This population-based cohort study examines patterns in tumor thickness–specific incidence in patients with cutaneous melanoma.
Key Points
Question
What patterns exist in the thickness-specific incidence of cutaneous melanoma in the US?
Findings
This population-based cohort study of 187 487 patients diagnosed with invasive cutaneous melanoma in the US between 2010 and 2018 found that overall and thinner melanoma incidence rates have stabilized, whereas the incidence of the thickest melanomas continued to increase. Individuals with lower socioeconomic status and members of minority groups were more likely to be diagnosed with thicker tumors.
Meaning
Findings of this study suggest that the incidence of the thickest cutaneous melanomas increased from 2010 to 2018, in contrast with thinner melanomas, and that overall melanoma incidence rates have potentially stabilized in the US after nearly a century of increase.
Abstract
Importance
The recent incidence of cutaneous melanoma of different thicknesses in the US is not well described.
Objective
To evaluate recent patterns in the incidence of melanoma by tumor thickness and examine associations of sex, race and ethnicity, and socioeconomic status with melanoma thickness-specific incidence.
Design, Setting, and Participants
This population-based cohort study analyzed data for 187 487 patients with a new diagnosis of invasive cutaneous melanoma from the Surveillance, Epidemiology, and End Results Registry from January 1, 2010, to December 31, 2018. The study was conducted from May 27 to December 29, 2021. Data were analyzed from June 21 to October 24, 2021.
Main Outcomes and Measures
Age-adjusted incidence rates of melanoma were calculated by tumor thickness (categorized by Breslow thickness) and annual percentage change (APC) in incidence rates. Analyses were stratified by sex and race and ethnicity. The associations with socioeconomic status were evaluated in 134 359 patients diagnosed with melanoma from 2010 to 2016.
Results
This study included 187 487 patients with a median (IQR) age of 62 (52-72) years and 58.4% men. Melanoma incidence was higher in men compared with women across all tumor thickness groups. Individuals in lower socioeconomic status quintiles and members of minority groups were more likely to be diagnosed with thicker (T4) tumors (20.7% [169 of 816] among non-Hispanic Black patients, 11.2% [674 of 6042] among Hispanic patients, and 6.3% [10 774 of 170 155] among non-Hispanic White patients). Between 2010 and 2018, there was no significant increase in incidence of cutaneous melanoma across the full population (APC, 0.39%; 95% CI, –0.40% to 1.18%). The incidence of the thickest melanomas (T4, >4.0 mm) increased between 2010 and 2018, with an APC of 3.32% (95% CI, 2.06%-4.60%) overall, 2.50% (95% CI, 1.27%-3.73%) in men, and 4.64% (95% CI, 2.56%-6.75%) in women.
Conclusions and Relevance
In this population-based cohort study, the incidence of the thickest cutaneous melanoma tumors increased from 2010 to 2018, in contrast with the incidence patterns for thinner melanomas. The findings suggest potential stabilization of overall melanoma incidence rates in the US after nearly a century of continuous increase in incidence. Patients with low socioeconomic status and Hispanic patients were more likely to be diagnosed with thick melanoma. The continued rise in incidence of thick melanoma is unlikely to be attributable to overdiagnosis given the stability of thin melanoma rates.
Introduction
Melanoma is the fifth most common cause of cancer in the US, with more than 106 000 expected new cases and 7000 estimated deaths in 2021.1 Consistent increases in melanoma incidence documented since the 1930s show that melanoma incidence is now 6 times as high as it was 40 years ago.2 It is unclear if this rising incidence is attributable to truly increased disease occurrence or overdiagnosis from frequent skin checks, biopsy results, and shifts in histopathologic criteria.2 Tumor thickness is a crucial risk factor in melanoma, with a 10-year survival of 75% for the thickest (T4, >4.0 mm) compared with 98% for the thinnest (T1, ≤1.0 mm) tumors.3 Understanding incidence patterns by thickness, sex, race and ethnicity, and socioeconomic status is important in quantifying the true burden of melanoma in the US.
Melanoma incidence patterns by tumor thickness were formerly investigated through 2009.4 We investigated thickness-specific patterns in melanoma incidence by sex, race and ethnicity, and socioeconomic status.
Methods
In this population-based cohort study, histologically confirmed cases of first primary invasive cutaneous melanoma (International Classification of Diseases for Oncology, Third Edition codes 8720-8790 and topographic codes C44.0-C44.9) with malignant behavior were identified using data from the Surveillance, Epidemiology, and End Results (SEER) Registry for 18 population-based registries from January 1, 2010, to December 31, 2018. The study was conducted from May 27 to December 29, 2021. Data were analyzed from June 21 to October 24, 2021. Four categories of Breslow thickness were distinguished: T1, 1.0 mm or less; T2, greater than 1.0 to 2.0 mm; T3, greater than 2.0 to 4.0 mm; and T4, greater than 4.0 mm. Patient characteristics included sex, age, self-reported race and ethnicity, SEER summary stage, and histologic subtype. Area-level socioeconomic status defined by the Yost Index,5 a composite socioeconomic status measure based on Census tract–level information, was available from 2010 to 2016 across all registries except Alaska. Socioeconomic status was classified into quintiles, with Q1 as the lowest socioeconomic status and Q5 as the highest socioeconomic status. Annual incidence rates per 100 000 person-years were age adjusted to the 2000 US standard population. Patterns in incidence rate were examined using annual percentage change (APC) calculated using the weighted least-squares method and 1-year percentage change. Sensitivity analyses for assessing the implications of unknown thickness are outlined in eMethods 1, eResults, and eFigures 1 through 3 in the Supplement. Statistical calculations used SEER*Stat, version 8.3.9 (Surveillance Research Program, National Cancer Institute). Further methods are described in eMethods 2 and 3 in the Supplement. The Stanford Institutional Review Board deemed this study exempt from review and waived the requirement for patient informed consent because only deidentified data were used.
Results
We identified 187 487 patients with newly diagnosed cutaneous melanoma from 2010 to 2018 (Table 1; eTable 1 in the Supplement). The patients had a median (IQR) age of 62 (52-72) years and included 109 500 (58.4%) men and 77 987 (41.6%) women, with 6042 (3.2%) Hispanic, 430 (0.2%) non-Hispanic American Indian or Alaska Native, 1145 (0.6%) non-Hispanic Asian or Pacific Islander, 816 (0.4%) non-Hispanic Black, and 170 155 (90.8%) non-Hispanic White patients (8899 patients [4.7%] were of unknown non-Hispanic race). Most patients, 75.1%, had thinner melanomas (T1 tumors, 116 952 [62.4%]; T2 tumors, 23 768 [12.7%]), 14.3% had thicker (T3 tumors, 14 728 [7.9%], T4 tumors, 11 928 [6.4%]), and 10.7% (20 111) had tumors of unknown thickness. Melanoma incidence was higher in men compared with women across all thickness groups. Patients from racial and ethnic minority groups were more likely to be diagnosed with the thickest (T4) melanomas (169 of 816 [20.7%] among non-Hispanic Black patients, 674 of 6042 [11.2%] among Hispanic patients) compared with non-Hispanic White patients (10 774 of 170 155 [6.3%]). Non-Hispanic White patients were more likely to be diagnosed with thin (T1) melanoma (106 099 of 170 155 [62.4%]) compared with Black patients (217 of 816 [26.6%]). Socioeconomic status data were available for 134 359 patients with melanoma. Individuals in lower socioeconomic status quintiles were more likely to have T4 melanoma compared with those in higher socioeconomic status quintiles (1157 of 11 304 [10.2%] in Q1 vs 1973 of 43 023 [4.6%] in Q5).
Table 1. Characteristics of Patients With Cutaneous Melanoma by Tumor Thickness From 2010 to 2018.
| Characteristic | No. (%) | Total (%) (n = 187 487) | ||||
|---|---|---|---|---|---|---|
| T1 tumors (n = 116 952)a | T2 tumors (n = 23 768)a | T3 tumors (n = 14 728)a | T4 tumors (n = 11 928)a | Tumors of unknown thickness (n = 20 111) | ||
| Sex | ||||||
| Men | 65 703 (56.2) | 14 176 (59.6) | 9459 (64.2) | 7776 (65.2) | 12 386 (61.6) | 109 500 (58.4) |
| Women | 51 249 (43.8) | 9592 (40.4) | 5269 (35.8) | 4152 (34.8) | 7725 (38.4) | 77 987 (41.6) |
| Race and ethnicity | ||||||
| Hispanic | 2996 (2.6) | 812 (3.4) | 643 (4.4) | 674 (5.7) | 917 (4.6) | 6042 (3.2) |
| Non-Hispanic American Indian or Alaska Native | 230 (0.2) | 60 (0.3) | 46 (0.3) | 40 (0.3) | 54 (0.3) | 430 (0.2) |
| Non-Hispanic Asian or Pacific Islander | 460 (0.4) | 164 (0.7) | 162 (1.1) | 183 (1.5) | 176 (0.9) | 1145 (0.6) |
| Non-Hispanic Black | 217 (0.2) | 114 (0.5) | 122 (0.8) | 169 (1.4) | 194 (1.0) | 816 (0.4) |
| Non-Hispanic White | 106 099 (90.7) | 22 257 (93.6) | 13 615 (92.4) | 10 774 (90.3) | 17 410 (86.6) | 170 155 (90.8) |
| Non-Hispanic unknown | 6950 (5.9) | 361 (1.5) | 140 (1.0) | 88 (0.7) | 1360 (6.8) | 8899 (4.7) |
| Socioeconomic status, quintileb | (n = 90 371) | (n = 18 351) | (n = 11 313) | (n = 8721) | (n = 13 651) | (n = 142 407) |
| Q1 (Lowest) | 5864 (6.5) | 1578 (8.6) | 1150 (10.2) | 1157 (13.3) | 1555 (11.4) | 11 304 (7.9) |
| Q2 | 11 469 (12.7) | 2661 (14.5) | 1912 (16.9) | 1528 (17.5) | 2148 (15.7) | 19 718 (13.8) |
| Q3 | 16 523 (18.3) | 3596 (19.6) | 2232 (19.7) | 1742 (20.0) | 2617 (19.2) | 26 710 (18.8) |
| Q4 | 21 885 (24.2) | 4347 (23.7) | 2536 (22.4) | 1810 (20.8) | 3026 (22.2) | 33 604 (23.6) |
| Q5 (Highest) | 29 605 (32.8) | 5135 (28.0) | 2878 (25.4) | 1973 (22.6) | 3432 (25.1) | 43 023 (30.2) |
| Unknown | 5025 (5.6) | 1034 (5.6) | 605 (5.3) | 511 (5.9) | 873 (6.4) | 8048 (5.7) |
Tumor thickness categories: T1, less than or equal to 1.0 mm; T2, more than 1.0 to 2.0 mm; T3, greater than 2.0 to 4.0 mm; T4, greater than 4 mm.
Patients are limited to those diagnosed from 2010 to 2016.
Table 2 shows melanoma incidence rates by thickness and sex in 2018 and the APC in incidence rate from 2010 to 2018. The overall incidence of melanoma did not increase significantly between 2010 and 2018 (APC, 0.39%; 95% CI, –0.40% to 1.18%). The incidence rate of melanoma in 2018 was 21.46 (95% CI, 21.17-21.75) for all tumors combined, 12.34 (95% CI, 12.12-12.56) for T1 tumors, and 1.56 (95% CI, 1.49-1.64) for T4 tumors. Incidence patterns differed by sex, with significant increases in incidence among women (APC, 0.92%; 95% CI, 0.01%-1.84%) but not among men (APC, –0.12%; 95% CI, –0.89% to 0.66%). From 2010 to 2018, there was a statistically significant increase in the incidence of T4 tumors (APC, 3.32%; 95% CI, 2.06%-4.60%), with similar patterns among men (APC, 2.50%; 95% CI, 1.27%-3.73%) and women (APC, 4.64%; 95% CI, 2.56%-6.75%) (Table 2).
Table 2. Annual Percentage Changes (APCs) From 2010 to 2018 in Incidence Rates of Cutaneous Melanoma and Incidence Rates in 2018, by Tumor Thickness and Sex.
| Tumor thickness | APC in incidence rate, % (95% CI) | Incidence rate per 100 000 person-years in 2018 (95% CI) | ||||
|---|---|---|---|---|---|---|
| Overall | Men | Women | Overall | Men | Women | |
| All | 0.39 (–0.40 to 1.18) | –0.12 (–0.89 to 0.66) | 0.92 (0.01 to 1.84)a | 21.46 (21.17 to 21.75) | 26.79 (26.32 to 27.27) | 17.51 (17.15 to 17.88) |
| T1, ≤1.0 mm | –0.28 (–1.98 to 1.46) | –0.69 (–2.44 to 1.09) | 0.09 (–1.60 to 1.81) | 12.34 (12.12 to 12.56) | 14.79 (14.44 to 15.14) | 10.60 (10.32 to 10.89) |
| T2, >1.0-2.0 mm | –0.70 (–1.67 to 0.29) | –1.43 (–2.44 to –0.41)a | 0.26 (–0.81 to 1.35) | 2.57 (2.47 to 2.68) | 3.26 (3.10 to 3.43) | 2.05 (1.93 to 2.18) |
| T3, >2.0-4.0 mm | –0.66 (–1.69 to 0.38) | –1.31 (–2.54 to –0.07)a | 0.27 (–1.22 to 1.78) | 1.58 (1.50 to 1.66) | 2.20 (2.06 to 2.34) | 1.12 (1.03 to 1.21) |
| T4, >4.0 mm | 3.32 (2.06 to 4.60)a | 2.50 (1.27 to 3.73)a | 4.64 (2.56 to 6.75)a | 1.56 (1.49 to 1.64) | 2.18 (2.05 to 2.33) | 1.07 (0.99 to 1.17) |
| Unknown | 5.00 (0.28 to 9.94)a | 3.91 (–0.39 to 8.40) | 6.59 (1.13 to 12.35)a | 3.40 (3.29 to 3.52) | 4.36 (4.17 to 4.56) | 2.67 (2.53 to 2.82) |
Statistically significant APC.
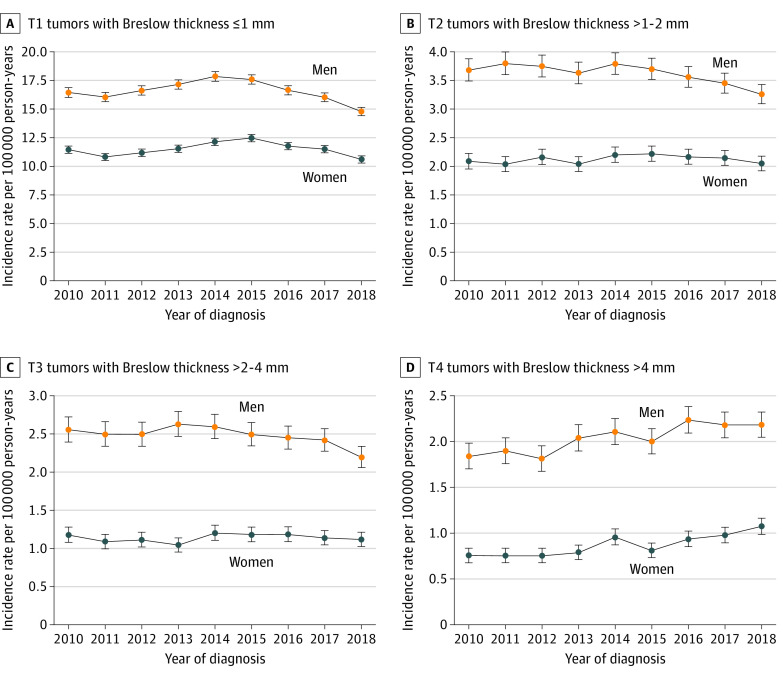
The Figure shows incidence rates of melanoma by tumor thickness and sex for each year. For T1 through T3 melanomas, incidence rates peaked between 2013 and 2015 and then decreased until 2018. In contrast, the incidence of T4 tumors increased from 2010 through 2018 for both men and women. eFigures 4 through 6 and eTables 2 through 4 in the Supplement show incidence rate patterns and APCs by race and ethnicity, socioeconomic status, and age. For example, the APC for non-Hispanic White patients for the thickest (T4) tumors was 3.99% (95% CI, 2.89%-5.09%) (eTable 2 in the Supplement). Thin (T1) melanomas had an APC of 3.77% (95% CI, 0.63%-7.01%) for the lowest socioeconomic status quintile, and thick (T4) melanomas had an APC of 4.02% (95% CI, 1.92%-6.17%) for the second socioeconomic status quintile.
Figure. Incidence Rates of Cutaneous Melanoma by Breslow Tumor Thickness and Patient Sex, 2010 to 2018.
Discussion
Using population-based SEER registry data for 187 487 patients with newly diagnosed cutaneous melanoma from 2010 to 2018, we assessed incidence patterns by tumor thickness, race and ethnicity, and Census tract–level socioeconomic status. We did not observe a significant increase in overall melanoma incidence; however, incidence for the thickest (T4) melanoma continued to rise with a significant APC of 3.32% between 2010 and 2018. This incidence differed from the pattern for thin (T1) melanoma, which peaked in incidence in 2013 through 2015 but steadily decreased since then. To our knowledge, this is the first study suggesting potential stabilization of melanoma incidence rates in the US after nearly a century of continuous increase in incidence.2,6,7
Interestingly, melanoma incidence decreased in Hungary from 2016 to 2019, mirroring the pattern we noted in the US.8 However, melanoma incidence continues to rise in Australia, the United Kingdom, and Germany.9,10,11 Although the overall melanoma incidence did not increase from 2010 through 2018, the continued increase of the incidence of thickest tumors during this period is concerning. Hispanic patients were more likely to be diagnosed with T4 melanoma compared with non-Hispanic White patients, suggesting persistent ethnic disparities.12 Patients in lower socioeconomic status quintiles were also more likely to be diagnosed with T4 melanomas compared with those in higher socioeconomic status quintiles and are known to have worse melanoma survival outcomes.13 These differences by socioeconomic status cannot be solely attributed to racial and ethnic disparities, as non-Hispanic White patients accounted for 90.8% of cases.
The finding that populations with both lower socioeconomic status and racial and ethnic minority status were more likely to have thicker melanomas has important implications for melanoma risk awareness and health care access in these groups. Evidence suggests that teledermatology can successfully identify malignant lesions and can facilitate care, especially in rural populations and for those with reduced health care access.14 Advances in artificial intelligence algorithms show promise in improving real-time skin disease diagnoses in a teledermatology setting.15 As adoption of these novel technologies grows, it is important to ensure that they do not exacerbate health disparities.
Our finding of thinner melanomas among individuals with higher socioeconomic status suggests increased screening and detection among those with better health care access may contribute to overdiagnoses of biologically indolent tumors and could lead to an apparent increasing incidence of thin melanoma.2 However, risk of thin melanoma is no longer increasing, and the increase in the incidence of the thickest melanomas suggests a true increase in the burden of prognostically worse melanoma cases in recent years.
Limitations
This study has limitations. First, melanoma thickness data were unknown for 10.7% of patients, similar to past SEER analyses.6 Second, non-Hispanic Black patients were a small fraction of the total reported patients, limiting generalizability of results for non-Hispanic Black patients. Third, the composite socioeconomic status measure used may not reflect an individual’s characteristics because individual socioeconomic status may vary within Census tracts. Fourth, the lack of socioeconomic status information after 2016 reduced the sample size for socioeconomic status analyses.
Conclusions
This population-based cohort study is, to our knowledge, the first study to suggest potential stabilization of overall melanoma incidence rates in the US after nearly a century of continuous increase in incidence. However, the continued increase in incidence of the thickest melanomas is concerning. Members of racial and ethnic minority groups and individuals of lower socioeconomic status were more likely to have thicker tumors, which may contribute to health disparities. The continued increase in the incidence of thick melanoma is unlikely to be because of overdiagnosis given the stability of thin melanoma incidence rates.
eMethods 1. Sensitivity Analysis Methods for Unknown Thickness Data.
eMethods 2. Statistical Methods
eMethods 3. Ethical Review of Study
eResults. Sensitivity Analysis for Unknown Thickness Data
eTable 1. Age, Stage, and Histologic Subtype Characteristics of Cutaneous Melanoma Cases by Tumor Thickness, 2010 to 2018
eTable 2. Annual Percentage Changes in Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Race and Ethnicity, 2010 to 2018
eTable 3. Annual Percentage Changes in Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Socioeconomic Status, 2010 to 2016
eTable 4. Annual Percentage Changes in Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Age, 2010 to 2018
eFigure 1. Incidence Rates of Cutaneous Melanoma by Sex and Race and Ethnicity for Tumors of Unknown Thickness, 2010 to 2018
eFigure 2. Incidence Rates of Cutaneous Melanoma for Unknown Thickness Tumors by SEER Registry Site, 2010 to 2018
eFigure 3. Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Sex Excluding Cancer Registries with Large Increases in Unknown Thickness Tumors, 2010 to 2018
eFigure 4. Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Race and Ethnicity, 2010 to 2018
eFigure 5. Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Socioeconomic Status, 2010 to 2016
eFigure 6. Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Age, 2010 to 2018
eReferences
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Welch HG, Mazer BL, Adamson AS. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med. 2021;384(1):72-79. doi: 10.1056/NEJMsb2019760 [DOI] [PubMed] [Google Scholar]
- 3.Gershenwald JE, Scolyer RA, Hess KR, et al. ; for members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform . Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472-492. doi: 10.3322/caac.21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaikh WR, Dusza SW, Weinstock MA, Oliveria SA, Geller AC, Halpern AC. Melanoma thickness and survival trends in the United States, 1989–2009. J Natl Cancer Inst. 2015;108(1):djv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703-711. doi: 10.1023/A:1011240019516 [DOI] [PubMed] [Google Scholar]
- 6.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129(7):1666-1674. doi: 10.1038/jid.2008.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31(33):4172-4178. doi: 10.1200/JCO.2012.47.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liszkay G, Kiss Z, Gyulai R, et al. Changing trends in melanoma incidence and decreasing melanoma mortality in Hungary between 2011 and 2019: a nationwide epidemiological study. Front Oncol. 2021;10:612459. doi: 10.3389/fonc.2020.612459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memon A, Bannister P, Rogers I, et al. Changing epidemiology and age-specific incidence of cutaneous malignant melanoma in England: an analysis of the national cancer registration data by age, gender and anatomical site, 1981-2018. Lancet Reg Health Eur. 2021;2:100024. doi: 10.1016/j.lanepe.2021.100024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer incidence. National Cancer Control Indicators. September 13, 2019. Accessed October 2, 2021. https://ncci.canceraustralia.gov.au/diagnosis/cancer-incidence/cancer-incidence
- 11.Cancer in Germany in 2015/2016. Robert Koch Institute, Association of Population-based Cancer Registries in Germany . 2019. Accessed October 2, 2021. https://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_Germany/cancer_in_germany.html
- 12.Qian Y, Johannet P, Sawyers A, Yu J, Osman I, Zhong J. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84(6):1585-1593. doi: 10.1016/j.jaad.2020.08.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Rahman O. Prognostic impact of socioeconomic status among patients with malignant melanoma of the skin: a population-based study. J Dermatolog Treat. 2020;31(6):571-575. doi: 10.1080/09546634.2019.1657223 [DOI] [PubMed] [Google Scholar]
- 14.Chuchu N, Dinnes J, Takwoingi Y, et al. ; Cochrane Skin Cancer Diagnostic Test Accuracy Group . Teledermatology for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12(12):CD013193. doi: 10.1002/14651858.CD013193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz-López C, Ramírez-Cornejo C, Marchetti MA, et al. Performance of a deep neural network in teledermatology: a single-centre prospective diagnostic study. J Eur Acad Dermatol Venereol. 2021;35(2):546-553. doi: 10.1111/jdv.16979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Sensitivity Analysis Methods for Unknown Thickness Data.
eMethods 2. Statistical Methods
eMethods 3. Ethical Review of Study
eResults. Sensitivity Analysis for Unknown Thickness Data
eTable 1. Age, Stage, and Histologic Subtype Characteristics of Cutaneous Melanoma Cases by Tumor Thickness, 2010 to 2018
eTable 2. Annual Percentage Changes in Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Race and Ethnicity, 2010 to 2018
eTable 3. Annual Percentage Changes in Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Socioeconomic Status, 2010 to 2016
eTable 4. Annual Percentage Changes in Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Age, 2010 to 2018
eFigure 1. Incidence Rates of Cutaneous Melanoma by Sex and Race and Ethnicity for Tumors of Unknown Thickness, 2010 to 2018
eFigure 2. Incidence Rates of Cutaneous Melanoma for Unknown Thickness Tumors by SEER Registry Site, 2010 to 2018
eFigure 3. Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Sex Excluding Cancer Registries with Large Increases in Unknown Thickness Tumors, 2010 to 2018
eFigure 4. Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Race and Ethnicity, 2010 to 2018
eFigure 5. Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Socioeconomic Status, 2010 to 2016
eFigure 6. Incidence Rates of Cutaneous Melanoma by Tumor Thickness and Age, 2010 to 2018
eReferences