Abstract
Liquid chromatography mass spectrometry (LC-MS) is a typical strategy for lipidomics analysis. Although capillary LC-MS is a common analytical technique for proteomics analysis, its application to lipidomics has been limited. In this study, we aim to improve lipid identifications achieved in a single LC-MS analysis by a three-fold approach: capillary LC and nanoelectrospray for enhanced ionization, ion trap for higher sensitivity tandem MS, and parallelization of mass analyzers for increased speed of acquisition on an Orbitrap hybrid system. By applying the methods to a complex lipid mixture of human plasma, we identified and performed relative quantification on over 1,500 lipids within a 60 min capillary LC-MS analysis.
Graphical Abstract

Lipids are important building blocks and mediators of metabolic processes in organisms.1,2 Owing to its ability for highly sensitive analysis of complex mixtures, most global lipid monitoring methods rely on mass spectrometry (MS).3–5 These experiments have diverse aims which broadly include full lipid structural characterization (e.g., sn-position, double bond localization, stereochemistry) and quantification of the complete set of lipids present within a biological sample.4–6 Many of the analytical challenges these aspirations present also exist in other MS-based ‘omics, for example, improving sampling depth of shotgun proteomics. Several technologies including capillary separations, nano-ESI, tandem MS, and parallel MS-acquisition for quantitative, high-throughput proteomics allow for near whole proteome analysis in short order.7–12 We conclude that some of these technical approaches might yield similar improvements for global lipidome characterization.
To better identify and quantify the various lipid classes and their associated isomers, chromatographic separations are frequently employed in lipidomics.3–5 Just as in proteome analysis, lipid separations also benefit from the reduced ion suppression that occurs when performing electrospray on complex mixtures.3,13,14 But unlike proteome analysis, where capillary columns (i.e., 25 to 100 μm I.D.) have been heavily utilized for decades,15,16 most LC-MS based lipidomics studies are conducted using high flow LC columns with normal- or micro-bore (i.e., 4.6 to 1 mm I.D.).3 The reason for this preference is because high-flow LC is robust, more broadly available, and more trouble-free as compared to capillary setups. Although high flow LC separations are more straightforward to implement, several benefits are offered by capillary LC: improved electrospray ionization efficiency, very low solvent and sample consumption, and increased tolerance to high salt concentration.13,14 Recently the potential benefits of capillary LC for lipidomics have been appreciated and started to gain more interest. These pioneering works have provided the first glimpses of how capillary separations can afford the aforementioned benefits.17–22 Here we build on these initial studies, that establish and benchmark capillary chromatographic conditions for lipid separation, and examine how optimizing mass spectrometer acquisition strategies can best leverage the improved separations offered by capillary LC.
For large-scale lipidome analysis, MS/MS duty cycle and mass analyzer sensitivity are two key Figures of Merit that directly affect sampling depth. Historically, lipidomic practitioners have mainly relied on more established mass spectrometers;3,25 however, despite their increased complexity and cost, tribrid Orbitrap systems have the potential to increase lipid identifications by increasing number of MS/MS per duty cycle. Tribrid systems with mass analyzers operating in parallel have been highly effective for increasing proteome depth when coupled with chromatography.24 For example, quadrupole linear ion trap (QLT)-based MS2 doubled the number of peptides identified as compared to Orbitrap-based MS2.8 Tradeoffs exist – Orbitraps provide much higher resolving power, while the sensitivity of the QLT allows for detection of lower level species and can scan up to five-fold faster (up to 71 Hz) as maximum parallelization can be achieved.24
Here we demonstrate improved depth and quality of large-scale lipidome analysis in a single LC-MS analysis by use of capillary chromatography coupled to a quadrupole Orbitrap linear ion trap hybrid mass spectrometer. We evaluate the number and quality of lipid identifications resulting from either Orbitrap MS2 or QLT MS2 scanning. We demonstrate the quadrupole linear ion trap offers increased MS/MS sensitivity for lipids and that parallelization of mass analyzers offers further increased acquisition speed. By applying these methods to a complex lipid mixture from human plasma, we identified and performed relative quantification on over 1,500 lipids within a 60-minute capillary LC-MS/MS analysis.
EXPERIMENTAL SECTION
Solvents and Reagents.
Solvents and reagents are listed in the Supporting Information with vendor and product number (Supplementary Table S1).
Sample Preparation.
Pooled human plasma (BioIVT) was thawed on ice. To 5 μL of plasma, 5 μL of stable isotope labeled internal standards (Avanti SPLASH mixture) were added. Lipids were extracted using 50 μL of methanol, 180 μL of methyl tert-butyl ether (MTBE), and 45 μL of water.25 The samples were vortexed for 10 s and centrifuged (12 000g, 5 min, 4 °C). 50 μL of the upper hydrophobic layer was aliquoted into a glass insert amber autosampler vial, dried, and reconstituted in 50 μL of n-butanol/ACN/H2O (8:23:69, v/v/v).17 NIST SRM 1950 plasma was prepared the same way above.
Capillary LC-MS.
LC separation was performed on the UltiMate 3000 RSLCnano System (Thermo Scientific) with an in-house packed C18 reversed-phase ethylene bridged hybrid (BEH) column (30 cm length × 75 μm inner diameter × 1.7 μm particle size) at 60 °C and 275 nL/min flow rate. Column packing procedure was reported previously.26 Mobile phase A consisted of 0.2% formic acid and 5 mM ammonium formate in ACN/H2O (60:40, v/v). Mobile phase B consisted of 0.2% formic acid and 5 mM ammonium formate in IPA/ACN (90:10, v/v). For an 1 h gradient, 1 μL resuspended lipids were loaded onto column at 0% of mobile phase B for 5 min. Gradient increased to 100% of mobile phase B for 30 min, followed by 25 min of washing and re-equilibration. Details of the 1 h gradient are listed in the Supporting Information (Supplementary Table S2).
Eluting lipids were ionized by a nanoelectrospray source and analyzed at positive or negative ion mode by an Orbitrap Eclipse Tribrid mass spectrometer (Thermo Scientific). Spray voltage was set to 1.75 kV. Ion transfer tube temperature was set to 275 °C. Source RF was set to 40. MS1 scan resolution varied from 60 000 to 240 000 with 250% automatic gain control (AGC) target, 200–1600 m/z range, and 50 ms maximum injection time. Precursor ions were isolated by quadrupole at 0.7 m/z width and fragmented by higher-energy collisional dissociation (HCD) at a 27 ± 5 of stepped normalized collision energy. MS2 scan was performed in either Orbitrap at varying resolution or ion trap at varying scan mode.
Data Processing.
Raw data files were processed using Compound Discoverer 3.1 (Thermo Scientific) and LipiDex.27 Chromatographic peaks were grouped and aligned using a 0.5 min retention time tolerance and 10 ppm mass tolerance. Peaks were detected using ≥ 1.5 signal-to-noise ratio, ≥ 50 000 intensity, and ≤ 0.75 min peak width. An in silico generated lipid spectral library (Lipidex_HCD_Formic) was used for MS2 spectra searching. Spectral matches were kept if they met the thresholds of > 500 dot product (DP) score and > 700 reverse DP score. MS2 spectra were annotated at molecular species level if the minimum spectral purity was at least 75%, otherwise sum compositions were reported. The lipid identifications were further filtered for adducts, dimers, in-source fragments, misidentified isotopes, and mismatched retention time.27 Relative standard deviations (RSDs) were calculated using injection replicates of the same extracts.
RESULTS AND DISCUSSION
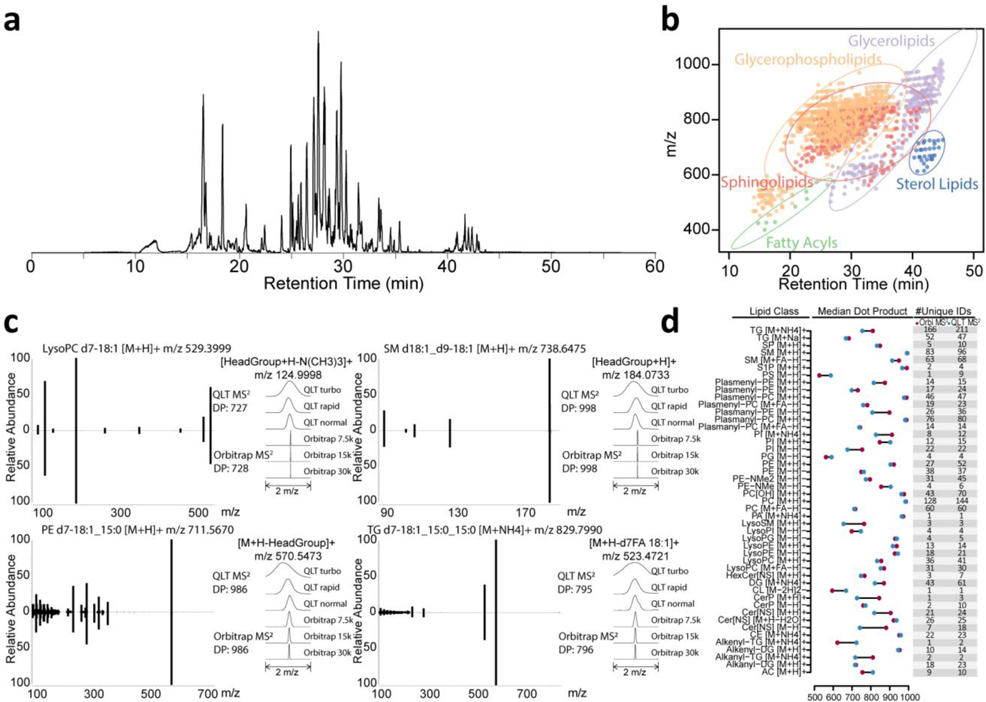
We aimed to improve the depth and quality of large-scale lipidome analysis by use of capillary separations coupled with a quadrupole-Orbitrap-dual cell linear ion trap hybrid MS system. The most widely used chromatographic method for lipidomics is reversed-phase liquid chromatography (RPLC), where lipids are separated based on their hydrophobicity. As such we constructed capillary columns using ethylene bridged hybrid (BEH) particles having a size of 1.7 μm and pore size of 130 Å. The particles were packed in a 75 μm inner diameter, 30 cm length fused silica capillary at ultrahigh pressures (30 kpsi).26,28 With 1 μL injection volume and 60 min gradient length, the full widths at half maximum (FWHM) of eluting lipids were in the range of 4–9 seconds for stable isotope labeled internal standards. These elution widths were similar to that observed from eluting lipids of complex mixtures on this setup and to those observed with high flow LC (data not shown). The retention times were reproducible as the median coefficient of variation (CV) was 0.014% for replicate injections. The base peak chromatogram and m/z-RT distribution of detected lipids are shown in Figure 1a–b.
Figure 1. Spectra obtained by QLT MS2 and Orbitrap MS2 return comparable dot product scores across lipid classes.
(a) capillary LC MS1 base peak chromatogram at positive mode for human plasma lipid extracts with stable isotope labeled internal standards. (b) m/z and retention time for lipids detected. The dots are colored by lipid categories with corresponding 95% confidence ellipse. (c) The spectra obtained from QLT MS2 and Orbitrap MS2 have high spectral similarity (representative spectra of internal standard lipids). (d) Median dot product for lipid spectral matches in different class and adduct forms.
QLT MS2 and Orbitrap MS2 of lipids generate comparable similarity scores.
Equipped with dual mass analyzers – Orbitrap and QLT – the MS system offers various ways to scan lipid product ions. Obviously Orbitrap MS/MS scanning offers much higher mass resolving power and accuracy as compared to the QLT. On the other hand, the QLT offers increased sensitivity and speed.
First, we sought to determine how MS/MS spectra produced by either analyzer from the same lipid precursor ion compared. To do this we calculated a dot product similarity score of HCD activated precursor populations (from lipid standards) and analyzed them using either the Orbitrap or QLT analyzers. As expected, tandem mass spectra collected by the two analyzers were near identical with the exception that the m/z peak widths created by the QLT were over an order of magnitude wider than those from the Orbitrap (i.e., low resolution, Figure 1c). These data demonstrate that even though the spectral resolutions are different, dot product scores were comparable. To further evaluate spectral match similarities between the MS2 acquisition strategies, we analyzed a complex mixture of plasma lipids using the capillary LC-MS/MS platform. QLT MS2 method resulted in the acquisition of 18,900 MS/MS spectra matches and 1,349 identified lipids, while Orbitrap MS2 method generates 11,058 MS/MS spectra matches and 1,052 identified lipids, with the dot product scores for lipid spectral matches calculated (Supplementary Figure 1, Supplementary Figure 2). Figure 1d summarizes these results and demonstrates the median dot products for each lipid class were similar, consistent with what was observed with the single species. Note for median dot products, we are comparing lipid spectral matches that are not filtered for adducts, in-source fragments, and retention times.
MS/MS acquisition speed key to maximize lipid identifications.
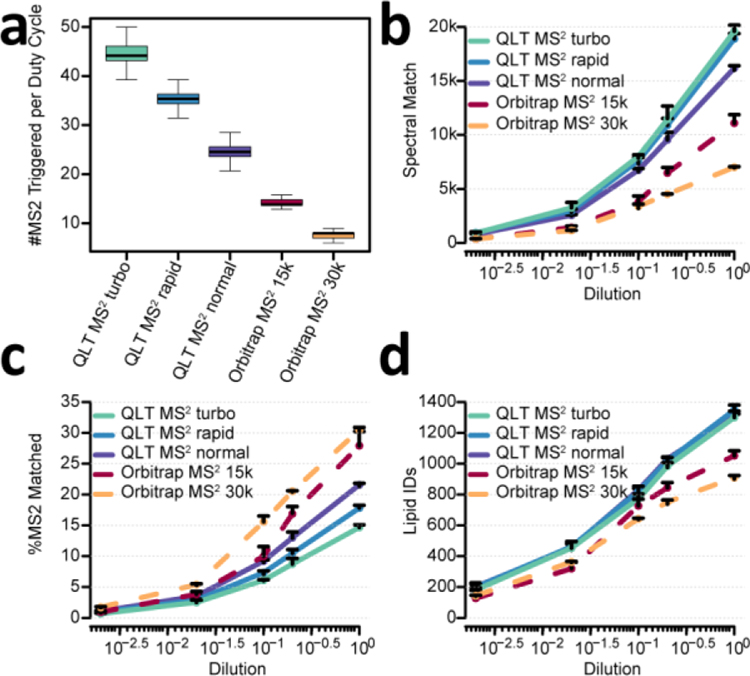
To evaluate the QLT and Orbitrap for MS2 acquisition, we tested several MS/MS methods (three QLT and two Orbitrap) against a series of complex lipid mixture dilutions (ranging from 18 nL to 36 pL injected on column). Note for all these methods the Orbitrap provided MS1 scanning. For the QLT methods, turbo (125 m/z per millisecond of scan rate), rapid (67 m/z per millisecond of scan rate), and normal (33 m/z per millisecond of scan rate) modes were tested. The parallelization of MS1 and QLT MS2 allows use of MS1 m/z resolving power of 240,000 at m/z 200 over the duration of 1.2 s cycle times. For the Orbitrap MS2 methods, 15,000 and 30,000 MS2 resolving powers were selected. As QLT scan rate was defined as m/z range scanned per unit time, the time needed for each QLT MS2 scan changes when the MS2 m/z range is not fixed, so it is not straightforward to obtain QLT MS2 acquisition speed before acquiring actual data from the complex mixture. Thus, to better demonstrate the MS2 acquisition speed across the five methods, the speed was shown as the number of MS2 scans per 1.2 s duty cycle in Figure 2a. The median number of MS2 scans per 1.2 s duty cycle across the gradient was 45, 36, 25, 14, and 8 for QLT MS2 turbo, QLT MS2 rapid, QLT MS2 normal, Orbitrap MS2 15k, and Orbitrap MS2 30k methods, respectively (Figure 2a). For the Orbitrap methods, the 240,000 resolving power MS1 scan consumes nearly half of the 1.2 second cycle (512 ms transient length), leading to reduced acquisition speed. To increase this acquisition speed, we also examined MS1 resolving powers of 120,000 and 60,000 (256 ms and 128 ms transient length respectively). Unfortunately, the increased speed was counteracted by the reduced precursor mass resolution and resulted in no improvement in terms of number of lipids identified (Supplementary Figure 3).
Figure 2. Comparison between QLT MS2 and Orbitrap MS2 across dilution series.
(a) #MS2 triggered per 1.2s duty cycle. (b) Number of lipid spectral matches across a range of injection amount. (c) Percentage of matched spectra across a range of injection amount. (d) Number of lipid identifications across a range of injection amount. Relative injection amount of one (100) equal to 1 μL out of 275 μL total extraction volume for 5 μL of human plasma, equivalent to 18 nL of human plasma. n=3, error bars represent standard deviation.
The QLT MS2 methods generated up to 5.8-fold more MS2 spectra and 2.8-fold more lipid spectral matches than Orbitrap MS2 methods (Figure 2b). Figure 2c displays that the percentage of spectra matching to a lipid was slightly higher for most lipid loads when using the Orbitrap analyzer, presumably because fewer lipids are being sampled for MS/MS scans and are of higher abundance and, thus, easier to identify species. This phenomenon is also observed in shotgun proteomics – that is, selection of lower-level precursors reduces identification rates. For all methods examined here, less than 35% of the MS2 spectra were assigned a lipid spectral match (Figure 2c). We attribute this observation to several reasons: 1) the rate of informative fragmentation events were limited by the existence of in-source fragment ions, dimers, and various adduct forms of the same lipid species;29,30 2) some lipid species were not included in the search library, e.g., lipids with modified fatty acyl chains; 3) low abundance ions were sampled for MS2 at high rate especially for QLT MS2 methods. These lower abundance species are more challenging to identify both because of low signal and they may not be biological in origin. But even with the lower identification rate, the sheer volume of increased MS/MS scans acquired demonstrated that the QLT speed outperforms the Orbitrap in the total number of identified lipids with relative quantification ( > 1,300 lipids per injection from QLT MS2 methods and ~ 1,000 lipids per injection from Orbitrap MS2 methods after chromatographic peak picking and filtering for adducts, dimers, in-source fragments, misidentified isotopes, and mismatched RT, Figure 2d). Interestingly, the number of identifications was quite comparable among the three QLT MS2 methods, while Orbitrap MS2 30k methods yielded 13.5% fewer identifications than the other Orbitrap MS2 15k methods, again underscoring the importance of MS/MS acquisition rate.
Further assessment of optimal acquisition methods.
To explore the performance between QLT and Orbitrap MS2 methods during the separation we selected the top performing settings for each analyzer – that is, Orbitrap MS1 at 240,000 resolving power followed by either QLT at rapid mode or Orbitrap at 15,000 resolving power. In these modes the scanning speed for MS2 of the Orbitrap MS2 method is 30% less than that of QLT MS2 method (Supplementary Figure 4). The maximum identification rate by QLT MS2 method is 110 lipids per minute, while the maximum identification rate by Orbitrap MS2 method is 90 lipids per minute (Figure 3a). With these optimal conditions the difference in identification rate led to 1,523 vs. 1,190 cumulative lipid identifications for QLT MS2 and Orbitrap MS2 methods from triplicates, respectively (Figure 3b). More lipids were identified by the QLT MS2 method for almost all major lipid classes that were detected in this study (Figure 3c). Although great overlap of lipid identification was obtained by both methods, QLT MS2 was able to capture more low abundance species (Figure 3d). As expected, the relative quantification of lipids that were identified by both methods was nearly identical (Figure 3e).
Figure 3. Comparison between optimal QLT MS2 and Orbitrap MS2 methods and their relative quantification precision.
(a) Identification rate of lipids across gradient. (b) Cumulative number of lipid identifications across gradient. (c) Number of lipid identifications within each lipid class from QLT MS2 and Orbitrap MS2 method. Lipid classes containing > 3 lipids were shown. (d) Histogram of log2 intensity of lipids for QLT MS2 and Orbitrap MS2 method. The Venn diagram on topright showed the overlap of lipids identified between QLT MS2 and Orbitrap MS2 method. (e) 2D density plot showing no difference for relative quantification of overlapped lipid identifications between QLT MS2 and Orbitrap MS2 method. Data were collected in positive mode.
NIST SRM 1950 plasma.
The same experiment was conducted using NIST SRM 1950, yielding 1493 cumulative lipid identifications (Supplementary data sheet 1). These compare well with the overall number of lipids annotated in 1950 plasma (~ 1500 reported by Bowden et al, 2017).23 The corresponding raw files are uploaded into the MassIVE repository (MSV000088741). Note because data reported here were collected using positive mode and HCD fragmentation, phosphatidylcholine lipids are identified by MS/MS spectra containing phosphocholine headgroup fragment ions (184, 125, 86 m/z); we acknowledge this strategy provides lower structural resolution. We are excited about the possibility of improved structural resolution when paired with alternative fragmentation and MSn experiments and this experiment is the subject of current investigations.
CONCLUSIONS
Here we implement capillary separations for discovery lipidome analysis. Further we evaluated the coverage achieved using either Orbitrap or QLT mass analyzers for tandem MS. These data demonstrate that the increased resolving power and mass accuracy generated by the Orbitrap mass analysis is not significant enough to outweigh the considerable gains in speed and sensitivity that are offered by the QLT for MS/MS. We expect further increases in lipid spectral identification rates as lipid spectral libraries are improved and expanded. Another way to extend lipidome coverage might be integrating iterative exclusion methods.31 Further, the use of the QLT offers the exciting possibility of conducting rapid MSn experiments for more enhanced lipid characterization.32–35
Note to keep things simple we only leveraged a single polarity (positive ion mode) throughout this study as our emphasis was on chromatography and assessing analyzer performance. Future work should consider negative mode operations and polarity switching. Aside from improved chromatography another motivation of this study was to create a lipidomic methodology that is on the same platform as shotgun proteomics – i.e., same capillary chromatography setup and same polarity as peptide analysis. The long-term objective of this work then is to expand our recent developments in integrated peptide and lipid analysis, Multi-Omic Single-shot Technology (MOST), from high flow to nanoflow.36 Since the reconstitution solvents are usually different for lipids and peptides. We are envisioning to inject lipids first and then peptides on a single capillary column for minimized interference of lipid solvent (high organic) to the chromatographic performance of peptides. The results presented here indicate that it will be possible to perform comprehensive proteome and lipidome profiling on a single capillary column coupled with a hybrid mass spectrometer in a single analysis.
Supplementary Material
ACKNOWLEDGMENT
We are grateful for support from NIH P41 GM108538 (J.J.C.).
Footnotes
Notes
J.J.C. is a consultant for Thermo Fisher Scientific.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Supplementary methods and results. (PDF).
Supplementary data sheet 1. (xlsx).
Data Availability
Raw data for 1950 plasma lipidomics analysis are available in MassIVE repository (MSV000088741).
REFERENCES
- (1).Shevchenko A; Simons K Lipidomics: Coming to Grips with Lipid Diversity. Nat Rev Mol Cell Biol 2010, 11 (8), 593–598. 10.1038/nrm2934. [DOI] [PubMed] [Google Scholar]
- (2).Wymann MP; Schneiter R Lipid Signalling in Disease. Nat Rev Mol Cell Biol 2008, 9 (2), 162–176. 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- (3).Cajka T; Fiehn O Comprehensive Analysis of Lipids in Biological Systems by Liquid Chromatography-Mass Spectrometry. TrAC Trends in Analytical Chemistry 2014, 61, 192–206. 10.1016/j.trac.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rustam YH; Reid GE Analytical Challenges and Recent Advances in Mass Spectrometry Based Lipidomics. Anal. Chem 2018, 90 (1), 374–397. 10.1021/acs.analchem.7b04836. [DOI] [PubMed] [Google Scholar]
- (5).Züllig T; Trötzmüller M; Köfeler HC Lipidomics from Sample Preparation to Data Analysis: A Primer. Anal Bioanal Chem 2020, 412 (10), 2191–2209. 10.1007/s00216-019-02241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lipidomics Standards Initiative Consortium. Lipidomics Needs More Standardization. Nat Metab 2019, 1 (8), 745–747. 10.1038/s42255-019-0094-z. [DOI] [PubMed] [Google Scholar]
- (7).Swaney DL; Wenger CD; Coon JJ Value of Using Multiple Proteases for Large-Scale Mass Spectrometry-Based Proteomics. J. Proteome Res 2010, 9 (3), 1323–1329. 10.1021/pr900863u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hebert AS; Richards AL; Bailey DJ; Ulbrich A; Coughlin EE; Westphall MS; Coon JJ The One Hour Yeast Proteome. Molecular & Cellular Proteomics 2014, 13 (1), 339–347. 10.1074/mcp.M113.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Grinias KM; Godinho JM; Franklin EG; Stobaugh JT; Jorgenson JW Development of a 45kpsi Ultrahigh Pressure Liquid Chromatography Instrument for Gradient Separations of Peptides Using Long Microcapillary Columns and Sub-2μm Particles. Journal of Chromatography A 2016, 1469, 60–67. 10.1016/j.chroma.2016.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Sorensen MJ; Anderson BG; Kennedy RT Liquid Chromatography above 20,000 PSI. TrAC Trends in Analytical Chemistry 2020, 124, 115810. 10.1016/j.trac.2020.115810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Senko MW; Remes PM; Canterbury JD; Mathur R; Song Q; Eliuk SM; Mullen C; Earley L; Hardman M; Blethrow JD; Bui H; Specht A; Lange O; Denisov E; Makarov A; Horning S; Zabrouskov V Novel Parallelized Quadrupole/Linear Ion Trap/Orbitrap Tribrid Mass Spectrometer Improving Proteome Coverage and Peptide Identification Rates. Anal. Chem 2013, 85 (24), 11710–11714. 10.1021/ac403115c. [DOI] [PubMed] [Google Scholar]
- (12).Eliuk S; Makarov A Evolution of Orbitrap Mass Spectrometry Instrumentation. Annual Rev. Anal. Chem 2015, 8 (1), 61–80. 10.1146/annurev-anchem-071114-040325. [DOI] [PubMed] [Google Scholar]
- (13).Wilm M; Mann M Analytical Properties of the Nanoelectrospray Ion Source. Anal. Chem 1996, 68 (1), 1–8. 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- (14).Juraschek R; Dülcks T; Karas M Nanoelectrospray—More than Just a Minimized-Flow Electrospray Ionization Source. J Am Soc Mass Spectrom 1999, 10 (4), 300–308. 10.1016/S1044-0305(98)00157-3. [DOI] [PubMed] [Google Scholar]
- (15).Kennedy RT; Jorgenson JW Preparation and Evaluation of Packed Capillary Liquid Chromatography Columns with Inner Diameters from 20 to 50 Micrometers. Anal. Chem 1989, 61 (10), 1128–1135. 10.1021/ac00185a016. [DOI] [Google Scholar]
- (16).Emmett MR; Caprioli RM Micro-Electrospray Mass Spectrometry: Ultra-High-Sensitivity Analysis of Peptides and Proteins. J. Am. Soc. Mass Spectrom 1994, 5 (7), 605–613. 10.1016/1044-0305(94)85001-1. [DOI] [PubMed] [Google Scholar]
- (17).Danne-Rasche N; Coman C; Ahrends R Nano-LC/NSI MS Refines Lipidomics by Enhancing Lipid Coverage, Measurement Sensitivity, and Linear Dynamic Range. Anal. Chem 2018, 90 (13), 8093–8101. 10.1021/acs.analchem.8b01275. [DOI] [PubMed] [Google Scholar]
- (18).Vasilopoulou CG; Sulek K; Brunner A-D; Meitei NS; Schweiger-Hufnagel U; Meyer SW; Barsch A; Mann M; Meier F Trapped Ion Mobility Spectrometry and PASEF Enable In-Depth Lipidomics from Minimal Sample Amounts. Nat Commun 2020, 11 (1), 331. 10.1038/s41467-019-14044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Zardini Buzatto A; Kwon BK; Li L Development of a NanoLC-MS Workflow for High-Sensitivity Global Lipidomic Analysis. Analytica Chimica Acta 2020, 1139, 88–99. 10.1016/j.aca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- (20).Lee GB; Lee JC; Moon MH Plasma Lipid Profile Comparison of Five Different Cancers by Nanoflow Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry. Analytica Chimica Acta 2019, 1063, 117–126. 10.1016/j.aca.2019.02.021. [DOI] [PubMed] [Google Scholar]
- (21).Bang DY; Lim S; Moon MH Effect of ionization modifiers on the simultaneous analysis of all classes of phospholipids by nanoflow liquid chromatography/tandem mass spectrometry in negative ion mode. J Chromatogr A. 2012, 1240:69–76. doi: 10.1016/j.chroma.2012.03.073. [DOI] [PubMed] [Google Scholar]
- (22).Bang DY; Kang D; Moon MH Nanoflow liquid chromatography-tandem mass spectrometry for the characterization of intact phosphatidylcholines from soybean, bovine brain, and liver. J Chromatogr A. 2006, 1104(1–2):222–229. doi: 10.1016/j.chroma.2005.12.005 [DOI] [PubMed] [Google Scholar]
- (23).Bowden JA; Heckert A; Ulmer CZ, et al. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950-Metabolites in Frozen Human Plasma. J Lipid Res. 2017;58(12):2275–2288. doi: 10.1194/jlr.M079012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Trujillo EA; Hebert AS; Brademan DR; Coon JJ Maximizing Tandem Mass Spectrometry Acquisition Rates for Shotgun Proteomics. Anal. Chem 2019, 91 (20), 12625–12629. 10.1021/acs.analchem.9b02979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Matyash V; Liebisch G; Kurzchalia TV; Shevchenko A; Schwudke D Lipid Extraction by Methyl-Tert-Butyl Ether for High-Throughput Lipidomics. J Lipid Res 2008, 49 (5), 1137–1146. 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Shishkova E; Hebert AS; Westphall MS; Coon JJ Ultra-High Pressure (>30,000 Psi) Packing of Capillary Columns Enhancing Depth of Shotgun Proteomic Analyses. Anal. Chem 2018, 90 (19), 11503–11508. 10.1021/acs.analchem.8b02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hutchins PD; Russell JD; Coon JJ LipiDex: An Integrated Software Package for High-Confidence Lipid Identification. Cell Systems 2018, 6 (5), 621–625.e5. 10.1016/j.cels.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).MacNair JE; Lewis KC; Jorgenson JW Ultrahigh-Pressure Reversed-Phase Liquid Chromatography in Packed Capillary Columns. Anal. Chem 1997, 69 (6), 983–989. 10.1021/ac961094r. [DOI] [PubMed] [Google Scholar]
- (29).Criscuolo A; Zeller M; Fedorova M Evaluation of Lipid In-Source Fragmentation on Different Orbitrap-Based Mass Spectrometers. J. Am. Soc. Mass Spectrom 2020, 31 (2), 463–466. 10.1021/jasms.9b00061. [DOI] [PubMed] [Google Scholar]
- (30).Gathungu RM; Larrea P; Sniatynski MJ; Marur VR; Bowden JA; Koelmel JP; Starke-Reed P; Hubbard VS; Kristal BS Optimization of Electrospray Ionization Source Parameters for Lipidomics To Reduce Misannotation of In-Source Fragments as Precursor Ions. Anal. Chem 2018, 90 (22), 13523–13532. 10.1021/acs.analchem.8b03436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Koelmel JP; Kroeger NM; Gill EL; et al. Expanding Lipidome Coverage Using LC-MS/MS Data-Dependent Acquisition with Automated Exclusion List Generation. J Am Soc Mass Spectrom 2017;28(5):908–917. doi: 10.1007/s13361-017-1608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ekroos K; Ejsing CS; Bahr U; Karas M; Simons K; Shevchenko A Charting Molecular Composition of Phosphatidylcholines by Fatty Acid Scanning and Ion Trap MS3 Fragmentation. Journal of Lipid Research 2003, 44 (11), 2181–2192. 10.1194/jlr.D300020-JLR200. [DOI] [PubMed] [Google Scholar]
- (33).McAnoy AM; Wu CC; Murphy RC Direct Qualitative Analysis of Triacylglycerols by Electrospray Mass Spectrometry Using a Linear Ion Trap. J Am Soc Mass Spectrom 2005, 16 (9), 1498–1509. 10.1016/j.jasms.2005.04.017. [DOI] [PubMed] [Google Scholar]
- (34).Rampler E; Criscuolo A; Zeller M; El Abiead Y; Schoeny H; Hermann G; Sokol E; Cook K; Peake DA; Delanghe B; Koellensperger G A Novel Lipidomics Workflow for Improved Human Plasma Identification and Quantification Using RPLC-MSn Methods and Isotope Dilution Strategies. Anal. Chem 2018, 90 (11), 6494–6501. 10.1021/acs.analchem.7b05382. [DOI] [PubMed] [Google Scholar]
- (35).Hartler J; Armando AM; Trötzmüller M; Dennis EA; Köfeler HC; Quehenberger O Automated Annotation of Sphingolipids Including Accurate Identification of Hydroxylation Sites Using MS n Data. Anal. Chem 2020, 92 (20), 14054–14062. 10.1021/acs.analchem.0c03016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).He Y; Rashan EH; Linke V; Shishkova E; Hebert AS; Jochem A; Westphall MS; Pagliarini DJ; Overmyer KA; Coon JJ Multi-Omic Single-Shot Technology for Integrated Proteome and Lipidome Analysis. Anal. Chem 2021, 93 (9), 4217–4222. 10.1021/acs.analchem.0c04764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data for 1950 plasma lipidomics analysis are available in MassIVE repository (MSV000088741).