borre Y, Hendriksen H, et al. Simvastatin improves learning and memory in control but not in olfactory bulbectomized rats. Psychopharmacology 2011; 216: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim HY, Kim JH, Dhong HJ, et al. Effects of statins on the recovery of olfactory function in a 3-methylindole–induced anosmia mouse model. Am J Rhinol Allergy 2012; 26: e81–e84. [DOI] [PubMed] [Google Scholar]
- 43. Lv L, Han X, Sun Y, et al. Valproic acid improves locomotion in vivo after SCI and axonal growth of neurons in vitro. Exp Neurol 2012; 233: 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogawa T, Takezawa K, Shimizu S, et al. Valproic acid promotes neural regeneration of olfactory epithelium in adult mice after methimazole-induced damage. Am J Rhinol Allergy 2014; 28: e95–e99. [DOI] [PubMed] [Google Scholar]
- 45. Iwata J, Hosokawa R, Sanchez-Lara PA, et al. Transforming growth factor-beta regulates basal transcriptional regulatory machinery to control cell proliferation and differentiation in cranial neural crest-derived osteoprogenitor cells. J Biol Chem 2010; 285: 4975–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishikawa T, Doi K, Ochi N, et al. Effect of intranasal administration of basic fibroblast growth factor on olfactory epithelium. Neuroreport 2009; 20: 764–769. [DOI] [PubMed] [Google Scholar]
- 47. Nota J, Takahashi H, Hakuba N, et al. Treatment of neural anosmia by topical application of basic fibroblast growth factor-gelatin hydrogel in the nasal cavity: an experimental study in mice. Otolaryngol Head Neck Surg 2013; 139: 396–400. [DOI] [PubMed] [Google Scholar]
- 48. Behnke O, Forer A. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur J Haematol Suppl 1998; 61: 3–23. [DOI] [PubMed] [Google Scholar]
- 49. Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent 2004; 13: 301–309. [DOI] [PubMed] [Google Scholar]
- 50. Cho HH, Jang S, Lee SC, et al. Effect of neural-induced mesenchymal stem cells and platelet-rich plasma on facial nerve regeneration in an acute nerve injury model. Laryngoscope 2010; 120: 907–913. [DOI] [PubMed] [Google Scholar]
- 51. Farrag TY, Lehar M, Verhaegen P, et al. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope 2007; 117: 157–165. [DOI] [PubMed] [Google Scholar]
- 52. Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents 2012; 26: 3S–22S. [PubMed] [Google Scholar]
- 53. Yasak AG, Yigit O, Araz Server E, et al. The effectiveness of platelet-rich plasma in an anosmia-induced mice model. Laryngoscope 2018; 128: E157–E162. [DOI] [PubMed] [Google Scholar]
- 54. Ramanathan S, Archunan G, Sivakumar M, et al. Theranostic applications of nanoparticles in neurodegenerative disorders. Int J Nanomedicine 2018; 13: 5561–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Flores AM, Ye J, Jarr KU, et al. Nanoparticle Therapy for Vascular Diseases. Arterioscler Thromb Vasc Biol 2019; 39: 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitchell MJ, Billingsley MM, Haley RM, et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021; 20: 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ekladious I, Colson YL, Grinstaff MW. Polymer-drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov 2019; 18: 273–294. [DOI] [PubMed] [Google Scholar]
- 58. Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 2010; 9: 615–627. [DOI] [PubMed] [Google Scholar]
- 59. Merkus FW, Verhoef JC, Schipper NG, et al. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev 1998; 29: 13–38. [DOI] [PubMed] [Google Scholar]
- 60. Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr Top Otorhinolaryngol Head Neck Surg 2010; 9: Doc07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eichner H, Behbehani AA, Hochstrasser K. Diagnostic value of nasal secretions, current state: normal values. 1. Laryngol Rhinol Otol 1983; 62: 561–565. [PubMed] [Google Scholar]
- 62. Sosnik A, das Neves J, Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog Polym Sci 2014; 39: 2030–2075. [Google Scholar]
- 63. Casettari L, Illum L. Chitosan in nasal delivery systems for therapeutic drugs. J Control Release 2014; 190: 189–200. [DOI] [PubMed] [Google Scholar]
- 64. Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta 2009; 1788: 892–910. [DOI] [PubMed] [Google Scholar]
- 65. Ramalingam P, Yoo SW, Ko YT. Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res Intern 2016; 84: 113–119. [Google Scholar]
- 66. Kumar M, Pandey RS, Patra KC, et al. Evaluation of neuropeptide loaded trimethyl chitosan nanoparticles for nose to brain delivery. Int J Biol Macromol 2013; 61: 189–195. [DOI] [PubMed] [Google Scholar]
- 67. Salade L, Wauthoz N, Deleu M, et al. Development of coated liposomes loaded with ghrelin for nose-to-brain delivery for the treatment of cachexia. Int J Nanomedicine 2017; 12: 8531–8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Clementino A, Batger M, Garrastazu G, et al. The nasal delivery of nanoencapsulated statins - an approach for brain delivery. Int J Nanomedicine 2016; 11: 6575–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sonvico F, Clementino A, Buttini F, et al. Surface-modified nanocarriers for Nose-to-Brain delivery: from bioadhesion to targeting. Pharmaceutics 2018; 10: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li C, Liu Z, Yan X, et al. Mucin-controlled drug release from mucoadhesive phenylboronic acid-rich nanoparticles. Int J Pharm 2015; 479: 261–264. [DOI] [PubMed] [Google Scholar]
- 71. Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. Int J Pharm 2013; 453: 56–64. [DOI] [PubMed] [Google Scholar]
- 72. Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev 2009; 61: 75–85. [DOI] [PubMed] [Google Scholar]
- 73. Vila A, Gill H, McCallion O, et al. Transport of PLA-PEG particles across the nasal mucosa: effect of particle size and PEG coating density. J Control Release 2004; 98(2): 231–244. [DOI] [PubMed] [Google Scholar]
- 74. Lai SK, Suk JS, Pace A, et al. Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials 2011; 32: 6285–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee M, Park CG, Huh BK, et al. Sinonasal delivery of resveratrol via mucoadhesive nanostructured microparticles in a nasal polyp mouse model. Sci Rep 2017; 7: 40249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sekerdag E, Lüle S, Bozdağ Pehlivan S, et al. A potential non-invasive glioblastoma treatment: nose-to-brain delivery of farnesylthiosalicylic acid incorporated hybrid nanoparticles. J Control Release 2017; 261: 187–198. [DOI] [PubMed] [Google Scholar]
- 77. Matsuyama T, Morita T, Horikiri Y, et al. Improved nasal absorption of salmon calcitonin by powdery formulation with N-acetyl-L-cysteine as a mucolytic agent. J Control Release 2006; 115: 183–188. [DOI] [PubMed] [Google Scholar]
- 78. Henke MO, Ratjen F. Mucolytics in cystic fibrosis. Paediatr Respir Rev 2007; 8: 24–29. [DOI] [PubMed] [Google Scholar]
- 79. Singh S. Nanomaterials exhibiting enzyme-like properties (Nanozymes): Current advances and future perspectives. Front Chem 2019; 7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hyun JK, Kim HW. Clinical and experimental advances in regeneration of spinal cord injury. J Tissue Eng 2010; 2010: 650857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Niu J, Wang K, Kolattukudy PE. Cerium oxide nanoparticles inhibit oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J Pharmacol Exp Ther 2011; 338: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pagliari F, Mandoli C, Forte G, et al. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano 2012; 6: 3767–3775. [DOI] [PubMed] [Google Scholar]
- 83. Li S, Jiang D, Ehlerding EB, et al. Intrathecal administration of nanoclusters for protecting neurons against oxidative stress in cerebral ischemia/reperfusion injury. ACS Nano 2019; 13: 13382–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tural K, Ozden O, Bilgi Z, et al. The protective effect of betanin and copper on spinal cord ischemia-reperfusion injury. J Spinal Cord Med 2021; 44: 704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mosselhy DA, Virtanen J, Kant R, et al. COVID-19 pandemic: What about the safety of anti-coronavirus nanoparticles? Nanomater 2021; 11: 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 2021; 24: 168–175. [DOI] [PubMed] [Google Scholar]
- 87. Yamagishi M, Fujiwara M, Nakamura H. Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology 1994; 32(3): 113–118. [PubMed] [Google Scholar]
- 88. Agrawal M, Saraf S, Saraf S, et al. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J Control Release 2020; 321: 372–415. [DOI] [PubMed] [Google Scholar]
- 89. Feng Y, He H, Li F, et al. An update on the role of nanovehicles in nose-to-brain drug delivery. Drug Discov Today 2018; 23: 1079–1088. [DOI] [PubMed] [Google Scholar]
- 90. Ugwoke MI, Agu RU, Verbeke N, et al. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev 2005; 57: 1640–1665. [DOI] [PubMed] [Google Scholar]
- 91. Chen D, Xia D, Li X, et al. Comparative study of Pluronic® F127-modified liposomes and chitosan-modified liposomes for mucus penetration and oral absorption of cyclosporine A in rats. Int J Pharm 2013; 449: 1–9. [DOI] [PubMed] [Google Scholar]
- 92. Li ST, Young TH, Huang TW. Regeneration of olfactory neuroepithelium in 3-methylindole-induced anosmic rats treated with intranasal chitosan. Biomaterials 2021; 271: 120738. [DOI] [PubMed] [Google Scholar]
- 93. Wang X, He H, Leng W, et al. Evaluation of brain-targeting for the nasal delivery of estradiol by the microdialysis method. Int J Pharm 2006; 317: 40–46. [DOI] [PubMed] [Google Scholar]
- 94. Fazil M, Md S, Haque S, et al. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci 2012; 47: 6–15. [DOI] [PubMed] [Google Scholar]
- 95. Alam S, Khan ZI, Mustafa G, et al. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int J Nanomedicine 2012; 7: 5705–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Md S, Khan RA, Mustafa G, et al. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur J Pharm Sci 2013; 48: 393–405. [DOI] [PubMed] [Google Scholar]
- 97. Raj R, Wairkar S, Sridhar V, et al. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: development, characterization and in vivo anti-Parkinson activity. Int J Biol Macromol 2018; 109: 27–35. [DOI] [PubMed] [Google Scholar]
- 98. Jafarieh O, Md S, Ali M, et al. Design, characterization, and evaluation of intranasal delivery of ropinirole-loaded mucoadhesive nanoparticles for brain targeting. Drug Dev Ind Pharm 2015; 41: 1674–1681. [DOI] [PubMed] [Google Scholar]
- 99. Javia A, Thakkar H. Intranasal delivery of tapentadol hydrochloride-loaded chitosan nanoparticles: formulation, characterisation and its in vivo evaluation. J Microencapsul 2017; 34: 644–658. [DOI] [PubMed] [Google Scholar]
- 100. Patel D, Naik S, Chuttani K, et al. Intranasal delivery of cyclobenzaprine hydrochloride-loaded thiolated chitosan nanoparticles for pain relief. J Drug Target 2013; 21: 759–769. [DOI] [PubMed] [Google Scholar]
- 101. Singh D, Rashid M, Hallan SS, et al. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif Cells Nanomed Biotechnol 2016; 44: 865–877. [DOI] [PubMed] [Google Scholar]
- 102. Chalikwar SS, Mene BS, Pardeshi CV, et al. Self-assembled, chitosan grafted PLGA nanoparticles for intranasal delivery: design, development and ex vivo characterization. Polym Plast Technol Eng 2013; 52: 368–380. [Google Scholar]
- 103. Sharma D, Sharma RK, Sharma N, et al. Nose-To-Brain delivery of PLGA-diazepam nanoparticles. AAPS PharmSciTech 2015; 16: 1108–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sharma D, Sharma RK, Bhatnagar A, et al. Nose to brain delivery of midazolam loaded PLGA nanoparticles: in vitro and in vivo investigations. Curr Drug Deliv 2016; 13: 557–564. [DOI] [PubMed] [Google Scholar]
- 105. Singletary M, Hagerty S, Muramoto S, et al. PEGylation of zinc nanoparticles amplifies their ability to enhance olfactory responses to odorant. PLoS One 2017; 12: e0189273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jain R, Nabar S, Dandekar P, et al. Micellar nanocarriers: potential nose-to-brain delivery of zolmitriptan as novel migraine therapy. Pharm Res 2010; 27: 655–664. [DOI] [PubMed] [Google Scholar]
- 107. Jain R, Nabar S, Dandekar P, et al. Formulation and evaluation of novel micellar nanocarrier for nasal delivery of sumatriptan. Nanomed 2010; 5: 575–587. [DOI] [PubMed] [Google Scholar]
- 108. Bhatt R, Singh D, Prakash A, et al. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv 2015; 22: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kimbrel EA, Lanza R. Next-generation stem cells — ushering in a new era of cell-based therapies. Nat Rev Drug Discov 2020; 19: 463–479. [DOI] [PubMed] [Google Scholar]
- 110. Pinho S, Macedo MH, Rebelo C, et al. Stem cells as vehicles and targets of nanoparticles. Drug Discov Today 2018; 23: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 111. Lee JH, Yoon JY, Lee JH, et al. Emerging biogenesis technologies of extracellular vesicles for tissue regenerative therapeutics. J Tissue Eng 2021; 12: 20417314211019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Teixeira AI, Duckworth JK, Hermanson O. Getting the right stuff: controlling neural stem cell state and fate in vivo and in vitro with biomaterials. Cell Res 2007; 17: 56–61. [DOI] [PubMed] [Google Scholar]
- 113. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007; 213: 341–347. [DOI] [PubMed] [Google Scholar]
- 114. Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 2002; 59: 514–523. [DOI] [PubMed] [Google Scholar]
- 115. Lu D, Mahmood A, Chopp M. Biologic transplantation and neurotrophin-induced neuroplasticity after traumatic brain injury. J Head Trauma Rehabil 2003; 18: 357–376. [DOI] [PubMed] [Google Scholar]
- 116. Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma 2004; 21: 33–39. [DOI] [PubMed] [Google Scholar]
- 117. Miwa T, Uramoto N, Ishimaru T, et al. Retrograde transport of nerve growth factor from olfactory bulb to olfactory epithelium. Neuroreport 1998; 9: 153–155. [DOI] [PubMed] [Google Scholar]
- 118. Aiba T, Mori J, Nakai Y. Nerve growth factor (NGF) and its receptor in rat olfactory epithelium. Acta Otolaryngol 1993; 113: 37–40. [DOI] [PubMed] [Google Scholar]
- 119. Lindsay RM. Neurotrophic growth factors and neurodegenerative diseases: therapeutic potential of the neurotrophins and ciliary neurotrophic factor. Neurobiol Aging 1994; 15(2): 249–251. [DOI] [PubMed] [Google Scholar]
- 120. Jo H, Jung M, Seo DJ, et al. The effect of rat bone marrow derived mesenchymal stem cells transplantation for restoration of olfactory disorder. Biochem Biophys Res Commun 2015; 467: 395–399. [DOI] [PubMed] [Google Scholar]
- 121. Kwon JW, Jo HG, Park SM, et al. Engraftment and regenerative effects of bone marrow stromal cell transplantation on damaged rat olfactory mucosa. Eur Arch Otorhinolaryngol 2016; 273: 2585–2590. [DOI] [PubMed] [Google Scholar]
- 122. Franceschini V, Bettini S, Pifferi S, et al. Transplanted human adipose tissue-derived stem cells engraft and induce regeneration in mice olfactory neuroepithelium in response to dichlobenil subministration. Chem Senses 2014; 39(7): 617–629. [DOI] [PubMed] [Google Scholar]
- 123. Franceschini VBS, Saccardi R, Revoltella RP. Stem cell transplantation supports the repair of injured olfactory neuroepithelium after permanent lesion. In: Baharvand H. (ed.) Trends in Stem Cell Biology and Technology. Totowa, NJ: Humana Press, 2009, pp.283–297. [Google Scholar]
- 124. Lee CH, Jeon SW, Seo BS, et al. Transplantation of neural stem cells in anosmic mice. Clin Exp Otorhinolaryngol 2010; 3: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kurtenbach S, Goss GM, Goncalves S, et al. Cell-based therapy restores olfactory function in an inducible model of hyposmia. Stem Cell Reports 2019; 12: 1354–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Joyce N, Annett G, Wirthlin L, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 2010; 5: 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cao L, Mu L, Qiu Y, et al. Diffusible, membrane-bound, and extracellular matrix factors from olfactory ensheathing cells have different effects on the self-renewing and differentiating properties of neural stem cells. Brain Res 2010; 1359: 56–66. [DOI] [PubMed] [Google Scholar]
- 128. Li Y, Zou T, Xue L, et al. TGF-β1 enhances phagocytic removal of neuron debris and neuronal survival by olfactory ensheathing cells via integrin/MFG-E8 signaling pathway. Mol Cell Neurosci 2017; 85: 45–56. [DOI] [PubMed] [Google Scholar]
- 129. Hong KH, Kim YM, Song SC. Fine-tunable and injectable 3D hydrogel for On-Demand Stem Cell Niche. Adv Sci 2019; 6: 1900597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Moore CA, Shah NN, Smith CP, et al. 3D bioprinting and stem cells. Methods Mol Biol 2018; 1842: 93–103. [DOI] [PubMed] [Google Scholar]
- 131. Zhang C, Yang Z, Dong DL, et al. 3D culture technologies of cancer stem cells: promising ex vivo tumor models. J Tissue Eng 2020; 11: 2041731420933407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Seo SJ, Mahapatra C, Singh RK, et al. Strategies for osteochondral repair: Focus on scaffolds. J Tissue Eng 2014; 5: 2041731414541850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chaudhuri O, Gu L, Klumpers D, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 2016; 15: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Re F, Sartore L, Moulisova V, et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J Tissue Eng 2019; 10: 2041731419845852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Matai I, Kaur G, Seyedsalehi A, et al. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020; 226: 119536. [DOI] [PubMed] [Google Scholar]
- 136. Koffler J, Zhu W, Qu X, et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med 2019; 25: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rider P, Kačarević ŽP, Alkildani S, et al. Bioprinting of tissue engineering scaffolds. J Tissue Eng 2018; 9: 2041731418802090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tang TT, Lv LL, Lan HY, et al. Extracellular vesicles: Opportunities and challenges for the treatment of renal diseases. Front Physiol 2019; 10: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Liu Y, Holmes C. Tissue regeneration capacity of extracellular vesicles isolated from bone marrow-derived and adipose-derived mesenchymal stromal/stem cells. Front Cell Dev Biol 2021; 9: 648098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Alqurashi H, Ortega Asencio I, Lambert DW. The emerging potential of extracellular vesicles in cell-free tissue engineering and regenerative medicine. Tissue Eng Part B Rev 2021; 27: 530–538. [DOI] [PubMed] [Google Scholar]