Abstract
Introduction:
Methotrexate (MTX) is included in the therapeutic armamentarium of Crohn’s disease (CD), although its positioning is currently uncertain in an era in which many effective biological drugs are available. No systematic reviews or meta-analysis have stratified the clinical outcomes of MTX according to the specific clinical scenarios of its use.
Methods:
Medline, PubMed and Scopus were used to extract eligible studies, from database inception to May 2021. A total of 163 studies were included. A systematic review was performed by stratifying the outcomes of MTX according to formulation, clinical indication and criteria of efficacy.
Results:
The use of MTX is supported by randomized clinical trials only in steroid-dependent CD, with similar outcomes to thiopurines. The use of MTX in patients with steroid-refractoriness, failure of thiopurines or in combination with biologics is not supported by high levels of evidence. Combination therapy with biologics can optimize the immunogenic profile of the biological drug, but the impact on long-term clinical outcomes is described only in small series with anti-TNFα. Other off-label uses, such as fistulizing disease, mucosal healing, postoperative prevention and extraintestinal manifestations, are described in small uncontrolled series. The best performance in most indications was shown by parenteral MTX, favouring higher doses (25 mg/week) in the induction phase.
Discussion:
Evidence from high-quality studies in favour of MTX is scarce and limited to the steroid-dependent disease, in which other drugs are the leading players today. Many limitations on study design have been found, such as the prevalence of retrospective underpowered studies and the lack of stratification of outcomes according to specific types of patients and formulations of MTX.
Conclusion:
MTX is a valid option as steroid-sparing agent in steroid-dependent CD. Numerous other clinical scenarios require well-designed clinical studies in terms of patient profile, drug formulation and dosage, and criteria of efficacy.
Keywords: Crohn’s disease, extraintestinal manifestations, methotrexate
Introduction
Methotrexate (MTX) has a long history as an effective therapy of oncological diseases, such as acute leukaemia, or rheumatologic diseases, such as rheumatoid arthritis and psoriasis. Over the past 30 years, several studies have also evaluated its efficacy in the treatment of inflammatory bowel disease (IBD).
The first description of MTX use in Crohn’s disease (CD) dates back to 1989 with the pilot study by Kozarek et al. 1 Since then, MTX has been included in international therapeutic guidelines,2–5 although its positioning is currently uncertain in an era in which new and effective drugs are available, in particular the anti-TNFα (infliximab – IFX, adalimumab – ADA, certolizumab pegol – CZP) or anti-integrin (vedolizumab – VDZ) and anti-IL12/23 (ustekinumab – UST) biologics.
Despite this, new interest has been raised about MTX in CD. In fact, the literature has been enriched with new studies aimed at delineating the best patient profile to benefit from this therapy according to age, concomitant or previous therapies, comorbidities and disease behaviour.
In this review, we will focus on the adult literature, although it should be noted that MTX is also emerging in the paediatric literature as the preferred immunosuppressant compared with thiopurines 6 because of concerns about rare cases of lymphoma in young males, treated with thiopurines in combination with anti-TNFα. 7 A recent systematic review of observational studies demonstrated the ability of MTX to induce clinical remission of disease in paediatric patients with CD in nearly 60% of cases at 3–6 months. 8 Thus, it is expected that more patients on MTX therapy will transit in the hands of the adult gastroenterologist.
Finally, MTX has been studied in the treatment of ulcerative colitis (UC),9,10 but the recent METEOR and MERIT-UC trials failed to demonstrate the efficacy of MTX in inducing and maintaining disease remission at 24 and 54 weeks, respectively.11,12
Methods
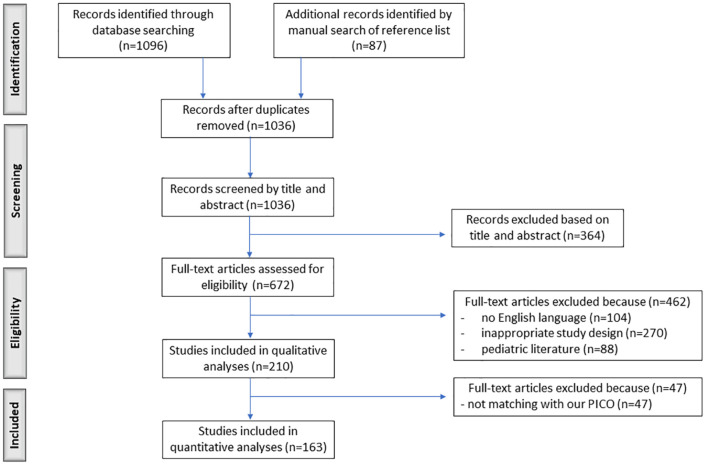
Medline, PubMed and Scopus were used to extract eligible studies, from database inception to May 2021. The terms ‘methotrexate’ AND (IBD OR Crohn’s) AND (combination therapy OR biologics OR infliximab OR adalimumab OR golimumab OR certolizumab OR vedolizumab OR ustekinumab OR extraintestinal manifestations OR erythema nodosum OR pyoderma gangrenosum OR arthritis OR spondylitis OR uveitis OR sclerosing cholangitis) were matched. The initial search yielded 1096 results, which were extracted by three independent gastroenterologists (A.C., M.P. and C.C.C.). Further selection was performed by the specialists in rheumatology (A.B.), dermatology (M.L.) and ophthalmology (P.R.) for each respective field of expertise, finding an additional 87 studies. After the exclusion of duplicates, overlapping and inappropriate records, a total of 163 studies were included (Figure 1). All authors finally analyzed the specific indications for which MTX was used. For each indication, formulation and dosage, we performed a systematic review following the rules of the Preferred Reporting Items for System-atic Reviews and Meta-Analysis (PRISMA) Statement. Clinical use of topical MTX was not included.
Figure 1.
PRISMA flow diagram of study screening and selection.
Results
Pharmacokinetics, formulation and dosage
The formulation and dosage of MTX may influence the clinical efficacy of the drug. MTX can be administered through oral, subcutaneous, intramuscular and intravenous routes. Its individual bioavailability varies from 45% to 100% depending not only on the route of administration but also on various patient-dependent factors and the indication for treatment (type, extent and activity of disease). For example, it is not clear whether established pharmacokinetic data demonstrated in non-gastroenterological (eg, rheumatologic) case series can be transferred to patients with CD, depending on the site and inflammatory activity of the disease, particularly with regard to small bowel disease. 13
In general, the bioavailability of the oral route is thought to be slightly lower than that of the parenteral route. 14 After oral intake, MTX is absorbed in the proximal jejunum by a saturable, dose-dependent process. 15 It follows that the bioavailability of the oral form is higher at low doses (up to 15 mg), whereas intestinal absorption may be relatively lower with higher doses of the drug. However, in patients with proximal small bowel CD, the absorption and, therefore, the bioavailability of the drug might be reduced, but specific studies are lacking.
In quiescent CD, two studies described a bioavailability of 73–86% for the oral form compared with the subcutaneous form.16,17 Despite this lower bioavailability than the parenteral formulation, some authors believe that, at least in patients with CD in remission, the oral form (preferred by patients for convenience, if tolerated) should not be discarded a priori, according to some positive results in uncontrolled series. However, as it will be shown below, the oral formulation is not supported by randomized controlled trials (RCTs) in CD.
To improve the bioavailability of the oral formulation of MTX, double split dose administration of oral MTX (halved doses 8 h apart, dose ranging 25–35 mg/week) has been proposed in rheumatoid arthritis, 18 but this strategy has not yet been tested in IBD. More recently, several formulations for targeted release have been developed exploiting the lymphatic route to increase drug bioavailability and thus reduce side effects, using micelles,19,20 microspheres, 21 nanoparticles,22,23 liposomes, 24 polymersomes, 25 nanoemulsions 26 and glucan particles, 27 but none of these formulations have been tested in IBD.
The use of intramuscular MTX in adult CD is supported by controlled studies of induction and maintenance of remission, in specific clinical scenarios (see below). The intramuscular formulation is associated with side effects, such as neuropathy, local irritation, pain, bleeding, fibrosis, abscesses, gangrene, and local contractures. However, the subcutaneous formulation of MTX is a viable alternative for parenteral use; although not used in controlled studies in adult CD, subcutaneous injections have shown similar pharmacokinetics to the intramuscular form, with comparable serum drug concentrations.28,29 In addition, the subcutaneous formulation is burdened with less local toxicity at the injection site and is suitable for self-administration by the patient.29,30
However, it is believed by some authors that the dose is more important than the formulation in determining the efficacy of MTX. In this regard, few studies comparing the various doses of MTX did not stratify the patient by the route of administration or indication for treatment. Egan et al., 9 in a small single-blind study, randomized patients with steroid-dependent IBD to two different doses of intramuscular MTX (25 versus 15 mg) plus steroids, but the authors included both patients with CD and UC in the analysis, thus not providing a picture for the specific scenario of CD.
Finally, no protocols for therapeutic monitoring of MTX are currently available.31,32 MTX is metabolized to active polyglutamates that accumulate in cells. The intracellular level of polyglutamates in red blood cells reflects systemic exposure, but two small studies described little correlation between these concentrations and the control of IBD.9,33
Efficacy in intestinal indications
In 1989, Kozarek et al. 1 were the first authors to describe the use of MTX in the induction of a clinical response in active CD. This was an open-label, pilot study, with 14 patients refractory to various therapies at that time (steroids, salazopyrin and metronidazole), which were treated with intramuscular MTX at a dose of 25 mg/week for 12 weeks, then switched to an oral maintenance dose of 15 mg/week in case of initial response; an unspecified proportion of these patients had also failed immunosuppressants. In total, 79% of patients reported a clinical response defined by the reduction in clinical activity index and significant reduction in steroid dose, but the steroid-free remission dropped to 50% at 12 weeks.
Several subsequent studies have described the use of MTX in other patients series (Tables 1–5), which are very heterogeneous in terms of disease behaviour (steroid-dependent or -refractory disease, intolerance or refractoriness to thiopurines, previous or concomitant biologic therapy), treatment regimen (formulation, dose and duration of therapy) and outcomes analyzed (response versus remission), thus providing some confusion in the generalization of results. Controlled studies are even few (Table 1), and published meta-analyses suffer from the limitations of the included studies, without focusing on the specific indication for treatment.34–38 Therefore, we report the available evidence sorted, where possible, by type of patient and indication for MTX use.
Table 1.
Prospective controlled studies using methotrexate in Crohn’s disease.
| Indication | Comparator | No. of patients | MTX formulation | MTX dosage (mg/week) | Concomitant steroids (% of patients) | Previous thiopurines | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Feagan et al. 39 | Chronically active CD despite daily dose of at least 12.5 mg of prednisone with at least one attempt to discontinue treatment | Placebo | 141 (MTX = 94; placebo = 47) | i.m. | 25 | 100% | Naive | 16-week clinical remission; 39.4% MTX versus 19.1% placebo (p = 0.025) |
| Oren et al. 41 | Chronic active CD (at least 7.5 mg/day for at least 4 months during the preceding 12 months) | Placebo | 84 (MTX = 26; 6-MP = 32; placebo = 26) | os | 12.5 | 80% (MTX) versus 79% (6-MP) versus 73% (placebo) | 12% (MTX), 27% (6-MP), 15% (placebo). Patients had to be off immunosuppressors for at least 3 months at the time of trial entry | 9-month clinical remission; 39% MTX versus 41% 6-MP versus 46% placebo (p = n.s.) |
| Arora et al. 42 | Steroid-dependent CD | Placebo | 28 (MTX = 13; placebo = 15) | os | 15–22.5 | 26.7% taking at least 20 mg/day prednisone (versus 55.6% in placebo group) | 46.7% (MTX), 22.2% (placebo) | 1-year clinical relapse; 46% MTX versus 80% placebo (p = n.s.) |
| Mate-Jimenez et al. 10 | Steroid-dependent CD | 6-MP 1.5 mg/kg/die, mesalazine 3 g/die | 38 (MTX = 15; 6-MP = 16; placebo = 7) | os | 15 | 100% | Naive | 30-week clinical remission; 93.7% 6-MP versus 80% MTX (p = n.s.) versus 14% 5-ASA (p = 0.01). 76-week remission (in early responders); 53.3% 6-MP versus 66.6% MTX p = n.s.) versus 0% 5-ASA (p < 0.001) |
| Feagan et al. 43 | Chronically active CD despite daily dose of at least 12.5 mg of prednisone with at least one attempt to discontinue treatment | Placebo | 76 (MTX = 40; placebo = 36) responders to MTX 25 mg/week for 16–24 weeks | i.m. | 15 | 0% | 2% | 40-week clinical remission; 65% MTX versus 39% placebo (p = 0.04) |
| Ardizzone et al. 40 | Chronic active CD despite daily dose of at least 10 mg of prednisone with at least one attempt to discontinue treatment | AZA | 54 (MTX = 27; AZA = 27) | i.v. for 3 months, then os for 3 months | 25 | 100% | 15% (MTX), 7% (AZA). Patients had to be off immunosuppressors for at least 3 months at the time of trial entry | 3-month clinical remission; 44% MTX versus 33% AZA (p = n.s.) 6-month clinical remission; 56% MTX versus 63% AZA (p = n.s.) |
Black-coloured cells, negative outcomes; CD, Crohn’s disease; grey-coloured cells, positive outcomes; i.m., intramuscular; i.v., intravenous; MTX, methotrexate; n.s., not significant.
Table 2.
Specific studies which included patients who failed thiopurines for intolerance or refractoriness.
| Author | Study design | No. of patients | MTX dosage (weekly) | Steroid-dependent versus steroid-refractory patients (%) | Failures to thiopurines (intolerant/refractory; %) | Failures to anti-TNFα (%) | Steroid-free clinical remission | Clinical remission (%) |
|---|---|---|---|---|---|---|---|---|
| Vandeputte et al. 46 | Retrospective | 20 | 25 mg i.m. for 12 weeks, then 12.5 mg i.m. | 65% /35% | 100% (25%/75%) | n.r. | Yes | 20% (3 months), 30% (6 months), 10% (1 year) |
| Soon et al. 50 | Retrospective | 66. Dropout 28% | Median 15 mg (5–30), os (97%) | n.r. | 100% (n.r.) | n.r. | Yes | 39.6% (6 months) |
| Domènech et al. 53 | Retrospective | 22 | 25 mg i.m./s.c. for 16 weeks + steroids for 3–4 months, then 10–15 mg i.m./s.c. (84%) or os (16%) | 100%/0% | 100% (55%/45%) | n.r. | Yes | 77% (1 year), 46% (2 years), 39% (3 years) |
| Wahed et al. 54 | Retrospective | 131 (99 CD, 32 UC) |
Mean 25 mg (7.5–25 mg) then 15 mg (15–25 mg) for median 72 weeks (7–208); i.m./s.c. 47% | n.r. | 100% (71%/29%) | n.r. | Yes | Intolerant: 62% (6 months) Refractory: 60% (6 months) |
| Hausmann et al. 56 | Retrospective | 63 | 25 mg i.m./s.c. (49%) or os (51%). Monotherapy 38% versus 43% combo IFX versus 19% combo steroids |
n.r. | 100% (49%/51%) | n.r. | n.r. | 79% (3 months), 75% (6 months), 71% (1 year), 50% (3 years) |
| Seinen et al. 61 | Retrospective | 174 | 25 mg s.c. for 3–4 months, then 15 mg s.c. (except for 15 patients) | n.r. | 100% (n.r.) | 23% | Not clear (‘Sustained clinical benefit’) | 86% (6 months), 63% (12 months), 47% (24 months), 20% (60 months) |
| Huang et al. 63 | Retrospective | 51 | 20 mg s.c. | 100%/0% | 100% (31.4%/ 68,6%) | 31.4% | Yes | 68.6% (16 weeks), 94.3% (24 weeks), 74.3% (36 weeks), 60% (48 weeks), 45.7% (60 weeks) |
| Wang et al. 65 | Retrospective | 27 | 15 or 25 mg, i.m. | n.r. | 100% (n.r.) | 29.6% | Yes | 48.1% (24 weeks) |
CD, Crohn’s disease; IFX, infliximab; i.m., intramuscular; MTX, methotrexate; n.r., not reported; s.c., subcutaneous; UC, ulcerative colitis; TNF, tumour necrosis factor.
Table 3.
Other uncontrolled studies using MTX for luminal active CD.
| Author | Study design | No. of patients | MTX dosage (weekly) | Steroid-dependent versus steroid-refractory patients (%) | Failures to thiopurines (intolerant/refractory; %) | Failures to anti-TNFα | Steroid-free clinical remission | Clinical remission (%) |
|---|---|---|---|---|---|---|---|---|
| Kozarek et al. 1 | Open, prospective | 21 (14 CD, 7 UC) | 25 mg i.m. for 12 weeks, then tapering to minimum 7.5 mg os | n.r. | 48% (including UC) | n.a. | n.r. | 50% (12 weeks) |
| Baron et al. 44 | Open, prospective | 19 (11 CD, 8 UC) | 15 mg os for 18 weeks | 100%/0% | 91% (n.r.) | n.a. | Yes | 20% (18 weeks) |
| Lemann et al. 45 | Retrospective | 39 | 25 mg i.m. for at least 3 months, then minimum 7.5 mg os | 38%/23% | 92% (41%/51%) | n.a. | Yes | 51% (1 month), 72% (3 months), 41% (1 year) |
| Lémann et al. 47 | Open, prospective | 49 | 25 mg i.m. for at least 3 months, then 7.5 mg os or 25 mg i.m. | Mixed, not specified | 86% (n.r.) | n.r. | n.r. | 84% (median 1.6 months, range 0–31) 59% (1 year), 49% (2 years), 43% (3 years) |
| Chong et al. 48 | Retrospective | 67 | Mean 20 mg i.m. or s.c. (78%) or os (22%) | 6%/94% | 85% (n.r.) | n.r. | Yes | 37% (mean 22 weeks, range 1–81). 75% (1 year), 31% (2 years), 21% (4 years) Only parenteral: 80% (1 year), 70% (2 years), 47% (4 years) |
| Fraser et al. 49 | Retrospective | 70 (48 CD, 22 UC) | Mean 20 mg (10–25 mg) os (89%). Dropout 21% | Mixed, not specified | Most patients, n.r. | n.r. | Yes | 62% (timing not specified) Among responders: 99% (1 year), 73% (2 years), 51% (3 years) |
| Hayee and Harris 51 | Retrospective | 24 | 25 mg i.m. (87%) for 16 weeks, then 15 mg i.m. | n.r. | 92% | n.r. | n.r. | 79% (16 weeks), 42% (1 year) |
| Nathan et al. 30 | Retrospective | 45 | Mean 21 (10–25 mg) s.c., duration not specified | n.r. | 91% (71%/20%) | 33% | Yes | 9% (timing not specified) |
| Din et al. 52 | Retrospective | 39 | 25 mg i.m. for 19 weeks, then 15 mg os | 53%/10% | 97% (61%/36%) | 61% | n.r. | 26% (16 weeks), 22% (50 weeks) |
| Parker et al. 56 | Retrospective | 37 | Median 20 mg (15–25), os 95% | n.r. | n.r. | n.r. | n.r. | ‘response’ 78% (12–18 weeks) |
| Saibeni et al. 58 | Retrospective | 112 (89 CD, 23 UC) | Median 20 mg (7.5–25) i.m. (82%), os (16%), s.c. (0.9%), i.v. (0.9%), including UC | 50%/5% | 76% (62%/15%) | n.r. | n.r. | ‘response’ 64.1% (timing not specified) |
| Chande et al. 57 | Retrospective | 79 | Median cumulative dose 1727 mg, i.m. or s.c. (87%) | n.r. | 53% | 38% | n.r. | 51% (timing not specified) |
| Suares et al. 59 | Retrospective | 66 | 25 mg s.c. for 4 months, then 15 mg s.c. (72.5%, range 7.5–25 mg; os or s.c.) | n.r. | 92.4% (27%/65%) | 27% | n.r. | ‘response’ 89.5% (4 months) |
| González-Lama et al. 60 | Retrospective | 77 (62 UC, 15 CD) |
Mean 21 mg, i.m. or s.c. (67%) or os (33%) | 94%/6% | 88% (61%/27%) | n.r. | Yes | 28% (timing not specified) |
| Kopylov et al. 62 | Retrospective | 118 | Induction: 23.75–25 os (31.4%) or parenteral (68.6%). Maintenance: 18 mg (15–25 mg), os 49.1% |
84%/n.r. | 49% (29%/20%) | 33.6% | Yes | Induction 37.2% (timing not specified) Maintenance 63.6% (median 12 months, range 3.5–18.5) |
| Mesonero et al. 68 | Retrospective | 110 | 25 mg/week (81%), 20 mg/week (4.5%), 15 mg/week (14.5%); parenteral 94% |
n.r./ n.r. (49% on steroids) |
77% (52%, 25%) | 100% (one drug 39%, two drugs 55.6%) | Yes | 30.9% (12–16 weeks) In initial responders, 82% (12 months), 74% (24 months) |
CD, Crohn’s disease; i.m., intramuscular; i.v., intravenous; MTX, methotrexate; n.a., not available; n.r., not reported; s.c., subcutaneous; UC, ulcerative colitis; TNF, tumour necrosis factor.
Table 4.
Mucosal healing and MTX.
| Author (year) | Study design | No. of patients | MTX formulation | MTX dosage | Mucosal healing |
|---|---|---|---|---|---|
| Kozarek et al. 1 | Prospective, open | 14 | Intramuscular | 25 mg/week | Total 5/11 (45%), only in colonic disease. |
| Manosa et al. 71 | Retrospective | 8, steroid-dependent | Parenteral (s.c./i.m.) | 25 mg/week for 16 weeks, then 15 mg/week on maintenance | Complete in 3/8 (37.5%), partial in 2/8 (25%) |
| Huang et al. 63 | Retrospective | 31 | Subcutaneous | 20 mg/week | Complete in 47.4% at 36 weeks |
| Laharie et al. 70 | Prospective, comparing MTX, AZA and IFX | 51, quiescent | Parenteral | 15–25 mg/week | Absence of ulcers in 11% MTX versus 50% AZA versus 60% IFX (p = 0.008 versus MTX) |
| Rouiller-Braunschweiga et al. 64 | Retrospective | 93 | n.r. | n.r. | 11.8% if MTX < 3 months 9.5% if MTX > 3 months |
| Vasudevan et al. 72 | Retrospective, comparing IFX/ADA and MTX/thiopurines | 269 (77 MTX, 192 thiopurines, 156 IFX 113 ADA) |
Not clear: s.c. in 58% of patients on at least 20 mg/week | Median 20 mg, IQR 10–25 mg/week. 71% of patients on at least 15 mg/week, 61% on at least 20 mg/week | 58% anti-TNF + thiopurines 17% anti-TNF + MTX (p < 0.01) at 12 months |
CD, Crohn’s disease; IFX, infliximab; IQR, interquartile range; MTX, methotrexate.
Table 5.
Studies using MTX in combination with anti-TNFα agents.
| Biological drug | Study design | No. of patients | MTX dosage and formulation | Previous anti-TNF (%) | Combo (MTX versus AZA) | Clinical benefit with combined treatment | |
|---|---|---|---|---|---|---|---|
| Schröder et al. 83 | IFX | Open, controlled | 19 | 20 mg/week for 5 weeks, then 20 mg/week os | 0 | 100% (n.r.) | Yes |
| Sokol et al. 80 | IFX | Prospective, registry (MICISTA) | 121 | n.r. | 86.8 | 100% (n.r.) | No |
| Feagan et al. 79 | IFX | RCT | 126 | 10 mg/week s.c., up to 25 mg/week | 0 | 100% (100%/0%) | No |
| Colman and Rubin 84 | IFX | Retrospective | 73 IBD (54 CD) | < 12.5 mg/week versus > 12.5 mg/week. Formulation not reported. | n.r. | 100% (100%/0%) | Same results |
| Borren et al. 85 | IFX | Retrospective | 222 IBD (163 CD) | 12.5 mg/week os versus > 12.5 mg/week, parenteral | 76 | 100% (100%/0%) | Same results |
| Targownik et al. 81 | IFX/ADA | Retrospective, population-based database | 852 (617 IFX, 235 ADA) | n.r. | n.r. | 52% (92%/8%) | Yes for some outcomes |
| Targownik et al. 82 | IFX/ADA | Retrospective, population-based database | 8129 (5050 IFX, 3079 ADA) | n.r. | n.r. | 39.1% (84%/16%) | No |
| Cosnes et al. 86 | IFX/ADA | Retrospettivo, multicentrico (MICISTA), comparing IFX/ADA combo versus mono | 906 [IFX 587 (374 combo), ADA 319 (152 combo), 442 thiopurines, 77 MTX] | 15 mg/week os | 0 | 58% (16% versus 84%) | Yes |
| Hanauer et al. 87 (CLASSIC-I) | ADA | RCT | 225 | n.r. | 0 | 29.8% (3.5%/26%) | No |
| Sandborn et al. 88 (CLASSIC-II) | ADA | RCT | 241 | n.r. | 0 | 34% (2.5%/27.8%) | No |
| Sandborn et al. 89 (GAIN) | ADA | RCT | 159 | n.r. | 100 | 46% (n.r.) | No |
ADA, adalimumab; AZA, azathioprine; black-coloured cells, negative outcomes; CD, Crohn’s disease; grey-coloured cells, positive outcomes; IBD, inflammatory bowel disease; IFX, infliximab; MTX, methotrexate; n.r., not reported; RCT, randomized controlled trial; TNF, tumour necrosis factor.
Induction of remission in steroid-dependent or refractory CD
Steroid-dependency is the only indication for which MTX currently has evidence from RCTs in CD, all performed in the pre-biological era (Table 1).10,39–43 Many other uncontrolled studies, mostly retrospective (Tables 2 and 3),9,44–65 are also available, but they have included heterogeneous populations of both steroid-refractory and steroid-dependent patients, thus preventing a clear interpretation of the performance of the various formulations of MTX in these two distinct clinical scenarios. In particular, the efficacy of MTX in steroid-refractory patients is not analyzed in any specific study.
In steroid-dependent CD, the placebo-controlled, double-blind, multicenter study by Feagan et al. 39 concerned a group of patients, naive to immunosuppressants and biologics, who according to a now ‘dated’ definition can be defined as steroid-dependent, 66 as they were active after at least one attempt to withdraw the steroid and therefore maintained on treatment with at least 12.5 mg of prednisolone/day. The authors demonstrated superiority of intramuscular MTX at a dose of 25 mg/week compared with placebo, with remission achieved at 16 weeks in more than one-third of patients (p = 0.025).
Opposite results were presented by two other placebo-controlled studies using oral MTX and lower doses ranging 12.5–22.5 mg/week, once again in steroid-dependent CD.41,42
Two further RCTs compared MTX with thiopurines, without significant differences as steroid-sparing agents in steroid-dependent CD.10,40 MTX was used, in addition to steroids, at the oral dose of 15 mg/week, in the first study by Matè-Jimenez 10 (induction of remission at 30 weeks = 80% MTX versus 93.7% 6-MP versus 14% mesalazine), while it was used at intravenous doses of 25 mg/week for 3 months, followed by the 25 mg/week oral dose for another 3 months, in the second study by Ardizzone et al. 40 (steroid-free remission = 44% MTX versus 33% AZA at 3 months; 56% MTX versus 63% AZA at 6 months).
The onset of clinical benefit with MTX, in terms of significant steroid reduction, is reported with variable latency, averaging 12 weeks. 67
Second-line immunosuppressive therapy in patient’s failures to thiopurines
No prospective controlled trials using MTX in patient’s failures to thiopurines monotherapy and naive to biologics are available. In adults, only eight retrospective studies, most using parenteral MTX, have described case series specifically limited to patients defined as failures to thiopurines,46,50,53,54,56,61,63,65 with variable remission rates: 30–86% at 6 months, 10–77% at 1 year and 20% at 5 years (Table 2); however, the outcome was not clearly stratified by the type of failure (ineffectiveness versus intolerance) and by immunosuppressant indication (steroid-dependency versus refractoriness), except in two cases. Domènech et al., 53 in 22 steroid-dependent patients (10 refractory and 12 intolerant to thiopurines), reported a steroid-free clinical remission with MTX (used parenterally in 84% of cases) of 77% at 16 weeks in the entire case series, which was maintained in 72%, 46% and 39% of cases at 1, 2 and 3 years, respectively. Even more specifically, Huang et al. 63 in a recent case series of 51 steroid-dependent CD patients reported steroid-free clinical remission at 16 weeks in 65.7% and 75% of patients refractory or intolerant to thiopurines, respectively.
Third-line therapy in patient’s failures to biologics
No controlled studies have been published for this clinical scenario. Some uncontrolled studies have included patients treated with MTX after failure of anti-TNFα drugs (Tables 2 and 3)30,52,56,57,59,61–63,65 but did not provide the outcomes of this specific subgroup. Recently, a retrospective multicenter Spanish study from the ENEIDA registry, specifically described 110 patients, with previous failure to at least one anti-TNFα agent, who had switched to MTX monotherapy. 68 Before switching to MTX, 77% of patients had already received a thiopurine; 54 patients (49%) were taking concomitant steroids. The induction dose of MTX was predominantly 25 mg/week parenteral. Short-term clinical remission (week 16) was achieved in 30.9% of cases; of these responders, long-term effectiveness was maintained in 82% and 74% at 12 and 24 months, respectively. In the multivariate analysis, non-remission at short-term was associated with long-term failure.
No studies are available on the use of MTX as rescue therapy after the failure of VDZ or UST.
Maintenance of remission
Once again, Feagan was the first author of the only randomized, placebo-controlled study that demonstrated the efficacy of parenteral MTX for the maintenance of steroid-induced clinical remission, specifically in steroid-dependent CD. 43
In this multicenter study on 76 patients, an induction dose of 25 mg/week intramuscular for 16–24 weeks was used, followed by a maintenance dose of 15 mg/week intramuscular, for 40 weeks. Of note, no other CD-related medication was allowed in the maintenance phase.
In responders at week 16, the study reported a steroid-free remission rate of 65% at 40 weeks in the MTX group, compared with 39% in the placebo group (p = 0.04). Interestingly, about half of the patients who relapsed on MTX 15 mg/week regained remission after reinduction with the 25 mg/week intramuscular dose (along with prednisone).
Comparing MTX with thiopurines as maintenance therapy in CD, three RCTs, in a total of 77 patients (mostly steroid-dependent) for 24–76 weeks, did not conclude for the superiority of one drug over the other.10,40,41
A further pletora of studies, mostly retrospective, have been published describing small case series of patients treated with maintenance MTX, with variable dosages, durations of treatment and percentages of patients failures to thiopurines or IFX (Tables 2 and 3): the maximum follow-up described is 5 years, with a maintained clinical remission in 20% of patients. 62
From these case reports, although heterogeneous, it is clear that, even for MTX, secondary loss of response becomes a problem over time, with remission rates progressively decreasing over the years. While the steroid-free remission is described in a range of 10–80% of patients at 1 year42,43,46,50,51,53,61,63 and 46–70% at 2 years,10,48,53,61 the same falls to 39% at 3 years 53 and 20% at 5 years. 61
Current guidelines do not define the duration of therapy with MTX. However, a retrospective study, which included 19 patients with CD and UC, reported a high relapse rate after discontinuation, most often within 1 year. Remission rate after treatment withdrawal at 6, 12 and 18 months were 42%, 21% and 16%, respectively. 49 There is more evidence on the withdrawal of MTX in patients with rheumatoid arthritis, where better outcomes have been found by distancing the weekly doses to every 2 weeks than with stopping the drug. 69
Mucosal healing
No controlled studies have analyzed the endoscopic and histological healing of MTX as primary endpoints in CD. The ability of MTX to induce mucosal healing in CD is reported in two prospective uncontrolled studies1,70 and in four small retrospective studies,63,64,71,72 ranging from 11% to 45% according to different definitions of complete endoscopic remission. The most favourable data have been described with parenteral formulations, starting with doses of 20–25 mg/week (Table 4). However, the only prospective comparison study by Laharie et al., 70 reported significantly better mucosal healing rates both with AZA or IFX than with parenteral MTX (50%, 60% and 11%, respectively).
Another recent, retrospective, study compared the mucosal healing rate induced by combination therapy with anti-TNFα and AZA versus anti-TNFα and MTX, reporting rates of 58% and 17% (p < 0.01), respectively, at 12 months. 72 This difference was even more evident when the anti-TNFα drug used in combination was IFX rather than ADA: endoscopic remission at 12 months was not achieved in any of the 11 patients treated with MTX + ADA compared with 4/12 (33%) patients treated with MTX + IFX. 72
Regarding histologic response, only the Laharie’s study is available; however, no significant differences between MTX, AZA and IFX were described, using D’Haens’ score. 70
Fistulizing CD
The efficacy of MTX monotherapy in fistulizing CD has been evaluated in small retrospective studies (ranging from 4 to 29 patients), which included various types of fistulas, both perianal and internal.45,50,59,73,74 The anatomic type of fistula and its complexity is not always specified and its response to previous therapies. When reported, complete closure of fistulas occurred in 22–50% of patients.45,50,74
The only RCT by Ardizzone et al., 40 controlled to thiopurines, was not designed for this purpose, but in six patients with CD it reported the complete closure of perianal fistulas in 50% of patients at 1 month and 67% at 3 and 6 months.
The response to a combined approach with MTX plus IFX and surgery led to complete closure rates ranging from 33% to 74% of patients.75–77 In the study by Roumeguère et al., an initial drainage with possible seton placement was performed and a second surgical step with seton removal (as well as possible procedures, such as fibrin glue and/or reconstructive flap) was planned between the second and third infusion of IFX. Parenteral MTX 25 mg/week was administered 2–3 months after the first surgical step, followed by IFX 1 week later. 76
Prevention of postoperative recurrence
The use of MTX in the prevention of postoperative recurrence of CD has so far been described by only one small prospective study that analyzed the combination of IFX 5 mg/kg and low doses of oral MTX (10 mg/week), the latter given to reduce the immunogenicity of IFX (see below). 78 The authors compared 7 patients on combo treatment with 16 control patients treated with oral mesalazine. No patients on combination therapy experienced endoscopic recurrence at 2 years, compared with 12/16 patients on mesalazine.
Combination therapy with biologics: clinical efficacy
Infliximab. IFX + MTX combination therapy has not demonstrated favourable efficacy results to date, either in a RCT using high parenteral doses, 79 or in some registries using MTX at unspecified dosages (Table 5).80–82
The only exception was an early small randomized, open-label study by Schröder et al., 83 which compared IFX 5 mg/kg monotherapy (n = 8) versus IFX + MTX 20 mg/week (n = 11) for 48 weeks in 19 patients refractory or intolerant to AZA. MTX was infused intravenously for the first 5 weeks then administered per os. Clinical remission at 48 weeks was 71% in the combination group, compared with 33% on monotherapy.
Some years later, the COMMIT trial did not confirm these good impression using smaller doses (10 mg, increased to 25 mg/week at week 5) of subcutaneous MTX in 126 patients who were immunosuppressors-naive and received prednisone 6 weeks before. 79 Steroid-free remission was comparable at both 14 weeks (76% versus 78%) and 50 weeks (56% versus 57%) in combination therapy compared with IFX monotherapy, respectively. However, the potential effect of concomitant steroids in the induction phase of both arms makes uncertain this short-term outcome. Another bias might have been the lower dose of MTX used by Feagan compared with Schroeder. However, discordant conclusions have been provided in two retrospective studies, aimed by the research of the best dose of MTX to combine with IFX (⩽ 12.5 mg/week or higher).84,85
Adalimumab. Table 5 summarizes the outcomes of studies describing MTX in combination with ADA. A recent meta-analysis of 24 studies in CD, regarding the efficacy of adding an immunosuppressor to ADA treatment, did not conclude in favour of combination therapy in terms of improved remission rates or clinical response. 90 However, this meta-analysis and other studies86–89 did not stratify outcomes according to the type of immunosuppressor (MTX versus AZA) or previous exposure to biologics (naive versus failures). Data regarding MTX are really limited in terms of number of patients included and heterogeneity of interpretation and, therefore, no firm conclusions can be drawn.
Regarding the most appropriate dose of MTX when combined with ADA, there are not comparative studies in IBD. In rheumatoid arthritis, however, there is even a large RCT that examined four different oral dosages (2.5, 5, 10 and 20 mg), in biologic-naive patients: similar benefit-risk profile was found for 10 and 20 mg/week of MTX, with increased ADA trough levels. 91
Certolizumab pegol. The effect of combination therapy with CZP and an immunosuppressant, including MTX, is unknown. The PRECISE trials, in fact, have not reported data about the clinical efficacy of combination therapy.92,93 A retrospective single-centre study of 222 IBD patients (163 with CD) treated with MTX in combination with various anti-TNFα drugs had included 6 patients on CZP but did not report the specific outcomes. 81
Vedolizumab. Also for VDZ, we do not have prospective controlled studies, specifically designed to evaluate the effect of combination therapy with MTX (Table 6).
Table 6.
Studies using MTX in combination with VDZ.
| Study design | No. of patients | MTX dosage and formulation | Previous anti-TNFα (%) | Combo (MTX versus AZA) | Clinical benefit with combined treatment | |
|---|---|---|---|---|---|---|
| Sandborn et al. 94 (GEMINI2) | RCT | 967 | n.r. | 59.5 | 52% (n.r.) | No |
| Shelton et al. 95 | Retrospective, multicenter | 107 | n.r. | 97.1 | 31.7% (17.3%/14.9%) | No |
| Dulai et al. 96 (VICTORY Consortium) | Retrospective, multicenter | 215 | n.r. | 91 | 40% (n.r.) | No |
| Baumgart et al. 97 | Prospective, multicenter | 97 | n.r. | 94.8 | 80.4% (n.r.) | No |
| Stallmach et al. 98 | Prospective, multicenter | 67 | n.r. | 91 | 14.9% (n.r.) | No |
| Amiot et al. 99 | Prospective, multicenter | 173 | 25 mg/week parenteral | 100 | 25% (n.r.) | No |
| Allegretti et al. 100 | Retrospective, bicentre cohort | 96 completing 14-week induction VDZ | n.r. | n.r. | 54% (n.r.) | Yes |
| Amiot et al. 101 (OBSERV-IBD) | Prospective, multicenter | 161 responders to 14-week induction VDZ | 25 mg/week parenteral | 99.4% (90.7% more than two previous biologics) | 25% (n.r.) | No |
| Gouynou and Peyrin-Biroulet 102 | Case series | 2, non-responders to 14-week induction VDZ | 25 mg/week sc for 3 months, then 15 mg/week | n.r. | 2 (100%) | No |
| Samaan et al. 103 | Retrospective, two-centre | 27 CD + 23 UC | n.r. | 76% both diseases | 42% (2%/40%) both diseases | No |
| Eriksson et al. 104 | Retrospective (SWIBREG registry) multicenter | 147 | n.r. | 86 | 35% (n.r.) | No |
| Kopylov et al. 105 | Multicentre, prospective | 130 | n.r. | 92.6 | 24.6% (n.r.) | No |
| Kopylov et al. 106 | Retrospective, multicenter, | 50 | n.r. | 0 | 10% (n.r.) | No |
| Lenti et al. 107 | Retrospective, multicenter | 135 | n.r. | 95.5 | 51.8% (n.r.) | No |
| Macaluso et al. 108 | Retrospective, multicenter | 84 | n.r. | 76.1 | 8% (n.r.) | No |
| Iborra et al. 109 | Retrospective, multicenter | 30 | n.r. | 90 | 37% (n.r.) | No |
| Ylisaukko-Oja et al. 110 | Retrospective, multicenter | 108 | n.r. | 95.4 | 46% (n.r.) | No |
| Macaluso et al. 111 | Prospective database | 1 | n.r. | 100 | 100% | No |
| Hoffmann et al. 112 | Retrospective, single centre | 28 | n.r. | 89.3 | 67.9% (4%/11%) | No |
| Ylisaukko-Oja et al. 113 | Retrospective | 23 | 15.7 ± 6.8 mg/week, formulation not reported | 95 | 100% | No |
| Hu et al. 114 | Retrospective | 53 | n.r. | n.r. | n.r. | No |
AZA, azathioprine; black-coloured cells, negative outcomes; CD, Crohn’s disease; grey-coloured cells, positive outcomes; IBD, inflammatory bowel disease; MTX, methotrexate; n.r., not reported; RCT, randomized controlled trial; TNF, tumour necrosis factor; UC, ulcerative colitis; VDZ, vedolizumab.
A population pharmacokinetic modelling showed that MTX had no clinically relevant effect on VDZ linear clearance. 115 Regarding clinical efficacy, the placebo-controlled, phase III, GEMINI-2 and GEMINI-3 trials did not analyze the specific role of MTX as combination therapy.94,116
Further open-label real-life experiences, which are often limited by the retrospective design and low numbers of patients included (Table 4), have not described significant differences between VDZ monotherapy and immunosuppressant combination therapy,95–99,101–112,114 except in two studies.100,113
Similar to other biologic agent trials, major limitations of these uncontrolled case series are the inability to differentiate the specific contribution of MTX compared with other immunosuppressants (especially thiopurines) and the limited number of patients included in MTX arms. Only three studies, all referring to patient’s predominant (91–95%) failures to anti-TNFα agents, analyzed the specific contribution of MTX, without observing a significant effect of the combined treatment, with regard to intestinal clinical endpoints.97,98,111 However, Macaluso et al., 111 in a small case series of four patients (three CD and one UC) in whom MTX (15–25 mg/week, unspecified formulation) was added to VDZ monotherapy in case of persistent joint manifestations, reported an unspecified joint response in two cases at the 15 mg/week dose.
Two studies, including biologic-naive patients, did not report benefit from combination therapy but results were not stratified for type of immunosuppressive drug.109,106
Ustekinumab. Current data from literature do not suggest superiority of combination therapy with UST and immunosuppressants, including MTX. As with other biologics, however, there are no controlled trials specifically designed (Table 7).
Table 7.
Studies reporting MTX use in combination UST in CD.
| Author | Study design | No. of patients | MTX dosage and formulation | Previous anti-TNFα (%) | Combo (MTX versus AZA) | Clinical benefit with combined treatment |
|---|---|---|---|---|---|---|
| Sandborn et al. 117 | RCT | 131 | n.r. | 58 | 37.4% (6.9%/30.5%) | No |
| Sandborn et al. 118 (CERTIFI) | RCT | 394 | n.r. | 100 | 24.4% (n.r.) | No |
| Kopylov et al. 119 | Retrospective, single centre | 38 | n.r. | 100 | 10.5% (5.3%/5.3%) | No |
| Feagan et al. 120 | RCT | 741 (UNITI-1) + 628 (UNITI-2) + 397 (IM-UNITI) | n.r. | UNITI-1 100%, UNITI-2 and IM-UNITI not available | UNITI-1 30.8% (?), UNITI-2 34.9% (?), IM-UNITI 36.4% (?) | No |
| Khorrami et al. 121 | Retrospective, multicenter | 116 | n.r. | 100 | 36.2% (n.r.) | No |
| Wils et al. 122 | Retrospettive, multicentre | 122 | n.r. | 100 | 15% (5.7%/9%) | Yes (AZA and MTX) |
| Ma et al. 123 | Retrospective, multicenter | 167 | n.r. | 95.2 | 43.7% (n.r.) | No |
| Ma et al. 124 | Retrospective, multicenter | 104 | n.r. | 92.3 | 42.3% (n.r.) | Yes (AZA and MTX) |
| Battat et al. 125 | Retrospective, single centre | 62 | n.r. | 100 | 25.8% (16.1%/9.7%) | No |
| Greenup et al. 126 | Retrospective, single centre | 69 | n.r. | 99 | 42% (n.r.) | No |
| Wils et al. 127 | Retrospective | 88 responders to 1-year UST | n.r. | 100 | 14.8% (6%/9%) | No |
| Hu et al. 114 | Retrospective | 63 | n.r. | n.r. | n.r. | No |
AZA, azathioprine; black-coloured cells, negative outcomes; grey-coloured cells, positive outcomes; MTX, methotrexate; n.r., not reported; RCT, randomized controlled trial; TNF, tumour necrosis factor; UST, ustekinumab.
Phase II and III trial (UNITI-1, UNITI-2 and IM-MUNITI) sub-analyses did not show benefit from combination treatment, but these are small subgroups that render these analysis underpowered and do not provide specific data for MTX.117,118,120
Uncontrolled published trials are also limited by the retrospective design, the small number of included patients (range 2–8) and the inability to stratify the immunosuppressant drug.119,121–127
Combination therapy with biologics: immunomodulatory effects and drug optimization
In the COMMIT study, 79 and in a prospective study by Vermeire et al., 128 the combination of MTX + IFX was associated with significantly lower levels of anti-IFX antibodies compared to IFX monotherapy as well as with higher circulating levels of IFX: it is known that these parameters influence long-term outcomes, such as secondary loss of response to IFX and the development of infusion reactions.129,130 Both studies used parenteral MTX, with doses ranging from 10–15 mg/week.
Other studies, both in CD and rheumatoid arthritis, confirm the ability of MTX to affect the immunogenicity not only of IFX131–133 but also of ADA88,134–137 and VDZ, 138 especially by reducing the development of anti-drug antibodies.
Few studies, limited to the anti-TNFα treatment, have analyzed whether this immunomodulatory effect is matched by a clinical benefit. A recent meta-analysis focused on the clinical response associated with the addition of an immunosuppressant (MTX or AZA) to anti-TNFα therapy (four studies), without specifying MTX (n = 19) versus AZA (n = 30) outcomes. 139
Concerning IFX, two small retrospective studies have provided some clinical data, with positive results:140,141 MTX use rather than AZA was significantly associated with the risk of relapse (HR 3.37, 95% CI 1.14–9.96) in 43 patients who stopped combination therapy, 140 while both MTX (n = 2) and AZA (n = 3) restored clinical response in five patients with secondary loss of response to IFX. 141 The addition of parenteral MTX was useful also in small series (range 5–21) of patients who lost clinical response to ADA, without differences with AZA.135,137,142
Finally, Kennedy et al. performed the largest prospective study of anti-TNFα therapy in IBD, by enrolling 1610 patients with active luminal CD treated with IFX or ADA. Clinical variables that were associated with treatment failure were week 14 drug concentrations and immunogenicity. Combination therapy with a thiopurine or MTX mitigated this risk. MTX was used in 59/955 patients treated with IFX and in 30/655 patients treated with ADA. No difference was measured in terms of immunogenicity between thiopurines or MTX. 136
Overall, these studies may suggest specific synergistic and/or additive effects between MTX and IFX or ADA, favouring the sustainability of long-term efficacy of the anti-TNFα drug. No studies, on the contrary, are available on MTX use as rescue therapy in failures to VDZ or UST monotherapy.
Cross indications
Artropathies
The most common extraintestinal manifestation in IBD patients is the articular one. In a simplified way, we can distinguish the axial form, characterized by sacroileitis and spondylitis, from the peripheral form marked by arthritis and/or dactilitis and/or entesitis.
While MTX is considered the anchor drug of rheumatoid arthritis treatment and the most commonly prescribed conventional synthetic disease-modifying anti-rheumatic drug (DMARD), either as monotherapy or in combination with biologic or targeted synthetic DMARDs,143,144 there are not specific prospective controlled trials for the treatment of IBD-associated arthritis.
The use of MTX monotherapy in the axial forms of enteropathic arthritis is not supported by a Cochrane meta-analysis, 145 and it is not endorsed by international guidelines,146,147 which instead favour anti-TNFα therapies. Notably, in the Cochrane review, 145 three small RCTs with a total of 116 patients were analyzed.148–150 MTX doses ranged from 7.5–10 mg/week orally for 12–24 weeks, while parenteral use was not explored. Instead, a small open-label study by Haibel consisting of 20 patients using MTX 20 mg/week subcutaneously demonstrated an ASAS20 response of 25%, which is similar to placebo response rates in some studies with anti-TNFα agents. 151
In peripheral form of enteropathic arthritis, MTX showed its efficacy according to treatment guidelines for spondyloarthritis, 152 although there is no evidence derived from ad hoc studies. Responses to the drug, variously defined, have been described in cases of spondylitis associated with peripheral involvement, either as monotherapy or in combination with salazopyrin.151,153–157
Also, the use of MTX in combination with anti-TNFα agents, which is reported in treatment of other rheumatic diseases,158,159,160 lacks ad hoc studies in IBD-associated arthopaties. Conflicting data on the impact of MTX co-treatment on anti-TNFα survival are present in literature, with some observational cohort studies showing positive results,161–164 and a number of other large studies which demonstrated no benefits.165–168
While, a single prospective monocentre study in 65 patients with CD and 15 patients with UC demonstrated MTX efficacy in patients with paradoxical articular manifestations during anti-TNFα treatment, without reporting its formulation. 169
Psoriasis and psoriasis induced by anti-TNFα agents
MTX remains as one of the first-line treatments used in patients with psoriasis, despite its lower efficacy compared with ADA and IFX.170,171 In the context of IBD, one proposed – but unsuccessful – use for MTX is its combination with anti-TNFα agents to control treatment-induced psoriatic lesions. The available literature is limited to small series or case reports.
In the first published report, Chu et al. described a case of palmoplantar pustular psoriasis that appeared during ADA treatment and was refractory to topical steroids but sensitive to cyclosporine. Not only MTX (in an unspecified formulation) but also various other agents failed to switch to an alternative maintenance therapy to cyclosporine in this notoriously difficult-to-treat form of psoriasis. 172
Buisson et al. described the effect of MTX in the treatment of psoriasiform lesions that arose during anti-TNF therapy in seven patients with CD. Six patients received 25 mg/week of MTX, whereas only one patient received 7.5 mg/week; the formulation was parenteral in all but one patient. At the time of MTX introduction, some were continuing anti-TNF (n = 2), some switched to other anti-TNF (n = 3) and some discontinued the drug (n = 2). After a follow-up of 20–45 months (median 29 months), only one patient had a response, that lasted 42 weeks and then relapsed. 173
In the most recent study by Mazloom et al. in eight patients treated with MTX (only one case in monotherapy and the other seven in combination with topical therapy), only 4/8 showed an unspecified improvement, whereas the other four, including the patient in monotherapy, had no improvement. These patients belonged to a larger case series of 102 cases of anti-TNF-induced psoriasis, and the indications for anti-TNF were heterogeneous, including not only IBD but also rheumatologic patients; moreover, treatment outcome was not stratified by pathology or by type of psoriasis. The most useful MTX dose, when effective, was greater than 15 mg per week, whereas no patient treated with dosages below 10 mg had a benefit; the formulation was not specified. 174
Regarding treatment ab initio in patients with both psoriasis and CD, only a recent safety analysis of UST in the various phase II/III registration trials is available, which reports no different outcomes between UST monotherapy and UST in combination with MTX. 175
Other cutaneous manifestations
With regard to other cutaneous manifestations in CD, the use of MTX is only anecdotally described in small case series of pyoderma gangrenosum (PG) and erythema nodosum (EN), refractory to steroids,176,177 and in one case report of metastatic CD in combination with IFX. 74 RCTs for PG or EN are not available.
No studies have described the use of MTX in Sweet’s syndrome and in oral CD; on the contrary, oral ulcerations can occur as a side effect of MTX therapy.
Schmidt et al., in describing the favourable outcome of 16 patients treated with pulse cyclophosphamide in combination with AZA or MTX, reported a ‘substantial improvement’ (in terms of pain and regression in size and/or number of lesions) within 8 weeks, in all eight patients with PG (n = 5) or EN (n = 3), refractory to steroids, four of whom treated with MTX (not specifying the type of skin lesion in this treatment group). After up to 30-month follow-up, all patients has achieved and maintained complete remission of their skin lesions, but the authors did not describe how many of these remained on MTX therapy. 176
More recently, Duarte-Chang and Visuetti 177 presented the case of a young man with PG in the setting of active CD, refractory to systemic steroid, who recovered a full clinical response 4 weeks after the addition of MTX, at a dose of 25 mg/week subcutaneously for 16 weeks, followed by 15 mg/week of maintenance treatment.
Very few other case reports of MTX used for PG, not associated with CD, showed mixed results (favourable with oral, 178 unfavourable with unspecified formulations). 179
In another case report by Tonkovic-Capin et al., 180 low doses of oral MTX had a beneficial effect on orofacial swelling in a case of cheilitis granulomatosa accompanied by CD with recurrence despite systemic glucocorticoids. Cheilitis granulomatosa is a rare idiopathic condition with painless lip swelling, characterized by non-necrotizing granulomatous inflammation which may precede the presentation of CD even after long-term follow-up. MTX 5 mg orally once weekly was initiated. Within 2 months, there was a marked reduction in the patient’s facial swelling; increasing MTX dose to 10 mg orally once weekly yielded almost complete resolution of facial swelling. This beneficial response has been maintained for 16 months, continuing MTX at the same dosage.
Equally anecdotal is the case of a 35-year-old woman with severe fistulizing CD presented with pyostomatitis vegetans affecting both the mouth and the vulva. Pyostomatitis vegetans is a rare non-microbial neutrophilic disease of the oral mucosa, associated with IBD. Three injections of IFX and maintenance therapy with MTX (25 mg weekly) resulted in rapid and complete regression of both the pyostomatitis vegetans and the CD, during 15 months of follow-up. 181
Ocular manifestations
MTX has been frequently employed to treat ocular inflammatory diseases, including uveitis, scleritis, and orbital inflammatory disease. 182 While the use of MTX is advocated at the forefront of paediatric guidelines for the treatment of children with chronic anterior uveitis and juvenile idiopathic arthritis requiring systemic immunosuppression (after failure/intolerance of topical steroids),183–186 there are not evidence-based guidelines or specific case series about its use in the treatment of adult IBD-associated uveitis.
In general, the therapeutic approach to uveitis has, however, differed minimally for different non-infectious etiologies. Most clinical trials for uveitis enrolled patients with a specific anatomic location for the uveal inflammation but not a specific etiology. 182 Most forms of anterior uveitis respond particularly well to topical steroids, which are not adequate to treat intermediate and posterior uveitis. If topical steroids are not adequate, the treating physicians will usually embark on a trial of oral corticosteroids.
The early use of corticosteroid-sparing immunosuppression has been advocated by a Delphi panel. 187 Traditionally, MTX was the most popular immunosuppressive for this indication.
The first report on the use of MTX in uveitis was published in 1965 by Wong and Hersh, 188 who described positive effects in 9 of 10 patients with a diagnosis of ‘cyclitis’ who were refractory to systemic steroid therapy. Since then, small series have reported MTX to be effective for ocular inflammation in general, 189 and for specific ocular inflammatory conditions, including uveitis associated to juvenile idiopathic arthritis,190–193 sarcoidosis, 194 Behcet’s disease, 195 mucous membrane pemphigoid, 196 and rheumatoid arthritis. 197
A recent systematic review analyzed the adult literature regarding the treatment of anterior uveitis, both idiopathic and associated with systemic disorders (mainly ankylosing spondylitis): 198 with regard to MTX, a single-centre prospective study in 19 patients, 199 and three retrospective studies in 36, 104 and 160 patients, respectively, are available.200–202 Another retrospective study in 46 patients with acute anterior uveitis associated with HLA-B27-positive ankylosing spondylitis (and UC in one patient) has been recently published. 203 The majority of these studies described the efficacy of MTX in patients predominantly naive to immunosuppressants and biologics, significantly reducing the number of relapses and uveitis activity, increasing the interval between relapses and reducing steroid consumption. The dose of MTX in these patients ranged from 7.5 to 25 mg/week per os or subcutaneously.
Primary sclerosing cholangitis
Both uncontrolled open-label studies and one RCT failed to demonstrate the efficacy of oral MTX in the treatment of primary sclerosing cholangitis (PSC).204,205 The empiric use of MTX in patients with PSC is therefore not recommended. The same authors of the controlled trial suggested continuing studies in patients with precirrhotic disease, without the signs of portal hypertension or liver failure, based on their previous small series of two patients, 206 and a preliminary study in 10 patients treated with low doses of oral MTX, which described biochemical and histologic improvement. 207 However, no further controlled studies followed in this specific setting.
Discussion
Our systematic review describes the evidences available to support the use of MTX in specific clinical scenarios of CD, with the aim to critically discuss the current indications described by the most recent guidelines, as well as its clinical off-label use, which is increasingly proposed as rescue therapy or optimization strategy in different clinical settings.
Our review shows that, despite more than one hundred published studies, there are very few evidences on the efficacy of MTX derived from RCTs. Moreover, several studies are limited by some methodological biases or were performed many years ago, according to different criteria of patient selection and treatment efficacy.
The latest guidelines recommend the use of MTX in patients with active CD as immunomodulator in the scenarios of steroid-dependency, steroid-failure, intolerance to thiopurines or in association with anti-TNFα treatment as combination therapy.2–5 In our review, we show that steroid-dependency is the only scenario supported by RCT so far.
On the contrary, the other ‘classic’ indications for MTX, indicated in previous guidelines and deleted or conditionally granted in more recent editions,2–5,208,209 do not find support from high level of evidence: steroid-refractoriness, failure to thiopurines and combination therapy with anti-TNFα drugs, although described in single uncontrolled case series, do not find an unequivocal favourable opinion from numerous, but heterogeneous, studies. Not surprisingly, the meta-analyses published to date describe the extreme heterogeneity of study populations, treatment regimens and outcome definitions.28–35,210 One seemingly redundant point, however, appears to be the better performance of the higher parenteral doses (25 mg per week) compared with the low oral doses, the first and only ones to be associated with a benefit over placebo in induction RCTs.39–42,210
Regarding the maintenance of remission, parenteral MTX appears to be effective in maintaining steroid-induced remission of CD, with controlled data for at least 1 year in steroid-dependent disease, in favour of the 15 mg/week dose. This has been confirmed by some meta-analyses that, although limited by the paucity of available ad hoc studies, have concluded for a favourable NNT = 4, comparable to that reported in meta-analyses concerning thiopurines.35–38
The role of the oral formulation as maintenance treatment remains uncertain: the unfavourable results of the two small placebo-controlled studies by Oren et al. 41 and Arora et al. 42 do not seem to support this formulation at least at the lowest dosages (12.5–15 mg) for 1 year of observation. However, the placebo-controlled performance of higher dosages (25 mg) is supported by the study of Ardizzone et al. 40 as an alternative to AZA but with higher rates of adverse events (asthenia, nausea and vomiting, not requiring drug withdrawal) than AZA, and without data from placebo-controlled trials.
In the most favourable studies to date,10,39,40 MTX was used in patients naive to immunosuppressants. More uncertain remains the role of MTX in second- and third-line after the failure of a first immunosuppressant (virtually thiopurines) or at least one biologic. However, it should be noted that the studies conducted in the so-called ‘failures’ actually describe a clinically heterogeneous context. In patients with early intolerance to AZA, in whom thiopurine has often not yet reach any clinical effect, it is not known whether a second-line drug, such as MTX, can achieve better results than the patient already refractory to AZA or other therapies since no controlled study has so far stratified the clinical outcome according to these clinical characteristics. Our review shows that MTX (preferably parenteral) may have a role as a second- or third-line therapy, but not precisely quantifiable by magnitude and sustainability of its effect and without clear evidence about the best dosage.
Our review then discusses some specific scenarios, such as fistulizing disease, postoperative prophylaxis, mucosal healing and cross-indications for extraintestinal manifestations.
In fistulizing disease and postoperative prophylaxis, small uncontrolled studies seem promising and interesting, but ad hoc controlled trials are needed.
MTX has been periodically tested in association with almost all currently available biologic drugs to optimize their efficacy and/or immunogenicity, but through small or uncontrolled series, or according to formulations and dosages that are probably not adequate. In some scenarios there is a complete lack of data, as in the case of MTX + ADA combination in patients naive to biologics. In other studies, MTX decreased the immunogenic profile of IFX, ADA and VDZ, favourably influencing levels of anti-drug antibodies and, in some cases, circulating biologic drug. Whether this immunomodulatory effect is matched by a clinical benefit remains unclear, but it appears that combination therapy does not improve the performance of biologics in terms of short-term clinical efficacy.
Instead, a challenge of current research is to understand whether MTX can be used to optimize long-term biological therapy efficacy. The possible scenarios would be its association ab initio, to prevent the appearance of antibodies to drugs, or during biological therapy to modulate the eventual profile of immunogenicity, for example in case of loss of response or in case of appearance of anti-drug antibodies. At the moment, we have few studies, underpowered or retrospective, and more focused on combination therapy with anti-TNFα agents. No specific data are available on the role of MTX in combination with VDZ or UST since current studies, reporting no benefit by adding immunosuppressants in general, did not stratify the outcome between MTX and thiopurines.
Even in extraintestinal manifestations, the role of MTX is mostly empirical and based on sharing similar approaches in other etiologies. In articular disease, the oral route had a bad performance, while parenteral MTX still deserves better analyses. In psoriasis induced by anti-TNFα agents, adding MTX is not useful, while oral MTX can be an option in some rare cutaneous manifestations. Finally, the use of MTX in uveitis seems interesting, but no data came from IBD-associated uveitis series.
The other side of the coin of the clinical use of MTX is safety. Within the standard dose range (subcutaneous or intramuscular, 15–25 mg weekly), up to one-third of patients discontinues MTX because of intolerance. Nausea and flu-like symptoms after parenteral administration are common. 32 At higher doses, myelotoxicity is possible, and long-term use has been associated with hepatic fibrosis that is more common in obese patients or with alcohol use. 211 Allergic pneumonitis is rare. MTX is also immunosuppressive and has been associated with an increase in infectious disease (e.g. viral infections, including herpes zoster). MTX is contraindicated during pregnancy and lactation. With the use of MTX, it has been shown that there is an elevated risk of NMSC, specifically squamous cell and basal cell carcinoma, especially in those patients with a prior history of NMSC. 212
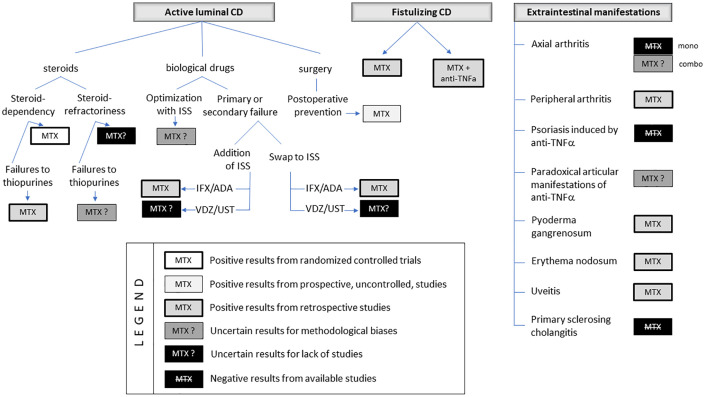
Finally, in Figure 2, we show a proposal of therapeutic use of MTX according to the specific evidences found in our review. Moreover, MTX could still be a therapeutic option in specific settings. First of all, there could be economic benefits. The burden of IBD is high, mostly owing to biological therapy. Hence, some health systems for which access to newer biologics is not an easy or affordable option could consider MTX therapy a viable option. Second, MTX could offer some advantages in patients with recent or active malignant disease.
Figure 2.
Positioning MTX use according to intestinal and extraintestinal indications in CD.
In conclusion, evidence from high-quality studies in favour of MTX in CD is scarce and limited to the steroid-dependent disease, in which other drugs are the leading players today. Numerous other clinical scenarios require well-designed clinical studies in terms of patient profile, drug formulation and dosage, and criteria of efficacy.
Footnotes
Author contributions: Andrea Cassinotti: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Writing – original draft.
Alberto Batticciotto: Data curation; Formal analysis; Writing – review & editing.
Marco Parravicini: Data curation; Writing – review & editing.
Maurizio Lombardo: Data curation; Formal analysis; Writing – review & editing.
Paolo Radice: Data curation; Formal analysis; Writing – review & editing.
Claudio Camillo Cortelezzi: Data curation; Writing – review & editing.
Simone Segato: Writing – review & editing.
Federico Zanzi: Writing – review & editing.
Antonella Cappelli: Supervision; Writing – review & editing.
Sergio Segato: Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrea Cassinotti  https://orcid.org/0000-0001-5733-048X
https://orcid.org/0000-0001-5733-048X
Contributor Information
Andrea Cassinotti, Gastroenterology Unit, ASST Sette Laghi, viale Borri 57, 21100, Varese, Italy.
Alberto Batticciotto, Rheumatology Unit, ASST Sette Laghi, Varese, Italy.
Marco Parravicini, Gastroenterology Unit, ASST Sette Laghi, Varese, Italy.
Maurizio Lombardo, Dermatology Unit, ASST Sette Laghi, Varese, Italy.
Paolo Radice, Ophtalmology Unit, ASST Sette Laghi, Varese, Italy.
Claudio Camillo Cortelezzi, Gastroenterology Unit, ASST Sette Laghi, Varese, Italy.
Simone Segato, Gastroenterology Unit, ASST Sette Laghi, Varese, Italy.
Federico Zanzi, Gastroenterology Unit, ASST Sette Laghi, Varese, Italy.
Antonella Cappelli, Rheumatology Unit, ASST Sette Laghi, Varese, Italy.
Sergio Segato, Gastroenterology Unit, ASST Sette Laghi, Varese, Italy.
References
- 1. Kozarek RA, Patterson DJ, Gelfand MD, et al. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med 1989; 110: 353–356. [DOI] [PubMed] [Google Scholar]
- 2. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020; 14: 4–22. [DOI] [PubMed] [Google Scholar]
- 3. Panaccione R, Steinhart H, Bressler B, et al. Canadian association of gastroenterology clinical practice guideline for the management of luminal Crohn’s disease. J Can Ass Gastroenterol 2019; 2: e1–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute Guideline on the use of thiopurines, methotrexate and, anti-TNF-α biologic Drugs for the induction and maintenance of remission inflammatory Crohn’s disease. Gastroenterology 2013; 145: 1459–1463. [DOI] [PubMed] [Google Scholar]
- 5. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68: s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Assa A, Avni I, Ben-Bassat O, et al. Practice variations in the management of inflammatory bowel disease between pediatric and adult gastroenterologists. J Pediatr Gastroenterol Nutr 2016; 62: 372–377. [DOI] [PubMed] [Google Scholar]
- 7. Thayu M, Markowitz JE, Mamula P, et al. Hepatosplenic T-cell lymphoma in an adolescent patient after immunomodulator and biologic therapy for Crohn’s disease. J Pediatr Gastroenterol Nutr 2005; 40: 220–222. [DOI] [PubMed] [Google Scholar]
- 8. Colman R, Lawton R, Dubinsky M, et al. Methotrexate for the treatment of pediatric Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2018; 24: 2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Egan LJ, Sandborn WJ, Tremaine WJ, et al. A randomized dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 1999; 13: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 10. Mate-Jimenez J, Hermida C, Cantero-Perona J, et al. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol 2000; 12: 1227–1233. [DOI] [PubMed] [Google Scholar]
- 11. Carbonnel F, Colombel JF, Filippi J, et al. Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterology 2016; 150: 380–388. [DOI] [PubMed] [Google Scholar]
- 12. Herfarth H, Barnes EL, Valentine JF, et al. Methotrexate is not superior to placebo in maintaining steroid-free response or remission in ulcerative colitis. Gastroenterology 2018; 155: 1098–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moshkovitz M, Oren R, Tishler M, et al. The absorption of low dose methotrexate in patients with inflammatory bowel disease. Aliment Pharmacol Ther 1997; 11: 569–573. [DOI] [PubMed] [Google Scholar]
- 14. Jundt JW, Browne BA, Fiocco GP, et al. A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J Rheumatol 1993; 20: 1845–1849. [PubMed] [Google Scholar]
- 15. Egan L, Sandborn W. Methotrexate for inflammatory bowel disease: pharmacology and preliminary results. Mayo Clin Proc 1996; 71: 69–80. [DOI] [PubMed] [Google Scholar]
- 16. Kurnik D, Loebstein R, Fishbein E, et al. Bioavailability of oral vs. subcutaneous low-dose methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther 2003; 18: 57–63. [DOI] [PubMed] [Google Scholar]
- 17. Wilson A, Patel V, Chande N, et al. Pharmacokinetic profiles for oral and subcutaneous methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther 2013; 37: 340–345. [DOI] [PubMed] [Google Scholar]
- 18. Hoekstra M, Haagsma C, Neef C, et al. Splitting high-dose oral methotrexate improves bioavailability: a pharmacokinetic study in patients with rheumatoid arthritis. J Rheumatol 2006; 33: 481–485. [PubMed] [Google Scholar]
- 19. Mahmoodzadeh F, Jannat B, Ghorbani M. Chitosan-based nanomicelle as a novel platform for targeted delivery of methotrexate. Int J Biol Macromol 2019; 126: 517–524. [DOI] [PubMed] [Google Scholar]
- 20. Singh A, Thotakura N, Kumar R, et al. PLGA-soya lecithin based micelles for enhanced delivery of methotrexate: cellular uptake, cytotoxic and pharmacokinetic evidences. Int J Biol Macromol 2017; 95: 750–756. [DOI] [PubMed] [Google Scholar]
- 21. Dhanka M, Shetty C, Srivastava R. Injectable methotrexate loaded polycaprolactone microspheres: physicochemical characterization, biocompatibility, and hemocompatibility evaluation. Mater Sci Eng C 2017; 81: 542–550. [DOI] [PubMed] [Google Scholar]
- 22. Ahmadi D, Zarei M, Rahimi M, et al. Preparation and in-vitro evaluation of pH-responsive cationic cyclodextrin coated magnetic nanoparticles for delivery of methotrexate to the Saos-2 bone cancer cells. J Drug Deliv Sci Technol 2020; 57: 101584. [Google Scholar]
- 23. Muntoni E, Martina K, Marini E, et al. Methotrexate-loaded solid lipid nanoparticles: protein functionalization to improve brain biodistribution. Pharmaceutics 2019; 11: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bahramizadeh M, Bahramizadeh M, Kiafar B, et al. Development, characterization and evaluation of topical methotrexate-entrapped deformable liposome on imiquimod-induced psoriasis in a mouse model. Int J Pharm 2019; 569: 118623. [DOI] [PubMed] [Google Scholar]
- 25. Nosrati H, Adinehvand R, Manjili HK, et al. Synthesis, characterization, and kinetic release study of methotrexate loaded mPEG–PCL polymersomes for inhibition of MCF-7 breast cancer cell line. Pharm Dev Technol 2019; 24: 89–98. [DOI] [PubMed] [Google Scholar]
- 26. Jang JH, Jeong SH, Lee YB. Enhanced lymphatic delivery of methotrexate using W/O/W nanoemulsion: in vitro characterization and pharmacokinetic study. Pharmaceutics 2020; 12: 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun Y, Duan B, Chen H, et al. A novel strategy for treating inflammatory bowel disease by targeting delivery of methotrexate through glucan particles. Adv Healthc Mater 2020; 9: e1901805. [DOI] [PubMed] [Google Scholar]
- 28. Balis FM, Mirro J, Reaman GH, et al. Pharmacokinetics of subcutaneous methotrexate. J Clin Oncol 1988; 6: 1882–1886. [DOI] [PubMed] [Google Scholar]
- 29. Brooks PJ, Spruill WJ, Parish RC, et al. Pharmacokinetics of methotrexate administered by intramuscular and subcutaneous injections in patients with rheumatoid arthritis. Arthritis Rheum 1990; 33: 91–94. [DOI] [PubMed] [Google Scholar]
- 30. Nathan DM, Iser JH, Gibson PR. A single center experience of methotrexate in the treatment of Crohn’s disease and ulcerative colitis: a case for subcutaneous administration. J Gastroenterol Hepatol 2008; 23: 954–958. [DOI] [PubMed] [Google Scholar]
- 31. McNeill RP, Barclay M. Cost-effectiveness of therapeutic drug monitoring in inflammatory bowel disease. Curr Opin Pharmacol 2020; 55: 41–46. [DOI] [PubMed] [Google Scholar]
- 32. Herfarth HH, Kappelman MD, Long MD, et al. Use of methotrexate in the treatment of inflammatory bowel diseases. Inflamm Bowel Dis 2016; 22: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brooks AJ, Begg EJ, Zhang M, et al. Red blood cell methotrexate polyglutamate concentrations in inflammatory bowel disease. Ther Drug Monit 2007; 29: 619–625. [DOI] [PubMed] [Google Scholar]
- 34. Nielsen OH, Steenholdt C, Juhl CB, et al. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: a systematic review and meta-analysis of randomized, controlled trials. EClinicalMedicine 2020; 20: 100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khan KJ, Dubinsky MC, Ford AC, et al. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 630–642. [DOI] [PubMed] [Google Scholar]
- 36. Patel V, Macdonald JK, McDonald JW, et al. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2009; 4: CD006884. [DOI] [PubMed] [Google Scholar]
- 37. Patel V, Wang Y, MacDonald JK, et al. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2014; 26: CD006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology 2015; 148: 344–354. [DOI] [PubMed] [Google Scholar]
- 39. Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med 1995; 332: 292–297. [DOI] [PubMed] [Google Scholar]
- 40. Ardizzone S, Bollani S, Manzionna G, et al. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn’ s disease: a randomised, investigator-blind study. Dig Liver Dis 2003; 35: 619–627. [DOI] [PubMed] [Google Scholar]
- 41. Oren R, Moshkowitz M, Odes S, et al. Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol 1997; 92: 2203–2209. [PubMed] [Google Scholar]
- 42. Arora S, Katkov W, Cooley J, et al. Methotrexate in Crohn’s disease: results of a randomized, double-blind, placebo-controlled trial. Hepatogastroenterology 1999; 46: 1724–1729. [PubMed] [Google Scholar]
- 43. Feagan BG, Fedorak R, Irvine J, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. N Engl J Med 2000; 342: 1627–1632. [DOI] [PubMed] [Google Scholar]
- 44. Baron TH, Truss CD, Elson CO. Low-dose oral methotrexate in refractory inflammatory bowel disease. Dig Dis Sci 1993; 38: 1851–1856. [DOI] [PubMed] [Google Scholar]
- 45. Lemann M, Chamiot-Prieur C, Mesnard B, et al. Methotrexate for the treatment of refractory Crohn’s disease. Aliment Pharmacol Ther 1996; 10: 309–314. [DOI] [PubMed] [Google Scholar]
- 46. Vandeputte L, D’Haens G, Baert F, et al. Methotrexate in refractory Crohn’s disease. Inflamm Bowel Dis 1999; 5: 11–15. [DOI] [PubMed] [Google Scholar]
- 47. Lémann M, Zenjari T, Bouhnik Y, et al. Methotrexate in Crohn’s disease: long-term efficacy and toxicity. Am J Gastroenterol 2000; 95: 1730–1734. [DOI] [PubMed] [Google Scholar]
- 48. Chong RY, Hanauer SB, Cohen RD. Efficacy of parenteral methotrexate in refractory Crohn’s disease. Aliment Pharmacol Ther 2001; 15: 35–44. [DOI] [PubMed] [Google Scholar]
- 49. Fraser AG, Morton D, McGovern D, et al. The efficacy of methotrexate for maintaining remission in inflammatory bowel disease. Aliment Pharmacol Ther 2002; 16: 693–697. [DOI] [PubMed] [Google Scholar]
- 50. Soon S, Ansari A, Yaneza M, et al. Experience with the use of low-dose methotrexate for inflammatory bowel disease. Eur J Gastroenterol Hepatol 2004; 16: 921–926. [DOI] [PubMed] [Google Scholar]
- 51. Hayee BH, Harris AW. Methotrexate for Crohn’s disease: experience in a district general hospital. Eur J Gastroenterol Hepatol 2005; 17: 893–898. [DOI] [PubMed] [Google Scholar]
- 52. Din S, Dahele A, Fennel J, et al. Use of methotrexate in refractory Crohn’s disease: the Edinburgh experience. Inflamm Bowel Dis 2008; 14: 756–762. [DOI] [PubMed] [Google Scholar]
- 53. Domènech E, Mañosa M, Navarro M, et al. Long-term methotrexate for Crohn’s disease: safety and efficacy in clinical practice. J Clin Gastroenterol 2008; 42: 395–399. [DOI] [PubMed] [Google Scholar]
- 54. Wahed M, Louis-Auguste J, Baxter L, et al. Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine⁄mercaptopurine. Aliment Pharmacol Ther 2009; 30: 614–620. [DOI] [PubMed] [Google Scholar]
- 55. Parker R, Dixit A, Fraser A, et al. Clinical experience of methotrexate in Crohn’s disease: response, safety and monitoring of treatment. Postgrad Med J 2010; 86: 208–211. [DOI] [PubMed] [Google Scholar]
- 56. Hausmann J, Zabel K, Herrmann E, et al. Methotrexate for maintenance of remission in chronic active Crohn’s disease: long-term single-center experience and meta-analysis of observational studies. Inflamm Bowel Dis 2010; 16: 1195–1202. [DOI] [PubMed] [Google Scholar]
- 57. Chande N, Abdelgadir I, Gregor J. The safety and tolerability of methotrexate for treating patients with Crohn’s disease. J Clin Gastroenterol 2011; 45: 599–601. [DOI] [PubMed] [Google Scholar]
- 58. Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis 2012; 44: 123–127. [DOI] [PubMed] [Google Scholar]
- 59. Suares N, Hamlin P, Greer D, et al. Efficacy and tolerability of methotrexate therapy for refractory Crohn’s disease: a large single-centre experience. Aliment Pharmacol Ther 2012; 35: 284–291. [DOI] [PubMed] [Google Scholar]
- 60. González-Lama Y, Taxonera C, López-Sanromán A, et al. Methotrexate in inflammatory bowel disease: a multicenter retrospective study focused on long-term efficacy and safety. The Madrid experience. Eur J Gastroenterol Hepatol 2012; 24: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 61. Seinen ML, Ponsioen CY, de Boer NK, et al. Sustained clinical benefit and tolerability of methotrexate monotherapy after thiopurine therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2013; 11: 667–672. [DOI] [PubMed] [Google Scholar]
- 62. Kopylov U, Katsanos KH, van der Woude CJ, et al. European experience with methotrexate treatment in Crohn’s disease: a multicenter retrospective analysis. Eur J Gastroenterol Hepatol 2016; 28: 802–806. [DOI] [PubMed] [Google Scholar]
- 63. Huang Z, Chao K, Li M, et al. Methotrexate for refractory Crohn’s disease compared with thiopurines: a retrospective non-head-to-head controlled study. Inflamm Bowel Dis 2017; 23: 440–447. [DOI] [PubMed] [Google Scholar]
- 64. Rouiller-Braunschweig C, Fournier N, Pittet V, et al. Efficacy, safety and mucosal healing of methotrexate in a large longitudinal cohort of inflammatory bowel disease patients. Digestion 2017; 96: 220–227. [DOI] [PubMed] [Google Scholar]
- 65. Wang T, Qiao Y, Zou D, et al. A single-center experience with methotrexate in the treatment of Chinese Crohn’s disease patients. J Dig Dis 2019; 19: 753–758. [DOI] [PubMed] [Google Scholar]
- 66. Bianchi Porro G, Cassinotti A, Ferrara E, et al. Review article: the management of steroid dependency in ulcerative colitis. Aliment Pharmacol Ther 2007; 26: 779–794. [DOI] [PubMed] [Google Scholar]
- 67. Vasudevan A, Gibson P, van Langenberg D. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: what should the clinician expect, what should patients be told? World J Gastroenterol 2017; 23: 6385–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mesonero F, Castro-Poceiro J, Benítez JM, et al. Effectiveness and safety of methotrexate monotherapy in patients with Crohn’s disease refractory to anti-TNF-α: results from the ENEIDA registry. Aliment Pharmacol Ther 2021; 53: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 69. Subesinghe S, Scott I. Key findings from studies of methotrexate tapering and withdrawal in rheumatoid arthritis. Expert Rev Clin Pharmacol 2015; 8: 751–760. [DOI] [PubMed] [Google Scholar]
- 70. Laharie D, Reffet, Belleannee G, et al. Mucosal healing with methotrexate in Crohn’s disease: a prospective comparative study with azathioprine and infliximab. Aliment Pharmacol Ther 2011; 33: 714–721. [DOI] [PubMed] [Google Scholar]
- 71. Manosa M, Naves JE, Leal C, et al. Does methotrexate induce mucosal healing in Crohn’s disease? Inflamm Bowel Dis 2010; 16: 377–378. [DOI] [PubMed] [Google Scholar]
- 72. Vasudevan A, Raghunath A, Anthony S, et al. Higher mucosal healing with tumor necrosis factor inhibitors in combination with thiopurines compared to methotrexate in Crohn’s disease. Dig Dis Sci 2019; 64: 1622–1631. [DOI] [PubMed] [Google Scholar]
- 73. Mahadevan U, Marion JF, Present DH. Fistula response to methotrexate in Crohn’s disease: a case series. Aliment Pharmacol Ther 2003; 18: 1003–1008. [DOI] [PubMed] [Google Scholar]
- 74. Konrad A, Seibold F. Response of cutaneous Crohn’s disease to infliximab and methotrexate. Dig Liver Dis 2003; 35: 351–356. [DOI] [PubMed] [Google Scholar]
- 75. Topstad DR, Panaccione R, Heine JA, et al. Combined seton placement, infliximab infusion, and maintenance immunosuppressives improve healing rate in fistulizing anorectal Crohn’s disease: a single center experience. Dis Colon Rectum 2003; 46: 577–583. [DOI] [PubMed] [Google Scholar]
- 76. Roumeguère P, Bouchard D, Pigot F, et al. Combined approach with infliximab, surgery, and methotrexate in severe fistulizing anoperineal Crohn’s disease: results from a prospective study. Inflamm Bowel Dis 2011; 17: 69–76. [DOI] [PubMed] [Google Scholar]
- 77. Schröder O, Blumenstein I, Schulte-Bockholt A, et al. Combining infliximab and methotrexate in fistulizing Crohn’s disease resistant or intolerant to azathioprine. Aliment Pharmacol Ther 2004; 19: 295–301. [DOI] [PubMed] [Google Scholar]
- 78. Sorrentino D, Terrosu G, Avellini C, et al. Infliximab with low-dose methotrexate for prevention of postsurgical recurrence of ileocolonic Crohn disease. Arch Intern Med 2007; 167: 1804–1807. [DOI] [PubMed] [Google Scholar]
- 79. Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014; 146: 681–688. [DOI] [PubMed] [Google Scholar]
- 80. Sokol H, Seksik P, Carrat F, et al. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut 2010; 59: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 81. Targownik L, Benchimol E, Bernstein C, et al. Upfront combination therapy, compared with monotherapy, for patients not previously treated with a biologic agent associates with reduced risk of inflammatory bowel disease-related complications in a population-based cohort study. Clin Gastroenterol Hepatol 2019; 17: 1788–1798. [DOI] [PubMed] [Google Scholar]
- 82. Targownik L, Benchimol E, Bernstein C, et al. Combined biologic and immunomodulatory therapy is superior to monotherapy for decreasing the risk of inflammatory bowel disease-related complications. J Crohn Colitis 2020; 14: 1354–1363. [DOI] [PubMed] [Google Scholar]
- 83. Schröder O, Blumenstein I, Stein J. Combining infliximab with methotrexate for the induction and maintenance of remission in refractory Crohn’s disease: a controlled pilot study. Eur J Gastroenterol Hepatol 2006; 18: 11–16. [DOI] [PubMed] [Google Scholar]
- 84. Colman R, Rubin D. Optimal doses of methotrexate combined with anti-TNF therapy to maintain clinical remission in inflammatory bowel disease. J Crohns Colitis 2015; 9: 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Borren N, Luther J, Colizzo F, et al. Low-dose methotrexate has similar outcomes to high-dose methotrexate in combination with anti-TNF therapy in inflammatory bowel diseases. J Crohns Colitis 2019: 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cosnes J, Sokol H, Bourrier A, et al. Adalimumab or infliximab as monotherapy, or in combination with an immunomodulator, in the treatment of Crohn’s disease. Aliment Pharmacol Ther 2016; 44: 1102–1113. [DOI] [PubMed] [Google Scholar]
- 87. Hanauer S, Sandborn W, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130: 323–333. [DOI] [PubMed] [Google Scholar]
- 88. Sandborn W, Hanauer S, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007; 56: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sandborn W, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab. A randomized trial. Ann Intern Med 2007; 146: 829–838. [DOI] [PubMed] [Google Scholar]
- 90. Chalhoub JM, Rimmani HH, Gumaste VV, et al. Systematic review and meta-analysis: adalimumab monotherapy versus combination therapy with immunomodulators for induction and maintenance of remission and response in patients with Crohn’s disease. Inflamm Bowel Dis 2017; 23: 1316–1327. [DOI] [PubMed] [Google Scholar]
- 91. Burmester GR, Kivitz AJ, Kupper H, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis 2015; 74: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 2007; 357: 239–250. [DOI] [PubMed] [Google Scholar]
- 93. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007; 357: 228–238. [DOI] [PubMed] [Google Scholar]
- 94. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 95. Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis 2015; 21: 2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol 2016; 111: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 97. Baumgart D, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice – a nationwide consecutive German cohort study. Aliment Pharmacol Ther 2016; 43: 1090–1102. [DOI] [PubMed] [Google Scholar]
- 98. Stallmach Langbein C, Atreya R, Bruns T, et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease – a prospective multicenter observational study. Aliment Pharmacol Ther 2016; 44: 1199–1212. [DOI] [PubMed] [Google Scholar]
- 99. Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2016; 14: 1593–1601. [DOI] [PubMed] [Google Scholar]
- 100. Allegretti JR, Barnes EL, Stevens B, et al. Predictors of clinical response and remission at 1 year among a multicenter cohort of patients with inflammatory bowel disease treated with vedolizumab. Dig Dis Sci 2017; 62: 1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Amiot A, Serrero M, Peyrin-Biroulet L, et al. OBSERV-IBD study group and the GETAID. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther 2017; 46: 310–321. [DOI] [PubMed] [Google Scholar]
- 102. Gouynou C, Peyrin-Biroulet L. Addition of methotrexate neither restores clinical response nor improbe the pharmacokinetic profile of vedolizumab-treated patients. Aliment Pharmacol Ther 2017; 46: 1019–1020. [DOI] [PubMed] [Google Scholar]
- 103. Samaan MA, Pavlidis P, Johnston E, et al. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol 2017; 8: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Eriksson C, Marsal J, Bergemalm D, et al. Long-term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish national quality registry for inflammatory bowel disease (SWIBREG). Scand J Gastroenterol 2017; 52: 722–729. [DOI] [PubMed] [Google Scholar]
- 105. Kopylov U, Ron Y, Avni-Biron I, et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the Israeli real-world experience. Inflamm Bowel Dis 2017; 23: 404–408. [DOI] [PubMed] [Google Scholar]
- 106. Kopylov U, Verstockt B, Biedermann L, et al. Effectiveness and safety of vedolizumab in anti-TNF-naive patients with inflammatory bowel disease – a multi center retrospective European study. Inflamm Bowel Dis 2018; 24: 2442–2451. [DOI] [PubMed] [Google Scholar]
- 107. Lenti MV, Levison S, Eliadou E, et al. A real-world, long-term experience on effectiveness and safety of vedolizumab in adult patients with inflammatory bowel disease: the Cross Pennine study. Dig Liver Dis 2018; 50: 1299–1304. [DOI] [PubMed] [Google Scholar]
- 108. Macaluso FS, Orlando R, Fries W, et al. The real-world effectiveness of vedolizumab on intestinal and articular outcomes in inflammatory bowel diseases. Dig Liver Dis 2018; 50: 675–681. [DOI] [PubMed] [Google Scholar]
- 109. Iborra M, Beltrán B, Maroto N, et al. Vedolizumab, an option in patients with inflammatory bowel disease intolerant to thiopurines and refractory to biological agents. Gastroenterol Hepatol 2018; 41: 535–543. [DOI] [PubMed] [Google Scholar]
- 110. Ylisaukko-Oja T, Aaltonen J, Nuutinen H, et al. High treatment persistence rate and significant endoscopic healing among real-life patients treated with vedolizumab – a Finnish Nationwide Inflammatory Bowel Disease Cohort Study (FINVEDO). Scand J Gastroenterol 2018; 53: 158–167. [DOI] [PubMed] [Google Scholar]
- 111. Macaluso F, Orlando R, Renna S, et al. Letter: the addition of an immunosuppressant in patients with unsatisfactory response to vedolizumab. Aliment Pharmacol Ther 2018; 47: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 112. Hoffmann P, Krisam J, Stremmel W, et al. Real-world outcomes of vedolizumab therapy in ulcerative colitis and Crohn’s disease at a tertiary referral center. Dig Dis 2019; 37: 33–44. [DOI] [PubMed] [Google Scholar]
- 113. Ylisaukko-Oja T, Torvinen S, Aaltonen J, et al. Characterization of inflammatory bowel disease management by vedolizumab and concomitant treatments in real-life clinical practice. Biologicals 2019; 58: 50–56. [DOI] [PubMed] [Google Scholar]
- 114. Hu A, Kotze P, Burgevin A, et al. Combination therapy does not improve rate of clinical or endoscopic remission in patients with inflammatory bowel diseases treated with vedolizumab or ustekinumab. Clin Gastroenterol Hepatol 2021; 19: 1366–1376. [DOI] [PubMed] [Google Scholar]
- 115. Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther 2015; 42: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014; 147: 618–627. [DOI] [PubMed] [Google Scholar]
- 117. Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of ustekinumab, a human interleukin-12/23 monocolonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008; 135: 1130–1141. [DOI] [PubMed] [Google Scholar]
- 118. Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012; 367: 1519–1528. [DOI] [PubMed] [Google Scholar]
- 119. Kopylov U, Afif W, Cohen A, et al. Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn’s disease – the McGill experience. J Crohns Colitis 2014; 8: 1516–1522. [DOI] [PubMed] [Google Scholar]
- 120. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375: 1946–1960. [DOI] [PubMed] [Google Scholar]
- 121. Khorrami S, Ginard D, Marín-Jiménez I, et al. Ustekinumab for the treatment of refractory Crohn’s disease: the Spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis 2016; 22: 1662–1669. [DOI] [PubMed] [Google Scholar]
- 122. Wils P, Bouhnik Y, Michetti P, et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn’s disease refractory to anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol 2016; 14: 242–250. [DOI] [PubMed] [Google Scholar]
- 123. Ma C, Fedorak RN, Kaplan GG, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohn’s disease: real-world experience from a multi center cohort study. Inflamm Bowel Dis 2017; 23: 833–839. [DOI] [PubMed] [Google Scholar]
- 124. Ma C, Fedorak RN, Kaplan GG, et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: real world experience from a multicentre color. Aliment Pharmacol Ther 2017; 45: 1232–1243. [DOI] [PubMed] [Google Scholar]
- 125. Battat R, Kopylov U, Bessissow T, et al. Association among ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2017; 15: 1427–1434. [DOI] [PubMed] [Google Scholar]
- 126. Greenup AJ, Rosenfeld G, Bressler B. Ustekinumab use in Crohn’s disease: a Canadian tertiary care centre experience. Scand J Gastroenterol 2017; 52: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 127. Wils P, Bouhnik Y, Michetti P, et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: a multicentre experience. Aliment Pharmacol Ther 2018; 47: 588–595. [DOI] [PubMed] [Google Scholar]
- 128. Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007; 56: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2019; 17: 1655–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cassinotti A, Travis S. Incidence and clinical significance of immunogenicity to infliximab in Crohn’s disease: a critical systematic review. Inflamm Bowel Dis 2009; 15: 1264–1275. [DOI] [PubMed] [Google Scholar]
- 131. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003; 348: 601–608. [DOI] [PubMed] [Google Scholar]
- 132. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 133. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004; 350: 876–885. [DOI] [PubMed] [Google Scholar]
- 134. West RL, Zelinkova Z, Wolbink GJ, et al. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther 2008; 28: 1122–1226. [DOI] [PubMed] [Google Scholar]
- 135. Strik AS, van den Brink GR, Ponsioen C, et al. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017; 45: 1128–1134. [DOI] [PubMed] [Google Scholar]
- 136. Kennedy N, Heap G, Green H, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019; 4: 341–353. [DOI] [PubMed] [Google Scholar]
- 137. Ungar B, Kopylov U, Engel T, et al. Addition of an immunomodulator can reverse antibody formation and loss of response in patients treated with adalimumab. Aliment Pharmacol Ther 2017; 45: 276–282. [DOI] [PubMed] [Google Scholar]
- 138. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017; 66: 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dhindsa B, Dhaliwal A, Mashiana HS, et al. Reversal of anti-drug antibodies against tumor necrosis factor inhibitors with addition of immunomodulators: a systematic review and meta-analysis. Indian J Gastroenterol 2020; 39: 153–160. [DOI] [PubMed] [Google Scholar]
- 140. Fischer M, Campbell S, Calley C, et al. Risk factors for rescue therapy in Crohn’s patients maintained on infliximab after withdrawal of the immunomodulator: a long-term follow-up. Dig Dis Sci 2017; 62: 3131–3137. [DOI] [PubMed] [Google Scholar]
- 141. Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013; 11: 444–447. [DOI] [PubMed] [Google Scholar]
- 142. Papamichael K, Karatzas P, Mantzaris GJ. Addition of an immunomodulator as a rescue therapy for loss of response to adalimumab dose escalation in patients with Crohn’s disease. J Crohns Colitis 2015; 9: 589–590. [DOI] [PubMed] [Google Scholar]
- 143. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76: 960–977. [DOI] [PubMed] [Google Scholar]
- 144. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016; 68: 1–26. [DOI] [PubMed] [Google Scholar]
- 145. Chen J, Veras MMS, Liu C, et al. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2013; 2: CD004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017; 76: 978–991. [DOI] [PubMed] [Google Scholar]
- 147. Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of AMERICA/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2016; 68: 282–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Altan L, Bing€ol U, Karakoç Y, et al. Clinical investigation of methotrexate in the treatment of ankylosing spondylitis. Scand J Rheumatol 2001; 30: 255–259. [DOI] [PubMed] [Google Scholar]
- 149. Roychowdhury B, Bintley-Bagot S, Bulgen D, et al. Is methotrexate effective in ankylosing spondylitis? Rheumatol 2002; 41: 1330–1332. [DOI] [PubMed] [Google Scholar]
- 150. Gonzalez-Lopez L, Garcia-Gonzalez A, Vazquez-Del-Mercado M, et al. Efficacy of methotrexate in ankylosing spondylitis: a randomized, double blind, placebo controlled trial. J Rheumatol 2004; 31: 1568–1574. [PubMed] [Google Scholar]
- 151. Haibel H, Brandt HC, Song IH, et al. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis 2007; 66: 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Armuzzi A, Felice C, Lubrano E, et al. Multidisciplinary management of patients with coexisting inflammatory bowel disease and spondyloarthritis: a Delphi consensus among Italian experts. Dig Liver Dis 2017; 49: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 153. Ostendorf B, Specker C, Schneider M, et al. Methotrexate lacks efficacy in the treatment of severe ankylosing spondylitis compared with rheumatoid and psoriasis arthritis. J Clin Rheumatol 1998; 4: 129–136. [DOI] [PubMed] [Google Scholar]
- 154. Ganapati A, Gowri M, Antonisamy B, et al. Combination of methotrexate and sulfasalazine is an efficacious option for axial spondyloarthritis in a resource-limited, real-world clinical setting: a prospective cohort study. Clin Rheumatol 2020; 40: 1871–1879. [DOI] [PubMed] [Google Scholar]
- 155. Biasi D, Carletto A, Caramaschi P, et al. Efficacy of methotrexate in the treatment of ankylosing spondylitis: a three-year open study. Clin Rheumatol 2000; 19: 114–117. [DOI] [PubMed] [Google Scholar]
- 156. Sampaio-Barros PD, Costallat LT, Bertolo MB, et al. Methotrexate in the treatment of ankylosing spondylitis. Scand J Rheumatol 2000; 29: 160–162. [DOI] [PubMed] [Google Scholar]
- 157. Dougados M. Methotrexate in peripheral spondyloarthitis including psoriasis arthritis: a need for further evaluation. Rheumatology 2012; 51: 1243–1244. [DOI] [PubMed] [Google Scholar]
- 158. Tornero-Molina J, Alperi-López M, Castellví I, et al. Experts document on methotrexate use in combined therapy with biological or targeted synthetic disease modifying drugs in patients with rheumatoid arthritis. Reumatol Clin. Epub ahead of print 8 October 2020. DOI: 10.1016/j.reuma.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 159. Ternant D, Mulleman D, Lauferon F, et al. Influence of methotrexate on infliximab pharmacokinetics and pharmacodynamics in ankylosing spondylitis. Br J Clin Pharmacol 2012; 73: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mulleman D, Lauferon F, Wendling D. Infliximab in ankylosing spondylitis: alone or in combination with methotrexate? A pharmacokinetic comparative study. Arthritis Res Ther 2011; 13: R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Marzo-Ortega H, McGonagle D, Jarrett S, et al. Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann Rheum Dis 2005; 64: 1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Heinonen AV, Aaltonen KJ, Joensuu JT, et al. Effectiveness and drug survival of TNF inhibitors in the treatment of ankylosing spondylitis: a prospective cohort study. J Rheumatol 2015; 42: 2339–2346. [DOI] [PubMed] [Google Scholar]
- 163. Nissen MJ, Ciurea A, Bernhard J, et al. The effect of comedication with a conventional synthetic disease-modifying antirheumatic drug on drug retention and clinical effectiveness of anti-tumor necrosis factor therapy in patients with axial spondyloarthritis. Arthritis Rheumatol 2016; 68: 2141–2150. [DOI] [PubMed] [Google Scholar]
- 164. Lie E, Kristensen LE, Forsblad-d’Elia H, et al. The effect of comedication with conventional synthetic disease modifying antirheumatic drugs on TNF inhibitor drug survival in patients with ankylosing spondylitis and undifferentiated spondyloarthritis: results from a nationwide prospective study. Ann Rheum Dis 2015; 74: 970–978. [DOI] [PubMed] [Google Scholar]
- 165. Sepriano A, Ramiro S, van der Heijde D, et al. Effect of comedication with conventional synthetic disease-modifying antirheumatic drugs on retention of tumor necrosis factor inhibitors in patients with spondyloarthritis: a prospective cohort study. Arthritis Rheumatol 2016; 68: 2671–2679. [DOI] [PubMed] [Google Scholar]
- 166. Glintborg B, Ostergaard M, Krogh NS, et al. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis 2010; 69: 2002–2008. [DOI] [PubMed] [Google Scholar]
- 167. Heiberg MS, Koldingsnes W, Mikkelsen K, et al. The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum 2008; 59: 234–240. [DOI] [PubMed] [Google Scholar]
- 168. Kristensen LE, Karlsson JA, Englund M, et al. Presence of peripheral arthritis and male sex predicting continuation of anti-tumor necrosis factor therapy in ankylosing spondylitis: an observational prospective cohort study from the South Swedish Arthritis Treatment Group Register. Arthritis Care Res (Hoboken) 2010; 62: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 169. Thiebault H, Boyard-Lasselin P, Guignant C, et al. Paradoxical articular manifestations in patients with inflammatory bowel diseases treated with infliximab. Eur J Gastroenterol Hepatol 2016; 28: 876–881. [DOI] [PubMed] [Google Scholar]
- 170. Coates LC, Merola JF, Grieb SM, et al. Methotrexate in psoriasis and psoriatic arthritis. J Rheumatol Suppl 2020; 96: 31–35. [DOI] [PubMed] [Google Scholar]
- 171. Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol 2020; 156: 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Chu DH, Van Voorhees AS, Rosenbach M. Treatment of refractory tumor necrosis factor inhibitor-induced palmoplantar pustulosis: a report of 2 cases. Arch Dermatol 2011; 147: 1228–1230. [DOI] [PubMed] [Google Scholar]
- 173. Buisson A, Cuny JF, Barbaud A, et al. Methotrexate for psoriasiform lesions associated with antitumour necrosis factor therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35: 1175–1180. [DOI] [PubMed] [Google Scholar]
- 174. Mazloom SE, Yan D, Hu JZ, et al. TNF-α inhibitor-induced psoriasis: a decade of experience at the Cleveland Clinic. J Am Acad Dermatol 2020; 83: 1590–1598. [DOI] [PubMed] [Google Scholar]
- 175. Ghosh S, Gensler L, Yang Z, et al. Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of phase II/III clinical development. Drug Saf 2019; 42: 751–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Schmidt C, Wittig BM, Moser C, et al. Cyclophosphamide pulse therapy followed by azathioprine or methotrexate induces long-term remission in patients with steroid-refractory Crohn’s disease. Aliment Pharmacol Ther 2006; 24: 343–350. [DOI] [PubMed] [Google Scholar]
- 177. Duarte-Chang C, Visuetti S. Successful treatment of gangrenous pyoderma with methotrexate in a patient with Crohn’s disease. Rev Gastroenterol Peru 2019; 39: 175–177. [PubMed] [Google Scholar]
- 178. Teitel AD. Treatment of pyoderma gangrenosum with methotrexate. Cutis 1996; 57: 326–328. [PubMed] [Google Scholar]
- 179. Ibrahim I, Shereef H, Hashim A, et al. Winning the battle after three years of suffering: a case of refractory pyoderma gangrenosum treatment challenge. Case Rep Rheumatol 2021; 2021: 8869914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tonkovic-Capin V, Galbraith SS, Rogers RS, III, et al. Cutaneous Crohn’s disease mimicking Melkersson-Rosenthal syndrome: treatment with methotrexate. J Eur Acad Dermatol Venereol 2006; 20: 449–452. [DOI] [PubMed] [Google Scholar]
- 181. Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol 2003; 149: 181–184. [DOI] [PubMed] [Google Scholar]
- 182. Ali A, Rosenbaum JT. Use of methotrexate in patients with uveitis. Clin Exp Rheumatol 2010; 28: S145–S150. [PubMed] [Google Scholar]
- 183. Angeles-Han S, Lo M, Henderson L, et al. Childhood arthritis and rheumatology research alliance consensus treatment plans for juvenile idiopathic arthritis–associated and idiopathic chronic anterior uveitis. Arthritis Care Res (Hoboken) 2019; 71: 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Constantin T, Foeldvari I, Anton J, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis 2018; 77: 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ferrara G, Mastrangelo G, Barone P, et al. Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Ped Rheumatol 2018; 46: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Heiligenhausa A, Mindenb K, Tappeiner C, et al. Update of the evidence based, interdisciplinary guideline for antiinflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Sem Arthritis Rheum 2019; 49: 43–55. [DOI] [PubMed] [Google Scholar]
- 187. Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000; 130: 492–513. [DOI] [PubMed] [Google Scholar]
- 188. Wong VG, Hersh EM. Methotrexate in the therapy of cyclitis. Tr Am Acad Ophth Otol 1965; 69: 279–293. [Google Scholar]
- 189. Okada AA. Immunomodulatory therapy for ocular inflammatory disease: a basic manual and review of the literature. Ocul Immunol Inflamm 2005; 13: 335–351. [DOI] [PubMed] [Google Scholar]
- 190. Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol 2005; 32: 362–365. [PubMed] [Google Scholar]
- 191. Shetty AK, Zganjar BE, Ellis GS, Jr, et al. Low-dose methotrexate in the treatment of severe juvenile rheumatoid arthritis and sarcoid iritis. J Pediatr Ophthalmol Strabismus 1999; 36: 125–128. [DOI] [PubMed] [Google Scholar]
- 192. Hemady RK, Baer JC, Foster CS. Immunosuppressive drugs in the management of progressive, corticosteroid-resistant uveitis associated with juvenile rheumatoid arthritis. Int Ophthalmol Clin 1992; 32: 241–252. [DOI] [PubMed] [Google Scholar]
- 193. Wallace CA, Bleyer WA, Sherry DD, et al. Toxicity and serum levels of methotrexate in children with juvenile rheumatoid arthritis. Arthritis Rheum 1989; 32: 677–681. [DOI] [PubMed] [Google Scholar]
- 194. Dev S, McCallum RM, Jaffe GJ. Methotrexate treatment for sarcoid-associated panuveitis. Ophthalmology 1999; 106: 111–118. [DOI] [PubMed] [Google Scholar]
- 195. Singal A, Chhabra N, Pandhi D, et al. Behcet’s disease in India: a dermatological perspective. Indian J Dermatol Venereol Leprol 2013; 79: 199–204. [DOI] [PubMed] [Google Scholar]
- 196. McCluskey P, Chang JH, Singh R, et al. Methotrexate therapy for ocular cicatricial pemphigoid. Ophthalmology 2004; 111: 796–801. [DOI] [PubMed] [Google Scholar]
- 197. Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 1985; 312: 818–822. [DOI] [PubMed] [Google Scholar]
- 198. Gómez-Gómez A, Loza E, Rosario MP, et al. Efficacy and safety of immunomodulatory drugs in patients with anterior uveitis. A systematic literature review. Medicine (Baltimore) 2017; 96: e8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Bachta A, Kisiel B, Tłustochowicz M, et al. High efficacy of methotrexate in patients with recurrent idiopathic acute anterior uveitis: a prospective study. Arch Immunol Ther Exp 2016; 65: 93–97. [DOI] [PubMed] [Google Scholar]
- 200. Kaplan-Messas A, Barkana Y, Avni I, et al. Methotrexate as a first-line corticosteroid-sparing therapy in a cohort of uveitis and scleritis. Ocul Immunol Inflamm 2003; 11: 131–139. [DOI] [PubMed] [Google Scholar]
- 201. Samson CM, Waheed N, Baltatzis S, et al. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology 2001; 108: 1134–1139. [DOI] [PubMed] [Google Scholar]
- 202. Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology 2009; 116: 2188–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Zu Hoerste MM, Walscheid K, Tappeiner C, et al. The effect of methotrexate and sulfasalazine on the course of HLA-B27-positive anterior uveitis: results from a retrospective cohort study. Graefes Arch Clin Exp Ophthalmol 2018; 256: 1985–1992. [DOI] [PubMed] [Google Scholar]
- 204. Lindor KD, Jorgensen RA, Anderson ML, et al. Ursodeoxycholic acid and methotrexate for primary sclerosing cholangitis: a pilot study. Am J Gastroenterol 1996; 91: 511–515. [PubMed] [Google Scholar]
- 205. Knox TA, Kaplan MM. A double-blind controlled trial of oral-pulse methotrexate therapy in the treatment of primary sclerosing cholangitis. Gastroenterology 1994; 106: 494–499. [DOI] [PubMed] [Google Scholar]
- 206. Kaplan MM, Arora S, Pincus SH. Primary sclerosing cholangitis and low-dose oral pulse methotrexate therapy. Clinical and histologic response. Ann Intern Med 1987; 106: 231–235. [DOI] [PubMed] [Google Scholar]
- 207. Knox TA, Kaplan MM. Treatment of primary sclerosing cholangitis with oral methotrexate. Am J Gastroenterology 1991; 86: 546–552. [PubMed] [Google Scholar]
- 208. Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis 2010; 4: 28–62. [DOI] [PubMed] [Google Scholar]
- 209. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 210. McDonald JW, Wang Y, Tsoulis DJ, et al. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev 2014; 8: CD003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Te HS, Schiano TD, Kuan SF, et al. Hepatic effects of long-term methotrexate use in the treatment of inflammatory bowel disease. Am J Gastroenterol 2000; 95: 3150–3156. [DOI] [PubMed] [Google Scholar]
- 212. Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol 2016; 152: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Li EK, Griffith JF, Lee VW, et al. Short-term efficacy of combination methotrexate and infliximab in patients with ankylosing spondylitis: a clinical and magnetic resonance imaging correlation. Rheumatology (Oxford) 2008; 47: 1358–1363. [DOI] [PubMed] [Google Scholar]
- 214. Breban M, Ravaud P, Claudepierre P, et al. Maintenance of infliximab treatment in ankylosing spondylitis: results of a one-year randomized controlled trial comparing systematic versus on-demand treatment. Arthritis Rheum 2008; 58: 88–97. [DOI] [PubMed] [Google Scholar]