Abstract
3-(1H-Indol-3-yl)benzofuran-2(3H)-ones were efficiently accessed via polyphosphoric acid-mediated condensation of 3-(2-nitrovinyl)-1H-indoles with phenols.
Keywords: nitroalkanes, Brønsted acid catalysis, indoles, rearrangements, cascade transformations
1. Introduction
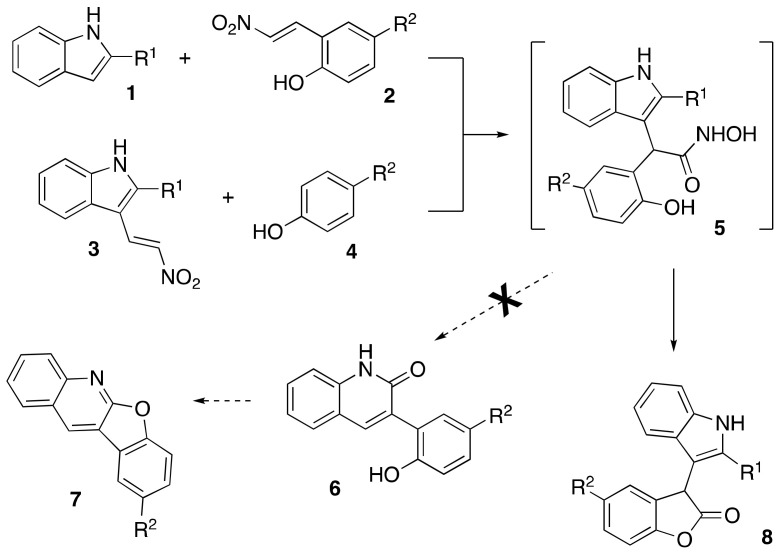
Since both indole and benzo–furanone structural fragments are omnipresent in nature and listed as privileged scaffolds for drug discovery, chimeric indigo-, or isoindigo-like molecules possessing both these moieties also attracted certain attention. There are several reports describing the preparation of these unique compounds and studies of their applications in medicinal chemistry and material sciences [1,2,3,4,5,6,7,8,9,10,11]. A common theme described in these reports is the metal-catalyzed C-C bond-forming reactions to accomplish the coupling between the indole and benzofuranone building blocks [1,5,8,10]. Other approaches used a Lewis acid-mediated reaction of indole derivatives with properly functionalized aromatic precursors (typically phenols or quinones) to furnish a five-membered lactone cycle in benzofurane [2,6,7]. We have recently established a synthetic approach towards benzofuro[2,3-b]quinoline cores employing a cascade transformation beginning with the condensation of indole and 1-methoxy-2-(2-nitrovinyl)benzenes in polyphosphoric acid (PPA). These studies ultimately resulted in a concise total synthesis of natural alkaloids norneocryptolepine and neocryptolepine [12,13]. During this investigation, the synthesis of benzofuro[2,3-b]quinoline 7 from 2-methylindole (1, R1 = Me) and 4-methyl-2-(2-nitrovinyl)phenol (2, R2 = Me) was attempted taking advantage of well-established protocol for PPA-mediated rearrangement of hydroxamic acid (5) into 2-quinolones (Scheme 1) [14,15,16]. However, the formation of compound 6 did not occur; instead, 3-(1H-Indol-3-yl)benzofuran-2(3H)-one 8 was formed in low yield, which was demonstrated on a single example [12]. We speculated that an alternative route towards the same compound could include the initial interaction of 3-(2-nitrovinyl)-1H-indoles (3) with phenols (4). Herein, we disclose an account of synthetic studies evaluating this idea and providing a reasonably efficient method for the preparation of 3-(1H-Indol-3-yl)benzofuran-2(3H)-ones 8 (Scheme 1).
Scheme 1.
[4 + 1]-Spirocyclization of nitroalkenes and nitriles and subsequent rearrangements.
2. Results and Discussion
It was anticipated that a Michael-like nucleophilic addition of phenol 4a across an electron-deficient alkene moiety in nitrovinylindole 3a would afford nitroalkane species 9aa. A substituent placed in the para-position would ensure the installation of a new C-C bond in an ortho-position of the phenol. Under acidic conditions, compound 9aa should exist in equilibrium with nitronate form, which is able to hydrolyze to afford hydroxamic acid of type 5 (Scheme 1). Subsequent intramolecular 5-exo-trig nucleophilic attack by phenol moiety with the release of hydroxylamine molecule would ensure the formation of the target structure 8aa. To test this idea, equimolar amounts of indole 3a and 4-chloro-3-methylphenol (4a) were subjected to reaction in 80% polyphosphoric acid at 80 °C, i.e., under conditions listed in our previous report [12]. The results were accurately reproduced, as compound 8aa was obtained as the sole isolable product, albeit in marginal yield (Table 1, entry 1). The material balance of this reaction seems low, as most of the starting material decomposed to provide polymeric side products. Speculating that the reaction conditions were too harsh, an attempt was made to carry out the same transformation in the presence of a weaker Brønsted acid. These tests revealed that the acidity of formic acid was not sufficient to trigger the requisite tautomerization into nitronate. While the formation of nitroalkane 9aa proceeded smoothly under these conditions, the subsequent transformation into 8aa did not occur at any tested temperature (entries 2–4). An attempt to perform the reaction in a catalytic fashion in the presence of small quantities of Lewis (anhydrous zinc chloride, entry 5) or strong Brønsted acid (MsOH, entry 6) did not prove successful leading to the consumption of starting materials without any formation of the target product. An improvement in yield was observed when the reaction was carried out in MsOH at 80 and 60 °C, where product 8aa was formed in 28% and 41% yield, respectively, while nitroalkane 9aa was not observed (entries 7, 8). This demonstrated that lowering the temperature allowed for a positive effect on the reaction performance. Testing the reactions at 40 °C afforded an improved yield of 8aa, though some unreacted nitroalkane 9aa was also detected (entry 9). At room temperature, 9aa was also formed, but its subsequent transformation into 8aa did not occur (entry 10). Increasing mesic acid load (4 mL per 1 mmol of 3a) also demonstrated a notable improvement in the reaction performance. Only nitroalkane 9aa was formed at room temperature (entry 12), but at 40 °C it was not detected, while material 8aa was formed as the sole product in 60% yield, as quantitated by 1H NMR. After isolation and purification on preparative scale, this translated into 55% yield (entry 11).
Table 1.
Optimization of the reaction conditions for a direct conversion of 3-(2-nitrovinyl)-1H-indole 3a and phenol 4a into 3-(1H-Indol-3-yl)benzofuran-2(3H)-ones 8aa.
| Acid (Volume in mL per mmol) a | Temperature (°C) | Yield of 8aa, % b | Yield of 9aa, % b | |
|---|---|---|---|---|
| 1 | PPA, 80% (2) | 80 | 15 | - |
| 2 | HCOOH (2) | 80 | - | 50 |
| 3 | HCOOH (2) | 40 | - | 40 |
| 4 | HCOOH (2) | 20 | - | 30 |
| 5 | Cat. ZnCl2 in EtOH c | 80 | - | - |
| 6 | Cat. MsOH in EtOH c | 80 | - | - |
| 7 | MsOH (2) | 80 | 28 | 0 |
| 8 | MsOH (2) | 60 | 41 | 0 |
| 9 | MsOH (2) | 40 | 45 | 10 |
| 10 | MsOH (2) | 20 | 0 | 50 |
| 11 | MsOH (4) | 40 | 60 (55) d | 0 |
| 12 | MsOH (4) | 20 | 0 | 53 |
| 13 | MsOH (4) e | 40 e | 34 | 12 |
| 14 | TsOH f | 40 | 0 h | 31 |
| 15 | TfOH (4) | 40 | 0 h | 0 |
| 16 | TfOH (2) g | 40 | 0 h | 0 |
| 17 | CF3COOH (4) | 40 | 0 h | 0 |
a Volume of acid in mL per 1 mmol of starting material 3a is listed. b NMR yields are reported unless specified otherwise. The best result is shown in bold. c 15 mg of ZnCl2 or 40 µL of MsOH in 2 mL of EtOH. Decomposition of the reaction mixture was observed due to polymerization. No low molecular mass products were detected. d Isolated yield of purified product 8aa is provided in parentheses. e The mixture was heated in microwave reactor maintaining constant temperature program. f 1 g of TsOH in 1 mL of CH2Cl2 was used. g In 2 mL of CH2Cl2. h Decomposition of the reaction mixture. No target product was detected.
An attempt to carry out the process under microwave irradiation (isothermic mode) was also made, but the outcome of the reaction was not impressive (entry 13). Most likely, this result can be attributed to poor accuracy in maintaining low-temperature settings by our microwave equipment. Other strong acids were also tested as promoters of the featured transformation. In the presence of tosic acid, the formation of nitroalkane 9aa in low yield was observed, while most starting material decomposed (entry 14). Triflic acid caused decomposition of the reaction mixture when used neat or in combination with CH2Cl2 as a co-solvent (entries 15, 16). Trifluoroacetic acid also caused decomposition, and no formation of the target product 8aa was detected (entry 17).
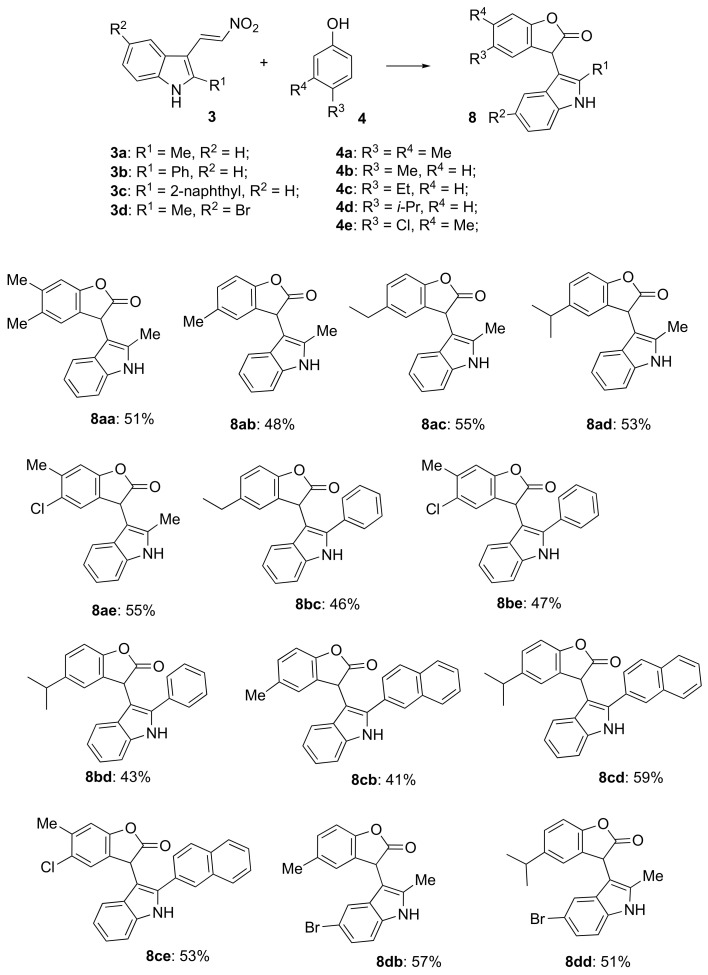
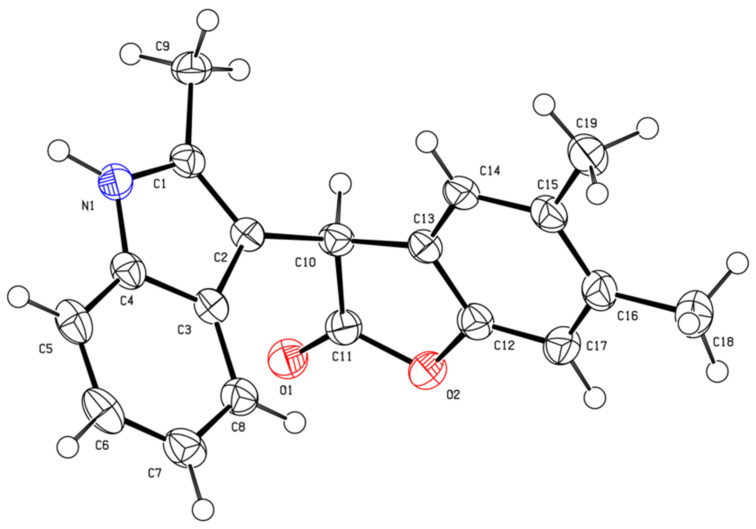
With optimized conditions in hand, the synthesis of a small, focused library of analogs with a scope and limitation studies progressed, results are summarized in Scheme 2. The standard reaction conditions afforded a meaningful preparative yield across a large spectrum of substrates. Most substituents and functional groups tested (Alk, Ar, Hal) were well tolerated. Formation of 3-(1H-indol-3-yl)benzofuran-2(3H)-one core was unambiguously confirmed by X-ray crystallography of compound 8aa (Figure 1).
Scheme 2.
Preparation of for preparation of 3-(1H-Indol-3-yl)benzofuran-2(3H)-ones 8.
Figure 1.
ORTEP drawing of X-ray structures of 5,6-dimethyl-3-(2-methyl-1H-indol-3-yl)benzofuran-2(3H)-one (6aa, CCDC #2154293). The thermal ellipsoids are shown at 50% probability.
3. Conclusions
An improved protocol for preparation of 3-(1H-Indol-3-yl)benzofuran-2(3H)-ones 8 via Brønsted acid-mediated cascade reaction of 3-(2-nitrovinyl)-1H-indoles (3) with phenols (4) was developed. This transformation involves initial nucleophilic addition of phenol across electron-deficient alkene moiety followed by hydrolysis of nitroalkane 9 into hydroxamic acid 5 and subsequent intramolecular 5-exo-trig nucleophilic attack to furnish the lactone cycle. This methodology was employed to synthesize a small, focused library of target molecules. Investigation of the biological activity of these new 3-(1H-indol-3-yl)benzofuran-2(3H)-ones is currently underway in our laboratories.
4. Experimental Part
General
NMR spectra (1H and 13C) were measured in solutions of CDCl3 or DMSO-d6 on Bruker AVANCE-III HD instrument (Bruker Biospin AG, Faellanden, Switzerland) (at 400.40 or 100.61 MHz, respectively). HRMS spectra were measured in MeCN solutions on a Bruker maXis impact (Bruker Daltonik GmbH, Bremen, Germany) (electrospray ionization, employing HCO2Na–HCO2H for calibration) mass spectrometer. See Supplementary Materials for NMR (Figures S1–S30) and HRMS (Figures S31–S45) spectral charts and X-ray crystallography data (Figure S46). IR spectra were measured on an FT-IR spectrometer Shimadzu IRAffinity-1S (Shimadzu Europa GmbH, Duisburg, Germany) equipped with an ATR sampling module. Reaction progress, purity of isolated compounds, and Rf values were assessed by TLC on Silufol UV-254 plates (Kavalier, Czech Republic). Column chromatography was performed on silica gel (32–63 μm, 60 Å pore size). Melting points were measured on a Stuart SMP30 apparatus. All reagents and solvents were purchased from commercial vendors and used as received.
(E)-5-Bromo-2-methyl-3-(2-nitrovinyl)-1H-indole (3d). This compound was prepared by a method described previously in the literature [17]. Red solid, mp (EtOH) 201–203 °C, Rf 0.48 (EtOAc/Hex, 2:1). Yield: 2.24 g (8 mmol, 80%). 1H NMR (400 MHz, DMSO) δ 12.34 (s, 1H), 8.25 (d, J = 13.3 Hz, 1H), 8.05 (d, J = 1.8 Hz, 1H), 7.96 (d, J = 13.3 Hz, 1H), 7.40–7.27 (m, 2H), 2.58 (s, 3H); 13C NMR (101 MHz, DMSO) δ 148.3, 135.2, 132.6, 130.8, 127.1, 125.4, 122.3, 114.7, 113.7, 104.7, 12.1. IR, vmax/cm−1: 3264, 1620, 1602, 1572, 1488, 1468, 1303, 1297, 1278, 1244, 1214, 1110. HRMS (ES TOF) calculated for (M + H)+ C11H10BrN2O2 280.9920, found 280.9911 (3.4 ppm).
Preparation of 3-(1H-indol-3-yl)benzofuran-2(3H)-ones (8aa–8dd) via Reaction of 3-(2-nitrovinyl)indoles with Phenols (General Procedure). A 5 mL round-bottomed flask equipped magnetic stirring bar was charged with 3-(2-nitrovinyl)indole (3a–d, 1 mmol), phenol (4a–e, 1.0 mmol), MeSO3H (4 mL). The mixture was vigorously stirred and heated to 40 °C for 1 h monitoring the reaction progress with TLC. After consumption of the starting 3-(2-nitrovinyl)indole, the reaction mass was neutralized with aqueous ammonia (25%) to pH 8 and extracted with ethyl acetate (3 × 25 mL). The combined organic extracts were washed with brine, dried with sodium sulphate, and concentrated in vacuum to obtain the crude material, which can be further purified by preparative column chromatography on silica gel.
5,6-Dimethyl-3-(2-methyl-1H-indol-3-yl)benzofuran-2(3H)-one (8aa). Colorless solid, mp (EtOH) 224.7–225.9 °C, Rf 0.49 (EtOAc/Hex, 1:2). Yield: 148 mg (0.51 mmol, 51%). 1H NMR (400 MHz, Chloroform-d) δ 8.01 (s, 1H), 7.28–7.24 (m, 1H), 7.14–7.04 (m, 1H), 7.02 (s, 1H), 6.98–6.89 (m, 2H), 6.85 (s, 1H), 5.05 (s, 1H), 2.34 (s, 3H), 2.31 (s, 3H), 2.14 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 177.0, 152.1, 137.7, 135.3, 133.6, 132.7, 127.1, 125.8, 124.8, 121.7, 120.0, 117.8, 111.8, 110.7, 105.6, 41.5, 20.5, 19.6, 12.0. IR, vmax/cm−1: 3372, 3101, 3053, 1779, 1491, 1455, 1302, 1238, 1115. HRMS (ES TOF) calculated for (M + Na)+ C19H17NNaO2 314.1151, found 314.1149 (0.8 ppm).
5-Methyl-3-(2-methyl-1H-indol-3-yl)benzofuran-2(3H)-one (8ab). Colorless solid, mp (EtOH) 175–175 °C, Rf 0.3 (EtOAc/Hex, 1:4). Yield: 134 mg (0.48 mmol, 48%). 1H NMR (400 MHz, CDCl3) δ 8.10 (s, 1H), 7.25 (d, J = 7.6 Hz, 1H), 7.16–7.07 (m, 3H), 6.93 (m, 3H), 5.08 (s, 1H), 2.27 (s, 3H), 2.25 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 176.9, 151.8, 135.3, 134.2, 133.8, 129.5, 127.7, 126.9, 125.6, 121.7, 119.9, 117.7, 110.8, 110.4, 105.2, 41.7, 21.2, 11.8.IR, vmax/cm−1:3364, 2974, 2934, 1817, 1789, 1620, 1480, 1465, 1307, 1230, 1132. HRMS (ES TOF) calculated for (M + Na)+ C18H15NNaO2 300.0995, found 300.0984 (3.8 ppm).
5-Ethyl-3-(2-methyl-1H-indol-3-yl)benzofuran-2(3H)-one (8ac). Colorless viscous liquid, Rf 0.51 (EtOAc/Hex, 1:2). Yield: 160 mg (0.55 mmol, 55%). 1H NMR (400 MHz, Chloroform-d) δ 8.10 (s, 1H), 7.25 (d, J = 5.6 Hz, 1H), 7.21–7.11 (m, 2H), 7.09 (t, J = 7.9 Hz, 1H), 6.94 (d, J = 7.2 Hz, 3H), 5.09 (s, 1H), 2.55 (q, J = 7.6 Hz, 2H), 2.27 (s, 3H), 1.15 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 176.9, 151.9, 140.8, 135.3, 133.8, 128.3, 127.7, 127.0, 124.5, 121.6, 119.9, 117.7, 110.7, 110.5, 105.2, 41.7, 28.6, 16.0, 11.8. IR, vmax/cm−1: 3396, 3061, 2966, 1813, 1783, 1733, 1481, 1461, 1242, 1135. HRMS (ES TOF) calculated for (M + Na)+ C19H17NNaO2 314.1151, found 314.1150 (0.5 ppm).
5-Isopropyl-3-(2-methyl-1H-indol-3-yl)benzofuran-2(3H)-one (8ad). Yellow oil, Rf 0.3 (EtOAc/Hex, 1:4). Yield: 162 mg (0.53 mmol, 53%). 1H NMR (400 MHz, Chloroform-d) δ 8.04 (s, 1H), 7.28 (s, 1H), 7.23–7.17 (m, 1H), 7.14 (d, J = 8.3 Hz, 1H), 7.09 (t, J = 8.0 Hz, 1H), 6.99–6.74 (m, 3H), 5.11 (s, 1H), 2.95–2.66 (m, 1H), 2.33 (s, 3H), 1.16 (t, J = 7.2 Hz, 6H).13C NMR (101 MHz, CDCl3) δ 176.7, 152.0, 145.5, 135.3, 133.7, 127.5, 127.2, 126.9, 123.2, 121.7, 120.0, 117.8, 110.7, 110.5, 105.3, 41.7, 34.0, 24.5, 24.1, 12.0. IR, vmax/cm−1: 3369, 2976, 2937, 1812, 1776, 1629, 1455, 1323, 1237, 1139. HRMS (ES TOF) calculated for (M + Na)+ C20H19NNaO2 328.1308, found 328.1295 (3.8 ppm).
5-Chloro-6-methyl-3-(2-methyl-1H-indol-3-yl)benzofuran-2(3H)-one (8ae). Yellow oil, Rf 0.28 (EtOAc/Hex, 1:4). Yield: 172 mg (0.55 mmol, 55%). 1H NMR (400 MHz, Chloroform-d) δ 8.06 (s, 1H), 7.28 (s, 1H), 7.17–7.06 (m, 3H), 6.96 (d, J = 7.3 Hz, 1H), 6.90 (d, J = 11.0 Hz, 1H), 5.08 (s, 1H), 2.43 (s, 3H), 2.32 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 175.9, 152.3, 137.4, 135.3, 133.8, 129.9, 126.8, 126.7, 125.5, 121.9, 120.2, 117.6, 113.1, 110.8, 104.8, 41.5, 20.8, 12.0. IR, vmax/cm−1: 3403, 2962, 1811, 1787, 1622, 1482, 1459, 1299, 1230, 1138, 1069, 1051, 906, 733. HRMS (ES TOF) calculated for (M + H)+ C18H15ClNO2 312.0786, found 312.0792 (−2.1 ppm).
5-Ethyl-3-(2-phenyl-1H-indol-3-yl)benzofuran-2(3H)-one (8bc). Colorless solid, mp (EtOH) 201.1–203.3 °C, Rf 0.68 (EtOAc/Hex, 1:2). Yield: 162 mg (0.46 mmol, 46%). 1H NMR (400 MHz, Chloroform-d) δ 8.29 (s, 1H), 7.82–7.66 (m, 2H), 7.56–7.42 (m, 3H), 7.38 (d, J = 8.1 Hz, 1H), 7.17 (d, J = 8.2 Hz, 3H), 7.00–6.86 (m, 2H), 6.76 (s, 1H), 5.28 (s, 1H), 2.52 (q, J = 7.6 Hz, 2H), 1.11 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 176.8, 151.9, 140.8, 138.3, 136.0, 132.0, 129.3 (2C), 128.9, 128.7 (2C), 128.4, 127.6, 127.0, 124.6, 122.9, 120.5, 119.1, 111.3, 110.5, 106.0, 42.1, 28.6, 16.0. IR, vmax/cm−1: 3432, 3050, 2927, 1815, 1618, 1487, 1457, 1310, 1270, 1236, 1137, 1049. HRMS (ES TOF) calculated for (M + Na)+ C24H19NNaO2 376.1308, found 376.1319 (−3.0 ppm).
5-Isopropyl-3-(2-phenyl-1H-indol-3-yl)benzofuran-2(3H)-one (8bd). Colorless solid, mp (EtOH) 212.5–214.2 °C, Rf 0.63 (EtOAc/Hex, 1:2). Yield: 158 mg (0.43 mmol, 43%). 1H NMR (400 MHz, Chloroform-d) δ 8.30 (s, 1H), 7.73 (s, 2H), 7.47 (dt, J = 24.5, 7.4 Hz, 3H), 7.38 (d, J = 8.2 Hz, 1H), 7.24–7.11 (m, 3H), 7.00–6.86 (m, 2H), 6.73 (s, 1H), 5.29 (s, 1H), 2.88–2.72 (m, 1H), 1.13 (d, J = 6.9 Hz, 3H), 1.11 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 176.9, 152.0, 145.5, 138.3, 136.0, 132.0, 129.3 (2C), 129.0, 128.7 (2C), 127.5, 127.0, 126.9, 123.3, 122.8, 120.4, 119.1, 111.2, 110.5, 105.9, 42.1, 33.9, 24.4, 24.1. IR, vmax/cm−1 3420, 3061, 2974, 1809, 1483, 1449, 1240, 1138, 1047. HRMS (ES TOF) calculated for (M + Na)+ C25H21NNaO2 390.1464, found 390.1468 (−0.9 ppm).
5-Chloro-6-methyl-3-(2-phenyl-1H-indol-3-yl)benzofuran-2(3H)-one (8be). Colorless solid, mp (EtOH) 186.2–188.2 °C, Rf 0.69 (EtOAc/Hex, 1:2). Yield: 175 mg (0.47 mmol, 47%). 1H NMR (400 MHz, Chloroform-d) δ 8.33 (s, 1H), 7.70 (d, J = 7.4 Hz, 2H), 7.56–7.43 (m, 3H), 7.39 (d, J = 8.1 Hz, 1H), 7.21–7.15 (m, 1H), 7.13 (s, 1H), 7.04 (s, 1H), 6.96 (t, J = 7.6 Hz, 1H), 6.76 (d, J = 7.9 Hz, 1H), 5.26 (s, 1H), 2.43 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 176.1, 152.3, 138.5, 137.5, 136.0, 131.7, 129.9, 129.4 (2C), 129.1, 128.7 (2C), 126.7, 126.7, 125.6, 123.0, 120.6, 118.8, 113.1, 111.4, 105.3, 41.9, 20.9. IR, vmax/cm−1: 3431, 3053, 2926, 1807, 1473, 1453, 1399, 1379, 1264, 1183. HRMS (ES TOF) calculated for (M + Na)+ C23H16ClNNaO2 396.0762, found 396.0759 (0.6 ppm).
5-Methyl-3-(2-(naphthalen-2-yl)-1H-indol-3-yl)benzofuran-2(3H)-one (8cb). Colorless viscous liquid, Rf 0.66 (EtOAc/Hex, 1:2). Yield: 159 mg (0.41 mmol, 41%). 1H NMR (400 MHz, Chloroform-d) δ 8.40 (s, 1H), 8.18 (s, 1H), 7.96 (d, J = 8.4 Hz, 1H), 7.90 (dd, J = 6.3, 3.3 Hz, 2H), 7.80 (d, J = 7.7 Hz, 1H), 7.61–7.53 (m, 2H), 7.39 (d, J = 8.2 Hz, 1H), 7.21–7.15 (m, 1H), 7.13 (s, 2H), 6.96 (t, J = 7.5 Hz, 1H), 6.90 (s, 1H), 6.81 (s, 1H), 5.37 (s, 1H), 2.22 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 176.9, 151.8, 138.3, 136.2, 134.2, 133.5, 133.2, 129.6, 129.3, 129.1, 128.4, 128.0, 127.9, 127.7, 127.1, 127.0, 126.9, 126.1, 125.7, 123.0, 120.5, 119.0, 111.3, 110.5, 106.4, 42.1, 21.2. IR, vmax/cm−1: 3372, 3065, 2986, 1809, 1783, 1735, 1489, 1242, 1140. HRMS (ES TOF) calculated for (M + Na)+ C27H19NNaO2 412.1308, found 412.1309 (−0.3 ppm).
5-Isopropyl-3-(2-(naphthalen-2-yl)-1H-indol-3-yl)benzofuran-2(3H)-one (8cd). Colorless solid, mp (EtOH) 21–216 °C, Rf 0.48 (EtOAc/Hex, 1:4). Yield: 246 mg (0.3 mmol, 59%). 1H NMR (400 MHz, Chloroform-d) δ 8.41 (s, 1H), 8.20 (s, 1H), 7.92 (m, 4H), 7.55 (dd, J = 6.3, 3.2 Hz, 2H), 7.40 (d, J = 8.2 Hz, 1H), 7.17 (q, J = 7.5 Hz, 3H), 6.94 (m, 2H), 6.81 (s, 1H), 5.38 (s, 1H), 2.93–2.61 (m, 1H), 1.10 (dd, J = 9.7, 6.8 Hz, 6H). 13C NMR (101 MHz, Chloroform-d) δ 177.0, 152.0, 145.5, 138.3, 136.2, 133.5, 133.2, 129.3, 129.1, 128.4, 128.0, 127.9, 127.5, 127.1, 127.90, 126.9, 126.9, 126.1, 123.3, 122.9, 120.5, 119.1, 111.3, 110.5, 106.3, 42.2, 33.9, 24.4, 24.1. IR, vmax/cm−1: 3418, 3078, 2936, 1845, 1612, 1456, 1399, 1365, 1323, 1214, 1178. HRMS (ES TOF) calculated for (M + H)+ C29H24NO2 418.1802, found 418.1793 (2.0 ppm).
5-Chloro-6-methyl-3-(2-(naphthalen-2-yl)-1H-indol-3-yl)benzofuran-2(3H)-one (8ce). Colorless solid, mp (EtOH) 218–219 °C, Rf 0.48 (EtOAc/Hex, 1:4). Yield: 224 mg (0.53 mmol, 53%). 1H NMR (400 MHz, Chloroform-d) δ 8.50 (s, 1H), 8.14 (s, 1H), 7.96–7.84 (m, 3H), 7.75 (d, J = 8.7 Hz, 1H), 7.55 (dt, J = 6.4, 3.5 Hz, 2H), 7.38–7.35 (m, 1H), 7.20–7.16 (m, 1H), 7.11 (s, 1H), 7.05 (d, J = 1.3 Hz, 1H), 6.98 (t, J = 7.6 Hz, 1H), 6.83 (s, 1H), 5.36 (s, 1H), 2.41 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 176.3, 152.2, 147.4, 138.4, 137.4, 136.1, 133.4, 133.2, 130.4, 129.9, 129.1, 128.4, 127.9, 127.0, 126.6, 126.0, 125.5, 124.4, 123.0, 120.7, 120.6, 118.7, 113.0, 111.5, 105.6, 42.0, 20.8. IR, vmax/cm−1: 3415, 3065, 2926, 1805, 1628, 1606, 1474, 1455, 1397, 1371, 1347, 1254, 1178, 1061, 906. HRMS (ES TOF) calculated for (M + Na)+ C27H18NNaO2 446.0918, found 446.0909 (2.2 ppm).
3-(5-Bromo-2-methyl-1H-indol-3-yl)-5-methylbenzofuran-2(3H)-one (8db). Yellowish viscous liquid, Rf 0.34 (EtOAc/Hex, 1:2). Yield: 202 mg (0.57 mmol, 57%). 1H NMR (400 MHz, Chloroform-d) δ 8.21 (s, 1H), 7.18–7.03 (m, 5H), 6.90–6.84 (m, 1H), 5.02 (s, 1H), 2.26 (s, 3H), 2.19 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 176.8, 151.6, 135.2, 134.5, 133.9, 129.8, 128.7, 127.1, 125.4, 124.5, 120.1, 113.2, 112.2, 110.6, 104.9, 41.5, 21.2, 11.8. IR, vmax/cm−1: 3376, 2998, 2898, 1813, 1779, 1737, 1485, 1463, 1220, 1131. HRMS (ES TOF) calculated for (M + H)+ C18H15BrNO2 356.0281, found 356.0282 (−0.4 ppm).
3-(5-Bromo-2-methyl-1H-indol-3-yl)-5-isopropylbenzofuran-2(3H)-one (8dd). Colorless viscous liquid, Rf 0.43 (EtOAc/Hex, 1:2). Yield: 195 mg (0.51 mmol, 51%). 1H NMR (400 MHz, Chloroform-d) δ 8.10 (s, 1H), 7.23 (dd, J = 8.3, 2.0 Hz, 1H), 7.20–7.09 (m, 3H), 6.95 (t, J = 1.6 Hz, 2H), 5.06 (s, 1H), 2.95–2.77 (m, 1H), 2.31 (s, 3H), 1.19 (d, J = 6.1 Hz, 3H), 1.17 (d, J = 6.2 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 176.4, 151.9, 145.6, 135.1, 133.8, 128.8, 127.2, 126.8, 124.4, 123.0, 120.3, 113.1, 112.0, 110.7, 104.8, 41.5, 33.9, 24.3, 24.1, 12.0. IR, vmax/cm−1: 3392, 2970, 1813, 1789, 1732, 1484, 1242, 1045. HRMS (ES TOF) calculated for (M + Na)+ C20H18BrNNaO2 406.0413, found 406.0412 (0.3 ppm).
Preparation of 4,5-dimethyl-2-(1-(2-methyl-1H-indol-3-yl)-2-nitroethyl)phenol (9aa) via Reaction of 2-methyl-3-(2-nitrovinyl)indole with 3,4-dimethylphenol (4ae). A 5 mL round-bottomed flask equipped magnetic stirring bar was charged with 2-methyl-3-(2-nitrovinyl)indole (3a, 1 mmol), 3,4-dimethylphenol (4a, 1.0 mmol), MeSO3H (4 mL). The mixture was vigorously stirred and heated to 20 °C for 1 h monitoring the reaction progress with TLC. After consumption of the starting 2-methyl-3-(2-nitrovinyl)indole, the reaction mass was neutralized with aqueous ammonia (25%) to pH 8 and extracted with ethyl acetate (3 × 25 mL). The combined organic extracts were washed with brine, dried with sodium sulphate, and concentrated in vacuum to obtain the crude material, which can be further purified by preparative column chromatography on silica gel.
Yellowish viscous liquid, Rf 0.43 (EtOAc/Hex, 1:2). Yield: 172 mg (0.53 mmol, 53%). 1H NMR (400 MHz, Chloroform-d) δ 7.88 (s, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.24–7.20 (m, 1H), 7.12 (ddd, J = 8.0, 7.1, 1.3 Hz, 1H), 7.09–7.02 (m, 2H), 6.52 (s, 1H), 5.40–5.22 (m, 2H), 5.09 (dd, J = 12.0, 9.1 Hz, 1H), 2.35 (s, 3H), 2.14 (s, 3H), 2.14 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 151.3, 136.9, 135.5, 133.5, 128.9, 128.8, 127.1, 122.6, 121.4, 119.8, 118.7, 117.7, 110.9, 107.5, 77.7, 35.4, 19.5, 19.2, 12.0. IR, vmax/cm−1: 3399, 2982, 1735, 1706, 1548, 1461, 1372, 1242. HRMS (ES TOF) calculated for (M + Na)+ C19H20N2NaO3 347.1366, found 347.1367 (−0.3 ppm).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27061902/s1, 1H and 13C NMR spectral charts.
Author Contributions
D.A.A. investigation; N.A.A. (Nikolai A. Arutiunov) investigation; A.Z.G. investigation; N.A.A. (Nicolai A. Aksenov) methodology, formal analysis; I.Y.G. investigation, formal analysis; A.V.A. conceptualization, supervision, funding acquisition; E.A.S. investigation, formal analysis; C.L. formal analysis, writing—review and editing; M.R. conceptualization, supervision, writing—original draft, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Foundation for Basic Research (grant #20-33-90049, by the President Grant for State Support of Young Scientists (grant #MK-2035.2021.1.3), and the Ministry of Education and Science of Russian Federation (grant #0795-2020-0031). The publication was prepared with the support of the RUDN University Strategic Academic Leadership Program (E.A.S).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supplementary Materials include NMR spectral charts.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Basnet P., Sebold M.B., Hendrick C.E., Kozlowski M.C. Copper Catalyzed Oxidative Arylation of Tertiary Carbon Centers. Org. Lett. 2020;22:9524–9528. doi: 10.1021/acs.orglett.0c03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadhav S.D., Singh A. Synthesis of Unsymmetrical α,α-Diarylacetates. J. Org. Chem. 2016;81:522–531. doi: 10.1021/acs.joc.5b02383. [DOI] [PubMed] [Google Scholar]
- 3.Jhulki S., Un H.-I., Ding Y.-F., Risko C., Mohapatra S.K., Pei J., Barlow S., Marder S.R. Reactivity of an air-stable dihydrobenzoimidazole n-dopant with organic semiconductor molecules. Chem. 2021;7:1050–1065. doi: 10.1016/j.chempr.2021.01.020. [DOI] [Google Scholar]
- 4.Kraner S., Prampolini G., Cuniberti G. Exciton Binding Energy in Molecular Triads. J. Phys. Chem. C. 2017;121:17088–17095. doi: 10.1021/acs.jpcc.7b03923. [DOI] [Google Scholar]
- 5.Lu H., Zhu G., Tang T., Ma Z., Chen Q., Chen Z. Anticancer Molecule Discovery via C2-Substituent Promoted Oxidative Coupling of Indole and Enolate. iScience. 2019;22:214–228. doi: 10.1016/j.isci.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz-Rojano L., Martinez-Mingo M., Garcia-Garcia C., Ribagorda M., Carreno M.C. Domino Reaction of Naphthoquinone and β-Arylpyruvic Acids: Synthesis of 3-(Naphthoquinonyl)naphthofuran-2(3H)-ones. Eur. J. Org. Chem. 2018;2018:1034–1040. doi: 10.1002/ejoc.201701656. [DOI] [Google Scholar]
- 7.Sharma N., Peddinti R.K. BF3·OEt2 Mediated Regioselective Reaction of Electron-Rich Arenes with 3-Ylidene Oxindoles. J. Org. Chem. 2017;82:918–924. doi: 10.1021/acs.joc.6b02395. [DOI] [PubMed] [Google Scholar]
- 8.Tang Z., Liu Z., Tong Z., Xu Z., Au C.-T., Qiu R., Kambe N. Cu-Catalyzed Cross-Dehydrogenative Coupling of Heteroaryl C(sp2)-H and Tertiary C(sp3)-H Bonds for the Construction of All-Carbon Triaryl Quaternary Centers. Org. Lett. 2019;21:5152–5156. doi: 10.1021/acs.orglett.9b01755. [DOI] [PubMed] [Google Scholar]
- 9.Tang Z., Peng L., Yuan Y., Li T., Qiu R., Kambe N. Synthesis of Triarylmethanes by Decarbonylation of 3,3-Diaryl Benzofuranones. J. Org. Chem. 2020;85:5300–5311. doi: 10.1021/acs.joc.9b03433. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji T., Tanaka T., Tanaka T., Yazaki R., Ohshima T. Catalytic aerobic cross-dehydrogenative coupling of azlactones en route to α,α-disubstituted α-amino acids. Org. Lett. 2020;22:4164–4170. doi: 10.1021/acs.orglett.0c01248. [DOI] [PubMed] [Google Scholar]
- 11.Yu Y., Xue N., Xiao C., Ravva M.K., Guo Y., Wu L., Zhang L., Li Z., Yue W., Wang Z. Effect of conjugation length on the properties of fused perylene diimides with variable isoindigos. J. Mater. Chem. C. 2019;7:12263–12269. doi: 10.1039/C9TC04078A. [DOI] [Google Scholar]
- 12.Aksenov D.A., Arutyunov N.A., Gasanova A.Z., Aksenov N.A., Aksenov A.V., Lower C., Rubin M. Synthetic studies towards benzofuro[2,3-b]quinoline and 6H-indolo[2,3-b]quinoline cores: Total synthesis of norneocryptolepine and neocryptolepine. Tetrahedron Lett. 2021;82:153395. doi: 10.1016/j.tetlet.2021.153395. [DOI] [Google Scholar]
- 13.Uyanik M., Tanaka H., Ishihara K. I+/TBHP Catalysis For Tandem Oxidative Cyclization To Indolo[2,3-b]quinolines. Asian J. Org. Chem. 2021;10:164–169. doi: 10.1002/ajoc.202000570. [DOI] [Google Scholar]
- 14.Aksenov A.V., Smirnov A.N., Aksenov N.A., Aksenova I.V., Bijieva A.S., Rubin M. Highly efficient modular metal-free synthesis of 3-substituted 2-quinolones. Org. Biomol. Chem. 2014;12:9786–9788. doi: 10.1039/C4OB02131B. [DOI] [PubMed] [Google Scholar]
- 15.Aksenov A.V., Smirnov A.N., Aksenov N.A., Aksenova I.V., Frolova L.V., Kornienko A., Magedov I.V., Rubin M. Metal-free transannulation reaction of indoles with nitrostyrenes: A simple practical synthesis of 3-substituted 2-quinolones. Chem. Commun. 2013;49:9305–9307. doi: 10.1039/c3cc45696j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aksenov A.V., Smirnov A.N., Aksenov N.A., Aksenova I.V., Matheny J.P., Rubin M. Metal-free ring expansion of indoles with nitroalkenes: A simple, modular approach to 3-substituted 2-quinolones. RSC Adv. 2015;5:8647–8656. doi: 10.1039/C4RA14406F. [DOI] [Google Scholar]
- 17.Canoira L., Rodriguez J.G., Subirats J.B., Escario J.-A., Jimenez I., Martinez-Fernandez A.R. Synthesis, structure and anti-fungal activity of 3-(2′-nitrovinyl)indoles. Eur. J. Med. Chem. 1989;24:39–42. doi: 10.1016/0223-5234(89)90161-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary Materials include NMR spectral charts.