Abstract
This study answers the question of whether the health care costs of managing COVID-19 in preexisting cardiovascular diseases (CVD) patients increased or decreased as a consequence of evidence-based efforts to optimize the initial COVID-19 management protocol in a CVD group of patients. A retrospective cohort study was conducted in preexisting CVD patients with COVID-19 in Hamad Medical Corporation, Qatar. From the health care perspective, only direct medical costs were considered, adjusted to their 2021 values. The impact of revising the protocol was a reduction in the overall costs in non-critically ill patients from QAR15,447 (USD 4243) to QAR4337 (USD 1191) per patient, with an economic benefit of QAR11,110 (USD 3051). In the critically ill patients, however, the cost increased from QAR202,094 (USD 55,505) to QAR292,856 (USD 80,433) per patient, with added cost of QAR90,762 (USD 24,928). Overall, regardless of critical care status, the optimization of the initial COVID-19 protocols in patients with preexisting CVD did not reduce overall health care costs, but increased it by QAR80,529 (USD 22,117) per patient.
Introduction
Multiple studies conducted in different countries have shown that the most common preexisting comorbid conditions among patients requiring intensive care unit (ICU) admission for the coronavirus disease 2019 (COVID-19) are cardiovascular diseases such as hypertension, heart failure, cardiomyopathy, and dyslipidemia, where high fatality rate is reported.1, 2, 3
Several recent studies have reported that the clinical manifestation of COVID-19 infection may exceed the respiratory system to involve other organs, including the cardiovascular system.4, 5, 6 While the exact pathogenesis of cardiovascular complications related to COVID-19 is not well-established yet, a wide range of injuries has been described in the literature, including arrhythmias, acute coronary syndrome, myocarditis, and heart failure.4 , 5 Furthermore, some of the medications currently used in the course of COVID-19 treatment have been linked to cardiovascular adverse events such as hydroxychloroquine.4, 5, 6 This situation is especially dire in patients with underlying CVD since they are a high-risk population ab initio with a propensity to more severe infections and subsequent higher mortality rates.2 , 3 The uncertainty about the exact therapeutic approach to managing COVID-19 clinical syndrome led to the hurried development of several national and international clinical guidelines that comprised antivirals, antibiotics, antiprotozoal, and immunosuppressant agents.7
Health care systems in many countries worldwide, including in Qatar, are facing unprecedented challenges to maintaining cost-effective medical care for patients with COVID-19. This is particularly problematic when the patients are older, with chronic diseases, and who are also at risk for life-threatening complications. Here, utilizing the evolving understanding of the nature of the disease, several successive evidence-based cost-cutting efforts were made to revise COVID-19 treatment protocols by the Communicable Disease Center (CDC) in Qatar, since the detection of the first cluster of patients.8 , 9 By 2020, in Qatar, there have been 12 revisions for the purpose of treatment optimization for COVID-19 clinical syndrome in patients, which related to reducing the number of medications and being more specific in relation to the underlying status, based on vitals, laboratory results, symptoms, and age. In this study, we have, for the first time, investigated whether these presumed cost-cutting revisions of the initial COVID-19 management protocol with preexisting CVD established in Qatar were indeed economically beneficial, quantifying their monetary value. The targeted revision was that before revisions became considering of the widespread of recent variants of the virus in 2021 (vide infra). To emphasize, the scope of the current study is primarily limited to the economic benefit of the protocol revisions. The study does not include analysis of the protocol revisions, and the interest in clinical benefits is secondary.
Materials and Methods
Ethics Approval
This study was approved in 2020 by the Medical Research Center of Hamad Medical Corporation (HMC), MRC-05-137.
Study Design and Setting
We retrospectively reviewed the medical records of patients admitted to Hazm Mebaireek General Hospital (HMGH), Mesaieed Hospital (MH), CDC, Ras Laffan Hospital (RLH), Rumailah Hospital (RH), and Cuban Hospital (CH), which were utilized to provide services to COVID-19 patients at HMC, the main public health care provider in Qatar.10
Study Population
We included COVID-19 patients with preexisting CVD admitted to the general and critical care units of HMGH, MH, CDC, RLH, RH, and CH with positive real-time reverse-transcriptase polymerase chain reaction and were managed with before- and after-optimization of the national management protocol for COVID-19 in 2020. We excluded COVID-19 patients without preexisting CVD, and patients managed with HMC national protocols outside the study period.
Study Groups
-
1.
Group 1 (initial study protocol): COVID-19 patients with preexisting CVD admitted to HMGH, MH, CDC, RLH, RH, and CH facilities between March-June 2020 and managed using the initial national protocol, which was released in March 2020.
-
2.
Group 2 (revised study protocol): COVID-19 patients with preexisting CVD admitted to HMGH, MH, CDC, RLH, RH, and CH facilities between September-December 2020 and managed using the September 2020 update of the national protocol.
A summary of the initial and latest COVID-19 management study protocols, including recommendations for changes, is available in Appendix 1.
Study Outcomes
Primary Outcome
The difference in total cost of management of COVID-19 per patient with preexisting CVD, according to the critical status, between the initial and revised study protocols.
Secondary Outcome
-
-
Adverse drug events (ADEs), defined as any events that occur after receiving treatments to manage COVID-19 patients with CVD, such as QT prolongation, torsade de point, gastrointestinal (GI) symptoms, increased liver enzymes, flu-like symptoms, injection site reactions, and hyperglycemia.11
-
-
All-cause death during hospitalization.
-
-
Length of hospital stay in the ICU and general ward.
-
-
Rate of discontinuation of therapy due to any reason.
Parameters Assessed
Patient parameters included age, gender, weight, risk factors such as preexisting diabetes, cancer, lung disease, chronic kidney disease, and liver disease, symptomatic status, immunosuppressive conditions, medications used to manage COVID-19, duration of treatment, ADEs, medications used to manage ADEs, rate of discontinuation of treatments, death, and duration of general ward and ICU stay.
Cost and Resource Utilization
The resources consumed and their pattern of use in the management of COVID-19 patients with preexisting CVD were drawn for each patient's medical record at HMC, which included: (1) medication cost, (2) radiology tests ordered during hospitalization, (3) laboratory tests ordered during hospitalization, (4) the length of hospital stay, by ward type (ICU or general medical ward), (5) and management of ADEs. Medication costs were collected from the pharmacy department and calculated based on the duration of therapy for each regimen from initiation to discontinuation of therapy. The costs of laboratory and radiology resources were obtained from the finance department at HMC, which provided the unit cost of each resource based on hospital charges. General ward and ICU stays were also obtained from the finance department, which included the cost of bed per patient, excluding other resources. The total length of stay was calculated by summing the length of stay in each ward. In this study, a micro-costing approach was followed for cost calculations, using the unit costs of each resource. The total costs of management were calculated by multiplying the average length of hospital stay in each ward by the total costs of management in each ward. Post-discharge costs were not included. All costs were based on the financial year 2021, utilizing the Qatari health Consumer Price Index,12 and were presented in Qatari Riyal (QAR) and United States dollar (USD).
Perspective
The economic analysis was performed from the HMC perspective, restricted to the direct medical costs only. Other types of costs, such as indirect and non-medical costs, were excluded.
Sample Size
We enrolled all COVID-19 patients with preexisting CVD admitted to HMGH, MH, CDC, RLH, RH, and CH facilities during the study period; March to December 2020. Therefore, no sample size was set for this study.
Statistical Analysis
Descriptive data were presented with numerical and percentage measures for categorical variables, while mean and standard deviation measures were used for continuous variables. Student t-test and Mann-Whitney U test were used to detect any significant difference between the 2 groups. A P-value of less than 0.05 was considered to signify the statistical significance. All statistical analyses were performed using the IBM SPSS (Statistical Package for the Social Sciences) version-24.
Sensitivity Analysis
Sensitivity analyses using one-way and probabilistic sensitivity analyses were conducted to explore the robustness of study outcomes. One-way sensitivity analysis was performed to target one uncertain input variable at a time. The input investigated was the cost of hospitalization including cost of general ward and ICU stay, using a ±10% range of uncertainty. Probabilistic sensitivity analysis was performed by targeting multiple inputs at once, where uncertainty range of ±15% was assigned to the base-case values of hospital stay and the probability of death. Random selection in all uncertainty ranges followed the triangular-type of distribution, using the Monte Carlo simulation, via @Risk-5.7 (Palisade Corporation, NY), based on 1000 iterations.
Results
Patient Demographics
A total of 535 (247 vs 288) COVID-19 patients with preexisting CVD, admitted to HMGH, MH, CDC, RLH, RH, and CH, were managed with the initial vs revised HMC COVID-19 national study protocol, respectively. The mean age of patients was 54.59 years (SD 12.43) and 55.60 years (SD 14.37) in the initial and revised protocols, respectively, and 197 (79.8%) and 186 (64.6%) of them were men, respectively. Older adults comprised the majority of patients in both groups (45.5% vs 50.4%), respectively. Patients in both groups had one or more history of diseases, of which diabetes was the most common comorbidity with 132 (53.4%) vs 149 (51.7%), respectively. At admission, 235 (95.1%) and 243 (84.4%) patients were symptomatic in the initial and revised protocol groups, respectively. Also, the majority of patients were classified as having non-ICU pneumonia with mild-moderate severity (49.8% vs 44.8%), respectively.
Overall, there were no significant differences in the baseline characteristics between the 2 study groups except in relation to receiving respiratory support, ex. cannula and mask (Table 1 ).
TABLE 1.
Demographic characteristics of the study participants
| Variable | Initial protocol (n = 247) |
Revised protocol (n = 288) |
P-value | |||||
|---|---|---|---|---|---|---|---|---|
| ICU (n = 61) | Non-ICU (n = 186) | ICU (n = 18) | Non-ICU (n = 270) | |||||
| Baseline characteristics | ||||||||
| Age (Years), Mean ± SD | 54.59 ± 12.43 | 55.60 ± 14.37 | 0.39 | |||||
| Gender (Male), n (%) | 197 (79.76) | 186 (64.58) | 0.12 | |||||
| Weight (Kg), Mean ± SD | 78.65 ± 15.21 | 80.58 ± 16.11 | 0.61 | |||||
| Ethnicity, n (%) | Qatari | 32 (12.96) | 79 (27.43) | 0.56 | ||||
| Arab (non-Qatari) | 30 (12.15) | 74 (25.69) | ||||||
| Others | 185 (74.90) | 135 (46.88) | ||||||
| Pregnancy, n (%) | 1 (0.41) | 7 (2.43) | 0.90 | |||||
| Breastfeeding, n (%) | 2 (0.81) | 0 (0) | - | |||||
| Risk factors, n (%) | ||||||||
| Older adults | 112 (45.4) | 145 (50.35) | 0.93 | |||||
| Smoking | 33 (13.36) | 28 (9.72) | 0.66 | |||||
| Diabetes | 132 (53.44) | 149 (51.74) | 0.67 | |||||
| Cancer | 4 (1.62) | 8 (2.78) | 0.71 | |||||
| Lung disease | 12 (4.86) | 105 (36.46) | 0.53 | |||||
| Chronic kidney disease | 36 (14.58) | 26 (9.03) | 0.10 | |||||
| Immunosuppressed patients | 4 (1.61) | 5 (1.74) | 0.77 | |||||
| Respiratory rate >24 BPM | 48 (19.43) | 31 (10.76) | 0.93 | |||||
| Heart rate >125 bpm | 2 (0.81) | 4 (1.39) | 0.86 | |||||
| SPO2 ≤94 on ambient air | 50 (17.18) | 48 (7.66) | 0.70 | |||||
| PaO2/FiO2 <300 mm Hg | 14 (5.67) | 8 (2.78) | 0.48 | |||||
| Liver disease | 5 (2.02) | 6 (2.08) | 0.72 | |||||
| CPK > twice upper normal | 8 (3.24) | 13 (4.51) | 0.52 | |||||
| ALC <0.8 | 21 (8.50) | 29 (10.07) | 0.40 | |||||
| Respiratory support | Nasal cannula | 77 (31.17) | 72 (25.00) | 0.04 | ||||
| Mask with reservoir bag | 11 (4.45) | 4 (1.39) | ||||||
| Simple face mask | 8 (3.24) | 4 (1.39) | ||||||
| None | 151 (61.13) | 208 (72.22) | ||||||
| Ventilation received | MV | 39 (15.79) | 8 (2.78) | 0.33 | ||||
| CPAP | 8 (3.24) | 4 (1.39) | ||||||
| BiPAP | 2 (0.81) | 0 (0) | ||||||
| HFMV | 0 (0) | 3 (1.01) | ||||||
| None | 198 (80.16) | 273 (94.79) | ||||||
| Patient status, n (%) | ||||||||
| Symptomatic | 235 (95.14) | 243 (84.38) | 0.55 | |||||
| National protocol classification | ICU Pneumonia | 68 (27.98) | 30 (11.19) | 0.10 | ||||
| Mild upper respiratory tract infection | 45 (18.51) | 94 (35.08) | ||||||
| Non-ICU pneumonia (mild-moderate) | 121 (49.79) | 120 (44.78) | ||||||
| Non-ICU pneumonia (mild-moderate) with pregnancy | 1 (00.41) | 0 (0) | ||||||
| Non-ICU pneumonia (severe) | 5 (2.06) | 23 (8.58) | ||||||
| Not reported | 3 (1.24) | 1 (0.37) | ||||||
ALC, absolute lymph count; BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; CPK, creatine phosphokinase; HFMV, high frequency mechanical ventilation; ICU, intensive care unit; MV, mechanical ventilation; SPO2, peripheral oxygen saturation, PaO2/FiO2, arterial oxygen partial pressure/fractional inspired oxygen.
Proportions of COVID-19 medications, which were used to manage the patients, are presented in Appendix 2.
Economic Outcomes
Non-ICU Patient Costs With the Initial Vs Revised Management Protocols
The overall cost associated with non-critically ill patients managed with the initial protocol was higher than the revised protocol with QAR 15,447 (USD 4243) vs QAR 4337 (USD 1,191) per patient, respectively. Non-critically ill patients managed with the initial protocol had lower medications costs, including COVID-19 medications and anticoagulants compared to the revised protocol, with QAR 161 (USD 44) vs QAR 208 (USD 57), and QAR 158 (USD 44) vs QAR 164 (USD 45), per patient, respectively. However, the cost of fluids and electrolytes was higher in the initial protocol with QAR 0.41 (USD 0.11) vs QAR 0.27 (USD 0.07) per patient, respectively. The cost of general ward stay in the initial protocol was higher compared to the revised protocol, QAR 12,424 (USD 3412) vs QAR 3731 (USD 1025), per patient, respectively. Additionally, the cost of laboratory and diagnostic tests ordered during hospitalization was higher in the initial protocol, QAR 2265 (USD 622) vs QAR 223 (USD 61) per patient, respectively. The cost of respiratory support in both protocols was the same, QAR 439 (USD 121) per patient. While none developed ADEs that required additional resources for management in the initial protocol, managing the ADEs among patients under the revised protocol can be seen in Table 3.
TABLE 3.
Adverse drug reactions and management of events between both management protocol groups
| Variable |
Initial study protocol (n = 247) |
Revised study protocol (n = 288) |
P-value | |||
|---|---|---|---|---|---|---|
| ICU (n = 61) |
Non-ICU (n = 186) |
ICU (n = 18) |
Non-ICU (n = 270) |
|||
| Adverse drug reactions, n (%) | ||||||
| QTc prolongation | 7 (11.48) | 19 (10.22) | 2 (11.11) | 17 (6.30) | 0.88 | |
| Torsade de pointes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - | |
| GI symptoms (nausea, vomiting, diarrhea, anorexia) | 0 (0) | 34 (18.28) | 1 (5.56) | 87 (32.22) | <0.001 | |
| Hemolytic anemia | 0 (0) | 13 (6.99) | 0 (0) | 20 (7.41) | 0.17 | |
| Injection site reaction | 0 (0) | 1 (0.54) | 0 (0) | 1 (0.37) | 0.92 | |
| Infusion related reaction | 0 (0) | 1 (0.54) | 0 (0) | 2 (0.74) | 0.84 | |
| Increase liver enzymes (ALT, ALP, T-bilirubin) | 0 (0) | 37 (19.89) | 0 (0) | 32 (11.85) | 0.38 | |
| Neutropenia | 0 (0) | 2 (1.08) | 0 (0) | 21 (7.78) | 0.14 | |
| Thrombocytopenia | 0 (0) | 8 (4.30) | 0 (0) | 14 (5.19) | 0.22 | |
| Leukopenia | 1 (1.64) | 5 (2.69) | 0 (0) | 2 (0.74) | 0.82 | |
| Flu-like symptoms | 0 (0) | 45 (24.19) | 0 (0) | 60 (22.22) | 0.002 | |
| Peripheral edema | 0 (0) | 2 (1.08) | 0 (0) | 5 (1.85) | 0.64 | |
| Increased Aminolaevulinic Acid (ALA) | 1 (1.64) | 1 (0.54) | 0 (0) | 14 (5.19) | 0.28 | |
| Increased aspartate aminotransferase (AST) | 0 (0) | 15 (8.07) | 0 (0) | 17 (6.30) | 0.12 | |
| Central nervous system (ataxia, chills, headache, hypertonia, insomnia, pain) | 3 (4.92) | 25 (13.44) | 0 (0) | 98 (36.30) | <0.001 | |
| Hyperuricemia | 0 (0) | 5 (2.69) | 0 (0) | 11 (4.07) | 0.34 | |
| Hyperbilirubinemia | 0 (0) | 6 (3.23) | 0 (0) | 5 (1.85) | 0.61 | |
| Hyperglycemia | 3 (4.92) | 50 (26.88) | 0 (0) | 48 (17.78) | 0.08 | |
| Hypoglycemia | 0 (0) | 1 (0.54) | 0 (0) | 3 (1.11) | 0.77 | |
| Acute Kidney injury (AKI) | 0 (0) | 16 (8.60) | 0 (0) | 14 (5.19) | 0.12 | |
| Others | Hypocalcemia | 1 (1.64) | 0 (0) | 0 (0) | 0 (0) | 0.41 |
| Ear pain | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | ||
| Hypertension | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | ||
| Sinus rhythm | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | ||
| Suicidal thoughts | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | ||
| Tachycardia | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | ||
| Management of adverse drug events, n (%) | ||||||
| Amlodipine addition | 0 (0) | 0 (0) | 0 (0) | 2 (0.74) | N/A | |
| Amoxicillin/Clavulanic acid stopped | 0 (0) | 2 (1.08) | 0 (0) | 0 (0) | N/A | |
| Azithromycin stopped | 0 (0) | 4 (2.15) | 0 (0) | 0 (0) | N/A | |
| Ciprofloxacin addition | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | N/A | |
| Diosmin/Hesperidin addition | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | N/A | |
| ECG assessment | 0 (0) | 2 (1.08) | 0 (0) | 0 (0) | N/A | |
| Electrolyte replacement | 0 (0) | 0 (0) | 0 (0) | 10 (3.70) | N/A | |
| Esomeprazole addition | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | N/A | |
| Fexofenadine addition | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | N/A | |
| Hydroxychloroquine stopped | 0 (0) | 11 (5.91) | 0 (0) | 0 (0) | N/A | |
| Levocetirizine addition | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | N/A | |
| Lopinavir/Ritonavir stopped | 0 (0) | 2 (1.08) | 0 (0) | 0 (0) | N/A | |
| AST, ALT, ALP, T-bilirubin follow up | 0 (0) | 1 (0.54) | 0 (0) | 0 (0) | N/A | |
| Loperamide | 0 (0) | 1 (0.54) | 0 (0) | 2 (0.74) | N/A | |
| Metformin | 0 (0) | 1 (0.54) | 0 (0) | 0 (0) | N/A | |
| Metoclopramide | 0 (0) | 1 (0.54) | 0 (0) | 10 (3.70) | N/A | |
| Nifedipine addition | 0 (0) | 0 (0) | 0 (0) | 5 (1.85) | N/A | |
| Ondansetron addition | 0 (0) | 0 (0) | 0 (0) | 10 (3.70) | N/A | |
| Oseltamivir stopped | 0 (0) | 1 (0.54) | 0 (0) | 0 (0) | N/A | |
| Pantoprazole addition | 0 (0) | 0 (0) | 0 (0) | 3 (1.11) | N/A | |
| Paracetamol addition | 0 (0) | 0 (0) | 0 (0) | 3 (1.11) | N/A | |
| Renal ultrasound | 0 (0) | 1 (0.54) | 0 (0) | 0 (0) | N/A | |
| Rivaroxaban | 0 (0) | 0 (0) | 0 (0) | 1 (0.37) | N/A | |
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; ECG, electrocardiogram; GI, gastrointestinal; ICU, intensive care unit.
The main contributors to the overall cost in the initial and revised protocols were general ward stay followed by the laboratory test, while fluids and electrolytes had the least effect on the outcome.
ICU Patient Costs With the Initial Vs Revised Management Protocols
The overall cost associated with critically ill patients in the initial protocol was lower than the revised protocol, QAR 202,094 (USD 55,505) vs QAR 292,856 (USD 80,433), per patient, respectively. Critically ill patients managed with the initial protocol had higher medications costs compared to the revised protocol, including COVID-19 medications, anticoagulants, and fluids and electrolytes with QAR 380 (USD 104) vs QAR 78 (USD 21), QAR 102 (USD 28) vs QAR 27 (USD 7), and QAR 0.8 (USD 0.2) vs QAR 0.6 (USD 0.16), per patient, respectively. However, the initial protocol had a lower general ward stay cost than the revised protocol, QAR 34,725 (USD 9537) vs QAR 99,604 (USD 27,356), per patient, respectively, and contributed to lower ICU stay with QAR 160,673 (USD 44,129) vs QAR 192,402 (USD 52,843), per patient, respectively. The cost of laboratory and diagnostic tests requested for the initial protocol was higher, QAR 5,774 (USD 1586) vs 744 (USD 204), per patient, respectively. Similar to the non-ICU patients, the cost of respiratory support in both protocols was the same, QAR 439 (USD 121) per patient. None developed ADEs that required additional resources for management in both protocols.
The main contributors to the overall cost in both protocols were the ICU stay followed by general ward stay, then laboratory tests, while receiving fluids and electrolytes contributed the least.
Overall Populations Costs With Initial Vs Revised Management Protocols
Regardless of ICU status, patients receiving the revised study protocol were associated with an overall increased cost by QAR 80,529 (USD 22,117) per patient, that is, (QAR 217,541 (USD 59,748) with the initial protocol vs QAR 298,070 (USD 81,865) with the revised protocol. The main driver of the cost increase was the increase in the length of stay in the ICU and general ward units, contributing to more than 90% of the total cost difference, followed by laboratory tests.
Utilized unit costs of HMC resources are available in Appendix 3. Table 2 shows the patient cost outcomes associated with the initial and revised CVD COVID-19 patient protocols in ICU and non-ICU patients.
TABLE 2.
Cost outcomes associated with the initial and revised management protocols
| Variable | Initial management protocol, QAR (USD) |
Revised management protocol, QAR (USD) |
||
|---|---|---|---|---|
| ICU | Non-ICU | ICU | Non-ICU | |
| COVID-19 medications | 380 (104) | 161 (44) | 78 (21) | 208 (57) |
| Anticoagulants | 102 (28) | 158 (44) | 27 (7) | 164 (45) |
| Fluids and electrolytes | 0.8 (0.2) | 0.41 (0.11) | 0.6 (0.16) | 0.27 (0.07) |
| Respiratory support | 439 (121) | 439 (121) | 439 (121) | 439 (121) |
| Medications to manage ADEs | 0 | 0 | 0 | 10 (3) |
| ICU stay | 160,673 (44,129 | 0 | 192,402 (52,843) | 0 |
| General ward stays | 34,725 (9537) |
12,424 (3412) | 99,604 (27,356) | 3731 (1025) |
| Laboratory and diagnostic tests | 5774 (1586) | 2265 (622) | 744 (204) | 223 (61) |
| Total cost | 202,094 (55,505) | 15,447 (4243) |
292,856 (80,433) |
4337 (1191) |
| 217,541 (59,748) | 298,070 (81,865) | |||
| Cost reduction per patient in the non-ICU group: 11,110 (3051) Cost increase per patient in the ICU group: 90,762 (24,928) | ||||
ADE, adverse drug event; ICU, intensive care unit, QAR, Qatari Riyal, USD, United States dollar.
Clinical Outcomes
Among 247 CVD patients managed with the initial COVID-19 protocol, 11.5% of critically ill patients and 10.2% of non-critically ill patients developed QT prolongation events, while in the revised protocol, 11.1% of critically ill patients and 6.3% of non-critically patients developed QT prolongation. More non-critically ill patients in both groups, compared to the critically ill, developed hemolytic anemia, infusion-related reactions, injection site reactions, neutropenia, thrombocytopenia, leukopenia, peripheral edema, increased liver enzymes, hyperuricemia, hyperbilirubinemia, hypoglycemia, and acute kidney injury. With regards to the ADEs, only GI symptoms, flu-like symptoms, central nervous system symptoms, such as ataxia, chills, headache, and hyperglycemia, were reported to be significantly different between the groups. Furthermore, only non-critically ill patients in both groups received medications to manage the ADEs. Comparison between both study groups in relation to the medications received, ADEs, and management of ADEs is detailed in Table 3 .
The length of hospital stay was comparable between both groups, with non-statistically longer stay observed among CVD patients managed with the initial protocol (mean 15.69 vs 11.63 days, P-value = 0.36). In addition, mortality was similar between both groups with 1.2% vs 1.0%, P-value = 0.85, of patients dying during hospitalization, respectively (Table 4 ).
TABLE 4.
Clinical outcomes associated with the initial and revised management protocols
| Variable |
Initial management protocol (n = 247) |
Revised management protocol (n = 288) |
P-value | ||
|---|---|---|---|---|---|
| ICU (n = 61) | Non-ICU (n = 186) | ICU (n = 18) | Non-ICU (n = 270) | ||
| Length of hospital stay (Days), Mean ± SD | 15.69 ± 18.81 | 11.63 ± 16.92 | 0.36 | ||
| Death, n (%) | 3 (1.22) | 3 (1.04) | 0.85 | ||
| Discontinuation of therapy, n (%) | 5 (2.1%) | 0 (0) | - | ||
ICU, intensive care unit.
With regards to the discontinuation of therapy, only 2.1% vs 0% discontinued their COVID-19 treatment among the CVD patients managed with the initial vs revised study treatment protocol, respectively.
Sensitivity Analysis
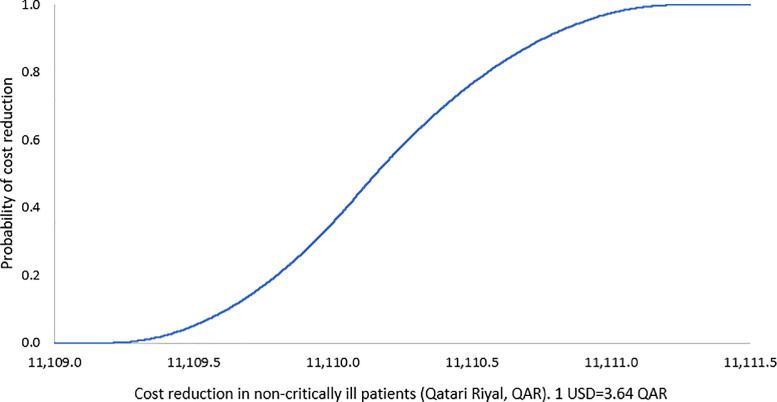
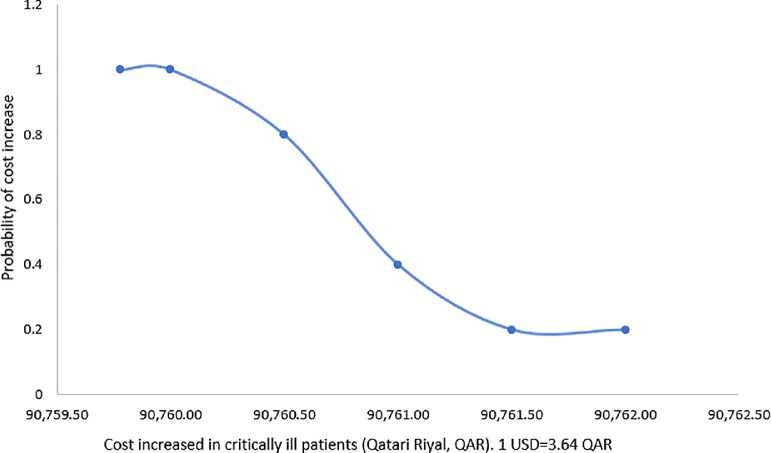
Based on the one-way sensitivity analysis, the base-case outcome was insensitive to the uncertainty in the cost of hospitalization. The probabilistic sensitivity analyses also showed that the base-case results were robust against the uncertainty in the values of clinical outcome probabilities. Results of sensitivity analyses are shown in Table 5 , and FIG. 1, FIG. 2 .
TABLE 5.
Patient cost outcomes of sensitivity analysis with their uncertainty distributions
| One-way sensitivity analysis | ||||||
| Variable |
Initial management protocol | Revised management protocol | ||||
| Variation range, QAR (USD) | Variation range, QAR (USD) | Mean cost reduction in non-critically ill patients with revised, compared to initial protocol, 95% CI | Mean cost increase in critically ill patients with revised, compared to initial protocol, 95% CI | |||
| Cost of ICU hospitalization | QAR 8830-10,792 (USD 2419-2957) | QAR 8830-10,792 (USD 2419-2,957) | QAR 11,110 (USD 3,051), 95% CI QAR 9656-QAR 12,559 (USD 2652-3449) |
QAR 90,762 (USD 24,928), 95% CI QAR 88,118-QAR 91,002 (USD 24,202-24,994) |
||
| Cost of general ward hospitalization | QAR 1494-1826 (USD 409-500) | QAR 1494-1826 (USD 409-500) | QAR 10,193 (USD 2972), 95% CI QAR 9645-QAR 12,755 (USD 2642-3495) |
QAR 91,174 (USD 24,979), 95% CI QAR 87,866-QAR 90,638 (USD 24,073-24,832) |
||
| Probabilistic sensitivity analysis | ||||||
| Variable |
Point estimate | Variation range | Point estimate | Variation range | Mean cost reduction in non-critically ill patients between initial and latest protocols, 95% CI | Mean cost increase in critically ill patients between initial and latest protocols, 95% CI |
| Length of hospital stay (Days, mean) |
15.69 | 13.34-18.04 | 11.63 | 9.89-13.37 | QAR 11,110 (USD 3051), 95% CI QAR 11,109-QAR 11,111 (USD 3050-3052) |
QAR 90,762 (USD 24,928), 95% CI QAR 90,758-QAR 90,763 (USD 24,927-24,929) |
| Death (n) | 3 | 2.55-3.55 | 3 | 2.55-3.55 | ||
CI, confidence interval; ICU, intensive care unit, QAR, Qatari Riyal, USD, United States dollar.
FIG. 1.
Probability curve of reduced cost in COVID-19 patients with preexisting cardiovascular disease in non-critically ill with revised protocol. (Color version of figure is available online.)
FIG. 2.
Probability curve of increased cost in COVID-19 patients with preexisting cardiovascular disease in critically ill with revised protocol. (Color version of figure is available online.)
Discussion
Achieving better performance in public health care systems requires strategy alignment. HMC COVID-19 management national protocols were forced to be redesigned to achieve optimum management while reducing the overall health care costs. Here, our study presents, to the best of our knowledge, the first attempt to investigate the economic consequences of an evidence-based, presumed cost-cutting, revision of an initial COVID-19 management protocol into a revised version of it in preexisting CVD patients in the literature. In the non-ICU preexisting CVD patients, the cost of COVID-19 management was reduced by QAR 11,110 (USD 3051), per patient. In the ICU preexisting CVD patients, however, the cost of managing COVID-19 increased by QAR 90,762 (USD 24,928) per patient. We report that managing COVID-19 in preexisting CVD patients with the revised protocol, regardless of their critical care status, was also associated with increased cost of QAR 80,529 (USD 22,117) with the protocol revision, per patient (Table 2). The main driver behind differences was the cost of hospitalization. While the initial therapy was insignificantly associated with longer overall hospital stay, the cost of ICU contributed to the revised protocol being more expensive.
The current study is not only the first to report the economic impact of protocol revision, but it is the first to report the cost of COVID-19 in preexisting CVD patients. Studies evaluated the cost among patients with COVID-19 regardless of the underlying medical condition. For example, Ismaila et al. showed that the average cost of managing COVID-19 patients was USD 11,925, ranging from USD 282 for patients with mild or asymptomatic status to USD 23,382 for ICU patients.13 Similar to our findings, where the increased in the length of stay in the ICU and general ward settings and laboratory tests contributed to more than 90% of the total cost increase, the main cost drivers in their study were personal protective equipment and transportation, which was due to the fact that all COVID-19 patients were transported from their homes or point of referral to the hospital and sent back upon discharge. In our study, we excluded the cost of personal protective equipment as these were mandatory to be used by all health care professionals regardless of the management protocol. Here, however, similar to our findings, the cost of medications accounted for less than 1% of patient costs. These findings were contradicted those by Xue-Zheng Li et al., where patients with preexisting CVD contributed to 30% of the total study population. In their study, the cost of medication acquisition and preexisting risk factors, including CVD, were the key driver, accounting for around 45% of the overall cost.14 This could be justified by the fact that many patients were admitted with severe symptoms and complex medical conditions, thus additional medications were used to manage the preexisting diseases, including CVD.
Rueda et al., in a multicenter study conducted in South Africa, Ethiopia, and Pakistan, revealed that the cost of managing COVID-19 patients ranged from USD 147 per day per non-critically ill patient to USD 1082 per day per critically ill patient.15 Our findings are in line with this study, where the cost of management in the intensive care setting significantly increased with both protocols but with higher estimations. This, however, was based on published sources to estimate the resource utilization, while, in our study, we used point of care resources obtained from medical records for the management of COVID-19 patients. Overall, variation in methods could contribute to differences in costs, in addition to the management protocol differences that may exist between the countries.
The literature studies and their results are particularly not comparable to our results because, in this study, we only included patients with preexisting CVD who were infected with COVID-19. CVD is the second most common disease affected by COVID-19.16 Patients with preexisting CVD are eight times more likely to die compared to other patients, and nearly 3.5 times more prone to transfer to the ICU.16 In fact, CVD does not only increase the risk of mortality, but also increases the risk of morbidity, such as sepsis and septic shock, which could lead to further complications and, consequently, more resource utilization. This may be a reason for a more extended stay in the hospital and/or an increased utilization of invasive devices such as mechanical ventilation to support the circulation.17
All patients regardless of their critical care status and types of protocol received anticoagulants (Table 2), which indicates the importance of these agents in COVID-19. Indeed, the development of coagulopathy among preexisting CVD patients with COVID-19 is associated with a worse prognosis.18 The risk of hypercoagulability in COVID-19 involves a wide range of complications that spans from localized microvascular thrombosis in the lungs, or pulmonary intravascular coagulopathy, to systemic venous and arterial thrombosis, including aortic thrombosis.19, 20, 21 Therefore, prophylactic agents with low molecular weight heparin or unfractionated heparin are recommended for all hospitalized COVID-19 patients to improve survival, unless there is a contraindication.18 Also, pulmonary complications were reported in our patients, and these should be considered in any COVID-19 patient who develops sudden deterioration of the clinical condition associated with a sharp drop in oxygen saturation or those with significantly elevated levels of D-dimer.
Remdesivir and systemic glucocorticoid have been recommended for patients with severe COVID-19 pneumonia.22 In our findings, we found that remdesivir was initiated among patients managed with the revised study protocol. Given that remdesivir has been shown to be an effective and cost-saving medicine, this may contribute to the overall cost saving of medications associated with the revised protocol.23
In the present study, the differences in number of patients between the study protocol groups, for each of ICU and non-ICU, have no impact on our findings. This is given the lack of significant differences in patient characteristics between both groups except with regards to receiving respiratory support; whereby the use of cannula and mask as respiratory support is relatively not costly, in addition to the fact that the main outcome of this study is the average economic impact per patient. In any case, unlike clinical research, economic evaluations like the current one is not concerned with hypothesis testing, but they are about making a cost estimation. Here, even if an economic evaluation is based on small sample size (underpowered), like in the current study, it still provides important information that guides decision making.24
This study has some inherent limitations that should be acknowledged. First, our findings are based on COVID-19 management protocol up to December 2020, and HMC released an updated protocol in November 2021. There are 2 justifications for this approach in the current study. The first is that the national campaign for the mandatory vaccination of the population in Qatar was launched in December 2020, which may considerably affect the incidence of infection and the underlying characteristics of the study patients if included, including in relation to severity. The second justification is that cases of recent COVID-19 variants were first reported in Qatar late in 2021 and, hence, including patients after the release of the November 2021 update of the protocol would have also considerably affected the characteristics of the study patients if to be included in analysis, including resource utilization and severity of disease. In addition, given that COVID-19 is a new disease, data and resource utilization could change and, consequently, the conclusions could change. However, the question in this study is whether local optimization revisions of the protocol did indeed produce economic benefits, and if so, at what value, which the study does certainly successfully answer. Furthermore, our study did not consider the potential COVID-19 progression over time, nor different preexisting medical conditions among the cohort, which may be associated with a prolonged hospital stay. Finally, we followed up the treatment consequences until discharge, assuming that there would not be any impact of COVID-19 on mortality or morbidity after hospital discharge, which may not be the case.
Conclusion
Based on the study perspective and assumptions, revising the initial national protocol of management of COVID-19 in preexisting CVD patients in HMC, and although presumably targeted cost minimization, increased the overall health care cost of therapy, mainly driven by the cost of hospitalization in the ICU ward. Cost cutting revision of the protocol by including lesser spending on medications, therefore, may not be effective in isolation from the consideration of the duration of ICU stay. Overall, our findings suggest that the changes in the use of medications, devices, and laboratory and diagnostic investigations, as well as the stratification of patients according to their critical care status, would likely affect resource consumption by preexisting CVD patients with COVID-19. Therefore, as new protocols emerge, there is a need to continually update the cost analysis of COVID-19 management for guiding decisions regarding any future resource consumption.
Footnotes
Conflict of Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding: This work was supported by the Qatar university [Emergency Response Grant 250]. Open Access funding was provided by the Qatar National Library, Qatar
References
- 1.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eastin C, Eastin T. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;58:711–712. doi: 10.1056/NEJMoa2002032. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 5.Ranard LS, Fried JA, Abdalla M, et al. Approach to Acute cardiovascular complications in COVID-19 infection. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farshidfar F, Koleini N, Ardehali H. Cardiovascular complications of COVID-19. JCI insight. 2021;6 doi: 10.1172/jci.insight.148980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jirjees F, Saad AK, Al Hano Z, Hatahet T, Al Obaidi H, Dallal Bashi YH. COVID-19 treatment guidelines: do they really reflect best medical practices to manage the pandemic? Infect Dis Rep. 2021;13(2):259–284. doi: 10.3390/idr13020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Communicable Disease Center COVID-19 Major Incident Command Centre Committee, March 2020. Suggested protocol for treatment of confirmed COVID-19 infection. Doha, Qatar.
- 9.Communicable Disease Center COVID-19 Scientific Committee, September 2020. Treatment protocol for confirmed COVID-19 infection. Doha, Qatar
- 10.Hamad Medical Corporation. https://www.hamad.qa/EN/Pages/default.aspx. Published 2021. Accessed October 15, 2021.
- 11.Sun J, Deng X, Chen X, et al. Incidence of adverse drug reactions in COVID-19 patients in China: an active monitoring study by hospital pharmacovigilance system. Clin Pharmacol Ther. 2020;108:791–797. doi: 10.1002/cpt.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qatar inflation rate, Trading Economics, October 2021. https://tradingeconomics.com/qatar/inflation-cpi. Accessed October 15, 2021.
- 13.Ismaila H, Asamani JA, Lokossou VK, Oduro-Mensah E, Nabyonga-Orem J, Akoriyea SK. The cost of clinical management of SARS-COV-2 (COVID-19) infection by level of disease severity in Ghana: a protocol-based cost of illness analysis. BMC Health Serv Res. 2021;21:1115. doi: 10.1186/s12913-021-07101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X-Z, Jin F, Zhang J-G, et al. Treatment of coronavirus disease 2019 in Shandong, China: a cost and affordability analysis. Infect Dis Poverty. 2020;9:78. doi: 10.1186/s40249-020-00689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Rueda S, Sweeney S, Bozzani F, Vassall A. The health sector cost of different policy responses to COVID-19 in low- and middle-income countries. medRxiv. 2020 doi: 10.1101/2020.08.23.20180299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2021;44:352–357. doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng J-H, Liu Y-X, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fogarty H, Townsend L, Ni Cheallaigh C, et al. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189:1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thachil J, Agarwal S. Understanding the COVID-19 coagulopathy spectrum. Anaesthesia. 2020;75:1432–1436. doi: 10.1111/anae.15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Arbelaez D, Ibarra-Sanchez G, Garcia-Gutierrez A, Comanges-Yeboles A, Ansuategui-Vicente M, Gonzalez-Fajardo JA. COVID-19-related aortic thrombosis: a report of four cases. Ann Vasc Surg. 2020;67:10–13. doi: 10.1016/j.avsg.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arthur Y Kim, Rajesh T Gandhi. COVID-19: management in hospitalized adults, January 24, 2022. In: UpToDate, Waltham, MA. Link: https://www.uptodate.com/contents/covid-19-management-in-hospitalized-adults. Accessed 4 February 2022.
- 23.Jo Y, Jamieson L, Edoka I, et al. Cost-effectiveness of remdesivir and dexamethasone for COVID-19 treatment in South Africa. Open forum Infect Dis. 2021;8:ofab040. doi: 10.1093/ofid/ofab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knox S. Cancer Research Economics Support Team, University of Technology Sydney; Sydney, NSW, Australia: 2012. Sample size calculation in economic evaluation.https://www.uts.edu.au/sites/default/files/2019-04/crest-factsheet-sample-size-in-economic-evaluation.pdf Accessed 28 January 2022. [Google Scholar]