Abstract
Congestive Heart Failure (CHF) is an emerging epidemic. Within one generation, the medical community has learned much of CHF syndromes. It has two distinct mechanisms, systolic and diastolic abnormalities, to account for the common CHF presentation. It is complex as it challenges the available health care services, resource, and funding models in providing an equitable service across the health continuum. Despite the improvement in many cardiovascular diseases, some CHF outcomes like readmissions and costs have increased. The reinvigoration of evidence-based medicine, the development of health services models of care, and standardisation of disease processes with taxonomies have also occurred within the same time span. These processes, however, need to be linked with health policy as presented in white papers. In this paper, we explore achieving optimal CHF guideline-recommended outcomes as the science approaches real- world translation.
Keywords: Congestive heart failure, cost-efficacy, health services, taxonomy, white-papers, health systems
1. INTRODUCTION
“A disruptive change is absolutely necessary because our current systems are failing in efficiencies, reproducibility, disease prevention, and affordability”. Gicklich et al. [1].
The burden of disease, health outcomes, and health system cost for Congestive Heart Failure (CHF) are projected to increase [2]. This syndrome appears to be headed toward a new phase between consensus guideline-based care and the ‘real world’ realities of translatable care, cost-effectiveness, and administrative oversight. This is an important consideration, as unchecked, the gap between governmental funding and expectations of ‘gold-standard care’ could deviate further apart. The main stakeholders of this process are clients, health professionals and health administrators. Peer-reviewed publications represent client and health services and white papers the health administrators. Traditional silos are gradually breaking down with overlapping areas between the stakeholders [3-9]. However, outcome models require a more intricate link between collaboration, innovation, and lobbying. In a recent white paper, this was highlighted as their third priority [10].
The OPTIMIZE-HF study [11] was a landmark in many ways; importantly it taught us about trial findings and the importance of governance. If we replicate clinical practice with a structured process similar to what trials do and deliver guideline standard care, we can reproduce outcomes achieved in trials. As we head into a new phase for CHF, in this mix, there are the changing landscape of digital information technologies, commercialisation of health care services, expanding patient demography, diminishing infrastructure and resource distribution, and funding constraints. In this environment, healthcare is becoming part of a broader economic strategy that requires a responsive system to innovate new models of care to balance the community need, infrastructure, and funding constraints [10, 12-14]. This review explores that boundary addressed in the OPTIMIZE-HF i.e. the hospital setting, to a broader community setting. We standardise the discussion around several domains described in Krumholtz’s taxonomy of chronic disease management (Table 1) [3]. To explore the wider considerations in achieving optimal guideline-based care, we explore three areas which are important, however, less frequently discussed:
Outcome measures: The link between evidence and health services.
Method of communication: The role of health data in the new phase of health services.
Wider considerations in exploring CHF disease management: We provide a narrative example of an evolving model of care.
2. HEALTH CLUSTERS, DISEASE TAXONOMY AND HEART FAILURE GUIDELINES
“Tomorrow's outcomes should be defined when translating the evidence in a defined area using a standardised framework within a funding model that is sustainable”.
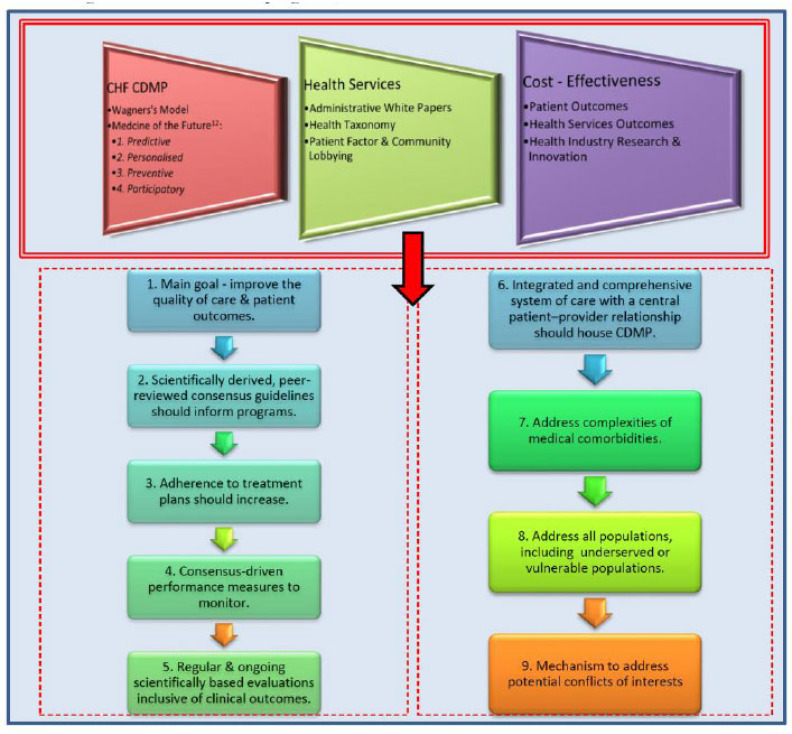
The Western evidence-based medical system is based on observations and a structured process to examine and interpret the findings. This structure pervades the health system, the diseases managed and the scientific process that gathers the evidence. Defining the components of these domains can shape the health systems we envisage (Fig. 1).
Fig. (1).
Chronic diseases programs, health services and cost-effectiveness. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Health administrators are increasingly looking for hybrid models that complement infrastructure rather than investing in new infrastructure. To achieve the main goal of optimal patient outcomes with an acceptable and sustainable budget, these four domains are important: 1. Congestive Heart Failure Chronic Disease Management Programs (CHF CDMP) - culminate from theoretical and researched concepts e.g., Wagner’s Model. Ongoing introspection and reinvention of the model, as community demography changes are important to keep them relevant; 2. Health Services - delivery of services to the public is shaped by an interplay between community need, administrative oversight and a Taxonomy framework that shapes the areas to service; 3. Health outcomes (cost-effectiveness) are achieved by translating guideline-based evidenced care. All systems invariably have push or pull factors that determine their shape; 4. The nine circles are principles and recommendations from the AHA’s expert panel on disease management and defining the foundations for health clusters. An important consideration universally is: firstly, reducing the utility of health services by improving self-management capacity; and having a broader look at local health care as an industry with data, life sciences, education, and employment capabilities to generate a local economy and finance. Models that can integrate existing infrastructure, and finding balance between evidence generation and translation, may increasingly achieve this goal [3, 15, 18, 19].
2.1. Defining Health Systems
Defining a health system today is difficult. New discoveries are frequent and individuals who subspecialise in these skills are housed in large institutions. As the population grows and are mobile, health services planning may not match the actual clinical needs, thus, the canvas on which division and compartmentalisation of health structure are an important point to base these discussions. We discuss this canvas from the concept of a ‘Health Clusters’. The Northern American Industry Classification System (NAICS) defines health care as constituting subsectors with: i. ambulatory services, ii. hospital operations, iii. nursing and residential care facilities, and iv. social assistance [12]. These nodes either through government or public-private initiatives, coalesce to form a degree of interdependence, clinically, economically, administratively and even share a future vision often within geographical or chronological boundaries . The shared goals encompass achieving optimal Key Performance Indicators (KPI) across the continuum of care with reduced capital expenditure, duplications, and integration where possible either cooperatively or competitively [14]. Thus, future thinking should envisage health clusters as part of a local economy as recipients and generators of funding [13].
2.2. Defining Disease Management
Taxonomy is the science and principles of classifying things or concepts into groups or hierarchies, including clusters, organisations, and diseases. Krumholtz et al., published a taxonomy of disease management, including 8 domains and more than 30 subdomains (Table 1). We believe this is a gold-standard document to standardise health care services, and evolving clusters [3]. Disease programs such as a heart failure program correspond to Standard Operating Protocols (SOP) for one disease within a service that uses the disease taxonomy’s foundation and populates it with evidenced care predominately from guidelines, consensus and other peer-reviewed publications. Administrative white papers are, in a sense, a blueprint of how and where governments see key areas within a taxonomy are funded. Lobbying is the final determinant of this process, particularly in regard to resourcing. The American Heart Association Expert Panel Principles and Recommendations on Disease Management [3, 15] provide a broad canvas when thinking of this journey as these also shaped the chronic disease taxonomy by Krumholtz et al., (Fig. 1).
Table 1.
Chronic disease taxonomy and model of care domains.
| Domain | Subdomains | Collaborate | Innovate | Lobby |
|---|---|---|---|---|
| 1. Patient Population | Risk Status Comorbid Condition Non-clinical Characteristics |
Pool Resources Pooling Data |
1. Data Analytics | VR |
| 2. Recipient | Patient/ Caregiver Care Provider |
Care coordination 2. Pool Resources |
1. Extension of existing works | VR |
| 3. Intervention Content | Patient Caregiver Education Medication Management Peer Support Remote Monitoring |
Standardise Document | 1. Remote Monitoring Systems 2. Medication Compliance |
VR |
| 4. Delivery Personnel | Nurses Physicians Pharmacists Social Workers Dieticians Physical Therapist Psychologist Case Manager Care Coordinators |
Pool Resources 2. Care Plans |
1. Patient Health Hub*, 2. Portable Data Storage - biometrics or patient controlled scannable technologies |
1. Case Management Structures, funding models |
| 5. Method of Communication | Face-to-face Individual Face-to-face Group Telephone In-person Telephone Mechanized Internet |
Stakeholders to regularly address options | 1. Extension of existing works 2. Translation of promising works |
1. Funding for inner-city telehealth to negate social isolation (disabled, elderly) |
| 6. Intensity and Complexity | Duration Frequency/ Periodicity Complexity |
VR | 1. Algorithms and computer-assisted learning | 1. Expansion of care plans beyond one shoe fits all 2. Self-management 3. Multidisciplinary clinics 4. Comorbidity clinics |
| 7. Environment | Hospital: Inpatient Hospital Outpatient Home-based |
Staged care - sharing of acute & convalescing capacities | 1. Extension of existing works | 1. Geographical factors may dictate |
| 8. Outcome Measure | Clinical Measures Process Measures |
Data Sharing | 1. Improve, reduce the number of KPI to better predict sensitive KPI | 1. Local guidelines and standardisations |
| 9. Others | Funding Models Future Projections Geopolitical and Economic Environments |
Define Stakeholders for health clusters | 1. Commissioned works - stakeholder input vital | 1. State 2. Federal 3. Local Council |
Clarifications: Innovation, collaboration or lobbying is a continual process and affects all domains. The points made are what appear as relevant to the arguments in this review. Number 9 ‘Others’ is a new addition to the taxonomy. We feel funding must be considered holistically as well as for each domain; however the former has greater weightage. *Patient health hub relates to storage and access of data. Patients are in control of their environment. Haemodynamic, medication changes can be updated and recorded in hubs e.g., council building, post office, which are linked to all health providers. This provides timely and accurate updates. Bar codes, biometrics. Abbreviations: C - collaborate; I - innovate; L - lobbying; VR - variable relevance. Table adapted from reference [3].
2.3. Defining Science and Standards
The scientific consensus which leads to guideline statements represents the general agreement of scientists in that field where they provide an opinion, judgement and take a collective position, that is not always unanimous. This is achieved through peer-reviewed publications, debates, rebuttals and finally, communication through conferences and scientific bodies. To this effect, the American College of Cardiology and American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) publish the most rigorous and used guidelines on CHF. Individual nation cardiac societies complement these works with guidelines that are often matched with these. As evidence is graded on the class and level of recommendation, it is hoped that individual nation guidelines will take stronger positions determining the class and level, to provide regional relevance [9, 16, 17]. Several questions are left unaccounted, firstly, the jurisdictions for which any evidence can be used to answer these (local or broader) questions; and secondly, the jurisdiction for advocacy and governance to influence these (local or broader) processes.
3. HEART FAILURE DATA - INVESTING IN DATA BANKS
“Medicine is at the beginning of a paradigm shift regarding how we store and process larger and larger amounts of data.” Seward [18].
3.1. Registry and Electonic Medical Records
Digital stored data is the new thread that weaves the health system continuum. However, the result has not been uniform, often seen as patchwork by those using the system. Regardless, in the future, the unifying link will be improved health systems data management. Let us explore some simple terminology. Clinical Registry, is a systematic or organised recording of uniform primary health information data in an observational methodology that is classified by the defined patient population, e.g. clinical quality (clinical), disease or conditions or a drug or device to monitor outcomes and other associations, within a governance, management structure. This requires a starting point and an end, defined by rigid parameters, producing user-friendly small data (Fig. 2). More importantly, data is stored on every clinical encounter on health service servers as big data. Registry data also have several levels of governance, firstly local institution human research ethics standards and secondly national commission on safety and quality in health care SOP and frameworks for governance, operation and technical requirements of clinical quality registries. This allows for standardisation of data from collection to data entry and storage, hence acceptable for research purposes [18-27]. Health systems also record secondary data. Creating a framework to utilise this data will present new horizons in healthcare and research.
Fig. (2).
The spectrum of health data. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
All information stored in servers can be considered as data. In social media, data use is quite advanced, and we can see the connectivity and other advantages of it. Electronic medical records (EMR) similarly store data; however only novel concepts and setups will allow data to overlap in its purpose (Fig. 2). If we assume registries are the gold standard for quality assurance in data management, we must also factor in the cost needed to achieve this and its potential uses. There is scope for industry here to bridge the EMR-registry gap by finding the opportunity-cost boundary for standardised high-quality data management for both clinical and research purposes. With the advent of assisted statistical learning, quantitative systems biology, correlations, and causality and with advancements in data extraction mechanisms we can progress the field further. Here with data analysis, systems (complex or complicated) analytics and algorithms, we have the ability to generate hypotheses and draw conclusions. One major gap in advancing machine learning is quality assurance, from human errors during data input and acquisition. The most recognised clinical quality assurance cycle remains the patient registry, as it prespecifies an algorithm or articulates its purpose for each scenario e.g. determining whether it is an appropriate means of addressing the research question, identifying stakeholders, defining the scope and target population, assessing feasibility, and securing funding. While the data is clean, as mentioned above, its precise scope is limited and costly.
3.2. Clinical and Biological Performance Measures
Performance measures are the term defining the gold standard parameter collected and uploaded. The process for EMR’s to replicate registries requires a transition and standardisation of these measures into routine clinical practice. The industry that develops from this will be a combination of what is readily translatable and innovative. The health cluster may be a reasonable jurisdiction to agree on minimum standards for variables and an acceptable margin of errors. Should this occur, the ability to forensically assess a patient’s journey through the health system across all domains and in real-time may be possible [28, 29]. An area that requires a quick mention is biological databanks or biobanks. The gradient of evidence is weighted towards advanced economies and limited to narrow population demography. However, the population attending medical care are increasingly heterogeneous. Just as EMR-registry data are used in an observational capacity, biobanks can be relied on to correlate variable post-trial translational outcomes observed from communities that were outside the trial criteria [30]. Health systems data will be a major factor and it is important, sufficient emphasis is given to understand this rapidly developing area.
4. CREATING A MODEL OF CARE
“This next decade belongs to distributed models, not centralized ones, to collaboration, not control, and to small data, not Big Data” [12].
4.1. Defining the Foundations of CHF Health Programs
The earlier discussions defined the ‘health system management or hardware’ by its boundary ‘heath cluster’; wired by the health domain “Taxonomy’; and populate it with evidence that must be relevant and translatable. ‘Data management or software’ is the programming that makes the machinery work. Table 1 fits these concepts into the other six more established disease management domains. Translating gold standard CHF guidelines [9, 16, 17] to ensure they are suitable for attending populations thus requires elevation, removal or innovation from this framework. By manipulating the building blocks and populating the gaps, disease management program or software then becomes a process that builds the relevant model of care. There are numerous published concepts, Wagner’s chronic disease model is well recognised . Using these principles, in a Chronic Disease Management Program (CDMP), we can prioritise and populate the subdomains within the Taxonomy framework based on an understanding of the 3 main stakeholders (participants) - patients, health services and administrative considerations. Understandably each stakeholder will have different perspectives and levels of input into the health system and equally varying abilities to influence its shape. How these three elements overlap will influence how large the disease management silos are. Thus from a realistic lens, ‘essentially disease management programs should be a consensus or generically acceptable view toward comprehensive, integrated, multidisciplinary, of standardised quality, chronic care for client’s illness, associated conditions and circumstances, with acceptable cost-efficacious clinical and outcome parameters, from a health network’.
In conventional models, higher costs were generated by utilisation of acute care resources and splintered post-discharge services contributing to high readmissions. It is probably the case, that cost can be saved by improving health systems that function in a more step-lock perspective as opposed to patchwork. Organised programs in CHF have unequivocally proven this for all outcome measures, including costs [2, 11]. There has been the discovery of key outcome contributors within health domains and utilisation and early reutilisation of hospital infrastructure assume a very significant cost. Identifying the preventable components by the expansion of ambulatory multidisciplinary services has been the subject of debate, research, and health policies. One such example is the design, services and infrastructure white paper plan for cardiac services, published by the Victoria health authorities [10]. While the first two priorities aimed at improving the existing system, as a third priority, the authors write,” Effective and innovative cardiac services will capitalise on opportunities to continuously improve how services are provided. Designated specialist services will provide clinical leadership in care and support for services across geographical areas through partnerships and innovative models of care.”. When looking at a section of the health map, the authors service, (Fig. 3) the paper refers to the realities including geography, demographics and volatile funding environments that have led to a fractured ability to access chronic disease care from hospitals alone, thus requiring the partnership and innovation aspects highly relevant. Increasing community health services have undoubtedly alleviated health system stress, with poor outcomes . Three examples of strategies previously used include: firstly, case management, which is a short intensive, usually nurse-led surveillance as client transitions to community life supported by medical, allied health and nonmedical care; next, coordinated care and multidisciplinary care with similarities to case management; and lastly the most comprehensive or chronic care model developed by Wagner which recognises multiple domains of care including 6 essential factors community resources and policies, healthcare organization, self- management support, delivery system design, decision support, and clinical information systems [3].
Fig. (3).
Map of Western Melbourne heart failure service. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.2. Considerations for CHF Programs for a Defined Jurisdiction
The science of medicine is invariably interwoven into the socio-political environment of health systems. Each stakeholder to achieve their goals has strengths and weaknesses. Patient as a consumer with a voice, health system through the science of evidence-based medicine and administrators through white-papers and policy, lobby the other to reach an acceptable cost-effectiveness equation. Looking at Fig. (3) and Table 2, let us summate the discussion by going through a process to formulate a theoretical framework for this jurisdiction. We explore three selective areas below:
Table 2.
Servicing and skill classification of hospitals for Western Melbourne.
| Hospitals | Beds | Cardiac Services | Distance from Werribee (km) | Population Served | Staffing (FTE) | aWIES (%) |
VAED
Emergency Disc 2019 |
NAWU
2011-14 |
|---|---|---|---|---|---|---|---|---|
| 1. The Alfred | 638 | 4C+, D+,E,R,S,T | 29.8 km | Variable – crosses clusters | >20 | TBA | 42,060 | $3700 |
| 2. RMH | 571 | 4C,D+,E,R,S,T | 30.2 km | Variable – crosses clusters | >20 | TBA | 43,916 | $3600 |
| 3. St Vincents | 504 | 4C,D+,E,R,S,T | 31.6 | Variable – crosses clusters | >20 | TBA | 22,936 | $4100 |
| 4. Footscray | 290 (Future 504) |
C,D+E,R,S,T | 21.4 | Footscray + | >10 | TBA | 21172 | $3300 |
| 5. Sunshine | 600 | C,D,E,R,S,T | 24.2 | Sunshine + | >10 | TBA | 33,698 | $3400 |
| 6. Williamstown | 90 | H | 20.5 | Williamstown | 0 | TBA | 73 | NA |
| 7. Werribee Mercy | 298 | H | NA | Werribee | 0.2 | TBA | 15,873 | $3600 |
| 8. St Vincents Private | 112 | C,D,E,R,S,T | 500m | Werribee | 6 | TBA | ? | NA |
| 9. Community Cardiology | NA | All subspecialties readily available | Good exposure to patients & service centres | Entire Western Region | >30 | NA | NA | NA |
Note: Western Melbourne Heart Failure mapping with Feasibility of Managing Two Worlds Together Patient JOURNEY Mapping Tool for Congestive Heart Failure (JOURNEY-HF study) is being developed to feed information into a model of care. This study will map a patients’ experience living with CHF, and help complete the loop along with service and administrative perspectives. Geography, and distance are common issues. The arrow highlights CHF patients' journey from Geelong to RMH. This policy could negate wider scenarios for comprehensive care at other sites. Abbreviations: aWIES - Acute Weighted inlier equivalent Separations. Capital - full service; small caption - limited service; C - cardiac catheterisation; C4 - advanced cardiac & cardiothoracic; C4+ - advanced cardiac, cardiothoracic & Transplant; D - device implantation ± limited electrophysiology; D+ Device & comprehensive electrophysiology; E - echocardiography; FTE - full time equivalent; H - holding capacity e.g. emergency triage, general admission, postop-rehab, limited intensive care; NA - not applicable; N/A - not available; PATS - patient assisted transport system; R - cardiac rehabilitation; RMH - Royal Melbourne Hospital; S - stress testing; T - transoesophageal echocardiography.
1. Patient Factors:
Proximity of Services - telecommunication bridges gaps . Communication and sharing of health information, remuneration models are areas to explore.
Self-Management - numerous studies have now highlighted the importance of this area for the future [31]. Attaining self-efficacy will drastically alter health trajectory outcomes across all domains. As behavioural conditioning is a critical component, medical education to the public must start early, and consistent engagement continues between providers, institutions and the public, including innovative research.
Participation - Wagner’s chronic disease model excels theoretically [5, 32]; however conceptually, silos have made successful application difficult. In participatory democracies, the patient’s perspective must be reflected in regional policy. Increasingly so as population demography is increasingly heterogeneous, medical associations could create lobbying platforms similar to examples like Change.comTM to help health clusters translate regional needs or findings quicker.
2. Health Services and Administration:
Collaboration and White Papers - the environment for collaboration is greatest when administrative white papers encourage it. For example, the State Government of Victorian released a design, service and infrastructure plan for Victoria’s cardiac system [10]. Their third priority outlines considerations for more efficiency and innovation. Whether collaborations entail individual aspects of CDMP and taxonomies or various models for health cluster reforms require a roundtable of stakeholders.
-
Cost Efficiency - all health clusters should universally target common denominators beyond individual, institutional goals. The model of care of tomorrow has to be data dependant with technical assistance. Cost efficacy is traditionally defined as a balance between cost of care and outcomes. As healthcare must factor administration and political aims, we must start attaching a value to a health care brand. When this occurs, the cost-efficacy equation can also have an emotional quotient or leverage to lobby for further improvements. We feel several areas worth considering are:
3. Cardiology as a Speciality - In the current era, regulatory bodies have been looking for increasing relevance beyond accreditation. Standardisation and lobbying capacity could be areas explored [34-42].
Medicolegal - collaboration in medicine is increasingly encouraged. However, the legal frameworks for the public, private, data storing, sharing and use are dealt with on a case by case basis. Medicare locals were an interesting concept that largely focused on general practices and could be reinvigorated to advocate local solutions across the health continuum [43].
Protocol Banks - reinvention is costly. Clinical and research gains should progress in a step-lock fashion. Investment in previous studies must be easily accessible for future medical endeavors.
Standards - performance measures have now been defined and published as consensus. The cost of utilising an additional measure in a study is cumulative. Regional grading of measures could help determine the power of one KPI over another to best gain insight into the relevant question [29].
Multidisciplinary clinic funding models [7, 35] - public funding in health systems often comes with a regulatory framework. Sometimes this is too rigid,
other times less so. The balance of these policies that best present a basket of choices are more likely to succeed in achieving cost-efficacy outcomes.
CONCLUSION
As the demography changes, as the interconnectedness increases, and as silos continue to break down, simple measures may have a great impact on health care, outcomes, and cost-effectiveness. Collaboration, innovation and lobbying of patients’ experiences, expanding evidence-based care and administrative white papers create an exciting environment for stakeholders to start dialogues. The convergence of health information technologies and data sciences is welcomed, and engagement is a priority. ‘The Health Cluster’ is probably a good framework to house for health services and health care models. Such a boundary balances the needs of quality services, research, income generating broader health industries and clinical governance. Priority 3, as documented in the Victorian health services white paper, is a welcomed recommendation for health systems globally [10]. Should health systems take up the ambitious challenge of ‘planning tomorrow’s healthcare with yesterday’s budget’ and add innovation to the mix, the results could be of interest.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Gliklich R., Dreyer N., Leavy M., editors. Registries for evaluating patient outcomes: a user’s guide. 3rd Ed. Rockville (MD): Agency for healthcare research and quality (US). . 2014 13 (14): EHC111. [PubMed] [Google Scholar]
- 2.Iyngkaran P, Liew D, Neil C, Driscoll A, Marwick TH, Hare DL. Moving From Heart Failure Guidelines to Clinical Practice: Gaps Contributing to Readmissions in Patients With Multiple Comorbidities and Older Age. Clin Med Insights Cardiol. 2018;12:1179546818809358. doi: 10.1177/1179546818809358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krumholz H.M., Currie P.M., Riegel B., Phillips C.O., Peterson E.D., Smith R., Yancy C.W., Faxon D.P. American Heart Association Disease Management Taxonomy Writing Group. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006;114(13):1432–1445. doi: 10.1161/CIRCULATIONAHA.106.177322. [DOI] [PubMed] [Google Scholar]
- 4.Iyngkaran P., Biddargardi N., Bastiampillai T., Beneby G. Making sense of health care delivery Where does the close to community health care worker fit in? - The case for congestive heart failure. Indian Heart J. 2015;67(3):250–258. doi: 10.1016/j.ihj.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner E.H., Austin B.T., Davis C., Hindmarsh M., Schaefer J., Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff. (Millwood) 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 6.Drolet B.C., Johnson K.B. Categorizing the world of registries. J. Biomed. Inform. 2008;41(6):1009–1020. doi: 10.1016/j.jbi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Takeda A., Martin N., Taylor R.S., Taylor S.J.C. Disease management interventions for heart failure. Cochrane Database Syst. Rev. 2019;1(1):CD002752. doi: 10.1002/14651858.CD002752.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonow R.O., Bennett S., Casey D.E., et al. Clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures. Circulation. 2005;112:1853–1887. doi: 10.1161/CIRCULATIONAHA.105.170072. [DOI] [PubMed] [Google Scholar]
- 9.Atherton J.J., Sindone A., De Pasquale C.G., Driscoll A., MacDonald P.S., Hopper I., Kistler P.M., Briffa T., Wong J., Abhayaratna W., Thomas L., Audehm R., Newton P., O’Loughlin J., Branagan M., Connell C. NHFA CSANZ Heart Failure Guidelines Working Group. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the Prevention, Detection, and Management of Heart Failure in Australia 2018. Heart Lung Circ. 2018;27(10):1123–1208. doi: 10.1016/j.hlc.2018.06.1042. [DOI] [PubMed] [Google Scholar]
- 10.Victoria State Government: Health and human services. Design, service and infrastructure plan for Victoria’s cardiac system. Available from: https://www2.health.vic.gov.au/hospitals-and-health-services/health-system-design-planning/cardiac-design-service-and-infrastructure-plan.
- 11.Fonarow G.C., Stough W.G., Abraham W.T., Albert N.M., Gheorghiade M., Greenberg B.H., O’Connor C.M., Sun J.L., Yancy C.W., Young J.B. OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 2007;50(8):768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 12.Camoin Associates. Available from: https://www.camoinassociates.com/healthcare-industry-critical- driver-cluster-development.
- 13.DMC. Creating a global destination for health and wellness. Available from: https://dmc.mn/
- 14.Thomas S., Kaltenbach K.M., Nath D. Health care clusters in hospitals. J. Hosp. Mark. 1993;7(2):61–76. doi: 10.1300/J043v07n02_07. [DOI] [PubMed] [Google Scholar]
- 15.Faxon D.P., Schwamm L.H., Pasternak R.C., Peterson E.D., McNeil B.J., Bufalino V., Yancy C.W., Brass L.M., Baker D.W., Bonow R.O., Smaha L.A., Jones D.W., Smith S.C., Jr, Ellrodt G., Allen J., Schwartz S.J., Fonarow G., Duncan P., Horton K., Smith R., Stranne S., Shine K. American Heart Association’s Expert Panel on Disease Management. Improving quality of care through disease management: principles and recommendations from the American Heart Association’s Expert Panel on Disease Management. Circulation. 2004;109(21):2651–2654. doi: 10.1161/01.CIR.0000128373.90851.7B. [DOI] [PubMed] [Google Scholar]
- 16.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr, Colvin M.M., Drazner M.H., Filippatos G.S., Fonarow G.C., Givertz M.M., Hollenberg S.M., Lindenfeld J., Masoudi F.A., McBride P.E., Peterson P.N., Stevenson L.W., Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail. 2017;23(8):628–651. doi: 10.1016/j.cardfail.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M.C., Ruilope L.M., Ruschitzka F., Rutten F.H., van der Meer P. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 18.Seward J.B. Paradigm shift in medical data management: big data and small data. JACC Cardiovasc. Imaging. 2017;10(11):1304–1306. doi: 10.1016/j.jcmg.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Alonso-Betanzos A., Bolón-Canedo V. Big-data analysis, cluster analysis, and machine-learning approaches. Adv. Exp. Med. Biol. 2018;1065:607–626. doi: 10.1007/978-3-319-77932-4_37. [DOI] [PubMed] [Google Scholar]
- 20.Effective Health Care Program. Available from: http://www.effectivehealthcare.ahrq.gov/registries-guide-3.cfm.
- 21.ICHOM. Heart failure data collection reference guide. V 1.1.4, 2017. Available from: https://ichom.org/files/medical-conditions/heart-failure/heart-failure-reference-guide.pdf.
- 22.American College of Cardiology. Heart Failure Toolkit. Available from: https://www.acc.org/tools-and-practice-support/clinical-toolkits/heart-failure-practice-solutions.
- 23.Health Cluster. Knowledge Bank. Available from: https://www.who.int/health-cluster/resources/publications/en/
- 24.Du X., Khamitova A., Kyhlstedt M., Sun S., Sengoelge M. Utilisation of real-world data from heart failure registries in OECD countries - A systematic review. Int J Cardiol Heart Vasc. 2018;19:90–97. doi: 10.1016/j.ijcha.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiweis B., Trinczek B., Köpcke F., Leusch T., Majeed R.W., Wenk J., Bergh B., Ohmann C., Röhrig R., Dugas M., Prokosch H.U. Comparison of electronic health record system functionalities to support the patient recruitment process in clinical trials. Int. J. Med. Inform. 2014;83(11):860–868. doi: 10.1016/j.ijmedinf.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Raman S.R., Curtis L.H., Temple R., Andersson T., Ezekowitz J., Ford I., James S., Marsolo K., Mirhaji P., Rocca M., Rothman R.L., Sethuraman B., Stockbridge N., Terry S., Wasserman S.M., Peterson E.D., Hernandez A.F. Leveraging electronic health records for clinical research. Am. Heart J. 2018;202:13–19. doi: 10.1016/j.ahj.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 27.De Moor G., Sundgren M., Kalra D., Schmidt A., Dugas M., Claerhout B., Karakoyun T., Ohmann C., Lastic P.Y., Ammour N., Kush R., Dupont D., Cuggia M., Daniel C., Thienpont G., Coorevits P. Using electronic health records for clinical research: the case of the EHR4CR project. J. Biomed. Inform. 2015;53:162–173. doi: 10.1016/j.jbi.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Cainzos-Achirica M., Rebordosa C., Vela E., Cleries M., Matsushita K., Plana E., Rivero-Ferrer E., Enjuanes C., Jimenez-Marrero S., Garcia-Rodriguez L.A., Comin-Colet J., Perez-Gutthann S. Challenges of evaluating chronic heart failure and acute heart failure events in research studies using large health care databases. Am. Heart J. 2018;202:76–83. doi: 10.1016/j.ahj.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Butler J., Stockbridge N., Gheorghiade M. Past, present, and future of acute heart failure clinical trials-a high-risk population in search of a strategy. Eur. J. Heart Fail. 2018;20(5):839–841. doi: 10.1002/ejhf.987. [DOI] [PubMed] [Google Scholar]
- 30.Baron T., Beskow A., James S., Lindahl B. Biobank linked to SWEDEHEART quality registry-routine blood sample collection opens new opportunities for cardiovascular research. Ups. J. Med. Sci. 2019;124(1):12–15. doi: 10.1080/03009734.2018.1498957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyngkaran P., Toukhsati S.R., Harris M., Connors C., Kangaharan N., Ilton M., Nagel T., Moser D.K., Battersby M. Self Managing Heart Failure in Remote Australia - Translating Concepts into Clinical Practice. Curr. Cardiol. Rev. 2016;12(4):270–284. doi: 10.2174/1573403X12666160703183001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner E.H., Austin B.T., Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. doi: 10.2307/3350391. [DOI] [PubMed] [Google Scholar]
- 33.Dierckx R., Inglis S.C., Clark R.A., Prieto-Merino D., Cleland J.G. Telemedicine in heart failure: new insights from the Cochrane meta-analyses. Eur. J. Heart Fail. 2017;19(3):304–306. doi: 10.1002/ejhf.759. [DOI] [PubMed] [Google Scholar]
- 34.Gandhi S., Mosleh W., Sharma U.C., Demers C., Farkouh M.E., Schwalm J.D. Multidisciplinary heart failure clinics are associated with lower heart failure hospitalization and mortality: systematic review and meta-analysis. Can. J. Cardiol. 2017;33(10):1237–1244. doi: 10.1016/j.cjca.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Cheema B., Ambrosy A.P., Kaplan R.M., Senni M., Fonarow G.C., Chioncel O., Butler J., Gheorghiade M. Lessons learned in acute heart failure. Eur. J. Heart Fail. 2018;20(4):630–641. doi: 10.1002/ejhf.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel Y.R., Robbins J.M., Kurgansky K.E., Imran T., Orkaby A.R., McLean R.R., Ho Y.L., Cho K., Michael Gaziano J., Djousse L., Gagnon D.R., Joseph J. Development and validation of a heart failure with preserved ejection fraction cohort using electronic medical records. BMC Cardiovasc. Disord. 2018;18(1):128. doi: 10.1186/s12872-018-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parissis J., Farmakis D., Triposkiadis F. Heart failure registries: how far can we go? Eur. J. Heart Fail. 2016;18(6):626–628. doi: 10.1002/ejhf.565. [DOI] [PubMed] [Google Scholar]
- 38.Du X., Khamitova A., Kyhlstedt M., Sun S., Sengoelge M. Utilisation of real-world data from heart failure registries in OECD countries - A systematic review. Int. J. Cardiol. Heart Vasc. 2018;19:90–97. doi: 10.1016/j.ijcha.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonsson A., Edner M., Alehagen U., Dahlström U. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur. J. Heart Fail. 2010;12(1):25–31. doi: 10.1093/eurjhf/hfp175. [DOI] [PubMed] [Google Scholar]
- 40.Aimo A., Seghieri C., Nuti S., Emdin M. Building medical knowledge from real world registries: The case of heart failure. Int. J. Cardiol. Heart Vasc. 2018;19:98–99. doi: 10.1016/j.ijcha.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spertus J.A., Bonow R.O., Chan P., Diamond G.A., Drozda J.P., Jr, Kaul S., Krumholz H.M., Masoudi F.A., Normand S.L., Peterson E.D., Radford M.J., Rumsfeld J.S. ACCF/AHA Task Force on Performance Measures. ACCF/AHA new insights into the methodology of performance measurement: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures. Circulation. 2010;122(20):2091–2106. doi: 10.1161/CIR.0b013e3181f7d78c. [DOI] [PubMed] [Google Scholar]
- 42.Riegel B., Lee C.S., Sochalski J. Developing an instrument to measure heart failure disease management program intensity and complexity. Circ. Cardiovasc. Qual. Outcomes. 2010;3(3):324–330. doi: 10.1161/CIRCOUTCOMES.109.877324. [DOI] [PubMed] [Google Scholar]
- 43.Primary health networks (PHNs). Available from: https://www.healthdirect.gov.au/primary-health-networks-phns. [Google Scholar]