Key Points
Question
Does chemotherapy with paclitaxel, cisplatin, and capecitabine (TPC) improve failure-free survival compared with cisplatin and fluorouracil (PF) as induction treatment prior to concurrent chemoradiotherapy for patients with stage IVA to IVB nasopharyngeal carcinoma?
Findings
In this phase 3 randomized clinical trial of 238 patients with stage IVA to IVB nasopharyngeal carcinoma, induction chemotherapy with 2 cycles of TPC followed by 2 cycles of concurrent chemoradiotherapy resulted in a statistically significant improvement in failure-free survival compared with 2 cycles of PF followed by 2 cycles of concurrent chemoradiotherapy, with no increase in the toxicity profile.
Meaning
Induction chemotherapy with TPC is more efficacious than that with PF for patients with stage IVA to IVB nasopharyngeal carcinoma.
Abstract
Importance
Induction chemotherapy added to concurrent chemoradiotherapy significantly improves survival for patients with locoregionally advanced nasopharyngeal carcinoma, but the optimal induction regimen remains unclear.
Objective
To determine whether induction chemotherapy with paclitaxel, cisplatin, and capecitabine (TPC) improves survival vs cisplatin and fluorouracil (PF) prior to chemoradiotherapy for patients with stage IVA to IVB nasopharyngeal carcinoma.
Design, Setting, and Participants
This randomized, open-label, phase 3 clinical trial recruited 238 patients at 4 hospitals in China from October 20, 2016, to August 29, 2019. Patients were 18 to 65 years of age with treatment-naive, nonkeratinizing stage IVA to IVB nasopharyngeal carcinoma and an Eastern Cooperative Oncology Group performance status of 0 to 1.
Interventions
Patients were randomly assigned (1:1) to receive induction chemotherapy with two 21-day cycles of TPC (intravenous paclitaxel [150 mg/m2, day 1], intravenous cisplatin [60 mg/m2, day 1], and oral capecitabine [1000 mg/m2 orally twice daily, days 1-14]) or PF (intravenous cisplatin [100 mg/m2, day 1] and fluorouracil [800 mg/m2 daily, days 1-5]), followed by chemoradiotherapy.
Main Outcomes and Measures
The primary end point was failure-free survival in the intention-to-treat population. Secondary end points included distant metastasis–free survival, locoregional relapse–free survival, overall survival, tumor response, and safety.
Results
Overall, 238 eligible patients (187 men [78.6%]; median age, 45 years [range, 18-65 years]) were randomly assigned to receive TPC (n = 118) or PF (n = 120). The median follow-up duration was 48.4 months (IQR, 39.6-53.3 months). Failure-free survival at 3 years was 83.5% (95% CI, 77.0%-90.6%) in the TPC group and 68.9% (95% CI, 61.1%-77.8%) in the PF group (stratified hazard ratio [HR] for recurrence or death, 0.47; 95% CI, 0.28-0.79; P = .004). Induction with the TPC regimen resulted in a significant reduction in the risk of distant metastases (stratified HR, 0.49 [95% CI, 0.24-0.98]; P = .04) and locoregional recurrence (stratified HR, 0.40 [95% CI, 0.18-0.93]; P = .03) compared with the PF regimen. However, there was no effect on early overall survival (stratified HR, 0.45 [95% CI, 0.17-1.18]; P = .10). The incidences of grade 3 to 4 acute adverse events and late-onset toxicities were 57.6% (n = 68) and 13.6% (16 of 118), respectively, in the TPC group and 65.8% (n = 79) and 17.9% (21 of 117), respectively, in the PF group. One treatment-related death occurred in the PF group.
Conclusions and Relevance
This randomized clinical trial found that induction chemotherapy with 2 cycles of TPC for patients with stage IVA to IVB nasopharyngeal carcinoma improved failure-free survival compared with 2 cycles of PF, with no increase in the toxicity profile.
Trial Registration
ClinicalTrials.gov Identifier: NCT02940925
This phase 3 randomized clinical trial examines whether induction chemotherapy with paclitaxel, cisplatin, and capecitabine improves survival vs cisplatin and fluorouracil prior to chemoradiotherapy for patients with stage IVA to IVB nasopharyngeal carcinoma.
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant neoplasm originating from the nasopharynx epithelium.1 Platinum-based concurrent chemoradiotherapy (CRT) represents the treatment backbone for locoregionally advanced NPC. Intensified chemotherapy in the induction or adjuvant setting, added to CRT, has improved survival outcomes.2 In the past decade, induction chemotherapy (IC) has regained the spotlight, with a series of randomized clinical trials demonstrating its clinical benefit.3,4,5,6,7,8 Induction chemotherapy in combination with CRT is the new standard of care recommended by the treatment guidelines for nonmetastatic stage III to IV NPC.9,10
Taxanes have shown promise in IC for advanced head and neck cancer, including NPC.3,11,12,13,14,15 A triplet induction regimen with cisplatin, fluorouracil, and a taxane might be more efficacious than cisplatin and fluorouracil (PF).13,14,15,16,17,18 Furthermore, replacing continuous fluorouracil infusion with oral capecitabine during IC has the advantages of convenience, good compliance, and favorable efficacy and safety.19 Capecitabine has shown robust antitumor activity against advanced NPC with fewer toxicities compared with fluorouracil, representing a promising chemotherapeutic agent for IC.20,21,22,23
After the landmark Intergroup 0099 trial,24 the PF regimen has emerged as the most used combination. This regimen is one of the treatment options in the guidelines.9,10 To date, the optimal induction regimen for NPC remains unclear. There is a lack of prospective data comparing 2 different IC regimens. Thus, we initiated a multicenter, open-label, randomized clinical phase 3 trial to compare the efficacy and safety of a paclitaxel, cisplatin, and capecitabine (TPC) regimen with that of a PF regimen as IC combined with CRT for patients with locoregionally advanced NPC. Given that the 5-year failure-free survival (FFS) of patients with stage III NPC exceeds 80%, we recruited only patients with stage IVA to IVB disease for this trial.25,26
Methods
Study Design
This multicenter, randomized, clinical, open-label, phase 3 trial (protocol in Supplement 1) recruited patients at 4 hospitals in China (eTable 1 in Supplement 2) from October 20, 2016, to August 29, 2019. A study design diagram is provided in eFigure 1 in Supplement 2. The Chinese Ethics Committee of Registering Clinical Trials approved the trial protocol. Written informed consent was obtained from all participating patients before enrollment. This trial was conducted according to the Declaration of Helsinki27 and the standards of Good Clinical Practice. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Patients who met the following criteria were eligible: aged 18 to 65 years, nonkeratinizing NPC, treatment naive, stage IVA to IVB disease according to the 7th edition of the American Joint Committee on Cancer staging system,28 Eastern Cooperative Oncology Group performance status of 0 or 1, and sufficient organ function. Key exclusion criteria included a history of other malignant neoplasms, previous anticancer treatment, distant metastasis, severe coexisting illness, and pregnancy or lactation. More information regarding inclusion and exclusion criteria are detailed in the trial protocol in Supplement 1.
Randomization and Masking
Randomization was performed centrally at the Sun Yat-sen University Cancer Center. A computer-generated randomization list was created using stratified block randomization. Blocks of variable size were adopted to guard against guessing.29 At study entry, patients were stratified by disease stage (IVA or IVB). Competitive recruitment was applied in this trial. After obtaining informed consent from eligible patients, an independent study coordinator unblinded treatment allocation information and notified investigators of interventions accordingly.
Procedures
All patients underwent comprehensive pretreatment evaluations within 4 weeks before randomization. Patients were randomly assigned (1:1) to receive IC with two 21-day cycles of either TPC or PF, followed by 6 to 7 weeks of CRT. The patients in the TPC group received the following regimen as induction therapy: paclitaxel was administered intravenously at a dose of 150 mg/m2 over 3 hours on day 1, cisplatin was administered at a dose of 60 mg/m2 on day 1, and capecitabine was taken orally twice a day at a dose of 1000 mg/m2 on days 1 to 14.11,12,18,22,23,30 The patients in the PF group received the following regimen as induction therapy: cisplatin was administered intravenously at a dose of 100 mg/m2 on day 1, and fluorouracil was administered as a continuous 120-hour infusion at a dose of 800 mg/m2 per day on days 1 to 5.23,31 Induction chemotherapy was administered every 3 weeks for 2 cycles. After completing 2 cycles of IC, both groups were to receive 2 cycles of concurrent chemotherapy (CC) with cisplatin, 100 mg/m2. All patients received intensity-modulated radiotherapy. Detailed information on dose modifications, and concomitant medication, and radiotherapy is listed in the trial protocol in Supplement 1.
Tumor responses were assessed using physical examination, flexible nasopharyngoscopy, and enhanced magnetic resonance imaging or computed tomographic scan of the nasopharynx and neck 1 week after IC and 12 weeks after CRT. Tumor responses were evaluated based on the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1).32 Acute toxic effects during treatment were graded with the Common Terminology Criteria for Adverse Events, version 4.0.33 Late radiation toxicities (complications occurring 3 months after radiotherapy) were assessed with the Late Radiation Morbidity Scoring Scheme of the Radiation Therapy Oncology Group.34 After completing scheduled treatment, each patient underwent assessment every 3 months in the first 3 years, every 6 months in the next 2 years, and annually after that until death.
Outcomes
The primary end point was FFS in the intention-to-treat population, defined as the interval between randomization and the first documented tumor recurrence (locoregional relapse or distant metastasis), death, or the last follow-up, whichever occurred first. Secondary end points included distant metastasis–free survival (the interval between randomization and distant metastasis), locoregional relapse–free survival (the interval between randomization and locoregional relapse), overall survival (OS; the interval between randomization and death from any cause), overall response rate after IC and after radiotherapy according to RECIST v1.1, safety profile, and treatment compliance.
Statistical Analysis
The trial sought to assess whether IC with the TPC regimen improved FFS compared with the PF regimen. We calculated that approximately 220 patients (110 patients per group) would need to undergo randomization, providing 80% power to detect the underlying hazard ratio (HR) of 0.50 for the primary analysis of FFS at a 2-sided α level of 5%.35 After allowing for an 8% dropout rate, we estimated that approximately 238 patients were needed.
The intention-to-treat population was used for efficacy analyses. We also performed efficacy analyses in the per-protocol population, including patients who completed 2 cycles of IC and 2 cycles of CC. We reported safety data for all patients who had started their randomly assigned treatment. Categorical variables were compared using the χ2 test or the Fisher exact test. Continuous variables were compared using the Mann-Whitney test. Time-to-event data were estimated using the Kaplan-Meier approach. Treatment groups were compared for time-to-event data stratified by disease stage using stratified log-rank tests. Hazard ratios and corresponding 95% CIs were calculated using a stratified Cox proportional hazards regression model to estimate the effect of the experimental treatment, stratified by disease stage. The proportional hazards assumption was examined using Schoenfeld residuals. Complementary analyses, such as those using restricted mean survival time, would be performed if the proportional hazards assumption was unmet.36 We also performed post hoc exploratory analyses to examine whether the treatment effect varied in specific subgroups. Statistical analyses were conducted with R software, version 4.1.0 (R Group for Statistical Computing) and were 2-sided at a significance level of P < .05.
Results
Patients and Treatment
Between October 20, 2016, and August 29, 2019, 238 eligible patients (187 men [78.6%]; median age, 45 years [range, 18-65 years]) with stage IVA to IVB NPC were recruited and randomly assigned to receive TPC (n = 118) or PF (n = 120) (Figure 1) as IC. Patient characteristics were balanced between groups (Table 1).
Figure 1. CONSORT Diagram of Participant Enrollment.
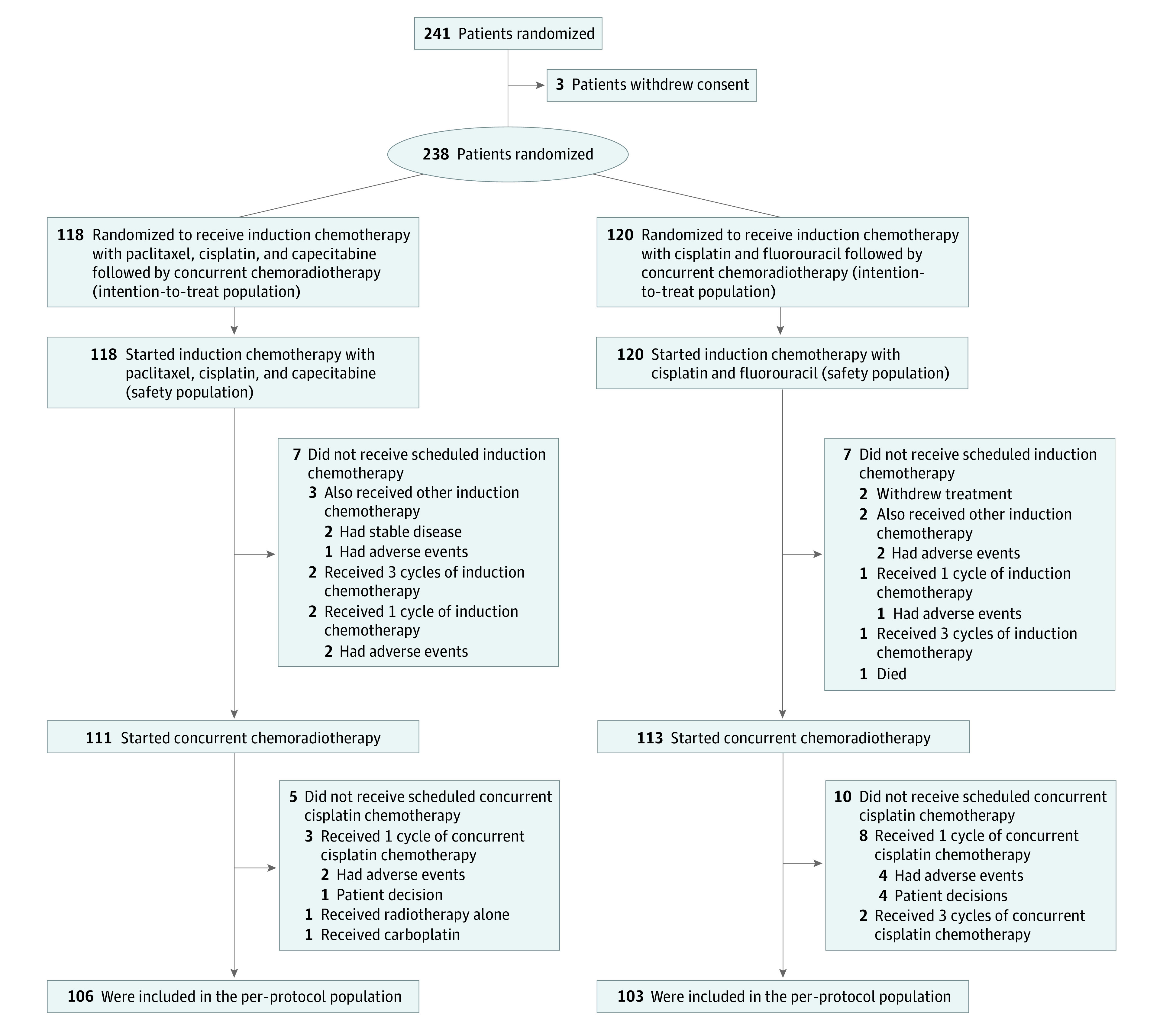
Table 1. Baseline Demographic and Disease Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Paclitaxel, cisplatin, and capecitabine (n = 118) | Cisplatin and fluorouracil (n = 120) | |
| Age, median (IQR), y | 45.5 (36.2-52.8) | 46.0 (39.0-52.0) |
| Sex | ||
| Female | 29 (24.6) | 22 (18.3) |
| Male | 89 (75.4) | 98 (81.7) |
| ECOG performance status | ||
| 0 | 101 (85.6) | 105 (87.5) |
| 1 | 17 (14.4) | 15 (12.5) |
| Tumor category | ||
| T1 | 1 (0.8) | 4 (3.3) |
| T2 | 5 (4.2) | 2 (1.7) |
| T3 | 34 (28.8) | 32 (26.7) |
| T4 | 78 (66.1) | 82 (68.3) |
| Node category | ||
| N0 | 4 (3.4) | 5 (4.2) |
| N1 | 26 (22.0) | 21 (17.5) |
| N2 | 34 (28.8) | 37 (30.8) |
| N3 | 54 (45.8) | 57 (47.5) |
| Disease stage | ||
| IVA | 64 (54.2) | 63 (52.5) |
| IVB | 54 (45.8) | 57 (47.5) |
| EBV DNA, copies/mL | ||
| Median (IQR) | 1340 (335-6915) | 1950 (319-12 400) |
| Missing | 0 | 1 (0.8) |
Abbreviations: EBV, Epstein-Barr virus; ECOG, Eastern Cooperative Oncology Group.
The relative dose intensity of chemotherapy in the 2 treatment groups is shown in eFigure 2 in Supplement 2. Overall, treatment groups were relatively comparable concerning treatment compliance (eTable 2 in Supplement 2). In the TPC group, 113 patients (95.8%) completed 2 cycles of IC, 3 patients (2.5%) received 1 cycle owing to adverse events, and 2 patients received 3 cycles. In the PF group, 113 patients (94.2%) completed 2 cycles of IC, 6 patients (5.0%) received 1 cycle (3 owing to adverse events, 2 owing to treatment withdrawal, and 1 owing to death caused by treatment-related acute renal failure), and 1 patient received 3 cycles. Protocol-defined IC doses were administered to 107 patients (90.7%) in the TPC group and 104 patients (86.7%) patients in the PF group. Dose reductions or cancellations of IC were more commonly seen in the PF group than in the TPC group (16 [13.3%] vs 7 [5.9%]; P = .05).
After completing IC, 111 patients (94.1%) in the TPC group started concurrent cisplatin chemotherapy: 106 patients completed 2 cycles of cisplatin, 3 received 1 cycle, 1 received carboplatin, and 1 received radiotherapy alone (eTable 2 in Supplement 2). In the PF group, 113 patients (94.2%) started CRT: 103 patients completed 2 cycles of cisplatin, 8 received 1 cycle, and 2 received 3 cycles. Protocol-defined concurrent cisplatin was administered to 101 of 111 patients (91.0%) in the TPC group and 95 of 113 patients (84.1%) in the PF group. Cisplatin dose reductions or cancellations were similar in the TPC and PF groups (10 of 111 [9.0%] vs 16 of 113 [14.2%]; P = .23). All 118 patients in the TPC group and 117 of 120 patients (97.5%) in the PF group completed protocol-defined intensity-modulated radiotherapy. The reasons for not receiving radiotherapy included treatment withdrawal (2 patients) and chemotherapy-related death (1 patient). No significant differences in radiotherapy parameters or treatment durations were observed. Finally, 106 of 118 patients (89.8%) in the TPC group and 103 of 120 patients (85.8%) in the PF group were included in the per-protocol population.
Efficacy
The response rates after IC and after the whole treatment are summarized in Table 2. There were no significant differences in the overall response rate at 1 week after IC between the TPC and PF groups (104 [88.1%] vs 96 [80.0%]; P = .09). At 12 weeks after CRT, more patients in the TPC group than in the PF group showed a complete response (111 of 118 [94.1%] vs 102 of 120 [85.0%]; P = .04).
Table 2. Efficacy of Study Treatment.
| End point | Paclitaxel, cisplatin, and capecitabine (n = 118) | Cisplatin and fluorouracil (n = 120) | HR (95% CI)a |
|---|---|---|---|
| Failure-free survival | |||
| Recurrence or death events, No. (%) | 22 (18.6) | 41 (34.2) | |
| Rate at 3 y, % (95% CI) | 83.5 (77.0-90.6) | 68.9 (61.1-77.8) | 0.47 (0.28-0.79) |
| Distant metastasis–free survival | |||
| Distant metastasis, No. (%) | 12 (10.2) | 23 (19.2) | |
| Rate at 3 y, % (95% CI) | 91.4 (86.4-96.6) | 80.4 (73.6-87.9) | 0.49 (0.24-0.98) |
| Locoregional relapse–free survival | |||
| Locoregional relapse events, No. (%) | 8 (6.8) | 18 (15.0) | |
| Rate at 3 y, % (95% CI) | 93.8 (89.5-98.4) | 87.4 (81.4-93.8) | 0.40 (0.18-0.93) |
| Overall survival | |||
| Death events, No. (%) | 6 (5.1) | 13 (10.8) | |
| Rate at 3 y, % (95% CI) | 94.7 (90.6-98.9) | 88.9 (83.4-94.8) | 0.45 (0.17-1.18) |
| Response to induction chemotherapy, No. (%) | |||
| Overall response | 104 (88.1) | 96 (80.0) | NA |
| Complete response | 2 (1.7) | 4 (3.3) | NA |
| Partial response | 102 (86.4) | 92 (76.7) | NA |
| Stable disease | 14 (11.9) | 21 (17.5) | NA |
| Not evaluable | 0 | 3 (2.5) | NA |
| Response to the whole treatment | |||
| Overall response | 118 (100) | 115 (95.8) | NA |
| Complete response | 111 (94.1) | 102 (85.0) | NA |
| Partial response | 7 (5.9) | 13 (10.8) | NA |
| Stable disease | 0 | 1 (0.8) | NA |
| Not evaluable | 0 | 4 (3.3) | NA |
Abbreviations: HR, hazard ratio; NA, not applicable.
Calculated using the stratified Cox proportional hazards regression model.
At the data cutoff date (August 28, 2021), the median follow-up time was 48.4 months (IQR, 39.6-53.3 months). Overall, 63 patients had disease recurrence or died (TPC group, 22; and PF group, 41). Information regarding disease failure patterns and subsequent salvage therapies are detailed in eTables 3 and 4, respectively, in Supplement 2. In the intention-to-treat population, the 3-year FFS rate was 83.5% (95% CI, 77.0%-90.6%) in the TPC group, compared with 68.9% (95% CI, 61.1%-77.8%) in the PF group (stratified HR, 0.47; 95% CI, 0.28-0.79; P = .004) (Table 2 and Figure 2A). The prespecified statistical criteria for the superiority of TPC vs PF were met. Treatment with the TPC regimen resulted in a reduction of 53% in the risk of disease failure, compared with treatment with PF. However, the proportional hazards assumption for FFS was unmet (eFigure 3 in Supplement 2); as additional analysis, the post hoc exploratory restricted mean survival time analysis was conducted to verify the clinical advantage of the TPC regimen. The estimated restricted mean survival time for FFS at the truncation time of 36 months was significantly longer in the TPC group than in the PF group (33.4 months [95% CI, 32.1-34.6 months] vs 29.1 months [95% CI, 27.1-31.1 months]; P < .001) (eFigure 4 in Supplement 2). In the per-protocol population, the clinical benefit of the TPC regimen vs the PF regimen was evident (eFigures 5A and 6 in Supplement 2). In the post hoc subgroup analysis for FFS, we observed a consistent benefit favoring the TPC regimen across all patient subgroups (eFigures 7, 8, and 9 in Supplement 2).
Figure 2. Kaplan-Meier Analysis of Survival in the Intention-to-Treat Population.
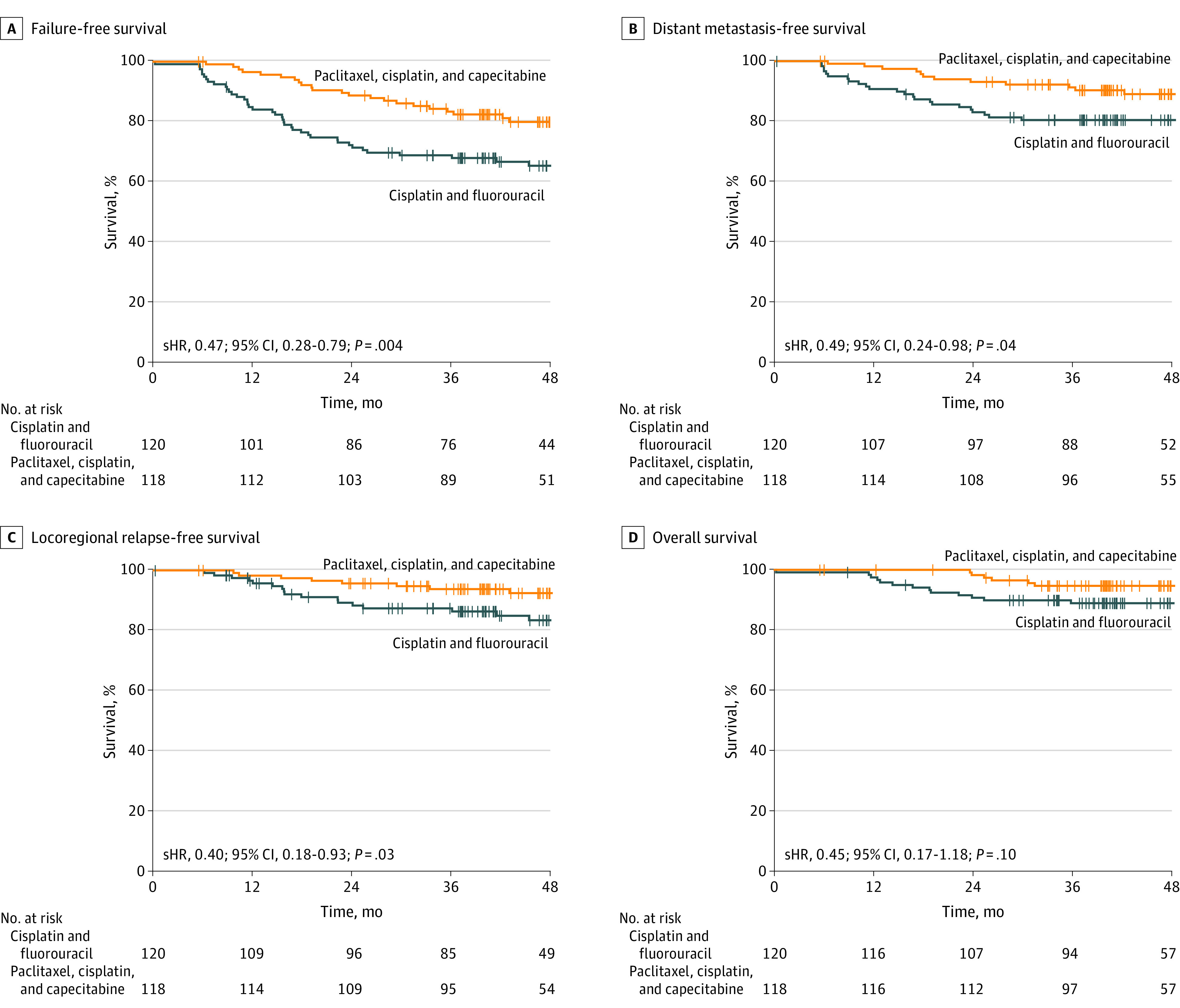
A, Failure-free survival. B, Distant failure–free survival. C, Locoregional failure–free survival. D, Overall survival. sHR indicates stratified hazard ratio.
In the intention-to-treat population, the 3-year distant metastasis–free survival rate was significantly higher in the TPC group (91.4% [95% CI, 86.4%-96.6%]) than in the PF group (80.4% [95% CI, 73.6%-87.9%]) (Table 2); 12 of 118 patients (10.2%) in the TPC group and 23 of 120 patients (19.2%) in the PF group showed distant metastasis (stratified HR, 0.49 [95% CI, 0.24-0.98]; P = .04) (Figure 2B). Similarly, the 3-year locoregional relapse–free survival rate was significantly higher in the TPC group than in the PF group (93.8% [95% CI, 89.5%-98.4%] vs 87.4% [95% CI, 81.4%-93.8%]) (Table 2); 18 of 120 patients (15.0%) in the PF group and 8 of 118 patients (6.8%) in the TPC group showed locoregional relapse (stratified HR, 0.40 [95% CI, 0.18-0.93]; P = .03) (Figure 2C). However, the 3-year OS rate was not significantly different between the TPC and PF groups (94.7% [95% CI, 90.6%-98.9%] vs 88.9% [95% CI, 83.4%-94.8%]) (Table 2); 6 of 118 patients (5.1%) in the TPC group and 13 of 120 patients (10.8%) in the PF group died (stratified HR, 0.45 [95% CI, 0.17-1.18]; P = .10) (Figure 2D). We observed similar results in the per-protocol analysis for the above-mentioned secondary end points (eFigure 4B, 4C, and 4D in Supplement 2).
Safety
During the entire treatment course, acute adverse events occurred in all patients. Details concerning adverse events during treatment are summarized in Table 3. The incidence of grade 2 or higher acute toxic effects was similar between the 2 groups. Grade 3 to 4 acute adverse events were reported in 68 patients (57.6%) in the TPC group compared with 79 patients (65.8%) in the PF group. The most frequent grade 3 to 4 adverse events were mucositis (TPC, 33 [28.0%] vs PF, 34 [28.3%]), nausea (TPC, 18 [15.3%] vs PF, 25 [20.8%]), vomiting (TPC, 22 [18.6%] vs PF, 19 [15.8%]), and neutropenia (TPC, 15 [12.7%] vs PF, 22 [18.3%]). We recorded 1 grade 5 adverse event in the PF group: death caused by treatment-related acute renal failure. The incidences of acute adverse events during IC and CC are summarized in eTables 5 and 6, respectively, in Supplement 2. The prevalence of late-onset toxicities was similar between the treatment groups (TPC, 16 of 118 [13.6%]; and PF, 21 of 117 [17.9%]; eTable 7 in Supplement 2).
Table 3. Treatment-Related Acute Adverse Events.
| Adverse event | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
| Paclitaxel, cisplatin, and capecitabine (n = 118) | Cisplatin and fluorouracil (n = 120) | |||||
| Grade 0-1 | Grade 2 | Grade 3-4 | Grade 0-1 | Grade 2 | Grade 3-4 | |
| Acute hematologic toxicity | ||||||
| Leukopenia | 60 (50.8) | 40 (33.9) | 18 (15.3) | 65 (54.2) | 28 (31.7) | 17 (14.2) |
| Neutropenia | 73 (61.9) | 30 (25.4) | 15 (12.7) | 70 (58.3) | 28 (23.3) | 22 (18.3) |
| Anemia | 68 (57.6) | 37 (31.4) | 13 (11.0) | 70 (58.3) | 39 (32.5) | 11 (9.2) |
| Thrombocytopenia | 108 (91.5) | 8 (6.8) | 2 (1.7) | 105 (87.5) | 8 (6.8) | 2 (1.7) |
| Acute nonhematologic toxicity | ||||||
| Dry mouth | 80 (67.8) | 31 (26.3) | 7 (5.9) | 73 (60.8) | 35(29.2) | 12 (10.0) |
| Mucositis | 53 (44.9) | 32 (27.1) | 33 (28.0) | 48 (40.0) | 38 (31.7) | 34 (28.3) |
| Dermatitis | 94 (79.7) | 22 (18.6) | 2 (1.7) | 87 (72.5) | 29 (24.2) | 4 (3.3) |
| Diarrhea | 111 (94.1) | 5 (4.2) | 2 (1.7) | 112 (93.3) | 6 (5.0) | 2 (1.7) |
| Nausea | 62 (52.5) | 38 (32.2) | 18 (15.3) | 63 (52.5) | 32 (26.7) | 25 (20.8) |
| Vomiting | 71 (60.2) | 25 (21.2) | 22 (18.6) | 66 (55.0) | 35 (29.2) | 19 (15.8) |
| Hepatotoxicity | 96 (81.4) | 19 (16.1) | 3 (2.5) | 101 (84.2) | 16 (13.3) | 3 (2.5) |
| Nephrotoxicitya | 112 (94.9) | 6 (5.1) | 0 | 112 (93.3) | 7 (5.8) | 1 (0.8) |
| Hand-foot syndrome | 116 (98.3) | 2 (1.7) | 0 | 119 (99.2) | 1 (0.8) | 0 |
| Allergic reaction | 112 (94.9) | 5 (4.2) | 1 (0.8) | 117 (97.5) | 3 (2.5) | 0 |
| Weight loss | 91 (77.1) | 25 (21.2) | 2 (1.7) | 90 (75.0) | 27 (22.5) | 3 (2.5) |
One grade 5 nephrotoxicity event occurred during treatment in the cisplatin and fluorouracil group.
Discussion
To our knowledge, this phase 3 randomized clinical trial of patients with stage IVA to IVB NPC is the first study to show the advantage of IC with TPC followed by CRT compared with IC with PF followed by CRT. Our study showed that IC with TPC improved FFS with no increase in toxicity. The TPC group had a lower incidence of distant metastases and locoregional relapse than the PF group, but the effect of TPC on early OS was not significant.
Induction chemotherapy in combination with CRT has become the new standard of care for locoregionally advanced NPC.9,10 The latest guidelines have provided more than 5 different induction regimens for patients with stage III to IVA NPC. There are no prospective trials available that compare 2 induction regimens head-to-head in a superiority design. Recently, an individual patient data meta-analysis involving 8214 patients indicated that IC with a taxane-based regimen ranked better than IC without taxanes for OS.37 Our trial suggested that the TPC regimen provided better disease control than the PF regimen did. Our findings are consistent with several trials that showed survival superiority of cisplatin and fluorouracil with a taxane compared with a PF regimen for advanced head and neck cancer.13,14,16
The cycles of IC and CC in this trial were deintensified from the traditional 3 cycles. The optimal cycle number of IC and CC when used in combination has not yet been established.1 The latest guideline recommends 2 or 3 cycles.10 Five randomized trials have used 2 cycles of IC in NPC.3,8,38,39,40 Two cycles of IC were well tolerated and associated with satisfactory subsequent delivery of cumulative concomitant cisplatin, 200 mg/m2.3,8,40 Furthermore, several retrospective studies also indicated that 2 and 3 cycles of IC were associated with similar survival in the era of intensity-modulated radiotherapy.41,42 Apart from IC, increasing evidence also indicates that 2 cycles of CC (cumulative cisplatin dose of 200 mg/m2) might be adequate for locoregionally advanced NPC. In the pooled analyses of NPC-9901 and NPC-9902 trials, there was no difference between cohorts receiving 2 cycles of concurrent cisplatin, 100 mg/m2, vs cohorts receiving 3 cycles.43 A series of subsequent studies also had similar findings.44,45,46,47,48,49 Given this evidence, updated reviews and guidelines recommended that a cumulative cisplatin dose of 200 mg/m2 may be the optimal threshold.1,10,50,51,52 A recent study has shown that 2 cycles of triweekly cisplatin, 100 mg/m2, was noninferior to standard weekly cisplatin, 40 mg/m2, for patients with locoregionally advanced NPC.53 Another updated randomized phase 2 noninferiority trial also revealed that 2 cycles of triweekly cisplatin, 100 mg/m2, was noninferior to 3 cycles for patients with Epstein-Barr virus DNA levels of less than 4000 copies/mL.54
This trial recorded similar acute toxicities and late radiotherapy toxicities in both the TPC and PF groups. When we designed and initiated this trial, there were no available standard doses of the TPC regimen. The dose and dosing schedule were based on several phase 2 studies investigating the efficacy of paclitaxel-based regimens in advanced NPC.11,12,22 The doses of 135 to 175 mg/m2 of paclitaxel were frequently used in these trials. Considering that the intensified triplet combination was used, a dose of 150 mg/m2 was selected. The cisplatin dose of 60 mg/m2 and capecitabine dose of 1000 mg/m2 twice daily on days 1 to 14 were used in previous trials.23,30 In our trial, adding the paclitaxel and replacing the fluorouracil with capecitabine did not increase toxicity. The FFS rate at 3 years was similar to that reported in the landmark trials examining the benefit of IC (docetaxel, cisplatin, and fluorouracil; and gemcitabine and cisplatin) when added to CRT in NPC,4,7 while we observed fewer grade 3 to 4 acute toxic effects in our trial. Patients with more advanced diseases were recruited in our trial, and they received fewer protocol-defined cycles of IC and CC. However, because of the absence of direct head-to-head comparative data, prospective randomized clinical trials are needed to define the best choice among these 3 regimens.
Limitations
This study has several limitations. First, all study participants came from endemic areas in China, where nonkeratinizing NPC accounts for more than 95% of cases.1 Whether the findings can be generalizable to nonendemic regions warrants further validation. Second, children, adolescents, and elderly patients with NPC were excluded from the current study. Therefore, our results should not be applied to these patients directly. Third, although the current trial has met the primary end point of FFS, the effect of the TPC regimen on early OS was not significant. A longer follow-up is needed to confirm whether there is a benefit of OS. Fourth, although 2 or 3 cycles of IC are recommended, 3 cycles are more commonly used.10 To date, the optimal cycle number of IC remains unclear. To address this issue, in 2018, we initiated a noninferiority phase 3 trial (ChiCTR1800018417) to compare 2 vs 3 cycles of IC followed by CRT for locoregionally advanced NPC.
Conclusions
This phase 3 randomized clinical trial found that IC with 2 cycles of TPC for patients with stage IVA to IVB NPC improved FFS compared with 2 cycles of PF, with no increase in toxicity profiles.
Trial Protocol
eTable 1. List of Participating Centers
eTable 2. Treatment Compliance
eTable 3. Distribution of Disease Recurrence or Death in the Two Treatment Groups
eTable 4. Summary of Salvage Treatments After Disease Recurrence
eTable 5. Treatment-Related Acute Adverse Events During Induction Chemotherapy
eTable 6. Treatment-Related Acute Adverse Events During Concurrent Chemoradiotherapy
eTable 7. Late Radiotherapy Toxicities After Treatment
eFigure 1. Study Design
eFigure 2. The Relative Dose Intensity of Chemotherapy Drugs in the Two Treatment Groups During Induction Chemotherapy and Concurrent Chemotherapy
eFigure 3. The Proportional-Hazards Assumption for Failure-Free Survival Was Tested Using Schoenfeld Residuals Test
eFigure 4. The Estimated Restricted Mean Survival Time for Failure-Free Survival at Truncation Time of 36 Months in the Intention-to-Treat Population
eFigure 5. Kaplan-Meier Analysis of Failure-Free Survival (A), Distant Metastasis-Free Survival (B), Locoregional Relapse-Free Survival (C), and Overall Survival (D) in the Two Treatment Groups in Per-Protocol Population
eFigure 6. The Estimated Restricted Mean Survival Time for Failure-Free Survival at Truncation Time of 36 Months in the Per-Protocol Population
eFigure 7. Subgroup Analysis of Failure-Free Survival in the Intention-to-Treat Population
eFigure 8. Kaplan-Meier Analysis of Failure-Free Survival in the Treatment Groups Stratified by the TNM Staging Classifications: (A) T1-3, (B) T4, (C) N0-1, (D) N2-3, (E) IVA, and (F) IVB
eFigure 9. Kaplan-Meier Analysis of Failure-Free Survival in the Treatment Groups Stratified by the Baseline EBV DNA: (A) ≤ 1500 copies/mL, (B) > 1500 copies/mL
Data Sharing Statement
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi: 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2.Ribassin-Majed L, Marguet S, Lee AWM, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? an individual patient data network meta-analysis. J Clin Oncol. 2017;35(5):498-505. doi: 10.1200/JCO.2016.67.4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242-249. doi: 10.1200/JCO.2008.18.1545 [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509-1520. doi: 10.1016/S1470-2045(16)30410-7 [DOI] [PubMed] [Google Scholar]
- 5.Hong RL, Hsiao CF, Ting LL, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma—Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol. 2018;29(9):1972-1979. doi: 10.1093/annonc/mdy249 [DOI] [PubMed] [Google Scholar]
- 6.Frikha M, Auperin A, Tao Y, et al. ; GORTEC . A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol. 2018;29(3):731-736. doi: 10.1093/annonc/mdx770 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124-1135. doi: 10.1056/NEJMoa1905287 [DOI] [PubMed] [Google Scholar]
- 8.Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14-23. doi: 10.1016/j.ejca.2016.12.039 [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . Head and neck cancers: version 3.2021. Accessed June 23, 2021. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 10.Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021;39(7):840-859. doi: 10.1200/JCO.20.03237 [DOI] [PubMed] [Google Scholar]
- 11.Yeo W, Leung T, Chan A, et al. A phase II study of combination paclitaxel and carboplatin in advanced nasopharyngeal carcinoma. Eur J Cancer. 1998;34(13):2027-2031. doi: 10.1016/S0959-8049(98)00280-9 [DOI] [PubMed] [Google Scholar]
- 12.Tan EH, Khoo KS, Wee J, et al. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol. 1999;10(2):235-237. doi: 10.1023/A:1008390929826 [DOI] [PubMed] [Google Scholar]
- 13.Posner MR, Hershock DM, Blajman CR, et al. ; TAX 324 Study Group . Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705-1715. doi: 10.1056/NEJMoa070956 [DOI] [PubMed] [Google Scholar]
- 14.Vermorken JB, Remenar E, van Herpen C, et al. ; EORTC 24971/TAX 323 Study Group . Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695-1704. doi: 10.1056/NEJMoa071028 [DOI] [PubMed] [Google Scholar]
- 15.Mody MD, Gill HS, Saba NF. The evolving and future role of taxanes in squamous cell carcinomas of the head and neck: a review. JAMA Otolaryngol Head Neck Surg. 2016;142(9):898-905. doi: 10.1001/jamaoto.2016.1238 [DOI] [PubMed] [Google Scholar]
- 16.Hitt R, López-Pousa A, Martínez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23(34):8636-8645. doi: 10.1200/JCO.2004.00.1990 [DOI] [PubMed] [Google Scholar]
- 17.Bae WK, Hwang JE, Shim HJ, et al. Phase II study of docetaxel, cisplatin, and 5-FU induction chemotherapy followed by chemoradiotherapy in locoregionally advanced nasopharyngeal cancer. Cancer Chemother Pharmacol. 2010;65(3):589-595. doi: 10.1007/s00280-009-1152-0 [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Wang FH, An X, et al. Triplet combination with paclitaxel, cisplatin and 5-FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2013;71(2):371-378. doi: 10.1007/s00280-012-2020-x [DOI] [PubMed] [Google Scholar]
- 19.Iqbal H, Pan Q. Capecitabine for treating head and neck cancer. Expert Opin Investig Drugs. 2016;25(7):851-859. doi: 10.1080/13543784.2016.1181747 [DOI] [PubMed] [Google Scholar]
- 20.Chua DT, Sham JS, Au GK. A phase II study of capecitabine in patients with recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy. Oral Oncol. 2003;39(4):361-366. doi: 10.1016/S1368-8375(02)00120-3 [DOI] [PubMed] [Google Scholar]
- 21.Chua DT, Yiu HH, Seetalarom K, et al. Phase II trial of capecitabine plus cisplatin as first-line therapy in patients with metastatic nasopharyngeal cancer. Head Neck. 2012;34(9):1225-1230. doi: 10.1002/hed.21884 [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Huang HQ, Bai B, Cai QC, Wang XX, Cai QQ. Treatment outcome of docetaxel, capecitabine and cisplatin regimen for patients with refractory and relapsed nasopharyngeal carcinoma who failed previous platinum-based chemotherapy. Expert Opin Pharmacother. 2014;15(2):163-171. doi: 10.1517/14656566.2014.866652 [DOI] [PubMed] [Google Scholar]
- 23.Lee AW, Ngan RK, Tung SY, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 2015;121(8):1328-1338. doi: 10.1002/cncr.29208 [DOI] [PubMed] [Google Scholar]
- 24.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310-1317. doi: 10.1200/JCO.1998.16.4.1310 [DOI] [PubMed] [Google Scholar]
- 25.Lee AWM, Ng WT, Chan LK, et al. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol. 2012;48(10):1007-1013. doi: 10.1016/j.oraloncology.2012.03.022 [DOI] [PubMed] [Google Scholar]
- 26.Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122(4):546-558. doi: 10.1002/cncr.29795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 28.Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC Cancer Staging Manual. Springer; 2010. [Google Scholar]
- 29.Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. Lancet. 2002;359(9310):966-970. doi: 10.1016/S0140-6736(02)08029-7 [DOI] [PubMed] [Google Scholar]
- 30.Guo L, Lin HX, Xu M, Chen QY, Wang CT, Huang PY. Phase I study of TPF neoadjuvant chemotherapy followed by radical radiotherapy in advanced nasopharyngeal carcinoma. Chin J Cancer. 2010;29(2):136-139. doi: 10.5732/cjc.009.10367 [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13(2):163-171. doi: 10.1016/S1470-2045(11)70320-5 [DOI] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute. Common terminology criteria for adverse events (CTCAE): version 4.03. National Cancer Institute, National Institutes of Health; 2010.
- 34.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346. doi: 10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 35.Chow SC, Shao J, Wang H, Lokhnygina Y, eds. Sample Size Calculations in Clinical Research. Marcel Dekker; 2003. [Google Scholar]
- 36.A’Hern RP. Restricted mean survival time: an obligatory end point for time-to-event analysis in cancer trials? J Clin Oncol. 2016;34(28):3474-3476. doi: 10.1200/JCO.2016.67.8045 [DOI] [PubMed] [Google Scholar]
- 37.Petit C, Lee AWM, Carmel A, et al. Network-meta-analysis of chemotherapy in nasopharyngeal carcinoma (MAC-NPC): an update on 8,221 patients. J Clin Oncol. 2020; 38(15):6523. doi: 10.1200/JCO.2020.38.15_suppl.6523 [DOI] [Google Scholar]
- 38.Chua DT, Sham JS, Choy D, et al. ; Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group . Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 1998;83(11):2270-2283. doi: [DOI] [PubMed] [Google Scholar]
- 39.Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19(5):1350-1357. doi: 10.1200/JCO.2001.19.5.1350 [DOI] [PubMed] [Google Scholar]
- 40.Lv X, Cao X, Xia WX, et al. Induction chemotherapy with lobaplatin and fluorouracil versus cisplatin and fluorouracil followed by chemoradiotherapy in patients with stage III-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):716-726. doi: 10.1016/S1470-2045(21)00075-9 [DOI] [PubMed] [Google Scholar]
- 41.He Y, Zhao Z, Wang Y, et al. Optimizing number of cycles of induction chemotherapy for patients with nasopharyngeal carcinoma: retrospective survival analysis. Head Neck. 2020;42(8):2067-2076. doi: 10.1002/hed.26141 [DOI] [PubMed] [Google Scholar]
- 42.Peng H, Chen L, Li WF, et al. Optimize the cycle of neoadjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: a propensity score matching analysis. Oral Oncol. 2016;62:78-84. doi: 10.1016/j.oraloncology.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 43.Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 trials. Eur J Cancer. 2011;47(5):656-666. doi: 10.1016/j.ejca.2010.10.026 [DOI] [PubMed] [Google Scholar]
- 44.Loong HH, Ma BB, Leung SF, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):300-304. doi: 10.1016/j.radonc.2011.12.022 [DOI] [PubMed] [Google Scholar]
- 45.Guo SS, Tang LQ, Zhang L, et al. The impact of the cumulative dose of cisplatin during concurrent chemoradiotherapy on the clinical outcomes of patients with advanced-stage nasopharyngeal carcinoma in an era of intensity-modulated radiotherapy. BMC Cancer. 2015;15:977. doi: 10.1186/s12885-015-1964-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv JW, Qi ZY, Zhou GQ, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients receiving additional induction chemotherapy. Cancer Sci. 2018;109(3):751-763. doi: 10.1111/cas.13474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu SL, Sun XS, Yan JJ, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on induction chemotherapy response. Radiother Oncol. 2019;137:83-94. doi: 10.1016/j.radonc.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 48.Peng L, Chen JL, Zhu GL, et al. Treatment effects of cumulative cisplatin dose during radiotherapy following induction chemotherapy in nasopharyngeal carcinoma: propensity score analyses. Ther Adv Med Oncol. 2020;12:1758835920937424. doi: 10.1177/1758835920937424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen DW, Li ZX, Chen FP, et al. Individualized cumulative cisplatin dose for locoregionally-advanced nasopharyngeal carcinoma patients receiving induction chemotherapy and concurrent chemoradiotherapy. Oral Oncol. 2020;107:104675. doi: 10.1016/j.oraloncology.2020.104675 [DOI] [PubMed] [Google Scholar]
- 50.Bossi P, Chan AT, Licitra L, et al. ; ESMO Guidelines Committee; EURACAN . Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(4):452-465. doi: 10.1016/j.annonc.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 51.Lee A, Chow JCH, Lee NY. Treatment deescalation strategies for nasopharyngeal cancer: a review. JAMA Oncol. 2021;7(3):445-453. doi: 10.1001/jamaoncol.2020.615 [DOI] [PubMed] [Google Scholar]
- 52.Wong KCW, Hui EP, Lo KW, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. 2021;18(11):679-695. doi: 10.1038/s41571-021-00524-x [DOI] [PubMed] [Google Scholar]
- 53.Xia WX, Lv X, Liang H, et al. A randomized controlled trial comparing two different schedules for cisplatin treatment in patients with locoregionally advanced nasopharyngeal cancer. Clin Cancer Res. 2021;27(15):4186-4194. doi: 10.1158/1078-0432.CCR-20-4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mai HQ, Li XY, Mo HY, et al. De-intensified chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma based on plasma EBV DNA: a phase 2 randomized noninferiority trial. J Clin Oncol. 2021;39(15_suppl):110. doi: 10.1200/JCO.2021.39.15_suppl.110 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. List of Participating Centers
eTable 2. Treatment Compliance
eTable 3. Distribution of Disease Recurrence or Death in the Two Treatment Groups
eTable 4. Summary of Salvage Treatments After Disease Recurrence
eTable 5. Treatment-Related Acute Adverse Events During Induction Chemotherapy
eTable 6. Treatment-Related Acute Adverse Events During Concurrent Chemoradiotherapy
eTable 7. Late Radiotherapy Toxicities After Treatment
eFigure 1. Study Design
eFigure 2. The Relative Dose Intensity of Chemotherapy Drugs in the Two Treatment Groups During Induction Chemotherapy and Concurrent Chemotherapy
eFigure 3. The Proportional-Hazards Assumption for Failure-Free Survival Was Tested Using Schoenfeld Residuals Test
eFigure 4. The Estimated Restricted Mean Survival Time for Failure-Free Survival at Truncation Time of 36 Months in the Intention-to-Treat Population
eFigure 5. Kaplan-Meier Analysis of Failure-Free Survival (A), Distant Metastasis-Free Survival (B), Locoregional Relapse-Free Survival (C), and Overall Survival (D) in the Two Treatment Groups in Per-Protocol Population
eFigure 6. The Estimated Restricted Mean Survival Time for Failure-Free Survival at Truncation Time of 36 Months in the Per-Protocol Population
eFigure 7. Subgroup Analysis of Failure-Free Survival in the Intention-to-Treat Population
eFigure 8. Kaplan-Meier Analysis of Failure-Free Survival in the Treatment Groups Stratified by the TNM Staging Classifications: (A) T1-3, (B) T4, (C) N0-1, (D) N2-3, (E) IVA, and (F) IVB
eFigure 9. Kaplan-Meier Analysis of Failure-Free Survival in the Treatment Groups Stratified by the Baseline EBV DNA: (A) ≤ 1500 copies/mL, (B) > 1500 copies/mL
Data Sharing Statement


