Abstract
Single-dose azithromycin therapy has recently been used in Uruguay for the treatment of uncomplicated gonococcal infections. As part of an active surveillance study to monitor the emergence of antibiotic resistance in gonococcal isolates, we examined the levels of azithromycin susceptibility in 51 consecutive isolates obtained from males with uncomplicated gonococcal urethritis. Isolates with decreased susceptibility to azithromycin (MICs, 0.25 to 0.5 μg/ml) were common, and these isolates often displayed cross-resistance to hydrophobic antimicrobial agents (erythromycin and Triton X-100). Resistance to erythromycin and Triton X-100 is frequently due to overexpression of the mtrCDE-encoded efflux pump mediated by mutations in the mtrR gene, which encodes a transcriptional repressor that modulates expression of the mtrCDE operon. Accordingly, we questioned whether clinical isolates that express decreased azithromycin susceptibility harbor mtrR mutations. Promoter mutations that would decrease the level of expression of mtrR as well as a missense mutation at codon 45 in the mtrR-coding region that would result in a radical amino acid replacement within the DNA-binding motif of MtrR were found in these strains. When these mutations were transferred into azithromycin-susceptible strain FA19 by transformation, the susceptibility of gonococci to azithromycin was decreased by nearly 10-fold. The mtrCDE-encoded efflux pump system was responsible for this property since insertional inactivation of the mtrC gene resulted in enhanced susceptibility of gonococci to azithromycin. We conclude that the mtrCDE-encoded efflux pump can recognize azithromycin and that the emergence of gonococcal strains with decreased susceptibility to azithromycin can, in part, be explained by mtrR mutations.
The emergence of strains of Neisseria gonorrhoeae that express clinically significant levels of resistance to penicillin and tetracycline is a global problem that is particularly acute in South America (9, 25). The loss of these relatively inexpensive antibiotics for the treatment of gonococcal infections has resulted in the use of alternative treatment regimens for effective clinical management of gonorrhea. For instance, azithromycin has been shown to achieve high and prolonged levels in tissues and cells, thereby allowing coverage against both gonococci and Chlamydia trachomatis (7). For these reasons, azithromycin has recently been used in Uruguay for the treatment of uncomplicated gonococcal infections in males.
Azithromycin is a 15-membered azalide derived from erythromycin by the replacement of the 9a carbonyl in the aglycone ring with a methyl-substituted nitrogen (35). Despite its improved pharmacokinetic properties and bactericidal capacity against gonococci in vitro, there is now evidence that clinical isolates with decreased azithromycin susceptibility can be recovered. Thus, we recently described (5) that for 161 consecutive genital isolates from males with acute gonococcal urethritis, the MIC at which 90% of isolates are inhibited (MIC90) was 0.5 μg/ml. This result suggested that azithromycin resistance in gonococci was present in Uruguay and could pose significant problems for the effective management of patients with gonococcal infections.
We have sought to determine the molecular basis for the decreased azithromycin susceptibility in strains of gonococci isolated from our patient population. In theory, resistance to azithromycin could arise due to mechanisms similar to those for resistance to other macrolides (10). These mechanisms include target site modification by methylases encoded by erm genes (18, 20, 21) or antibiotic inactivation due to the actions of different enzymes (1–4, 6, 17, 19, 22, 32) or the actions of efflux pumps (16, 36, 43). Little is known about the mechanisms of macrolide resistance in gonococci, but the recently described mtrCDE-encoded efflux pump has been suggested to be one mechanism by which certain strains could express decreased susceptibility to erythromycin (8, 12, 13, 23, 24, 33). This efflux pump has been shown to mediate energy-dependent export of structurally diverse hydrophobic antimicrobial agents. The mtrCDE genes constitute a single transcriptional unit that is negatively regulated by the product of the adjacent but divergent mtrR gene. The mtrR gene product (MtrR) is a transcriptional repressor that binds to a 31-nucleotide region that encompasses the promoter element that drives transcription of mtrCDE (23). Mutations within the mtrR-coding region or a 13-bp inverted repeat sequence within the mtrR promoter can result in decreased susceptibility of gonococci to erythromycin as well as other hydrophobic agents. We questioned whether such mutations might explain the decreased azithromycin susceptibility property that seems to be increasing in prevalence in isolates obtained from infected individuals in Uruguay. We now report that mtrR promoter and coding region mutations occur frequently in strains that express decreased susceptibility to azithromycin and that their presence can explain this property.
MATERIALS AND METHODS
Strains of N. gonorrhoeae used and growth conditions.
A sample of 51 consecutive clinical isolates of N. gonorrhoeae with no known epidemiologic relationship were recovered from male patients with urethritis and were received in one of our laboratories (the laboratory of G.B. and L.Z.) for national (Uruguay) antimicrobial susceptibility surveillance studies. These strains were collected in Uruguay during 1996 and 1997. All isolates were identified as N. gonorrhoeae by conventional methods and were frozen at −70°C. Control strains (ATCC 49226, WHO III, WHO V, WHO VII, FA19, KH12, and KH15) with known profiles of susceptibility to the antimicrobial agents used in this study were included. Strain FA19 is considered a wild type with respect to the mtrCDE-encoded efflux pump system (13). Strains KH12 and KH15 are isogenic transformants of strain FA19. KH12 contains an insertional mutation in the mtrC gene (12) that renders it hypersusceptible to hydrophobic agents. Strain KH15 is hyperresistant to hydrophobic agents and contains a single-base-pair deletion in a 13-bp inverted repeat sequence within the promoter region used for transcription of the mtrR gene (13).
Antimicrobial testing.
The MICs of azithromycin, crystal violet, erythromycin, Triton X-100, and tetracycline were determined by the agar dilution method as specified in the National Committee for Clinical Laboratory Standards protocol (29). Penicillinase production was tested with the chromogenic cephalosporin nitrocefin (Oxoid Ltd., Basingstoke, United Kingdom). High-level resistance to tetracycline was shown to be due to possession of tetM by PCR as described previously (26). By using the criteria of Morse et al. (28), strains that express high-level resistance to hydrophobic agents due to the mtrCDE-encoded system (Mtr phenotype) were identified with respect to their susceptibilities to crystal violet (MIC, >1.0 μg/ml), erythromycin (MIC, >2.0 μg/ml), and Triton X-100 (MIC, >2,000 μg/ml), as determined by the method of Shafer et al. (37).
PCR amplification and DNA sequencing studies.
Chromosomal DNA was prepared from test strains as described previously (27). These DNA samples were used in PCRs to amplify the mtrR gene, including the promoter region, as described previously (23). The PCRs used the oligonucleotide primers used by Lucas et al. (23): RPMAL#2 (5′-ACTGAAGCTTATTTCCGGCGCAGGCAGGG-3′) and KH9#3 (5′-GACGACAGTGCCAATGCAACG-3′). The PCR products were purified by use of the QIAquick PCR purification kit protocol supplied by the manufacturer. Automatic DNA sequencing was performed at the Emory University DNA Sequencing Core Facility and used oligonucleotide primer RPMAL #2, KH9#1 (5′-GTCGCAGATACGTTGGAACAACG-3′), or KH9#3.
Transformation studies.
In order to inactivate the mtrCDE-encoded efflux pump system in strains that express decreased susceptibility to azithromycin, four clinical isolates that express the Mtr phenotype were transformed with 0.5 μg of chromosomal DNA from strain KH12 (mtrC::Kmr) per ml. The transformation reaction was as described previously (12), and recombinants were selected for their resistance to kanamycin (50 μg/ml). Insertional inactivation of mtrC in transformants was confirmed by PCR with oligonucleotide primers KH9#2 (5′-CGTTTCGGGTCGGTTTGACG-3′) and KH9#3. The mtrR mutations in clinical isolate 9638 was introduced into strain FA19 by transformation with chromosomal DNA and selection for erythromycin-resistant transformants with 0.5 μg of erythromycin per ml. The susceptibilities of the transformants to antimicrobial agents along with that of the respective parental strain were determined as described above.
Statistical analyses.
Linear correlations between logarithmic values of the MICs of azithromycin, erythromycin, and tetracycline were evaluated with the Pearson coefficient (r) with EPI INFO Statistical Software.
RESULTS AND DISCUSSION
Antibiotic susceptibilities of gonococcal isolates.
We determined (Table 1) the susceptibilities of 51 consecutive isolates obtained from our patient population in Uruguay to azithromycin, erythromycin, and tetracycline; due to the high proportion (>50%) of β-lactamase-producing gonococci in Uruguay, penicillin susceptibility testing was not conducted. Chromosomally mediated resistance to tetracycline was observed in 62.7% (32 of 51) of the isolates, and seven additional isolates displayed higher levels of resistance to tetracycline (MIC, >16 μg/ml) due to a plasmid-borne tetM determinant. A high proportion of isolates displayed a multiple-drug-resistance pattern. There was a significant linear correlation between logarithmic values of the MICs of tetracycline or the MICs of azithromycin and logarithmic values of the MICs of erythromycin (r = 0.77 [P < 0.01] and r = 0.84 [P < 0.01], respectively) (data not shown).
TABLE 1.
Susceptibilities of 51 clinical isolates of N. gonorrhoeae to tetracycline, erythromycin, and azithromycin
| Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Tetracyclinea | 0.25–8.0 | 2.0 | 8.0 |
| Erythromycin | 0.063–4 | 2.0 | 2.0 |
| Azithromycin | 0.032–0.5 | 0.25 | 0.5 |
Isolates with plasmid-mediated resistance were excluded (MICs, ≥16 μg/ml).
Expression of Mtr phenotype in clinical isolates.
Using the criteria of Morse et al. (28), we established that the Mtr phenotype (elevated resistance to crystal violet and Triton X-100) was expressed by nearly 50% of strains that displayed decreased susceptibility to azithromycin, erythromycin, and tetracycline (Table 2). It is relevant to note that those gonococci that displayed the Mtr phenotype also showed elevated resistance to azithromycin, erythromycin, and tetracycline compared to the level of resistance of those strains that did not display the Mtr phenotype.
TABLE 2.
Susceptibilities of clinical isolates of N. gonorrhoeae with different cell envelope phenotypea
| Antimicrobial agent and cell envelope phenotype | No. of isolates | No. of isolates for which MIC (μg/ml) was:
|
MIC (μg/ml)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.032 | 0.063 | 0.125 | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | 50% | 90% | ||
| Tetracyclineb | ||||||||||||
| Mtr | 21 | 1 | 4 | 8 | 8 | 4.0 | 8.0 | |||||
| Non-Mtr | 23 | 1 | 1 | 8 | 9 | 3 | 1 | 2.0 | 4.0 | |||
| Erythromycin | ||||||||||||
| Mtr | 23 | 22 | 1 | 2.0 | 2.0 | |||||||
| Non-Mtr | 28 | 1 | 4 | 5 | 10 | 3 | 5 | 0.5 | 2.0 | |||
| Azithromycin | ||||||||||||
| Mtr | 23 | 2 | 9 | 12 | 0.5 | 0.5 | ||||||
| Non-Mtr | 28 | 2 | 4 | 7 | 13 | 2 | 0.25 | 0.25 | ||||
The Mtr cell envelope phenotype was defined for isolates for which the erythromycin MIC was ≥2 μg/ml, the Triton X-100 MIC was ≥2,000 μg/ml, and the crystal violet MIC was ≥1 μg/ml (28).
Isolates with plasmid-mediated resistance were excluded (MICs, ≥16 μg/ml).
Identification of mtrR mutations in clinical isolates.
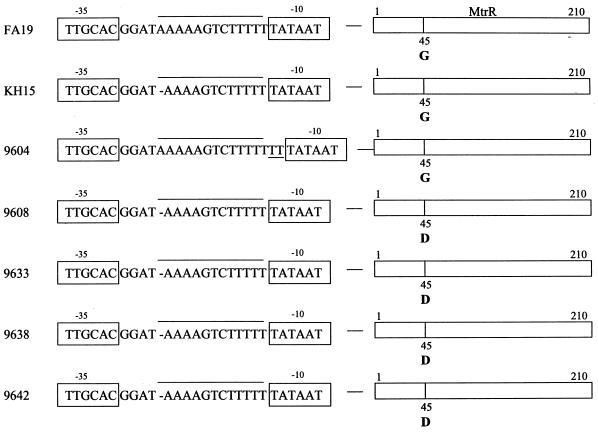
Previous studies (13, 33, 38) revealed that clinical isolates or laboratory-derived mutants that display resistance to hydrophobic agents frequently contain loss-of-function mutations in the mtrR-coding sequence or a single-base-pair deletion in a 13-bp inverted repeat within the mtrR promoter. In order to determine whether the strains used in this study might contain mutations in the mtrR region, we randomly selected four strains (strains 9608, 9633, 9638, and 9642) that exhibited decreased susceptibility to azithromycin and erythromycin for molecular analysis. DNA was prepared from these isolates for PCR amplification of a nearly 1-kb sequence that encompassed the mtrR-coding and promoter sequences. DNA sequencing studies of these PCR products revealed (Fig. 1) that all four strains contained the previously described single-base-pair deletion in the mtrR promoter region. This mutation is known to abrogate transcription of mtrR while it enhances transcription of the mtrCDE efflux pump operon (14, 23). These effects result in high-level resistance to Triton X-100 (12, 38). In addition to this promoter mutation, each strain contained a missense mutation at codon 45, which results in a radical amino acid replacement (Gly-45 to Asp-45) within the helix-turn-helix motif of MtrR (Fig. 1); this mutation, in the absence of the promoter mutation, has been observed previously (13, 33, 38) in other gonococcal isolates that display intermediate levels of resistance to hydrophobic agents.
FIG. 1.
mtrR mutations in clinical isolates of N. gonorrhoeae. Shown is the nucleotide sequence information of the mtrR promoter region from strains FA19 and KH15 and five clinical isolates that display decreased levels of azithromycin susceptibility. The −10 and −35 hexamers are shown inside the boxes, the presence of the 13-bp inverted repeat is shown with a line over the sequence, the single-base-pair deletion is shown with a dash, and the dinucleotide (TT) insertion in strain 9604 is underlined. Also presented is the Gly-45 (G)-to-Asp-45 (D) replacement in the helix-turn-helix motif of the MtrR proteins (210 amino acids in length) of strains 9608, 9633, 9638, and 9642.
Transformation studies.
In order to establish whether the mtrCDE-encoded efflux pump was responsible for the decreased azithromycin susceptibility expressed by strains 9608, 9633, 9638, and 9642, we inactivated their mtrC genes by transformation with the mtrC::Kmr determinant from strain KH12 (12). Insertional inactivation of mtrC in representative strains was confirmed by PCR analysis, as described in Materials and Methods (data not shown). In all cases, representative transformants of each clinical isolate displayed hypersusceptibility to azithromycin, erythromycin, and Triton X-100 (Table 3). This result confirmed that an intact mtrCDE operon was required for the hydrophobic agent resistance profiles expressed by these clinical isolates.
TABLE 3.
Results of MtrC inactivation in multiple-drug-resistant clinical isolates of N. gonorrhoeae
| Isolate | MIC (μg/ml)
|
||
|---|---|---|---|
| Triton X-100 | Erythromycin | Azithromycin | |
| 9608 | >16,000 | 2 | 0.25 |
| 9608 (mtrC::Kmr)a | 16 | 0.032 | 0.032 |
| 9633 | >16,000 | 2 | 0.25 |
| 9633 (mtrC::Kmr)a | 16 | 0.064 | 0.032 |
| 9638 | >16,000 | 2 | 0.25 |
| 9638 (mtrC::Kmr)a | 16 | 0.064 | 0.032 |
| 9642 | >16,000 | 2 | 0.5 |
| 9642 (mtrC::Kmr)a | 16 | 0.064 | 0.064 |
| 9604 | 250 | 2 | 0.5 |
| 9604 (mtrC::Kmr)a | 16 | 0.064 | 0.064 |
| FA19 (wild type) | 125 | 0.25 | 0.064 |
| FA19 (mtrR-9638)b | >16,000 | 2 | 0.25 |
| KH12 (mtrC::Kmr)c | 16 | 0.032 | 0.032 |
| KH15 (mtrR-171)d | >16,000 | 2 | 0.5 |
Transformant of each clinical isolate obtained by transformation with DNA from strain KH12.
Transformant of strain FA19 obtained by transformation with DNA from strain 9638.
Strain KH12 is a transformant of FA19 in which the mtrC gene is inactivated by insertion of the 1.3-kb Kmr cassette, and it is hypersusceptible to a panel of hydrophobic agents (12).
In order to verify that the mutations within mtrR are important in determining the decreased levels of azithromycin susceptibility, we transformed strain FA19 with chromosomal DNA from strain 9638. We selected erythromycin-resistant transformants and scored them for cross-resistance to azithromycin and Triton X-100. Representative transformants (see FA19 [mtrR-9838], Table 3) also had decreased susceptibility to azithromycin and Triton X-100. DNA sequencing analysis of the mtrR region from transformant strain FA19 (mtrR-9638) revealed that it had acquired both the single-base-pair deletion in the promoter and the missense mutation at codon 45 that are present in donor strain 9638 (data not shown). It is important that the single-base-pair deletion in the 13-bp inverted repeat sequence of the mtrR promoter was sufficient to decrease the level of susceptibility of gonococci to azithromycin by nearly 10-fold. This conclusion was drawn from a comparison of azithromycin MICs for isogenic strains FA19 and KH15 (Table 3), which differ at their mtrR regions by only the single base pair in the mtrR promoter (13).
Identification of a novel mtrR promoter mutation.
The mtrR mutations in the clinical isolates described above were identical to those reported for other strains of gonococci that express resistance to multiple hydrophobic compounds (14, 39). During our screening of erythromycin-resistant clinical isolates, we noticed rare strains (e.g., strain 9604, Table 3) that, compared to strain FA19, displayed a nearly 10-fold decrease in susceptibility to azithromycin but only a 2-fold decrease in susceptibility to Triton X-100. We analyzed the importance of the mtrCDE-encoded efflux pump in determining levels of azithromycin and erythromycin susceptibility in strain 9604 by constructing an insertional mutation within its mtrC gene using donor DNA from strain KH12. A representative transformant of strain 9604 bearing mtrC::Kmr displayed significantly enhanced susceptibility to azithromycin, erythromycin, and Triton X-100 (Table 3). DNA sequence analysis of the mtrR region of strain 9604 revealed that it contained a heretofore undescribed mutation in its promoter. This mutation represented a dinucleotide insertion (TT) into the 13-bp inverted repeat sequence (Fig. 1). This mutation would increase the spacing between the −10 and −35 hexamers from an optimal 17 nucleotides to an unfavorable 19 nucleotides (14).
The combined genetic and molecular results obtained in this investigation implicate the mtrCDE-encoded efflux pump as a mechanism by which gonococci can express decreased susceptibility to azithromycin. Through mutations that are known (13) to abrogate transcription of the gene (mtrR) that encodes a transcriptional repressor of mtrCDE or loss-of-function mutations in the repressor-encoding gene, gonococci can overproduce the MtrC-MtrD-MtrE efflux pump to increase their capacity to export hydrophobic agents. These hydrophobic agents are structurally diverse and include dyes (e.g., crystal violet), detergents (Triton X-100), and drugs (erythromycin and azithromycin). That azithromycin can be recognized by this efflux pump system is not surprising given its structural similarity to erythromycin. Other bacterial efflux pumps (30, 31, 34), including the MexA-MexB-OprM pump of Pseudomonas aeruginosa, which displays similarity at the amino acid level to the MtrC-MtrD-MtrE pump of gonococci (8, 13, 14, 40), can also recognize macrolide antibiotics.
Over the past decade, several studies have evaluated the efficacy of a single, oral dose of azithromycin for treatment of bacterial sexually transmitted diseases, including gonorrhea. Although a single, oral dose of 1.0 g of azithromycin proved effective for treatment of uncomplicated chlamydial infections, treatment of uncomplicated gonococcal infections produced variable results (15, 41, 45). In this respect, failure of azithromycin therapy for patients with uncomplicated gonococcal urethritis has been reported (11, 41, 42, 44, 46). The azithromycin MICs for some gonococcal strains isolated from patients who have failed azithromycin therapy were, in fact, similar to the MICs obtained for the isolates used in this investigation (0.25 to 0.5 μg/ml). Our results demonstrate that mtrR mutations that are known to result (12, 14, 23) in overproduction of the MtrC-MtrD-MtrE efflux pump are sufficient to result in these unfavorable azithromycin MICs. The acquisition of additional mutations outside of the mtr gene complex that modify the azithromycin structure or its target could further enhance azithromycin resistance to levels that would abolish the effectiveness of azithromycin as an alternative antibiotic in the treatment of uncomplicated gonorrhea. Continued surveillance and monitoring of antibiotic susceptibility patterns are needed to detect the emergence of such strains.
ACKNOWLEDGMENTS
We thank L. Pucko for help in manuscript preparation, Q. F. Carolina Marquez for the tetM amplification data, J.-R. Dillon (Director of WHO/PAHO GASP-Americas Centre) for providing susceptibility reference strains, and the external quality control program.
This work was supported by funds from the Program of Development in Basic Sciences (PEDECIBA) from Uruguay (to G.B.) and by Public Health Service grant AI-21150 (to W.M.S.) from the National Institutes of Health. W.M.S. was supported by a Research Career Scientist award from the Veterans Affairs Research Service.
REFERENCES
- 1.Andremont A, Gerbaud G, Courvalin P. Plasmid-mediated high-level resistance to erythromycin in Escherichia coli. Antimicrob Agents Chemother. 1986;29:515–518. doi: 10.1128/aac.29.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur M, Andremont A, Courvalin P. Distribution of erythromycin esterase and rRNA methylase genes in members of the family Enterobacteriaceae highly resistant to erythromycin. Antimicrob Agents Chemother. 1987;31:404–409. doi: 10.1128/aac.31.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur M, Autissier D, Courvalin P. Analysis of the nucleotide sequence of the ermB gene encoding the erythromycin esterase type II. Nucleic Acids Res. 1986;14:4987–4999. doi: 10.1093/nar/14.12.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthélémy P, Autissier D, Gerbaud G, Courvalin P. Enzymatic hydrolysis of erythromycin by a strain of Escherichia coli: a new mechanism of resistance. J Antibiot. 1984;37:1692–1696. doi: 10.7164/antibiotics.37.1692. [DOI] [PubMed] [Google Scholar]
- 5.Borthagaray G, Marquez C, Zarantonelli L, Acevedo A. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Macrolide susceptibility patterns in N. gonorrhoeae isolates from Uruguay, abstr. A-15; p. 40. [Google Scholar]
- 6.Brisson-Noël A, Delrieu P, Samain D, Courvalin P. Inactivation of lincosamide antibiotics in Staphylococcus aureus. J Biol Chem. 1988;263:15880–15887. [PubMed] [Google Scholar]
- 7.Chevalier B, Crenn Y, Cavallo J D, Plotton N, Meyran M. Comparative activity of azithromycin against 100 strains of Neisseria gonorrhoeae. Pathol Biol. 1995;43:281–283. [PubMed] [Google Scholar]
- 8.Delahay R M, Robertson B D, Balthazar J T, Shafer W M, Ison C A. Involvement of the gonococcal MtrE protein in the resistance of Neisseria gonorrhoeae to toxic hydrophobic agents. Microbiology. 1997;143:2127–2133. doi: 10.1099/00221287-143-7-2127. [DOI] [PubMed] [Google Scholar]
- 9.Dillon J R, Li H the GASP Network in the Americas. Program and abstracts of the 12th Meeting of the International Society of Sexually Transmitted Diseases. 1997. High burden of antibiotic resistant Neisseria gonorrhoeae isolates in the Americas and the Caribbean (1993–1995), abstr. O147; p. 85. [Google Scholar]
- 10.Eady E A, Ross J I, Cove J H. Multiple mechanism of erythromycin resistance. J Antimicrob Chemother. 1990;26:461–471. doi: 10.1093/jac/26.4.461. [DOI] [PubMed] [Google Scholar]
- 11.Ehret J M, Nims L J, Judson F N. A clinical isolate of Neisseria gonorrhoeae with in vitro resistance to erythromycin and decreased susceptibility to azithromycin. Sex Transm Dis. 1996;23:270–272. doi: 10.1097/00007435-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hagman K E, Pan W, Spratt B G, Balthazar J T, Judd R C, Shafer W M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 13.Hagman K E, Lucas C E, Balthazar J T, Snyder L, Nilles M, Judd R C, Shafer W M. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology. 1997;131:2117–2125. doi: 10.1099/00221287-143-7-2117. [DOI] [PubMed] [Google Scholar]
- 14.Hagman K E, Shafer W. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J Bacteriol. 1995;177:4162–4165. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handsfield H H, Dalu Z A, Martin D J, Douglas J M, Jr, McCarty J M, Schlossberg D the Azithromycin Gonorrhea Study Group. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Sex Transm Dis. 1994;21:107–111. doi: 10.1097/00007435-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Janosi L, Nakajima Y, Hashimoto H. Characterization of plasmids that confer inducible resistance to 14-membered macrolides and streptogramin type B antibiotics in Staphylococcus aureus. Microbiol Immunol. 1990;34:723–735. doi: 10.1111/j.1348-0421.1990.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 17.Kono M, O’Hara K, Ebisu T. Purification and characterization of macrolide 2′-phosphotransferase type II from a strain of Escherichia coli highly resistant to macrolide antibiotics. FEMS Microbiol Lett. 1992;97:89–94. doi: 10.1016/0378-1097(92)90369-y. [DOI] [PubMed] [Google Scholar]
- 18.Lai C, Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci USA. 1971;68:856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq R, Brisson-Noël A, Duval J, Courvalin P. Phenotypic expression and genetic heterogeneity of lincosamide inactivation in Staphylococcus spp. Antimicrob Agents Chemother. 1987;31:1887–1891. doi: 10.1128/aac.31.12.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leclercq R, Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991;35:1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Goffic F, Capmau M L, Abbe J, Cerceau C, Dublanchet A, Duval J. Plasmid-mediated pristinamycin resistance: PH1A, a pristinamycin 1A hydrolase. Ann Inst Pasteur (Paris) 1977;128:471–474. [PubMed] [Google Scholar]
- 23.Lucas C E, Balthazar J T, Hagman K E, Shafer W M. The MtrR repressor binds the DNA sequence between the mtrR and mtrC genes of Neisseria gonorrhoeae. J Bacteriol. 1997;179:4123–4128. doi: 10.1128/jb.179.13.4123-4128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas C E, Hagman K E, Levin J C, Stein D C, Shafer W M. Importance of lipooligosaccharide structure in determining gonococcal resistance to hydrophobic antimicrobial agents resulting from the mtr efflux system. Mol Microbiol. 1995;16:1001–1009. doi: 10.1111/j.1365-2958.1995.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 25.Márquez C, Castro M, Chan H J, Borthagaray G. Program and abstracts of the 3rd National Meeting of the Uruguayan Society of Microbiology. 1996. Antimicrobial resistance phenotypic association in Neisseria gonorrhoeae, abstr; p. 31. [Google Scholar]
- 26.Márquez C, Roberts M C, Borthagaray G, Xia M, Alen C, Acevedo A, Castro M. The first molecular characterization of tetracycline resistant N. gonorrhoeae from Uruguay. J Antimicrob Chemother. 1996;37:839–841. doi: 10.1093/jac/37.4.839. [DOI] [PubMed] [Google Scholar]
- 27.McAllister C F, Stephens D S. Analysis in Neisseria meningitidis and other Neisseria species of genes homologous to the FKBP immunophilin family. Mol Microbiol. 1993;10:13–24. doi: 10.1111/j.1365-2958.1993.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 28.Morse, S. A., P. G. Lysko, L. McFarland, J. S. Knapp, E. Sandstrom, C. Critchlow, and K. K. Holmes. 1982. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. 37:432–438. [DOI] [PMC free article] [PubMed]
- 29.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 4th ed. Approved standard. M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 30.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;64:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 31.Nikaido H. Multidrug efflux pumps of Gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Hara K, Kanda T, Ohmiya K, Ebisu T, Kono M. Purification and characterization of macrolide 2′-phosphotransferase from a strain of Escherichia coli that is highly resistant to erythromycin. Antimicrob Agents Chemother. 1989;33:1354–1357. doi: 10.1128/aac.33.8.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan W, Spratt B G. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol. 1994;11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 34.Paulsen F T, Brown M H, Skurry R A. Proton dependent efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Retsema J, Girard A, Schelkly W, Manousos M, Anderson M, Bright G, Borovoy R, Brennan L, Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987;31:1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross J I, Eady E A, Cove J H, Clunliffe W J, Baumberg S, Wootton J. Inducible erythromycin resistance in staphylococci is encoded by a member of a ATP-binding transport super gene family. Mol Microbiol. 1990;4:1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 37.Shafer W M, Guymon M, Lind L F, Sparling P F. Identification of an envelope mutation (env-10) resulting in increased antibiotic susceptibility and pyocin resistance in a clinical isolate of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1984;25:767–769. doi: 10.1128/aac.25.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shafer W M, Balthazar J T, Hagman K E, Morse S A. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology. 1995;141:907–911. doi: 10.1099/13500872-141-4-907. [DOI] [PubMed] [Google Scholar]
- 39.Slaney L, Chubb H, Ronald A, Brunham R. In-vitro activity of azithromycin, erythromycin, ciprofloxacin and norfloxacin against Neisseria gonorrhoeae, Haemophilus ducreyi, and Chlamydia trachomatis. J Antimicrob Chemother. 1990;25(Suppl. A):1–5. doi: 10.1093/jac/25.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 40.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steingrimsson O, Ólafsson J H, Thórarinsson H, Ryan R W, Johnson R B, Tilton R C. Azithromycin in the treatment of sexually transmitted disease. J Antimicrob Chemother. 1990;25(Suppl. A):109–114. doi: 10.1093/jac/25.suppl_a.109. [DOI] [PubMed] [Google Scholar]
- 42.Steingrimsson O, Ólafsson J H, Thórarinsson H, Ryan R W, Johnson R B, Tilton R C. Single dose azithromycin treatment of gonorrhea and infections caused by C. trachomatis and U. urealyticum in men. Sex Transm Dis. 1994;21:43–46. doi: 10.1097/00007435-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Sutcliffe J, Tait Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tapsall J W, Limnios E A, Donovan B, Lum G, Mulhall B P. Failure of azithromycin therapy in gonorrhea and discorrelation with laboratory test parameters. Sex Transm Dis. 1998;25:505–508. doi: 10.1097/00007435-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Waugh M A. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhea in men and women. J Antimicrob Chemother. 1993;31(Suppl. E):193–198. doi: 10.1093/jac/31.suppl_e.193. [DOI] [PubMed] [Google Scholar]
- 46.Young H, Moyes A, McMillan A. Azithromycin and erythromycin resistant Neisseria gonorrhoeae following treatment with azithromycin. Int J Sex Transm Dis AIDS. 1997;8:299–302. doi: 10.1258/0956462971920127. [DOI] [PubMed] [Google Scholar]