Abstract
Our paper presents detailed evolutionary analyses of narcissus viruses from wild and domesticated Narcissus plants in Japan. Narcissus late season yellows virus (NLSYV) and narcissus degeneration virus (NDV) are major viruses of Narcissus plants, causing serious disease outbreaks in Japan. In this study, we collected Narcissus plants showing mosaic or striped leaves along with asymptomatic plants in Japan for evolutionary analyses. Our findings show that (1) NLSYV is widely distributed, whereas the distribution of NDV is limited to the southwest parts of Japan; (2) the genomes of NLSYV isolates share nucleotide identities of around 82%, whereas those of NDV isolates are around 94%; (3) three novel recombination type patterns were found in NLSYV; (4) NLSYV comprises at least five distinct phylogenetic groups whereas NDV has two; and (5) infection with narcissus viruses often occur as co-infection with different viruses, different isolates of the same virus, and in the presence of quasispecies (mutant clouds) of the same virus in nature. Therefore, the wild and domesticated Narcissus plants in Japan are somewhat like a melting pot of potyviruses and other viruses.
Keywords: Narcissus plants, co-infection, narcissus late season yellows virus, narcissus degeneration virus, potyvirus
1. Introduction
Studies of the co-infection of plant viruses in wild and domesticated plants are important for understanding plant virus epidemiology, evolution, and virus–virus interactions; consequently, these investigations will contribute to developing efficient and durable control strategies [1,2,3,4,5]. Co-infections by two or more plant viruses [6,7,8,9,10,11], different isolates of the same viruses [12,13], and the presence of quasispecies of the same viruses [12,14,15,16,17] in wild and domesticated plants seem likely to be common in nature [18,19,20]. Plants of the genus Narcissus in the family Amaryllidaceae are known as wild or domesticated plants, although it is difficult to draw a boundary distinguishing between these plants in Japan. Narcissus plants are also known to be infected with more than twenty different virus species [13,21,22,23,24,25,26,27,28]. For instance, many viruses that infect Narcissus plants belong to the genus Potyvirus; cyrtanthus elatus virus A (CyEVA), iris severe mosaic virus (ISMV), lily mottle virus, narcissus late season yellows virus (NLSYV), narcissus yellow stripe virus (NYSV), narcissus degeneration virus (NDV), Indian narcissus virus, and ornithogalum mosaic virus. Other major viruses known to infect wild and domesticated Narcissus plants worldwide are narcissus latent virus (NLV, macluravirus), narcissus symptomless virus (carlavirus), narcissus common latent virus (NCLV, carlavirus), nerine latent virus (NeLV, carlavirus) [13,27,28,29,30,31,32]. To date, 26 virus species and three tentative species were also reported to infect Narcissus plants but some of them were distributed in the limited countries of the world [29].
Viruses in the genus Potyvirus in the family Potyviridae are distributed worldwide and infect a wide range of both mono- and dicotyledonous plant species, including Narcissus plants [3,33,34]. They are transmitted non-persistently by many aphid species [35] and are flexuous filamentous particles 700–750 nm long, each of which contains a single copy of the genome. The genome is a single-stranded, positive-sense RNA of about 10,000 nucleotides with one open reading frame (ORF) that is translated into one large polyprotein which is known to be post-translationally cleaved by virus-encoded proteases, and with a small overlapping ORF named pretty interesting potyviridae ORF (PIPO) [3,36].
Narcissus plants are likely to harbor co-infections of potyviruses and other viruses in several areas of China, European countries, India, New Zealand, and Japan [13,22,23,31,37,38,39,40,41,42] but not in the sites surrounding the Perth metropolitan area in Western Australia [43]. Furthermore, there is a report that NLSYV was singly infected [39]. Co-infections of narcissus viruses have been reported on Kyushu Island in Japan [13], and whether and how they might be distributed in other parts of Japan is still largely unknown. Only a taxonomic study of NYSV-like viruses has been reported so far [28]. The term NYSV-like virus refers to the narcissus viruses in the turnip mosaic virus (TuMV) phylogenetic group that does not include NLSYV. Note that TuMV phylogenetic group includes NLSYV, NYSV, Japanese yam mosaic virus (JYMV), scallion mosaic virus (ScaMV) and TuMV [28].
In this study, we collected wild and domesticated Narcissus plants throughout Japan and screened for the presence of potyvirus infections using potyvirus-specific primers. The Japanese Narcissus plant (we call Nihon-zuisen, Narcissus tazetta var. chinensis) is known to differ from western Narcissus (daffodil). Furthermore, we focused on the distribution and evolution of narcissus potyviruses of NLSYV and NDV because these two appeared to be major viruses in Japan in the earlier study [13].
2. Materials and Methods
2.1. Narcissus Plant Samples
One-hundred eighty-nine wild and domesticated Narcissus plant leaves showing mosaic or chlorotic stripe and asymptomatic plant leaves were collected from different sites on the fields and banks of rivers in Japan, including home gardens and flower beds, during the winter and spring seasons of 2009–2015. Details of the plants and isolates, their place of origin, collection dates, host plants, and symptoms are shown in Table S1.
2.2. Detection of NLSYV and NDV by RT-PCR and Sequencing
Because the sap from the collected Narcissus plant leaves in Japan did not induce local lesions on Tetragonia tetragonioides (formerly T. expansa), Chenopodium amaranticolor, C. quinoa, Nicotiana benthamiana, or N. tabacum plants, we could not clone the viruses biologically. Therefore, the viruses were directly identified from the sampled symptomatic or asymptomatic Narcissus plant leaves by reverse-transcription polymerase chain reaction (RT-PCR). We used potyvirus-specific primer pairs that were expected to amplify all potyviruses (Table S2) and determined the partial nucleotide sequences of the cloned RT-PCR products as described below. Total RNA was extracted using Isogen RNA extraction reagent (Nippon Gene, Tokyo, Japan) from the plant leaves (Table S1). We reverse-transcribed the viral RNAs using PrimeScript II Moloney murine leukemia virus reverse transcriptase (Takara Bio, Shiga, Japan), and amplified the potyvirus cDNAs using high-fidelity Prime STAR GXL DNA Polymerase with a PrimeScript II High Fidelity One-Step RT-PCR Kit (TaKaRa Bio, Shiga, Japan). The RT-PCR conditions were: 45 °C for 10 min for RT, one cycle of 94 °C for 2 min, and 40 cycles of 98 °C for 10 s, 45 °C for 15 s, and 68 °C for 35 s. RT-PCR products of 1850–2100 bp for NLSYV, NDV, and some other potyviruses were amplified from Narcissus plant leaves using potyvirus-specific primer pairs [13,28] (Table S2), POTYNIbNOT4P (forward, 5′-GGGGCGGCCGCATATGGGGTGAGAGAGGTNTGYGTNGAYGAYTTYAAYAA-3′) and Tu3T9M (reverse, 5′-GGGGCGGCCGCT15-3′), or onion yellow dwarf virus (OYDV) phylogenetic group-specific primer pairs, RGNDNIBNOT4P (forward, 5′-GGGGCGGCCGCATATGGGGTGAGAGAGGRTAYSRWGGGAAGAAGAAGGA-3′) and Tu3T9M for virus genome amplification (underlined nucleotides; position of POTYNIB5P forward primer, see later). These primer pairs amplify most of the potyviruses and viruses in the OYDV phylogenetic group. The OYDV phylogenetic group consists of CyEVA, ISMV, OYDV, and shallot yellow stripe virus (SYSV). We separated the amplified cDNAs by electrophoresis in agarose gels and purified them using a QIAquick Gel Extraction Kit (Qiagen K.K., Tokyo, Japan). The nucleotide sequences of the amplified RT-PCR products from nuclear inclusion b (NIb) protein coding region to the 3′ end region (NIb-3′NCR region) from symptomatic and asymptomatic Narcissus plant leaves were firstly determined using a direct sequencing method. However, we often found multiple peaks at positions in the nucleotide sequence signals, indicating co-infections of different potyviruses, different isolates of the same virus, or the presence of quasispecies of the same virus in a single Narcissus plant. Therefore, only cloning was considered feasible for determining the genomic sequences of each virus in a single Narcissus plant. The RT-PCR products were cloned into the NotI site of plasmid pZErO-2 (Invitrogen, ThermoFisher Scientific, Tokyo, Japan). Three to 20 independent clones from each virus-infected Narcissus plant were obtained.
Firstly, the nucleotide sequences (600–700 bp) of parts of the NIb-3′NCR regions of all clones obtained from 120 isolates were first determined using POTYNIB5P forward primer (5′-CGCATATGGGGTGAGAGAGG-3′), a part of POTYNIbNOT4P or RGNDNIBNOT4P, using a BigDye Terminator v3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) and an Applied Biosystems Genetic Analyzer DNA model 3130. Because large numbers of clones were obtained (Table S1), we first aimed to identify the viruses in each Narcissus plant and selected clones using the 600–700 nucleotide sequences obtained by POTYNIB5P primer for further sequencing. As a result, BLAST searches showed that the cloned partial NIb and coat protein (CP) sequences are closely related to one or other of the sequences of potyviruses of CyEVA, NDV, NLSYV, OrMV, and NYSV, or a macluravirus of NLV. The sequences of NIb-3′NCR clones from the NLSYV and NDV genomes were also determined in both directions using the narcissus virus-specific primers (Table S2) and primer walking. Sequence data were assembled using BioEdit version 5.0.9 [44].
2.3. Sequencing of Full Genomics of NLSYV and NDV
Since the information of consensus sequences in the internal genomic regions of NLSYV and NDV was insufficient to design primers, we applied the primer walking method to obtain the full genomic sequences of NLSYV and NDV isolates. The appropriate primers were designed based on the full genomic sequences of the TuMV or OYDV phylogenetic group viruses available from GenBank (Table S2). Finally, three to five fragments (Figure S1) of RT-PCR products were used to obtain the full genomic sequences of NLSYV and NDV. The RT-PCR products were cloned into the NotI site of plasmid pZErO-2. We obtained at least three independent clones for each fragment. We overlapped the regions between RT-PCR products at least 450 nucleotides. The clones that had no mismatch in the overlapping regions were concatenated to obtain full genomic sequences. These overlapping fragments ensured no artificial recombination events in the NLSYV or NDV genomes during the sequencing and evolutionary analyses. The nucleotide sequences of clones were determined in both directions by primer walking using approximately 80 primers (Table S2) because of variations between the sequences of each isolate. Finally, we determined the full genomic sequences of 25 NLSYV isolates and five NDV isolates.
2.4. Neighbor-Net Phylogenetic Network of CP Coding Regions
We inferred the phylogenetic network of NLSYV and NDV using Neighbor-net methods implemented in SPLITSTREE v4.11.3 [45]. A total of 248 CP coding nucleotide sequences of NLSYV isolates and 125 CP coding nucleotide sequences of NDV (Table S1) obtained by the cloning methods were aligned with those of the same viruses available from GenBank with outgroup taxa. The outgroup included the CP coding sequences of the isolates of JYMV [46] (acc # KJ701427), TuMV [47] (acc # AB701690), NYSV [26] (acc # JQ911732), and ScaMV [48] (acc # NC_003399). For NLSYV, the outgroup included those of the isolates of CyEVA [27] (acc # NC_017977), ISMV [49] (acc # NC_029076), OYDV [50] (acc # JX433020), and SYSV [51] (acc # AM267479) for NDV. The alignments were made using CLUSTAL_X2 [52] with TRANSALIGN [53] to maintain the degapped alignment of the encoded amino acids. The aligned nucleotide sequences of CP coding regions used to infer the phylogenetic network of NLSYV with TuMV phylogenetic group viruses were 810 nucleotides in length, whereas those of NDV with OYDV group viruses were 750 nucleotides.
2.5. Maximum Likelihood Phylogenetic Tree of Polyprotein Coding Regions
A total of 25 polyprotein coding nucleotide sequences of NLSYV isolates determined in this study with those of three sequences from GenBank (Marijiniup8 acc # NC_02362, Marijiniup9 acc # JX156421, and Zhangzhou acc # JQ326210), and five polyprotein coding sequences of NDV determined in this study with those of two sequences from GenBank (Zhangzhou acc # NC_008824 and Marijiniup2 acc # JQ395041) were used to construct maximum likelihood phylogenetic trees.
The isolates of JYMV [46] (acc # KJ701427), TuMV [47] (acc # AB701690), NYSV [23,26,27] (acc # NC_011541, JQ911732 and JQ395042), and ScaMV [48] (acc # NC_003399) were used as outgroup taxa for NLSYV polyprotein coding sequences, and the isolates of CyEVA [27] (acc # NC_017977), ISMV [49] (acc # NC_029076), OYDV [50] (acc # JX433020), and SYSV [51] (acc # AM267479) were used as outgroup taxa for NDV polyprotein coding sequences. The alignments were performed using CLUSTAL_X2 [52] with TRANSALIGN [53] to maintain the degapped alignment of the encoded amino acids. The aligned sequences of the polyprotein coding regions were 8970 nucleotides for NLSYV and 9093 nucleotides for NDV after removing gaps. Note that incomplete nucleotide sequences with ambiguous nucleotides from GenBank were not included in the analyses.
Partial regions of polyprotein coding sequence (nt 2076-7471) were used for NLSYV maximum likelihood analysis because the recombination sites were identified at positions around nt 2000 and nt 7500 (see later). Conversely, the complete polyprotein coding sequences were used for NDV maximum likelihood analysis because no evidence of recombination was found.
Phylogenetic relationships were inferred using the maximum likelihood method implemented in PhyML version 3.1 [54] under the general time-reversible substitution model with a proportion of invariable sites and gamma-distributed site rates (GTR+I+Γ4). We used jModeltest version 2.1.2 [55] to determine the best-fit model of nucleotide substitution for each dataset. Branch support was evaluated by the bootstrap method based on 1000 pseudoreplicates. The inferred trees were visualized using TREEVIEW [56].
2.6. Recombination Analysis
We reassembled the aligned 5′ and 3′ NCR sequences with both ends of the aligned NLSYV and NDV polyprotein coding sequences to form nearly complete genomic sequences of 9556 nucleotides for NLSYV and 9786 nucleotides for NDV, excluding the 24 nucleotides that were used to design the primer for RT-PCR amplification. The nearly complete genomic sequences were assessed for evidence of recombination, especially for recombination sites in both polyproteins and in the NCRs. Putative recombination sites in all sequences were identified using RDP [57], GENECONV [58], BOOTSCAN [59], MAXCHI [60], CHIMAERA [61], and SISCAN [62] programs implemented in the RDP4 version 101 software package [63] and the original SISCAN version 2 program [62]. First, the incongruent relationships were checked using the programs implemented in RDP4. These analyses were performed using default settings (except to use ‘sequences are linear’) for the different detection programs and a Bonferroni-corrected p-value cutoff of 0.01. All isolates that were identified as likely recombinants by the programs in RDP4, supported by three different methods with an associated p-value of <1.0 × 10−4 (i.e., the most likely recombination sites), were re-checked using the original SISCAN version 2 with all nucleotide sites. We checked all sequences with a 100 and 50 nucleotide sliding window for evidence of recombination using the SISCAN program. These analyses also assessed which non-recombinant sequences had regions that were closest to those from the recombinant sequences, indicating the likely lineages of the donor sequence that provided those regions of the recombinant genomes. For convenience, we called them the “parental isolates” of recombinants. Second, we also aligned NLSYV or NDV sequences without outgroup taxa sequences and directly checked for evidence of recombination using the programs. Finally, GARD [64] was used to assess sites for any evidence of recombination.
2.7. Genetic Diversity
The nucleotide diversities and identities of 28 NLSYV and seven NDV were estimated using MEGA version 7 [65], EMBOSS Needle [66], and the Sequence Demarcation Tool (SDT) v1.2 [67]. We evaluated the degree of mutational saturation in the polyprotein coding sequences using the substitution saturation (Iss) statistic in DAMBE version 6.4.81 [68].
2.8. Spatial Diffusion of NLSYV and NDV
We applied a continuous phylogeographic model [69] to reconstruct the spatial diffusion of NLSYV and NDV in Japan. We used 25 NLSYV isolates of recombination-free partial polyprotein coding sequence (nt 2076–7471) and five NDV isolates of complete polyprotein coding sequence after removing isolates sampled outside Japan. The polyprotein coding sequences of each isolate were assigned two-dimensional geographic coordinates (i.e., latitude and longitude) (Table S3) and analyzed using BEAST v1.10.4 [70]. The posterior distribution of each parameter was inferred based on the Markov chain Monte Carlo run for 100 million steps each, sampled every 10,000 steps. Tracer v1.7.1 [71] was used to check for convergence and satisfactory mixing, based on the effective sample size exceeding 200 for each parameter. We carried out the phylogeographic reconstruction using the gamma-relaxed random walk model that was supported by marginal likelihood estimation based on path sampling and stepping-stone sampling methods [72]. The 95% credible intervals area of the inferred location for each lineage was visualized using SpreaD3 [73] based on 1000 subsampled trees after removing burn-in states.
2.9. Detection of Carlaviruses
We inoculated NLSYV and NDV, NCLV, and NeLV-free asymptomatic Narcissus plants with the saps of Narcissus plant leaves infected with NLSYV (NY-HO42, NY-A65, and NY-HR39 plants) or NDV (NY-KG11 and NY-FI23 plants). We inoculated the virus-infected saps when the Narcissus plant leaves were germinated and very small, namely before the leaves expand in the winter and spring season. The potyvirus- and carlavirus-free Narcissus plants were confirmed for the presence or absence of potyviruses and carlaviruses using the narcissus potyvirus- and carlavirus-specific primer pairs (Table S2). For calravirus detection, we used NCLV-specific forward primer, CARNCLVCP4P, 5′-GCGGCCGCCTGACCCCAGCAATCCTTACAA-3′ or NeLV-specific forward primer, CARNLVCP5P, 5′-GCGGCCGCARAARGGKTGGAGRCCTTCYTC-3′ and carlavirus-specific reverse primer Tu3T9M for amplifying the RT-PCR products of the CP coding regions of carlaviruses and used NCLV-specific forward primer CARNCLVCP1P, NeLV-specific forward primer CARNLVCP2P, and NCLV and NeLV-specific forward primer CARNACP3P forward primers for their sequencing. We applied RT-PCR, cloning, and sequencing methods similarly to for narcissus potyviruses. We focused on the carlaviruses of NCLV and NeLV because they were important narcissus viruses detected in Narcissus plants in the earlier studies [27,74].
3. Results and Discussion
3.1. Co- and Single-Infection of Narcissus Viruses
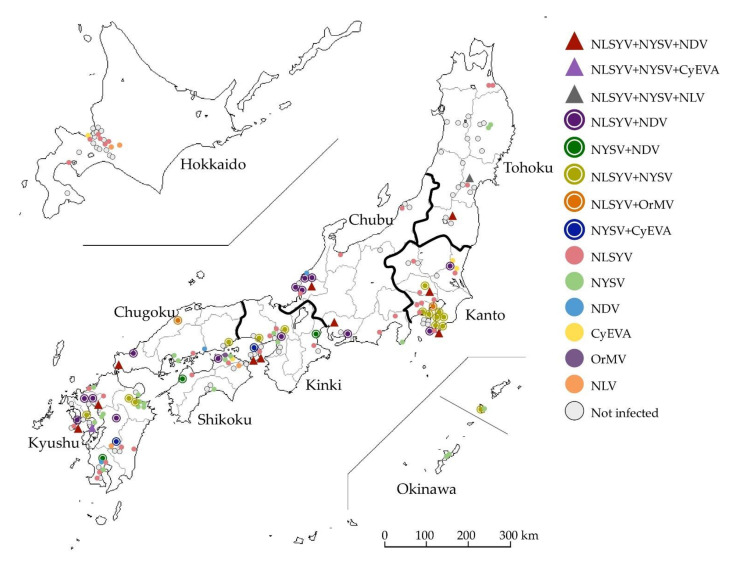
We determined parts of nucleotide sequences (approximately 600–700 bp) in the NIb protein-coding regions of 1174 clones obtained from 189 symptomatic and asymptomatic Narcissus plant leaves (Table S1) using POTYNIB5P primer. Of the 189 plants, 120 (64%) were infected with NLSYV, NDV, NYSV, and other narcissus viruses of the genus Potyvirus and Macluravirus in the family Potyviridae (Figure 1, Table 1). Of 120 Narcissus plants, 12 (10%) were co-infected with three viruses and all were co-infected with NLSYV and NYSV. We found that 36 (30%) plants were co-infected with two different viruses, and 15 (13%) were co-infected with NLSYV and NYSV. Therefore, 48 (40%) plants in total were co-infected. A total of 72 (60%) plants were singly infected, and 37 (31%), 23 (19%), and 3 (3%) plants were infected with NLSYV, NYSV, and NDV, respectively; therefore, the major narcissus potyviruses in Japan are NLSYV, NYSV, and NDV. We focused on the distribution and evolution of NLSYV and NDV in this study because the epidemiology of NYSV-like viruses has already been reported in an earlier study [28]. Note that no RT-PCR product was obtained from the amplification by potyvirus-specific primers of 69 Narcissus plants, even though many showed virus-like symptoms (Table S1). This indicates that these Narcissus plants were infected with viruses that do not belong to the Potyviridae family.
Figure 1.
Collection map of wild and domesticated Narcissus plants in Japan. The Narcissus plants showing mosaic and chlorotic stripe and asymptomatic plants were collected from different sites on the banks of rivers and fields in Japan, including home gardens and flower beds, during the winter and spring seasons of 2009–2015. Color and shape of point on the map indicate the combination of detected virus, as shown in Table 1 and Table S1. Cyrtanthus elatus virus A (CyEVA), narcissus degeneration virus (NDV), narcissus late season yellows virus (NLSYV), narcissus latent virus (NLV), narcissus yellow stripe virus (NYSV), and ornithogalum mosaic virus (OrMV). The map was obtained from http://www.craftmap.box-i.net/ (accessed on 10 January 2022).
Table 1.
Detection of viruses in the family Potyviridae, including narcissus degeneration virus and narcissus late season yellows virus, in Japan.
| Numbers of Plants (%, Number of Plants Detected out of 120 Detected Plants) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| District (Island) |
Plants Examined (n) |
Plants Detected (n) (%) 1 |
Co-Infected with Three Viruses |
Co-Infected with Two Viruses |
Singly Infected with | |||||||||||
| Potyvirus 2 | Maclura-Virus | |||||||||||||||
| TuMV Group | OYDV Group | |||||||||||||||
| NLSYV NYSV NDV |
NLSYV NYSV CyEVA |
NLSYV NYSV NLV |
NDV NLSYV |
NDV NYSV |
NLSYV NYSV |
NLSYV OrMV |
CyEVA NYSV |
NLSYV | NYSV | NDV | CyEVA | OrMV | NLV | |||
| Hokkaido | 24 | 9 (37.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 1 | 0 | 2 |
| Tohoku | 25 | 7 (28.0) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 |
| Kanto | 30 | 23 (76.7) | 2 | 0 | 0 | 2 | 0 | 8 | 1 | 0 | 8 | 0 | 0 | 2 | 0 | 0 |
| Chubu | 19 | 14 (73.7) | 2 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 5 | 1 | 1 | 0 | 0 | 0 |
| Kinki | 21 | 13 (61.9) | 2 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 4 | 2 | 0 | 0 | 0 | 0 |
| Chugoku | 9 | 8 (88.9) | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 |
| Shikoku | 15 | 10 (66.7) | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 3 | 0 | 1 | 1 | 1 |
| Kyushu and Okinawa | 46 | 36 (78.3) | 2 | 1 | 0 | 4 | 1 | 4 | 0 | 1 | 8 | 13 | 1 | 0 | 0 | 1 |
| Total | 189 | 120 (63.5) | 10 (8.3) | 1 (0.8) | 1 (0.8) | 14 (11.7) | 3 (2.5) | 15 (12.5) | 2 (1.7) | 2 (1.7) | 37 (30.8) | 23 (19.2) | 3 (2.5) | 4 (3.3) | 1 (0.8) | 4 (3.3) |
1 This includes cyrtanthus elatus virus A (CyEVA), narcissus degeneration virus (NDV), narcissus late season yellows virus (NLSYV), narcissus latent virus (NLV), narcissus yellow stripe virus (NYSV), and/or ornithogalum mosaic virus (OrMV). Percentage (%); number of plants detected/number of plants examined for virus infection in each district. 2 TuMV group: turnip mosaic virus phylogenetic group and OYDV group: onion yellow dwarf virus phylogenetic group.
3.2. 3′ Terminal Region Sequences
We determined the remaining nucleotide sequences of the NIb-3′NCR regions of 248 clones of NLSYV and 125 clones of NDV (Table S1). A total of approximately 658,000 nucleotides were sequenced. The nucleotide sequences of the NIb-3′NCR regions of each NLSYV and NDV clone determined in this study were approximately 2250 nucleotides in length.
The CP coding regions of NLSYV reported from Australia and China and those sequenced from Japan in this study were all 822 nucleotides (274 amino acids) in length. By contrast, the length of 3′NCR varied: Australian Marijiniup8 and Marijiniup9 isolates (acc # NC_023628 and JX156421, respectively) were 289 and 158 nucleotides in length, respectively; the Chinese Zhangzhou isolate (acc # JQ326210) and all 248 clones from the 25 Japanese NLSYV isolates were 210 nucleotides in length. Conversely, the CP coding regions and the 3′ NCR of NDV reported from all the countries including Japan were all 780–783 nucleotides (260–261 amino acids) and 148 nucleotides in length, respectively.
3.3. Phylogenetic Relationships and Nucleotide Identities Assessed by Coat Protein Coding Sequences
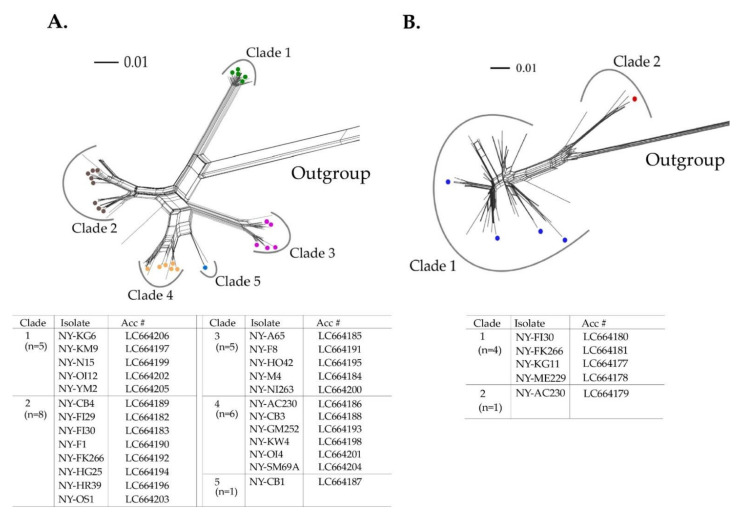
We inferred the phylogenetic network using 248 NLSYV and 125 NDV CP coding sequences. NLSYV CP coding sequences fell into at least five major clades in the phylogenetic network (Figure 2A), whereas the NDV CP coding sequences fell into two major clades (Figure 2B). Therefore, we chose 25 NLSYV and 5 NDV isolates for subsequent full genomic sequencing, so that they represented all the major clades found in the CP phylogenetic network.
Figure 2.
Phylogenetic networks of the nucleotide sequences of the complete coat protein (CP) coding regions from the plasmid clones of narcissus viruses. (A) Narcissus late season yellows virus (NLSYV). The nucleotide sequences of CP coding regions of Japanese yam mosaic virus (JYMV) [46], narcissus yellow stripe virus (NYSV) [26], scallion mosaic virus (ScaMV) [48], and turnip mosaic virus (TuMV) [47] in the TuMV phylogenetic group were used as outgroup taxa. (B) Narcissus degeneration virus (NDV). The nucleotide sequences of CP coding regions of cyrtanthus elatus virus A (CyEVA) [27], iris severe mosaic virus (ISMV) [49], onion yellow dwarf virus (OYDV) [50], and shallot yellow stripe virus (SYSV) [51] in the OYDV phylogenetic group were used as outgroup taxa. Dots in each clade show the isolates whose genomic sequences were fully sequenced. The full genomic sequences of the isolates determined in this study are listed in the tables below the phylogenetic networks of NLSYV and NDV. Acc # are also listed in the tables. The NY-CB1 isolate was found to have recombination site in CP coding sequence (see Section 3.6. Inference of Recombination).
3.4. Co-Infection with Different Isolates and Quasispecies
One of the difficulties in this study was obtaining the full genomic sequences of NLSYV and NDV using several cloned plasmids that cover partial genomic regions because we were unable to isolate either virus from single lesion infections. Therefore, we needed to characterize the sequences from a single RNA molecule in the infected Narcissus plants. We amplified the longest possible RT-PCR products with the aim of obtaining full genomic sequences from the cloned fragments with no mismatches in the overlapping regions (Figure S1). As a result, the full genomic sequences of NLSYV and NDV isolates were obtained from three to five overlapping clone fragments. We found many nucleotide mismatches among the cloned fragment sequences, and a single Narcissus plant was co-infected with different isolates of the same virus or quasispecies of the same virus (Table S1).
For instance, among 23 NLSYV isolates, five isolates (NY-CB3, NY-CB4, NY-KW4, NY-M4, and NY-FK266) were co-infected with different isolates (65–300 mismatches between the clones), 16 were co-infected with quasispecies (1–17 mismatches between the clones), and only two isolates (NY-F1 and NY-AC230) had no mixed nucleotides between the overlapping fragment clones. Similarly, we found many nucleotide mismatches between the sequences of NDV overlapping fragments. Narcissus are bulbous plants, and narcissus potyviruses are transmitted by aphids; hence, the plants have numerous chances to be co-infected [13,75]. Note that we used high-fidelity Taq DNA polymerase for amplifying RT-PCR products. The fidelity of the polymerase was also checked, as potentially contributing to the observed mismatches, by amplifying and then sequencing 12,000 nucleotides of PCR products from cloned cDNA of known sequence [15], and no mismatches were found compared with the original sequences. Finally, we found clones with no mismatches in the overlapping regions of each NLSYV and NDV isolate and successfully obtained the full genomic sequences of all the isolates from a single RNA molecule but not from a different RNA molecule of the same virus in the infected Narcissus plants.
3.5. Full Genomic Sequences
The NLSYV genomes of Japanese isolates were 9625–9628 nucleotides in length, excluding the 5′ end 24 nucleotide primer sequences. All 25 Japanese isolates of the polyprotein coding regions were 9315 nucleotides with 3105 amino acids in length. The lengths of 5′ and 3′ NCRs of NLSYV were 99 nucleotides, excluding the 5′ end primer sequences and 211–214 nucleotides, respectively. The lengths of encoding protein 1 (P1), helper-components proteinase (HC-Pro), P3, overlapping ORF (PIPO), 6K1, CI, 6Kda 2 protein (6K2), genome-linked viral protein (VPg), NIa-Pro, NIb, and CP proteins were 957, 1374, 1062, 195, 156, 1932, 159, 573, 729, 1551, and 822 nucleotides, respectively. Therefore, the lengths of the genomes and protein-coding regions are similar to those of NYSV and NYSV-like isolates, which belong to the TuMV phylogenetic group [28]. The lengths of P1 and P3 varied among narcissus viruses in the TuMV and OYDV phylogenetic groups. All the motifs reported for different potyvirus encoded proteins were found. The CP region of NY-CB4 and NY-OI4 clones (Table S1) belonged to NLSYV Clades 2 and 4 respectively (Figure 2A), whereas all the full and partial genomic sequences including the CP region of both isolates belong to Group 1 after we obtained the full genomic sequences (Figure S2). This indicates that the full genomic sequence of NY-CB4 and NY-OI4 was obtained from the different viral populations from the CP sequences in Clades 2 and 4 of both plants.
The NDV genomes of Japanese isolates were 9789–9792 nucleotides in length, excluding the 5′-end 24 nucleotide primer sequences. Five Japanese isolates of polyprotein coding regions were 9552–9555 nucleotides with 3184–3185 amino acids in length. The lengths of 5′ and 3′ NCRs of NDV were 89 nucleotides, excluding the 5′-end 24 nucleotide primer sequences, and 148 nucleotides. The lengths of encoding P1, HC-Pro, P3, overlapping ORF (PIPO), 6K1, CI, 6K2, VPg, NIa-Pro, NIb, and CP proteins were 1182, 1374, 1152, 234, 156, 1908, 159, 555, 723, 1560, and 780–783 nucleotides, respectively. The nucleotide sequence data reported in this paper are available in the GenBank/ENA/DDBJ database as accession LC664177-LC664181 for NDV isolates and LC664182-LC664206 for NLSYV isolates.
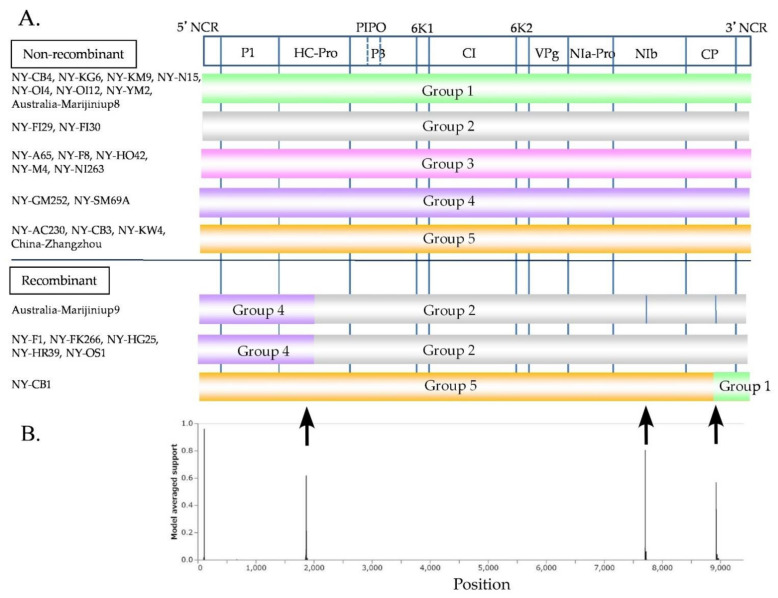
3.6. Inference of Recombination
The aligned polyprotein coding sequences of NLSYV were 8970 nucleotides in length. The phylogenetic network of the polyprotein coding sequences showed reticulated phylogenetic relationships, reflecting conflicts in the phylogenetic signal that are possibly due to the presence of recombinant sequences (data not shown). Recombination is an important source of genetic variation for potyviruses [3]. The 25 polyprotein coding sequences determined in this study, together with three sequences (Marijiniup8 acc # NC_02362, Marijiniup9 acc # JX156421, and Zhangzhou acc # JQ326210) obtained from GenBank, were assessed for evidence of recombination. Three recombination sites were identified by RDP4 in the NLSYV genomes: one located at the middle of the HC-Pro coding region (genome position around nt 2000) and the other two located in the CP coding region (Figure 3). Two (around nt 2000 and nt 7500) of three recombination sites were clearly supported by more than five programs in RDP4 (Table S4). The recombination site in the HC-Pro coding region was found in both Japanese and Australian isolates. The recombination site at positions around nt 9000 was found in both Japanese and Australian NLSYV, but both seemed to be tentative, and the different parental sequences were identified by RDP4. On the other hand, no identical recombination type pattern of the genome was found between these three countries. Moreover, identical non-recombinants were found in China and Japan, and in Australia and Japan, but not in Australia and China. Fewer recombination sites were found in NLSYV compared to other potyvirus species [76,77]. However, no evidence of recombination sites was found in the NDV genome. The genomes of NDV isolates shared high nucleotide identity throughout their genomes. Therefore, we cannot exclude the possibility of recombination in NDV genomes, awaiting the discovery of supporting evidence using a large number of isolates collected over the world. We also used GARD [64] software to confirm the evidence of recombination sites (Figure 3B). The results of NLSYV also showed evidence of recombination at almost the same recombination sites as detected by RDP4. We also inferred phylogenetic trees using the regions between the recombination sites (Figure S2); 5′ end–nt 1800, nt 2200–7400, and nt 7700–3′end. The topology of the trees clearly indicated that there might be recombination sites between these genomic regions. Similarly, none of the recombination sites were found in NDV genomes. A more detailed collection of Narcissus plants from around the world would clarify the evidence of common and different recombination sites in the NLSYV and NDV genomes.
Figure 3.
Recombination analysis. (A) Recombination genome map of the narcissus late season yellows virus (NLSYV) genomes of each isolate. Vertical solid lines show estimated approximate recombination sites. (B) The best placement of recombination sites (breakpoints) inferred by the algorithm for each site considered by GARD. The nucleotide positions correspond to the genome of the Zhangzhou isolate [26]. Note that recombination site around nt 9000 in Marijiniup9 genome is tentative.
We compared the number of clades obtained by CP coding regions in the NLSYV phylogenetic network (Figure 2A) and that of groups in non-recombinant and recombinant genomes (Figure 3 and Figure S2). As a result, Clade 2 split into Group 2 and recombinant group (NY-F1, NY-FK266, NY-HG25, NY-HR39, and NY-OS1), and Clade 4 split into Groups 4 and 5. The NY-CB1 isolate fell into the independent clade (Clade 5) in the CP phylogenetic network and has a recombination site in CP coding region with Group 1 and 5 parents.
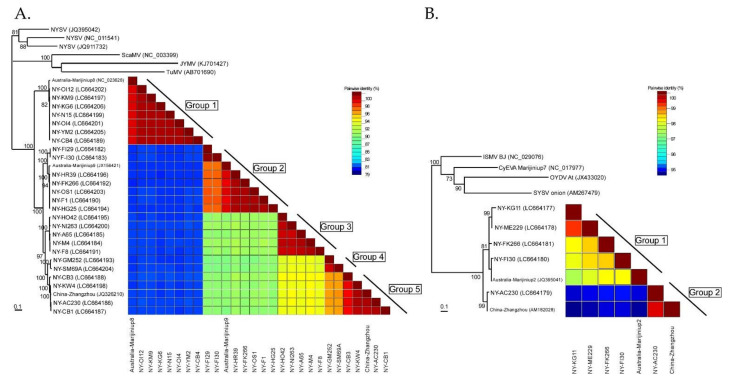
3.7. Inference of Phylogenetic Relationships and Nucleotide Diversities Assessed by Polyprotein Coding Sequences
The NLSYV partial polyprotein coding sequences (genome position nt 2076–7471) fell into five major phylogenetic groups (Figure 4A), and this grouping was also supported by pairwise identity comparisons by SDT v1.2. Phylogenetic analysis of the NLSYV polyprotein coding region shows that NLSYV has at least five phylogenetic groups. The groupings are also supported by full and partial genomic sequences of NLSYV (Figure S2). The grouping of isolates did not correspond well to their geographical origins. The pairwise identities of NLSYV sequences are higher than 81%. There are two clusters for NDV supported by high bootstrap values. Therefore, NDV polyprotein coding sequences seem to form two groups (Figure 4B) and the pairwise identities of NDV sequences are higher than 95%. Although we still need additional collections of NDV isolates from around the world to assess the diversity of the NDV population, our results indicate that in Japan the genetic diversities of NLSYV are higher than those of NDV.
Figure 4.
Phylogenetic relationships and pairwise identities of two narcissus viruses. (A) Twenty-eight partial polyprotein coding sequences (genome position nt 2076–7471) of narcissus late season yellows virus (NLSYV) were used to infer maximum likelihood phylogenetic trees. The polyprotein coding sequences of Japanese yam mosaic virus (JYMV) [46], narcissus yellow stripe virus (NYSV) [23,26,27], scallion mosaic virus (ScaMV) [48], and turnip mosaic virus (TuMV) [47] in the TuMV phylogenetic group were used as outgroup taxa. (B) Seven complete polyprotein coding sequences of narcissus degeneration virus (NDV) were used to infer maximum likelihood phylogenetic trees. The polyprotein coding sequences of cyrtanthus elatus virus A (CyEVA) [27] (acc # NC_017977), iris severe mosaic virus (ISMV) [49] (acc # NC_029076), onion yellow dwarf virus (OYDV) [50] (acc # JX433020), and shallot yellow stripe virus (SYSV) [51] (acc # NC_007433) in the OYDV phylogenetic group were used as outgroup taxa for NDV. Number at each node indicate bootstrap percentages based on 1000 pseudoreplicates for both trees and the values >70% are only shown.
The nucleotide sequence identities of complete polyprotein coding sequences of 28 NLSYV (9294–9315 nucleotides) and 7 NDV (9552–9555 nucleotides) were also calculated by EMBOSS Needle. The nucleotide identities of polyprotein coding sequences between each isolate are higher than 82% for NLSYV and higher than 94% for NDV. The identities are similar in most of the protein coding regions, whereas the P1 protein coding region is the most diverse, as previously reported for some potyviruses [4,78,79]. The nucleotide identities of different narcissus viruses are different: NLSYV, NYSV [28], and CyEVA [27,80] are genetically diverse, whereas NDV is the least variable. NYSV was first reported in 1908 in the UK [81], whereas NLSYV was first reported in N. pseudonarcissus in 1988 from the UK [39]. CyEVA, as a vallota speciosa virus, was first reported in 1980 from Vallota speciosa in The Netherlands [82,83], and NDV was first reported in N. tazetta in 1970 from the UK [84]. NLV is a species of macluravirus first reported in N. pseudonarcissus from the UK in 1967 [85]. Since NYSV, NLSYV, CyEVA, and NDV have narrow host ranges and are mostly limited to Amaryllidaceae host plants, as NLSYV has been reported from Sternbergia lutea, Clivia miniata, and NDV from Lycoris, the extent of nucleotide diversity might be related to the time of emergences of each virus. However, to validate this hypothesis, we need to compare them using the viruses collected from over the world.
3.8. Timescale Analysis, Migration of Non-Recombinants and Recombinants
We evaluated the degree of mutational saturation for NLSYV and NDV populations using Iss statistics. The estimated Iss was six to 23 times lower than the critical Iss value (Iss.c) for all datasets (p < 0.05). Therefore, there is little saturation across the NLSYV and NDV sequences in the datasets of each polyprotein coding region. We then attempted to infer the evolutionary timescale for NLSYV and NDV. However, a lack of temporal signals was suggested by the date-randomization test (data not shown) in both the NLSYV and NDV datasets of some genomic regions (e.g., HC-Pro, P3, NIb, and CP) and polyprotein coding regions. More serial collections of NLSYV and NDV isolates would allow us to measurably observe the evolving population.
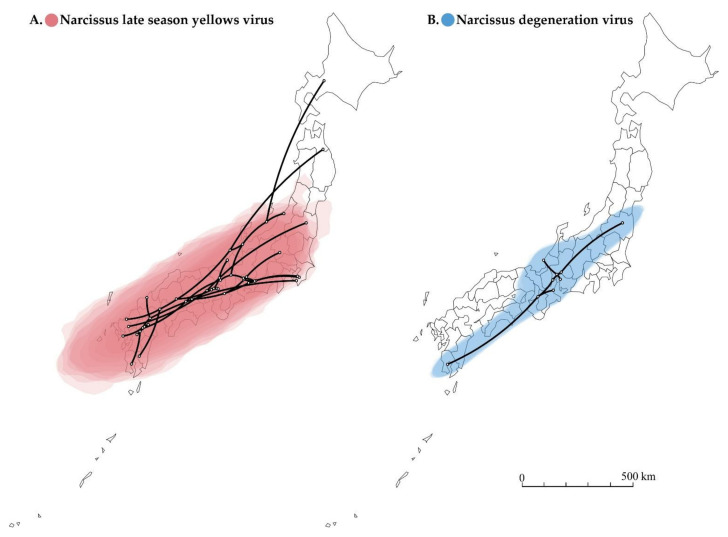
The reconstructed spatial diffusion of NLSYV and NDV isolates in Japan were examined using the polyprotein coding region (Figure 5). The inferred distribution areas of NLSYV and NDV seem different in terms of the range of statistical uncertainty, even though NLSYV and NDV were often found in the same host plant as co-infections. The distributed area of statistical uncertainty for NLSYV is broader (Figure 5A), whereas that of NDV is more limited (Figure 5B), although both exist in the southwest parts of Japan. This is possibly due to the different periods of introduction into Japan for NLSYV and NDV. NDV might have been more recently introduced into Japan than NLSYV, considering the difference of the expanding area and nucleotide diversities of the two viruses collected in Japan. However, we still need further sampling of NLSYV and NDV both in Japan and neighboring countries to determine their time of introduction into Japan.
Figure 5.
Spatial diffusion of narcissus viruses. (A) Twenty-five partial polyprotein coding sequences (genome position nt 2076–7471) of narcissus late season yellows virus (NLSYV). (B) Five complete polyprotein coding sequences of narcissus degeneration virus (NDV) were used. The points indicate the location of ancestral internal nodes and external tips. The 95% areas of credible intervals based on 1000 trees subsampled from the post-burn-in posterior distribution are shown as colored shadows. The map was obtained from https://gist.github.com/minikomi/4043986 (accessed on 10 January 2022).
Our findings also raise an additional question as to why co- or single infection of viruses occurs in different host plants. For instance, our earlier studies of ScaMV from wild Japanese garlic plants (Chinese garlic or no-biru, a species of wild onion, Allium macrostemon Bunge) [77,86] showed almost no co-infection with other potyviruses, no co-infection with different viruses of the same isolates, and even the absence of quasispecies of the same virus. Japanese garlic and Narcissus are both monocotyledonous plants, and the infected potyviruses seem to have a narrow host range. The origins of domesticated populations of Narcissus are still unclear. They appear to have arisen in the area of the Iberian Peninsula, southern France, and northwestern Italy [87]. The Japanese Narcissus plant (Nihon-zuisen, Narcissus tazetta var. chinensis) was first described in the kanji dictionary as Kagakushu, which was published in 1444 CE in Japan. It is speculated that Japanese Narcissus plants drifted along oceanic currents from the Chinese continent to Japan before the Muromachi period (1336–1573 CE) at least three times or could have been introduced to Japan through trade with China, but the introduction of Narcissus plants to Japan is still unclear (Echizen Town, Ota Museum of Culture History, https://www.town.echizen.fukui.jp/otabunreki/ accessed on 10 January 2022). It is also speculated that Narcissus plants dispersed from Mediterranean countries via Silk Road to Eastern China [88]. Therefore, the Narcissus plant has a relatively long history in Japan. There are three main colonies of wild Japanese Narcissus plants: Echizen Seacoast (Fukui Prefecture), Kyonan-machi (Chiba Prefecture), and Nadakuroiwa (Hyogo Prefecture). The Narcissus plants Narcissus poeticus and Narcissus jonquilla were introduced from European countries to Japan after Meiji-Ishin (1868 CE). Japanese garlic plants are considered to be naturally part of the country’s flora. The epidemiological and evolutionary studies of co-infections of plant viruses have only recently started because it is difficult to collect co-infected plants from a wide area, so more such studies are needed in the near future [11,20,89].
3.9. Co-Infection with Carlaviruses
The potyvirus-free Narcissus plant leaves inoculated with the sap of NY-A65, NY-HO42, and NY-HR49 plants (infected with NLSYV) or NY-KG11 and NY-FI23 plants (infected with NDV) (Table S1) showed chlorotic stripes and mosaic symptoms on Narcisuss (N. tazzeta var. chinensis) plant leaves one-month post-inoculation. The presence of NLSYV, NDV, NCLV, and NeLV in the inoculated plants was confirmed by RT-PCR amplification and sequencing. The Narcissus plants inoculated with the sap of NY-A65, NY-HO42, NY-HR49, NY-KG11, and NY-FI23 plant leaves were found to be infected with not only NLSYV or NDV but also NCLV. Therefore, many Narcissus plants in nature seem to be co-infected with not only potyviruses but also with carlaviruses [13,23,27,38,90]. A more detailed global analysis of narcissus carlaviruses will be reported in a future publication.
4. Conclusions
We presented a detailed evolutionary investigation of co-infections of narcissus viruses from wild and domesticated Narcissus plants in Japan. Our findings are that (1) NLSYV is widely distributed whereas the distribution of NDV is limited to the southwest parts of Japan; (2) the nucleotide sequence identities of the genomes between NLSYV isolates are higher than 82%, whereas those between NDV isolates are higher than 94%; (3) three novel recombination type patterns were found in the NLSYV population; (4) NLSYV has at least five distinct phylogenetic groups whereas NDV has two; and (5) infection with narcissus viruses often occur as co-infections of different viruses, different isolates of the same viruses, and in the presence of quasispecies (mutant clouds) of the same virus in nature. Our findings also illustrate that it is important to understand the co-infections of potyviruses in nature when designing control strategies in the future, as the wild and domesticated Narcissus plants in Japan are somewhat like a melting pot of potyviruses.
Acknowledgments
We thank Rei Nomiyama, Yuki Honda, Kenta Soejima, Shinichiro Mitoma, Kaoru Tominaga, Miho Fujita, Yuichiro Hirosue, and Mai Yoshida (Laboratory of Plant Virology, Saga University, Japan) for their careful technical assistance for sequencing virus genomes; and Kazuo Yamashita (Fukuchi Garlic R&S, Aomori, Japan) for sample collection. Computations were partly performed on the National Institute of Genetics (NIG) supercomputer at the Research Organization of Information and Systems (ROIS) National Institute of Genetics, Japan. Genomic sequences of narcissus virus isolates were determined at the Laboratory of Plant Virology and Analytical Research Center for Experimental Sciences, Saga University.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v14030582/s1, Figure S1: Workflow for the full genomic sequencing of narcissus late season yellows virus and narcissus degeneration virus, Figure S2: Maximum likelihood phylogenetic trees inferred using full and partial genomic sequences of narcissus late season yellows virus (NLSYV). Table S1: Collection sites and the results of genetic diagnosis of Narcissus plants in this study, Table S2: Primers used for RT-PCR amplification and sequencing in this study, Table S3: Location of narcissus late season yellows virus and narcissus degeneration virus Japanese isolates, Table S4: Tentative and clear recombination sites in narcissus late season yellows virus genomes.
Author Contributions
Conceptualization, K.O.; methodology, K.O.; software, W.P. and S.K.; validation, W.P., S.K. and K.O.; formal analysis, W.P., S.K. and K.O.; investigation, W.P., S.K. and K.O.; resources, K.O.; data curation, W.P., S.K. and K.O.; writing—original draft preparation, K.O.; writing—review and editing, W.P., S.K. and K.O.; visualization, W.P., S.K. and K.O.; supervision, K.O.; project administration, K.O.; funding acquisition, K.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was in part funded by Saga University and Kagoshima University and supported by the Japanese Society for the Promotion of Science (JSPS) KAKENHI, grant numbers 16K14862, 18K05653, and 21K05601.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data obtained in this research is available upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.García-Arenal F., Fraile A., Malpica J.M. Variability and genetic structure of plant virus populations. Annu. Rev. Phytopathol. 2001;39:157–186. doi: 10.1146/annurev.phyto.39.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Gibbs A.J., Mackenzie A.M., Wei K.J., Gibbs M.J. The potyviruses of Australia. Arch. Virol. 2008;153:1411–1420. doi: 10.1007/s00705-008-0134-6. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs A.J., Ohshima K. Potyviruses and the digital revolution. Annu. Rev. Phytopathol. 2010;48:205–223. doi: 10.1146/annurev-phyto-073009-114404. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs A.J., Hajizadeh M., Ohshima K., Jones R.A.C. The potyviruses: An evolutionary synthesis is emerging. Viruses. 2020;12:132. doi: 10.3390/v12020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno Gonçalves A.B., López-Moya J.J. When viruses play team sports: Mixed infections in plants. Phytopathology. 2020;110:29–48. doi: 10.1094/PHYTO-07-19-0250-FI. [DOI] [PubMed] [Google Scholar]
- 6.Redinbaugh M.G., Stewart L.R. Maize lethal necrosis: An emerging, synergistic viral disease. Annu. Rev. Virol. 2018;5:301–322. doi: 10.1146/annurev-virology-092917-043413. [DOI] [PubMed] [Google Scholar]
- 7.Klap C., Luria N., Smith E., Hadad L., Bakelman E., Sela N., Belausov E., Lachman O., Leibman D., Dombrovsky A. Tomato brown rugose fruit virus contributes to enhanced pepino mosaic virus titers in tomato plants. Viruses. 2020;12:879. doi: 10.3390/v12080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ontiveros I., Lopez-Moya J.J., Díaz-Pendón J.A. Co-infection of tomato plants with Tomato yellow leaf curl virus and Tomato chlorosis virus affects the interaction with host and whiteflies. Phytopathology. 2021;26:341. doi: 10.1094/PHYTO-08-21-0341-R. [DOI] [PubMed] [Google Scholar]
- 9.Vinodhini J., Rajendran L., Abirami R., Karthikeyan G. Co-existence of chlorosis inducing strain of Cucumber mosaic virus with tospoviruses on hot pepper (Capsicum annuum) in India. Sci. Rep. 2021;11:8796. doi: 10.1038/s41598-021-88282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart L.R., Willie K. Maize yellow mosaic virus interacts with Maize chlorotic mottle virus and Sugarcane mosaic virus in mixed infections, but does not cause maize lethal necrosis. Plant Dis. 2021;105:3008–3014. doi: 10.1094/PDIS-09-20-2088-RE. [DOI] [PubMed] [Google Scholar]
- 11.Kavalappara S.R., Milner H., Konakalla N.C., Morgan K., Sparks A.N., McGregor C., Culbreath A.K., Wintermantel W.M., Bag S. High throughput sequencing-aided survey reveals widespread mixed infections of whitefly-transmitted viruses in cucurbits in Georgia, USA. Viruses. 2021;13:988. doi: 10.3390/v13060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Z., Yasaka R., Li W., Li S., Ohshima K. Genetic structure of populations of sugarcane streak mosaic virus in China: Comparison with the populations in India. Virus Res. 2015;211:103–116. doi: 10.1016/j.virusres.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Ohshima K., Nomiyama R., Mitoma S., Honda Y., Yasaka R., Tomimura K. Evolutionary rates and genetic diversities of mixed potyviruses in Narcissus. Infect. Genet. Evol. 2016;45:213–223. doi: 10.1016/j.meegid.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 14.French R., Stenger D.C. Population structure within lineages of Wheat streak mosaic virus derived from a common founding event exhibits stochastic variation inconsistent with the deterministic quasi-species model. Virology. 2005;343:179–189. doi: 10.1016/j.virol.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Ohshima K., Akaishi S., Kajiyama H., Koga R., Gibbs A.J. The evolutionary trajectory of turnip mosaic virus populations adapting to a new host. J. Gen. Virol. 2010;91:788–801. doi: 10.1099/vir.0.016055-0. [DOI] [PubMed] [Google Scholar]
- 16.Ali A., Roossinck M.J. Analysis of quasispecies variation in single and mixed viral infection. Virus Evol. 2017;3:vex037. doi: 10.1093/ve/vex037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo Y., Choi H., Kim S.M., Kim S.L., Lee B.C., Cho W.K. The pepper virome: Natural co-infection of diverse viruses and their quasispecies. BMC Genom. 2017;18:453. doi: 10.1186/s12864-017-3838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syller J. Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol. Plant Pathol. 2012;13:204–216. doi: 10.1111/j.1364-3703.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLeish M., Sacristán S., Fraile A., García-Arenal F. Coinfection organizes epidemiological networks of viruses and hosts and reveals hubs of transmission. Phytopathology. 2019;109:1003–1010. doi: 10.1094/PHYTO-08-18-0293-R. [DOI] [PubMed] [Google Scholar]
- 20.Allen L.J.S., Bokil V.A., Cunniffe N.J., Hamelin F.M., Hilker F.M., Jeger M.J. Modelling vector transmission and epidemiology of co-infecting plant viruses. Viruses. 2019;11:1153. doi: 10.3390/v11121153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langeveld S.A., Derks A.F.L.M., Konicheva V., Muñoz D., Zhin-nan C., Denkova S.T., Lemmers M.E.C., Boonekamp P.M. Molecular identification of potyviruses in Dutch stocks of Narcissus. Acta Hortic. 1997;430:641–648. doi: 10.17660/ActaHortic.1997.430.101. [DOI] [Google Scholar]
- 22.Chen J., Chen J.P., Langeveld A.F., Derks A.F.L.M., Adams M.J. Molecular characterization of carla- and potyviruses from Narcissus in China. J. Phytopathol. 2003;151:26–29. doi: 10.1046/j.1439-0434.2003.00674.x. [DOI] [Google Scholar]
- 23.Chen J., Shi Y.H., Lu Y.W., Adams M.J., Chen J.P. Narcissus symptomless virus: A new carlavirus of daffodils. Arch. Virol. 2006;151:2261–2267. doi: 10.1007/s00705-006-0801-4. [DOI] [PubMed] [Google Scholar]
- 24.Chen J., Shi Y.H., Adams M.J., Zheng H.Y., Qin B.X., Chen J.P. Characterisation of an isolate of Narcissus degeneration virus from Chinese narcissus (Narcissus tazetta var. chinensis) Arch. Virol. 2007;152:441–448. doi: 10.1007/s00705-006-0841-9. [DOI] [PubMed] [Google Scholar]
- 25.Monger W.A., Mumford R.A. Vallota mosaic virus infecting nerine in the UK. Plant Pathol. 2008;57:768. doi: 10.1111/j.1365-3059.2008.01850.x. [DOI] [Google Scholar]
- 26.Lin S.Q., Shen J.G., Gao F.L., Cai W., Huang Z., Xie L.Y., Wu Z.J. Complete genome sequence of Narcissus late season yellows virus infecting Chinese narcissus in China. Arch. Virol. 2012;157:1821–1824. doi: 10.1007/s00705-012-1328-5. [DOI] [PubMed] [Google Scholar]
- 27.Wylie S.J., Jones M.G.K. Complete genome sequences of seven carlavirus and potyvirus isolates from Narcissus and Hippeastrum plants in Australia, and proposals to clarify their naming. Arch. Virol. 2012;157:1471–1480. doi: 10.1007/s00705-012-1319-6. [DOI] [PubMed] [Google Scholar]
- 28.Ohshima K., Mitoma S., Gibbs A.J. The genetic diversity of narcissus viruses related to turnip mosaic virus blur arbitrary boundaries used to discriminate potyvirus species. PLoS ONE. 2018;13:e0190511. doi: 10.1371/journal.pone.0190511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunt A.A. Narcissus. In: Loebenstein G., Lawson R.H., Brunt A.A., editors. Virus and Virus-Like Diseases of Bulb and Flower Crops. John Wiley and Sons; Chichester, UK: 1995. pp. 322–334. [Google Scholar]
- 30.Wylie S.J., Li H., Sivasithamparam K., Jones M.G.K. Complete genome analysis of three isolates of narcissus late season yellows virus and two of narcissus yellow stripe virus: Three species or one? Arch. Virol. 2014;159:1521–1525. doi: 10.1007/s00705-013-1969-z. [DOI] [PubMed] [Google Scholar]
- 31.Raj R., Kaur C., Srivastava A., Kumar S., Raj S.K. Sequence analyses of RT-PCR products obtained from seven infected leaf samples revealed existence of three potyvirus species in Indian narcissus (Narcissus tazetta L.) 3 Biotech. 2020;10:428–430. doi: 10.1007/s13205-020-02418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valouzi H., Shahmohammadi N., Golnaraghi A. Genetic diversity and evolutionary analyses of potyviruses infecting narcissus in Iran. J. Plant Pathol. 2021;7:237–250. doi: 10.1007/s42161-021-00985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibbs A.J., Nguyen H.D., Ohshima K. The emergence of turnip mosaic virus was probably a gene-forquasi-gene event. Curr. Opin. Virol. 2015;10:20–26. doi: 10.1016/j.coviro.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Fuji S., Mochizuki T., Okuda M., Tsuda S., Kagiwada S., Sekine K.T., Ugaki M., Natsuaki K.T., Isogai M., Maoka T., et al. Plant viruses and viroids in Japan. J. Gen. Plant Pathol. 2022;88:105–127. doi: 10.1007/s10327-022-01051-y. [DOI] [Google Scholar]
- 35.Lefkowitz E.J., Dempsey D.M., Hendrickson R.C., Orton R.J., Siddell S.G., Smith D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV) Nucleic Acids Res. 2018;46:708–717. doi: 10.1093/nar/gkx932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung B.Y., Miller W.A., Atkins J.F., Firth A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA. 2008;105:5897–5902. doi: 10.1073/pnas.0800468105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter D.A., Fletcher J.D., Davies K.M., Zhang H. Colour break in reverse bicolour daffodils is associated with the presence of Narcissus mosaic virus. Virol. J. 2011;8:412. doi: 10.1186/1743-422X-8-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward L.I., Veerakone S., Tang J., Clover G.R.G. First report of Narcissus degeneration virus, Narcissus late season yellows virus, and Narcissus symptomless virus on Narcissus in New Zealand. Plant Dis. 2009;93:964. doi: 10.1094/PDIS-93-9-0964A. [DOI] [PubMed] [Google Scholar]
- 39.Mowat W.P., Duncan G.H., Dawson S. Narcissus late season yellows potyvirus: Symptoms, properties and serological detection. Ann. Appl. Biol. 1988;113:531–544. doi: 10.1111/j.1744-7348.1988.tb03330.x. [DOI] [Google Scholar]
- 40.Chandel V., Singh M.K., Hallan V.Z., Aijaz A. Evidence for the occurrence of a distinct potyvirus on naturally growing Narcissus tazetta. Arch. Phytopathol. Plant Prot. 2010;43:209–214. doi: 10.1080/03235400701722145. [DOI] [Google Scholar]
- 41.Sochacki D., Chojnowska E. The frequency of viral infections on two narcissus plantations in central Poland. J. Hortic. Res. 2016;24:19–24. doi: 10.1515/johr-2016-0016. [DOI] [Google Scholar]
- 42.Agoston J., Almasi A., Nemes K., Salanki K., Palkovics L. First report of hippeastrum mosaic virus, narcissus late season yellows virus, narcissus latent virus and narcissus mosaic virus in daffodils from Hungary. J. Plant Pathol. 2020;102:1275–1276. doi: 10.1007/s42161-020-00556-9. [DOI] [Google Scholar]
- 43.Wylie S.J., Nouri S., Coutts B.A., Jones M.G.K. Narcissus late season yellows virus and Vallota speciosa virus found infecting domestic and wild populations of Narcissus species in Australia. Arch. Virol. 2010;155:1171–1174. doi: 10.1007/s00705-010-0682-4. [DOI] [PubMed] [Google Scholar]
- 44.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 45.Huson D.H., Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 46.Lan P., Li F., Wang M., Li R. Complete genome sequence of a divergent strain of Japanese yam mosaic virus from China. Arch. Virol. 2015;160:573–576. doi: 10.1007/s00705-014-2231-z. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen H.D., Tomitaka Y., Ho S.Y.W., Duchêne S., Vetten H.J., Lesemann D., Walsh J.A., Gibbs A.J., Ohshima K. Turnip mosaic potyvirus probably first spread to Eurasian brassica crops from wild orchids about 1000 years ago. PLoS ONE. 2013;8:e55336. doi: 10.1371/journal.pone.0055336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J., Zheng H., Chen J., Adams M. Characterisation of a potyvirus and a potexvirus from Chinese scallion. Arch. Virol. 2002;147:683–693. doi: 10.1007/s007050200018. [DOI] [PubMed] [Google Scholar]
- 49.Li Y., Deng C., Shang Q., Zhao X., Liu X., Zhou Q. The first complete genome sequence of iris severe mosaic virus. Arch. Virol. 2016;161:1069–1072. doi: 10.1007/s00705-015-2743-1. [DOI] [PubMed] [Google Scholar]
- 50.Celli M.G., Torrico A.K., Kiehr M., Conci V.C. Striking differences in the biological and molecular properties of onion and garlic isolates of onion yellow dwarf virus. Arch. Virol. 2013;158:1377–1382. doi: 10.1007/s00705-012-1597-z. [DOI] [PubMed] [Google Scholar]
- 51.Chen J., Wei C.B., Zheng H.Y., Shi Y.H., Adams M.J., Lin L., Zhang Q.Y., Wang S.J., Chen J.P. Characterisation of the welsh onion isolate of Shallot yellow stripe virus from China. Arch. Virol. 2005;150:2091–2099. doi: 10.1007/s00705-005-0580-3. [DOI] [PubMed] [Google Scholar]
- 52.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 53.Weiller G.F. TransAlign. Research School of Biological Sciences; Canberra, Australia: 1999. Version 1.0. [Google Scholar]
- 54.Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 55.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Page R.D.M. Treeview: An application to display phylogenetic trees on personal computer. Bioinformatics. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 57.Martin D., Rybicki E. RDP: Detection of recombination amongst aligned sequences. Bioinformatics. 2000;16:562–563. doi: 10.1093/bioinformatics/16.6.562. [DOI] [PubMed] [Google Scholar]
- 58.Sawyer S.A. GENECONV: A Computer Package for the Statistical Detection of Gene Conversion. Department of Mathematics, Washington University; St. Louis, MO, USA: 1999. [Google Scholar]
- 59.Salminen M.O., Carr J.K., Burke D.S., McCutchan F.E. Identification of break points in intergenotypic recombinants of HIV type 1 by bootscanning. World J. Gastroenterol. 1995;11:1423–1425. doi: 10.1089/aid.1995.11.1423. [DOI] [PubMed] [Google Scholar]
- 60.Smith M.J. Analyzing the mosaic structure of genes. J. Mol. Evol. 1992;34:126–129. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- 61.Posada D., Crandall K.A. Evolution of methods for detecting recombination from DNA sequence: Computer simulations. Proc. Natl. Acad. Sci. USA. 2001;98:13757–13762. doi: 10.1073/pnas.241370698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibbs M.J., Armstrong J.S., Gibbs A.J. Sister-scanning: A monte calro procedure for assessing signals in recombinant sequences. Bioinformatics. 2000;16:573–582. doi: 10.1093/bioinformatics/16.7.573. [DOI] [PubMed] [Google Scholar]
- 63.Martin D.P., Murrell B., Golden M., Khoosal A., Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1:vev003. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosakovsky Pond S.L., Posada D., Gravenor M.B., Woelk C.H., Frost S.D. Automated phylogenetic detection of recombination using a genetic algorithm. Mol. Biol. Evol. 2006;23:1891–1901. doi: 10.1093/molbev/msl051. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rice P., Longden I., Bleasby A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2001;16:276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 67.Muhire B.M., Varsani A., Martin D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE. 2014;9:e108277. doi: 10.1371/journal.pone.0108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013;30:1720–1728. doi: 10.1093/molbev/mst064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lemey P., Rambaut A., Welch J.J., Suchard M.A. Phylogeography takes a relaxed random walk-in continuous space and time. Mol. Biol. Evol. 2010;27:1877–1885. doi: 10.1093/molbev/msq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suchard M.A., Lemey P., Baele G., Ayres D.L., Drummond A.J., Rambaut A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4:vey016. doi: 10.1093/ve/vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baele G., Li W.L., Drummond A.J., Suchard M.A., Lemey P. Accurate model selection of relaxed molecular clocks in Bayesian phylogenetics. Mol. Biol. Evol. 2013;30:239–243. doi: 10.1093/molbev/mss243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bielejec F., Baele G., Vrancken B., Suchard M.A., Rambaut A., Lemey P. SpreaD3: Interactive visualization of spatiotemporal history and trait evolutionary processes. Mol. Biol. Evol. 2016;33:2167–2169. doi: 10.1093/molbev/msw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng H.Y., Chen J., Adams M.J., Chen J.P. Complete nucleotide sequence and affinities of the genomic RNA of Narcissus common latent virus (genus Carlavirus) Arch. Virol. 2006;151:1667–1672. doi: 10.1007/s00705-006-0727-x. [DOI] [PubMed] [Google Scholar]
- 75.Chen J., Chen J., Adams M.J. Molecular characterisation of a complex mixture of viruses in garlic with mosaic symptoms in China. Arch. Virol. 2001;146:1841–1853. doi: 10.1007/s007050170037. [DOI] [PubMed] [Google Scholar]
- 76.Ohshima K., Tomitaka Y., Wood J.T., Minematsu Y., Kajiyama H., Tomimura K., Gibbs A.J. Patterns of recombination in turnip mosaic virus genomic sequences indicate hotspots of recombination. J. Gen. Virol. 2007;88:298–315. doi: 10.1099/vir.0.82335-0. [DOI] [PubMed] [Google Scholar]
- 77.Ohshima K., Kawakubo S., Muraoka S., Gao F., Ishimaru K., Kayashima T., Fukuda S. Genomic epidemiology and evolution of Scallion mosaic potyvirus from asymptomatic wild Japanese garlic. Front. Microbiol. 2021;12:789596. doi: 10.3389/fmicb.2021.789596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montarry J., Doumayrou J., Simon V., Moury B. Genetic background matters: A plant-virus gene-for-gene interaction is strongly influenced by genetic contexts. Mol. Plant Pathol. 2011;12:911–920. doi: 10.1111/j.1364-3703.2011.00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nigam D., LaTourrette K., Souza P.F.N., Garcia-Ruiz H. Genome-wide variation in potyviruses. Front. Plant Sci. 2019;12:1439–1442. doi: 10.3389/fpls.2019.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raj R., Kaur C., Agrawal L., Chauhan P.S., Kumar S., Raj S.K. Full-length genome sequence of Cyrtanthus elatus virus—An isolated from Narcissus tazetta in India. 3 Biotech. 2018;8:168–173. doi: 10.1007/s13205-018-1189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Darlington H.R. Yellow-stripe of daffodils. J. R. Hortic. Soc. 1908;34:161. [Google Scholar]
- 82.Inouye N., Hakkaart F.A. Preliminary description of a potyvirus from Vallota speciosa. Neth. J. Plant Pathol. 1980;68:265–275. doi: 10.1007/BF01977302. [DOI] [Google Scholar]
- 83.Kumar S., Raj R., Kaur C., Raj S.K. First report of Cyrtanthus elatus virus A in Narcissus tazetta in India. Plant Dis. 2015;99:1658. doi: 10.1094/PDIS-04-15-0492-PDN. [DOI] [Google Scholar]
- 84.Brunt A.A. Narcissus viruses. Rep. Glasshouse Crops Res. Inst. 1970:134. [Google Scholar]
- 85.Brunt A.A., Atkey P.T. Rapid detection of Narcissus yellow stripe virus and two other filamentous viruses in crude negatively stained narcissus sap. Rep. Glasshouse Crops Res. Inst. 1967:155–159. [Google Scholar]
- 86.Ohshima K., Muraoka S., Yasaka R., Adachi S., Tokuda M. First report of Scallion mosaic virus on wild Japanese garlic (Allium macrostemon) in Japan. J. Gen. Plant Pathol. 2016;82:61–64. doi: 10.1007/s10327-015-0636-5. [DOI] [Google Scholar]
- 87.Santos-Gally R., Vargas P., Arroyo J. Insights into neogene Mediterranean biogeography based on phylogenetic relationships of mountain and lowland lineages of Narcissus (Amaryllidaceae) J. Biogeogr. 2012;39:782–798. doi: 10.1111/j.1365-2699.2011.02526.x. [DOI] [Google Scholar]
- 88.Matsumoto A., Hori D. Echizen Narcissus Mystery. Echizen-Board Education; Fukui, Japan: 2011. [(accessed on 1 March 2022)]. Available online: https://www.town.echizen.fukui.jp/otabunreki/panel/15.html. [Google Scholar]
- 89.Park C.H., Song E.G., Ryu K.H. Detection of co-infection of Notocactus leninghausii f. cristatus with six virus species in South Korea. Plant Pathol. J. 2018;34:65–70. doi: 10.5423/PPJ.NT.08.2017.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao F., Shen J., Liao F., Cai W., Lin S., Yang H., Chen S. The first complete genome sequence of narcissus latent virus from Narcissus. Arch. Virol. 2018;163:1383–1386. doi: 10.1007/s00705-018-3741-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data obtained in this research is available upon request.