Abstract
HMR3647 is a semisynthetic representative of a new group of drugs, the ketolides, derived from erythromycin A. Since macrolides have been shown to accumulate in human polymorphonuclear cells (PMNs), we have investigated the ability of the molecule HMR3647 to enter human PMNs as well as other cell types, such as peripheral blood mononuclear cells and cell lines of hematopoietic and nonhematopoietic origin. In these experiments, HMR3647 was compared to erythromycin A, azithromycin, clarithromycin, and roxithromycin. Our results show that HMR3647 is specifically trapped in PMNs, where it is concentrated up to 300 times. In addition, it is poorly released by these cells, 80% of the compound remaining cell associated after 2 h in fresh medium. By contrast, it is poorly internalized and quickly released by the other cell types studied. This differs from the results obtained with the macrolide molecules, which behaved similarly in the different cells studied. In addition, subcellular fractionation of PMNs allowed us to identify the intracellular compartment where HMR3647 was trapped. In PMNs, more than 75% of the molecule was recovered in the azurophil granule fraction. Similarly, in NB4 cells differentiated into PMN-like cells, almost 60% of the molecules accumulated in the azurophil granule fraction. In addition, when HMR3647 was added to disrupted PMNs, 63% accumulated in the azurophil granules. Therefore, this study shows that the ketolide HMR3647 specifically accumulates in PMN azurophil granules, thus favoring its delivery to bacteria phagocytosed in these cells.
HMR3647 is a member of a new group of drugs called ketolides, in which the l-cladinose moiety of erythromycin A is replaced by a 3-keto group (1). HMR3647 displays a broad spectrum of activity against common respiratory and intracellular pathogens and gram-positive coccus isolates resistant to erythromycin A (2, 18). Macrolides have been shown to enter and concentrate within human polymorphonuclear cells (PMNs) (for a review, see references 13 and 22). Depending on the molecule, the degree of accumulation varies; azithromycin is the molecule that is most concentrated by neutrophils (3, 19, 26).
PMNs constitute the first line of host defense, since they migrate from the circulation to fight infectious microorganisms in tissues. In addition to their capacity to engulf infectious particles and produce toxic oxygen derivatives, they exert a bactericidal action through the release of their granule content into phagosomes and into the extracellular medium (4). PMNs contain different granule populations; the azurophil granules, which are specialized lysosomes, and the specific and gelatinase granules. Differential mobilization of these granules has been reported. For instance, particulate stimuli trigger the fusion of the three granule populations while soluble stimuli, which bind to plasma membrane receptors, do not induce the mobilization of azurophil granules but trigger the exocytosis of specific and gelatinase granules (5). Retention of antibiotics within the PMNs would favor cellular delivery of these drugs to the site of infection in vivo. In addition, accumulation in a granule population which is preferentially routed to phagosomes would facilitate their delivery upon contact with bacteria and therefore their efficiency.
It is largely agreed that macrolides accumulate in the granules of PMNs. However, the precise subcellular localization of these antibiotics has rarely been reported. Studies with cell sonication in the presence of detergent and sucrose have shown that various amounts of these drugs can be found in neutrophil granules or in macrophage lysosomes (35 to 45% for erythromycylamin, erythromycin, or roxithromycin and 80% for dirithromycin [7, 16]). In addition, HMR3004, another member of the ketolide family, has been shown to be massively incorporated by PMNs and concentrated in the granular fraction (23).
In the present work we have investigated whether the new ketolide HMR3647 was able to enter PMNs and other cell types. The uptake of HMR3647 was compared to that of erythromycin A, azithromycin, clarithromycin, and roxithromycin, whose behavior in PMNs has been extensively studied. In addition, the precise localization of HMR3647 was investigated by subcellular fractionation of PMNs by differential centrifugation procedures and Percoll gradient separation.
MATERIALS AND METHODS
Antibiotics.
Azithromycin, clarithromycin, and erythromycin were purchased from their respective manufacturers. Roxithromycin and HMR3647 (11,12-dideoxy-3-de(2,6-dideoxy-3-C-methyl-3-O-methyl-alpha-l-ribohexopyranosyl) oxy)-6-O-methyl-3-oxo-12,11-(oxycarbonyl((4-(4-(3-pyridinyl)-1H-imidazol-1-yl) butyl)imino))-erythromycin) were from Hoechst Marion Roussel, Romainville, France. They were dissolved at 200 mg/ml in H2O with 0.1% CH3COOH (vol/vol), immediately diluted in phosphate-buffered saline to 20 mg/ml, and then aliquoted and stored at −80°C. The radiolabelled drugs [3H]HMR3647 (35.97 Ci/mmol), [3H]azithromycin (23.48 Ci/mmol), [3H]clarithromycin (22.20 Ci/mmol), [3H]erythromycin (20.01 Ci/mmol), and [3H]roxithromycin (21.89 Ci/mmol) were prepared by Hoechst Marion Roussel. Tritiated antibiotics were mixed with the unlabelled drugs to be used at 1 μCi/ml and at the desired concentration (0.1 to 10 μg/ml).
Cells.
THP1, Jurkat, K562, NB4, and Colo205 cell lines were cultivated in RMPI 1640 supplemented medium (glutamine, pyruvate, and HEPES) containing 10% fetal calf serum. Human PMNs and peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Paque centrifugation from the venous blood of healthy volunteers. The PMNs were further purified by 2% dextran sedimentation and osmotic lysis of residual erythrocytes.
Determination of cellular volumes was performed with a Coulter Counter ZM equipped with a Channelyzer. The mean cell volume was 0.361 × 10−6 μl for PMNs. For PBMC suspensions, the mean cell volume was calculated according to the percentage of lymphocytes (cell volume, 0.195 × 10−6 μl) and monocytes (cell volume, 0.39 × 10−6 μl) in the cell suspension. The cell volumes measured for cell lines were 1.2 × 10−6 μl for THP1, 0.765 × 10−6 μl for Jurkat, 1.72 × 10−6 μl for K562, 1.04 × 10−6 μl for NB4, and 1.44 × 10−6 μl for Colo205 cells.
In some experiments, NB4 cells were differentiated into neutrophils (25). The cells were maintained in culture in the presence of 1 μM all-trans retinoic acid for 5 days. The differentiation of nonadherent cells was assessed in each experiment by testing their ability to generate O2− in response to phorbol myristate acetate before their use (17).
Antibiotic uptake.
Cells were washed and resuspended in RPMI 1640 medium supplemented with glutamine, pyruvate, and HEPES, without serum. The cells (2 × 106; final concentration, 5 × 106/ml) were incubated in triplicate at 37°C for various periods (10 to 180 min) with the radiolabelled drugs at concentrations ranging from 0.1 to 10 μg/ml. The cells were then centrifuged at 12,000 × g for 3 min at 4°C, and the cell pellet was solubilized in ice-cold hypotonic buffer (20 mM Tris base, 10% Triton X-100, 1 mM EDTA, and 10 μg of trypsin inhibitor/ml). The supernatant and cell-associated radioactivity were quantified by liquid scintillation counting. Radioactive counts were recorded as disintegrations per minute in a calibrated counter. Cell-associated antibiotic concentrations were expressed as (i) the intracellular amount of antibiotic (nanograms of drug per 106 cells), (ii) the intracellular concentration of antibiotic (micromoles of drug per cellular volume of 106 cells), or (iii) the ratio of the intracellular concentration to the extracellular concentration (C/E) of antibiotic. The viability of the cells during the experiment was assessed by measuring lactate dehydrogenase release in the supernatant after incubation.
Antibiotic release.
Cells were incubated for 2 h with 10 μg of the radiolabelled drugs/ml as described above. They were then centrifuged, washed in ice-cold medium, and resuspended at 5 × 106/ml in medium without antibiotic. After various periods of incubation (0 to 120 min) at 37°C, they were centrifuged and treated as described above for liquid scintillation counting. Data analysis was performed as described for the uptake experiments.
Subcellular fractionation of PMNs and NB4 cells by differential centrifugation.
Fractionation of PMNs was performed as previously described (15). Briefly, the PMNs were resuspended at 108/ml in relaxation buffer (100 mM KCl, 3 mM NaCl, 10 mM PIPES (piperazine-N,N′-bis(2-ethanesulfonic acid), 3.5 mM MgCl2, pH 7.2) and cavitated in a nitrogen bomb for 4 min at 2,588 kPa (375 lb/in2). With this method, 10% of the cells remained intact. Debris, nuclei, and remaining cells were sedimented for 10 min at 250 g. The granules were sedimented from the postnuclear supernatant at 15,000 × g for 10 min at 4°C. The cytosol was separated from the postgranular membranes by centrifugation at 100,000 × g for 45 min at 4°C. The radioactivity associated with the different fractions was quantified by liquid scintillation counting.
Fractionation of NB4 cells was performed as previously described (25). Briefly, the cells were cavitated in a nitrogen bomb and the nuclei, cell debris, and intact cells were centrifuged at 300 × g for 10 min. A fraction enriched in azurophil granules was obtained by centrifugation of the postnuclear supernatant at 1,000 × g for 10 min followed by separation of the granule-free membranes and the cytosol at 100,000 × g for 45 min.
Marker proteins (β2 microglobulin for the plasma membrane, myeloperoxidase for azurophil granules, and lactoferrin for specific granules) were measured by enzyme-linked immunosorbent assay (15) in subcellular fractions to check the quality of the fractionation. Fractions obtained from both cell types were separated with an efficiency comparable to that previously described (15, 25).
Subcellular fractionation of PMNs on a discontinuous Percoll gradient.
PMNs were disrupted, and nuclei and debris were removed as described above. The postnuclear supernatant was centrifuged on a discontinuous isotonic Percoll gradient at 48,000 × g for 20 min as previously described (15). Four fractions, corresponding to the cytosol, the nongranular membranes, the specific granules, and the azurophil granules, were recovered, and the Percoll was removed by centrifugation at 250,000 × g for 90 min. All steps were performed at 4°C. The radioactivity associated with the different fractions was quantified by liquid scintillation counting. The extent of cross-contamination between the granule fractions was evaluated by measuring myeloperoxidase and lactoferrin (15).
RESULTS
Incorporation of HMR3647 into PMNs and release from the cells.
Human PMNs were incubated with radiolabelled HMR3647 to measure the drug uptake into the cells. The radioactivity associated with the cell pellets and supernatants and the mean cell volume were used to calculate the C/E ratio of the drug. The uptake of azithromycin, clarithromycin, erythromycin, and roxithromycin was assessed in parallel with tritiated compounds.
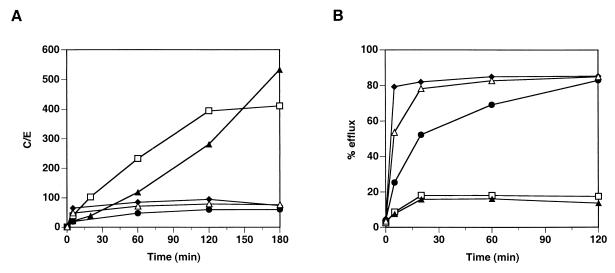
Figure 1A shows the results obtained for the drugs in a representative experiment. Two groups of drugs could be clearly distinguished based on their ability to be incorporated into PMNs. In the first group, HMR3647 and azithromycin were massively concentrated in PMNs. Their uptake was slow and continuous over 2 h, reaching a C/E ratio of around 300 by 2 h. Upon further incubation of the cells (up to 3 h), incorporation of HMR3647 reached a plateau whereas azithromycin uptake continued to increase. Clarithromycin, roxithromycin, and erythromycin were in a second group of drugs exhibiting different behavior. For these three macrolides, the uptake into PMNs was moderate (maximum C/E, around 50) and reached a plateau within 10 min (60 min for erythromycin). Further incubation of the cells for up to 3 h did not enhance the uptake of the drugs.
FIG. 1.
Incorporation and release of HMR3647 and macrolide comparators into PMNs. Human PMNs were incubated with radiolabelled HMR3647 (open squares), azithromycin (solid triangles), clarithromycin (solid diamonds), erythromycin (solid circles), and roxithromycin (open triangles) at 10-μg/ml final concentration. (A) Drug uptake was monitored at different time points and is expressed as a C/E ratio. (B) Drug release from the cells was monitored after 2 h of incubation with the radiolabelled compounds and is expressed as the percentage of drug found in the supernatant (% efflux). The results correspond to the mean of three independent experimental points obtained with cells from the same blood donor. Standard errors of the mean were always below 10%.
The efflux of the drugs from the cells was also monitored in the same experiment. After incubation of the cells with the radiolabelled compounds for 2 h, the cells were quickly washed and resuspended in medium devoid of antibiotics. The release of radiolabelled drugs in the medium was monitored at different time points. As shown in Fig. 1B, HMR3647 and azithromycin remained trapped in the cells. Indeed, only 20% of the drugs were recovered in the supernatants after 30 min. Further incubation of the cells for up to 2 h did not increase the drug efflux, and 80% of the drugs remained cell associated. On the contrary, erythromycin, clarithromycin, and roxithromycin showed a massive and rapid release from the cells. Around 80% of roxithromycin and clarithromycin were exported into the medium after 20 min. The efflux of erythromycin was somewhat slower, but 70% of the drug was recovered in the supernatant after 1 h.
Incorporation of HMR3647 into PBMC and efflux.
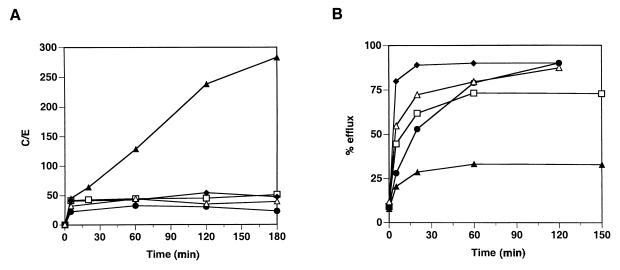
Similar experiments with drug uptake and efflux were performed with PBMC. After incubation of PBMC with the radiolabelled drugs, incorporation into the cells was monitored for all five antibiotic compounds. The results obtained for PBMC were similar to those obtained with PMNs for all drugs except HMR3647 (Fig. 2). Incorporation of erythromycin, clarithromycin, and roxithromycin into PBMC was moderate (maximum C/E ratio, around 50), as in PMNs (Fig. 2A). In addition, azithromycin was strongly concentrated in PBMC, although the C/E ratio obtained after 3 h was a little lower in PBMC than in PMNs (300 versus 500). On the contrary, HMR3647 uptake was low and reached a plateau at a C/E of around 50 by 10 min with no further increase.
FIG. 2.
Incorporation and release of HMR3647 and macrolide comparators into PBMC. Human PBMC were incubated with radiolabelled HMR3647 (open squares), azithromycin (solid triangles), clarithromycin (solid diamonds), erythromycin (solid circles), and roxithromycin (open triangles) at 10-μg/ml final concentration. (A) Drug uptake was monitored as described in the legend to Fig. 1. The results correspond to the mean of three independent experimental points obtained with cells from the same blood donor. Standard errors of the mean were always below 10%.
The same trend was observed when drug release was monitored. As shown in Fig. 2B, erythromycin, clarithromycin, and roxithromycin were rapidly released from PBMC whereas azithromycin remained strongly cell associated. These results were similar to those obtained with PMNs. By contrast, HMR3647 incorporated into PBMC was quickly released into the medium: as shown in Fig. 2B, more than 70% of the drug was released into the supernatant after 1 h with only 25% of the drug remaining cell associated. These results were in contrast to those obtained with PMNs, where the compound HMR3647 remained cell associated (Fig. 1B).
Variability among donors.
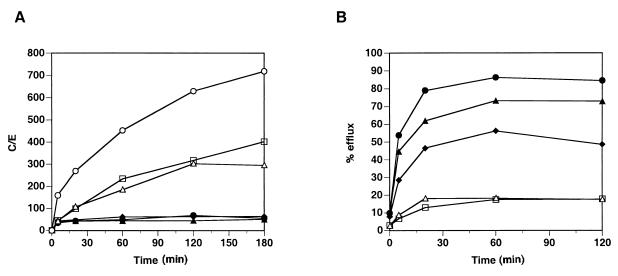
In order to determine the variability of the results obtained with HMR3647, the experiments were repeated with different blood donors. Figure 3 shows the results of independent experiments performed with PMNs or PBMC obtained from different individuals.
FIG. 3.
Incorporation and release of HMR3647 in PMNs and PBMC from different individuals. Human PMNs (open symbols) and PBMC (solid symbols) were obtained from different blood donors. HMR3647 uptake (A) and release (B) were monitored as described in the legend to Fig. 1. Each datum point corresponds to the mean of three independent experimental points obtained with cells from the same blood donor. Standard errors of the mean were always below 10%.
Figure 3A shows the different results obtained for uptake of the drug. Although some variability was observed in the maximum uptake observed with PMNs, the incorporation of HMR3647 had a trend similar to that shown in Fig. 1A. In addition, the uptakes observed in PBMC were similar in all three individuals tested. Figure 3B shows the efflux observed for the drug. As described in Fig. 1B and 2B, the efflux of the drug was quick and massive in all PBMC tested whereas the efflux was slow and minimal in the different PMNs studied.
Incorporation of HMR3647 into human cell lines.
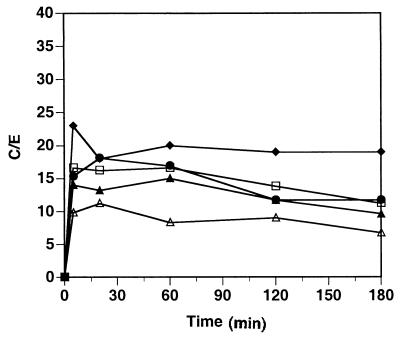
To establish whether HMR3647 preferentially accumulates in PMNs, we analyzed the uptake of HMR3647 in several cell lines of human origin. The cell lines used were a T-lymphocytic cell line (Jurkat), a monocytic cell line (THP1), an erythroid lineage progenitor cell type (K562), a promyelocytic cell line (NB4), and a colon carcinoma cell line (Colo205).
As shown in Fig. 4, HMR3647 uptake was similar in all of the cell lines studied. A rapid accumulation was observed within 5 to 10 min, reaching a plateau at a C/E ratio around 15 to 25. Further incubation of the cells with the radiolabelled compound did not show any increase in drug uptake. On the contrary, the plateau slowly decreased with time (up to 3 h). Incorporation of HMR3647 into the cell lines was even less than that observed in PBMC with this compound. The C/E ratio was similar in all cell types examined, independent of their tissue origin. In particular, cell lines of nonhematopoietic origin, such as Colo205, did not differ from hematopoietic cell lines. In addition, NB4 cells, which are arrested at the promyelocytic stage of the myeloid differentiation towards polymorphonuclear cells (14), did not accumulate HMR3647 either. Taken together, these results confirm that HMR3647 preferentially accumulates in polymorphonuclear cells.
FIG. 4.
Incorporation of HMR3647 into human cell lines. Jurkat (solid squares), K562 (solid triangles), THP-1 (open circles), NB4 (solid diamonds), and Colo205 (open squares) cells were incubated with radiolabelled HMR3647 (10-μg/ml final concentration). Drug uptake by the cells was monitored at different time points and is expressed as the C/E ratio. The results correspond to the means of three independent experimental points obtained with cells from the same blood donor. Standard errors of the mean were always below 10%.
Effect of different extracellular concentrations of HMR3647 on PMN drug incorporation.
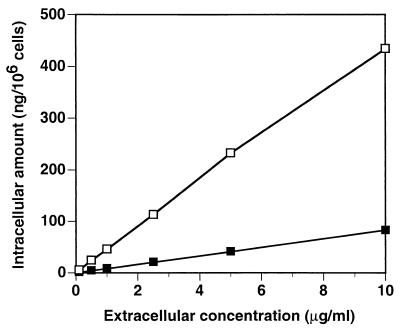
Since plasmatic concentrations of HMR3647 measured in vivo were shown to be lower than 10 μg/ml (24), it was of interest to examine the accumulation of this compound in PMNs at different extracellular concentrations. Human PMNs were therefore incubated with HMR3647 at concentrations ranging from 0.1 to 10 μg/ml. Two different time points were analyzed: 5 min and 2 h. These time points corresponded to the initial fast uptake and to the plateau observed in initial experiments, respectively (Fig. 1A). The results are expressed as intracellular drug quantities (nanograms/106 cells), calculated from the counts and the isotopic dilution of the compound.
As shown in Fig. 5, intracellular quantities of HMR3647 increased linearly with the extracellular concentration of the compound. After 5 min of incubation, the intracellular amount of HMR3647 was strictly proportional to the extracellular concentration of the drug. Therefore, the C/E ratio was constant and equal to 50. After 2 h of incubation, the amount of intracellular drugs was also found to be proportional to the extracellular concentration, with a small decrease at the highest extracellular concentration (10 μg/ml). Thus, the C/E ratio decreased somewhat with increasing HMR3647 extracellular concentrations, ranging from 613 at 0.1 μg/ml to 457 at 10 μg/ml. The results obtained for 10 μg/ml at 5 min and 2 h are in accordance with the results shown in Fig. 1A.
FIG. 5.
Incorporation of HMR3647 in PMNs: effect of extracellular drug concentration. Human PMNs were incubated for 5 min (solid squares) or 120 min (open squares) with radiolabelled HMR3647 at different concentrations (0.1, 1, 2.5, 5, and 10 μg/ml). Drug incorporation into the cells is expressed as the amount of drug (in nanograms) present in 106 cells. The results correspond to the means of three independent experimental points obtained with cells from the same blood donor. Standard errors of the mean were always below 10%.
From these experiments, we conclude that the intracellular-to-extracellular ratio of HMR3647 in PMNs after 5 min of incubation is not related to the extracellular concentration of the drug. The massive accumulation of HMR3647 observed in PMNs can therefore occur even at 10- or 100-times-lower concentrations of the drug in the extracellular medium. Finally, the results depicted in Fig. 5 show that the accumulation of the drug does not reach saturation over the concentrations tested. Indeed, intracellular quantities of the compound reach as much as 0.4 pg per cell at these concentrations.
HMR3647 accumulates in azurophil granules of human PMNs.
In light of the massive uptake of HMR3647 by PMNs, its subcellular localization was examined. After 120 min of incubation with the radiolabelled drug (10-μg/ml final concentration), the neutrophils were disrupted. The radioactivity incorporated into the nuclear fraction, which also contains cell debris and unbroken cells, was counted and found to be 25.9% of the total. The postnuclear supernatant was further fractionated, and the radioactivity present in the cytosol (5.4%), in the plasma membrane fraction (0.4%), and in the granular fraction (58.1%) was determined. Most of the drug was found in the granule fraction, while the cell membranes and cytosol contained only a small percentage of the incorporated radioactivity.
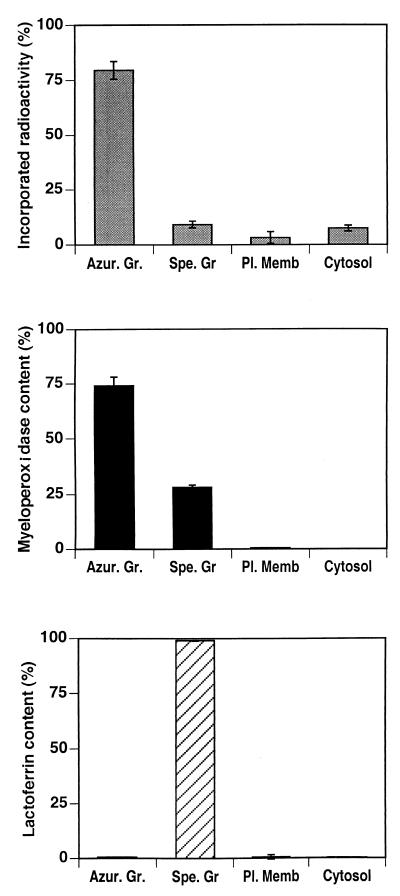
To identify the type of granule in which HMR3647 was trapped, the postnuclear supernatant from the PMNs was fractionated on a discontinuous Percoll gradient, which provides an efficient separation of azurophil granules from specific and gelatinase granules (25). Under these conditions, four fractions were obtained: the cytosol, the plasma membrane-enriched fraction, the specific and gelatinase granules, and the azurophil granules. According to the results shown above, Percoll-separated cytosolic and plasma membrane fractions contained only minor amounts of radioactive HMR3647 (Fig. 6). Furthermore, most of the drug (74 and 80% in two separate experiments) was detected in the azurophil granule fraction, while only 10% of the drug was in the specific-granule fraction. The azurophil and specific-granule markers, myeloperoxidase and lactoferrin, were measured in the fractions to check the separation of these granules. While the azurophil granule fraction was not contaminated by the specific-granule marker, lactoferrin, a contamination of specific granules by azurophil granules was revealed by the presence of myeloperoxidase in this fraction. About 25% of the myeloperoxidase cell content was present in specific granules (Fig. 6), while it does not usually exceed 10 to 15% after Percoll fractionation (15). This suggests that the presence of radiolabelled antibiotic in the specific-granule fraction could be, at least in part, due to the presence of contaminating azurophil granules.
FIG. 6.
Accumulation of HMR3647 in PMN azurophil granules. Human PMNs were incubated for 120 min with radiolabelled HMR3647 (10-μg/ml final concentration) and then disrupted by nitrogen cavitation and fractionated on a discontinuous Percoll gradient. The radioactivity incorporated into the membrane fractions (Azur. Gr, azurophil granules; Spe. Gr, specific granules; Pl. Memb, plasma membrane) and the cytosol was counted. Markers for azurophil granules (myeloperoxidase) and specific granules (lactoferrin) were assayed in each fraction by enzyme-linked immunosorbent assay. The data are expressed as mean ± standard error of the mean of four experiments.
Next, we investigated whether the specific incorporation of the drug in azurophil granules persisted when neutrophils were disrupted prior to exposure to HMR3647. Postnuclear supernatants were incubated with radiolabelled HMR3647 for 20 min at 37°C and then fractionated on Percoll gradient. Three fractions were recovered: 63.4% of the radioactivity associated with these fractions was found in the azurophil granules, 18.5% was found in specific granules, and 18.1% was found in the plasma membrane fraction. Again, we observed a contamination of specific granules by azurophil granules (30% of them sedimented at the specific-granule density). These results show that HMR3647 preferentially accumulates in the azurophil granules whether cells are intact or disrupted.
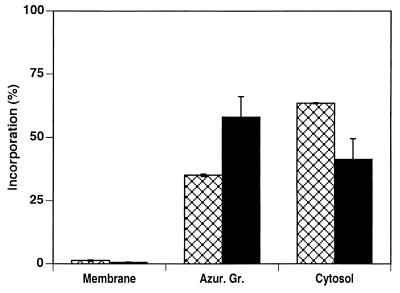
The promyelocytic cell line NB4 contains azurophil granules but not specific granules (10, 14). We have recently shown an increase in the expression of β-glucuronidase, an enzyme of the azurophil granule matrix, and Hck, an Src family tyrosine kinase associated with the granule membrane, during the process of NB4 cell differentiation into neutrophils (10, 25). This suggests that, at the promyelocytic stage, azurophil granules are not mature and acquire additional proteins during the process of differentiation into neutrophil-like cells. Therefore, we investigated whether differentiated NB4 cells would be able to accumulate HMR3647 in their azurophil granules as observed above with PMNs. Nondifferentiated and all-trans retinoic acid-differentiated NB4 cells were exposed to HMR3647 under the experimental conditions used for PMNs. First, we observed a 3.7-fold increase in the C/E ratio in differentiated NB4 cells compared to that in nondifferentiated cells (data not shown). Second, in nondifferentiated cells, the bulk of radioactivity was found in the cytosol and only 35% was in the azurophil granule fraction. In contrast, in differentiated NB4 cells, almost 60% of the antibiotic was present in the azurophil granule fraction and 40% was present in the cytosol. In both cases, only traces of HMR3647 were detected in the granule-free membrane fraction (Fig. 7). These results indicate that HMR3647 accumulates in azurophil granules only in mature granulocytes, such as differentiated NB4 cells or PMNs.
FIG. 7.
HMR3647 accumulates in azurophil granules only in NB4 cells differentiated into PMN-like cells. NB4 cells were differentiated for 5 days in the presence of all-trans retinoic acid. Differentiated (solid bars) and nondifferentiated (crosshatched bars) cells were exposed to HMR3647 as described in the legend to Fig. 6 and fractionated by differential centrifugation. The radioactivity incorporated into the different fractions was counted, and the results are expressed as percentages of the radioactivity present in the postnuclear supernatant. The data are expressed as mean ± standard error of the mean of two experiments.
DISCUSSION
The experiments presented here show that the compound HMR3647 is specifically trapped in mature PMNs. Within these cells, the compound has a defined tropism for azurophil granules, in which it accumulates even when disrupted cells are used for incubation. The compound does not enter the specific and gelatinase granules, and only a small fraction (5%) remains in the cytosol. This tropism of the compound for PMN granules probably explains its specific cell distribution. Indeed, in other cells, such as lymphocytes, monocytes, and the nonhematopoietic Colo205 cells, HMR3647 is poorly internalized and quickly released. When compared to azithromycin and to macrolides such as erythromycin, clarithromycin, and roxithromycin, HMR3647 has a distinct behavior. Indeed, molecules of the macrolide class do not exhibit cell specificity, as they are internalized with comparable efficiencies in different cell types (9, 20). In our experiments, the results obtained with these molecules were comparable to those already reported (8, 11, 21).
Although the general mechanism underlying macrolide uptake by cells is poorly understood, one of the proposed hypotheses is that macrolides are weak bases which accumulate in acidic compartments (13, 22). Lysosomes and azurophil granules are acidic compartments with similar pH values of around 5 (12), but HMR3647 preferentially accumulates in cells containing azurophil granules rather than in cells containing lysosomes. This indicates that the existence of an acidic compartment could be necessary but is not sufficient. In addition, in our experiments with undifferentiated NB4 cells, which contain azurophil granules, the uptake of HMR3647 remains at a low level and the drug stays in the cytosol with only a small fraction entering the granules. Upon differentiation of these cells into PMNs, we observe the accumulation of the drug in the cells and preferential uptake into azurophil granules. This suggests that proteins involved in the preferential accumulation of HMR3647 are expressed during the cell differentiation process. One can hypothesize that a plasma membrane carrier or a protein associated with azurophil granules is involved in this process. Further investigations with PMNs and differentiated NB4 cells will be needed to identify such proteins as well as the exact mechanism of drug entry into the granules.
The function of azurophil granules is to fuse with phagosomes and deliver proteases and bactericidal proteins upon contact with bacteria. HMR3647 can be exposed to pH values as low as 4 and still retain its bactericidal activity (6). Specific accumulation of the molecule into azurophil granules will favor its delivery upon contact with a large spectrum of intracellular bacteria, which, together with its large antibiotic spectrum, suggests that it will demonstrate a potent antibiotic activity in vivo.
ACKNOWLEDGMENTS
We thank C. Agouridas, C. Bonnat, A. Denis, P. Mauvais, and C. Fridman-Sautes for helpful discussions during this work. We acknowledge G. Touyer and J.-N. Veltz for the supply of radiolabelled antibiotics. We also thank M. C. Decoen for her assistance in drawing figures and C. Raynaud, P. Taine, and M. Rémy for their help in obtaining blood samples.
REFERENCES
- 1.Agouridas C, Benedetti Y, Denis A, Le Martret O, Chantot J-F. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Ketolides: a new distinct class of macrolide antibacterials. Synthesis and structural characteristics of RU 004, abstr. 157; p. 140. [Google Scholar]
- 2.Barry A L, Brown S D, Fuchs P C. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Antipneumococcal activity of a ketolide (HMR3647) and seven related drugs in vivo, abstr. 107; p. 164. [Google Scholar]
- 3.Bonnet M, Van der Auwera P. In vitro and in vivo intraleukocytic accumulation of azithromycin (CP-62,993) and its influence on ex vivo leukocyte chemiluminescence. Antimicrob Agents Chemother. 1992;36:1302–1309. doi: 10.1128/aac.36.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borregaard N. Current concepts about neutrophil granule physiology. Curr Opin Hematol. 1996;3:11–18. doi: 10.1097/00062752-199603010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Borregaard N, Cowland J B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 6.Bryskier A. New semi-synthetic 14-membered-ring macrolides. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides and streptogramins. New York, N.Y: Marcel Dekker; 1997. pp. 39–50. [Google Scholar]
- 7.Carlier M B, Zenebergh A, Tulkens P M. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J Antimicrob Chemother. 1987;20(Suppl. B):47–56. doi: 10.1093/jac/20.suppl_b.47. [DOI] [PubMed] [Google Scholar]
- 8.Fietta A, Merlini C, Gialdroni Grassi G. Requirements for intracellular accumulation and release of Clarithromycin and Azithromycin by human phagocytes. J Chemother. 1997;9:23–31. doi: 10.1179/joc.1997.9.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Gladue R P, Snider M E. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob Agents Chemother. 1990;34:1056–1060. doi: 10.1128/aac.34.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grégoire C, Welch H, Astarie-Dequeker C, Maridonneau-Parini I. Expression of azurophil and specific protein during differentiation of NB4 cells in neutrophils. J Cell Physiol. 1998;175:203–210. doi: 10.1002/(SICI)1097-4652(199805)175:2<203::AID-JCP10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Ishiguro M, Koga H, Kohno S, Hayashi T, Yamaguchi K, Hirota M. Penetration of macrolides into human polymorphonuclear leucocytes. J Antimicrob Chemother. 1989;24:719–729. doi: 10.1093/jac/24.5.719. [DOI] [PubMed] [Google Scholar]
- 12.Klempner M S, Styrt B. Clindamycin uptake by human neutrophils. J Infect Dis. 1981;4:472–479. doi: 10.1093/infdis/144.5.472. [DOI] [PubMed] [Google Scholar]
- 13.Labro M T. Intraphagocytic penetration of macrolide antibiotics. In: Bryskier A J, Butzler J-P, Neu H C, Tulkens P M, editors. Macrolides. Chemistry, pharmacology and clinical uses. Paris, France: Arnette Blackwell; 1993. pp. 379–388. [Google Scholar]
- 14.Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- 15.Möhn H, Le Cabec V, Fischer S, Maridonneau-Parini I. The src-family protein-tyrosine kinase p59hck is located on the secretory granules in human neutrophils and translocates towards the phagosome during cell activation. Biochem J. 1995;309:657–665. doi: 10.1042/bj3090657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mtairag E L, Abdelghaffar H, Douhet C, Labro M T. Role of extracellular calcium in in vitro uptake and intraphagocytic location of macrolides. Antimicrob Agents Chemother. 1995;39:1676–1682. doi: 10.1128/aac.39.8.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.N’Diaye E-N, Vaissiere C, Gonzalez-Christin J, Grégoire C, Le Cabec V, Maridonneau-Parini I. Expression of NADPH oxidase is induced by all-trans retinoic acid but not by phorbol myristate acetate and 1,25 dihydroxyvitamin D3 in the human promyelocytic cell line NB4. Leukemia. 1997;11:2131–2136. doi: 10.1038/sj.leu.2400855. [DOI] [PubMed] [Google Scholar]
- 18.Negri M C, Morosini M I, Loza E, Meseguer M A, Almaraz F, Baquero F. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. The novel ketolide HMR3647 is highly active on antibiotic-susceptible and -resistant streptococcus pneumoniae, abstr. 110; p. 164. [Google Scholar]
- 19.Panteix G, Guillaumond B, Harf R, Desbos A, Sapin V, Leclercq M, Perrin-Fayolle M. In vitro concentration of Azithromycin in human phagocytic cells. J Antimicrob Chemother. 1993;31(Suppl. E):1–4. doi: 10.1093/jac/31.suppl_e.1. [DOI] [PubMed] [Google Scholar]
- 20.Pascual A, Rodriguez-Bano J, Ballesta S, Garcia I, Perea E J. Azithromycin uptake by tissue cultured epithelial cells. J Antimicrob Chemother. 1997;39:293–295. doi: 10.1093/jac/39.2.293. [DOI] [PubMed] [Google Scholar]
- 21.Raghoebar M, Lindeyer E, Van Den Berg W B, Van Ginneken C A M. On the mechanisms of association of the macrolide antibiotic Erythromycin with isolated human polymorphonuclear leucocytes. Biochem Pharmacol. 1988;37:3221–3227. doi: 10.1016/0006-2952(88)90631-4. [DOI] [PubMed] [Google Scholar]
- 22.Silverstein S C, Kabbash C. Activity of antibiotics within phagocytes. Curr Opin Hematol. 1994;1:85–91. [PubMed] [Google Scholar]
- 23.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the new ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vesga O, Bonnat C, Craig A. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vivo pharmacolodynamic activity of HMR3647, a new ketolide, abstr. 255; p. 189. [Google Scholar]
- 25.Welch H, Maridonneau-Parini I. Hck is activated by opsonized zymosan and A23187 in distinct subcellular fractions of human granulocytes. J Biol Chem. 1997;272:102–109. doi: 10.1074/jbc.272.1.102. [DOI] [PubMed] [Google Scholar]
- 26.Wildfeuer A, Laufen H, Zimmermann T. Distribution of orally administered Azithromycin in various blood compartments. Int J Clin Pharmacol Ther. 1994;32:356–360. [PubMed] [Google Scholar]