Abstract
Listeria (L.) monocytogenes is a foodborne pathogen that can cause disease, mainly in elderly, pregnant or immunocompromised persons through consumption of contaminated food, including pork products. It is widespread in the environment and can also be found in asymptomatic carrier animals, for example, in different tissues of pigs. To learn more about their nature, 16 Listeria spp. isolates found in tonsils and intestinal content of pigs and 13 isolates from the slaughterhouse environment were characterized using next-generation sequencing (NGS). A wide distribution of clonal complexes was observed in pigs, as well as in the pork production chain, suggesting multiple sources of entry. Hypervirulent clones were found in pig tonsils, showing the potential risk of pigs as source of isolates causing human disease. The presence of closely related isolates along the production chain suggests a cross-contamination in the slaughterhouse or recontamination from the same source, strengthening the importance of efficient cleaning and disinfection procedures. The phenotypical antimicrobial resistance status of L. monocytogenes isolates was examined via broth microdilution and revealed a low resistance level. Nevertheless, genotypical resistance data suggested multiple resistances in some non-pathogenic L. innocua isolates from pig samples, which might pose a risk of spreading resistances to pathogenic species.
Keywords: Listeria monocytogenes, Listeria innocua, Listeria welshimeri, pork production, food safety, next-generation sequencing, MLST, SNP, antimicrobial resistance, food contamination
1. Introduction
Listeria (L.) monocytogenes, a Gram-positive bacterium, is the cause of human listeriosis, a rare foodborne illness with a high hospitalization and case-fatality rate [1]. Currently, 20 Listeria species are known [2], among which L. monocytogenes and L. ivanovii are considered mammalian pathogens. While infections caused by L. ivanovii are seldom and mainly affect ruminants, L. monocytogenes is associated with most human and animal listeriosis disease cases [3]. Other Listeria species are generally considered non-pathogenic, although there are some rare cases of disease reported caused by L. innocua [4,5] and L. seeligeri [6].
The species L. monocytogenes is subdivided into four evolutionary lineages [7,8,9,10] and 13 serovars. Almost all human disease cases are associated with one of three serovars: 4b, 1/2a or 1/2b [11]. The bacterium is further classified into sequence types (STs) via multilocus sequence typing (MLST) and into clonal complexes (CCs). STs are defined as the unique collective of alleles from seven housekeeping genes, and CCs are defined as clusters of STs sharing at least six alleles [12,13]. MLST is a reference method that allows for comparison of isolates and demonstrates that only a few frequent L. monocytogenes clones are globally distributed [14].
Its resilience against a wide range of environmental stressors, such as low nutrient availability, acidic conditions, high salinity, and a broad temperature range from 0 °C to 45 °C [11] give L. monocytogenes the ability to survive in different food sources. In addition, L. monocytogenes is widespread in the environment, requiring constant control of this pathogen in food production facilities [1]. It is also present in the intestines of asymptomatic animals and humans [15]. Regarding pigs, studies have confirmed the presence of the pathogen on carcasses and in different tissues [16], as well as in the farm environment and in feed [17]. The prevalence of Listeria spp. in pigs in German slaughterhouses was described by Oswaldi et al. [18]. L. monocytogenes is frequently found in pork products worldwide [19,20], especially in products intended to be eaten raw (ready-to-eat products); this poses a threat to public health [21]. Some studies have confirmed living pigs as the origin of L. monocytogenes found in pork [17,22]; others identified the slaughter and processing environment as the source of pork contamination [23,24]. Demaître et al. [25] reasoned that persistent isolates in the slaughterhouse environment are more likely the source of contamination than less common, presumably transient and sporadically introduced isolates, as routine cleaning and sanitizing have become ineffective. Next-generation sequencing (NGS) makes it possible to investigate the origin of isolates and provides information on virulence and resistance [26].
L. monocytogenes is a highly heterogeneous species in terms of pathogenicity and contains hypovirulent and hypervirulent clones, which are most likely to cause disease. Thus, knowledge about the clonal structure of isolates is needed to assess their potential to cause disease [27]. The major genes associated with virulence in Listeria are involved in the cell infectious cycle: prfA, plcA, hly, mpl, actA and plcB for pathogenicity island 1 and inlA and inlB for pathogenicity island 2 [28]. Isolates considered non-pathogenic do not possess these virulence genes [29].
Knowledge about the antimicrobial resistance (AMR) status of isolates that cause human disease is crucial for treatment and the prevention of fatal outcomes. L. monocytogenes is susceptible to a wide range of antimicrobial agents that are effective against Gram-positive bacteria, such as tetracyclines, erythromycin, ampicillin and gentamicin [30], and shows intrinsic resistance to fosfomycin and fusidic acid [31]. The multiple antimicrobial resistance of a L. monocytogenes isolate was first shown in 1988 [32], followed by various resistant strains from different sources, including food, as well as environmental and human clinical samples [33]. Poyart-Salmeron et al. [32] showed that resistance genes can be transferred between Listeria species and other bacteria by self-transmissible plasmids.
The aim of this study was to perform NGS on Listeria spp. isolates found in pigs and along the corresponding pork processing chain to gain knowledge about their MLST types, differences in SNPs, virulence genes and antimicrobial resistance genes. Moreover, we identified the presence of phenotypic resistance against antimicrobial agents in these L. monocytogenes via broth microdilution. These results may provide insights into the relatedness of Listeria spp. found in pigs and along the corresponding pork production chain, as well as their relevance for human disease.
2. Materials and Methods
2.1. Origin of Isolates
The isolates were recovered by sampling 430 fattening pigs and the slaughterhouse environment in two industrial high-capacity pig slaughterhouses in Germany, as described by Oswaldi et al. [18], later referred to as slaughterhouses A and B. Overall, 16 isolates of Listeria spp. originating from pigs and 13 isolates originating from the slaughterhouse environment were isolated. In slaughterhouse A, samples were collected from pig tonsils and intestinal content on four dates in the winter period of 2018/2019, later referred to as dates 1 to 4. On sampling dates 3 and 4, with two months in between, environmental samples were also taken from slaughter, cutting and processing environments during processing. These environmental samples were collected with sponges (Whirl-Pak™ Speci-Sponge™ Environmental Surface Sampling Bags, Nasco, Fort Atkinson, WI, USA) moisturized with 10 mL sterile 0.85% saline solution. The samples from pig tonsils in slaughterhouse B were taken in October 2019 (sampling dates 5 and 6); no environmental samples were taken due to organizational reasons. Sample transportation and examination, as well as confirmation of presumptive isolates, were performed as described by Oswaldi et al. [18]. All confirmed isolates were stored under cryopreservation conditions at −80 °C in cryovials (ROTI®Store cryovials, Carl Roth GmbH + Co. KG, Karlsruhe, Germany).
2.2. Sequencing and Bioinformatic Analysis of Sequences
Next-generation sequencing (NGS) was performed at the German Federal Institute for Risk Assessment (BfR) in Berlin, Germany. The DNA was isolated with a PureLinkTM Genomic DNA Mini Kit (InvitrogenTM, Carlsbad, CA, USA). For lysis, the PulseNet protocol for Gram-positive bacteria was used (https://www.cdc.gov/pulsenet/pdf/pnl32-miseq-nextera-xt.pdf; accessed on 22 April 2021). DNA concentration was quantified using a QubitTM dsDNA BR assay kit with a QubitTM 2.0 fluorometer (InvitrogenTM, Carlsbad, CA, USA). The sequencing library was prepared with an Illumina DNA prep kit (Illumina Inc., San Diego, CA, USA). Sequencing was performed in paired-end mode at 2 × 150 bp with an Illumina NextSeq 500 or at 2 × 300 bp with an Illumina MiSeq (Illumina Inc., San Diego, CA, USA). Trimming of sequencing raw reads and overall quality assessment was conducted using the pipeline AQUAMIS [34].
For single-nucleotide polymorphism (SNP) analysis, the pipeline snippySnake (https://gitlab.com/bfr_bioinformatics/snippySnake; accessed on 25 May 2021), based on Snippy (https://github.com/tseemann/snippy; accessed on 25 May 2021), was used. Three separate SNP analyses were carried out for the three different Listeria species found so as not to distort the analysis with interspecies variability. Optimal reference genomes for SNP analysis were automatically identified via the mash-based search included in the snippySnake workflow. Accordingly, NZ_CP026043.1 (strain FDAARGOS_58) was used as reference for the analysis of L. mono cy togenes strains, NC_003212.1 (strain Clip11262) was used as reference for the analysis of L. innocua strains, and NZ_LT906444.1 (strain NCTC11857) was used as reference for the analysis of L. welshimeri strains. Complete linkage clustering of SNP distance matrices was performed in R. Trees were exported using the phylogram package and visualized in iTOL [35]. Strains with single-digit SNP differences were rated as likely to be related to one another.
The BakCharak pipeline (https://gitlab.com/bfr_bioinformatics/bakcharak; accessed on 27 May 2021) was used for MLST determination (database: pubMLST), as well as for screening of sequences for antimicrobial resistance genes (database: NCBI resistance gene database) and virulence factors (database: VFDB).
2.3. Antimicrobial Susceptibility Testing
For all L. monocytogenes isolates, antimicrobial susceptibility testing was performed by broth microdilution according to the recommendations of the Clinical and Laboratory Standards Institute given in the document VET06 [36]. Bacterial suspensions with a turbidity equivalent to McFarland 0.5 were prepared. Subsequently, 5 µL of the suspension was mixed per mL CAMHB II (cation-adjusted Mueller-Hinton-II) broth (Oxoid, Wesel, Germany) supplemented with 5% (v/v) lysed horse blood. Then, 50 µL of this suspension was pipetted in each well of the four microtiter plates (Thermo Fisher Scientific, Basingstoke, UK) with a multichannel pipet and incubated at 35 °C ± 2 °C for 24 h. To count the colony-forming units (cfu), 100 µL of the suspension was inoculated on Columbia blood agar. Streptococcus pneumoniae ATCC® 49619 was used as a quality control strain. The resulting minimal inhibitory concentrations (MICs) of penicillin, ampicillin and sulfamethoxazole/trimethoprim were classified as susceptible, intermediate or resistant according to the clinical breakpoints available in CLSI documents M45 [37] and VET06 [36]. For the remaining antimicrobial agents, erythromycin, clindamycin, ciprofloxacin, gentamicin, tetracycline and vancomycin, the clinical breakpoints for staphylococci listed in the CLSI document M100 [38] were used. Furthermore, based on CLSI document VET01S [39], breakpoints for staphylococci of animal origin were applied for amoxicillin/clavulanic acid, enrofloxacin, marbofloxacin, pirlimycin and doxycycline. For streptomycin and neomycin, the breakpoints described by Troxler et al. [31] were used.
3. Results
3.1. Presence of Listeria spp. in Pigs in Slaughterhouse A and B
Sixteen isolates of Listeria spp. (seven L. monocytogenes and nine L. innocua) used in this study were found in porcine samples; their distribution and prevalence in pigs were reported by Oswaldi et al. [18]. Two L. monocytogenes and five L. innocua isolates originated from slaughterhouse A, taken on sampling dates 1 to 4, whereas the remaining five L. monocytogenes and four L. innocua isolates came from slaughterhouse B on sampling dates 5 and 6.
3.2. Presence of Listeria spp. in Environmental Samples in Slaughterhouse A
The numbers of slaughter and processing environmental samples positive for Listeria spp. are shown in Table 1.
Table 1.
Results of environmental samples taken on date 3 (n = 36) and date 4 (n = 41) in slaughterhouse A in absolute numbers.
| Place of Sampling | Number of Samples Taken | Samples Positive for L. monocytogenes | Samples Positive for L. innocua | Samples Positive for L. welshimeri | ||||
|---|---|---|---|---|---|---|---|---|
| Date 3 | Date 4 | Date 3 | Date 4 | Date 3 | Date 4 | Date 3 | Date 4 | |
| Saws | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tonsil removal device | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Floor drains | 5 | 6 | 1 | 1 | 0 | 0 | 0 | 0 |
| Rubber boots (sole) | 7 | 8 | 1 | 0 | 1 | 0 | 1 | 0 |
| Equipment in slaughter hall | 8 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cutting plant (product contact surfaces/devices) | 5 | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Processing plant (product contact surfaces/devices) | 5 | 7 | 0 | 2 | 0 | 1 | 2 | 1 |
| Total | 77 | 7 | 2 | 4 | ||||
No Listeria spp. was found in samples of saws (n = 8), tonsil removal devices (n = 4) or other equipment in the slaughter hall with product contact (n = 16). On date 3, L. monocytogenes, L. innocua and L. welshimeri were found on soles of rubber boots used within the slaughter hall. L. monocytogenes was isolated from the same drain on date 3 and date 4. On date 4, we found samples positive for L. monocytogenes in the cutting and processing plant in places with direct product contact, including the mincer. In the cutting plant, L. monocytogenes-positive samples were found on the conveyor belt and a cutting tool. Other Listeria species, L. innocua and L. welshimeri, were found in the processing plant on both sampling dates.
3.3. MLST Analyses
The distribution of STs and CCs of the L. monocytogenes isolates is presented in Table 2. There were lineage I isolates with ST5 = CC5 (n = 2) and ST6 = CC6 (n =3), as well as lineage II isolates with ST7 = CC7 (n = 1), ST9 = CC9 (n =1), ST18 = CC18 (n =1), ST20 = CC20 (n = 1), ST37 = CC37 (n =1), ST325 = CC31 (n =1), ST412 = CC412 (n =2) and ST451 = CC11 (n = 1). Altogether, there were ten different STs and CCs present among the 14 isolates.
Table 2.
Characteristics of L. monocytogenes isolates found in pigs and in the environment 1.
| Sampled Matrix (Sample Number) |
Slaughterhouse | Sampling Date | Lineage | MLST ST | MLST CC | Virulence Genes (Total Number) |
|---|---|---|---|---|---|---|
| Pig tonsil (21-LI00365-0) |
A | 2 | II | 451 | 11 | actA, ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lapB, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (32) |
| Pig tonsil (21-LI00512-0) |
A | 3 | II | 37 | 37 | actA, ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lapB, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2 (31) |
| Pig tonsil (21-LI00523-0) |
B | 5 | I | 6 | 6 | actA, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlK, lap, lapB, llsA, llsB, llsD, llsG, llsH, llsP, llsX, llsY, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (37) |
| Pig tonsil (21-LI00524-0) |
B | 5 | II | 325 | 31 | ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2 (29) |
| Pig tonsil (21-LI00525-0) |
B | 5 | I | 6 | 6 | actA, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlK, lap, lapB, llsA, llsB, llsD, llsG, llsH, llsP, llsX, llsY, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (37) |
| Pig tonsil (21-LI00526-0) |
B | 5 | II | 7 | 7 | actA, ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lapB, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2 (31) |
| Pig tonsil (21-LI00527-0) |
B | 5 | II | 18 | 18 | actA, ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lapB, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (32) |
| Floor drain (21-LI00513-0) |
A | 3 | II | 412 | 412 | actA, ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2 (30) |
| Sole of rubber boot (21-LI00517-1) |
A | 3 | II | 9 | 9 | actA, ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lapB, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (32) |
| Floor drain (21-LI00519-0) |
A | 4 | II | 412 | 412 | actA, ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2 (30) |
| Cutting plant (21-LI00520-0) |
A | 4 | I | 5 | 5 | ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlK, lap, lapB, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (30) |
| Cutting plant (21-LI00521-0) |
A | 4 | I | 5 | 5 | ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlK, lap, lapB, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (30) |
| Processing plant (21-LI00522-0) |
A | 4 | I | 6 | 6 | actA, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlK, lap, lapB, llsA, llsB, llsD, llsG, llsH, llsP, llsX, llsY, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (37) |
| Processing plant (21-LI00368-0) |
A | 4 | II | 20 | 20 | actA, ami, aut, bsh, clpC, clpE, clpP, fbpA, gtcA, hly, hpt, iap/cwhA, inlA, inlB, inlC, inlF, inlJ, inlK, lap, lapB, lntA, lpeA, lpA1, lspA, mpl, oatA, pdgA, plcA, plcB, prfA, prsA2, vip (32) |
1 All the listed isolates showed AMR genes fosX and vga(G), as well as phenotypical resistance to clindamycin and pirlimycin.
3.4. SNP Analyses
The results of the SNP analyses showed great genetic differences between the isolates, consistent with MLST types. Intra-CC diversity showed SNP differences, most likely related to the isolates’ epidemiological or evolutionary relationship.
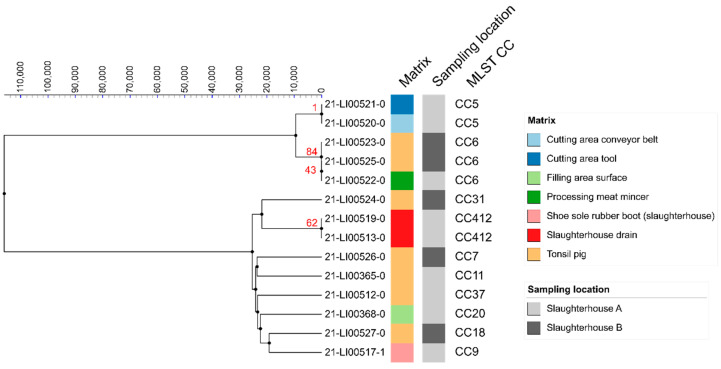
The two L. monocytogenes isolates found in the same drain in the slaughter hall of slaughterhouse A on two different dates both belong to CC412 and showed a difference of 62 SNPs, which makes a direct relation unlikely (Figure 1). In the cutting plant, two L. monocytogenes isolates found on a conveyor belt and a cutting tool further down the line belong to CC5 and had only one pairwise SNP difference, indicating a possible connection (Figure 1).
Figure 1.
Clustering of 14 L. monocytogenes isolates found in pigs and the slaughterhouse environment in a linkage tree with differences of less than 1000 SNPs indicated.
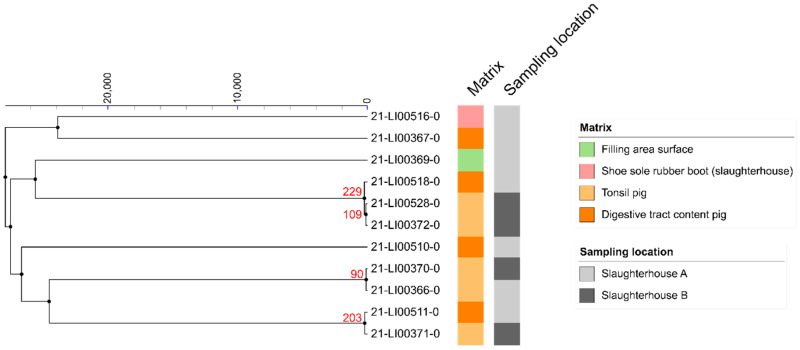
The two CC6 isolates found in pig tonsils in slaughterhouse B on the same date but originating from different farms have a pairwise SNP difference of 84, making a direct relation unlikely (Figure 1). In the processing plant of slaughterhouse A, another CC6 isolate was isolated from the meat mincer. The difference in SNPs was 43 to the nearer related CC6 isolate, so no close relationship was assumed. None of the isolated L. innocua showed close relationships with one another, as they all differed from each other by more than 90 SNPs (Figure 2).
Figure 2.
Clustering of 11 L. innocua isolates found in pigs and the slaughterhouse environment in a linkage tree with differences of less than 1000 SNPs indicated.
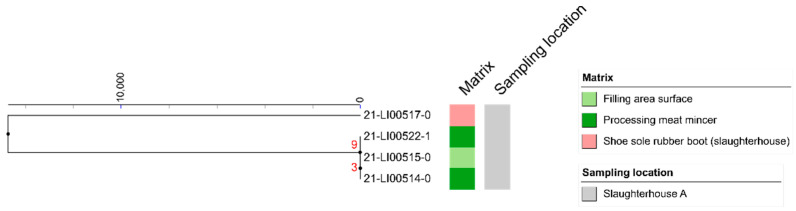
Three clones of L. welshimeri isolated from the processing plant (mincer on dates 3 and 4, working surface in filling area on date 3) showed a close relationship to one another, with differences of only three and nine SNPs, respectively (Figure 3).
Figure 3.
Clustering of 4 L. welshimeri isolates found in the slaughterhouse environment in a linkage tree with differences of less than 1000 SNPs indicated.
3.5. Antimicrobial Resistance Genes
All tested Listeria spp. isolates carried the AMR gene fosX (Table 2 and Table 3), which indicates genotypic resistance to fosfomycin.
Table 3.
Characteristics of Listeria species other than L. monocytogenes found in pigs and the slaughterhouse environment.
| Sampled Matrix (Laboratory Number) |
Slaughterhouse | Sampling Date | Species | AMR Genes | Virulence Genes (Total Number) |
|---|---|---|---|---|---|
| Pig intestinal content (21-LI00510-0) |
A | 1 | L. innocua | fosX; tet(S) | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (13) |
| Pig intestinal content (21-LI00511-0) |
A | 2 | L. innocua | fosX | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, llsA, llsG, llsH, llsX, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (17) |
| Pig intestinal content (21-LI00518-0) |
A | 4 | L. innocua | fosX; tet(M) | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (13) |
| Pig intestinal content (21-LI00367-0) |
A | 4 | L. innocua | fosX | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, llsA, llsG, llsH, llsX, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (17) |
| Pig tonsil (21-LI00366-0) |
A | 2 | L. innocua | fosX | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (13) |
| Pig tonsil (21-LI00528-0) |
B | 6 | L. innocua |
fosX; dfrG; tet(M); ant(6)-Ia |
clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (13) |
| Pig tonsil (21-LI00370-0) |
B | 6 | L. innocua | fosX | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (13) |
| Pig tonsil (21-LI00371-0) |
B | 6 | L. innocua | fosX | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, llsA, llsG, llsH, llsX, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (17) |
| Pig tonsil (21-LI00372-0) |
B | 6 | L. innocua |
fosX; tet(M); ant(6)-Ia |
clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (13) |
| Processing plant (21-LI00514-0) |
A | 3 | L. welshimeri | fosX | clpC, clpE, clpP, fbpA, lap, lpeA, lpA1, lspA, prsA2 (9) |
| Processing plant (21-LI00515-0) |
A | 3 | L. welshimeri | fosX | clpC, clpE, clpP, fbpA, lap, lpeA, lpA1, lspA, prsA2 (9) |
| Sole of rubber boot (21-LI00516-0) |
A | 3 | L. innocua | fosX | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, llsA, llsG, llsH, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (16) |
| Sole of rubber boot (21-LI00517-0) |
A | 3 | L. welshimeri | fosX | clpC, clpE, clpP, fbpA, lap, lpeA, lpA1, lspA, prsA2 (9) |
| Processing plant (21-LI00522-1) |
A | 4 | L. welshimeri | fosX; vga(G) | clpC, clpE, clpP, fbpA, lap, lpeA, lpA1, lspA, prsA2 (9) |
| Processing plant (21-LI00369-0) |
A | 4 | L. innocua | fosX | clpC, clpE, clpP, fbpA, gtcA, iap/cwhA, lap, llsA, llsG, llsH, llsX, lpeA, lpA1, lspA, oatA, pdgA, prsA2 (17) |
In addition, all the L. monocytogenes isolates carried the gene vga(G), which confers resistance to lincosamides, streptogramin A antimicrobial agents and possibly pleuromutilins [40,41]. This gene has previously been referred to as lmo0919 [40] and was later tentatively designated vga(L) [41]. The designation vga(G) was recently approved by the Nomenclature Center for Macrolide–Lincosamide–Streptogramin (MLS) Genes (https://faculty.washington.edu/marilynr/; accessed on 9 December 2021). As all L. monocytogenes share the same AMR genes, fosX and vga(G), and there was no difference in AMR gene content between L. monocytogenes of lineage I and II.
Among the 11 L. innocua isolates, seven harbored only the fosX resistance gene, whereas two isolates carried two, one isolate carried three and one isolate carried four AMR genes. One of these additional resistance genes was the gene tet(S), which codes for resistance to the tetracyclines doxycycline, tetracycline and minocycline. Three isolates carried the tet(M) gene, which confers the same resistance phenotype as tet(S). Two of these isolates additionally had the streptomycin resistance gene ant(6)-Ia, also known as ant6 or aadE. One isolate harbored the trimethoprim resistance gene dfrG as a fourth resistance gene.
In one L. welshimeri isolate, the resistance gene vga(G) was found in addition to the fosX gene.
3.6. Virulence Genes
All L. monocytogenes isolates found in our study harbored the major virulence genes prfA, plcA, hly, mpl, plcB, inlA and inlB. Three isolates—two CC5s found in the cutting plant and one CC31 in a pig tonsil—carried a truncated version of actA. The truncation was of 1024/1920 nt and 1026/1920 nt for CC5 and CC31 strains, respectively, compared to EGD-e actA (NCBI gene ID: 987035). The gene actA is one of the major virulence genes of pathogenicity island 1. Table 2 and Table 3 list the virulence genes for each isolate.
Neither L. innocua nor L. welshimeri isolates in this study showed any of the abovementioned major virulence genes.
3.7. Antimicrobial Susceptibility Testing Results
The results of antimicrobial susceptibility testing of 14 L. monocytogenes isolates are listed in Table 4. All tested isolates were susceptible to penicillin, ampicillin, erythromycin, ciprofloxacin, gentamicin, streptomycin, neomycin, tetracycline, sulfamethoxazole/trimethoprim and vancomycin. For amoxicillin/clavulanic acid, 57% (n = 8) of the L. monocytogenes isolates tested susceptible, whereas 43% (n = 6) were classified as intermediate. All isolates, except of one intermediate isolate, were susceptible to the fluoroquinolone enrofloxacin according to the clinical breakpoints for staphylococci in VET01S. The majority (86%, n = 12) of the isolates tested susceptible for marbofloxacin, another fluoroquinolone, two isolates (14%) were classified as intermediate and 11 isolates (79%) had intermediate results recorded for doxycycline, whereas only 21% (n = 3) were susceptible. Using the clinical breakpoints for staphylococci in the CLSI documents M100 or VET01S, all tested isolates proved to be resistant to the lincosamides clindamycin and pirlimycin, which is in agreement with the carriage of the resistance gene vga(G).
Table 4.
Susceptibility testing results of the 14 L. monocytogenes isolates for antimicrobial agents with existing clinical breakpoints.
| Antimicrobial Agent(s) | Susceptible | Intermediate | Resistant | |||
|---|---|---|---|---|---|---|
| no. | % | no. | % | no. | % | |
| Penicillin | 14 | 100 | 0 | 0 | 0 | 0 |
| Ampicillin | 14 | 100 | 0 | 0 | 0 | 0 |
| Amoxicillin/clavulanic acid | 8 | 57 | 6 | 43 | 0 | 0 |
| Erythromycin | 14 | 100 | 0 | 0 | 0 | 0 |
| Clindamycin | 0 | 0 | 0 | 0 | 14 | 100 |
| Pirlimycin | 0 | 0 | 0 | 0 | 14 | 100 |
| Ciprofloxacin | 14 | 100 | 0 | 0 | 0 | 0 |
| Enrofloxacin | 13 | 93 | 1 | 7 | 0 | 0 |
| Marbofloxacin | 12 | 86 | 2 | 14 | 0 | 0 |
| Gentamicin | 14 | 100 | 0 | 0 | 0 | 0 |
| Streptomycin | 14 | 100 | 0 | 0 | 0 | 0 |
| Neomycin | 14 | 100 | 0 | 0 | 0 | 0 |
| Tetracycline | 14 | 100 | 0 | 0 | 0 | 0 |
| Doxycycline | 3 | 21 | 11 | 79 | 0 | 0 |
| Sulfamethoxazole/trimethoprim | 14 | 100 | 0 | 0 | 0 | 0 |
| Vancomycin | 14 | 100 | 0 | 0 | 0 | 0 |
4. Discussion
We were able to demonstrate a wide distribution of Listeria spp.-positive samples within the slaughterhouse and the associated meat production chain, with only five out of 29 Listeria spp. isolates being genetically related, including two L. monocytogenes and three L. welshimeri isolates, in contrast to 24 Listeria spp. isolates not being genetically related. This assumes several different sources of entry along the slaughter and processing line. Isolates of L. monocytogenes were found in pig tonsil samples, as well as in the slaughter, cutting and processing environment. L. innocua was isolated in samples of pig intestinal content, whereas isolates of L. welshimeri, in contrast, were only present in samples from the slaughter and processing environment.
In our study, equipment in the slaughter hall was not found to be a carrier of Listeria spp., whereas isolates were detected on the soles of rubber boots worn within the slaughter hall. Positive samples in floor drains support the risk of spreading clones within the slaughterhouse via boots or via air during washdown. Berrang and Frank [42] showed that contaminated floor drains in poultry processing plants can be the source of airborne spread of Listeria and can cross-contaminate food contact surfaces, equipment, and exposed products. Therefore, the authors recommended that workers act with caution so as to not spray hoses directly into drains.
Maury et al. [27] grouped prevalent CC types into food-associated (CC9 and CC121), infection-associated (CC1, CC2, CC4 and CC6) and intermediate clonal complexes (others), with various origins. In our study, infection-associated isolates (belonging to CC6) were found in two pig tonsil samples originating from separate farms. We found another CC6 isolate in the mincer, which constitutes a threat for the consumer if products get contaminated. These isolates have the same CC but differences in SNPs, which indicates no close relationship. Therefore, we were not able to detect a direct connection between isolates found in pigs and along the slaughter, cutting and processing chain. CC6 and CC5, which we found in the cutting environment, were considered by Félix et al. [24] to be ubiquitous and evenly spread CCs in the pork production chain. In another study, Maury et al. [43] suggested that hypovirulent isolates belonging to CC9 and CC121 are better adapted to the food processing environment, whereas hypervirulent isolates of CC6 are better adapted to the mammalian gut environment. CC9 and CC121 were both reported as the most frequent CCs in the pork processing environment, confirming their adaptation to the conditions of the production environment [24,44]. Demaître et al. [25] suggested the continuous introduction and repeated contamination with the most common CCs via incoming carcasses and/or that they persist in the concerned cutting plants, likely by genetic determinants contributing to their establishment. We found no CC121 isolate in our study and one CC9 isolate on a sole of a rubber boot. Félix et al. [24] connected CC9 to raw meat processing, from which isolates of this CC may have originated. On a rubber boot, isolates can be easily distributed throughout the whole slaughterhouse and possibly contaminate food. Our finding of a CC37 isolate in a pig tonsil is in accordance with the results of Félix et al. [24], who reported that this CC type is better adapted to pig farms than to the pork production environment. CC11 (ST451) is considered a hypervirulent clonal complex specific to central Europe, as it is found frequently there, mainly in dairy but also in meat products [45], and has also been reported as the cause of human listeriosis cases in Germany [46]. We found one CC11 isolate in a pig tonsil in slaughterhouse A. These findings highlight the potential risk of pigs as a source of pathogenic L. monocytogenes in food, as also other studies have suggested [47,48]. Looking at the SNP results, we found closely related L. monocytogenes distributed in the cutting plant, indicating a carryover along the processing line, from the conveyor belt to a cutting tool further down the cutting line.
In the processing plant, two L. welshimeri clones isolated on date 3 indicate a cross-contamination from a working surface to the mincer. On date 4, the same clone was again isolated from the same mincer. Despite daily disinfection, this clone showed a persistence over a period of two months, or it was reintroduced from the same source. Stoller et al. [49] showed a possible persistence of L. monocytogenes isolates belonging to CC9, CC121 and CC204 in meat production plants for at least four years, suggesting disinfectant failures and biofilm formation of the pathogen as possible causes of persistence. Even though L. welshimeri is considered non-pathogenic [50], it has similar growth characteristics and can thereby be considered a model of distribution for L. monocytogenes [28] and illustrate the risk of persistence and cross contamination in the slaughterhouse environment.
The virulence genes analyzed support the pathogenicity of 11 out of the 14 L. monocytogenes isolates found in this study. The three exceptions were due to the truncation of the actA, as recently described [51]. The actA gene plays a role in intracellular motility and intercellular spreading, a key determinant of L. monocytogenes virulence [52]. Domann et al. [52] demonstrated that strains without this gene are incapable of infecting adjacent cells and are distinctly less virulent. In our study, two CC5 isolates found in the cutting plant and a CC31 isolate found in a pig tonsil in slaughterhouse B harbored only a truncated rather than the full-length gene.
All L. innocua and L. welshimeri isolates found in this study can be considered non-pathogenic. In contrast, atypical, pathogenic L. innocua isolates able to cause human disease were shown to have the genes encoding for the pathogenicity island 1 and inlA [53]. A study in Brazil [54] found such isolates in the environment of pork processing plants.
The genotypic AMR results showed fosfomycin resistance in all Listeria spp. isolates, which is not remarkable, as intrinsic resistance is known [31]. In addition, in all L. monocytogenes isolates, a genotypic resistance to lincosamides was found, which has been shown to be a common native resistance in L. monocytogenes [33]. Four L. innocua isolates in our study had a genetic resistance to tetracyclines, and two were also resistant to the aminoglycoside streptomycin. In addition, one isolate had a fourth resistance gene determining trimethoprim resistance. The L. innocua isolates with multiple resistance genes in our study all originated from samples from pigs. Tetracycline is one of the most often used antimicrobial agents in pig production in Germany, and aminoglycosides are also commonly used [55]; therefore, subinhibitory levels of these antimicrobials may be expected in the gastrointestinal tract and promote the occurrence of resistances [33]. Consequently, L. innocua isolates can be of concern, as reservoirs of these antimicrobial resistance genes can be transferred to L. monocytogenes, for example, in the gastrointestinal tract.
The MIC values of the L. monocytogenes isolates of this study provide information about the phenotypic situation of resistance against antimicrobial agents. However, our interpretation of these values was limited, as specific clinical breakpoints for L. monocytogenes do not exist for all antimicrobial agents. Therefore, we used existing clinical breakpoints for erythromycin, clindamycin, ciprofloxacin, gentamicin, tetracycline and vancomycin that are applicable to staphylococci, although not necessarily for staphylococci of porcine origin. As a consequence, the classifications obtained with these breakpoints have to be considered with caution. No L. monocytogenes isolate in our study showed a phenotypic resistance against antimicrobial agents commonly used for treatment of human listeriosis, including ampicillin or penicillin alone or in combination with gentamicin, whereas for patients allergic to β-lactams, the combination of trimethoprim and a sulfonamide is recommended. For treatment of listeriosis in pregnant woman, erythromycin is usually used, whereas bacteraemia is usually treated with vancomycin. Other antimicrobial agents, such as rifampicin, tetracycline, chloramphenicol and fluoroquinolones are used to a lesser extent [56]. A small percentage of isolates in our study were classified as intermediate, and no isolate was shown to be resistant. However, Alonso-Hernando et al. [56] reported increasing resistances to the fluoroquinolones enrofloxacin and ciprofloxacin and other antimicrobial agents, such as gentamicin, which is commonly used for treating human listeriosis. These emerging resistances, particularly multidrug resistances, represent a public health concern, as they may result in unsuccessful treatment of human disease cases [33].
5. Conclusions
In this study, we analyzed Listeria spp. isolates in the pork production chain, beginning from tonsils and intestinal content of fattening pigs, through the slaughter hall to the cutting and the processing plant in Germany. We found 14 L. monocytogenes isolates belonging to ten different clonal complexes. The isolates found in floor drains risk cross-contamination of pork products. Closely related isolates were identified in the cutting plant, suggesting contamination along the cutting line. Additionally, we found hypervirulent CC6 isolates of L. monocytogenes, known for causing a high risk of human listeriosis, in pig tonsils, which verifies pigs as a potential entry source into pork products. Closely related L. welshimeri isolates found on a working surface and in a nearby mincer on two different sampling dates indicate a risk of cross-contamination and persistence of Listeria spp. over a longer period. This highlights the importance of proper cleaning and disinfection procedures.
In our study, L. monocytogenes isolates were found to have a low level of antimicrobial resistance, as demonstrated by genotypical analyses and phenotypical antimicrobial resistance tests. No isolate of L. monocytogenes from pigs or the slaughterhouse environment showed resistance to commonly used antimicrobials for treatment of human listeriosis. However, we found non-pathogenic L. innocua had multiple resistance genes, which poses a risk for public health, as bacteria are able to pass on their resistance genes to related and unrelated species through horizontal transfer mechanisms. Therefore, monitoring of the resistance situation of non-pathogenic species is also required.
In conclusion, for the successful control and treatment of human listeriosis, an one health approach is needed, considering the origin of the food contamination and possible causes of resistances.
Acknowledgments
The authors thank the slaughterhouses for their cooperation in sampling and the Institut Pasteur teams for curation and maintenance of BIGSdb-Pasteur databases at http://bigsdb.pasteur.fr/; accessed on 23 January 2022. A big thank you to the whole team of the working group Meat Hygiene, FU Berlin for the laboratory work and help in sampling. Furthermore, many thanks to Jan-Robin Redel at the German Federal Institute for Risk Assessment for his technical assistance and Yves van der Stede from EFSA for his critical comments on the draft article. The publication of this article was funded by Freie Universität Berlin.
Author Contributions
Conceptualization, D.M., V.O. and S.L. (Susann Langforth); methodology, V.O., S.L. (Susann Langforth), S.S., A.T.F. and S.L. (Stefanie Lüth); validation, B.F. and S.L. (Stefanie Lüth); formal analysis, V.O., D.M., S.L. (Susann Langforth), B.F. and S.L. (Stefanie Lüth); investigation, V.O., J.D., A.T.F., S.L. (Stefanie Lüth); resources, D.M., S.S. and B.F.; data curation, V.O., S.L. (Stefanie Lüth), A.T.F. and B.F.; writing—original draft preparation, V.O.; writing—review and editing, all authors; visualization, S.L. (Stefanie Lüth), V.O., S.S. and A.T.F.; supervision, D.M. and S.L. (Susann Langforth); project administration, D.M., S.L. (Susann Langforth) and V.O.; funding acquisition, D.M. and V.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the QS Qualität und Sicherheit GmbH science fund, Freie Universität Berlin Contract Number: 2018000308. We acknowledge support by the Open Access Publication Fund of the Freie Universität Berlin.
Institutional Review Board Statement
Not applicable as no interventions or handling of live animals were conducted in this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw NGS data can be found for downloading at: https://www.ncbi.nlm.nih.gov/sra/PRJNA797842; accessed on 23 January 2022 (BioProject accession number PRJNA797842).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. This work is presenting the author’s own views and does not represent an EFSA position.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buchanan R.L., Gorris L., Hayman M.M., Jackson T.C., Whiting R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control. 2017;75:1–13. doi: 10.1016/j.foodcont.2016.12.016. [DOI] [Google Scholar]
- 2.Leclercq A., Moura A., Vales G., Tessaud-Rita N., Aguilhon C., Lecuit M. Listeria thailandensis sp. nov. Int. J. Syst. Evol. Microbiol. 2019;69:74–81. doi: 10.1099/ijsem.0.003097. [DOI] [PubMed] [Google Scholar]
- 3.Orsi R.H., den Bakker H.C., Wiedmann M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011;301:79–96. doi: 10.1016/j.ijmm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Perrin M., Bemer M., Delamare C. Fatal Case of Listeria innocua Bacteremia. J. Clin. Microbiol. 2003;41:5308–5309. doi: 10.1128/JCM.41.11.5308-5309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favaro M., Sarmati L., Sancesario G., Fontana C. First case of Listeria innocua meningitis in a patient on steroids and eternecept. JMM Case Rep. 2014;1:e003103. doi: 10.1099/jmmcr.0.003103. [DOI] [Google Scholar]
- 6.Rocourt J., Hof H., Schrettenbrunner A., Malinverni R., Bille J. Méningite purulente aiguë à Listeria seeligeri chez un adulte immunocompétent. Schweiz. Med. Wochenschr. 1986;116:248–251. (In French) [PubMed] [Google Scholar]
- 7.Liu D., Lawrence M.L., Wiedmann M., Gorski L., Mandrell R.E., Ainsworth A.J., Austin F.W. Listeria monocytogenes Subgroups IIIA, IIIB, and IIIC Delineate Genetically Distinct Populations with Varied Pathogenic Potential. J. Clin. Microbiol. 2006;44:4229–4233. doi: 10.1128/JCM.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward T.J., Ducey T., Usgaard T., Dunn K., Bielawski J. Multilocus Genotyping Assays for Single Nucleotide Polymorphism-Based Subtyping of Listeria monocytogenes Isolates. Appl. Environ. Microbiol. 2008;74:7629–7642. doi: 10.1128/AEM.01127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen O.F., Skouboe P., Dons L., Rossen L., Olsen J.E. Listeria monocytogenes exists in at least three evolutionary lines: evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology. 1995;141:2053–2061. doi: 10.1099/13500872-141-9-2053. [DOI] [PubMed] [Google Scholar]
- 10.Roberts A., Nightingale K., Jeffers G., Fortes E., Kongo J.M., Wiedmann M. Genetic and phenotypic characterization of Listeria monocytogenes lineage III. Microbiology. 2006;152:685–693. doi: 10.1099/mic.0.28503-0. [DOI] [PubMed] [Google Scholar]
- 11.Allerberger F., Huhulescu S. Pregnancy related listeriosis: treatment and control. Expert Rev. Anti-Infec. Ther. 2014;13:395–403. doi: 10.1586/14787210.2015.1003809. [DOI] [PubMed] [Google Scholar]
- 12.Ragon M., Wirth T., Hollandt F., Lavenir R., Lecuit M., Le Monnier A., Brisse S. A New Perspective on Listeria monocytogenes Evolution. PLOS Pathog. 2008;4:e1000146. doi: 10.1371/journal.ppat.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salcedo C., Arreaza L., Alcalá B., de la Fuente L., Vázquez J.A., Kazor C.E., Mitchell P.M., Lee A.M., Stokes L.N., Loesche W.J., et al. Development of a Multilocus Sequence Typing Method for Analysis of Listeria monocytogenes Clones. J. Clin. Microbiol. 2003;41:558–563. doi: 10.1128/JCM.41.2.757-762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chenal-Francisque V., Lopez J., Cantinelli T., Caro V., Tran C., Leclercq A., Lecuit M., Brisse S. Worldwide Distribution of Major Clones of Listeria monocytogenes. Emerg. Infect. Dis. 2011;17:1110–1112. doi: 10.3201/eid1706.101778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iida T., Kanzaki M., Nakama A., Kokubo Y., Maruyama T., Kaneuchi C. Detection of Listeria monocytogenes in Humans, Animals and Foods. J. Veter- Med Sci. 1998;60:1341–1343. doi: 10.1292/jvms.60.1341. [DOI] [PubMed] [Google Scholar]
- 16.Kanuganti S.R., Wesley I.V., Reddy P.G., Mckean J., Hurd H.S. Detection of Listeria monocytogenes in Pigs and Pork. J. Food Prot. 2002;65:1470–1474. doi: 10.4315/0362-028X-65.9.1470. [DOI] [PubMed] [Google Scholar]
- 17.Hellström S., Laukkanen-Ninios R., Siekkinen K.-M., Ranta J., Maijala R., Korkeala H. Listeria monocytogenes Contamination in Pork Can Originate from Farms. J. Food Prot. 2010;73:641–648. doi: 10.4315/0362-028X-73.4.641. [DOI] [PubMed] [Google Scholar]
- 18.Oswaldi V., Dzierzon J., Thieme S., Merle R., Meemken D. Slaughter pigs as carrier of Listeria monocytogenes in Germany. J. Consum. Prot. Food Saf. 2021;16:109–115. doi: 10.1007/s00003-021-01322-4. [DOI] [Google Scholar]
- 19.Figueroa-López A.M., Maldonado-Mendoza I., López-Cervantes J., Verdugo-Fuentes A.A., Ruiz-Vega D.A., Cantú-Soto E.U. Prevalence and characterization of Listeria monocytogenes isolated from pork meat and on inert surfaces. Braz. J. Microbiol. 2019;50:817–824. doi: 10.1007/s42770-019-00073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Ji Q., Li S., Liu M. Prevalence and Genetic Diversity of Listeria monocytogenes Isolated from Retail Pork in Wuhan, China. Front. Microbiol. 2021;12:459. doi: 10.3389/fmicb.2021.620482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duranti A., Sabbatucci M., Blasi G., Acciari V.A., Ancora M., Bella A., Busani L., Centorame P., Cammà C., Conti F., et al. A severe outbreak of listeriosis in central Italy with a rare pulsotype associated with processed pork products. J. Med Microbiol. 2018;67:1351–1360. doi: 10.1099/jmm.0.000785. [DOI] [PubMed] [Google Scholar]
- 22.Giovannacci I., Ragimbeau C., Queguiner S., Salvat G., Vendeuvre J.-L., Carlier V., Ermel G. Listeria monocytogenes in pork slaughtering and cutting plants: use of RAPD, PFGE and PCR–REA for tracing and molecular epidemiology. Int. J. Food Microbiol. 1999;53:127–140. doi: 10.1016/S0168-1605(99)00141-5. [DOI] [PubMed] [Google Scholar]
- 23.Boerlin P., Piffaretti J.C. Typing of human, animal, food, and environmental isolates of Listeria monocytogenes by multilocus enzyme electrophoresis. Appl. Environ. Microbiol. 1991;57:1624–1629. doi: 10.1128/aem.57.6.1624-1629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Félix B., Feurer C., Maillet A., Guillier L., Boscher E., Kerouanton A., Denis M., Roussel S. Population Genetic Structure of Listeria monocytogenes Strains Isolated from the Pig and Pork Production Chain in France. Front. Microbiol. 2018;9:684. doi: 10.3389/fmicb.2018.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demaître N., Rasschaert G., De Zutter L., Geeraerd A., De Reu K. Genetic Listeria monocytogenes Types in the Pork Processing Plant Environment: from Occasional Introduction to Plausible Persistence in Harborage Sites. Pathogens. 2021;10:717. doi: 10.3390/pathogens10060717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deurenberg R.H., Bathoorn E., Chlebowicz M.A., Couto N., Ferdous M., García-Cobos S., Kooistra-Smid A.M., Raangs E.C., Rosema S., Veloo A.C., et al. Application of next generation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2016;243:16–24. doi: 10.1016/j.jbiotec.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Maury M.M., Tsai Y.-H., Charlier C., Touchon M., Chenal-Francisque V., Leclercq A., Criscuolo A., Gaultier C., Roussel S., Brisabois S.R.A., et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016;48:308–313. doi: 10.1038/ng.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsi R.H., Wiedmann M. Characteristics and distribution of Listeria spp., including Listeria species newly described since 2009. Appl. Microbiol. Biotechnol. 2016;100:5273–5287. doi: 10.1007/s00253-016-7552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchrieser C., Rusniok C., Kunst F., Cossart P., Glaser P. The Listeria Consortium Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: clues for evolution and pathogenicity. FEMS Immunol. Med. Microbiol. 2003;35:207–213. doi: 10.1016/S0928-8244(02)00448-0. [DOI] [PubMed] [Google Scholar]
- 30.Teuber M. Spread of antibiotic resistance with food-borne pathogens. Exp. 1999;56:755–763. doi: 10.1007/s000180050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troxler R., von Graevenitz A., Funke G., Wiedemann B., Stock I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin. Microbiol. Infect. 2000;6:525–535. doi: 10.1046/j.1469-0691.2000.00168.x. [DOI] [PubMed] [Google Scholar]
- 32.Poyart C., Carlier C., Trieu-Cuot P., Courvalin P., Courtieu A.-L. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet. 1990;335:1422–1426. doi: 10.1016/0140-6736(90)91447-i. [DOI] [PubMed] [Google Scholar]
- 33.Olaimat A.N., Al-Holy M.A., Shahbaz H.M., Al-Nabulsi A.A., Abu Ghoush M.H., Osaili T.M., Ayyash M.M., Holley R.A. Emergence of Antibiotic Resistance in Listeria monocytogenes Isolated from Food Products: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018;17:1277–1292. doi: 10.1111/1541-4337.12387. [DOI] [PubMed] [Google Scholar]
- 34.Deneke C., Brendebach H., Uelze L., Borowiak M., Malorny B., Tausch S. Species-Specific Quality Control, Assembly and Contamination Detection in Microbial Isolate Sequences with AQUAMIS. Genes. 2021;12:644. doi: 10.3390/genes12050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letunic I., Bork P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CLSI . Methods for Antimicrobial Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria Isolated from Animals, VET06. 1st ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2017. [Google Scholar]
- 37.CLSI . Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, M45. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. [Google Scholar]
- 38.CLSI . Performance Standards for Antimicrobial Susceptibility Testing, M100. 31st ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CLSI . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, VET01S. 5th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. [Google Scholar]
- 40.Chesneau O., Ligeret H., Hosan-Aghaie N., Morvan A., Dassa E. Molecular Analysis of Resistance to Streptogramin a Compounds Conferred by the Vga Proteins of Staphylococci. Antimicrob. Agents Chemother. 2005;49:973–980. doi: 10.1128/AAC.49.3.973-980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowe-McAuliffe C., Murina V., Turnbull K.J., Kasari M., Mohamad M., Polte C., Takada H., Vaitkevicius K., Johansson J., Ignatova Z., et al. Structural basis of ABCF-mediated resistance to pleuromutilin, lincosamide, and streptogramin A antibiotics in Gram-positive pathogens. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-021-23753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berrang M.E., Frank J.F. Generation of Airborne Listeria innocua from Model Floor Drains. J. Food Prot. 2012;75:1328–1331. doi: 10.4315/0362-028X.JFP-12-021. [DOI] [PubMed] [Google Scholar]
- 43.Maury M.M., Bracq-Dieye H., Huang L., Vales G., Lavina M., Thouvenot P., Disson O., Leclercq A., Brisse S., Lecuit M. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-10380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin B., Perich A., Gómez D., Yangüela J., Rodriguez A., Garriga M., Aymerich T. Diversity and distribution of Listeria monocytogenes in meat processing plants. Food Microbiol. 2014;44:119–127. doi: 10.1016/j.fm.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Kubicová Z., Roussel S., Félix B., Cabanová L. Genomic Diversity of Listeria monocytogenes Isolates from Slovakia (2010 to 2020) Front. Microbiol. 2021;12:3223. doi: 10.3389/fmicb.2021.729050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halbedel S., Prager R., Fuchs S., Trost E., Werner G., Flieger A. Whole-Genome Sequencing of Recent Listeria monocytogenes Isolates from Germany Reveals Population Structure and Disease Clusters. J. Clin. Microbiol. 2018;56 doi: 10.1128/JCM.00119-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meloni D., Piras F., Mureddu A., Fois F., Consolati S.G., Lamon S., Mazzette R. Listeria monocytogenes in Five Sardinian Swine Slaughterhouses: Prevalence, Serotype, and Genotype Characterization. J. Food Prot. 2013;76:1863–1867. doi: 10.4315/0362-028X.JFP-12-505. [DOI] [PubMed] [Google Scholar]
- 48.Lariviére-Gauthier G., Letellier A., Kérouanton A., Bekal S., Quessy S., Fournaise S., Fravalo P. Analysis of Listeria monocytogenes Strain Distribution in a Pork Slaughter and Cutting Plant in the Province of Quebec. J. Food Prot. 2014;77:2121–2128. doi: 10.4315/0362-028X.JFP-14-192. [DOI] [PubMed] [Google Scholar]
- 49.Stoller A., Stevens M.J.A., Stephan R., Guldimann C. Characteristics of Listeria Monocytogenes Strains Persisting in a Meat Processing Facility over a 4-Year Period. Pathogens. 2019;8:32. doi: 10.3390/pathogens8010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zakrzewski A.J., Chajęcka-Wierzchowska W., Zadernowska A., Podlasz P. Virulence Characterization of Listeria monocytogenes, Listeria innocua, and Listeria welshimeri Isolated from Fish and Shrimp Using In Vivo Early Zebrafish Larvae Models and Molecular Study. Pathogens. 2020;9:1028. doi: 10.3390/pathogens9121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lake F.B., van Overbeek L.S., Baars J.J., Koomen J., Abee T., Besten H.M.D. Genomic characteristics of Listeria monocytogenes isolated during mushroom (Agaricus bisporus) production and processing. Int. J. Food Microbiol. 2021;360:109438. doi: 10.1016/j.ijfoodmicro.2021.109438. [DOI] [PubMed] [Google Scholar]
- 52.Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volokhov D.V., Duperrier S., Neverov A.A., George J., Buchrieser C., Hitchins A.D. The Presence of the Internalin Gene in Natural Atypically Hemolytic Listeria innocua Strains Suggests Descent from L. monocytogenes. Appl. Environ. Microbiol. 2007;73:1928–1939. doi: 10.1128/AEM.01796-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno L.Z., Paixão R., Gobbi D.D., Raimundo D.C., Ferreira T.P., Hofer E., Matte M.H., Moreno A.M. Characterization of atypical Listeria innocua isolated from swine slaughterhouses and meat markets. Res. Microbiol. 2012;163:268–271. doi: 10.1016/j.resmic.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 55.QS—Qualitätssicherung 2. Statusbericht zum Antibiotikamonitoring im QS-System. 2019. [(accessed on 14 November 2021)]. Available online: https://www.q-s.de/services/files/mediencenter/publikationen/statusbericht-antibiotika/Statusbericht_QS-Antibiotikamonitoring_2019.pdf. (In German)
- 56.Alonso-Hernando A., Prieto M., García-Fernández C., Alonso-Calleja C., Capita R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control. 2012;23:37–41. doi: 10.1016/j.foodcont.2011.06.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw NGS data can be found for downloading at: https://www.ncbi.nlm.nih.gov/sra/PRJNA797842; accessed on 23 January 2022 (BioProject accession number PRJNA797842).