Abstract
Isobavachalcone (IBC) is a natural prenylated chalcone with a broad spectrum of pharmacological properties. In this work, we newly synthesized and investigated the antibacterial activity of IBC against Gram-positive, Gram-negative and mycobacterial species. IBC was active against Gram-positive bacteria, mainly against Methicillin-Susceptible Staphylococcus aureus (MSSA) and Methicillin-Resistant Staphylococcus aureus (MRSA), with minimum inhibitory concentration (MIC) values of 1.56 and 3.12 µg/mL, respectively. On the other hand, IBC was not able to act against Gram-negative species (MIC > 400 µg/mL). IBC displayed activity against mycobacterial species (MIC = 64 µg/mL), including Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium kansasii. IBC was able to inhibit more than 50% of MSSA and MRSA biofilm formation at 0.78 µg/mL. Its antibiofilm activity was similar to vancomycin, which was active at 0.74 µg/mL. In order to study the mechanism of the action by fluorescence microscopy, the propidium iodide (PI) and SYTO9 fluorophores indicated that IBC disrupted the membrane of Bacillus subtilis. Toxicity assays using human keratinocytes (HaCaT cell line) showed that IBC did not have the capacity to reduce the cell viability. These results suggested that IBC is a promising antibacterial agent with an elucidated mode of action and potential applications as an antibacterial drug and a medical device coating.
Keywords: chalcone, membrane, natural product, antibacterial, biofilm
1. Introduction
Approximately 1 million people died due to antibiotic-resistant infections between 2014 and 2016 around the world, and there is a projection that multidrug resistance will lead 300 million people to premature deaths until 2050 [1,2]. The fast and global spread of multidrug-resistant bacteria has been recognized as a great challenge to be overcome in the 21st century. Thus, strong efforts are necessary to investigate new antibacterial drugs, which must be active against resistant pathogens to current anti-infective therapy [2].
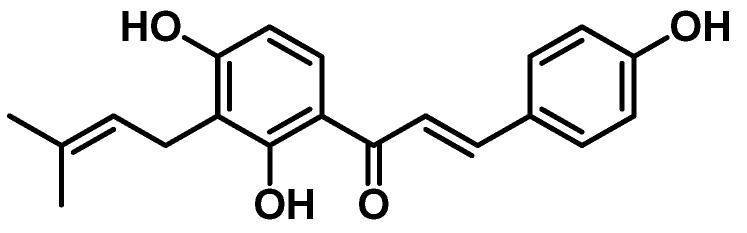
Natural products from plants and microorganisms are sources of new antibacterial compounds [3]. Among the 162 antibacterial agents approved by FDA between 1981 and 2019, about 55% are natural products and their derivatives, highlighting their relevance to modern drug discovery [3,4]. Among the promising natural products, isobavachalcone (IBC) is a prenylated chalcone isolated from plants of the Fabaceae, Clusiaceae, Moraceae, Schisandraceae and Apiaceae families (Figure 1) [5,6]. Moreover, the concise synthesis of IBC has been described by several groups [7,8,9,10,11]. IBC is a privileged compound due to its extensive pharmacological properties, including antibacterial [6], anti-cancer [12], antifungal [13], antioxidant [14], neuroprotective [15] and anti-inflammatory [16] properties.
Figure 1.
Structure of Isobavachalcone (IBC).
As part of our continuing search for novel antibacterial drugs that act on bacterial membranes, we newly synthesized IBC and evaluated its antibacterial activity against Gram-positive, Gram-negative and Mycobacterium planktonic cells. Additionally, we investigated the antibacterial activity of IBC against biofilms of Methicillin-susceptible Staphylococcus aureus (MSSA) and Methicillin-resistant Staphylococcus aureus (MRSA). IBC was tested regarding its effects on the membrane of Bacillus subtilis, as well as its toxicity toward human skin cells.
2. Materials and Methods
2.1. Isobavachalcone (IBC)
The synthesis of IBC was performed according to Sugamoto and collaborators, using six steps [8]. The structure of IBC was confirmed by 1H and 13C nuclear magnetic resonance (NMR) and mass spectrometry (MS) data analyses. The purity of IBC was determined by high-performance liquid chromatography with a photodiode array detector (HPLC-PAD). Detailed synthetic experimental procedures, NMR spectra, mass spectrum and the HPLC-PAD chromatogram are presented in the Supplementary Material.
2.2. Antibacterial and Antimycobacterial Assays
The strains were directly purchased from the American Type Culture Collection (ATCC) and were maintained in the culture collection of the Laboratory of Antimicrobial Testing of the Federal University of Uberlândia state of Minas Gerais, Brazil.
The antibacterial activity was determined against Methicillin-susceptible Staphylococcus aureus (ATCC 6538), Methicilin-resistant Staphylococcus aureus (ATCC BAA44), Streptococcus pneumoniae (ATCC 6305), Streptococcus sanguinis (ATCC 10556), Streptococcus sobrinus (ATCC 33478), Streptococcus mutans (ATCC 25175), Klebsiella pneumoniae (ATCC 10031) and Pseudomonas aeruginosa (ATCC 15442). The antimycobacterial activity was determined against Mycobacterium tuberculosis H37Rv (ATCC 27294), Mycobacterium avium (ATCC 25291) and Mycobacterium kansasii (ATCC 12478).
The minimal inhibitory concentration (MIC) against Gram-positive and Gram-negative bacterial species was determined in triplicate using the broth microdilution method in 96-well microplates as previously reported, using resazurin as a colorimetric indicator of cell viability [17]. IBC was dissolved in DMSO at 1 mg/mL, followed by dilution in brain heart infusion to achieve the final concentrations ranging from 400.0 to 0.195 μg/mL. The final DMSO content was 5% (v/v), and this solution was used as the negative control. The inoculum was adjusted for each microorganism to reach a cell suspension of 5.0 × 105 colony-forming units per mL (CFU/mL), as preconized by the Clinical and Laboratory Standards Institute, with modifications in the culture medium [18]. Growth control (inoculated well) and sterility control (non-inoculated well free of antimicrobial agent) were also included. Tetracycline and chlorhexidine were used as positive controls and were tested in concentrations ranging from 0.0115 μg/mL to 5.9 μg/mL and 0.115 to 59 μg/mL, respectively. The 96-well microplates were sealed with plastic film and incubated at 37 °C for 24 h. After this period, 30 μL of aqueous solution of resazurin (0.02%) was added to the microplates and was incubated for 15 min at 37 °C. MIC value was defined as the lowest concentration able to inhibit the microorganism growth indicated by the color change of resazurin from blue to pink.
MIC values of IBC against mycobacteria species were determined according to Palomino and collaborators protocol, with slight modifications [19,20]. A stock solution of IBC was prepared in DMSO and diluted in Middlebrook 7H9 broth to achieve final concentrations ranging from 7.8 to 1000 µg/mL. Isoniazid was dissolved in DMSO and used as positive control in concentrations ranging from 0.015 to 1.0 µg/mL. The inoculum was prepared by introducing a range of colonies grown in Ogawa-Kudoh in a tube containing glass beads with 500 μL of sterile water. An aliquot of 200 μL was transferred to a tube containing 2 mL of 7H9 broth, incubated at 37 °C for 7 days and compared with McFarland scale 1 (3.0 × 108 cells/mL). Inoculum was suspended in 96-well plates at a 1:25 ratio with 7H9 broth. The growth controls (without antibiotic) and sterility controls (without inoculation) were also included. The 96-well plates were incubated at 37 °C for 7 days. After this period, 30 µL resazurin 0.02% aqueous solution was added to each plate well. The MIC value was defined as the lowest drug concentration able to inhibit the mycobacterial growth, which was expressed in µg/mL. The assay was conducted in triplicate.
2.3. Checkerboard Assay
The combination effect of IBC with vancomycin was evaluated against MSSA and MRSA using microdilution broth checkerboard assay according to White and collaborators, adapted from the standard procedure established by CLSI [18,21]. The fractional inhibitory concentration (FIC) of the combination between IBC and vancomycin was determined using Mueller–Hinton broth culture medium into 96-well plates, with a final inoculum suspension of 5.0 × 105 CFU/mL. The plates were incubated at 37 °C for 24 h. After incubation, 0.02% aqueous resazurin solution was added to the wells. Fractional inhibitory concentration index (FICI) was calculated using the Equation (1):
| ΣFICI = FICA + FICB | (1) |
where the FIC is the ratio between the MIC of the drug in combination with the MIC alone. The combination was classified as synergistic (FICI ≤ 0.5), additive (1> FICI > 0.5), indifferent (4 > FICI > 1) and antagonistic (FICI ≥ 4) [21]. The assays were performed in triplicate on independent experiments.
2.4. Antibiofilm Assay
The inhibition of IBC against MSSA and MRSA biofilm formation was evaluated using broth microdilution methodology proposed by CLSI (2012) [18]. The minimum biofilm inhibitory concentration (MBIC) was established as the concentration of IBC able to inhibit 50% or more of biofilm formation [22]. The MBIC values were determined using two protocols, including biomass assessment by optical density (OD) reading and determination of viable biofilm cells by counting colony-forming units per milliliter (CFU/mL). Two microplates were used for each protocol. Assays were performed in triplicate in three independent experiments. The inoculum concentration and optimal incubation time for this assay were determined by standardizing biofilm formation (data not shown).
Briefly, in 96-well flat-bottom microplates containing brain heart infusion (BHI) broth supplemented with 2% glucose, serial dilutions of the samples were made from the stock solution (1600 µg/mL), obtaining a final concentration between 0.195 to 400 µg/mL. From a 24 h culture on BHI agar plates, the inoculum of S. aureus was prepared in BHI broth supplemented with 2% glucose, with equivalent turbidity on a spectrophotometer operating at 625 nm, to match 0.5 in the McFarland scale (1.5 × 108 CFU/mL). The bacterial suspensions were diluted to the final concentration of 1.0 × 106 CFU/mL. The microplates were incubated at 37 °C for 24 h. Subsequently, the contents of the wells were aspirated, and non-adhered cells were removed by washing with a phosphate-buffered saline (PBS) buffer (pH = 7.2). The biofilm formed was fixed with methanol for 15 min, dried at room temperature and stained with a crystal violet solution (0.2%) for 20 min. After removing the crystal and washing the wells with the PBS buffer, 33% acetic acid was added for 30 min to solubilize the crystal retained in the biofilm. The absorbance of the wells was determined in a spectrophotometer at 595 nm. The determination of MBIC was performed using Equation (2) [22,23].
| MBIC = (A595 of the test ÷ A595 of the untreated control) × 100 | (2) |
For the determination of viable cells, plates were prepared following the same methodology described above. After the incubation period, the contents of each well were aspirated and washed with PBS buffer to remove non-adhered cells. Then, 200 µL of BHI broth with 2% glucose was added to the wells and the microplate was subjected to an ultrasound bath for 15 min. The content of the wells was homogenized, and decimal dilutions were performed (100 to 10−7). After that, 50 µL aliquots of each dilution were plated on BHI agar plates and incubated at 37 °C for 24 h. Finally, the colonies were counted, and the results were expressed in log10 scale (CFU/mL).
Vancomycin was used as positive control and was tested in concentrations ranging from 0.0115 to 5.9 µg/mL. Bacterial cells were evaluated in the absence of the antibacterial compounds and were used as negative control. Antibiofilm assay data were analyzed by nonparametric Kruskal–Wallis one-way analysis of variance (ANOVA) with a Steel–Dwass–Critchlow–Fligner pairwise comparison test. Results were considered statistically significant with p < 0.05.
2.5. Membrane Disruption Assay
For the membrane permeabilization assay, Bacillus subtilis strain 168 was kindly donated by Dr. Frederico Gueiros-Filho, Department of Biochemistry, Institute of Chemistry, São Paulo University.
B. subtilis was cultivated in LB/LB-agar at 30 °C, with stirring at 200 rpm for liquid medium. A stock solution of IBC at 10 mg/mL was diluted into the wells of a 96-multiwell plate to furnish the final concentrations ranging from 100 to 0.781 µg/mL, in total volumes of 100 µL/well (NYG medium) [24,25]. The bacteria were inoculated at 1.0 × 105 cells per 100 µL of LB medium per well. Plates were incubated for 12 h at 30 °C. 15 µL of 0.1 mg/mL resazurin Sigma-Aldrich (Taufkirchen, Germany) was added into each well, followed by a 2 h incubation at 30 °C. The post-reaction plates were evaluated through excitation and emission wavelengths at 530 and 590 nm, respectively, using the Fluorescence Synergy H1N1. The data obtained were used to plot the concentration of the compound versus cell growth inhibition, and through a polynomial curve regression, it was possible to determine the percentages able to inhibit cellular metabolism [26].
The minimum bactericidal concentration (MBC) was established by inoculating the contents of each REMA well into a 15 cm Petri dish with solid LB medium before the resazurin addition. The microbial transfer was aided by a stamping replicator fit for a 96-well microtiter plate. The cells were incubated in triplicates at 30 °C for 24 h to quantify their growth.
Cells of B. subtilis were exposed to the IBC at its MBC. In 1 microcentrifuge tubes, 100 μL of 1.0 × 105 cells were used per treatment. After 15 min, 900 μL of saline solution (0.85%) were added to each tube to dilute the compound and stop the contact reaction. For the membrane integrity analyses, cells were stained using the Live/Dead BacLight kit following the instructions of the manufacturer. Cells treated with 1% DMSO and nisin (5 µg/mL) were used as negative control and positive control, respectively [26]. Cells were immobilized on agarose-covered slides before the microscope observations with Olympus BX-61 microscope, equipped with a monochromatic OrcaFlash-2.8 camera. Image acquisition and processing were performed with the software CellSens version 11 (Olympus). Data analyses were conducted with a minimum of 100 cells per treatment [27].
2.6. Cytotoxicity Assay
Human keratinocytes cells (HaCaT) were cultivated in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU/mL), streptomycin (100 μg/mL), and glutamine (2 mmol/L) (ThermoFisher, Waltham, MA, USA) in an incubator with 5% CO₂ at 37 °C (Isotemp Fisher Scientific, Pittsburgh, PA, USA), being subcultured every 2 days. The cells were seeded (5 × 105 cells/well) and pre-incubated for 24 h. After that, the cells were treated with IBC and standard drug (chlorhexidine) at concentrations ranging from 25 to 0.39 μg/mL in 96 wells microplates for 24 h. Then, the culture medium was aspirated, and the cells were incubated with resazurin (70 μM, Sigma Aldrich) in the culture medium and re-incubated for another 4 h. Cell viability was read in a spectrophotometer (Biotek, Winooski, VT, USA) at wavelengths of 570 and 600 m. The values were converted into a percentage of cell viability in comparison with the negative control (DMEM), which was defined as having 100% cell metabolism. The means were determined for each compound [28].
3. Results and Discussion
3.1. Isobavachalcone (IBC)
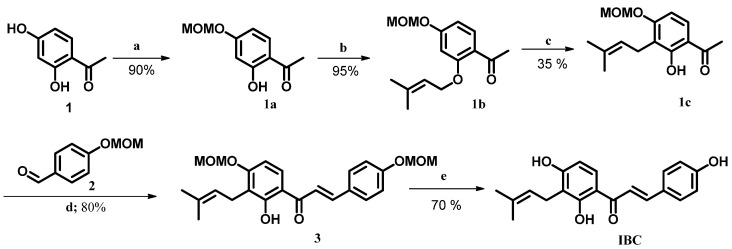
Scheme 1 shows our synthetic route for synthesis of IBC. As illustrated, resacetophenone (1) was used as accessible starting material. A set of six steps, including MOM protection/desprotection, [1,3]-sigmatropic rearrangement and Claisen–Schmidt reactions furnished IBC with an overall yield of 12% (Scheme 1). NMR parameters, including chemical shifts, coupling constants and multiplicities as well as molecular weight corresponded to IBC structure and were compared with former literature reports [8,11]. HPLC-PAD analysis indicated 99% purity according to area peak at 372 nm.
Scheme 1.
Synthesis of IBC. Reagents and conditions: (a) MOMCl, K2CO3, acetone, rt, 2 h; (b) isoprenyl bromide, K2CO3, acetone, rt, 24 h; (c) montmorillonite K10, DCM, rt, 0.5 h; (d) KOH 60%, EtOH, rt, 2 h; (e) HCl 1 mol L−1, MeOH:THF (1:1), 55 °C, 6 h. MOM = methoxymethyl.
3.2. Antibacterial and Antimycobacterial Activities
In order to assess the antibacterial and antimycobacterial activities, IBC was evaluated against five Gram-positive, two Gram-negative and three Mycobacterium species (Table 1). Among these species, five species are in the World Health Organization (WHO) priority list, justifying the discovery of innovative antibacterial agents [18]. IBC was active against S. aureus, S. pneumoniae, S. sanguinis, S. sobrinus and S. mutans planktonic cells, exhibiting MIC values ranging from 1.56 to 50.0 μg/mL. Among these, IBC displayed potent activity against MSSA and MRSA, demonstrating MIC values of 1.56 and 3.12 μg/mL. These data are according to studies on anti-Staphylococcus aureus effect of IBC against standard strains and clinical isolates [29,30,31,32]. IBC was inactive against P. aeruginosa and K. pneumoniae planktonic cells (MIC > 400 µg/mL).
Table 1.
Antibacterial and antimycobacterial activities of IBC.
| Species | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| IBC | Tetracycline | Chlorhexidine | Isoniazid | ||
| Gram-positive | MSSA | 1.56 | 0.20 | - | - |
| MRSA | 3.12 | >5.90 | - | - | |
| Streptococcus pneumoniae | 50.0 | 0.40 | - | - | |
| Streptococcus sanguinis | 3.12 | - | 1.82 | - | |
| Streptococcus sobrinus | 6.25 | - | 3.64 | - | |
| Streptococcus mutans | 6.25 | - | 0.91 | - | |
| Mycobacterium avium | 62.5 | - | - | 0.50 | |
| Mycobacteria | Mycobacterium kansasii | 62.5 | - | - | 1.00 |
| Mycobacterium tuberculosis | 62.5 | - | - | >1.00 | |
| Gram-negative | Pseudomonas aeruginosa | >400 | 5.90 | - | - |
| Klebsiella pneumoniae | >400 | 2.95 | - | - | |
MSSA = Methicillin-susceptible Staphylococcus aureus; MRSA = Methicillin-resistant Staphylococcus aureus.
Antimycobacterial assays indicated MIC values of 62.5 μg/mL against M. avium, M. kansasii and M. tuberculosis. Two studies described the antitubercular activity of IBC against M. tuberculosis [33,34]. In addition, IBC was described as an inhibitor of extracellular nuclease Rv0888 from M. tuberculosis H37Rv, which acts as a virulence factor and could be related to the persistence of M. tuberculosis in the host [35].
3.3. Checkerboard Assay
In anti-infective drug therapy, the association of drugs is a useful strategy to treat bacterial infections, enhancing the antibacterial potency and decreasing side effects [36,37]. In this context, hydroxychalcones and aminochalcones showed synergistic association with vancomycin [38,39]. Considering the potent activity of IBC against S. aureus, we used this species for the checkerboard assay. We evaluated the effect of the combination of IBC with vancomycin against MSSA and MRSA planktonic cells (Table 2). The association between IBC and vancomycin displayed FICI values of 2.5 and 2.0 against MSSA and MRSA, respectively, indicating an indifferent association.
Table 2.
Antibacterial effect of combination of IBC and vancomycin against MSSA and MRSA.
| S. aureus Strain | Combination | MIC (µg/mL) | FICI | Type of Combination | |||
|---|---|---|---|---|---|---|---|
| Alone | Combined | FICIBC + FICVAN | |||||
| IBC | VAN | IBC | VAN | ||||
| MRSA | IBC + VAN | 3.12 | 0.73 | 1.56 | 1.47 | 2.5 | indifferent |
| MSSA | IBC + VAN | 1.56 | 0.73 | 1.56 | 0.73 | 2.0 | indifferent |
VAN = vancomycin.
3.4. Antibiofilm Assay
Biofilm can be described as a highly organized sessile community of cells, which are attached to a substratum, interface and complex polymeric matrix [40]. S. aureus has been known to infect and form a chronic biofilm infection in medical devices, being the most common pathogen associated with nosocomial infections [41,42]. Several chronic infections are associated with S. aureus biofilms, including osteomyelitis, periodontitis, chronic wound infections, chronic rhinosinusitis, endocarditis and ocular infections. After the attachment stage, S. aureus biofilms are difficult to eradicate with conventional antibacterial agents and the host response [41].
Current antibacterial drugs have not been effective against S. aureus biofilms, requiring its surgical removal [43]. Some therapeutic alternatives have been investigated, including antibacterial peptides, vaccines, matrix-degrading enzymes, modulation of quorum-sense system and small molecules inhibitors. Most of them are in preclinical development stage [41,43]. Xanthohumol, a prenylated chalcone from hop extracts, showed a potent anti-adherent and antibiofilm activities against S. aureus [44,45]. As part of our efforts in the discovery of novel antibiofilm agents, we described the effect of chalcones against bacterial and fungal biofilms [38,46,47].
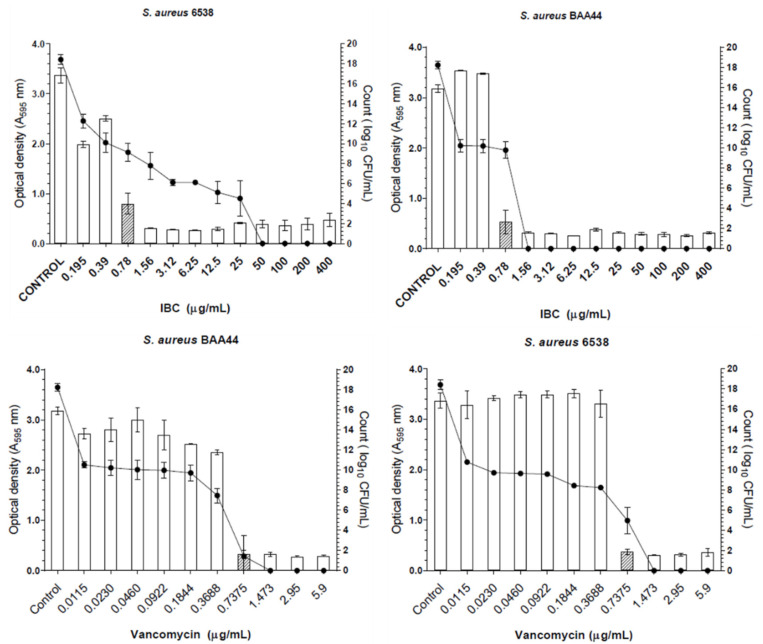
IBC and vancomycin were evaluated in concentrations ranging from 0.195 to 400 µg/mL and 0.0115 to 5.9 µg/mL, respectively. IBC displayed antibiofilm activity with MBIC values of 0.78 µg/mL against MSSA and MRSA (Figure 2). At this concentration, IBC was able to inhibit 75% biofilm formation. Vancomycin displayed MBIC value of 0.74 µg/mL against MSSA and MRSA, demonstrating 90% biofilm formation inhibition. Moreover, in the presence of IBC at MBIC value, the number of viable cells of MSSA and MRSA decreased about 9 CFU/mL when compared with the untreated control.
Figure 2.
Effect of IBC and vancomycin on MSSA (S. aureus 6538) and MRSA (S. aureus BAA44) biofilm formation. The hatched bars represent the MBIC values. The lines represent the log10 CFU/mL.
3.5. Membrane Disruption Assay
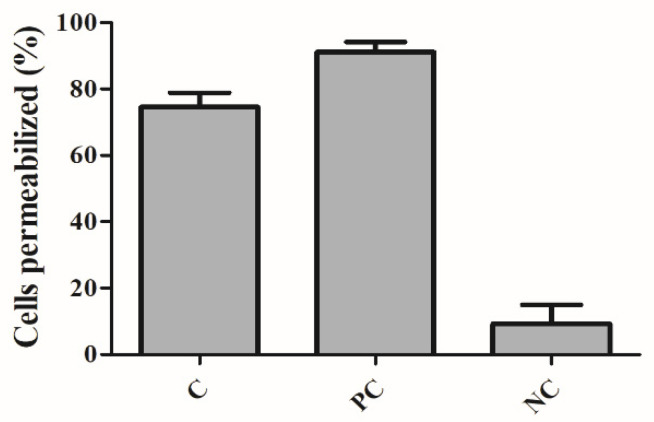
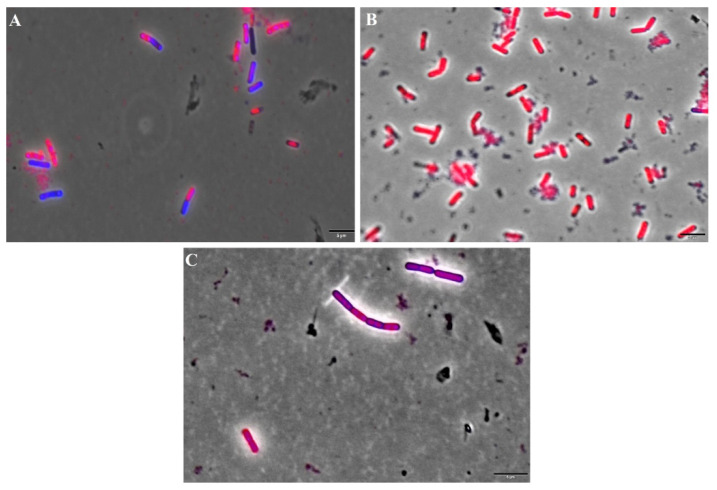
In order to investigate the action of IBC on the bacterial membrane, we used B. subtilis, which is a non-pathogenic Gram-positive bacteria used for studies of antibacterial mode of action [26]. Bacterial cells were treated with IBC at its MBC value (3.13 µg/mL) for 15 min, as well as with nisin (positive control), an antibiotic that targets the bacterial membrane, producing pores [48]. Two fluorescent dyes propidium iodide (PI) and SYTO9 were added, which stain cells with disrupted membranes (in red) and intact membranes (in blue) [49]. Representative fluorescence microscopy images of the negative control (cells treated with 1% DMSO), the positive control (nisin at 5.0 µg/mL) and IBC were presented in Figure 3. The percentages of cells with damaged membranes were quantified from the microscope images (Figure 4). Cultures treated with 1% DMSO had approximately 10% of the cells with membranes permeabilized. Treatment with nisin demonstrated 95% of the cells stained with PI/SYTO9 due to its ability to makes pores in the bacterial membrane. Treatment of IBC displayed damage percentages of 75%.
Figure 3.
Percentage of disrupted Bacillus subtilis cells. C: cells treated with IBC; PC: cells treated with nisin (positive control) and NC: cells treated with 1% DMSO (negative control).
Figure 4.
Fluorescence microscopy of Bacillus subtilis stained with propidium iodide and SYTO9. (A) negative control (cells treated with 1% DMSO); (B) positive control (cells treated with nisin at 5.0 µg/mL). (C) cells treated with IBC. Magnification 100×; Scale bar 5 μm.
Our finds are corroborated by other studies, which reported that the antibacterial mode of action of IBC is related to the disrupting action on bacterial membranes. IBC was able to cause damage to the MSSA membrane, evidenced by diS-C3-(5) dye experiments, leading to macromolecular biosynthesis inhibition [29]. Song and collaborators described how IBC binds to the phospholipids of MSSA membrane, resulting in the dissipation of proton motive force and metabolic perturbations [50]. Palko-Labuz and coauthors identified IBC as membrane-perturbing agent of human colorectal adenocarcinoma cells. IBC was able to be intercalated into model membranes, affecting phospholipid phase transition [51]. Additionally, the antibacterial effect of IBC has been correlated with the leakage of alkaline phosphatase (AKP) due to the impairment of the cell wall and cell membrane damage, the inhibition of protein and nucleic acids biosynthesis as well as the inhibition of energy metabolism [30,52].
3.6. Cytotoxicity Assay
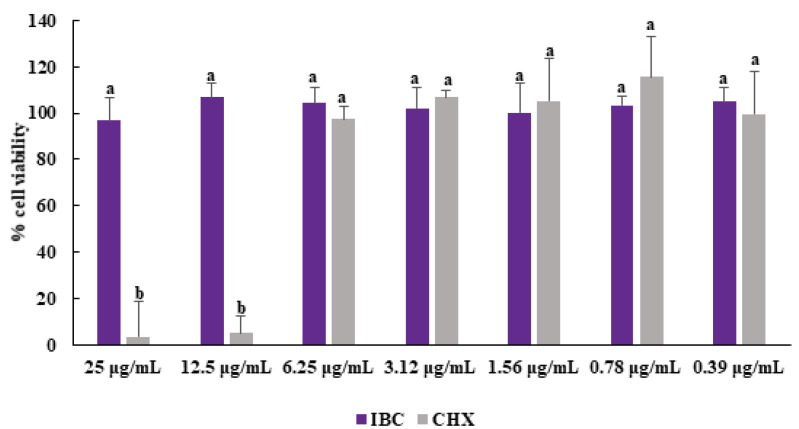
The evaluation of new antibacterial compounds against human cells is an important step for investigations of their selectivity and safety. The toxicity of IBC was tested against epidermal human keratinocytes (HaCaT cell line), which was chosen because skin is a typical site of S. aureus colonization [53]. IBC was not able to reduce the cell viability of HaCaT cells at the highest concentrations (25 µg/mL) after 24 h. (Figure 5). This concentration is approximatively 20 times higher than the MIC values obtained from assays MSSA and MRSA, indicating IBC has selectivity at its antibacterial concentration. Moreover, IBC demonstrated to be less cytotoxic than chlorhexidine at concentrations equal to or higher than 12.5 µg/mL.
Figure 5.
Effect of IBC and chlorhexidine on the viability of HaCaT cells. The results are expressed as means ± SDs. Different lowercase letters (a, b) show statistical differences between IBC and CHX according to ANOVA and Tukey’s test (p <0.05).
In spite of the non-toxic effect of IBC against skin cells, several studies have reported its toxicity against leukemic cells [12] and solid tumor cells [54], including colorectal (HCT116) [55], tongue (Tca 8113) [56], liver (HepG2) [57], breast (MCF-7) [58], prostate (PC-3) [59], gastric (MGC803) [60], cervical (HeLa) [61], ovarian (OVCAR-08) [62] and neuroblastoma (IMR-32) [63]. The mechanism of IBC cytotoxicity is related to apoptosis induction via the mitochondrial pathway, decreasing its transmembrane potential [55,57,60,61,62,63].
4. Conclusions
In summary, we newly synthesized and evaluated IBC as part of our ongoing search for antibacterial agents. IBC has potent activity against Gram-positive (MIC = 1.56–50.0 µg/mL) and Mycobacterium species (MIC = 62.5 µg/mL). The combination of IBC and vancomycin exhibited indifferent effects against MSSA and MRSA planktonic cells. Antibiofilm activity of IBC was equipotent to vancomycin, displaying similar MBIC values. The mode of action of IBC involved membrane disruption, which is a crucial target for the bacterial survival. Furthermore, investigations of toxicity against human keratinocytes indicated that IBC is a selective compound. Altogether, our findings open new avenues for IBC as an antibacterial agent, with potential applications as a drug candidate and medical device coating.
Acknowledgments
The authors would like to thank the Multiuser Centre for Biomolecular Innovation (CMIB/Fapesp Grant 2009/53989-4) for NMR experiments. LRA thanks CAPES (Finance code 001) for her scholarship.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/membranes12030269/s1, Figure S1: HPLC-PAD chromatogram of IBC. Methanol:Water (3:1), 372 nm, Figure S2. UV-Vis spectra of IBC, Figure S3. 1H NMR spectrum of IBC (acetone-d6; 600 MHz), Figure S4. 13C NMR spectrum of IBC (acetone-d6; 150 MHz), Figure S5. Mass spectrum (MS) of IBC (electrospray, positive mode).
Author Contributions
L.R.d.A., R.d.S.T., M.B.S.C., J.A.S.N., M.D.d.R., M.A.d.S.B., R.d.P.M., G.D., G.B.H. and V.R.d.S.; data curation, formal analysis, methodology, writing—original draft. C.D., H.F., C.H.G.M. and L.O.R.; Resources, funding acquisition, investigation, supervision, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES), the National Research Council (CNPq) (Grants 471129/2013-5; 306251/2016-7, 429322/2018-6 and 309957/2019-2), and the São Paulo Research Foundation (FAPESP) (Grants 2014/18330-0 and 2018/15083-2). The APC was funded by FAPESP.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vikesland P., Garner E., Gupta S., Kang S., Maile-Moskowitz A., Zhu N. Differential Drivers of Antimicrobial Resistance across the World. Acc. Chem. Res. 2019;52:916–924. doi: 10.1021/acs.accounts.8b00643. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Government of the United Kingdom; London, UK: 2016. The Review on Antimicrobial Resistance; pp. 1–80. [Google Scholar]
- 3.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from January 1981 to September 2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 4.Rossiter S.E., Fletcher M.H., Wuest W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017;117:12415–12474. doi: 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuete V., Sandjo L.P. Isobavachalcone: An overview. Chin. J. Integr. Med. 2012;18:543–547. doi: 10.1007/s11655-012-1142-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang M., Lin L., Lu J.-J., Chen X. Pharmacological review of isobavachalcone, a naturally occurring chalcone. Pharmacol. Res. 2021;165:105483. doi: 10.1016/j.phrs.2021.105483. [DOI] [PubMed] [Google Scholar]
- 7.Dong X., Chen J., Jiang C., Liu T., Hu Y. Design, synthesis, and biological evaluation of prenylated chalcones as vasorelaxant agents. Arch. Pharm. 2009;342:428–432. doi: 10.1002/ardp.200800229. [DOI] [PubMed] [Google Scholar]
- 8.Sugamoto K., Matsusita Y.I., Matsui K., Kurogi C., Matsui T. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron. 2011;67:5346–5359. doi: 10.1016/j.tet.2011.04.104. [DOI] [Google Scholar]
- 9.Grealis J.P., Müller-Bunz H., Ortin Y., Casey M., McGlinchey M.J. Synthesis of isobavachalcone and some organometallic derivatives. Eur. J. Org. Chem. 2013:332–347. doi: 10.1002/ejoc.201201063. [DOI] [Google Scholar]
- 10.Wang H., Yan Z., Lei Y., Sheng K., Yao Q., Lu K., Yu P. Concise synthesis of prenylated and geranylated chalcone natural products by regiospecific iodination and Suzuki coupling reactions. Tetrahedron Lett. 2014;55:897–899. doi: 10.1016/j.tetlet.2013.12.044. [DOI] [Google Scholar]
- 11.Wang H., Zhang L., Liu J., Yang Z.L., Zhao H.Y., Yang Y., Shen D., Lu K., Fan Z.C., Yao Q.W., et al. Synthesis and anti-cancer activity evaluation of novel prenylated and geranylated chalcone natural products and their analogs. Eur. J. Med. Chem. 2015;92:439–448. doi: 10.1016/j.ejmech.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Yang L., Song L., Zhao S., Ma C., Wu D., Wu Y.L. Isobavachalcone reveals novel characteristics of methuosis-like cell death in leukemia cells. Chem. Biol. Interact. 2019;304:131–138. doi: 10.1016/j.cbi.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 13.ElSohly H.N., Joshi A.S., Nimrod A.C., Walker L.A., Clark A.M. Antifungal Chalcones from Maclura tinctoria. Planta Med. 2001;67:87–89. doi: 10.1055/s-2001-10621. [DOI] [PubMed] [Google Scholar]
- 14.Abdullah S.A., Jamil S., Basar N., Abdul Lathiff S.M., Mohd Arriffin N. Flavonoids from the leaves and heartwoods of Artocarpus lowii King and their bioactivities. Nat. Prod. Res. 2017;31:1113–1120. doi: 10.1080/14786419.2016.1222387. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q.X., Hu Y., Li G.Y., Xu W., Zhang Y.T., Yang X.W. Multi-target anti-Alzheimer activities of four prenylated compounds from Psoralea fructus. Molecules. 2018;23:614. doi: 10.3390/molecules23030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin H.J., Shon D.H., Youn H.S. Isobavachalcone suppresses expression of inducible nitric oxide synthase induced by Toll-like receptor agonists. Int. Immunopharmacol. 2013;15:38–41. doi: 10.1016/j.intimp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Leandro L.F., Cardoso M.J.O., Silva S.D.C., Souza M.G.M., Veneziani R.C.S., Ambrosio S.R., Martins C.H.G. Antibacterial activity of Pinus elliottii and its major compound, dehydroabietic acid, against multidrug-resistant strains. J. Med. Microbiol. 2014;63:1649–1653. doi: 10.1099/jmm.0.081711-0. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute (CLSI) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 9th ed. Volume 32 CLSI; Wayne, PA, USA: 2012. [Google Scholar]
- 19.Palomino J.C., Martin A., Camacho M., Guerra H., Swings J., Portaels F. Resazurin Microtiter Assay Plate: Simple and Inexpensive Method for Detection of Drug Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002;46:2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves J.A., Abrão F., Moraes T.D.S., Damasceno J.L., da Silva Moraes M.F., Veneziani R.C.S., Ambrósio S.R., Bastos J.K., Miranda M.L.D., Martins C.H.G. Investigation of Copaifera genus as a new source of antimycobaterial agents. Futur. Sci. OA. 2020;6:FSO587. doi: 10.2144/fsoa-2020-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White R.L., Burgess D.S., Manduru M., Bosso J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 1996;40:1914–1918. doi: 10.1128/AAC.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei G.X., Campagna A.N., Bobek L.A. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J. Antimicrob. Chemother. 2006;57:1100–1109. doi: 10.1093/jac/dkl120. [DOI] [PubMed] [Google Scholar]
- 23.Vieira R.G.L., Moraes T.S., Silva L.O., Bianchi T.C., Veneziani R.C.S., Ambrósio S.R., Bastos J.K., Pires R.H., Martins C.H.G. In vitro studies of the antibacterial activity of Copaifera spp. oleoresins, sodium hypochlorite, and peracetic acid against clinical and environmental isolates recovered from a hemodialysis unit. Antimicrob. Resist. Infect. Control. 2018;7:14. doi: 10.1186/s13756-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning: A Laboratory Manual. 4th ed. Volume 1 Cold Spring Harbor Protocols; New York, NY, USA: 2012. [Google Scholar]
- 25.Silva I.C., Regasini L.O., Petrônio M.S., Silva D.H.S., Bolzani V.S., Belasque J., Sacramento L.V.S., Ferreira H. Antibacterial activity of alkyl gallates against Xanthomonas citri subsp. Citri. J. Bacteriol. 2013;195:85–94. doi: 10.1128/JB.01442-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morão L.G., Polaquini C.R., Kopacz M., Torrezan G.S., Ayusso G.M., Dilarri G., Cavalca L.B., Zielińska A., Scheffers D.J., Regasini L.O., et al. A simplified curcumin targets the membrane of Bacillus subtilis. Microbiologyopen. 2019;8:e00683. doi: 10.1002/mbo3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins P.M.M., Lau I.F., Bacci M., Belasque J., Amaral A.M., Taboga S.R., Ferreira H. Subcellular localization of proteins labeled with GFP in Xanthomonas citri ssp. citri: Targeting the division septum. FEMS Microbiol. Lett. 2010;310:76–83. doi: 10.1111/j.1574-6968.2010.02047.x. [DOI] [PubMed] [Google Scholar]
- 28.Kreling P.F., Aida K.L., Massunari L., Caiaffa K.S., Percinoto C., Bedran T.B.L., Spolidorio D.M.P., Abuna G.F., Cilli E.M., Duque C. Cytotoxicity and the effect of cationic peptide fragments against cariogenic bacteria under planktonic and biofilm conditions. Biofouling. 2016;32:995–1006. doi: 10.1080/08927014.2016.1218850. [DOI] [PubMed] [Google Scholar]
- 29.Dzoyem J.P., Hamamoto H., Ngameni B., Ngadjui B.T., Sekimizu K. Antimicrobial Action Mechanism of Flavonoids from Dorstenia Species. Drug Discov. Ther. 2013;7:66–72. doi: 10.5582/ddt.2013.v7.2.66. [DOI] [PubMed] [Google Scholar]
- 30.He N., Zhou J., Hu M., Ma C., Kang W. The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L. Open Chem. 2018;16:882–889. doi: 10.1515/chem-2018-0091. [DOI] [Google Scholar]
- 31.Cui Y., Taniguchi S., Kuroda T., Hatano T. Constituents of Psoralea corylifolia fruits and their effects on methicillin-resistant Staphylococcus aureus. Molecules. 2015;20:12500–12511. doi: 10.3390/molecules200712500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osório T.M., Monache F.D., Chiaradia L.D., Mascarello A., Stumpf T.R., Zanetti C.R., Silveira D.B., Barardi C.R.M., Smânia E.D.F.A., Viancelli A., et al. Antibacterial activity of chalcones, hydrazones and oxadiazoles against methicillin-resistant Staphylococcus aureus. Bioorganic Med. Chem. Lett. 2012;22:225–230. doi: 10.1016/j.bmcl.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 33.Kuete V., Ngameni B., Mbaveng A.T., Ngadjui B., Meyer J.J.M., Lall N. Evaluation of flavonoids from Dorstenia barteri for their antimycobacterial, antigonorrheal and anti-reverse transcriptase activities. Acta Trop. 2010;116:100–104. doi: 10.1016/j.actatropica.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Chiang C.C., Cheng M.J., Peng C.F., Huang H.Y., Chen I.S. A novel dimeric coumarin analog and antimycobacterial constituents from Fatoua pilosa. Chem. Biodivers. 2010;7:1728–1736. doi: 10.1002/cbdv.200900326. [DOI] [PubMed] [Google Scholar]
- 35.Dang G., Cao J., Cui Y., Song N., Chen L., Pang H., Liu S. Characterization of Rv0888, a Novel Extracellular Nuclease from Mycobacterium tuberculosis. Sci. Rep. 2016;6:19033. doi: 10.1038/srep19033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuuren S.V., Viljoen A. Plant-based antimicrobial studies methods and approaches to study the interaction between natural products. Planta Med. 2011;77:1168–1182. doi: 10.1055/s-0030-1250736. [DOI] [PubMed] [Google Scholar]
- 37.Cottarel G., Wierzbowski J. Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol. 2007;25:547–555. doi: 10.1016/j.tibtech.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Garcia M.A.R., Theodoro R.S., Sardi J.C.O., Santos M.B., Ayusso G.M., Pavan F.R., Costa A.R., Santa Cruz L.M., Rosalen P.L., Regasini L.O. Design, synthesis and antibacterial activity of chalcones against MSSA and MRSA planktonic cells and biofilms. Bioorg. Chem. 2021;116:105279. doi: 10.1016/j.bioorg.2021.105279. [DOI] [PubMed] [Google Scholar]
- 39.Tran T.D., Nguyen T.T.N., Do T.H., Huynh T.N.P., Tran C.D., Thai K.M. Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics. Molecules. 2012;17:6684–6696. doi: 10.3390/molecules17066684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin W., Wang Y., Liu L., He J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019;20:3423. doi: 10.3390/ijms20143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Archer N.K., Mazaitis M.J., Costerton J.W., Leid J.G., Powers M.E., Shirtliff M.E. Staphylococcus aureus biofilms: Properties, regulation and roles in human disease. Virulence. 2011;2:445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otto M. Staphylococcal Biofilms. Microbiol. Spectr. 2018;6 doi: 10.1128/microbiolspec.GPP3-0023-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhattacharya M., Wozniak D.J., Stoodley P., Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti. Infect. Ther. 2015;13:1499–1516. doi: 10.1586/14787210.2015.1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rozalski M., Micota B., Sadowska B., Stochmal A., Jedrejek D., Wieckowska-Szakiel M., Rozalska B. Antiadherent and antibiofilm activity of Humulus lupulus L. derived products: New pharmacological properties. Biomed Res. Int. 2013 doi: 10.1155/2013/101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bogdanova K., Röderova M., Kolar M., Langova K., Dusek M., Jost P., Kubelkova K., Bostik P., Olsovska J. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018;169:127–134. doi: 10.1016/j.resmic.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Sardi J.C.O., Polaquini C.R., Freires I.A., de Carvalho Galvão L.C., Lazarini J.G., Torrezan G.S., Regasini L.O., Rosalen P.L. Antibacterial activity of diacetylcurcumin against Staphylococcus aureus results in decreased biofilm and cellular adhesion. J. Med. Microbiol. 2017;66:816–824. doi: 10.1099/jmm.0.000494. [DOI] [PubMed] [Google Scholar]
- 47.de Almeida Sayão de Emeri F.T., Rosalen P.L., Paganini É.R., Garcia M.A.R., Nazaré A.C., Lazarini J.G., de Alencar S.M., Regasini L.O., de Cassia Orlandi Sardi J. Antimicrobial activity of nitrochalcone and pentyl caffeate against hospital pathogens results in decreased microbial adhesion and biofilm formation. Biofouling. 2019;35:129–142. doi: 10.1080/08927014.2019.1574763. [DOI] [PubMed] [Google Scholar]
- 48.Wiedemann I., Benz R., Sahl H.G. Lipid II-Mediated Pore Formation by the Peptide Antibiotic Nisin: A Black Lipid Membrane Study. J. Bacteriol. 2004;186:3259–3261. doi: 10.1128/JB.186.10.3259-3261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navarro M.O.P., Dilarri G., Simionato A.S., Grzegorczyk K., Dealis M.L., Cano B.G., Barazetti A.R., Afonso L., Chryssafidis A.L., Ferreira H., et al. Determining the Targets of Fluopsin C Action on Gram-Negative and Gram-Positive Bacteria. Front. Microbiol. 2020;11:1076. doi: 10.3389/fmicb.2020.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song M., Liu Y., Li T., Liu X., Hao Z., Ding S., Panichayupakaranant P., Zhu K., Shen J. Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. 2021;8:2100749. doi: 10.1002/advs.202100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palko-Labuz A., Blaszczyk M., Sroda-Pomianek K., Wesolowska O. Isobavachalcone as an active membrane perturbing agent and inhibitor of ABCB1 multidrug transporter. Molecules. 2021;26:4637. doi: 10.3390/molecules26154637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cushnie T.P.T., Lamb A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Gong J.Q., Lin L., Lin T., Hao F., Zeng F.Q., Bi Z.G., Yi D., Zhao B. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: A double-blind multicentre randomized controlled trial. Br. J. Dermatol. 2006;155:680–687. doi: 10.1111/j.1365-2133.2006.07410.x. [DOI] [PubMed] [Google Scholar]
- 54.Kuete V., Mbaveng A.T., Zeino M., Fozing C.D., Ngameni B., Kapche G.D.W.F., Ngadjui B.T., Efferth T. Cytotoxicity of three naturally occurring flavonoid derived compounds (artocarpesin, cycloartocarpesin and isobavachalcone) towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2015;22:1096–1102. doi: 10.1016/j.phymed.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Li Y., Qin X., Li P., Zhang H., Lin T., Miao Z., Ma S. Isobavachalcone isolated from Psoralea corylifolia inhibits cell proliferation and induces apoptosis via inhibiting the AKT/GSK-3β/β-catenin pathway in colorectal cancer cells. Drug. Des. Dev. Ther. 2019;13:1449–1460. doi: 10.2147/DDDT.S192681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Y., Wu W., Huo A., Zhou W., Zhu Z. Inhibitory effect of isobavachalcone on migration and invasion of Tca8113 cells and its mechanism. Chin. Pharmacol. Bull. 2015;3:1741–1745. doi: 10.369/j.issn.1001-1978.201.12.022. [DOI] [Google Scholar]
- 57.Li B., Xu N., Wan Z., Ma L., Li H., Cai W., Chen X., Huang Z.H. Isobavachalcone exerts anti-proliferative and pro-apoptotic effects on human liver cancer cells by targeting the ERKs/RSK2 signaling pathway. Oncol. Rep. 2019;41:3355–3366. doi: 10.3892/or.2019.7090. [DOI] [PubMed] [Google Scholar]
- 58.Shi J., Chen Y., Chen W., Tang C., Zhang H., Chen Y., Yang X., Xu Z., Wei J., Chen J. Isobavachalcone sensitizes cells to E2-induced paclitaxel resistance by down-regulating CD44 expression in ER+ breast cancer cells. J. Cell. Mol. Med. 2018;11:5220–5230. doi: 10.1111/jcmm.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li K., Zheng Q., Chen X., Wang W., Wang D., Wang J. Isobavachalcone induces ROS-mediated apoptosis via targeting thioredoxin reductase 1 in human prostate cancer PC-3 cells. Oxid. Med. Cell. Longev. 2018;2018:1915828. doi: 10.1155/2018/1915828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin X., Shi Y. Isobavachalcone induces the apoptosis of gastric cancer cells via inhibiton of Akt and Erk pathways. Exp. Ther. Med. 2016;11:403–408. doi: 10.3892/etm.2015.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szliszka E., Jaworska D., Ksek M., Czuba Z., Krol W. Targeting death receptor TRAIL-R2 by chalcones for TRAIL-induced apoptosis in cancer cells. Int. J. Mol. Sci. 2012;13:15343–15359. doi: 10.3390/ijms131115343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jing H., Zhou X., Dong X., Cao J., Zhu H., Lou J., Hu Y., He Q., Yang B. Abrogation of Akt signaling by isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett. 2010;294:167–177. doi: 10.1016/j.canlet.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 63.Nishimura R., Tabata K., Arakawa M., Ito Y., Kimura Y., Akihisa T., Nagai H., Sakuma A., Kohno H., Suzuki T. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 2007;30:1878–1883. doi: 10.1248/bpb.30.1878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.