Abstract
Introduction
Glioblastoma multiforme (GBM) makes 60–70% of gliomas and 15% of primary brain tumors. Despite the availability of standard multimodal therapy, 2 years, 3 years, and 5 years survival rate of GBM are still low. Active immunotherapy is a relatively new treatment option for GBM that seems promising.
Methods
An electronic database search on PubMed, Cochrane, Scopus, and clinicaltrials.gov was performed to include all relevant studies. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). Reported parameters are OS, PFS, AEs, post treatment KPS, and 2 year mortality.
Results
Active immunotherapy provided better OS (HR = .85; 95% CI = .71–1.01; P = .06) and PFS (HS = .83; 95% CI= .66 – 1.03; P = .11) side albeit not statistically significant. Active immunotherapy reduces the risk of 2 year mortality as much as 2.5% compared to control group (NNT and RRR was 56.7078 and 0,0258, respectively).
Conclusion
Active immunotherapy might be beneficial in terms of survival rate in patients with GBM although not statistically significant. It could be a treatment option for GBM in the future.
Keywords: active immunotherapy, vaccine, high-grade glioma, glioblastoma, glioblastoma multiforme
Introduction
Background
Glioblastoma Multiforme (GBM) is the most common form of primary brain malignancy. 1 GBM makes 60–70% of gliomas and 15% of primary brain tumor. 2 Optimal treatment with maximal tumor resection followed by adjuvant radiotherapy (RT) and temozolomide (TMZ), does not guarantee a good survival rate nor avoid recurrence of GBM,2,3 as proven by the low 2 years, 3 years, and 5 years survival rate in population-based study (18%, 11% and 4% respectively). 4
Researches on new therapeutic modalities for GBM have been done, among which is on targeted immunotherapy.5,6 The immunosuppressive trait of its microenvironment, and its ability to interfere microglial immune activity, laid basis for immunotherapy researches for GBM. 7 In general, immunotherapy is classified into active and passive immunotherapy. 8
The idea of immunotherapy in GBM is to trigger immune system to be more reactive to GBM and therefore able to kill the tumor cells. 9 However, the impact of immunotherapeutic advances on improvement of patients’ survival is still unknown. Therefore, a well-designed systematic review and data synthesis would be invaluable to conclude the overall efficacy of active immunotherapies for the treatment of GBM.
Objective
This study was performed in order to draw conclusion about efficacy of active immunotherapy for the treatment of the patients with GBM.
Materials and Methods
Criteria for Considering Studies for This Review
Type of Participants
Eligible participants are adults aged ≥18 year-old with confirmed histopathological diagnosis of newly diagnosed or recurrent GBM (WHO Grade IV) who have received either active immunotherapy or conventional therapy.
Type of Intervention
The intervention arms of eligible studies were active immunotherapy. Active immunotherapy included (1) dendritic cell vaccination, (2) peptide vaccination, (3) DNA vaccine, (4) viral vector-based vaccine, (5) antigen non-specific vaccine, and (6) autologous tumor cell therapy. Standard therapy stands for combination of surgical resection, radiotherapy or chemotherapy.
Type of Comparators
Standard therapy (surgery, chemotherapy, and radiotherapy) with or without placebo.
Type of Outcome Measures
Studies assessing both the primary and secondary outcomes were included in this study.
Primary Outcome
The primary outcomes of this review are:
(1)Overall survival (OS)
(2)Progression-free survival (PFS)
Secondary Outcome
The secondary outcomes of this review are:
(1)Karnofsky performance scale (KPS)
(2)Adverse events (AEs)
(3)Two year mortality
Type of Studies
Randomized controlled trials (RCTs) phase II or later on active immunotherapy
Exclusion Criteria:
(1)Patients younger than 18 or diagnosed with other type of tumor
(2)Study in which non-active immunotherapy were used
(3)Study in which treatment other than standard therapy is used as comparison
(4)Studies in which neither OS nor PFS are measured
(5)Case series, non-RCT, review studies, irrelevant articles
(6)Studies published beyond the last 10 years or not written in English
Search Methods for the Identification of Studies
This systematic review and meta-analysis was conducted according to the PRISMA. 10 Literature search was done on PubMed, Cochrane, Scopus, and clinicaltrials.gov. The keywords used on each electronic database in this review were (“active immunotherapy” OR “targeted therapy” OR “dendritic cell vaccine” OR “peptide vaccine” OR “DNA vaccine” OR “Viral vector-based vaccine” OR “antigen non-specific vaccines” OR “autologous tumour cell therapy”) AND (glioblastoma OR GBM OR “high-grade glioma” OR HGG) AND (RCT OR “Randomized controlled trial”). The search result from all databases was imported to Mendeley. Duplicate results were detected using “check for duplicate” in Mendeley and were excluded. We have registered this systematic review and meta-analysis in PROSPERO with ID number 276332
Data Collection and Analysis
Selection of studies
All articles’ titles and abstracts were scanned independently by all authors. The results of independent searches were matched in order to find the common result; 2 physician reviewed unmatched findings once more, in order to check if they met the inclusion eligibility criteria.
Data Appraisal and Extraction
IBIH, MRA, BS, RM, AR, and RIS independently reviewed the full texts in order to confirm their eligibility according to the predefined participants, intervention, comparison, outcome, and study type (PICOS). In case of disagreement between the authors, the issue was discussed until full agreement was reached. The required data were extracted and put into a table accordingly.
Assessment of Risk of Bias in Included Studies
Risk of bias was assessed by IBIH, MRA, BS, RM, AR, and RIS. Should conclusion not be met, JW and CRSP would be asked to give their opinion. The biases assessed in this study were those mentioned in The Cochrane Collaboration Tool for Assessing Risk of Bias in Randomized Trials published in 2011. 11 Accordingly, all studies were classified as “low risk”, “high risk,” or “unclear risk” of bias.
Measures of Treatment Effect
OS and PFS were collected as median and range, and presented as dichotomous outcome. KPS are presented in mean differences (MDs) while 2 Year Mortality are presented as Number-Needed to Treat (NNT). AEs are presented as pooled incidence. Only serious AEs are included in the calculation.
Dealing With Missing Data
Data were extracted as they were reported in the studies. Missing data were noted and reported as bias or explained in the result and/or discussion.
Assessment of Heterogeneity
Heterogeneity is addressed according to the I2 value reported on the forest plot made using RevMan 5.4.
Data Synthesis
Data of OS and PFS were collected and presented in the form of forest plot.
Result
Description of Included Studies
Search results
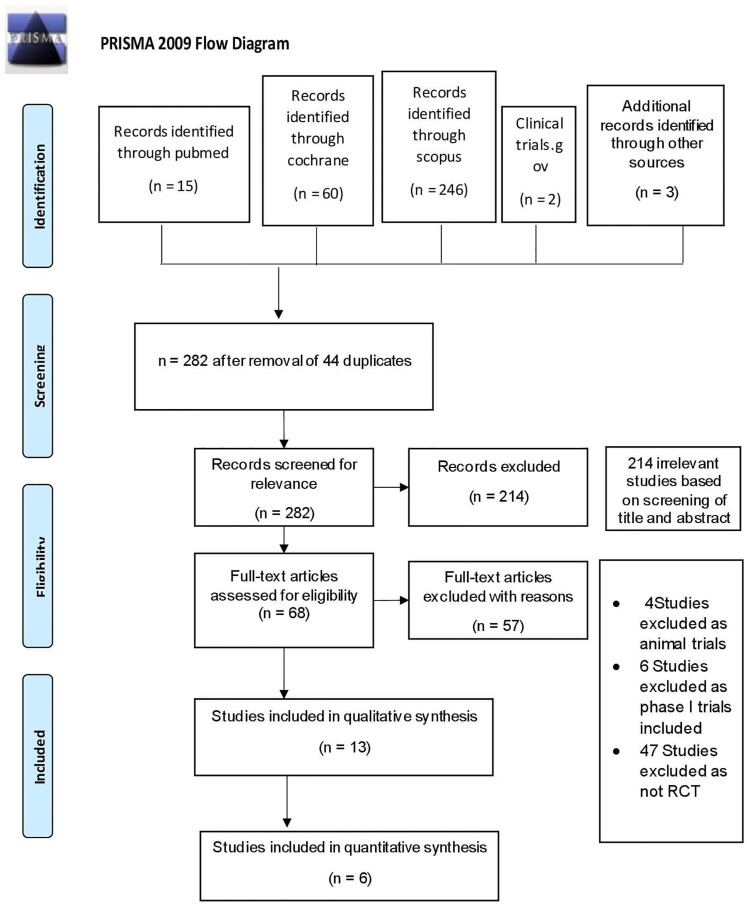
A systematic search was done on July–August 2020. Initially, a total of 326 publications were identified. However, 44 studies were excluded due to articles duplication. From a total number of 282 studies, further 214 studies were excluded after abstract screening. Most of those excluded were either not a phase-II/above RCTs or did not report the outcome that we look upon. At final screening, 57 of 68 studies were excluded due to one or more of the following: (1) no available full text, (2) not written in English, and (3) meeting our exclusion criteria. Thirteen studies were included for qualitative synthesis, and six studies were included for quantitative synthesis. The PRISMA flow diagram was presented in Figure 1.
Figure 1.
PRISMA flowchart.
Included Studies
Six studies were included in this review. In detail, 2 evaluated DCV (Yao et al, 2018 and Buchroitner et al, 2018), 1 WLDCV (Cho et al, 2012), 1 CIK (Kong et al, 2017), 1 autologous dendritic cell (ICT-107) (Wen et al, 2019), and 1 Rindopepimut (Weller et al, 2017). All results of included studied were summarized in Table 1.
Table 1.
Included studies.
| Author, year | Type of Study | Patients (n) | Patients Characteristic | Length of Follow-Up | Type of Intervention (n) | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|
| OS* | PFS* | Ae (n) | KPS Post Treatment | ||||||
| Kong, 2017 11 | RCT, Phase III | 180 | 53.3 ± 10.8 y.o for intervention 52.8 ± 10.5 y.o for control ND GBM KPS≥60 | 10.26 (2.48–41.7) | Standard + CIK (91) | 22.47 (17.2–23.85) | 8.1 (5.8–8.5) | 43 | n/a |
| Standard (89) | 16.88 (13.91–21.94) | 5.4 (3.3–7.9) | 32 | n/a | |||||
| Cho, 2012 12 | RCT, Phase II | 34 | 53.8 ± 12.0 y.o for intervention 55.8 ± 11.1 y.o for control ND GBM KPS >70 | 26 (17–56) | Standard + WLDCV (18) | 31.9 (20–56) | 8.5 (3–56) | 0 | 70 (50–100) |
| Standard (16) | 15 (5–27) | 8 (2–18) | n/a | 50 (0–50) | |||||
| Yao, 2018 13 | RCT, Phase II | 43 | 48 ± n/a y.o for intervention 50 ± n/a y.o for control ND or rGBM KPS ≥60 | 14 (9–23) | Standard + DCV (22) | 13.7 (n/a) | 7.7 (n/a) | 0 | n/a |
| Standard (21) | 10.7 (n/a) | 6.9 (n/a) | n/a | n/a | |||||
| Wen, 2019 14 | RCT, Phase II | 124 | 57.4 ± n/a y.o for intervention 57.4 ± n/a y.o for control ND GBM KPS≥70 | 11.4 (1.4–28.4) | Standard + ICT-107 (81) | 17 (13.68–20.61) | 11.2 (8.22 – 13.05) | 47 | n/a |
| Standard (43) | 15 (12.33–23.05) | 9 (5.52 – 10.29) | 35 | n/a | |||||
| Buchroitner, 2018 15 | RCT phase II | 76 | Median 54.6 (n/a) for intervention Median 54 (n/a) for control ND GBM KPS n/a | 18.73 (1.4–53.3) | DCV + standard (34) | 18.8 (14.53–22.37) | 6.8 (4.6 – 9.3) | 18 | n/a |
| Standard (42) | 18.93 (11.63–22.67) | 7 (5.97 – 9.53) | 12 | n/a | |||||
| Weller, 2017 16 | RCT, Phase III | 745 | Median age 59 (51–64) for intervention Median age 58 (52-–64) for control ND GBM KPS n/a | 17.12 (.5–48) | Standard therapy → rindopepimut + TMZ (371) | 17.4 (16.1–19.4) | 7.1 (5.4 – 7.9) | 337 | n/a |
| Standard therapy → Placebo + Standard (374) | 17.4 (16.2–18.1) | 5.6 (5.1–7.1) | 343 | n/a | |||||
| AE: Adverse eventsCIK: Cytokine-induced killerDCV: Dendritic cell vaccine*Presented in median (range) month | KPS: Karnofsky performance score ND GBM: Newly diagnosed GBM rGBM: Recurrent GBM | Wl: Whole-cell lysate OS and PFS presented in median (CI) Length of follow-up presented in median (range) | |||||||
Risk of Bias in Included Studies
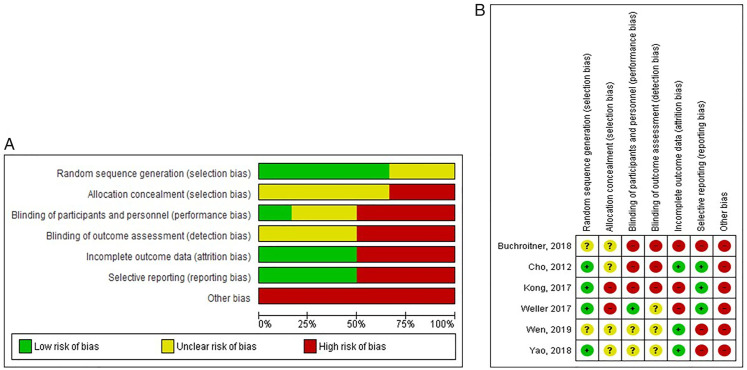
In general, the studies mostly (more than 50%) had low risk of selection bias as 4 of 6 studies reported the randomization process. However, in terms of detection bias, attrition bias, reporting bias, and other bias, 50% or more of the studies were considered high risk (Figures 2(A) and (B)).
Figure 2. Risk of bias (A) across all included studies, (B) eachinc luded study.
Summary of Findings
Summary of findings of included studies and quality of evidence of this study are listed on Table 1 and Table 2, respectively. Difference of baseline characteristics between studies can be seen on Table 3.
Table 2.
Quality of evidence.
| Outcomes | Hazard Ratio (95% CI) | Number of Participants (studies) | Quality of Evidence (GRADE) | Comments |
|---|---|---|---|---|
| OS | HR .85 (.71 to 1.01) | 1202 (6) | ⊕⊕⊝⊝ Low | Presence of Bias, Imprecision Indicated by the Range of Confidence Interval (CI) |
| PFS | HR .83 (.66 to 1.03) | 1202 (6) | ⊕⊕⊝⊝ low | Presence of bias, imprecision indicated by the range of confidence interval (CI) |
Table 3.
Baseline characteristics of each study.
| Author | Mean Age | Mutation (%) | GTR (%) | Mean Baseline KPS |
|---|---|---|---|---|
| Kong, 2017 11 | 53.3 | Not checked | 48.4% | 84.4 |
| 52.8 | Not checked | 53.9% | 85.7 | |
| Cho, 2012 12 | 52.11 | MGMT 55.5% | 77.7% | n/a |
| 55.81 | MGMT 56.25% | 68.75% | n/a | |
| Yao, 2018 13 | 50 | IDH1MTTERTWT 4.76% IDH1WTTERTWT 47.6% IDH1WTTERTMT 47.6% MGMT 57.14% |
n/a | 79 |
| 48 | IDH1MTTERTWT 13.63% IDH1WTTERTWT 45.45% IDH1WTTERTMT 40.9% MGMT 40.9% |
n/a | 83 | |
| Wen, 2019 14 | 57.4 | MGMT 34.6% HLA-A1 40.7% HLA-A2 51.9% HLA-A1 and A2 7.4% |
71.6% | 24.7% KPS <90 |
| 57.5 | MGMT 41.9% HLA-A1 32.6% HLA-A2 51.2% HLA-A1 and A2 16.3% |
74.4% | 39.5% KPS <90 | |
| Buchroitner, 2018 15 | 54.6 | MGMT 20.58% | 70.58% | n/a |
| 54 | MGMT 14.28% | 83.3% | n/a | |
| Weller, 2017 16 | 59$ | MGMT 33% | n/a | 84.4 |
| 58$ | MGMT 35% | n/a | 85.7 |
Row in green: interventionRow in yellow: control$Median
Effects of Intervention
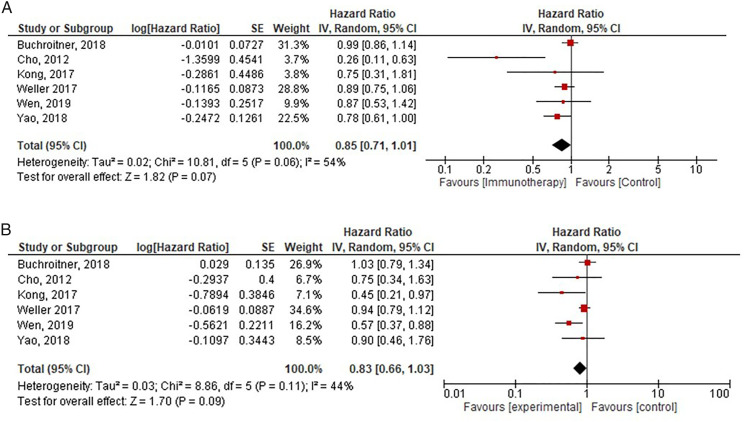
The effect of the intervention on OS and PFS can be seen on Figures 3(A) and (B). The median follow-up of the studies was 15.56 (10.26–26) months. Changes in KPS were unfortunately not reported in most studies, thus MDs was not possible to be analyzed.
Figure 3.
(A) The effect of intervention towards OS, (B) The effect of intervention towards PFS.
Overall Survival
Kong et al compared autologous CIK cells and standard therapy against standard therapy alone. The median OS in the treatment group was 22.47 (95% CI, 17.2–23.85) against 16.88 (95% CI, 13.91–21.94) in the control group. The median OS in Cho et al was 31.9 (95% CI, 20–56) for intervention group against 15 (95% CI, 5–27) in control group. Median OS in Yao’s study was 13.7 in the treatment group against 10.7 in the control group. Median OS in intervention group and control group of Buchroitner’s study was 18.8 (95% CI, 14.53–22.37) and 18.93 (95% CI, 11.63–22.67), respectively. Wen et al reported median OS for intervention group was 17 (95% CI, 13.68–20.61), and in controlled group was 15 (95% CI, 12.33–23.05). The median OS in Weller et al was 17.4 (95% CI, 16.1–19.4) for intervention group and 17.4 (95% CI, 16.2–18.1).
The cumulative hazard ratio (HR) for OS in this study was .85 (95% CI, .71–1.01). This indicated that active immunotherapy was favorable despite not statistically significant. The studies were heterogenous in terms of OS (I2 = 54%, P = .06). Only one study had CI which did not cross or touch value of 1 (Figure 3(A)).
Progression Free Survival
The median PFS in intervention and control group of Kong’s study was 8.1 (95% CI, 5.8–8.5) and 5.4 (95% CI, 3.3–7.9), respectively. The median PFS in Cho et al was 8.5 (95% CI, 3–56) for intervention group and 8 (95% CI, 2–18) in control group. Yao et al compared DC vaccine for patients with new or recurrent GBM who had the tumor resected ≥95% prior to study. Median PFS for intervention group in this study was 7.7, while for control group was 6.9. Wen et al reported median PFS for intervention group was 11.2 (95% CI, 8.22–13.05), and in controlled group was 9 (95% CI, 5.52–10.29). In Buchroitner’s study, the median PFS in intervention group and control group was 6.8 (95% CI, 4.6–9.3) and 7 (95% CI, 5.97–9.53), respectively. The median OS in Weller et al was 7.1 (95% CI, 5.4–7.9) for intervention group and 5.6 (95% CI, 5.1–7.1).
The cumulative hazard ratio (HR) for PFS was .83 (95% CI, .66–1.03). This indicated that active immunotherapy was favorable despite not statistically significant. The studies were heterogenous in terms of OS (I2=44%, P = .11). Only 2 studies had CI which did not cross value of 1, yet they were barely significant (Figure 3(B)).
Two Year Mortality
We extracted the number of events of 2 year mortality from each study based on the given rate or inferred from the provided Kaplan–Meier graphs. One study 14 did not reach 2 year follow-up and thus was not included in the calculation. In total, there were 411 events of 2 year mortality out of 617 total subjects in the intervention arm. On the other hand, there were 400 similar events out of 585 total subjects in the control arm. We calculated the number-needed to treat (NNT), control event rate (CER), experimental event rate (EER), absolute risk reduction (ARR), and relative risk reduction (RRR) based on cumulative number of subjects from all included studies (Table 4).
Table 4.
Calculation of EER, CER, ARR, RRR, and NNT based on 2-year mortality.
| Intervention Group Size | 617 |
|---|---|
| Control group size | 585 |
| Events in experimental group | 411 |
| Events in control group | 400 |
| EER (experimental event rate) | .666 126 |
| CER (control event rate) | .683 761 |
| ARR (absolute risk reduction) | .0176 343 |
| RRR (relative risk reduction) | .0257 901 |
| NNT (number-needed to treat) | 56.7078 |
The cumulative value of NNT was 56.7078, which means that 56–57 patients need to be treated with active immunotherapy to prevent 1 mortality. The RRR of .0257 901 signifies that patients gain a small benefit of reduction of 2 year mortality risk by 2.5%. Absolute risk reduction of .0176 343 signifies that there really is only 1.76% risk difference between control group and treatment group.
Adverse Events
Kong et al reported 43 grade III–IV AEs in the intervention group and 32 in control group. However, there were only 3 AEs which were thought to be related to the CIK. No AEs were reported in Cho’s and Yao’s studies. Wen et al reported 47 and 35 serious AEs in intervention and control group, respectively. Serious AEs in Buchroitner’s study occured in 18 and 12 subjects of intervention and control group, respectively. Weller et al reported 337 serious AEs in intervention group and 343 in control group
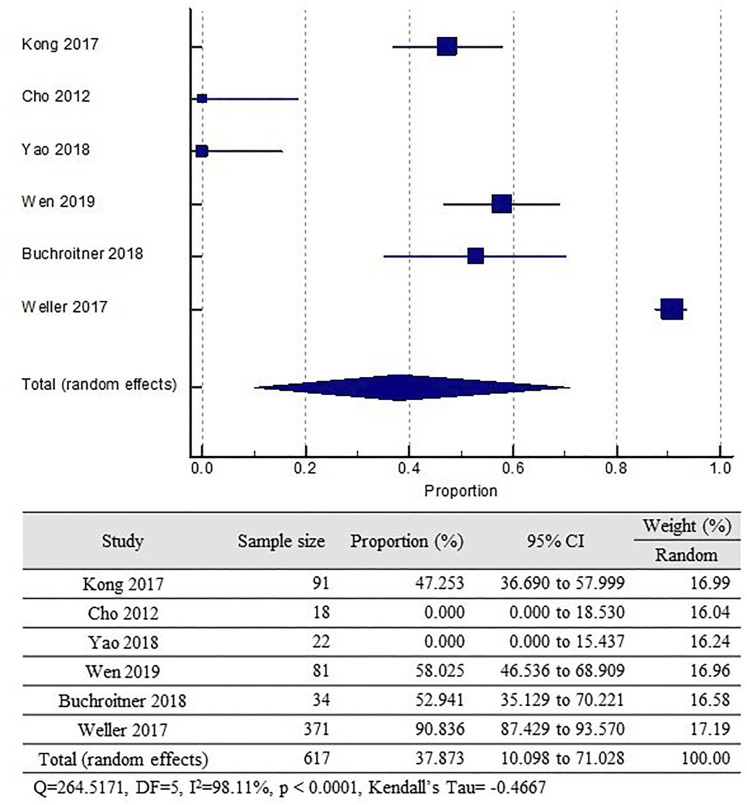
The pooled incidence of AEs among the treatment group was 37.87% (95% CI, 10.098–71.028). This finding, however, is severely heterogenous (I2 = 98%, P < .0001). It is also important to note that some studies did not clearly report if more than 2 AEs were present in a single subject. Two of the included studies did not report any AEs. (Figure 4)
Figure 4.
Pooled incidence of grade III/IV AEs among treatment group.
Discussion
Up to this point, standard therapy for GBM includes surgery, radiotherapy, and chemotherapy. 18 However, it is well known that GBM would still recur despite maximum effort with standard therapy.19-22 Studies investigating the standard therapy of GBM reported that the survival rate of GBM was less than 2 years.23-25 Patients with GBM was found to have a median survival time of 15–17 months. 26 Only ≤5% of patients were said to be alive at 5 year follow-up. 25
Previous study shows that immunotherapy in general could be beneficial for glioblastoma treatment. 8 Here, we specifically review the efficacy of active immunotherapy for glioblastoma from recent RCTs phase II or above that met the criteria. The principle of immunotherapy for cancer is amplifying the body’s immune system to react towards the tumor through humoral and cytotoxic mechanism. 6 Active immunotherapy works by training the immune system to attack the tumor based on specific antigenicity 6 and also generates tumor-specific T-cells and immunological memory. 27
Of all 6 studies reviewed, 2 evaluated DCV, 1 WLDCV, 1 CIK, 1 autologous dendritic cell (ICT-107), and 1 Rindopepimut. Active immunotherapy has been reported to be promising in other published studies.28-30 Similarly, in our meta-analysis we found that the OS and PFS were in favor of active immunotherapy (.85 (95% CI, .71–1.01) and .83 (95% CI, .66–1.03), respectively). Our finding on OS and PFS of active immunotherapy indicated that this treatment modality is favorable despite not statistically significant.
This present study analyzed the mortality rate in the first 2 years since randomization in each study. Follow-up in Yao et al 31 did not reach 2 years so that it was excluded from 2 year mortality calculation. We found an NNT of 56.7078, which means that 56–57 patients need to be treated with active immunotherapy to prevent 1 mortality. The RRR of .0257 901 signifies that the patients gain a small benefit of reduction of 2 year mortality risk by 2.5%. Absolute risk reduction of .0176 343 signifies that there really is only 1.76% risk difference between the 2 groups. This calculation, however, was possibly biased as some studies did not provide censored events in their respective Kaplan–Meier graphs. In such cases, mortality rate was calculated based on either survival or mortality percentage provided by the authors from the related study.
Serious AEs from the included studies were 411 and 400 in immunotherapy and in control group, respectively. Two studies13,14 did not provide data on AEs. It is important to note that this present study only gathered data on AEs of grade III or above. None of the studies made clear if the AEs were exclusively related to the immunotherapy agents. Study which best elaborated their AEs finding was that of Weller et al. 17 Most common grade III–IV AEs for all 369 treated patients in the rindopepimut group vs 372 treated patients in the control group were: thrombocytopenia (32 (9%) vs 23 (6%)), fatigue (6 (2%) vs 19 (5%)), brain edema (8 (2%) vs 11 (3%)), seizure (9 (2%) vs 8 (2%)), and headache (6 (2%) vs 10 (3%)). Serious AEs included seizure (18 (5%) vs 22 (6%)) and brain edema (7 (2%) vs 12 (3%)). 32 However, since the treatment group also received standard therapy in all studies, it is impossible to draw conclusion if the AEs were truly due to immunotherapy.
Reported AEs of immunotherapy for cancer treatment includes autoimmunity. 33 This phenomenon mainly occurred in immune-checkpoint types of immunotherapy. The induction of autoimmunity is thought to occur from enhancement of subclinical autoimmune disease which had presented previously in the patient, altering commensal microbiota in the GI tract and/or immune interactions with environmental microorganisms, leading to pathology where there was previously tolerance, bystander tissue injury due to antitumor response, or aberrant response to the immunotherapy agents themselves. 33 In a retrospective study on patients with melanoma receiving DCV, Most common AEs were flu-like symptoms (67%) and injection site reactions (50%). Both of these AEs significantly correlated with the presence of tetramer-positive CD8 T-cells. 34
Significant progress has been made in understanding how cancer cells elude the immune system by expressing immunological checkpoints that limit T cell activity and proliferation during the last several decades. The cytotoxic T lymphocyte antigen 4 (CTLA-4) receptor and its ligand (PD-L1) are currently the most clinically relevant immunological checkpoints. However, immune checkpoint inhibition is unable to demonstrate efficacy in GBM patients. A systematic review by Brahm et al, showed that majority of clinical trials involving GBM patients showed minimal clinical activity. Ongoing clinical trials by CheckMate studies with nivolumab have also failed to prove its efficacy. Thus, the role of immune checkpoint inhibitors currently is not applicable in glioblastoma patients.
Excluded Studies
A study by Buchroitner et al 35 was excluded as both JW and RIS deemed the authors did not report the study’s method clearly enough. Akiyama et al published a phase I clinical trial. 36 Ardon et al published a clinical trial which was unfortunately not randomized. 37 We also identified trials which were still in pilot phase.38,39 Bota et al compared ERC1671 (Gliovac), granulocyte-macrophage stimulating factor (GM-CSF), cyclophosphamide, and bevacizumab against placebo and bevacizumab for recurrent GBM. The study was excluded due to comparing patients not currently on standard therapy. 40 Similarly, study by Narita et al was excluded as they did not investigate patients on standard therapy. 41 Liau et al studied dendritic cell (DC) vaccine for 331 newly diagnosed GBM subjects aged. Despite amassing one of the biggest number of subjects for this topic, this study was a cross-over trial, which means that in the end all patients received the DC vaccine and, therefore, was excluded. 42 A study by Inoges et al was excluded for not being an RCT.
Quality, Bias, and Limitations of the Study
Quality of evidence for OS and PFS in this study was low grade as there is imprecision indicated by the range of CI and presence of bias.
Bias
Bias in each study was summarized in Supplementary Figure 1.
Randomization and Allocation Concealment
Kong et al, Cho et al, and Yao et al randomized the patients using a computer-based randomization, but the allocation concealment was not made clear in all 3.12-14 Allocation concealment in Weller’s study was prone to bias as there was MGMT methylation-based stratification. 17 Wen et al and Buchroitner et al did not mention the randomization and allocation concealment method.15,16
Blinding
Buchroitner et al 16 did not make mention of any blinding processes. Wen et al 15 and Yao et al 14 did not explain how blinding was kept throughout the study. Cho et al 13 and Kong et al 12 was an open-label study. Weller et al kept the participants and personnel blinded by assigning someone not involved in the study to prepare the drugs and label them only with patient’s ID. 17
Incomplete Outcome Data
There were some subjects who failed to complete the intervention in three studies.12,16,17
Selective Reporting
Wen et al, 15 Yao et al, 14 and Buchroitner et al 16 did not mark any censored events on the Kaplan–Meier graph.
Other Potential Sources of Bias
Five studies received fundings.12,14-17 Buchroitner et al 16 provided survival data in days instead of months. Therefore, we converted the given data to months by dividing them by 30. A patient in Cho et al did not complete the standard radiation therapy.
Limitations
Other limitations in our study are different types of active immunotherapy, unspecified mutational characteristic, probable dissimilar baseline KPS between studies, and differences in extent of resection among subjects. Besides that, inconsistent reporting in most of the studies made conclusion regarding KPS impossible to infer. Our search of literature was limited to one language which was English. It is possible that other trials may be available in other languages or on other Websites. Not all RCTs included in this study discussed factors that related to immunity, so we could not analyze those factors that might affect the patients immunity. More comprehensive RCTs about active immunotherapy for glioblastoma are needed.
Conclusion
Active immunotherapy might be beneficial in terms of survival rate in patients with GBM although not statistically significant. Further high quality RCT with bigger number of samples are required to draw better conclusion. Severe AEs reported, yet none of the studies made clear if the AEs were exclusively related to the immunotherapy agents. As most of the studies did not report post-intervention KPS, we could not report the conclusion regarding KPS in this review. Randomized controlled trial on GBM with specific mutation characteristics will be beneficial for future researches and will direct future GBM treatments to be more patients-specific.
Acknowledgments
The Authors would like to acknowledge Dr Soetomo General Academic Hospital and Surabaya Neuroscience Institute for its support in this study.
Appendix
Abbreviation
- AA
Anaplastic astrocytoma;
- ADCTA
Autologous dendritic cell/tumour antigen vaccine;
- AE
Adverse events;
- ARR
Absolute risk reduction;
- CER
Control event rate;
- CIK
Cytokine-induced killer;
- DC
Dendritic cell;
- DCV
dendritic cell vaccine;
- EER
Experimental event rate;
- GBM
Glioblastoma Multiforme;
- GM-CSF
Granulocyte-macrophage stimulating factor;
- HR
Hazard Ratio;
- KPS
Karnofsky Performance Scale;
- LAK
Lymphokine activated killer;
- ND GBM
Newly diagnosed GBM;
- NNT
Number-Needed to Treat;
- OS
Overall Survival;
- PD-1
Programmed Death-1;
- PFS
Progression-free survival;
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis;
- RCTs
Randomized controlled trials;
- rGBM
Recurrent GBM;
- RRR
Relative Risk Reduction;
- RT
Adjuvant Radiotherapy;
- TMZ
Temozolomide;
- TILs
Tumour-infiltrating lymphocytes;
- WL
Whole-cell Lysate.
Footnotes
Authors’ Contribution: All authors contributed to the study conception and writing manuscript. Should conclusion not be met, JW and CRSP would be asked to give their opinion. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclaimers: The information and views presented in this review reflect the views of the authors of this review, not an official position of institution or funder.
ORCID iDs
Joni Wahyuhadi https://orcid.org/0000-0003-2053-1805
Bagus Sulistyono https://orcid.org/0000-0001-7776-8788
Rizki Meizikri https://orcid.org/0000-0002-8243-9951
References
- 1.Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37:E1. [DOI] [PubMed] [Google Scholar]
- 2.Huang B. Advances in Immunotherapy for Glioblastoma Multiforme; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R, Deng X, Wu H, et al. Combined immunotherapy with dendritic cells and cytokine-induced killer cells for malignant tumors: A systematic review and meta-analysis. Int Immunopharm. 2014;22:451-464. [DOI] [PubMed] [Google Scholar]
- 4.Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM. Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: A systematic review and meta-analysis. Sci Rep. 2020;10:11622-11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vatu BI, Artene S-A, Staicu A-G, et al. Assessment of efficacy of dendritic cell therapy and viral therapy in high grade glioma clinical trials. A meta-analytic review. J Immunoassay Immunochem. 2019;40:70-80. [DOI] [PubMed] [Google Scholar]
- 6.Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ. Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev. 2013;39:891-907. [DOI] [PubMed] [Google Scholar]
- 7.Safdari H, Hochberg FH, Richardson EP. Prognostic value of round cell (lymphocyte) infiltration in malignant gliomas. Surg Neurol. 1985;23:221-226. [DOI] [PubMed] [Google Scholar]
- 8.Hanaei S, Afshari K, Hirbod-Mobarakeh A, Mohajer B, Amir Dastmalchi D, Rezaei N. Therapeutic efficacy of specific immunotherapy for glioma: A systematic review and meta-analysis. Rev Neurosci. 2018;29:443-461. [DOI] [PubMed] [Google Scholar]
- 9.Weenink B, French PJ, Sillevis Smitt PAE, Debets R, Geurts M. Immunotherapy in glioblastoma: Current shortcomings and future perspectives. Cancers. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong D-S, Nam D-H, Kang S-H, et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in korea. Oncotarget. 2017;8:7003-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho D-Y, Yang W-K, Lee H-C, et al. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: A phase II clinical trial. World Neurosurgery. 2012;77:736-744. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Luo F, Tang C, et al. Molecular subgroups and B7-H4 expression levels predict responses to dendritic cell vaccines in glioblastoma: An exploratory randomized phase II clinical trial. Cancer Immunol Immunother. 2018;67:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen PY, Reardon DA, Armstrong TS, et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;25:5799-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchroithner J, Erhart F, Pichler J, et al. Audencel immunotherapy based on dendritic cells has no effect on overall and progression-free survival in newly diagnosed glioblastoma: A phase II randomized trial. Cancers. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weller M, Butowski N, Tran DD, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373-1385. [DOI] [PubMed] [Google Scholar]
- 18.Komotar RJ, Otten ML, Moise G, Connolly ES. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma-a critical review. Clin Med Oncol. 2008;2:421-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander BM, Cloughesy TF. Adult glioblastoma. J Clin Oncol. 2019;35:2902-2909. [DOI] [PubMed] [Google Scholar]
- 20.Burri SH, Gondi V, Brown PD, Mehta MP. The evolving role of tumor treating fields in managing glioblastoma. Am J Clin Oncol. 2018;41:191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji X, Zhu H, Dai X, et al. Overexpression of GBP1 predicts poor prognosis and promotes tumor growth in human glioblastoma multiforme. Cancer Biomarkers. 2019;25:275-290. [DOI] [PubMed] [Google Scholar]
- 22.Tang X, Xu P, Wang B, et al. Identification of a specific gene module for predicting prognosis in glioblastoma patients. Front Oncol. 2019;9:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegi ME, Diserens A-C, Gorlia T, et al. MGMTGene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003. [DOI] [PubMed] [Google Scholar]
- 24.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [DOI] [PubMed] [Google Scholar]
- 25.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466. [DOI] [PubMed] [Google Scholar]
- 26.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17(suppl 4):iv1-iv62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinman RM. Decisions about dendritic cells: Past, present, and future. Annu Rev Immunol. 2012;30:1-22. [DOI] [PubMed] [Google Scholar]
- 28.Chodon T, Koya RC, Odunsi K. Active immunotherapy of cancer. Immunol Invest. 2015;44:817-836. [DOI] [PubMed] [Google Scholar]
- 29.Jin J, Joo KM, Lee SJ, et al. Synergistic therapeutic effects of cytokine-induced killer cells and temozolomide against glioblastoma. Oncol Rep. 2011;25:33-39. [PubMed] [Google Scholar]
- 30.Rapp M, Grauer OM, Kamp M, et al. A randomized controlled phase II trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard radiochemotherapy of newly diagnosed glioblastoma (GlioVax): Study protocol for a randomized controlled trial. Trials. 2018;19:293-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao M, Li S, Wu X, et al. Cellular origin of glioblastoma and its implication in precision therapy. Cell Mol Immunol. 2018;15:737-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse events following cancer immunotherapy: Obstacles and opportunities. Trends Immunol. 2019;40:511-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boudewijns S, Westdorp H, Koornstra RHT, et al. Immune-related adverse events of dendritic cell vaccination correlate with immunologic and clinical outcome in stage III and IV melanoma patients. J Immunother. 2016;39:241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchroithner J, Pichler J, Marosi C, et al. Vascular endothelia growth factor targeted therapy may improve the effect of dendritic cell-based cancer immune therapy. Int J Clin Pharm Ther. 2014;52:76-77. [DOI] [PubMed] [Google Scholar]
- 35.Akiyama Y, Oshita C, Kume A, et al. α-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: A phase I clinical trial. BMC Cancer. 2012;12:623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ardon H, Van Gool SW, Verschuere T, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: Results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother 2012;61:2033-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Cai L, Zhang K, et al. A pilot study on EGFR-targeted molecular imaging of PET/CT With 11C-PD153035 in human gliomas. Clin Nucl Med. 2014;39:e20-6. [DOI] [PubMed] [Google Scholar]
- 38.Reap EA, Suryadevara CM, Batich KA, et al. Dendritic cells enhance polyfunctionality of adoptively transferred t cells that target cytomegalovirus in glioblastoma. Cancer Res. 2018;78:256-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bota DA, Chung J, Dandekar M, et al. Phase II study of ERC1671 plus bevacizumab versus bevacizumab plus placebo in recurrent glioblastoma: Interim results and correlations with CD4+ T-lymphocyte counts. CNS Oncology. 2018;7:CNS22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narita Y, Arakawa Y, Yamasaki F, et al. A randomized, double-blind, phase III trial of personalized peptide vaccination for recurrent glioblastoma. Neuro Oncol. 2019;21:348-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liau LM, Ashkan K, Tran DD, et al. Correction to: First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inogés S, Tejada S, de Cerio AL-D, et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J Transl Med. 2017;15:1-12. doi: 10.1186/s12967-017-1202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]