Abstract
Although combination therapy with antimicrobial agents is often used, no available method explains or predicts the efficacies of these combinations satisfactorily. Since the efficacies of antimicrobial agents can be described by pharmacodynamic indices (PDIs), such as area under the concentration-time curve (AUC), peak level, and the time that the concentration is above the MIC (time>MIC), it was hypothesized that the same PDIs would be valid in explaining efficacy during combination therapy. Twenty-four-hour efficacy data (numbers of CFU) for Pseudomonas aeruginosa in a neutropenic mouse thigh model were determined for various combination regimens: ticarcillin-tobramycin (n = 41 different regimens), ceftazidime-netilmicin (n = 60), ciprofloxacin-ceftazidime (n = 59), netilmicin-ciprofloxacin (n = 38) and for each of these agents given singly. Multiple regression analysis was used to determine the importance of various PDIs (time>MIC, time>0.25× the MIC, time>4× the MIC, peak level, AUC, AUC/MIC, and their logarithmically transformed values) during monotherapy and combination therapy. The PDIs that best explained the efficacies of single-agent regimens were time>0.25× the MIC for beta-lactams and log AUC/MIC for ciprofloxacin and the aminoglycosides. For the combination regimens, regression analysis showed that efficacy could best be explained by the combination of the two PDIs that each best explained the response for the respective agents given singly. A regression model for the efficacy of combination therapy was developed by use of a linear combination of the regression models of the PDI with the highest R2 for each agent given singly. The model values for the single-agent therapies were then used in that equation, and the predicted values that were obtained were compared with the experimental values. The responses of the combination regimens could best be predicted by the sum of the responses of the single-agent regimens as functions of their respective PDIs (e.g., time>0.25× the MIC for ticarcillin and log AUC/MIC for tobramycin). The relationship between the predicted response and the observed response for the combination regimens may be useful for determination of the presence of synergism. We conclude that the PDIs for the individual drugs used in this study are class dependent and predictive of outcome not only when the drugs are given as single agents but also when they are given in combination. When given in combination, there appears to be a degree of synergism independent of the dosing regimen applied.
Since the advent of antimicrobial agents, methods that describe and predict the efficacies of the use of these agents in combination have been sought (1, 2, 7, 12, 14, 16, 20, 28). In most attempts the results of in vitro experiments have been applied to predict in vivo efficacy, for instance, by using checkerboards and/or time-kill curves (5, 6, 13, 14, 26). However, these in vitro methods are limited by the fact that they measure effects at static concentrations only, while in vivo concentrations fluctuate over a wide range due to different dosing regimens, absorption rates, and elimination rates. In addition, combination therapy also results in continuous variations in the concentration ratios of the two (or more) agents. Several attempts have recently been made to define some kind of universal predictor of efficacy or method for determination of the efficacy of combination therapy in both in vitro (3, 4, 9, 13, 24) and in vivo models (17, 21, 24).
For antimicrobial agents given singly, it is now recognized that their antibacterial activities are dependent on the dosing regimen (22, 27). For instance, while the efficacies of beta-lactam antibiotics are primarily dependent on the time that the concentration remains above the MIC (time>MIC) and therefore the frequency of dosing, the antibacterial activities of aminoglycosides and quinolones are mainly dependent on the cumulative daily dose of the drug or the area under the concentration-time curve (AUC). We therefore set out to explore whether the same pharmacodynamic indices (PDIs) that explain efficacy during monotherapy would explain efficacy during combination therapy. Two approaches were applied to evaluations of efficacy in a Pseudomonas aeruginosa animal infection model. First, the efficacies of various dosing regimens with combinations were determined and multiple regression analysis was used to determine which PDIs most contributed significantly to the model. Alternatively, the efficacies of agents given singly were described as a function of the PDI most appropriate for each agent in a regression model. The efficacies of the combination regimens were then predicted on the basis of a linear combination of these regression models for the single agents and predictions were compared with the outcome of combination therapy.
(Part of these results were presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy of the American Society for Microbiology, San Diego, Calif., 24 to 27 September 1998.)
MATERIALS AND METHODS
Strain and animal model.
P. aeruginosa ATCC 27853 was used in all experiments. The MICs were 8 mg/liter for ticarcillin, 2 mg/liter for ceftazidime, 0.5 mg/liter for tobramycin, 1 mg/liter for netilmicin, and 0.5 mg/liter for ciprofloxacin. Efficacy experiments with a neutropenic thigh model were performed as described earlier (8). Briefly, neutropenic mice were inoculated with approximately 106 CFU of P. aeruginosa in the exponential phase of growth. Two hours (time zero) after infection, treatment was started with antibiotics, either alone or in combination; control animals received no treatment. Animals were killed at 0 h (controls) or after 24 h of therapy. The thighs were removed and homogenized, and aliquots of serial 10-fold dilutions were plated. Efficacy was then determined as the difference between the number of CFU expressed as the change in the log10 CFU (ΔCFU) at time zero and 24 h. Thus, a negative ΔCFU value indicates a decrease in the number of bacteria after the start of therapy. All regimens were performed with two mice, while the efficacy obtained for one mouse was the mean result for two infected thighs.
Dosing regimens.
For ceftazidime, netilmicin, and ciprofloxacin, the single-agent regimens were designed to cover the ranges of time>MIC, peak level, and AUC with minimum interdependence. This would allow optimal discrimination of the importance of the indices in the regression analyses. For the combination regimens the same approach was taken, but in addition, the combinations were chosen to minimize interdependence between the indices (six in total, three for each drug) of the two drugs in the combination. Thus, dosing intervals were 1, 4, 12, and 24 h, while the total daily doses varied from low to high for each drug.
The single-agent regimens used were 25 mg/kg of body weight every 1 h (q1h) to 2,400 mg/kg every 12 h (q12h) for ticarcillin (n = 14 different regimens), 3.12 mg/kg q1h to 600 mg/kg q12h for ceftazidime (n = 12), 1 mg/kg q1h to 48 mg/kg every 24 h (q24h) for tobramycin (n = 14), 0.83 mg/kg q1h to 160 mg/kg q24h for netilmicin (n = 12), and 1.04 mg/kg q1h to 200 mg/kg q24h for ciprofloxacin (n = 15). These regimens were combined, yielding various combination regimens: ceftazidime-netilmicin (n = 60 different combination regimens), ceftazidime-ciprofloxacin (n = 59), netilmicin-ciprofloxacin (n = 38), and ticarcillin-tobramycin (n = 41).
Pharmacokinetics in mice.
The pharmacokinetics of the antimicrobial agents were determined in mice by taking serum samples after the administration of various doses as described previously (8, 26). A one-compartment open model with an absorption phase (Kinfit; Mediware, Groningen, The Netherlands) was fit to the data. The values obtained were used to simulate the concentrations over time for the dosing regimens used and then to calculate the various PDIs.
Analysis.
The following indices were determined for each regimen: time>MIC, time>0.25× the MIC, time>4× the MIC, peak, level, AUC/MIC, dosing frequency, dose, total dose, and their logarithmically transformed values. Multiple regression analysis was used to determine the R2 values and regression coefficients with respect to efficacy by using the SAS program (23). Forward and backward selection procedures were used to determine the two (or more) indices that best explained the efficacies of the combination regimens or the interaction between indices. The two indices that best explained efficacy were used to obtain a three-dimensional plot to describe the respective relationships. A three-dimensional surface plot was fit by using a fifth-order polynomial and a low stiffness (Statistica; Statsoft, Tulsa, Okla.) to obtain a qualitative impression of the linearity of the relationship. Prediction of the efficacies of combination regimens was done by linear combination of the various regression models for single-agent therapy (11). The predicted values were then compared with the measured values.
RESULTS
Single-agent regimens.
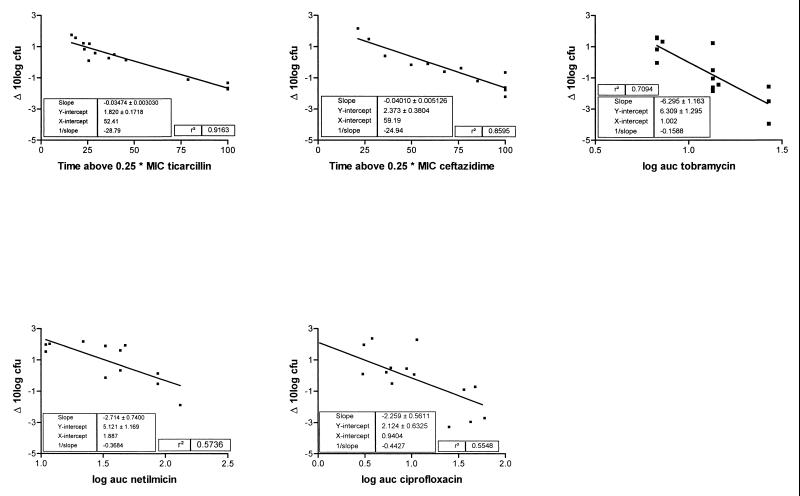
Figure 1 shows the plots of ΔCFU as a function of the PDIs that best explained the efficacy of the antibiotic, as well as the coefficients for the models after linear regression. The PDIs that best explained the efficacy of each drug were obtained by multiple regression analysis of the single-agent data and were time>0.25× the MIC for ticarcillin and ceftazidime and log AUC for tobramycin, netilmicin, and ciprofloxacin. It must be noted that for ceftazidime, time>MIC was as explanatory as time>0.25× the MIC.
FIG. 1.
Scatter plots and linear regressions for five antibiotics and their PDIs that best explain efficacy.
Combination therapy.
Multiple regression analysis of the efficacies of the combination regimens showed that for each combination, two PDIs could explain most of the variation. These were, by and large, the same PDIs that best explained the variations for each of the agents given singly (Table 1). Thus, for the combination ticarcillin and tobramycin, R2 was 0.517 if time>0.25× the MIC for ticarcillin was entered in the model and increased to 0.850 if the log AUC of tobramycin was entered next. Although in some cases additional PDIs could be entered into the model with significance (F test), no clear pattern was distinguishable and their additional value was limited. Interaction terms were not significant.
TABLE 1.
Results of multiple regression analysis of various dosing regimens
| Combination regimen | Variable entered in model | Partial R2 | Model R2 |
|---|---|---|---|
| Ticarcillin-tobramycin (n = 41) | Time>0.25× the MIC of ticarcillin | 0.517 | 0.517 |
| Log AUC for tobramycin | 0.333 | 0.850 | |
| Ceftazidime-netilmicin (n = 60) | Time>0.25× the MIC of ceftazidime | 0.522 | 0.522 |
| Log AUC for netilmicin | 0.193 | 0.715 | |
| Ceftazidime-ciprofloxacin (n = 59) | Log AUC for ciprofloxacin | 0.614 | 0.614 |
| Time>0.25× the MIC of ceftazidime | 0.157 | 0.771 | |
| Ciprofloxacin-netilmicin (n = 38) | AUC for ciprofloxacin | 0.525 (0.598)a | 0.525 (0.598) |
| AUC for netilmicin | 0.172 (0.217) | 0.697 (0.815) |
The values in parentheses are the results of regression analysis without the outlying datum point.
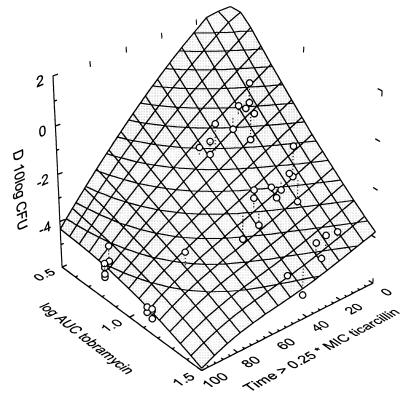
Three-dimensional plot analysis confirmed these results. An example of such a three-dimensional plot is shown in Fig. 2 for tobramycin and ticarcillin. The datum points are concentrated in a nearly flat surface plane as a function of the two PDIs that each explains the efficacy of the single agent. The flatness of the plane indicates that the efficacy of the combination is dependent on a linear combination of the efficacies of the single agents, as explained by their respective predictive PDIs, in this case, time>0.25× the MIC for ticarcillin and log AUC for tobramycin. It also indicates that there is little interaction between the PDIs. In the case of ciprofloxacin and netilmicin one datum point was clearly outside the scatter region. This was the result of a regimen in which both agents were administered q24h, thus, only once each. Because the results of an analysis without this point yielded a superior final model, the respective values of the model without this point are given in parentheses in Table 1.
FIG. 2.
Three-dimensional plot of efficacy (in ΔCFU [indicated as D 10log CFU in the figure]) as a function of time>0.25× the MIC of ticarcillin and log AUC of tobramycin.
Prediction of efficacy.
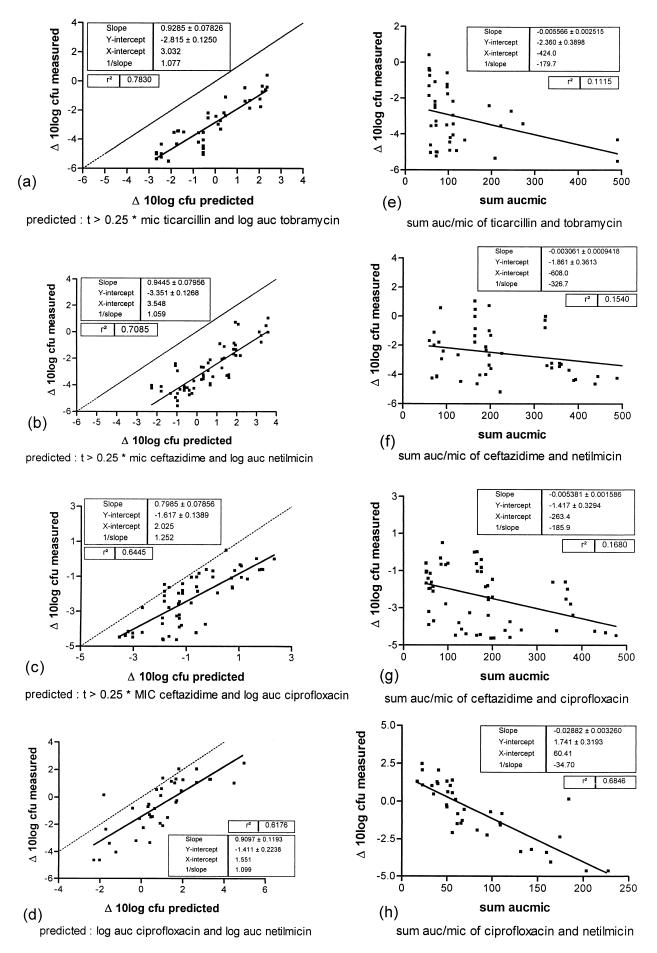
From the results described above, it was hypothesized that the efficacies of the combinations could be predicted or described by a linear combination of the efficacy functions of the single-agent therapies, in which the independent variable and the parameter values were those of the PDI that best describes the efficacy of each drug. Thus, the parameter values shown in Fig. 1 for each drug were used to predict the efficacy of the combination. Figures 3a to d show the plots of the predicted efficacy of each combination regimen versus the actually observed values. Alternatively, for each combination, a similar prediction was made on the basis of AUC/MIC which has been advocated by some investigators (24) as a sort of universal predictor for combination therapy. It can be observed that for the beta-lactam combinations, time>0.25× the MIC clearly explains efficacy during combination therapy (as is the case for monotherapy) but AUC/MIC does not. Use of the latter value results in correlations which are hardly, if at all, significant.
FIG. 3.
Observed versus predicted values of various dosing regimens for four antimicrobial combinations. (a to d) Predicted values based on the PDI that best explains the efficacy of each antibiotic. (e to h) For the same combinations in panels a to d, respectively, predicted values based on the sum of values of AUC/MIC (sum aucmic). t, time.
DISCUSSION
In this report we show that the PDI of a certain antimicrobial agent which is predictive of efficacy in an in vivo model of infection is also predictive of efficacy of combination therapy. Multiple regression analysis of the various PDIs with respect to the efficacies of the various combination regimens showed that two PDIs explained most of the variation for each combination. These were the same two indices that each characterized the efficacy of single-agent therapy. Alternatively, the results of combination therapy were adequately predicted by the efficacies of each of the single-agent therapies in that there was a good correlation between the predicted values and the observed values.
In the regression analyses of the combination regimens containing a beta-lactam, the time>0.25× the MIC was consistently more predictive than time>MIC. This could have been because the MIC of the beta-lactam for the P. aeruginosa strain is lower in the presence of another antibiotic. By applying the recently described method of White et al. (28) with the E test, it was found for several strains that the MIC of ceftazidime was indeed approximately 0.25× the MIC in the presence of tobramycin (9) and that this phenomenon could explain the results obtained with various combination regimens in an in vitro model. For the strain used in this study, the E test produced similar results (data not shown). Thus, the presence of another antibiotic in addition to the beta-lactam infers some degree of synergism, resulting in a lower MIC. However it must be noted that while time>MIC and time>0.25× the MIC were equally predictive of efficacy of the single-agent regimen for ceftazidime, for ticarcillin time>0.25× the MIC best explained the variation during both combination therapy and monotherapy.
Regression analysis of the combination netilmicin and ciprofloxacin yielded somewhat different results. The single most explanatory index for that combination was the sum of the values of AUC/MIC. This is not surprising, since AUC has been shown to be an important explanatory variable for both agents (27). Without this index, forward selection of the various variables resulted in the AUCs for both agents being selected in the model.
From Fig. 3d and h it can be observed that one datum point is clearly an outlier. This point is the efficacy of a once-daily dose of both antibiotics, and the observed efficacy is less than would be expected from the combination. Analysis without this datum point yields a significantly better model (Table 1). The most likely explanation is that q24h regimens result in concentrations which are too low for too long a time due to rapid elimination of the antibiotics in mice, and regrowth of bacteria occurs after a certain time (18). The use of agents in combination in q24h regimens has little additional effect since regrowth will still occur. In pharmacodynamic terms, this means that for dosing regimens with long intervals relative to the elimination half-life it not only is the total dose or the AUC which is predictive of the outcome but is also the time>MIC (or better, the time<MIC). This is confirmed in other analyses, in which it has been shown for aminoglycosides that time>MIC is an additional important factor for prediction of the efficacies of q24 regimens (19, 27).
If the results of combination therapy were solely a linear combination of each of the expected efficacies of the single agents, it would be expected that the intercepts of the regression lines would intersect the y (and x) axis at zero. However, if Fig. 3a to d are closely observed, it appears that none of the intercepts intersect the y axis at zero but intersect it at a negative ΔCFU significantly different from zero. This is an indication that the agents have some synergistic action with each other, as was already indicated by the observation that time>0.25× the MIC was more predictive of efficacy than time>MIC for beta-lactams in the combination regimens. Definitions of synergism between antimicrobial agents which both have killing effects has always been problematic (2, 7, 10, 15, 20, 25). In vitro, a fractional inhibitory concentration index greater than 2 for checkerboard titration studies and more than a 2-log killing of the most active agent in time-kill curve studies are being used for that purpose (25), but both methods have the limitation that synergism is determined with static drug concentrations and the methods are difficult to apply to agents with high killing rates. The method used in the study described in this report offers another way to define synergism between two antimicrobial agents which have different (or similar) modes of action but in which the efficacy of the combination can be predicted by the response of each of the agents given singly. In this model two antimicrobial agents show synergy if the intercept of the regression line of the predicted and observed responses is significantly different from zero. Although we are aware that this method is perhaps not an ideal solution for all combinations of antibiotics, this definition has the advantage that synergism can be expressed quantitatively; it has the additional capability of taking the combined effects of declining concentrations of the two drugs with different half-lives into account and is based on the in vivo outcome.
The major disadvantage of this method is the labor involved in the study, and at present, there is no easy laboratory test to determine whether synergism against clinical strains is present. For that purpose, more strains and animal models should be analyzed. The method does, however, provide insight into the general presence of synergy between certain combinations of antibiotics.
Several investigators have tried to describe a universal approach of synergism. An excellent review can be found in the work of Greco et al. (12). The two frameworks most widely used are those of Bliss and the one originally described by Loewe and later by Berenbaum and Greco et al. (12). Basically, by both approaches the effect is described as a function of the concentrations of the two drugs, either as an interaction model or as additivity with an interaction term. The interpretation of the fractional inhibitory concentration index is based on the latter model. Incorporation of our results in these frameworks is difficult, because by our approach the final effect is described by the addition of two effects which are described by two different variables instead of one variable. Moreover, the two frameworks mentioned above are based on a concentration-effect relationship, while by our approach the time factor also plays a role. Intuitively, the approach taken resembles the Loewe additivity model (12), in that we look at a term that describes a difference between predicted values (obtained by the addition of two values) and actually measured values. The difference, then, would be the interaction term or synergy. The significant difference that was found, however, was not dependent on the value of the underlying variables but was dependent on its mere presence, as indicated by the fact that the intercept of the predicted versus measured relationship was significantly different from zero (Fig. 3a to d) and the fact that interaction terms did not significantly contribute to the model. Another way to look at it is to see Fig. 3 as a two-dimensional projection of the three-dimensional graph exemplified in Fig. 2 and perpendicular to that plane. The fact that this results in a more or less linear relationship indicates the dosing regimen independence of the synergism.
We conclude that the methods and results described in this report provide a tool for determination of the presence of synergism between antimicrobial agents and that dosing regimens with combinations of antimicrobial agents can be optimized in a manner similar to that used for single-agent regimens. The results indicate that the synergy found is primarily dependent on the presence of the second drug, irrespective of the dosing regimen.
REFERENCES
- 1.Andriole V T. Antibiotic synergy in experimental infection with Pseudomonas. II. The effect of carbenicillin, cephalothin, or cephanone combined with tobramycin and gentamicin. J Infect Dis. 1974;129:124–133. doi: 10.1093/infdis/129.2.124. [DOI] [PubMed] [Google Scholar]
- 2.Berenbaum M C. Synergy, additivism, and antagonism in immunosuppression. Clin Exp Immunol. 1977;28:1–18. [PMC free article] [PubMed] [Google Scholar]
- 3.Berenbaum M C. Correlations between methods for measurement of synergy. J Infect Dis. 1980;142:476–480. doi: 10.1093/infdis/142.3.476. [DOI] [PubMed] [Google Scholar]
- 4.Berenbaum M C. What is synergy? Pharmacol Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- 5.Blaser J. Interactions of antimicrobial combinations in vitro: the relativity of synergism. Scand J Infect Dis. 1991;74:71–79. [PubMed] [Google Scholar]
- 6.Bustamante C L, Wharton R C, Wade J C. In vitro activity of ciprofloxacin in combination with ceftazidime, aztreonam, and azlocillin against multiresistant isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990;34:1814–1815. doi: 10.1128/aac.34.9.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalkey L J, Koornhof H J. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus determined by the killing curve method: antibiotic comparisons and synergistic interactions. Antimicrob Agents Chemother. 1985;28:331–342. doi: 10.1128/aac.28.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig W A, Redington J, Ebert S C. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J Antimicrob Chemother. 1991;27(Suppl. C):29–40. doi: 10.1093/jac/27.suppl_c.29. [DOI] [PubMed] [Google Scholar]
- 9.den Hollander J G, Mouton J W, Verbrugh H A. Use of pharmacodynamic parameters to predict efficacy of combination therapy by using fractional inhibitory concentration kinetics. Antimicrob Agents Chemother. 1998;42:744–748. doi: 10.1128/aac.42.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elion G B, Singer S, Hitchings G H. Antagonists of nucleic acid derivates. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1953;208:477–488. [PubMed] [Google Scholar]
- 11.Graphpad Software Inc. Instat 2 program manual. San Diego, Calif: Graphpad Software Inc.; 1990. [Google Scholar]
- 12.Greco W R, Bravo G, Parsons J C. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 13.Hallender H O, Dornbusch K, Gezelius L, Jacobson K, Karisson I. Synergism between aminoglycosides and cephalosporins with antipseudomonal activity: interaction index and killing curve method. Antimicrob Agents Chemother. 1982;22:743–752. doi: 10.1128/aac.22.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyatt J M, Nix D E, Stratton C W, Schentag J J. In vitro pharmacodynamics of piperacillin, piperacillin-tazobactam, and ciprofloxacin alone and in combination against Staphylococcus aureus, Klebsiella pneumoniae, Enterobacter cloacae, and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1711–1716. doi: 10.1128/aac.39.8.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer R D, Liu S. Synergy studies in vitro of the effect of ciprofloxacin and selected β-lactam agents and aminoglycosides upon multidrug-resistant Pseudomonas aeruginosa. Rev Infect Dis. 1989;11(Suppl. 5):1037–1038. doi: 10.1016/0732-8893(88)90017-x. [DOI] [PubMed] [Google Scholar]
- 16.Moellering R C., Jr Antimicrobial synergy—an elusive concept. J Infect Dis. 1979;140:639–641. doi: 10.1093/infdis/140.4.639. [DOI] [PubMed] [Google Scholar]
- 17.Mordenti J J, Quiniliani R, Nightingale C H. Combination antibiotic therapy: comparison of constant infusion and intermittent bolus dosing in an experimental animal model. J Antimicrob Chemother. 1985;15(Suppl. A):313–321. doi: 10.1093/jac/15.suppl_a.313. [DOI] [PubMed] [Google Scholar]
- 18.Mouton J W, Vinks A A T M M, Punt N, Craig W A. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Pharmacokinetic modelling of bacterial killing in vivo, abstr. A22; p. 5. [Google Scholar]
- 19.Mouton J W, Leggett J, Craig W A. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Dynamics of pharmacodynamic parameters over time, abstr. A23; p. 5. [Google Scholar]
- 20.Norden C W, Wenzel H, Keleti E. Comparison of techniques for measurement of in vitro antibiotic synergism. J Infect Dis. 1979;140:629–633. doi: 10.1093/infdis/140.4.629. [DOI] [PubMed] [Google Scholar]
- 21.Renneberg J. Definitions of antimicrobial interactions in animal infection models. J Antimicrob Chemother. 1993;31(Suppl. D):167–175. doi: 10.1093/jac/31.suppl_d.167. [DOI] [PubMed] [Google Scholar]
- 22.Roosendaal R, Bakker-Woudenberg I A J M, van den Berghe-Raffe M A, et al. Impact of the dose schedule on the efficacy of ceftazidime, gentamicin and ciprofloxacin in Klebsiella pneumoniae pneumonia and septicaemia in leukopenic rats. Eur J Clin Microbiol Infect Dis. 1989;8:878–887. doi: 10.1007/BF01963774. [DOI] [PubMed] [Google Scholar]
- 23.SAS Institute Inc. SAS user’s guide. Cary, N.C: SAS Institute Inc.; 1990. [Google Scholar]
- 24.Schentag, J. J., L. C. Strenkoski-Nix, D. E. Nix, and A. Forrest. Pharmacodynamic interactions of antibiotics alone and in combination. Clin. Infect. Dis. 27:40–46. [DOI] [PubMed]
- 25.Stratton C W, Cooksey R C. Susceptibility tests: special tests. In: Balows A, Hausler W J Jr, Hermann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 1153–1166. [Google Scholar]
- 26.Stratton C W, Franke J J, Weeks L S, Manion F A. Comparison of the bactericidal activity of ciprofloxacin alone and in combination with selected antipseudomonal β-lactam agents against clinical isolates of Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 1998;11:41–52. doi: 10.1016/0732-8893(88)90072-7. [DOI] [PubMed] [Google Scholar]
- 27.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–874. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 28.White R L, Burgess D S, Manduru M, Bosso J A. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother. 1996;40:1914–1918. doi: 10.1128/aac.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]