Abstract
The increase of penicillin-resistant Streptococcus pneumoniae (PRSP) pneumonia results in a greater risk of antibiotic treatment failure. In vitro data are not sufficient predictors of clinical efficacy, and animal models may be insufficiently contributive, since they often use immunocompromised animals and do not always respect the human pharmacokinetics of antibiotics. We developed an experimental PRSP pneumonia model in immunocompetent rabbits, by using intrabronchial instillation of PRSP (MIC = 4 mg/liter), without any adjuvant. This reproducible model was used to assess amoxicillin efficacy by reproducing human serum pharmacokinetics following 1-g oral or intravenous administrations of amoxicillin every 8 h. Evaluation was performed by using clinical, CT scan, macroscopic, histopathologic, and microbiological criteria. Experimental pneumonia in untreated rabbits was similar to untreated severe human bacteremic untreated pneumonia; in both rabbits and humans, (i) cumulative survival was close to 50%, (ii) red or gray lung congestion and pleuritis were observed, and (iii) lung and spleen concentrations reached 5 and 4 log10 CFU/g. A 48-h treatment resulted in a significant bacterial clearance in the lungs (1.53 versus 5.07 log10 CFU/ml, P < 0.001) and spleen (1.00 versus 4.40 log10 CFU/ml, P < 10−6) and a significant decrease in mortality (0% versus 50%, P = 0.02) in treated versus untreated rabbits. No difference was observed on macroscopic and histopathologic lesions between treated and untreated rabbits (P = 0.36 and 0.78, respectively). Similar results were obtained by using a fully penicillin-susceptible S. pneumoniae strain (MIC = 0.01 mg/liter). Our findings suggest that (i) this new model can be contributive in the evaluation of antibacterial agents and (ii) 1 g of amoxicillin three times a day may be sufficient to treat PRSP pneumonia in immunocompetent humans.
Invasive Streptococcus pneumoniae infection is a worldwide problem. S. pneumoniae is the most common cause of bacterial pneumonia, leading to significant morbidity and mortality rates which vary around 25% (5, 37, 51). Since the first reports three decades ago of strains of S. pneumoniae with a decreased susceptibility to penicillin, there have been increasing reports of pneumococcal infections caused by strains with high levels of resistance to penicillin and to multiple antibiotics (5, 25, 36, 37, 51). Clinical treatment failures in patients with infections caused by penicillin-resistant S. pneumoniae point out the interest for more evaluation of therapeutic efficacy. Indeed, in vitro data are only mildly helpful because of their incapacity to predict clinical therapeutic success (5). Furthermore, human therapeutic trials are very difficult to conduct, because of the impossibility of clinically evaluating the situation, due to penicillin-resistant pneumococcal infection, and the great prevalence of precessive antibiotherapy, which reduces the probability of isolating penicillin-resistant S. pneumoniae, even after treatment failure. Consequently, animal models could contribute to predicting antibiotic treatment efficacy in such infections.
Several penicillin-resistant S. pneumoniae animal models exist (1–3, 6, 9, 14, 17, 18, 33, 34, 39, 41–43, 45, 46). However, the greatest difficulty for these models was the inability to infect healthy animals with penicillin-resistant S. pneumoniae strains. Consequently most of the available models were developed in immunocompromised mice (1–3, 6, 9, 14, 17, 33, 34, 42, 43) and young rodents (9, 41, 45, 46) or used adjuvant to enhance bacterial virulence (9, 18, 39, 41). Moreover, differences between animal and human pharmacokinetics constituted another important limit.
In the present study, we developed a model of experimental penicillin-resistant S. pneumoniae pneumonia using nonimmunosuppressed animals (adult New Zealand rabbits), reproducing human pathology with an inoculum free of any adjuvant. We also reproduced human serum pharmacokinetics following amoxicillin administration. In a third phase, we conducted an experimental therapeutic study in order to evaluate the amoxicillin (3 g/day) efficacy on bacterial clearance in penicillin-resistant S. pneumoniae pneumonia, corresponding to the dose recommended in France for pneumonia treatment (44).
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 1998.)
MATERIALS AND METHODS
Microorganisms.
Two S. pneumoniae strains isolated from the blood of patients with pneumonia were used (kindly provided by Geslin of the Centre National de Référence des Pneumocoques, Créteil, France). The first strain (strain 195, serotype 19) was fully susceptible to penicillin (MIC = 0.01 mg/liter). The second strain (strain 16089, serotype 9V) was highly resistant to penicillin (MIC = 4 mg/liter) and less susceptible to amoxicillin (MIC = 2 mg/liter) and ceftriaxone (MIC = 1 mg/liter). Purity was confirmed throughout the study by Gram staining and colony morphology. Working stock cultures were kept frozen at −70°C in a 15% glycerol supplemented brain heart infusion (BioMérieux Laboratories, Marcy l’Etoile, France). In order to maintain virulence, stock cultures were changed every month by using the colonies isolated from rabbits with untreated S. pneumoniae pneumonia.
Before each experiment, several S. pneumoniae strains from one aliquot (per strain) were inoculated into brain heart infusion, cultured on agar plates, and incubated for 24 h at 37°C in 5% CO2. Twenty-five to 30 colonies were taken and inoculated into 9 ml of brain heart infusion, incubated for 6 h at 37°C, and then cultured on agar plates for 18 h at 37°C in 5% CO2. This culture was diluted in physiologic saline in order to obtain final concentrations of 7, 8.5, and 10 log10 CFU/ml. No adjuvant was used. These concentrations were first determined by using optic density measure, in reference to a standard curve, and confirmed by using successive dilution cultures.
Animals.
Male New Zealand White rabbits (body weight, 2.7 to 3.0 kg) were obtained from Elevage Scientifique des Dombes (Romans, France). These animals were not immunosuppressed and had a sanitary status of virus antibody free and specific pathogen free. They were placed in individual cages and were nourished ad libitum with drinkable water and feed, according to current recommendations.
Experimental pneumococcal pneumonia in rabbits.
The animals were anesthetized intramuscularly with 1.5 ml of a mixture of ketamine (500 mg/ml) and xylazine (2.75 mg/ml). A silicone catheter was introduced into the jugular vein, through a lateral incision of the neck, and then subcutaneously tunneled through the interscapular area (50). This catheter was introduced in order to subsequently infuse antibiotics at human pharmacokinetic rates.
Twenty-four hours later, the rabbits were anesthetized intravenously by using 0.8 ml of the ketamine-plus-xylazine mixture and then by a few milliliters of propofol as needed. Under view control, a silicone catheter (Sigma Medical, Nanterre, France) was introduced through the vocal cords into the trachea and pulled till it reached the bronchia. Freshly prepared pneumococcal inoculum (0.5 ml) was then gently flushed through this catheter. The endobronchial catheter was then immediately removed after the inoculum instillation, and the animals were placed upright for 15 s to facilitate distal alveolar migration by gravity. Using the same experimental conditions, some rabbits were inoculated with heat-killed penicillin-resistant S. pneumoniae as negative controls.
Experimental pneumonia examination.
For each strain and inoculum, experimental pneumonia was evaluated by using invasive and noninvasive criteria. For a few animals, a thoracic evaluation CT scan was also performed. For each rabbit, the main evaluation took into account pulmonary injury levels and microbiological findings in each lobe of the lungs and the spleen. These organs were taken either after sacrifice or after pneumonia-related death. Animal sacrifice was performed after anesthesia by using overdoses of thiopental. For each dead rabbit (sacrificed or pneumonia related), an exsanguination by heart puncture was performed. The thorax was opened, and the existence of pleural effusion was noted. The lungs were then dissected, free from the trachea and other structures, in sterile fashion and put on a sterile gauze for at least 5 min, to allow residual pulmonary blood absorption. A laparotomy was then performed, and the spleen was aseptically removed.
For each pulmonary lobe, the macroscopic aspect was noted by using a scoring grid based upon human morphologic findings (Table 1) (32). An overall macroscopic score was calculated as the sum of all lobar macroscopic scores, plus 2 points in the case of pleural effusion (range, 0 to 39 points).
TABLE 1.
Macroscopic scoring grid
| Score | Aspect |
|---|---|
| 0 | Normal |
| 1 | Scar |
| 2 | Slight congestion |
| 3 | Red congestion |
| 4 | Gray congestion |
| 5 | Yellowish congestion |
Two parts of each lobe were taken, fixed in 10% neutral buffered formalin, and thereafter embedded in paraffin. Hematoxylin-eosin-safranin staining was applied to 5-μm-thick sections. Light microscopy examination was performed by a pathologist who was not in possession of the experimental, macroscopic, and microbiological data. A scoring grid system was also used, based upon human histopathologic data (31, 40) (Table 2). An overall histopathologic score was calculated for each rabbit as the sum of each lobar histopathologic score (range, 0 to 35 points).
TABLE 2.
Histopathologic scoring
| Score | Aspect |
|---|---|
| 0 | Normal |
| 1 | Vascular lesions |
| 2 | Bronchiolitis |
| 3 | Focal bronchopneumonia |
| 4 | Confluent bronchopneumonia |
| 5 | Lung abscess |
Each pulmonary lobe was weighed and homogenized in sterile water. The spleen was prepared under the same conditions. Bacteria were counted in a sample of this crude homogenate by plating 10-fold dilutions on sheep blood agar and incubating the plates for 24 to 48 h at 37°C. Samples of pleural effusions were directly plated and cultured in the same conditions. Bacterial concentrations in each lobe or in the spleen were determined after adjusting for weight. The threshold value was 1 log10 CFU/ml. For each rabbit, the mean pneumococcal pulmonary concentration was calculated according to each lobar bacterial concentration with lobar weight (e.g., mean concentration = Σ [lobar concentration × lobar weight]/Σ lobar weights).
Simulation of human amoxicillin pharmacokinetics in rabbits.
Amoxicillin (Clamoxyl; SmithKline Beecham Laboratories, Nanterre, France) was reconstituted from laboratory powder of known potency, according to the manufacturer’s instructions, just before each experiment. A 20-mg/kg of body weight bolus was infused intravenously in four rabbits. Arterial blood punctures were performed at 0, 5, 10, 15, 20, 30, 45, 60, 90, and 120 min after injection. Sera from these blood samples were stored at −70°C till assay. Amoxicillin concentrations in the blood and lungs were determined by the disk plate bioassay method (10). The bioassay microorganism was Micrococcus luteus ATCC 9341, and the growth medium was antibiotic medium no. 11 (Difco Laboratories, Detroit, Mich.). Standard curves were established with solutions of amoxicillin (progression from 0.5 to 8 mg/liter) in sterile water. The linearity of the standard curves used for the disk plate bioassays was at least 0.98 (r2). The amoxicillin concentrations in the serum and lungs were derived from the standard curves. The serum and lung samples were diluted in sterile water, as their concentrations would be within range of those on the standard curve. Results were expressed as micrograms per milliliter of blood or per gram of lung. New batches of standard samples were assayed for each experiment, and concentrations were assayed in duplicate. The between-day and within-day coefficients of variation for replicates were equal to 3.8 and 7.0%, respectively.
For each rabbit, a logarithmic regression of measured concentrations versus time during the elimination phase (on the basis of an open bicompartmental model) was performed by using the least-squares method. Such a regression led to the determination of the β slope and the B constant of the elimination phase. The same method was used to determine the α slope and the A constant of the distribution phase, with the exception that values calculated according to the elimination phase equation were withdrawn from measured concentrations (30). The correlation coefficient (r) and the observed versus expected area under the curve ratio (calculated by using the trapezoidal rule) were used to validate the obtained model (30). Concentrations in serum following an intravenous injection of amoxicillin could be calculated from the following equation: concentration in serum = A.e−αt + B.e−βt, where t corresponded to the time elapsed since the bolus was injected. From these data, the following constants were deduced: apparent volumes of distribution (vascular and extravascular), elimination constant, and intercompartmental rate constants (22, 27).
The objectives were to simulate human pharmacokinetics following the administration of 1 g of amoxicillin, either orally (oral simulation) (13) or intravenously (intravenous simulation) (12, 24). Because of faster amoxicillin elimination in rabbits than in humans, a variable flow rate infusion with successive levels was used. Briefly, by using the amoxicillin pharmacokinetics constants in rabbits, determined as described above, it was possible to calculate both vascular and extravascular concentrations following each constant rate infusion, given any initial condition (i.e., with an empty model or not), by using the model developed by Hull (27). Indeed, intercepts A and B from the plasmatic concentration equation depend in part on the antibiotic dose given either in bolus or by continuous infusion. Thus, it was possible, by reversing the formulas, to calculate the infusion rate necessary to yield a specific plasmatic concentration. This method has already been successfully used in humans (26). We developed a computer program to facilitate and to automate calculations.
For each experiment, a computer-controlled pump containing amoxicillin was connected to the central venous catheter. This protected connection allowed free circulation and free food access to the rabbits. Infusion rates were controlled by programmable computer software (Softpump; World Precision Instruments, Sarasota, Fla.). Infusion rates were modified every 30 min (oral simulation) and every 5 min (intravenous simulation).
Twenty-two rabbits were used for the simulation of amoxicillin human pharmacokinetics, most of them being infected with S. pneumoniae. To control the quality of simulations, arterial blood samples were regularly taken during simulation at 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 120, 150, and 180 min for intravenous simulation and at 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, and 360 min for oral simulation. Rabbits were sacrificed at variable times, and lung samples were taken for amoxicillin assay. Serum and lung homogenates were stored at −70°C until assay. Comparison between observed and expected values was performed by using both a correlation coefficient and the expected versus observed area under curve ratios.
Human-simulated amoxicillin treatment.
Eighteen rabbits were randomly assigned just before inoculation with 0.5 ml of 10 log10 CFU of penicillin-resistant S. pneumoniae (strain 16089) per ml to three arms: (i) untreated (n = 8), (ii) oral amoxicillin treated (n = 5), and (iii) intravenous amoxicillin treated (n = 5). The same procedure was used in a second group inoculated with 0.5 ml of 10 log10 CFU of penicillin-susceptible S. pneumoniae (strain 195) per ml. Human-like amoxicillin treatment was started 4 h after inoculation. Four doses of 1 g of amoxicillin (every 8 h) were simulated in the oral-treated arms, while five doses of 1 g of amoxicillin (every 8 h) were simulated in the intravenous-treated arm. Blood samples were taken in treated rabbits to assess the quality of the human pharmacokinetics simulation. All treated rabbits still alive were sacrificed 10 h (intravenous simulation, five cycles) to 14 h (oral simulation, four cycles) after the last computer-controlled amoxicillin infusion, i.e., around 48 h after inoculation. Untreated rabbits still alive were also sacrificed 48 h after inoculation. Amoxicillin concentrations in the serum and lungs were systematically assayed at death time in treated rabbits. Evaluation criteria were survival, macroscopic, and histopathologic scores and pneumococcal concentrations in lungs and spleen at death time for all the rabbits (sacrificed or dead from pneumonia).
Statistics.
The results were expressed as the mean or percentage ± standard deviation (SD). Differences between quantitative values were analyzed by using the Mann-Whitney nonparametric test. To compare relationships between quantitative values, the r and r2 values were calculated by the linear regression model.
Survival analysis was performed using the Kaplan-Meier method. Significant events were pneumonia-related deaths, and sacrifices were considered as censored events. Comparisons between curves were made by using the log-rank test or Peto’s Khi2 test as needed.
In the experimental pneumonia treatment phase, proportions were analyzed as quantitative values by using angular transformation. After verification of variance homogeneity by using Hartley’s table, continuous variables were analyzed with one-way analysis of variance. In case of a significant test, post hoc analysis comparing results for each treated arm versus the untreated arm was conducted by using Dunnett’s test. For all the tests, a P value of <0.05 was considered significant.
RESULTS
Experimental pneumococcal pneumonia in rabbits.
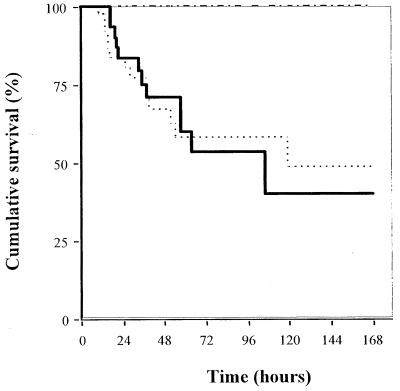
Cumulative survival rates of rabbits with experimental pneumonia produced by inoculation of penicillin-resistant S. pneumoniae (strain 16089) at 7, 8.5, and 10 log10 CFU/ml, compared with penicillin-susceptible S. pneumoniae (strain 195) at 10 log10 CFU/ml, are shown in Fig. 1. With the inoculum of 10 log10 CFU/ml, the first pneumonia-related deaths occurred 6 h after inoculation, most of them during the first 72 h, with very few events after this time. With the inocula of 7 and 8.5 log10 CFU/ml, no pneumonia-related deaths were observed. So there was a significant difference between the cumulative survival observed after inoculation of 7 or 8.5 log10 CFU/ml and that observed with 10 log10 CFU of penicillin-resistant S. pneumoniae per ml (7 versus 10 log10 CFU/ml, P < 0.05, log-rank test, and 8.5 versus 10 log10 CFU/ml, P < 0.05, log-rank test). There was no difference between cumulative survival observed after inoculation with 10 log10 CFU of penicillin-resistant S. pneumoniae per ml and 10 log10 CFU of penicillin-susceptible S. pneumoniae per ml (P = 0.46, Peto’s Khi2 test).
FIG. 1.
Cumulative survival of rabbits with penicillin-resistant (strain 16089) and penicillin-susceptible (strain 195) S. pneumoniae experimental pneumonia, according to inoculum concentration (10 log10 versus 7 or 8.5 log10 CFU/ml, P < 0.05, log-rank test). Symbols: -----, strain 16089 (inoculum, 10 log10); ——, strain 195 (inoculum, 10 log10); –-–-, strain 16089 (inoculums, 7 and 8.5 log10).
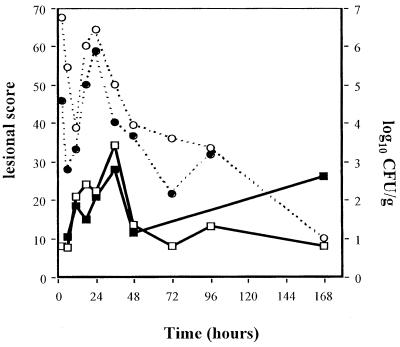
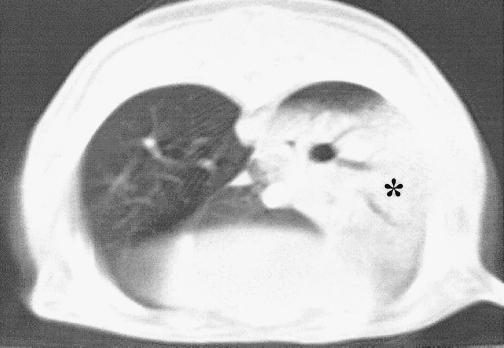
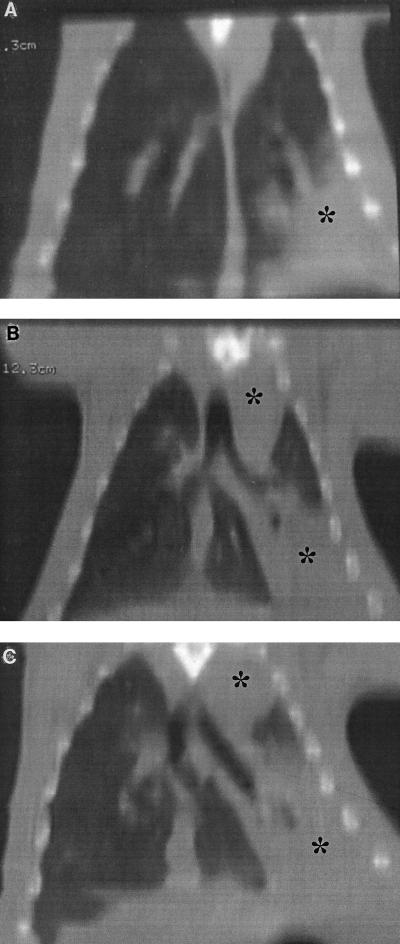
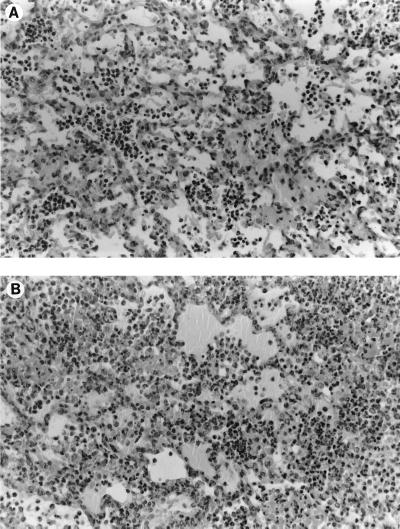
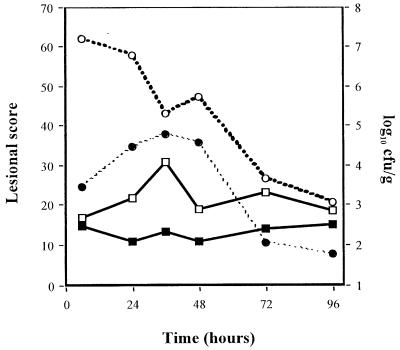
Inoculation of 7 and 8.5 log10 CFU of penicillin-resistant S. pneumoniae per ml induced few to moderate macroscopic and histopathologic lesions and mild bacterial concentrations (data not shown). Evolutions of macroscopic, histopathologic, and bacterial concentrations observed with 10 log10 CFU of penicillin-resistant S. pneumoniae per ml inocula are shown in Fig. 2. Four hours after inoculation, a significant bacteremic pneumonia was already observed, with pneumococcal concentrations reaching 5 log10 CFU/g in lungs and 3 log10 CFU/g in the spleen. Histopathologic and macroscopic scores were close to 10 at this time. Bacteremia (as spleen pneumococcal culture) then reached a peak concentration around 24 h. Lung pneumococcal concentrations followed the same pattern. CT scan examinations showed a lobar condensation first in a lower lobe (Fig. 3), with rapid extension to the other lobes within 72 h (Fig. 4A to C). These CT scan aspects are very close to those observed in humans. The main macroscopic aspects were red congestion at 12 h and gray congestion at 24 h. Lung histopathologic examination at 24 h showed a slight increase of leukocytes and a few erythrocytes within the alveoli and bronchiolar lumen (Fig. 5A). At 48 h, the alveolar spaces were filled up with a large number of polymorphonuclear leukocytes and fibrinous exudate (Fig. 5B). Pneumococcal pleural effusion was constantly seen at 36 h. Major pathological lesions and bacterial concentrations occurred between 24 and 48 h and progressively evolved to fibrosis in 2 weeks.
FIG. 2.
Pulmonary lesion scores and pneumococcal concentration evolution in rabbits with penicillin-resistant S. pneumoniae (strain 16089) experimental pneumonia by using 10 log10 CFU/ml inoculum. Symbols:  , macroscopic score;
, macroscopic score;  , histopathologic score; ---○---, lung pneumococcal concentrations; ---●---, spleen pneumococcal concentrations.
, histopathologic score; ---○---, lung pneumococcal concentrations; ---●---, spleen pneumococcal concentrations.
FIG. 3.
CT scan examination of penicillin-resistant S. pneumoniae (strain 16089) experimental pneumonia at 24 h. The alveolar condensation of the entire left lower lobe (∗) is due to active pneumonia, in contrast with normal aspects of the right lower and median lobes.
FIG. 4.
CT bidimensional coronal reconstruction of transaxial sections. Shown is the evolution of penicillin-resistant S. pneumoniae (strain 16089) experimental pneumonia at 12 h (A), 36 h (B), and 60 h (C). Also shown is alveolar condensation of the left lower lobe (∗) 12 h after inoculation, with a progressive extension to the entire left lung at 60 h. In this case, the right lung does not present radiographic signs of pneumonia.
FIG. 5.
Lung histopathologic examination of penicillin-resistant S. pneumoniae (strain 16089) experimental pneumonia at 24 h (A) and 48 h (B). Pneumonia with polymorphonuclear leukocytes and fibrinous exudate fill up the alveoli. Hematoxylin-eosin-safranin stain was applied to the sections. Original magnification, ×250.
As a comparison, pulmonary lesions and bacterial evolution observed after instillation of 10 log10 CFU of penicillin-susceptible S. pneumoniae per ml are shown in Fig. 6. A similar evolution was observed, even if lung lesions seemed to be less important than with the penicillin-resistant S. pneumoniae strain. Thus, this inoculum of 10 log10 CFU/ml was used for the experiments for both penicillin-resistant S. pneumoniae and penicillin-susceptible S. pneumoniae pneumonia.
FIG. 6.
Pulmonary lesions and pneumococcal concentration evolution in rabbits with penicillin-susceptible S. pneumoniae (strain 195) experimental pneumonia by using 10 log10 CFU/ml. Symbols:  , macroscopic score;
, macroscopic score;  , histopathologic score; ---○---, lung pneumococcal concentrations; ---●---, spleen pneumococcal concentrations.
, histopathologic score; ---○---, lung pneumococcal concentrations; ---●---, spleen pneumococcal concentrations.
Differences observed between sacrificed rabbits and rabbits killed by pneumonia at 24 and 48 h are summarized in Table 3. Lung and spleen pneumococcal concentrations were significantly different at 24 h, according to cause of death, whereas no difference was observed at 48 h. On the other hand, macroscopic and histopathologic lesions were not different at 24 h, but a significant difference existed at 48 h. Thoracic CT scan examination at 12 h did not show any difference between rabbits which survived pneumonia and those which died of it at 24 h.
TABLE 3.
Pulmonary lesions and pneumococcal concentrations in rabbits with penicillin-resistant S. pneumoniae (strain 16089) experimental pneumonia, according to death circumstances
| Parameter | Result or concentration for death at the following time by the indicated causea
|
|||
|---|---|---|---|---|
| 24 h
|
48 h
|
|||
| Pneumonia-related death | Sacrificed (P) | Pneumonia-related death | Sacrificed (P) | |
| No. | 5 | 5 | 5 | 5 |
| Macroscopic score | 22.60 ± 7.27 | 22.40 ± 5.18 (1.00) | 24.00 ± 6.20 | 11.60 ± 6.54 (0.04) |
| Histopathological score | 12.50 ± 2.12 | 17.00 ± 1.51 (0.88) | 21.67 ± 3.21 | 9.00 ± 4.16 (0.03) |
| Pneumococcal concentration in lungsb | 6.87 ± 0.25 | 3.93 ± 2.35 (0.009) | 4.10 ± 1.88 | 3.61 ± 1.51 (0.92) |
| Pneumococcal concentration in spleenb | 5.26 ± 1.71 | 3.83 ± 2.79 (0.35) | 2.98 ± 2.15 | 3.34 ± 2.19 (0.92) |
Each value represents the mean ± SD or number and percentage. P values were determined by the Mann-Whitney test.
Log10 CFU/g.
There was a clear and significant correlation between macroscopic and histopathologic scores (r = 0.57, P < 10−6), as well as between bacterial contents in lungs and spleen (r = 0.67, P < 10−6). There was also a weak but significant correlation between pneumococcal pulmonary concentrations and macroscopic or histopathologic scores (r = 0.21, P < 10−6, and r = 0.12, P < 10−4, respectively).
As expected, neither deaths nor pulmonary lesions (either macroscopic or histopathologic) were induced by inoculation of heat-killed penicillin-resistant S. pneumoniae (negative controls).
Simulation of human amoxicillin pharmacokinetics in rabbits.
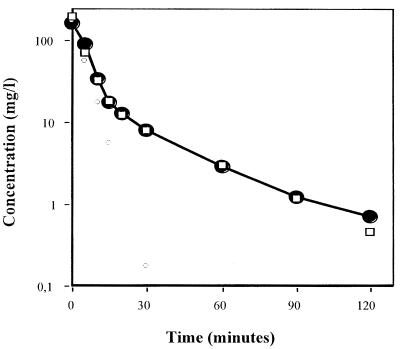
Amoxicillin concentrations in serum following the infusion of a 20-mg/kg bolus in four rabbits fitted into a bicompartmental model, as shown in Fig. 7. Constants were as follows: A, 155 mg/liter; B, 12 mg/liter; α, 15 h−1; and β, 1.5 h−1. The observed area under the curve was equal to 15.55 mg · h−1 · liter−1. The correlation coefficient between observed and calculated values was 0.996. The time above MIC in serum was 45 and 150 min for penicillin-resistant S. pneumoniae and penicillin-susceptible S. pneumoniae, respectively.
FIG. 7.
Pharmacokinetics of amoxicillin in rabbits and open bicompartmental modelization. Symbols: ---□---, calculated concentrations; —●—, measured concentrations.
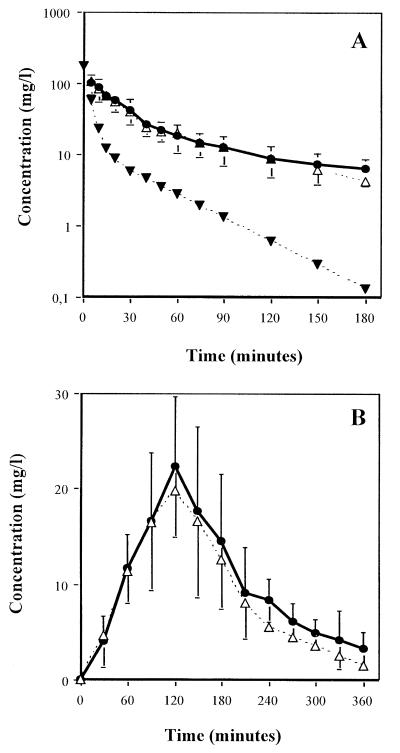
Simulations of human pharmacokinetics following oral (n = 14 rabbits) or intravenous (n = 8 rabbits) amoxicillin (1 g) administration are shown in Fig. 8A and B. Cumulative daily doses of amoxicillin used to reproduce both intravenous and oral human profiles in rabbits were close to 170 mg/kg. Mean areas under curves were equal to 60.36 for oral and 63.58 mg · h−1 · liter−1 for intravenous administration, and times above MIC for the penicillin-resistant S. pneumoniae strain were 360 and 190 min, respectively. Correlation coefficients and area under the curve ratios, between observed and expected values, were 0.988 and 1.08 for oral simulation and 0.998 and 1.15 for intravenous simulation, respectively. The mean measured pulmonary amoxicillin concentration 1 h after intravenous simulation initiation was 22.3 mg/liter.
FIG. 8.
Human amoxicillin pharmacokinetics simulation in rabbits, reproducing human serum profiles following 1-g dose administered intravenously (A) or orally (B). Symbols:  , obtained concentrations under the human pharmacokinetics simulation; ---▾---, native concentrations without the controlled infusions; ---▵---, expected human concentrations.
, obtained concentrations under the human pharmacokinetics simulation; ---▾---, native concentrations without the controlled infusions; ---▵---, expected human concentrations.
Human-simulated amoxicillin treatment of experimental penicillin-resistant pneumococcal pneumonia in rabbits.
For the penicillin-resistant S. pneumoniae pneumonia model (strain 16089), observed peak amoxicillin concentrations in serum were 14.21 mg/liter for oral simulation and 92.85 mg/liter for intravenous simulation, with expected values of 19.7 and 101 mg/liter, respectively. Correlation coefficients and area under the curve ratios between observed and expected values were 0.917 and 0.66 (oral simulation) and 0.997 and 0.89 (intravenous simulation), respectively. In all the treated rabbits, measured amoxicillin pulmonary concentrations were always below 1 mg/liter at the time of the sacrifice (around 48 h after inoculation). Pneumococcal concentrations in lungs and spleen were significantly lower in treated arms than in untreated arms (orally treated versus untreated, P = 0.005 and P < 10−6, and intravenously treated versus untreated, P = 0.004 and P < 10−6, respectively; Dunnett’s test) (Table 4). There was no difference between the two treated arms. On the other hand, macroscopic and histopathologic evaluations were not different within the three arms. Results observed for the three arms 48 h after inoculation of the penicillin-resistant S. pneumoniae (strain 16089) group are summarized in Table 4.
TABLE 4.
Effects of treatment by amoxicillin (oral and intravenous simulation) on penicillin-resistant (strain 16089) and penicillin-susceptible (strain 195) S. pneumoniae experimental pneumonia
| Parameter | Result or concentration for the following straina
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Penicillin-resistant S. pneumoniae (strain 16089)
|
Penicillin-susceptible S. pneumoniae (strain 195)
|
|||||||
| Untreated | Treated
|
Pd | Untreated | Treated
|
Pd | |||
| Oralb | Intravenousc | Oralb | Intravenousc | |||||
| No. | 8 | 5 | 5 | 8 | 5 | 5 | ||
| Death at 48 h (%) | 4/8 (50) | 0/5 (0) | 0/5 (0) | 0.02 | 4/8 (50) | 0/5 (0) | 0/5 (0) | 0.02 |
| Macroscopic score | 17.5 ± 10.4 | 12.0 ± 6.8 | 11.8 ± 1.3 | 0.36 | 17.8 ± 7.5 | 22.6 ± 5.9 | 16.4 ± 5.4 | 0.31 |
| Histopathologic score | 14.0 ± 8.3 | 16.6 ± 8.3 | 15.2 ± 5.0 | 0.78 | 13.1 ± 5.4 | 17.6 ± 2.6 | 15.6 ± 4.3 | 0.24 |
| Sterilized lungs (%) | 0/8 (0) | 3/5 (60) | 3/5 (60) | 0.01 | 0/8 (0) | 4/5 (80) | 5/5 (100) | <0.001 |
| Pneumococcal concentration in lungse | 5.07 ± 2.05 | 1.63 ± 1.02 | 1.44 ± 0.81 | <0.001 | 6.49 ± 1.33 | 1.05 ± 0.11 | 1.00 ± 0.00 | <10−6 |
| Pneumococcal concentration in spleene | 4.40 ± 2.37 | 1.00 ± 0.00 | 1.00 ± 0.00 | <10−6 | 5.00 ± 2.10 | 1.00 ± 0.00 | 1.00 ± 0.00 | <10−6 |
Each value represents the mean ± SD or number and percentage.
Simulation of four amoxicillin (1-g) oral administrations every 8 h.
Simulation of five amoxicillin (1-g) intravenous administrations every 8 h.
One-way variance analysis.
Log10 CFU/g.
In the penicillin-susceptible S. pneumoniae pneumonia model (strain 195), observed concentrations in serum at peak were 19.93 mg/liter in oral simulation and 126.36 mg/liter in intravenous simulation, with expected values of 19.7 and 101 mg/liter, respectively. Correlation coefficients and area under the curve ratios between observed and expected values were 0.953 and 0.80 (oral simulation) and 0.992 and 1.46 (intravenous simulation), respectively. As for the penicillin-resistant S. pneumoniae pneumonia model, in all the treated rabbits measured amoxicillin pulmonary concentrations were always below 1 mg/liter at the time of the sacrifice. Pneumococcal concentrations in lungs and spleen were also significantly different between treated and untreated arms (orally or intravenously treated versus untreated; macroscopic or histopathologic score, P < 10−6; Dunnett’s test). There was no difference between the two treated arms. On the other hand, macroscopic and histopathologic evaluations were not different within the three arms. Results observed for the three arms 48 h after inoculation in the penicillin-susceptible S. pneumoniae (strain 195) group are summarized in Table 4.
DISCUSSION
Experimental penicillin-resistant S. pneumoniae pneumonia in rabbits.
The primary purpose of our study was to develop a penicillin-resistant S. pneumoniae pneumonia model in parallel with a penicillin-susceptible S. pneumoniae pneumonia model. Several animal models have been published (1–3, 6, 9, 14, 17, 18, 33, 34, 39, 41–43, 45, 46). However, although these models are contributive, our model exhibits several advantages. The strains used in our study were clinical isolates with common serotypes. The use of conventional adult male New Zealand White rabbits offered the following two advantages. (i) They were not naturally susceptible to S. pneumoniae infections (4), which guarantees that the observed pathologies were induced by experiments. (ii) They were not immunosuppressed, in contrast to most of the animals used in other penicillin-resistant S. pneumoniae pneumonia models (1–3, 6, 9, 14, 17, 33, 34, 42, 43). Inoculation through natural airways into bronchia was more reproducible than aerosols or intratracheal instillation (38) and less aggressive than pertracheal or transthoracic inoculation (9, 18, 33, 39, 41). However, the final inoculum concentration used for the experiments (10 log10 CFU/ml) was higher than those used in most other experimental models (6 to 9.3 log10 CFU/ml) (9, 18, 33, 39, 41, 45). Several parameters may explain such a difference. First, a different virulence may already exist according to animals and serotypes of strains (7, 8). Second, the penicillin-resistant S. pneumoniae strains used in our model were diluted in physiologic serum, without any adjuvant, and inoculated to nonimmunosuppressed animals. Third, the inoculum concentration was determined to reproduce findings observed in severe pneumococcal pneumonia in humans.
The different and numerous evaluation criteria used in this model constitute another interesting advantage. Indeed, survival, radiological, anatomic, and microbiological data allowed a precise evaluation of pneumonia and precise comparison with human pathology. The correlation between these criteria was quite good, but differences observed in some cases underline their individual importance.
The most important interest of this model is its ability to closely reproduce severe human pneumococcal pneumonia, with the exception that intrabronchial inoculation of a highly concentrated S. pneumoniae suspension resulted in the absence of a true incubation phase. Illness began directly with an invasion phase, quickly followed by bacteremic pneumonia, possibly evolving in 2 to 3 weeks to a cure (data not shown). Cumulative mortality of rabbits inoculated with 10 log10 CFU of penicillin-resistant S. pneumoniae per ml (around 50%) was slightly higher than the 38% observed in penicillin-resistant S. pneumoniae pneumonia in humans (37) and close to the 45 to 85% observed in untreated bacteremic pneumococcal pneumonia (47, 48). In humans, the severity of pneumococcal pneumonia is correlated with the existence of bacteremia, which was constantly observed in our experimental model. The macroscopic and histopathological evolution observed in both penicillin-resistant and penicillin-susceptible S. pneumoniae pneumonia models was very similar to human pneumonia (31, 32, 40). Experimental infection begins with unilobar lesions. A rapid extension to the other lobes occurs within a few hours, accompanied by pleural effusion and bacteremia. Finally, the lesions evolve to fibrosis. Pneumococcal lung concentrations in dead rabbits (5 to 8 log10 CFU/ml) were very similar to these observed in human postmortem lung cultures (29). There was a weak correlation between bacterial concentrations and pulmonary lesions, as described in nosocomial pneumonia (28, 40, 49), even taking into account the possible lack of reproducibility by pathologists (11).
Another point of interest was that comparisons between sacrificed and pneumonia-related dead rabbits pointed out that in the first 24 h the main predictive factor of ulterior evolution was the bacterial concentration (and bacteremia); after this delay, pulmonary injuries were the most important factor. So, it is tempting to speculate that inflammatory phenomena induced by either penicillin-resistant or penicillin-susceptible S. pneumoniae evolve in an autonomous fashion, without strict correlation with in situ residual bacterial inoculum. Furthermore, the absence of macroscopic and histopathological differences observed between treated and untreated rabbits, while bacterial clearance was obtained, seems to reinforce this hypothesis.
Simulation of human amoxicillin pharmacokinetics in rabbits.
Considering that the experimental pneumonia in our model closely reproduced human pathology, the humanization of antibiotic treatment appeared interesting and important. So, the second purpose of our study was to reproduce human serum pharmacokinetics of amoxicillin in rabbits. To do so, we chose an adaptation of antibiotic administration rather than a modification of the antibiotic elimination. Most of the studies simulating human pharmacokinetics by adaptation of antibiotic administration reproduced plasmatic kinetics following intravenous administration alone and/or for only a few hours (15, 16, 19, 20, 52, 53). In our study, a total of 25 simulations of human amoxicillin pharmacokinetics were performed. Results obtained after simulation were very close to the expected (human) concentrations, which were themselves far from (native) concentrations observed in rabbits after a single bolus without subsequent infusion (23). So, by developing the Hull mathematical model (27), we were able to reproduce human pharmacokinetics fitting into an open bicompartmental model and, moreover, to mimic human serum profiles following not only intravenous but also oral administration of amoxicillin. Another theoretical advantage of this mathematical model was to calculate the extravascular antibiotic concentrations, thus allowing comparisons with measured intrapulmonary concentrations. Indeed, it was of particular interest that the measured amoxicillin pulmonary concentrations were close to the extravascular concentrations calculated from the model. Moreover, these pulmonary concentrations were near those observed in humans, even if these latter are infrequently evaluated and variable (20.8 to 43.1 mg/liter 1 h after intravenous injection of amoxicillin [1 g]) (12, 21).
Human-simulated amoxicillin treatment of experimental penicillin-resistant pneumococcal pneumonia in rabbits.
The third purpose of our study was to evaluate antibiotic efficacy in our penicillin-resistant S. pneumoniae pneumonia model by simulating human pharmacokinetics of amoxicillin.
Time between inoculation and treatment initiation is an important prognostic factor, even if it cannot really be appreciated in humans. It seems overall relatively short in severe pneumonia (35). In our experimental therapeutic study, we chose a brief but sufficient delay (4 h after inoculation) to observe consolidated pulmonary pathological lesions and high pneumococcal concentrations and to start the treatment before the first untreated pneumonia-related deaths occurred (8 h after inoculation). Lung lesions and spleen pneumococcal concentrations observed 4 h after inoculation in our pneumonia model ensure that amoxicillin had a therapeutic and not a prophylactic effect. Treatment duration (between 32 and 34 h) allowed an evaluation 48 h after inoculation, without any carryover effect, at a time when bacterial concentrations are important in untreated rabbits.
Our experimental therapeutic study has shown that amoxicillin treatment (either four oral or five intravenous administrations of 1 g every 8 h) resulted in a very significant and similar pneumococcal clearance in lungs and spleen, for both penicillin-susceptible S. pneumoniae and penicillin-resistant S. pneumoniae pneumonia. These results were obtained reproducing current recommendations for S. pneumoniae pneumonia treatment in France (44). Similar findings have already been reported by other studies, in immunosuppressed mice or guinea pigs, by using amoxicillin doses theoretically higher than that in our work (150, 600, and 1,200 mg/kg/day), without human pharmacokinetics simulation, on penicillin-resistant S. pneumoniae pneumonia (penicillin MIC = 1 to 4 mg/liter) (33, 39, 42). In fact, pharmacokinetic data in these studies were close to those observed in humans after a 1.5-to-2-g amoxicillin treatment three times a day, i.e., approximately twice the dose we evaluated with our procedure in rabbits. Lung lesions were not significantly different between arms, probably because inflammatory responses when triggered continued in spite of pneumococcal clearance.
The lack of difference in efficacy observed between oral and intravenous simulated treatments was correlated to the global pharmacokinetic equivalence between them. The time above MIC was always greater than 40%, although it was longer in the oral arm than in the intravenous one (87.5% versus 50% for the penicillin-resistant strain and 95% versus 66% for the penicillin-susceptible strain), whereas the total area under the curve was greater in intravenous than in oral arms (320 versus 240 mg · h−1 · liter−1).
In conclusion, we developed a penicillin-resistant S. pneumoniae experimental pneumonia in immunocompetent rabbits, which is easy to reproduce and very close to bacteremic pneumococcal pneumonia in humans. Human amoxicillin serum profile simulation in rabbits, corresponding to an open bicompartmental model, was realized in an easy and reproducible fashion. This experimental therapeutic study permitted the assessment of the efficacy of amoxicillin (reproducing a 3-g/day dose) to obtain pneumococcal clearance and survival improvement at 48 h, even with penicillin-resistant S. pneumoniae (MIC = 4 mg/liter). This work may permit a better understanding of pneumonia physiopathology, including inflammatory response study, and an evaluation of different therapeutic approaches to penicillin-resistant S. pneumoniae pneumonia.
ACKNOWLEDGMENT
This study was supported by a grant from MEDEX Society.
REFERENCES
- 1.Azoulay-Dupuis E, Bedos J, Vallée E, Hardy D J, Swanson R N, Pocidalo J. Antipneumococcal activity of ciprofloxacin, ofloxacin, and temafloxacin in an experimental mouse pneumonia model at various stages of the disease. J Infect Dis. 1991;163:319–324. doi: 10.1093/infdis/163.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay-Dupuis E, Moine P, Bedos J, Rieux V, Vallée E. Amoxicillin dose-effect relationship with Streptococcus pneumoniae in a mouse pneumonia model and roles of in vitro penicillin susceptibilities, autolysis, and tolerance properties of the strains. Antimicrob Agents Chemother. 1996;40:941–946. doi: 10.1128/aac.40.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azoulay-Dupuis E, Vallée E, Veber B, Bedos J, Bauchet J, Pocidalo J. In vivo efficacy of a new fluoroquinolone, sparfloxacin, against penicillin-susceptible and -resistant and multiresistant strains of Streptococcus pneumoniae in a mouse model of pneumonia. Antimicrob Agents Chemother. 1992;36:2698–2703. doi: 10.1128/aac.36.12.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker D. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev. 1998;11:231–266. doi: 10.1128/cmr.11.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett J, Breiman R, Mandell L, File T. Community-acquired pneumonia in adults: guidelines for management. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 6.Bédos J, Rieux V, Bauchet J, Muffat-Joly M, Carbon C, Azoulay-Dupuis E. Efficacy of trovafloxacin against penicillin-susceptible and multiresistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1996;40:2829–2834. doi: 10.1128/aac.42.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bédos J, Rolin O, Bouanchaud D, Pocidalo J. Relation entre virulence et résistance aux antibiotiques des pneumocoques. Apport des données expérimentales sur un modèle animal. Pathol Biol. 1991;39:984–990. [PubMed] [Google Scholar]
- 8.Briles D. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candiani G, Abbondi M, Borgonovi M, Williams R. Experimental lobar pneumonia due to penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae in immunocompetent and neutropenic rats: efficacy of penicillin and teicoplanin treatment. J Antimicrob Chemother. 1997;39:199–207. doi: 10.1093/jac/39.2.199. [DOI] [PubMed] [Google Scholar]
- 10.Chapin-Robertson K, Edberg S. Measurement of antibiotics in human body fluids: techniques and significance. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: Williams and Wilkins; 1991. pp. 295–366. [Google Scholar]
- 11.Corley D, Kirtland S, Winterbauer R, Hammar S, Dail D, Bauermeister D, Bolen J. Reproducibility of the histologic diagnosis of pneumonia among a panel of four pathologists—analysis of a gold standard. Chest. 1997;112:458–465. doi: 10.1378/chest.112.2.458. [DOI] [PubMed] [Google Scholar]
- 12.Cox A, Meewis J, Horton R. Penetration into lung tissue after intravenous administration of amoxycillin/clavulanate. J Antimicrob Chemother. 1989;24:S87–S91. doi: 10.1093/jac/24.suppl_b.87. [DOI] [PubMed] [Google Scholar]
- 13.Croydon E A P, Sutherland R. α-Amino-p-hydroxybenzylpenicillin (BRL 2333), a new semisynthetic penicillin: absorption and excretion in man. 1971. pp. 427–430. . Antimicrob. Agents Chemother. 1970. [PubMed] [Google Scholar]
- 14.Darras-Joly C, Bédos J, Sauve C, Moine P, Vallee E, Carbon C, Azoulay-Dupuis E. Synergy between amoxicillin and gentamicin in combination against a highly penicillin-resistant and -tolerant strain of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1996;40:2147–2151. doi: 10.1128/aac.40.9.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flückiger U, Moreillon P, Blaser J, Bickle M, Glauser M, Francioli P. Simulation of amoxicillin pharmacokinetics in humans for the prevention of streptococcal endocarditis in rats. Antimicrob Agents Chemother. 1994;38:2846–2849. doi: 10.1128/aac.38.12.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flückiger U, Segessenmann C, Gerber A. Integration of pharmacokinetics and pharmacodynamics of imipenem in a human-adapted mouse model. Antimicrob Agents Chemother. 1991;35:1905–1910. doi: 10.1128/aac.35.9.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuoka T, Kawada H, Kitayama A, Koga T, Kubota M, Harasaki T, Kamai Y, Ohya S, Yasuda H, Iwata M, Kuwahara S. Efficacy of CS-834 against experimental pneumonia caused by penicillin-susceptible and -resistant Streptococcus pneumoniae in mice. Antimicrob Agents Chemother. 1998;42:23–27. doi: 10.1128/aac.42.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavalda J, Capdevila J A, Almirante B, Otero J, Ruiz I, Laguarda M, Allende H, Crespo E, Pigrau C, Pahissa A. Treatment of experimental pneumonia due to penicillin-resistant Streptococcus pneumoniae in immunocompetent rats. Antimicrob Agents Chemother. 1997;41:795–801. doi: 10.1128/aac.41.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber A, Brugger H, Feller C, Stritzko T, Stadler B. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J Infect Dis. 1986;153:90–97. doi: 10.1093/infdis/153.1.90. [DOI] [PubMed] [Google Scholar]
- 20.Gerber A, Stritzko T, Segessenmann C, Stalder B. Simulation of human pharmacokinetic profiles in mice, and impact on antimicrobial efficacy of netilmicin, ticarcillin and ceftazidime in the peritonitis-septicemia model. Scand J Infect Dis. 1991;74:195–203. [PubMed] [Google Scholar]
- 21.Gould I M, Harvey G, Golder D, Reid T M, Watt S J, Friend J A, Legge J S, Douglas J G. Penetration of amoxycillin/clavulanic acid into bronchial mucosa with different dosing regimens. Thorax. 1994;49:999–1001. doi: 10.1136/thx.49.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenblatt D, Koch-Weser J. Clinical pharmacokinetics. N Engl J Med. 1975;297:702–705. doi: 10.1056/NEJM197510022931406. [DOI] [PubMed] [Google Scholar]
- 23.Hekster Y, Baars A, Vree T, van Klingeren B, Rutgers A. Comparison of the determination of β-lactam antibiotic drugs in plasma of rabbits by means of HPLC and of a microbiological assay. Pharm Wbl (Sci Ed) 1979;1:695–700. [Google Scholar]
- 24.Hill S, Jones K. Pharmacokinetics of parenterally administered amoxycillin. J Infect. 1980;2:320–332. doi: 10.1016/s0163-4453(80)92720-6. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann J, Cetron M, Farley M, Baughman W, Facklam R, Elliott J, Deaver K, Breiman R. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 26.Hull C, McLeod K. Pharmacokinetic analysis using an electrical analogue. Br J Anaesth. 1976;48:677–686. doi: 10.1093/bja/48.7.677. [DOI] [PubMed] [Google Scholar]
- 27.Hull C J. General principles of pharmacokinetics. In: Prys-Roberts C, Hug C, editors. Pharmacokinetics of anesthesia. Oxford, England: Blackwell; 1984. pp. 1–24. [Google Scholar]
- 28.Kirtland S, Corley D, Winterbauer R, Springmeyer S, Casey K, Hampson N, Dreis D. The diagnosis of ventilator-associated pneumonia—a comparison of histologic, microbiologic, and clinical criteria. Chest. 1997;112:445–457. doi: 10.1378/chest.112.2.445. [DOI] [PubMed] [Google Scholar]
- 29.Knapp B, Kent T. Postmortem lung cultures. Arch Pathol. 1968;85:200–203. [PubMed] [Google Scholar]
- 30.Labaune J. Pharmacocinétique. 2nd ed. Paris, France: Masson; 1988. [Google Scholar]
- 31.Marquette C, Wallet F, Copin M, Wermet D, Desmidt A, Ramon P, Courcol R, Tonnel A. Relationship between microbiologic and histologic features in bacterial pneumonia. Am J Respir Crit Care Med. 1996;154:1784–1787. doi: 10.1164/ajrccm.154.6.8970371. [DOI] [PubMed] [Google Scholar]
- 32.Menten M, Bailey S, DeBone F. Pneumonia in children. J Infect Dis. 1932;51:254–267. [Google Scholar]
- 33.Moine P, Sauve C, Vallee E, Bedos J, Azoulay-Dupuis E. In vivo efficacy of cefotaxime and amoxicillin against penicillin-susceptible, penicillin-resistant and penicillin-cephalosporin-resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Clin Microbiol Infect. 1997;3:608–615. doi: 10.1111/j.1469-0691.1997.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 34.Moine P, Vallée E, Azoulay-Dupuis E, Bourget P, Bédos J P, Bauchet J, Pocidalo J J. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1994;38:1953–1958. doi: 10.1128/aac.38.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moine P, Vercken J, Chevret S, Gajdos P. Severe community-acquired pneumococcal pneumonia. Scand J Infect Dis. 1995;27:201–206. doi: 10.3109/00365549509019009. [DOI] [PubMed] [Google Scholar]
- 36.Nava J M, Bella F, Garau J, Lite J, Morera M, Marti C, Fontanals D, Font B, Pineda V, Uriz S, Deulofeu F, Calderon A, Duran P, Grau M, Agudo A. Predictive factors for invasive disease due to penicillin-resistant Streptococcus pneumoniae: a population-based study. J Infect Dis. 1994;19:884–890. doi: 10.1093/clinids/19.5.884. [DOI] [PubMed] [Google Scholar]
- 37.Pallares R, Lineares J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martin R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 38.Percy D H, Bhasin J L, Rosendal S. Experimental pneumonia in rabbits inoculated with strains of Pasteurella multocida. Can J Vet Res. 1986;50:36–41. [PMC free article] [PubMed] [Google Scholar]
- 39.Ponte C, Parra A, Nieto E, Soriano F. Development of experimental pneumonia by infection with penicillin-insensitive Streptococcus pneumoniae in guinea pigs and their treatment with amoxicillin, cefotaxime, and meropenem. Antimicrob Agents Chemother. 1996;40:2698–2702. doi: 10.1128/aac.40.12.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouby J, Martin de Lassale E, Poete P, Nicolas M, Bodin L, Jarlier V, Le Charpentier Y, Grosset J, Viars P. Nosocomial bronchopneumonia in the critically ill. Am Rev Respir Dis. 1992;146:1059–1066. doi: 10.1164/ajrccm/146.4.1059. [DOI] [PubMed] [Google Scholar]
- 41.Saladino R, Stack A, Fleisher G, Thompson C, Briles D, Kobzik L, Siber G. Development of a model of low-inoculum Streptococcus pneumoniae intrapulmonary infection in infant rats. Infect Immun. 1997;65:4701–4704. doi: 10.1128/iai.65.11.4701-4704.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauve C, Azoulay-Dupuis E, Moine P, Darras-Joly V, Rieux V, Carbon C, Bédos J. Efficacies of cefotaxime and ceftriaxone in a mouse model of pneumonia induced by two penicillin- and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2147–2151. doi: 10.1128/aac.40.12.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith G M, Abbott K H. Development of experimental respiratory infections in neutropenic rats with either penicillin-resistant Streptococcus pneumoniae or β-lactamase-producing Haemophilus influenzae. Antimicrob Agents Chemother. 1994;38:608–610. doi: 10.1128/aac.38.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Société de Pathologie Infectieuse de Langue Française (SPILF) Quatrième conférence de consensus sur le traitement des infections respiratoires. Med Mal Infect. 1992;22:44–47. [Google Scholar]
- 45.Takashima K, Tateda K, Matsumoto T, Ito T, Iizawa Y, Nakao M, Yamaguchi K. Establishment of a model of penicillin-resistant Streptococcus pneumoniae pneumonia in healthy CBA/J mice. J Med. 1996;45:319–322. doi: 10.1099/00222615-45-5-319. [DOI] [PubMed] [Google Scholar]
- 46.Tateda K, Takashima K, Miyazaki H, Matsumoto T, Hatori T, Yamaguchi K. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob Agents Chemother. 1996;40:1520–1525. doi: 10.1128/aac.40.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson R, Ruegsegger J, Blankenhorm M, Hamburger M. Primary pneumococcic pneumonia in the Cincinnati General Hospital, 1936–1950. J Lab Clin Med. 1951;37:73–87. [PubMed] [Google Scholar]
- 48.Tilghman R, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med. 1937;59:602–619. [Google Scholar]
- 49.Torres A, El-Ebiary M, Padro L, Gonzales J, Puig de la Belcasa J, Ramirez J, Xaubet A, Ferrer M, Rodriguez-Roisin R. Validation of different techniques for the diagnosis of ventilator-associated pneumonia: comparison with immediate postmortem pulmonary biopsy. Am J Respir Crit Care Med. 1994;149:324–331. doi: 10.1164/ajrccm.149.2.8306025. [DOI] [PubMed] [Google Scholar]
- 50.Walsh T, Bacher J, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci. 1988;38:467–471. [PubMed] [Google Scholar]
- 51.Watanakunakorn C, Bailey T. Adult bacteremic pneumococcal pneumonia in a community hospital, 1992–1996. Arch Intern Med. 1997;157:1965–1971. [PubMed] [Google Scholar]
- 52.Woodnutt G, Berry V, Mizen L. Simulation of human serum pharmacokinetics of cefazolin, piperacillin, and BRL 42715 in rats and efficacy against experimental intraperitoneal infections. Antimicrob Agents Chemother. 1992;36:1427–1431. doi: 10.1128/aac.36.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodnutt G, Catherall E, Kernutt I, Mizen L. Temocillin efficacy in experimental Klebsiella pneumoniae meningitis after infusion into rabbit plasma to simulate antibiotic concentrations in human serum. Antimicrob Agents Chemother. 1988;32:1705–1709. doi: 10.1128/aac.32.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]