Abstract
The present review aims at analyzing the current evidence regarding probiotic administration for non-alcoholic fatty liver disease (NAFLD) management. Additionally, the involved mechanisms of action modulated by probiotic administration, as well as the eventual limitations of this therapeutic approach and potential alternatives, are discussed. Preclinical studies have demonstrated that the administration of single-strain probiotics and probiotic mixtures effectively prevents diet-induced NAFLD. In both cases, the magnitude of the described effects, as well as the involved mechanisms of action, are comparable, including reduced liver lipid accumulation (due to lipogenesis downregulation and fatty acid oxidation upregulation), recovery of gut microbiota composition and enhanced intestinal integrity. Similar results have also been reported in clinical trials, where the administration of probiotics proved to be effective in the treatment of NAFLD in patients featuring this liver condition. In this case, information regarding the mechanisms of action underlying probiotics-mediated hepatoprotective effects is scarcer (mainly due to the difficulty of liver sample collection). Since probiotics administration represents an increased risk of infection in vulnerable subjects, much attention has been paid to parabiotics and postbiotics, which seem to be effective in the management of several metabolic diseases, and thus represent a suitable alternative to probiotic usage.
Keywords: probiotics, microbiota, liver steatosis, NAFLD, inflammation, parabiotics, postbiotics
1. Introduction
The prevalence of chronic metabolic diseases has been on the rise in the last decades, becoming a major health problem worldwide. Despite the amount of attention that has been paid to obesity, millions of deaths (up to 2 million by the year 2010) have also been attributed to liver diseases such as cirrhosis and hepatocellular carcinoma [1]. In this line, non-alcoholic fatty liver disease (NAFLD), also known as metabolic (dysfunction)-associated fatty liver disease (MAFLD), has become the most prevalent hepatic alteration in the last years [2]. Indeed, it is estimated that the prevalence of NAFLD is 20–30% in adults, and that this prevalence may well be higher in industrialized countries [3]. This hepatic condition includes relatively benign and reversible steatosis, characterized by excessive triglyceride (TG) accumulation in the liver, along with the more harmful stage known as non-alcoholic steatohepatitis (NASH), that can progress to cirrhosis or even hepatocellular carcinoma [4]. In this regard, besides the aforementioned excessive hepatic lipid accumulation leading to simple steatosis, further events such as inflammation, oxidative stress and fibrosis are also involved in the progression of the disease [5,6].
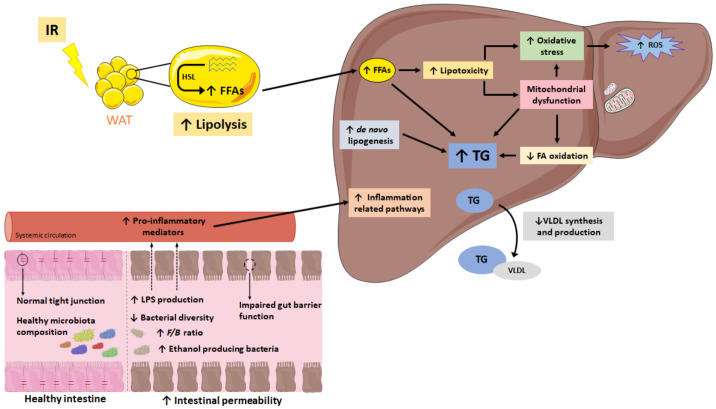
Due to the array of processes that have been identified to participate in NAFLD development, the once widely assumed “two-hit theory” has been replaced by the “multiple-hit theory” [6]. According to the latter, white adipose tissue insulin resistance plays a major role, impairing lipolysis and triggering inflammation. All these impairments result in a greater release of free fatty acids to the blood stream, which end up in the liver, thus contributing to excessive hepatic lipid accumulation. Additionally, this increased hepatic fatty acid deposition also results in lipotoxicity and subsequent mitochondrial dysfunction, which in turn increases reactive oxygen species (ROS) production and oxidative stress, and activates endoplasmic reticulum stress [6]. Moreover, gut microbiota alterations have also been described as contributing factors to NAFLD development. Impaired gut microbiota composition results in a greater production of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF α) and interleukin 6 (IL-6), as well as microbial products with pro-inflammatory properties, including lipopolysaccharides (LPS) and unmethylated CpG DNA [7]. Moreover, increased intestinal permeability, resulting from altered tight junctions, leads to greater translocation of these pro-inflammatory mediators into circulation, which once reaching the liver, trigger the activation of pro-inflammatory pathways in the organ, thus contributing to the progression of NAFLD in NASH (Figure 1) [8]. As far as the causes leading to NAFLD development are concerned, excessive dietary fat and/or sugar intake (specially fructose) are considered among the main contributors [9,10]. Indeed, this kind of dietary pattern not only promotes excessive hepatic lipid accumulation (due to enhanced de novo lipogenesis and impaired mitochondrial fatty-acid oxidation), but it also induces liver inflammation, oxidative stress and mitochondrial dysfunction, all of which leads to the progression of hepatic damage [9,10]. Nevertheless, other factors such as food processing or polyphenol content can also have a role.
Figure 1.
Simplified schematic representation of the events considered in the multiple-hit theory leading to NAFLD development. F/B: Firmicutes/Bacteroidetes ratio; FA: fatty acid; FFA: free fatty acid; HSL: hormone-sensitive lipase; IR: insulin resistance; ROS: reactive oxygen species; TG: triglyceride; VLDL: very-low-density lipoprotein; WAT: white adipose tissue. ↑: increase; ↓: decrease.
The high prevalence of NAFLD, as well as its potential implications in health, highlights the necessity for effective approaches in the prevention and treatment of this liver condition. However, since no specific treatment has been designed so far, conventional interventions based on dietary treatment and enhanced physical activity leading to body weight reduction are still widely prescribed [11,12]. One of the main reasons for using such an approach for NAFLD management relies on the higher prevalence of this hepatic condition in obese subjects. Indeed, according to recent data, it is estimated, that NAFLD is present in up to 50–90% of subjects featuring obesity [13]. Notwithstanding that the effectiveness of these approaches has been demonstrated, a common low adherence requires further therapeutic tools that may be prescribed as complementary or alternative treatments. In this scenario, the administration of probiotics for NAFLD has gained much attention, especially due to the involvement of gut microbiota alterations in the development of this liver alteration. By definition, probiotics are viable microorganisms that exert health benefits when consumed in sufficient amounts [14]. Thus, probiotic consumption may help normalize gut microbiota composition in patients with NAFLD, which in turn could result in improved gut barrier function and decreased pro-inflammatory cytokine production and release. Additionally, the recovery of gut microbiota eubiosis will also be helpful in restoring the production and levels of gut microbiota-derived metabolites with described health benefits, such as short-chain fatty acids (SCFA) [15].
In this context, the aim of this narrative review is to summarize the available evidence regarding probiotic usefulness in NAFLD prevention. In addition, the mechanisms of action described so far underlying the potential hepatoprotective effects of probiotics are also discussed. For this purpose, the first part of this manuscript is focused on the results obtained in preclinical studies (rodent models), whereas the second part summarizes the current evidence obtained from clinical trials. Additionally, limitations related to probiotic intake, as well as potential alternatives, are also discussed. With regard to the criteria followed to include or exclude articles in this narrative review, those using probiotics alone (single strain or mixtures) for NAFLD (not NASH) management, and studying variables such as liver fat content, liver histologic analysis (liver lipid content and/or inflammation) and transaminase levels, were selected. In the contrary, articles where none of these variables were analyzed or probiotics were administered along with other ingredients (unsaturated fatty acids or polysaccharides, for instance) were excluded. This article-selection task was carried out by two different persons.
2. Effects of Probiotic Administration (Single Strain and Mixtures) on NAFLD Prevention: Evidence from Preclinical Studies
When analyzing the potential usefulness of a molecule/compound in NAFLD prevention, both the molecule/compound and the stressor leading to the development of this hepatic condition are administered together. In preclinical studies, this liver alteration is commonly induced by using unbalanced diets characterized by a high content of fat and/or processed sugar. These feeding conditions not only result in an impaired nutrient intake, but they can also lead to an excessive caloric consumption. Moreover, diets lacking specific nutrients, such as choline-deficient diets, are also an effective approach when generating diet-induced NAFLD.
2.1. Preclinical Studies Using Single Strain Probiotics
Different studies have been carried out using a single-strain probiotic (Table 1). In general, the majority of these studies have addressed the effects of specific probiotic strains in animals challenged by diets leading to NAFLD. In this line, the administration of several probiotic strains (mainly Lactobacillus and Bifidobacterium) has been shown to be effective in reducing liver lipid accumulation under dietary conditions providing 40 to 65% of energy as fat [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Moreover, this effect is also maintained when excessive hepatic lipid accumulation is mediated by dietary conditions providing high sugar intakes (10 to 30% of energy as fructose). In this case, probiotic administration prevented liver lipid accumulation when standard diets were supplemented with fructose [32,33,34,35], as well as when high-fat and fructose intakes occurred concomitantly [33,34]. Furthermore, probiotic-administration-mediated liver fat accumulation prevention was also reported in a study in which NAFLD was induced using a choline-deficient diet [36]. It is worth noting that the aforementioned effects were described in both mice and rats receiving different probiotic doses (from 1 × 107 to 1 × 1010 CFU/day) and during different treatment periods (from 4 to 42 weeks) (Table 1).
Table 1.
Preclinical studies (rodent models) addressing the effects of different single-strain probiotics on diet-induced NAFLD.
| Reference | Animal Model | Experimental Conditions | Probiotic Treatment | Effects on Liver | Mechanisms of Action |
|---|---|---|---|---|---|
| [32] | Female C57BL/J6 mice 6-week-old |
STD diet with 30% fructose in drinking water. |
L. rhamnosus GG—LGG Daily administration Dose: 5.2 × 107 CFU/bw g/d. Diluted in drinking water Treatment length: 8 w. |
↓ Liver fat accumulation ↓ Liver TG content ↓ Serum ALT levels ↓ Liver inflammation |
Down-regulation of lipogenic markers in the liver: ↓ Gene expression of Acc, Fas and Chrebp. Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Gene expression of Il-1β, Il-8R, Tnfα and Il-12. Decreased portal levels of LPS. Up-regulation of markers of intestinal mucosa integrity: ↑ Protein expression of Occludin-1 and Claudin-1. |
| [16] | Male C57BL/6 mice 4-week-old |
HFD (60% energy from fat). |
L. rhamnosus GG—LGG Oral daily administration Dose: 1 × 108 CFU/day Treatment length: 13 w. |
↓ Liver weight ↓ Liver fat accumulation ↓ Liver inflammation |
Down-regulation of lipogenic markers in the liver: ↓ Gene expression of Srebp-1 and Ppar-γ. Down-regulation of genes related to long-chain fatty acid uptake and lipoprotein synthesis: ↓ Gene expression of Cd36 and ApoB100. Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Gene expression of Il-6, Il-12, F4/80 and Cd11b. Modulation of gut microbiota composition: ↑ Proportion of Bacteroidetes. |
| [17] | Male C57BL/6 mice 4-week-old |
HFD. |
L. paracasei N1115 Oral daily administration Dose: 2.2 × 109 CFU/mL diluted in normal saline (0.5 mL/day). Treatment length: 16 w. |
↓ Liver fat accumulation ↓ Liver inflammation ↓ Liver fibrosis |
Decreased content of hepatic inflammatory mediators (Tnfα and IL-1β). Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Gene expression of Nf-κB, Tlr-4 and Lps. Decreased serum levels of liver fibrosis markers (MAO). Up-regulation of markers of intestinal mucosa integrity: ↑ Protein expression of Occludin-1 and Claudin-1. |
| [33] | Female C57BL/6N mice 6–8-week-old |
STD diet with 30% fructose in drinking water. |
L. rhamnosus Oral daily gavage Dose:1 × 109 CFU/day. Treatment length: 5 or 12 w. |
↓ Liver fat accumulation ↓ Liver TG, TC and VLDL content ↓ Liver inflammation ↓ Liver apoptotic cells |
Down-regulation of liver injury protection markers: ↑ Gene expression of Fgf21. Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Gene expression of Tnfα and Cxcl10. Down-regulation of lipogenic markers in the liver: ↓ Gene expression of Fas, Srebp1c and Scd1. ↓ Protein expression of SREBP1c and ChREBP. Up-regulation of fatty acid oxidation markers in the liver: ↑ Gene expression of CPT1 and PPARα. Down-regulation of markers involved in hepatic ceramide content: ↓ Methylation of PP2AC. |
| [34] | Male C57BL/6N mice 8-week-old |
HFD/F (65% energy from fat and 30% fructose solution). |
L. plantarum NA136 group Oral daily administration Dose: 1 × 109 CFU/day. Treatment length: 16 w. |
↓ Liver fat accumulation ↓ Liver FFA content ↓ Liver inflammation ↓ Liver ALT and AST levels |
Down-regulation of lipogenic markers in the liver: ↓ Protein expression of FAS and SREBP-1. ↑ Phosphorylation of ACC. Up-regulation of energy yielding pathways in the liver: ↑ Phosphorylation of AMPK. Down-regulation of oxidative stress in the liver: ↓ Content of MDA. ↑ Protein expression of HO-1 andNrf2. ↑ Content of CAT. ↑ Activity of SOD. |
| [18] | Male SPF C57BL/6J mice 6-week-old |
Normal or Western diet (42% energy from fat). |
L. bulgaricus L. casei L. helveticus P. pentosaceus KID7 Daily administration Dose: 1 × 109 CFU/g suspended in distilled water. Treatment length: 8 w. |
↓ Liver steatosis grade (all treated groups) ↓ Liver inflammation (all treated groups except animals receiving L. casei) ↓ Liver/bw ratio (groups treated with L. bulgaricus, L. helveticus and P. pentosaceus) ↓ Liver AST levels (groups treated with L. bulgaricus and L. helveticus) ↓ Liver ALT levels (group treated with L. bulgaricus) ↓ NAS (groups treated with L. bulgaricus, L. helveticus and P. pentosaceus) |
Down-regulation of macrophage markers in the liver: ↓ Expression of Cd68 (groups treated with L. bulgaricus, L. helveticus, L. casei and P. pentosaceus). Modulation of gut microbiota composition: ↓ F/B ratio (groups treated with L. bulgaricus, L. helveticus, P. pentosaceus and L. casei). ↑ Content of A. muciniphila (groups treated with L. bulgaricus, L. helveticus and L. casei). Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Gene expression of Tnfα Il-6 and Il-1β (in all the treated groups). |
| [19] | Male Swiss mice 4-week-old |
HFD (61% energy from fat). |
B. longum Daily oral gavage Dose: 5 × 109 CFU/kg bw/d Treatment length: 4 w. |
↓ Liver lipid droplet size | Up-regulation of RAS related genes in the liver: ↑ Gene expression of Ace2 and Masr. |
| [20] | Male C57BL/6N mice 6-week-old |
HFD. Animals also received a 10% fructose solution. |
L. fermentum—CQPC06 L. delbrueckii subsp. Bulgaricus—LDSB Daily oral gavage Dose of 1 × 109 CFU/kg bw/d (L. fermentum—CQPC06) or 1 × 1010 CFU/kg bw/d (L. fermentum—CQPC06 and L. delbrueckii subsp. Bulgaricus—LDSB) suspended in sterile saline. Treatment length: 8 w. |
↓ Liver weight and index ↓ Liver TG ↓ Serum and liver AST and ALT levels ↓ Serum and liver AKP levels |
Decreased ROS levels in the livers of animals receiving L. fermentum—CQPC06 (at both doses) and L. delbrueckii subsp. Bulgaricus—LDSB. Up-regulation of fatty acid oxidation markers in the liver (L. fermentum—CQPC06 (at both doses)): ↑ Gene expression of Cpt1 and Ppar-α. ↑ Protein expression of CPT1 and PPAR-α. Down-regulation of lipogenic markers in the liver (L. fermentum—CQPC06 (at both doses)): ↓ Gene expression of C/ebp-α and Ppar-γ. ↓ Protein expression of C/EBP-α and PPAR-γ. Up-regulation of markers of intestinal mucosa integrity (L. fermentum—CQPC06 (at both doses)): ↑ Protein expression of ZO-1, Occludin and Claudin-1. Modulation of gut microbiota composition (L. fermentum—CQPC06 (at both doses) and L. delbrueckii subsp. Bulgaricus—LDSB): ↓ F/B ratio. ↑ Content of Akkermansia. |
| [21] | Female C57BL/6 mice |
WSD (40% energy from fat). |
L. rhamnosus GG ATCC 53103 L. lactis subsp. cremoris ATCC 19257 Oral gavage Thrice weekly Dose: 1 × 109 CFU Treatment length: 16 w. |
↓ Liver weight (group treated with L. cremoris) ↓ Liver PC, PS, TG and TG content (group treated with L. cremoris) ↓ Liver lipid droplet area (group treated with L. cremoris) ↓ Liver inflammation (group treated with L. cremoris) |
Down-regulation of hepatic content of lipids related to pro-inflammatory response: ↓ Levels of ARA containing lipids (group treated with L. cremoris). Down-regulation of inflammation associated metabolites in the liver: ↓ Levels of Resolvin E1, 9-HETE and 9HpODE (group treated with L. cremoris). |
| [22] | Male C57BL/6J mice 6-week-old |
HFD (45% energy from fat). |
L. reuteri 6475 L. reuteri VPL3461 Daily oral gavage (in a volume of 100 μL) Dose of 1 × 109 CFU/mL Treatment length: 8 w. |
↓ Liver TG content (all groups) | Not specified. |
| [23] | Male C57BL/6N mice 3–4-weeks-old |
HFD (42% energy from fat). |
L. rhamnosus GG Daily administration Dose: 1 × 108 CFU/day mixed in the experimental diet. Treatment length: 17 w. |
↓ Liver weight ↓ Liver TG content |
Modulation of SCFA levels in the cecum: ↑ Acetate levels. Modulation of SCFA in the liver: ↑ Acetate levels. Modulation of anti-inflammatory lipid mediator levels: ↓ ώ6/ώ 3 PUFA ratio. |
| [37] | Male C57BL/6N mice 8-week-old |
HFD/F (65% energy from fat and 30% dietary volume provided as fructose solution). |
L. plantarum NA136 Daily oral administration daily Dose: 1 × 109 CFU/day. Treatment length: 16 w. |
↓ Liver lipid content | Modulation of gut microbiota composition: ↑ Bacterial richness and diversity. Up-regulation of intestinal mucosa integrity markers: ↑ Protein expression of tight-junction markers (ZO-1, Occludin, Claudin-1). ↓ Protein expression mucosal dysfunction markers (HIF-1α). Decreased serum levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and LPS. Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Protein expression of NF-κB. ↓ Phosphorylation of p38. |
| [24] | Male SPF C57BL/6J mice 6-week-old |
WSD (42% energy from fat). |
L. acidophilus L. fermentum L. paracasei L. plantarum Daily administration Dose: 1 × 109 CFU suspended in drinking water. Treatment length: 8 w. |
↓ Liver steatosis score (groups treated with L. paracasei, L. plantarum and L. acidophilus) ↓ Liver TG content (groups treated with L. acidophilus, L. fermentum and L. paracasei) |
Modulation of microbiota composition: ↑ Bacteroidetes content (group treated with L. paracasei). ↓ Firmicutes content (group treated with L. paracasei). |
| [25] | Male Sprague-Dawley rats |
HFD |
L. acidophilus CGMCC 2106. B. longum CGMCC 2107. Daily administration Dose: 1 × 1010 CFU/mL suspended in drinking water. Treatment length: 12 w. |
↓ Liver fat accumulation (group treated with B. longum) | Modulation of fecal microbiota composition: ↑ Bifidobacterium content (group treated with B. longum). ↑ Lactobacillus content (group treated with L. acidophilus). |
| [36] | Male Fischer 344 rats | CDAA diet (30% energy from fat). Animals were fed ad libitum and had free access to drinking water during the whole experiment. |
C. butyricum Daily administration Dose: 8.5 × 109 CFU/g mixed in the diet. Treatment length: 42 w. |
↓ Liver total lipid and TG content ↓ Liver inflammation ↓ NAFLD progression (fibrosis) ↓ Serum ALT levels ↓ Liver lipid peroxidation ↓ Oxidative stress |
Up-regulation of energy yielding pathways in the liver: ↑ Phosphorylation of AMPK. Up-regulation of fatty acid oxidation markers in the liver: ↑ Protein expression of PPARα. Down-regulation of lipogenic markers in the liver: ↓ Protein expression of SREBP-1c and PPAR-γ. Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Protein expression of NF-kB and TNF-α. Down-regulation of lipid peroxidation markers in the liver: ↓ Content of 4-HNE and MDA. Up-regulation of antioxidant markers in the liver: ↑ Protein expression of Nrf2 and HO-1. Up-regulation of markers of intestinal mucosa integrity: ↑ Protein expression of ZO1 and Ocln. |
| [26] | Male Sprague-Dawley rats |
HFD. |
L. plantarum NCU116-L L. plantarum NCU116-H Daily administration Dose: 1 × 108 CFU/mL (L. plantarum NCU116-L) or 1 × 109 CFU/mL (L. plantarum NCU116-H) suspended in a sterile saline solution. Treatment length: 5 w. |
↓ Liver AST levels (group treated with L. plantarum NCU116-H) ↓ Liver oxidative stress ↓ Liver TC and TG content ↓ Liver inflammation |
Down-regulation of oxidative stress markers in liver: ↓ MDA content (group treated with L. plantarum NCU116-H). Up-regulation of antioxidant markers in the liver: ↑ Activity of SOD and GPx (all groups). ↑ Activity of CAT (group treated with L. plantarum NCU116-H). ↑ T-AOC (all groups). Down-regulation of serum pro-inflammatory cytokines: ↓ Levels of LPS and IL-6 (all groups). ↓ Levels of TNFα (L. plantarum NCU116-H). Up-regulation of fatty acid oxidation and lipolysis markers in the liver: ↑ Gene expression of Pparα, Pparγ, Pparδ, Pgc1α and Cpt1α (all groups). Down-regulation of lipogenic markers in the liver: ↓ Gene expression of Fas, Acc and Scd1 (all groups). Modulation of colonic microbiota composition: ↓ Gene expression of Bacteroides (all groups). ↑ Gene expression of Lactobacillus spp. and Bifidobacterium spp. (all groups). |
| [24] | Male Wistar rats |
HFD (60% energy from fat). |
L. paracasei Jlus66 Daily oral administration Doses: 1, 2 or 4 × 1010 CFU/d. Treatment length: 20 w. |
↓ Liver weight ↓ Liver fat accumulation ↓ Liver inflammation ↓ Serum ALT levels (high dose) |
Not specified. |
| [35] | Male Sprague-Dawley Rats 42-day-old |
STD plus 20% fructose in drinking water. |
L. acidophilus B. coagulans L. casei L. reuteri Daily administration Dose: 1 × 109 CFU/mL suspended in drinking water. Treatment length: 16 w. |
↓ Liver TG content (groups treated with L. acidophilus and L. reuteri) ↓ Serum ALT levels (all groups) ↓ Liver oxidative stress (all groups) |
Up-regulation of antioxidant response in the liver: ↑ Content of glutathione (groups treated with L. acidophilus and L. casei). ↓ Liver ROS formation (groups treated with L. acidophilus, L. casei and B. coagulans). ↓ Liver protein-carbonylation (all groups). ↓ Liver lipid peroxidation (all groups). |
| [28] | Sprague-Dawley rats 8-week-old |
HFD 54% energy from fat). Animals were injected with 600 mg/kg/day of D-galactose daily. |
L. fermentum DR9 L. plantarum DR7 L. reuteri 8513d Daily administration Dose: 1 × 1010 CFU/day dissolved in 100 μL of saline and mixed into 1 g of experimental diet. Treatment length: 12 w. |
↓ Liver lipid content (groups treated with L. fermentum DR9, L. plantarum DR7 and L. reuteri 8513d) ↓ Liver inflammation (groups treated with L. fermentum DR9, L. plantarum DR7 and L. reuteri 8513d) ↓ Liver ALP content (groups treated with L. fermentum DR9 and L. plantarum DR7) |
Down-regulation of lipogenic markers in the liver: ↓ Gene expression of Scd1 gene expression (groups treated with L. fermentum DR9 and L. plantarum DR7). Decreased liver content of pro-inflammatory cytokines: ↓ IL-4 levels (groups treated with L. fermentum DR9 and L. plantarum DR7). Up-regulation of energy yielding pathways in the liver: ↑ Gene expression of Ampkα1 (groups treated with L. fermentum DR9 and L. plantarum DR7) and Ampkα2 gene expression (group treated with L. plantarum DR7). |
| [16] | Male Wistar Rats 6-week-old |
HFD (45% of energy from fat). Animals also received 10% fructose in drinking water. |
L. Plantarum strain ATG-K2 L. Plantarum strain ATG-K6 Daily oral gavage Dose: 5 × 108 CFU/d. Treatment length: 8 w. |
↓ Liver TG and TC content ↓ Serum AST and ALT levels (all groups) ↓ Serum ALP levels (all groups) ↓ Liver lipid peroxidation |
Down-regulation of lipogenic markers in the liver: ↓ Gene expression of Srebp-1c and Fas (all groups). ↓ Protein expression of SREBP-1c (group treated with L. Plantarum strain ATG-K6). ↓ Protein expression of FAS (all groups). ↓ Protein expression of C/EBP (group treated with L. Plantarum strain ATG-K2). ↑ Phosphorylation of ACC (group treated with L. Plantarum strain ATG-K2). Up-regulation of energy yielding pathways in the liver: ↑ Phosphorylation of AMPK (group treated with L. Plantarum strain ATG-K2). Up-regulation of fatty acid oxidation markers in the liver: ↑ Protein expression of CPT-1 (group treated with L. Plantarum strain ATG-K2). Decreased liver MDA content. Modulation of gut microbiota composition: ↓ Relative abundance of Firmicutes (all groups). ↑ Relative abundance of Bacteroidetes (all groups). |
| [30] | Male Wistar rats | HFD (60% energy from fat). |
B. animalis subsp. Lactis
V9 Daily oral gavage Dose: 1 × 109 CFU/mL. Treatment length: 4 w. |
↓ Liver TG and FFA content ↓ Serum AST and ALT levels ↓ Liver inflammation ↓ Progression to NASH |
Down-regulation of lipogenic markers in the liver: ↓ Gene expression of Srebp-1c and Fas. Up-regulation of fatty acid oxidation markers in the liver: ↑ Gene expression of Pparα. Up-regulation of energy yielding pathways in the liver: ↑ Phosphorylation of AMPK. Down-regulation of NASH progression markers in the liver: ↓ Gene expression of Nlrp3, Asc, Tlr-4 and Tlr-9. Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Gene expression of Tnfα, IL-1β and IL-6. ↓ Phosphorylation of JNK, NF-kB, ERK and AKT. |
| [31] | Male Sprague-Dawley rats |
HFD (45% energy from fat). |
Eosinophil-Lactobacillus Daily oral gavage (312 mg/kg). Dose: 1 × 107 CFU/g. Treatment length: 8 w. |
↓ Liver lipid content ↓ Liver inflammation ↓ Serum and liver ALT and AST levels |
Modulation of gut microbiota composition: ↑ Bacterial diversity. ↓ Pathogenic bacteria content. Up-regulation of liver lipogenesis inhibitors: ↑ Protein expression of FGF15. |
ACC: acetyl-CoA carboxylase; ACE2: angiotensin-converting enzyme 2; Akt: protein kinase B; ALP: alkaline phosphatase; ALT: alanine transaminase; AMPK: AMP-activated protein kinase; ApoB100: apolipoprotein B100; ARA: arachidonic acid; ASC: Apoptosis-associated speck-like protein containing a caspase recruitment domain; AST: aspartate transaminase; bw: body weight; d: day; CAT: catalase; CD36: cluster of differentiation 36; CD11b: cluster of differentiation molecule 11B; CD68: cluster of differentiation 68; CDAA: choline-deficient/L-amino acid-defined; C/EBP-α: CCAAT/enhancer binding protein α; CFU: colony-forming unit; ChREBP: carbohydrate-responsive element-binding protein; CPT1: carnitine palmitoyltransferase 1; CXCL10: C-X-C Motif Chemokine Ligand 10; ERK: extracellular-signal-regulated kinase; F4/80: EGF-like module-containing mucin-like hormone receptor-like 1; FAS: fatty acid synthase; F/B: Firmicutes/Bacteroidetes; FGF15: fibroblast growth factor-15; FGF21: fibroblast growth factor-21; GPx: glutathione peroxidase; HFD: high-fat diet; HFD/F: high-fat and fructose diet; HIF-1α: hypoxia Inducible factor 1 Subunit α; HO-1: heme oxygenase 1; IL-1β: interleukin 1β; IL-4: interleukin 4; IL-6: interleukin 6; IL-8R: interleukin 8 receptor; IL-12: interleukin 12; JNK: janus kinase; LDL-c: LDL cholesterol; LPS: lipopolysaccharide; MAO: monoamino oxidase; MASR: Mas receptor; MDA: malondialdehyde; NAS: NAFLD activity score; NASH: non-alcoholic steatohepatitis; NF-κB: nuclear factor kappa B; NLRP3: nod-like receptor protein 3; Nrf2: nuclear factor erythroid 2–related factor 2; Ocln: Occludin; p38: p38 MAP kinase; PBS: phosphate buffered saline; PGC1α: peroxisome proliferator-activated receptor gamma coactivator 1-α; PP2AC: protein phosphatase 2 catalytic subunit α; PPAR-α: peroxisome proliferator-activated receptor α; PPAR-γ: peroxisome proliferator-activated receptor γ; PPAR-δ: peroxisome proliferator-activated receptor δ; PUFA: polyunsaturated fatty acids; RAS: renin–angiotensin system; ROS: reactive oxygen species; SCD1: stearoyl-CoA desaturase; SCFA: short-chain fatty acids; SOD: superoxide dismutase; SPF: specific pathogen-free; SREBP-1: Sterol regulatory element-binding protein 1; STD: standard; T-AOC: total antioxidant capacity; TC: total cholesterol; TG: triglycerides; TLR-4: toll-like receptor 4; TLR-9: toll-like receptor 9; Tnfα: tumor necrosis factor α; w: weeks; WSD: western-style diet; ZO1: Zonula Occludens 1; 4-HNE: 4-hydroxynonenal; 9-HETE: 9-hydroxy-5Z,7E,11Z,14Z-eicosatetraenoic acid; 9HpODE: 9-hydroperoxy-10E,12Z-octadecadienoic acid; ↓: significant reduction; ↑: significant increase.
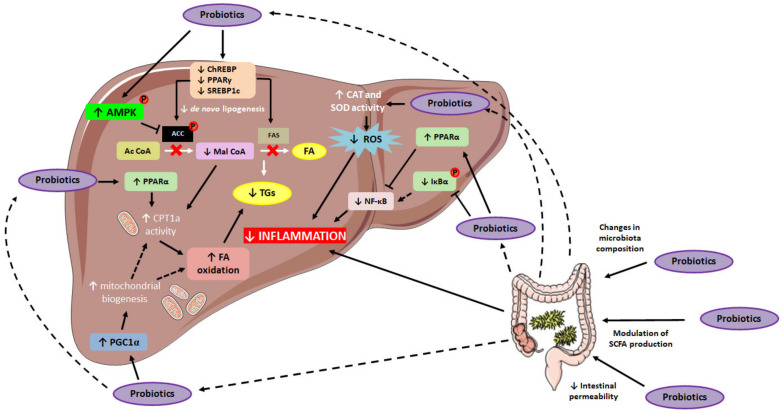
Regarding the mechanisms of action underlying the aforementioned probiotic-mediated effects on TG accumulation, several studies have described the down-regulation of lipogenesis [16,20,26,28,30,32,33,34,36]. In this regard, decreased liver gene and protein expressions, as well as diminished activation of de novo lipogenesis key-mediators such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) have been reported [16,26,30,32,33,34]. Additionally, the down-regulation of transcriptional factors regulating the expression of these enzymes, including carbohydrate response element-binding protein (ChREBP), sterol regulatory element-binding transcription factor 1 (SREBP-1) and peroxisome proliferator-activated receptor γ (PPARγ) has also been described in studies using single-strain probiotics [16,20,30,32,33,34,36]. Thus, it seems that one of the mechanisms of action by which probiotic administration may prevent excessive hepatic lipid accumulation relies on their ability to reduce the expression and/or the activity of lipogenic mediators (Figure 2). Interestingly, the effects of single-strain probiotics in terms of hepatic lipid “output” have also been described. In this regard, several studies have highlighted the capacity of this intervention to modulate liver fatty acid oxidation, mainly by enhancing the activity of carnitine palmitoyltransferase-1a (CPT-1a), the enzyme that mediates the entrance of long-chain fatty acids into the mitochondria for their subsequent oxidation, and thus, is considered the rate-limiting step in long-chain fatty acid oxidation [16,20,33]. Moreover, some studies have also reported enhanced expression (gene and protein) of PPARα, which is known to control the activity of enzymes involved in mitochondrial β-oxidation (including CPT-1a) [20,30,33,36,38]. In addition, increased activation of AMP protein kinase (AMPK) has also been reported when administering single-strain probiotics [16,28,30,34,36]. In this line, the AMPK mediated ACC inhibition results in a lower production of malonyl-CoA, which is an inhibitor of CPT-1a, resulting in a greater activation of the latter. Finally, alongside the up-regulation of markers involved in lipid oxidation, higher peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) gene expression was reported under probiotic administration [26]. Since this transcriptional co-activator is considered as the master regulator of mitochondrial biogenesis [39], its up-regulation could result in an enhanced mitochondrial biogenesis, and thus, in an increased fatty acid oxidation.
Figure 2.
Schematic representation of single-strain probiotics-mediated effects in hepatic lipid accumulation. ACC: acetyl-CoA carboxylase; AMPK: AMP protein kinase; Ac Coa: acetyl CoA; CAT: catalase; ChREBP: carbohydrate-responsive element-binding protein; CPT1a: carnitine palmitoyltransferase 1a; FA: fatty acid; FAS: fatty acid synthase; Iκβα: NF-kappa-β inhibitor α; Mal CoA: malonyl CoA; NF-κB: nuclear factor kappa B; PGC1α: peroxisome proliferator-activated receptor gamma coactivator 1-α; PPAR-α: peroxisome proliferator-activated receptor α; PPAR-γ: peroxisome proliferator-activated receptor γ; ROS: reactive oxygen species; SCFA: short-chain fatty acids; SOD: superoxide dismutase; SREBP-1: Sterol regulatory element-binding protein 1; TG: triglyceride. ↑: up-regulation; ↓: down-regulation.
Concerning the mechanisms of action underlying probiotic administration-mediated anti-inflammatory effects, several studies have described decreased gene and/or protein expressions of pro-inflammatory markers and mediators such as interleukin-1β, -4, -6 and -12 (IL-1β, IL-4, IL-6, IL-12), TNFα or nuclear factor kappa B (NF-κB) [16,17,18,26,28,30,32,33,36,37]. In addition, the aforementioned up-regulation of PPARα, which is known to negatively regulate NF-κB, may be involved in the anti-inflammatory effect of probiotic administration. Another process that is directly implicated in liver inflammation is the oxidative status of the organ, by cause of the tissue damage that occurs as a result of the imbalance between pro-oxidant production and antioxidant defense [40]. In this line, reduced liberation of ROS and production of lipid peroxidation and oxidative stress markers, such as malonaldehyde (MDA) or 4-hydroxynonenal (4-HNE), as well as enhanced activity of antioxidant enzymes, including catalase (CAT) and superoxide dismutase (SOD), have been described in several studies using single-strain probiotics [26,29,34,35,36]. Moreover, the up-regulation of the nuclear factor erythroid 2-elated factor 2 (Nrf2) pathway, including a greater expression of encoded antioxidant genes such as heme oxygenase 1 (HO-1), has also been described as a result of probiotic administration [36].
There are also a number of studies that have addressed the effects induced by probiotic administration on gut microbiota composition, microbial metabolite production and intestinal barrier integrity, all processes involved in NAFLD pathogenesis. Thus, the majority of the studies have reported that single-strain probiotic administration prevented diet-induced gut microbiota dysbiosis, mainly by increasing bacterial diversity and Bacteroidetes relative abundance and/or decreasing the Firmicutes/Bacteroidetes ratio [16,18,20,24,25,29,31,37]. In addition, the enhanced gene and protein expression of intestinal tight-junction markers, such as Occludin-1, Zonula Occludens-1 or Claudin, suggests that single-strain probiotic administration is an effective approach preventing high-fat and/or high-sugar diets induced gut barrier function impairment [17,20,32,36,37]. In this context, diet-induced impairment in gut microbiota composition, as well as the enhanced intestinal permeability, may well result in both a greater microbial production of LPS and its translocation to the liver. Indeed, once reaching the liver, LPS is known to bind receptors such as toll-like receptor 4 (TLR-4), releasing inflammatory mediators, and thus contributing to liver inflammation and NAFLD progression [13]. In this regard, the reduction induced by single-strain probiotic administration in TLR-4 expression may well account for the liver anti-inflammatory effect described for this intervention [17,30]. Finally, some authors have reported that the levels of SCFA in the cecum and in the liver are also modulated by probiotic administration. In this regard, it has been reported that the levels of acetate, a SCFA with known appetite modulating and hepato-protective properties [17,20,32,36,37,41,42,43], are increased after single-strain probiotic administration [23].
2.2. Preclinical Studies Using Probiotic Mixtures
As it has been demonstrated in studies using single-strain probiotics, the administration of probiotic mixtures as a preventive approach in rodent models featuring NAFLD has also been reported as effective. Thus, reductions in liver weight, fat accumulation and TG content, as well as decreased liver inflammation, have been described in studies using mixtures of different probiotic strains, administered in a wide range of doses and treatment periods (Table 2) [18,35,44,45,46,47,48,49,50,51].
Table 2.
Preclinical studies (rodent models) addressing the effects of different probiotic mixtures in diet-induced NAFLD.
| Reference | Animal Model | Experimental Conditions | Probiotic Treatment | Effects in Liver | Mechanisms of Action |
|---|---|---|---|---|---|
| [44] | Male C57BL/6J mice 5-week-old |
HFD (60% energy from fat). |
Probiotic mixtures: Bacillus mixture: B. sonorensis JJY12–3, B. paralicheniformis JJY12–8, B. sonorensis JJY13–1, B. sonorensis JJY 13–3 and B. sonorensis JJY 13–8. VSL#3: L. acidophilus, L. plantarum, L. casei, L. delbrueckii subspecies bulgaricus, B. breve, B. longum, B. infantis and S. salivarius subspecies thermophilus Daily administration Dose of 1 × 108 CFU/day Treatment length: 13 w. |
↓ Liver weight (group treated with Bacillus mixture) ↓ Liver fat accumulation (group treated with Bacillus mixture) ↓ Liver TG content (group treated with Bacillus mixture) ↓ Liver inflammation (group treated with Bacillus mixture) |
Up-regulation of markers related to fatty acid oxidation in the liver (group treated with Bacillus mixture): ↑ Gene expression of Acox1 and Cpt1. ↑ Protein expression of PCG1α. Down-regulation of pro-inflammatory markers and mediators in the liver (group treated with Bacillus mixture): ↓ Gene expression of Tnfα, Infγ, Mcp-1 and Il-12. Up-regulation of markers of intestinal mucosa integrity (group treated with Bacillus mixture): ↑ Gene expression of Zo1 and Ocln. ↑ Protein expression of Occludin. Modulation of cecum SCFA content and hepatic receptors: ↓ Cecum acetate levels (group treated with Bacillus mixture). ↓ Gene expression of acetate receptor Gpr43 (group treated with Bacillus mixture). |
| [45] | Male C57BL/6 mice 6-week-old |
HFD (60% energy from fat). |
Probiotic mixture: L. plantarum LC27 B. longum LC67 Daily oral gavage 3:1 proportion (0.75 × 109 CFU of L. plantarum and 0.25 × 109 CFU of B. longum) Treatment length: 4 w. |
↓ Liver weight ↓ Liver lipid accumulation ↓ Liver TG content (group treated with L. plantarum LC27) ↓ NAS (all groups) ↓ Serum AST and ALT levels |
Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Levels of TNFα. ↓ Activity of MPO. ↓ Protein expression of iNOS and COX-2. ↓ Protein expression of p65. ↓ Phosphorylation of p65. ↑ Protein expression of Iκβα. ↓ Phosphorylation of Iκβα. Up-regulation of energy yielding pathways in the liver: ↑ Phosphorylation of AMPK. Down-regulation of periportal fibrogenesis markers in the liver: ↓ Protein expression of α-SMA. ↓ Phosphorylation of α-SMA. Up-regulation of markers of intestinal mucosa integrity: ↑ Protein expression of Occludin-1 and Claudin-1. Modulation of gut microbiota composition: ↓ Proportion of Firmicutes, Bacteroidetes, Proteobacteria, Deferribacteria. ↓ F/B ratio. ↓ P/B ratio. ↑ Proportion of Actinobacteria. ↓ Gut content of LPS. |
| [46] | Male C57BL/6N mice >78-week-old |
HFD (60% energy from fat). |
Human origin probiotic mixture: 5 strains of Lactobacillus 5 strains of Enterococcus Daily administration Dose: 1 × 109 CFU/mL suspended in the drinking water. Treatment length: 10 w. |
↓ Liver fat accumulation ↓ Liver inflammation |
Enhanced microbial diversity enriched in beneficial commensal bacteria. Up-regulation of markers of intestinal mucosa integrity: ↑ Gene expression of Zo1 and Ocln. Modulation of microbial metabolites in the gut: ↑ Abundance of butyrate and propionate. |
| [18] | Male SPF C57BL/6J mice 6-week-old |
Normal or a Western diet (42% energy from fat). |
Probiotic mixtures: Mix 1: L. casei + L. helveticus Mix 2: L. casei + L. helveticus + P. pentosaceus KID7 Mix 3: L. casei + L. helveticus + L. bulgaricus Daily administration Dose of 1 × 109 CFU/g suspended in distilled water. Treatment length: 8 w. |
↓ Liver steatosis grade (all groups) ↓ Liver inflammation (all groups) ↓ Liver/bw ratio (groups treated with Mix 1 and Mix 2) ↓ NAS (all groups) |
Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ Gene expression of Tnfα (all groups). ↓ Gene expression of Il-6 (groups treated with Mix 2 and Mix 3). ↓ Gene expression of Il-1β (group treated with Mix 3). |
| [47] | Male SPF C57BL/6J mice 3–4-week-old |
HFD (60% energy from fat). |
Probiotic mixture: L. plantarum KLDS1.0344 L. plantarum KLDS1.0386 Daily oral gavage Dose: 108 CFU Treatment length: 8 w. |
↓ Serum ALT and AST levels ↓ Liver TG content |
Down-regulation of oxidative stress markers in the liver: ↓ Content of MDA. ↑ Content of GSH-Px. ↑ Content of CAT. ↑ Content of SOD. Modulation of gut microbiota composition: - Relative abundance of Parabacteroides, Eubacterium xylanophilum group, GCA-900066575, Lachnoclostridium, Lachnospiraceae UCG-006 and Rombustia. ↑ Richness of Lachnospiraceae NK4A136 group and Bacteroides. Modulation of gut SCFA production: ↑ Levels of acetic and butyric acids. |
| [48] | Male C57BL/6J mice 5-week-old |
HFD (60% energy from fat). |
Probiotic mixture: B. subtilis (1.4 × 109 CFU) E. faecium (1.55 × 1010 CFU) Daily oral gavage. Treatment length: 16 w. |
↓ Liver index ↓ Serum ALT and AST levels ↓ Liver lipid accumulation |
Up-regulation of fatty acid oxidation markers in the liver: ↑ Protein expression of CPT1 and PPARα. Down-regulation of pro-inflammatory markers and mediators in the liver: ↓ LPS levels. ↓ Gene expression of Il-1β, Il-6 and Tnf-α. ↓ Protein expression of TLR-4 and NF-κβ. Up-regulation of markers of intestinal mucosa integrity: ↑ Protein expression of Occludin-1 and Claudin-1. Modulation of gut microbiota composition: ↓ F/B ratio. ↓ Relative abundance of Firmicutes. ↑ Relative abundance of Bacteroidetes, Verrucomicrobia, Akkermansia and Oscillibacter. |
| [49] | Male Zucker-Leprfa/fa rats | Chow diet. | Probiotic mixture: L. paracasei CNCM I-4034 B. breve CNCM I-4035 Daily oral gavage Dose: 1010 CFU. Treatment length: 30 days. |
↓ Liver TG content | Decreased serum levels of pro-inflammatory markers cytokines and mediators: ↓ Levels of TNF-α. ↓ Levels of LPS. |
| [50] | Male albino rats 6-week-old |
HFSD (59% energy from fat). |
Probiotic mixture: L. acidophilus (10 × 108 CFU/g). L. plantarum (9.8 × 107 CFU/g). B. bifidum (2 × 106 CFU/g). B. subtilis fermentation extract (50 g per kg of product). A. oryzae fermentation extract (50 g per kg of product). Daily administration Dose: 1 g of probiotic mixture/kg diet Treatment length: 4 w. |
↓ Serum ALT levels ↓ NAS ↓ Liver inflammation |
Not specified. |
| [51] | Male Sprague-Dawley rats 7-week-old |
HCD (15% energy from fat). |
Probiotic mixture: B. longum CBG-C11 B. lactis CBG-C10 B. breve CBG-C2 L. reuteri CBG-C15 L. plantarum CBG-C21 Daily by oral gavage Doses: Low dose (1.65 × 109 CFU/kg/d) Medium dose (5.5 × 109 CFU/kg/d) High dose (1.65 × 1010 CFU/kg/d) Treatment length: 8 w. |
↓ Hepatic steatosis score (group treated with the high dose) ↓ Liver TG and TC content (all groups) ↓ Liver AST and ALT levels (all groups) |
Down-regulation of lipogenic markers in the liver: ↓ Protein expression of FAS, ACC and SREBP-1 (all doses). |
| [35] | Male Sprague-Dawley rats 42-day-old |
STD + 20% fructose in drinking water. | Probiotic mixture: L. acidophilus B. coagulans L. casei L. reuteri Daily administration Dose: 1 × 109 CFU/mL suspended in drinking water. Treatment length: 16 w. |
↓ Liver TG content ↓ Serum ALT levels ↓ Liver oxidative stress |
Up-regulation of antioxidant response in the liver: ↑ Content of glutathione. ↓ Liver ROS formation. ↑ Liver total antioxidant level. ↓ Liver protein-carbonylation. ↓ Liver lipid peroxidation. |
ACC: acyl-CoA-carboxylase; Acox 1: acyl-CoA oxidase 1; ALT: alanine transaminase; AMPK: AMP-activated protein kinase; α-SMA: smooth muscle alpha-actin; bw: body weight; CAT: catalase; CFU: colony-forming unit; COX-2: cyclooxygenase-2; CPT1: carnitine palmitoyltransferase 1; FAS: fatty acid synthase; F/B: Firmicutes/Bacteroidetes ratio; GPR-43: G-protein coupled receptor 43; GSH-Px: plasma glutathione peroxidase; HCD: high-cholesterol diet; HFD: high-fat diet; HFSD: high-fat sucrose diet; Iκβα: NF-κβ inhibitor; IL-1β: interleukin 1β; IL-2: interleukin 2; IL-6: interleukin 6; IL-12: interleukin 12; INFγ: interferon γ; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharides; MCP-1: monocyte chemoattractant protein-1; MDA: malonaldehyde; MPO: myeloperoxidase; NAS: NAFLD activity score; NF-κβ: nuclear factor kappa-light-chain-enhancer of activated B cells; Ocln: Occludin; PBS: phosphate buffered saline; P/B: Proteobacteria/Bacteroidetes ratio; PCG1α: peroxisome proliferator-activated receptor gamma coactivator 1-α; PPARα: peroxisome proliferator-activated receptor alpha; ROS: reactive oxygen species; SCFA: short-chain fatty acids; SOD: superoxide dismutase; SPF: specific pathogen-free; SREBP-1: sterol regulatory element-binding transcription factor-1; STD: standard; TG: triglycerides; TLR-4: toll-like receptor 4; Tnfα: tumor necrosis factor α; w: weeks; ZO-1: Zonula Occludens; ↓: significant reduction; ↑: significant increase.
The studies that analyze the effects of probiotic mixtures in NAFLD prevention are more limited than those conducted using single-strain probiotics. Interestingly, the described effects, as well as the involved mechanisms of action, seem to be similar. In this regard, the down-regulation of de novo lipogenesis, along with the upregulation of energy yielding pathways and fatty acid oxidation, seem to be among the mechanisms of action modulated by probiotic mixture administration that can prevent lipid accumulation in the liver [44,45,48,51]. Concerning liver inflammation, the administration of mixtures of probiotic strains effectively decreased the expression (gene or protein) and levels of pro-inflammatory markers and mediators, such as TNFα, myeloperoxidase (MPO), Il-6, Il-12, Il-1 β, interferon γ (Inf-γ), monocyte chemoattractant protein-1 (Mcp-1), inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX) [18,44,45,48,49]. In addition, as occurred in studies using single-strain probiotics, it has been reported that probiotic mixtures decreased the expression of LPS and TLR-4 [45,48,49]. In this regard, LPS is known to interact with TLR-4, triggering cytokine cascades and inflammation [52] and, thus, their down-regulation might be regarded as having an anti-inflammatory effect.
In this context, and similarly to what has been described in studies using single-strain probiotics, the administration of probiotic mixtures seems to exert anti-inflammatory effects by modulating the NF-κβ pathway (Figure 2). Thus, the administration of probiotic mixtures not only down-regulates the protein expression of NF-κβ, but it also prevents its nuclear translocation, as suggested by the reduced phosphorylation found in its p65 subunit [45]. Furthermore, the administration of probiotic mixtures can also modulate this pathway by preventing the phosphorylation and subsequent degradation of the NF-κβ inhibitor (Iκβα), thus averting the nuclear translocation of NF-κβ [45]. Moreover, according to the reported results, the anti-inflammatory effects exerted by the administration of probiotic mixtures in the liver can be potentiated, at least partially, by the reduction in hepatic oxidative stress induced by this approach (Figure 2). In this regard, the up-regulation of enzymes with antioxidant capacity, such as CAT, SOD and glutathione peroxidase (GSH-Px), has been described, as well as a reduction in ROS levels and markers of lipid peroxidation including MDA [35,47].
Besides the aforementioned effects and underlying mechanisms of action, probiotic mixtures have also shown to effectively regulate an array of processes in the gut. For instance, several studies have reported a modulation of gut microbiota composition, resulting in an enhanced microbial diversity (enriched in beneficial commensal bacteria), as well as reduced Firmicutes/Bacteroidetes ratio values or enhanced relative abundances of microbial species such as Bacteroidetes or Akkermansia [45,46,47,48]. In addition, a modulation of the levels of microbial metabolites such as SCFA have also been described, which may be related to the aforementioned effects induced by probiotic mixture administration in gut microbiota composition. Thus, the enhanced abundances of butyrate and propionate, which are SCFA with well-known anti-inflammatory properties [41,43], have been described [46,47]. Finally, the administration of probiotic strain mixtures also seems to be an effective approach in terms of gut barrier function maintenance. In this line, this therapeutic approach resulted in increased gene and protein expression of intestinal tight-junction markers, including Zonula Occludens-1, Occludin-1 and Claudin-1 [44,45,46,48], which suggests an improvement in intestinal mucosa integrity, which in turn may prevent/reduce the delivery of pro-inflammatory mediators into the circulation.
Altogether, and based on the studies analyzed within this section, it could be concluded that the administration of probiotics, as single strains or mixtures, results in an effective intervention in diet-induced NAFLD prevention/management. Likewise, according to the published results, it may also be concluded that the effects produced by both types of interventions are similar, and that they are mediated by the same metabolic pathways. Therefore, it seems that the administration of a mixture of different probiotic strains does not represent an advantage compared to single-strain probiotic administration. Finally, it is worth mentioning that these effects have been described when the probiotics had been administered along with the stressors that lead to NAFLD development. Therefore, much attention should be paid to the analysis and interpretation of such data, since these experimental conditions are not likely to be reproduced in humans for obvious ethical reasons.
3. Effects of Probiotic Administration (Single Strain and Mixtures) in NAFLD Prevention: Evidence from Clinical Trials
The potential of probiotics for NAFLD management has also been investigated in humans. In this regard, the available studies addressing the effects of probiotics in NAFLD are more limited than those carried out in animals. Unlike preclinical studies, in which the preventive effect of probiotics on steatosis has been analyzed, studies in humans have addressed their therapeutic effects. In some studies, probiotics were combined with other compounds/molecules, thus creating a synbiotic, and consequently, the reported effects in NAFLD treatment cannot be attributed solely to probiotics. This represented a limitation when selecting suitable studies to be included in this narrative review article. Moreover, besides the probiotic treatment, the participants also received some sort of dietary advice and/or were encouraged to practice physical activity.
According to the majority of the clinical trials included in this review article, probiotic administration seems to be effective in the treatment of NAFLD (Table 3). Interestingly, and contrary to that observed in preclinical studies, most of this research has been conducted using probiotic strain mixtures, instead of single-strain probiotics. Decreased hepatic lipid content, reduced steatosis grade and lowered serum transaminase levels have been reported in studies using different probiotic strain combinations (including from 2 to 8 different probiotic bacteria strains), doses (from 5 × 108 CFU/day to 22.5 × 1010 CFU/day) and administration periods (from 8 weeks to 12 months) in patients featuring NAFLD [53,54,55,56,57,58]. It is worth noting that these effects were described even in studies where all the participants (including the control group) received dietary advice (aimed at inducing body weight reduction), and also included physical activity programs or pharmaceutical treatment for further health alterations (statins and fibrates) [56,57]. In addition, similar hepatoprotective effects were also described in a study in which probiotics were administered mixed in a yogurt, instead as a supplement (capsule or sachet) [59]. Nevertheless, there are also studies in which the administration of probiotics did not result in the improvement of markers of liver injury in patients with NAFLD, despite the fact that the doses used and the administration periods were similar to those studies in which significant improvements were reported (Table 3) [60,61]. In this regard, according to Mohamed Nor et al. [60], the reduced sample size, along with the apparent higher variability of Malaysians’ gut microbiota composition (due to a more diverse dietary intake), may have influenced the obtained results. Moreover, the authors also pointed to a change in dietary fat observed in the group receiving the probiotic, which could have somehow blunted the potential beneficial effects of the probiotic intervention [60]. As far as the study carried out by Chong et al. [61] is concerned, differences in baseline characteristics between the probiotic and the placebo groups, as well as the impossibility to determine the participants’ NAFLD severity, were pointed out by the authors as potential factors influencing the outcomes of the study. Moreover, the participants in the studies in which no probiotic-administration-derived benefits in NAFLD were reported were older than in the rest of the studies. Since age-related variations in gut microbiota composition have been identified [62], it cannot be ruled out that this variable may have also influenced the outcomes of these studies.
Table 3.
Studies conducted in humans addressing the effects of different probiotics (single strain and mixtures) in NAFLD treatment (PICO format).
| Reference | Population | Intervention | Comparison | Outcome |
|---|---|---|---|---|
| [53] | 28 adults with NAFLD 20 men 8 women. |
Daily consumption Probiotic mixture (5 × 108 CFU): L. bulgaricus S. thermophilus Treatment period: 3 months. |
Placebo. | ↓ Serum ALT, AST and GGT levels. |
| [59] | 72 obese adults with NAFLD 33 men 39 women Age: 23–63 years old. BMI: 25–40 kg/m2 |
Daily consumption Probiotic-enriched yogurt (300 g/d): L. bulgaricus S. thermophilus L. acidophilus (6.46 × 106 CFU/g) B. lactis Bb12 (4.97 × 106 CFU/g) Treatment period: 8 weeks |
Daily consumption Conventional yogurt (300 g/d) L. bulgaricus S. thermophilus |
↓ Serum ALT and AST levels. |
| [54] | 64 obese adolescents with NAFLD Age: 10–18 years old BMI >85th percentile (age and sex specific) |
Daily consumption Probiotic mixture (1 capsule): L. acidophilus ATCC B3208 (3 × 109 CFU) B. lactis DSMZ 32269 (6 × 109 CFU) B. bifidum ATCC SD6576 (2 × 109 CFU) L. rhamnosus DSMZ 21690 (2 × 109 CFU) Treatment period: 12 weeks |
Placebo. | ↓ Serum ALT and AST levels. ↓ Fatty liver grade (sonographic grading). |
| [55] | 58 adult patients with NAFLD and T2DM Age: 18–65 years old BMI > 25 kg/m2 |
Daily consumption Probiotic mixture (1 sachet of 10 g). Lactobacillus + Lactococcus (6 × 1010 CFU/g) Bifidobacterium (1 × 1010 CFU/g) Propionibacterium (3 × 1010 CFU/g) Acetobacter (1 × 106 CFU/g) Treatment period: 8 weeks |
Placebo. | ↓ FLI and LS. ↓ Serum AST and GGT levels. Decreased circulating levels of proinflammatory markers: ↓ Serum TNF-α and IL-6 levels. |
| [56] | 65 obese adults with NAFLD 33 men 32 women Age: 19–75 years old BMI >25 kg/m2 Mean hepatic MRI-PDFF 16.2% |
Daily consumption Probiotic mixture (containing 1 × 109 CFU/1.4 g) L. acidophilus CBT LA1 L. rhamnosus CBT LR5 (human feces) L. paracasei CBT LPC5 (Korean fermented food—jeotgal) P. pentosaceus CBT SL4 (Korean fermented vegetable product—kimchi) B. lactis CBT BL3 B. breve CBT BR3 (Korean infant feces) Treatment period: 12 weeks |
Placebo. | ↓ IHF fraction. Modulation of gut microbiota composition: ↑ Relative abundances of L. acidophilus L. rhamnosus P. pentosaceus B. lactis B. breve |
| [57] | 30 adults with NAFLD: [ALT] and [AST] >1.5-fold normal levels |
Daily consumption Probiotic mixture (2 capsules containing 11.25 × 1010 CFU, each) L. paracasei DSM 24733, L. plantarum DSM 24730 L. acidophilus DSM 24735 L. delbrueckii subsp. Bulgaricus DSM 24734 B. longum DSM 24736 B. infantis DSM 24737 B. breve DSM 24732 S. thermophilus DSM 24731 Treatment period: 12 months |
Placebo. | Improvement of liver histology: ↓ Hepatocyte ballooning. ↓ Lobular inflammation. ↓ NAS score. ↓ Serum ALT levels. ↓ Serum ALP levels. ↓ Serum pro-inflammatory cytokines: Il-1β, IL-6 and TNF-α. |
| [58] | 60 adults with NAFLD 43 men 17 women Age: 20–60 years old BMI: 20–40 kg/m2 |
Daily consumption Probiotic mixture (1 capsule containing 5 × 109 CFU) L. casei L. rhamnosus L. acidophilus B. longum B. breve Treatment period: 12 weeks |
Placebo. | ↓ Serum ALT, AST and GGT levels ↓ Serum ALP levels. |
| [60] | 35 adults with NAFLD 28 men 7 women Age: 25–70 years old Mean BMI 32.6 ± 5.0 kg/m2 |
Daily consumption Probiotic mixture (2 sachets of VSL#3 twice daily) S. thermophilus B. breve B. infantis B. longum L. acidophilus L. plantarum L. paracasei L delbrueckii subsp. bulgaricus Treatment period: 10 weeks |
Placebo. | No significant improvements in markers of liver injury. |
| [61] | 39 obese adults 28 men 11 women with NAFLD: Fatty liver score >263 dB/m |
Daily consumption Probiotic mixture (1 sachet containing 3 × 1010 CFU twice daily) L. acidophilus BCMC 12,130 (107 mg) L. lactis MCMC 12,451 (107 mg) B. bifidum BCMC 02290 (107 mg) B. infantis BCMC 02129 (107 mg) B. longum BCMC 02120 (107 mg) Treatment period: 6 months |
Placebo. | No significant improvements in liver fibrosis parameters or serum markers of inflammation. |
ALP: alkaline phosphatase; ALT: alanine transaminase; AST: aspartate aminotransferase; BMI: body mass index; CFU: colony-forming units; FLI: fatty liver index; GGT: γ glutamyl transferase; HbA1c: glycated hemoglobin; IHF: intrahepatic fat; IL-1β: interleukin 1β; IL-6: interleukin 6; m: men; LS: liver stiffness; MRI-PDFF: magnetic-resonance-imaging-derived proton density fat fraction; NAFLD: non-alcoholic fatty liver disease; NAS: NAFLD activity score; TNF-α: tumor necrosis factor α; w: women; ↓: significant reduction; ↑: significant increase.
Besides the aforementioned effects on liver TG accumulation, anti-inflammatory properties have also been described in studies addressing the effects of probiotic administration in NAFLD patients. In this regard, decreased circulating levels of pro-inflammatory mediators such as TNF-α, IL-1β or IL-6, as well as lowered hepatocyte ballooning and liver lobular fibrosis (assessed by histological analysis) have been observed [55,57]. Similarly, and in line with the outcomes found in preclinical studies, probiotic administration also resulted in gut microbiota modulation in patients with NAFLD. In this case, increased relative abundances of L. acidophilus, L. rhamnosus, P. pentosaceus, B. lactis and B. breve were found in obese NAFLD patients receiving a probiotic mixture (containing six different strains) for 12 weeks [56]. By contrast, none of the studies included in this review section have addressed the effects of probiotic administration on SCFA levels and/or intestinal integrity.
Based on the results reported in clinical trials, it could be concluded that in general terms, probiotic administration effectively improves markers of liver injury in patients with NAFLD. Indeed, the major effects that have been described to date, such as lower intrahepatic lipid content, decreased liver injury, as well as decreased circulating transaminase and pro-inflammatory cytokine levels, are compatible to those reported in preclinical studies. However, one of the main limitations of studies conducted in humans relies on the difficulty to obtain samples that may make it possible to investigate the mechanisms of action involved in these hepatoprotective effects. Notwithstanding that different non-invasive imaging techniques including ultrasound, computer tomography or magnetic resonance imaging have been demonstrated to be effective detecting liver fat infiltration, these are not appropriate to assess liver inflammation or fibrosis [63]. Furthermore, these techniques are not suitable to explore the pathways and mechanisms of action modulated by probiotic administration. In this regard, liver biopsies represent the gold standard to study hepatic inflammation and fibrosis, as well as the mechanisms of action involved in the effects mediated by probiotics. Unfortunately, since the procedures needed to obtain such samples happen to be very invasive, markers that can be more easily studied (such as serum transaminase or cytokine levels) are usually selected to elucidate the effectiveness of these approaches in NAFLD.
4. Limitations of Probiotic Administration and Potential Alternatives
According to the studies included in this review, as well the ones found in the literature that address the effects of probiotics on diseases other than NAFLD, probiotic administration represents an effective therapeutic tool for the management of an array of metabolic alterations including obesity, diabetes or dyslipidemia [64,65,66,67,68]. In this regard, besides the more “conventional” probiotics such as Bifidobacterium and Lactobacillus, much attention has also been paid to other microorganisms referred to as next-generation probiotics (NGP) as potential therapeutic approaches for NAFLD management. These “new” probiotics, resulting from improved culture methods, bioinformatics and next-generation sequencing, include such species as Akkermansia muciniphila, Faecalibacterium prausnitzii, Eubacterium hallii, Propionibacterium, Bacteroides fragilis and genus belonging to the Clostridia clusters IV, XIVa and XVIII [69]. For instance, lower Akkermansia muciniphila abundances have been related to metabolic disorders such as obesity and NAFLD [70,71]. Interestingly, the administration of this bacteria was found to be effective in ameliorating obesity and related metabolic disorders, but without affecting gut microbiota composition [65]. Moreover, the administration of heat-treated Akkermansia muciniphila was also shown to exert metabolic benefits, similar to those produced by the administration of viable bacteria [72]. In addition, it was reported that in NAFLD patients, the abundance of Faecalibacterium prausnitzii tends to be low. Since this bacteria is known to produce butyrate, its usefulness for NAFLD prevention was proposed [69]. Similarly, Roseburia spp. are butyrate-producing bacteria, and as in the case of Faecalibacterium prausnitzii, may be effective for NAFLD management. Indeed, it was reported that Roseburia spp. administration reduces hepatic steatosis and inflammation, mainly by restoring the gut microbiota environment and intestinal integrity [73]. Therefore, current available data suggest that the NGP may represent an additional therapeutic tool for NAFLD prevention and treatment.
Despite the aforementioned probiotic health benefits, their administration also involves some hazards, since this therapeutic approach is based on the administration of life/viable microorganisms to vulnerable subjects, which in turn results in an increased risk of systemic infection and/or immune system overstimulation [74]. In this line, much attention has been paid to the usage of parabiotics and postbiotics as alternative approaches to probiotics [75].
In the case of parabiotics, also referred to as paraprobiotics or ghost probiotics, these are usually obtained by inactivation of probiotic bacteria, mainly by means of thermic treatment [76]. In this regard, the efficacy of parabiotics relies on the molecules and compounds contained in inactivated bacterial cells, and not in their viability [77]. In comparison to probiotics (live bacteria), parabiotics represent several potential advantages, which include a lower risk of infection and antibiotic resistance acquisition/transfer, as well as an easier storage and handling [78]. Even though the available data regarding the usage of parabiotics in the management of different diseases are still scarce, it was described that administration of heat-inactivated probiotic bacteria (Streptococcus thermophilus MN-ZLW-002) is effective in preventing high-fat diet feeding induced body weight gain, insulin resistance and dyslipidemia in mice [79]. In addition, according to data reported in clinical trials, the continuous administration of fragmented Lactobacillus amylovorus CP1563 (heat inactivated, lyophilized and then milled) for 12 weeks significantly reduces whole body and visceral fat, ameliorates markers related to glycaemic control (reduced fasting blood glucose and insulin levels) and improves dyslipidemia (reducing blood TG, and total and LDL cholesterol levels) in subjects featuring class I obesity [80]. Indeed, in a recent systematic review addressing the efficacy of parabiotics in the prevention and treatment of different diseases, compared to probiotics, no significant differences were reported regarding the effectiveness of parabiotics in the majority of the preventive and treatment trials analyzed (86% and 69%, respectively) [81]. Thus, although the available evidence concerning parabiotic use as therapeutic approach is limited, the results reported so far suggests that overall, their efficacy is similar to that attributed to probiotics.
In respect to postbiotics, these encompasses a wide spectrum of non-viable bacterial products and cell components with potential bioactive activity in the host, including certain vitamins (A and K, for instance), bile acids, SCFAs, polyamines, branched-chain amino acids or components of bacterial cell wall, such as teichoic acids [77,82]. As occurs for parabiotics, data regarding the efficacy of postbiotics is still scant and mainly limited to preclinical studies. According to the studies that have been published so far, in older mice, the administration of lipoteichoic acid from heat-inactivated Lactobacillus paracasei D3-5 prevented high-fat diet feeding-induced metabolic dysfunction [83]. Similarly, the administration of the polyamine spermidine has been reported to effectively prevent high-fat diet feeding-induced body weight gain, liver lipid accumulation or insulin resistance in mice [84,85]. In these cases, the administration of the postbiotics resulted in the amelioration of gut microbiota dysbiosis and inflammation, as well as in the recovery of intestinal integrity [83,84,85].
Altogether, and despite the fact that further research is warranted, these data suggest that the administration of parabiotics and postbiotics may also prove effective in the management of certain diseases, as well as highlighting that the functionality of such compounds is beyond microbial viability.
5. Conclusions
The aim of the present narrative review article was to summarize the evidence available regarding the effectiveness of probiotic administration in NFALD management. In this context, studies conducted in rodent models have revealed that both the administration of single-strain probiotics, as well as the administration of probiotic mixtures, represent an effective approach in the prevention of this liver condition. Based on the magnitude of the observed effects, along with the described mechanisms of action, it could be suggested that compared to the usage of single-strain probiotics, the combination of different probiotic strains does not represent an advantage. In the case of studies conducted in humans, in the majority of them, probiotic administration also resulted in the amelioration of markers of liver injury in NAFLD patients.
With regard to the mechanisms of action underlying the effects that have been described so far, preclinical studies have demonstrated that probiotics (single or mixed strains) act in the liver, down-regulating lipid synthesis, activating lipid oxidation and down-regulating pro-inflammatory pathways, as well as in the gut, modulating microbiota composition, intestinal integrity and the production of microbial metabolites. As for clinical studies, data describing such mechanisms are scarce, mainly due to limitations in terms of obtaining samples. In this regard, metagenomics and metabolomics may represent a useful tool to better assess the effects of probiotic administration using samples such as blood, urine or feces. Similarly, further research is warranted in order to elucidate whether the administration of parabiotics or postbiotics constitutes a real alternative to the usage of probiotics for NAFLD management, and thus, to overcome the limitation that represents the administration of viable microorganisms to vulnerable subjects.
Acknowledgments
Iñaki Milton-Laskibar acknowledges financial support from the Juan de la Cierva Programme-Training Grants of the Spanish State Research Agency of the Spanish Ministerio de Ciencia e Innovación y Ministerio de Universidades (FJC2019-038925-I).
Author Contributions
Conceptualization, I.M.-L., L.A.-G., J.A.M. and M.P.P.; writing—original draft preparation, I.M.-L., L.A.-G. and M.P.P.; writing—reviewing and editing, I.M.-L., L.A.-G., J.A.M. and M.P.P.; supervision, J.A.M. and M.P.P.; funding acquisition, M.P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CIBEROBN under Grant CB12/03/30007 and the Community of Madrid under Grant Y2020/BIO-6600.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Byass P. The global burden of liver disease: A challenge for methods and for public health. BMC Med. 2014;12:159. doi: 10.1186/s12916-014-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engin A. Non-Alcoholic Fatty Liver Disease. In: Engin A.B., Engin A., editors. Obesity and Lipotoxicity. Springer International Publishing; Cham, Switzerland: 2017. pp. 443–467. [Google Scholar]
- 3.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 5.Brunt E.M., Wong V.W.S., Nobili V., Day C.P., Sookoian S., Maher J.J., Bugianesi E., Sirlin C.B., Neuschwander-Tetri B.A., Rinella M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 6.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X., Zheng J., Zhang S., Wang B., Wu C., Guo X. Advances in the Involvement of Gut Microbiota in Pathophysiology of NAFLD. Front. Med. 2020;7:361. doi: 10.3389/fmed.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gómez-Zorita S., Aguirre L., Milton-Laskibar I., Fernández-Quintela A., Trepiana J., Kajarabille N., Mosqueda-Solís A., González M., Portillo M.P. Relationship between changes in microbiota and liver steatosis induced by high-fat feeding—A review of rodent models. Nutrients. 2019;11:2156. doi: 10.3390/nu11092156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Softic S., Cohen D.E., Kahn C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian C., Zhai Z., Li Z., Wang L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem.-Biol. Interact. 2020;330:109199. doi: 10.1016/j.cbi.2020.109199. [DOI] [PubMed] [Google Scholar]
- 11.Larson-Meyer D.E., Newcomer B.R., Heilbronn L.K., Volaufova J., Smith S.R., Alfonso A.J., Lefevre M., Rood J.C., Williamson D.A., Ravussin E., et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity. 2008;16:1355–1362. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias M.C., Parise E.R., de Carvalho L., Szejnfeld D., Netto J.P. Effect of 6-month nutritional intervention on non-alcoholic fatty liver disease. Nutrition. 2010;26:1094–1099. doi: 10.1016/j.nut.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Divella R., Mazzocca A., Daniele A., Sabbà C., Paradiso A. Obesity, Nonalcoholic Fatty Liver Disease and Adipocyto-kines Network in Promotion of Cancer. Int. J. Biol. Sci. 2019;15:610–616. doi: 10.7150/ijbs.29599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenplas Y., Huys G., Daube G. Probiotics: An update. J. Pediatr. 2015;91:6–21. doi: 10.1016/j.jped.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Perumpail B.J., Li A.A., John N., Sallam S., Shah N.D., Kwong W., Cholankeril G., Kim D., Ahmed A. The Therapeutic Implications of the Gut Microbiome and Probiotics in Patients with NAFLD. Diseases. 2019;7:27. doi: 10.3390/diseases7010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim B., Park K., Ji Y., Park S., Holzapfel W., Hyun C. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem. Biophys. Res. Commun. 2016;473:530–536. doi: 10.1016/j.bbrc.2016.03.107. [DOI] [PubMed] [Google Scholar]
- 17.Yao F., Jia R., Huang H., Yu Y., Mei L., Bai L., Ding Y., Zheng P. Effect of Lactobacillus paracasei N1115 and fructooligosaccharides in nonalcoholic fatty liver disease. Arch. Med. Sci. 2019;15:1336–1344. doi: 10.5114/aoms.2019.86611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee N.Y., Yoon S.J., Han D.H., Gupta H., Youn G.S., Shin M.J., Ham Y.L., Kwak M.J., Kim B.Y., Yu J.S., et al. Lactobacillus and Pediococcus ameliorate progression of non-alcoholic fatty liver disease through modulation of the gut microbiome. Gut Microbes. 2020;11:882–899. doi: 10.1080/19490976.2020.1712984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado A.S., Oliveira J.R., Lelis D.D.F., de Paula A.M.B., Guimarães A.L.S., Andrade J.M.O., Brandi I.V., Santos S.H.S. Oral Probiotic Bifidobacterium Longum Supplementation Improves Metabolic Parameters and Alters the Expression of the Renin-Angiotensin System in Obese Mice Liver. Biol. Res. Nurs. 2021;23:100–108. doi: 10.1177/1099800420942942. [DOI] [PubMed] [Google Scholar]
- 20.Mu J., Tan F., Zhou X., Zhao X. Lactobacillus fermentum CQPC06 in naturally fermented pickles prevents non-alcoholic fatty liver disease by stabilizing the gut-liver axis in mice. Food Funct. 2020;11:8707–8723. doi: 10.1039/D0FO01823F. [DOI] [PubMed] [Google Scholar]
- 21.Naudin C.R., Maner-Smith K., Owens J.A., Wynn G.M., Robinson B.S., Matthews J.D., Reedy A.R., Luo L., Wolfarth A.A., Darby T.M., et al. Lactococcus lactis Subspecies cremoris Elicits Protection Against Metabolic Changes Induced by a Western-Style Diet. Gastroenterology. 2020;159:639–651.e5. doi: 10.1053/j.gastro.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Oh J., Schueler K.L., Stapleton D.S., Alexander L.M., Yen C.E., Keller M.P., Attie A.D., van Pijkeren J.P. Secretion of Recombinant Interleukin-22 by Engineered Lactobacillus reuteri Reduces Fatty Liver Disease in a Mouse Model of Diet-Induced Obesity. mSphere. 2020;5:e00183-20. doi: 10.1128/mSphere.00183-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yau Y.F., El-nezami H., Galano J., Kundi Z.M., Durand T., Lee J.C. Lactobacillus rhamnosus GG and Oat Beta-Glucan Regulated Fatty Acid Profiles along the Gut-Liver-Brain Axis of Mice Fed with High Fat Diet and Demonstrated Antioxidant and Anti-Inflammatory Potentials. Mol. Nutr. Food Res. 2020;64:2000566. doi: 10.1002/mnfr.202000566. [DOI] [PubMed] [Google Scholar]
- 24.Lee N.Y., Shin M.J., Youn G.S., Yoon S.J., Choi Y.R., Kim H.S., Gupta H., Han S.H., Kim B.K., Lee D.Y., et al. Lactobacillus attenuates progression of nonalcoholic fatty liver disease by lowering cholesterol and steatosis. Clin. Mol. Hepatol. 2021;27:110–124. doi: 10.3350/cmh.2020.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu R., Wan Y., Fang Q., Lu W., Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J. Clin. Biochem. Nut. 2012;50:72–77. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C., Nie S., Zhu K., Ding Q., Li C., Xiong T., Xie M. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014;5:3216–3223. doi: 10.1039/C4FO00549J. [DOI] [PubMed] [Google Scholar]
- 27.Ye H., Li Q., Zhang Z., Sun M., Zhao C., Zhang T. Effect of a novel potential probiotic Lactobacillus paracasei Jlus66 isolated from fermented milk on nonalcoholic fatty liver in rats. Food Funct. 2017;8:4539–4546. doi: 10.1039/C7FO01108C. [DOI] [PubMed] [Google Scholar]
- 28.Lew L.-C., Hor Y.-Y., Jaafar M.-H., Lau A.-S.-Y., Lee B.-K., Chuah L.-O., Yap K.-P., Azlan A., Azzam G., Choi S.-B., et al. Lactobacillus Strains Alleviated Hyperlipidemia and Liver Steatosis in Aging Rats via Activation of AMPK. Int. J. Mol. Sci. 2020;21:5872. doi: 10.3390/ijms21165872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park E.-J., Lee Y.-S., Kim S.M., Park G.-S., Lee Y.H., Jeong D.Y., Kang J., Lee H.-J. Beneficial Effects of Lactobacillus plantarum Strains on Non-Alcoholic Fatty Liver Disease in High Fat/High Fructose Diet-Fed Rats. Nutrients. 2020;12:542. doi: 10.3390/nu12020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan Y., Liu C., Zhao S., Wang X., Wang J., Zhang H., Wang Y., Zhao G. Probiotic Bifidobacterium lactis V9 attenuates hepatic steatosis and inflammation in rats with non-alcoholic fatty liver disease. AMB Express. 2020;10:101. doi: 10.1186/s13568-020-01038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo M., Yan J., Wu L., Wu J., Chen Z., Jiang J., Chen Z., He B. Probiotics Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats via Gut Microbiota/FXR/FGF15 Signaling Pathway. J. Immunol. Res. 2021;2021:2264737. doi: 10.1155/2021/2264737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritze Y., Bárdos G., Claus A., Ehrmann V., Bergheim I., Schwiertz A., Bischoff S.C. Lactobacillus rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao C., Liu L., Liu Q., Li F., Zhang L., Zhu F., Shao T., Barve S., Chen Y., Li X., et al. Fibroblast growth factor 21 is required for the therapeutic effects of Lactobacillus rhamnosus GG against fructose-induced fatty liver in mice. Mol. Metab. 2019;29:145–157. doi: 10.1016/j.molmet.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Z., Wang C., Zhang L., Zhao Y., Duan C., Zhang X., Gao L., Li S. Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl. Microbiol. Biotechnol. 2019;103:5843–5850. doi: 10.1007/s00253-019-09703-4. [DOI] [PubMed] [Google Scholar]
- 35.Azarang A., Farshad O., Ommati M.M., Jamshidzadeh A., Heidari R., Abootalebi S.N., Gholami A. Protective Role of Probiotic Supplements in Hepatic Steatosis: A Rat Model Study. Biomed. Res. Int. 2020;2020:5487659. doi: 10.1155/2020/5487659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo H., Niioka M., Kobayashi N., Tanaka M., Watanabe T. Butyrate-Producing Probiotics Reduce Nonalcoholic Fatty Liver Disease Progression in Rats: New Insight into the Probiotics for the Gut-Liver Axis. PLoS ONE. 2013;8:e63388. doi: 10.1371/journal.pone.0063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Z., Chen L., Zhao Y., Wang C., Duan C., Yang G., Niu C., Li S. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl. Microbiol. Biotechnol. 2020;104:5273–5282. doi: 10.1007/s00253-020-10633-9. [DOI] [PubMed] [Google Scholar]
- 38.Rakhshandehroo M., Knoch B., Müller M., Kersten S. Peroxisome Proliferator-Activated Receptor Alpha Target Genes. PPAR Res. 2010;2010:612089. doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantó C., Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016;2016:5276130. doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin C.J., Sellmann C., Engstler A.J., Ziegenhardt D., Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH) Br. J. Nutr. 2015;114:1745–1755. doi: 10.1017/S0007114515003621. [DOI] [PubMed] [Google Scholar]
- 42.Chambers E.S., Preston T., Frost G., Morrison D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dangana E.O., Omolekulo T.E., Areola E.D., Olaniyi K.S., Soladoye A.O., Olatunji L.A. Sodium acetate protects against nicotine-induced excess hepatic lipid in male rats by suppressing xanthine oxidase activity. Chem.-Biol. Interact. 2020;316:108929. doi: 10.1016/j.cbi.2019.108929. [DOI] [PubMed] [Google Scholar]
- 44.Kim B., Kwon J., Kim M., Park H., Ji Y., Holzapfel W., Hyun C. Protective effects of Bacillus probiotics against high-fat diet-induced metabolic disorders in mice. PLoS ONE. 2018;13:e0210120. doi: 10.1371/journal.pone.0210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H., Kim J., Kim J., Jang S., Han M.J., Kim D.H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate high-fat diet-induced colitis, endotoxemia, liver steatosis, and obesity in mice. Nutr. Res. 2019;67:78–89. doi: 10.1016/j.nutres.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Ahmadi S., Wang S., Nagpal R., Wang B., Jain S., Razazan A., Mishra S.P., Zhu X., Wang Z., Kavanagh K., et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight. 2020;5:e132055. doi: 10.1172/jci.insight.132055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H., Liu F., Lu J., Shi J., Guan J., Yan F., Li B., Huo G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020;11:512. doi: 10.3389/fmicb.2020.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J., Xiong J., Ni J., Chen C., Wang K. Live Combined B. subtilis and E. faecium Alleviate Liver Inflammation, Improve Intestinal Barrier Function, and Modulate Gut Microbiota in Mice with Non-Alcoholic Fatty Liver Disease. Med. Sci. Monit. 2021;27:e931143. doi: 10.12659/MSM.931143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plaza-Diaz J., Gomez-Llorente C., Abadia-Molina F., Saez-Lara M.J., Campaña-Martin L., Muñoz-Quezada S., Romero F., Gil A., Fontana L. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on Hepatic Steatosis in Zucker Rats. PLoS ONE. 2014;9:e98401. doi: 10.1371/journal.pone.0098401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Muzafar H.M., Amin K.A. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complement. Altern. Med. 2017;17:43. doi: 10.1186/s12906-016-1540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S.-J., Park S.H., Sin H.-S., Jang S.-H., Lee S.-W., Kim S.-Y., Kwon B., Yu K.-Y., Kim S.Y., Yang D.K. Hypocholesterolemic effects of probiotic mixture on diet-induced hypercholesterolemic rats. Nutrients. 2017;9:293. doi: 10.3390/nu9030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Candelli M., Franza L., Pignataro G., Ojetti V., Covino M., Piccioni A., Gasbarrini A., Franceschi F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021;22:6242. doi: 10.3390/ijms22126242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aller R., De Luis D.A., Izaola O., Conde R., Gonzalez Sagrado M., Primo D., De La Fuente B., Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 54.Famouri F., Shariat Z., Hashemipour M., Keikha M., Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2017;64:413–417. doi: 10.1097/MPG.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 55.Kobyliak N., Abenavoli L., Mykhalchyshyn G., Kononenko L., Boccuto L., Kyriienko D., Dynnyk O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointest. Liver Dis. 2018;27:41–49. doi: 10.15403/jgld.2014.1121.271.kby. [DOI] [PubMed] [Google Scholar]
- 56.Ahn S.B., Jun D.W., Kang B., Lim J.H., Lim S., Chung M.J. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019;9:5688. doi: 10.1038/s41598-019-42059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duseja A., Acharya S.K., Mehta M., Chhabra S., Rana S., Das A., Dattagupta S., Dhiman R.K., Chawla Y.K. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): A randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019;6:e000315. doi: 10.1136/bmjgast-2019-000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Behrouz V., Aryaeian N., Zahedi M.J., Jazayeri S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: A randomized clinical trial. J. Food Sci. 2020;85:3611–3617. doi: 10.1111/1750-3841.15367. [DOI] [PubMed] [Google Scholar]
- 59.Nabavi S., Rafraf M., Somi M.H., Homayouni-Rad A., Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy Sci. 2014;97:7386–7393. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 60.Chong P.L., Laight D., Aspinall R.J., Higginson A., Cummings M.H. A randomised placebo controlled trial of VSL#3® probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. 2021;21:144. doi: 10.1186/s12876-021-01660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohamad Nor M.H., Ayob N., Mokhtar N.M., Raja Ali R.A., Tan G.C., Wong Z., Shafiee N.H., Wong Y.P., Mustangin M., Nawawi K.N.M. The Effect of Probiotics (MCP® BCMC® Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients. 2021;13:3192. doi: 10.3390/nu13093192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu C., Zhu H., Qiu P. Aging progression of human gut microbiota. BMC Microbiol. 2019;19:236. doi: 10.1186/s12866-019-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han A., Zhang Y.N., Boehringer A.S., Montes V., Andre M.P., Erdman J.W., Jr., Loomba R., Valasek M.A., Sirlin C.B., O’Brien W.D., Jr. Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease by Using Quantitative US. Radiology. 2020;295:106–113. doi: 10.1148/radiol.2020191152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yadav H., Lee J., Lloyd J., Walter P., Rane S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013;288:25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minami J., Kondo S., Yanagisawa N., Odamaki T., Xiao J., Abe F., Nakajima S., Hamamoto Y., Saitoh S., Shimoda T. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J. Nutr. Sci. 2015;4:e17. doi: 10.1017/jns.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Firouzi S., Majid H.A., Ismail A., Kamaruddin N.A., Barakatun-Nisak M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017;56:1535–1550. doi: 10.1007/s00394-016-1199-8. [DOI] [PubMed] [Google Scholar]
- 68.Asad F., Anwar H., Yassine H.M., Ullah M.I., Rahman A., Kamran Z., Sohail M.U. White Button Mushroom, Agaricus bisporus (Agaricomycetes), and a Probiotics Mixture Supplementation Correct Dyslipidemia without Influencing the Colon Microbiome Profile in Hypercholesterolemic Rats. Int. J. Med. Mushrooms. 2020;22:235–244. doi: 10.1615/IntJMedMushrooms.2020033807. [DOI] [PubMed] [Google Scholar]
- 69.Yao M., Qv L., Lu Y., Wang B., Berglund B., Li L. An Update on the Efficacy and Functionality of Probiotics for the Treatment of Non-Alcoholic Fatty Liver Disease. Engineering. 2021;7:679–686. doi: 10.1016/j.eng.2020.01.017. [DOI] [Google Scholar]
- 70.Etxeberria U., Hijona E., Aguirre L., Milagro F.I., Bujanda L., Rimando A.M., Martínez J.A., Portillo M.P. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 2017;61:1500906. doi: 10.1002/mnfr.201500906. [DOI] [PubMed] [Google Scholar]
- 71.Moreira G.V., Azevedo F.F., Ribeiro L.M., Santos A., Guadagnini D., Gama P., Liberti E.A., Saad M.J.A., Carvalho C.R.O. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J. Nutr. Biochem. 2018;62:143–154. doi: 10.1016/j.jnutbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Cani P.D., de Vos W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seo B., Jeon K., Moon S., Lee K., Kim W.K., Jeong H., Cha K.H., Lim M.Y., Kang W., Kweon M.N., et al. Roseburia spp. Abundance Associates with Alcohol Consumption in Humans and Its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell Host Microbe. 2020;27:25–40.e6. doi: 10.1016/j.chom.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Doron S., Snydman D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015;60:S129–S134. doi: 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cuevas-González P.F., Liceaga A.M., Aguilar-Toalá J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020;136:109502. doi: 10.1016/j.foodres.2020.109502. [DOI] [PubMed] [Google Scholar]
- 76.Nataraj B.H., Ali S.A., Behare P.V., Yadav H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020;19:168. doi: 10.1186/s12934-020-01426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vallejo-Cordoba B., Castro-López C., García H.S., González-Córdova A.F., Hernández-Mendoza A. Postbiotics and paraprobiotics: A review of current evidence and emerging trends. Adv. Food Nutr. Res. 2020;94:1–34. doi: 10.1016/bs.afnr.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Piqué N., Berlanga M., Miñana-Galbis D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang X., Liang H., Luo Y., Li Z., He F., Han X., Zhang L. Anti-adipogenesis and metabolism-regulating effects of heat-inactivated Streptococcus thermophilus MN-ZLW-002. Lett. Appl. Microbiol. 2020;72:677–687. doi: 10.1111/lam.13398. [DOI] [PubMed] [Google Scholar]
- 80.Nakamura F., Ishida Y., Aihara K., Sawada D., Ashida N., Sugawara T., Aoki Y., Takehara I., Takano K., Fujiwara S. Effect of fragmentedLactobacillus amylovorusCP1563 on lipid metabolism in overweight and mildly obese individuals: A randomized controlled trial. Microb. Ecol. Health Dis. 2016;27:30312. doi: 10.3402/mehd.v27.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zorzela L., Ardestani S.K., Mcfarland L.V., Vohra S. Is there a role for modified probiotics as beneficial microbes: A systematic review of the literature. Benef. Microbes. 2017;8:739–754. doi: 10.3920/BM2017.0032. [DOI] [PubMed] [Google Scholar]
- 82.Peluzio M.D.C.G., Martinez J.A., Milagro F.I. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 2021;108:11–26. doi: 10.1016/j.tifs.2020.12.004. [DOI] [Google Scholar]
- 83.Wang S., Ahmadi S., Nagpal R., Jain S., Mishra S.P., Kavanagh K., Zhu X., Wang Z., McClain D.A., Kritchevsky S.B., et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. GeroScience. 2020;42:333–352. doi: 10.1007/s11357-019-00137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma L., Ni Y., Wang Z., Tu W., Ni L., Zhuge F., Zheng A., Hu L., Zhao Y., Zheng L., et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes. 2020;12:1832857. doi: 10.1080/19490976.2020.1832857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma L., Ni Y., Hu L., Zhao Y., Zheng L., Yang S., Ni L., Fu Z. Spermidine ameliorates high-fat diet-induced hepatic steatosis and adipose tissue inflammation in preexisting obese mice. Life Sci. 2021;265:118739. doi: 10.1016/j.lfs.2020.118739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.