Abstract
Background
Coronavirus disease 2019 (Covid-19) pandemic is a global health crisis that has culminated in thousands of deaths. In order to reduce the spread of the Sars-CoV-2 virus, governments of several countries have adopted social isolation as a strategy. However, social isolation has culminated in deleterious effects on the population’s health, including increased physical inactivity, stress and, consequently, adverse changes in body composition, cardiorespiratory capacity, muscle strength, physical functionality, and vascular events, which are increasingly pointed out as the main determinants of cardiovascular health. Staying physically active during lockdown is a challenge, especially for the population with a higher risk of mortality from COVID-19, who are still encouraged to maintain social distance until there is a vaccine available. Strategies to avoid physical inactivity and reduce stress levels can promote cardiovascular protection and must be considered during COVID-19 time.
Objective
The aim of this paper is to discuss the risks of physical inactivity and stress for the cardiovascular system during the COVID-19 pandemic and propose strategies to protect cardiovascular health.
Conclusion
A home-based training protocol could be an interesting and effective strategy for the population who need to remain physically active and safe at home.
Keywords: Sedentary behavior, physical inactivity, cardiovascular diseases, exercise, stress, coronavirus
1. INTRODUCTION
Coronavirus disease 2019 (COVID-19) pandemic is a globally impacting health crisis that has resulted in high mortality and morbidity [1]. To date (November 15, 2020), over 53.7 million cases have been reported, and more than 1.3 million deaths have been confirmed [2] worldwide. Among the effective strategies to minimize contagion, social isolation has been found as one of the main ways to decrease viral spread [3]. However, prolonged quarantine may result in unfavorable sedentary behavior (i.e., physical inactivity, 'too much sitting' behavior and energy expenditure of ≤1.5 metabolic equivalent method - METs [4]) and chronic stress, increasing the risk of chronic disease [5]. In fact, recent studies have already pointed out potential risks related to physical inactivity and social isolation [6-12]. Physical inactivity and sedentary behavior are associated with adverse changes in body composition, cardiorespiratory capacity, muscle strength, physical functionality, and vascular events [13], which are increasingly pointed out as the main determinants of cardiovascular health [14]. Especially in elderly individuals, physical inactivity results in increased age-related muscle waste, accelerating the progression of sarcopenia and associated comorbidities [11]. Chronic stress is also associated with the development of cardiovascular diseases [15], with the stress-related reduction in the physical activity being an important factor for cardiovascular risk. In addition, the long-term development of an atherosclerotic process and subclinical coronary heart disease also occur with stress and acute exposure to stress can trigger cardiac events in people with advanced coronary heart disease [15]. Stress can also affect the autonomic nervous system, decreasing heart rate variability, thus increasing the cardiovascular risk [16]. In Brazil, a study assessed the changes in the habits of the participants engaged in physical activities in relation to their practices due to the social distancing during the COVID-19 period. Importantly, 79.4% of the participants reported that the quarantine had an impact on their physical activities, and many had to interrupt or decrease the frequency of physical activity. Participants who felt a higher impact of social isolation on physical activity tended to show symptoms of anxiety, depression and poor mental health [17].
Recommendations for the social isolation period include maintaining physical activity and avoid prolonged periods of time spent sitting. Therefore, strategies to avoid physical inactivity and reduce stress levels can promote cardiovascular protection, and must be considered during COVID-19 time [18]. This paper aims to discuss the risks of physical inactivity and stress during the COVID-19 pandemic for the cardiovascular system and to propose strategies to protect cardiovascular health.
2. IMPACT OF PHYSICAL INACTIVITY ON CARDIOVASCULAR HEALTH
Data from the World Health Organization indicate that 31% of individuals over 15 years of age are physically inactive [19]. Even short periods of reduced physical activity can result in deleterious effects in many biological systems [7]. Sedentary behavior leads to a positive energy balance resulting in a fat deposition which is related to systemic inflammation [19], favoring the prevalence of obesity and type 2 diabetes. A recent study found that even short-term changes in physical activity and sedentary behavior in response to COVID-19 restrictions can become permanent, leading to an increased risk of obesity, diabetes and cardiovascular disease in children living in the United States [12]. Another study carried out in Italy showed that the lockdown culminated in a lower rate of physical activity and was correlated with significantly higher weight gain in obese individuals [20]. This scenario increases the risk of mortality from COVID-19 and cardiovascular diseases [21]. It is estimated that 3.2 million deaths per year are related to physical inactivity [22]. In parallel, individuals with cardiovascular diseases may present the most severe symptoms of COVID-19 [18]. Thus, a significant concern arises with the deleterious effects of physical inactivity on the cardiovascular system during the COVID-19 era [23].
Cardiovascular disease is the leading cause of death worldwide, reaching around 17.9 million deaths each year. Much of the burden and mortality from cardiovascular disease is related to unhealthy behaviors, including physical inactivity and low cardiorespiratory capacity [24]. A sedentary lifestyle usually results in increased inflammation associated with atrial fibrillation [25], visceral adiposity, sarcopenia, atherogenic dyslipidemia, arterial hypertension, endothelial dysfunction, type 2 diabetes, and insulin resistance [14]. For instance, reduced VO2 max and muscle volume are evident after two weeks of bed [26] rest. It is essential to highlight that a reduction of 1 MET in VO2 max is associated with an 18% increase in cardiovascular disease incidence [27, 28], and the vast majority of these risk factors (i.e., arterial hypertension, type 2 diabetes, visceral adiposity) also imply the severity of the COVID-19 symptoms [29].
Another relevant impact of a sedentary lifestyle occurs on the autonomic nervous system (ANS) control [30]. ANS is responsible for controlling all parts of the body, except skeletal muscles. Through ANS, the physiological integrity of cells, tissues, and organs is maintained, ensuring body homeostasis [31]. Importantly, ANS is the main regulator of circulatory adjustments in the face of acute hemodynamic changes [32]. The nerves in the carotid sinus are responsible for detecting changes in blood pressure and humoral factors, triggering sympathetic and parasympathetic autonomic reflexes [32, 33]. These nerves influence cardiovascular homeostasis in two main ways: inhibiting the sympathetic impulse through their activation, which prevents an increase in blood pressure, or increasing sympathetic activity when blood pressure decreases [32]. Consequently, autonomic imbalance might be related to vascular diseases and culminates in target organ damage, cardiovascular events, and aggravate disability deficits [34].
In particular, impairment in autonomic control is associated with the onset of hypertension, and hypertensive patients are more predisposed to cardiovascular events and stroke than individuals with normal blood pressure [35]. In this sense, stroke patients usually present decreased heart rate variability, autonomic imbalance, and impaired cardiac vagal modulation [34]. The onset and progression of stroke can be determined by afferent and efferent vagal pathways, especially by central and peripheral inflammation, which may either lead to stroke or be stroke-induced [34, 36]. Considering that arterial hypertension and vascular injuries are important causes of morbidity and mortality due to COVID-19 [18], controlling blood pressure, and managing damage to organs is crucial to reduce this risk [35].
It is worth mentioning that muscle inactivity can result in a condition in the legs called deep vein thrombosis [ 37 ]. Pulmonary embolism is commonly a consequence of deep vein thrombosis and it is associated with greater mortality [ 38 ]. This risk must be taken into account, especially by patients with COVID-19 who must remain in isolation [ 39 ]. A form of diagnosis of venous thromboembolism is the investigation of D-dimer levels (i.e., a breakdown product of soluble fibrin that results from the ordered breakdown of thrombi by the fibrinolytic system) [ 40 ]. Interestingly, a recent meta-analysis found that mean D-dimer levels, were significantly increased in patients with severe COVID-19 infection. This evidence suggests that patients with COVID-19 presenting elevated D-dimer levels have an elevated risk of mortality [ 39 ].
Sedentary behavior is also related to increased inflammation [14], which is associated with ANS [41]. Studies have further strengthened the hypothesis of a direct relationship between ANS and immune responses. Pieces of evidence show that the cholinergic system influences the inflammatory response, controlling the release of TNF-α, IL1β, and IL-6, and the non-neuronal acetylcholine synthesis and release machinery is unregulated in the inflammatory process [41]. Additionally, cardiovascular damage caused by excessive stimulation of the sympathetic nervous system and the renin-angiotensin system, as well as its receptors (ɑ- and β-adrenergic receptors and AT1), appears to be mediated through activation of pro-inflammatory response. The migration of innate or adaptive immune cells to the central nervous system can induce excitation of hypothalamic and paraventricular nuclei and caudal and ventrolateral medullary neurons that cause central sympathetic excitation [42, 43]. Interestingly, vagal activation may suppress inflammation [44], and physical exercise can contribute to greater vagal control [45], thus preventing important cardiovascular risk factors.
Considering that levels of cardiorespiratory fitness and muscle strength are inversely associated with the incidence of cardiovascular disease and all-cause mortality [27, 28], increasing levels of physical activity is crucial for the integrity of cardiovascular health, especially during the COVID-19 pandemic.
3. IMPACT OF STRESS ON CARDIOVASCULAR HEALTH
COVID-19 pandemic is associated with adverse consequences for mental health [46]. This situation significantly increased levels of panic, stress, and anxiety [47]. One study evaluated and compared several unhealthy behaviors during the lockdown period with the negative mood scale. The authors pointed out that poorer diet, poorer sleep quality, and physical inactivity were linked to a more-negative mood [10]. In Brazil, the cluster of unhealthy behaviors increased substantially and was associated with poorer mental health during quarantine [8]. In Spain, younger individuals with chronic diseases reported elevated symptoms of anxiety, stress and depression [48]. Social isolation, combined with fear of contagion and information overload, can also culminate in chronic stress related to a mental health burden. In addition, social isolation has been pointed out as a harmful factor to the autonomic regulation of the heart. This impairment is associated with high levels of cortisol and epinephrine, the main molecular markers of stress [15, 49]. It is already established that chronic stress is an important modulator of immunity; therefore, this factor can directly influence the probability of infection [50], as well as cardiovascular health. Stress in adulthood can trigger cardiovascular diseases in individuals who already have a high burden of atherosclerotic plaque. Besides, chronic stress can be a determining factor in the prognosis of pre-existing cardiovascular diseases [51]. High stress is also associated with an increase in the number of several infections, including influenza and other agents, and with cardiovascular damage. Evidence shows that chronic stress predicts coronary heart disease and socially isolated or lonely individuals have an increased risk of the first event of this type of disease [15]. Short-term emotional stress can also act as a precursor to cardiac injuries in individuals with advanced atherosclerosis [15].
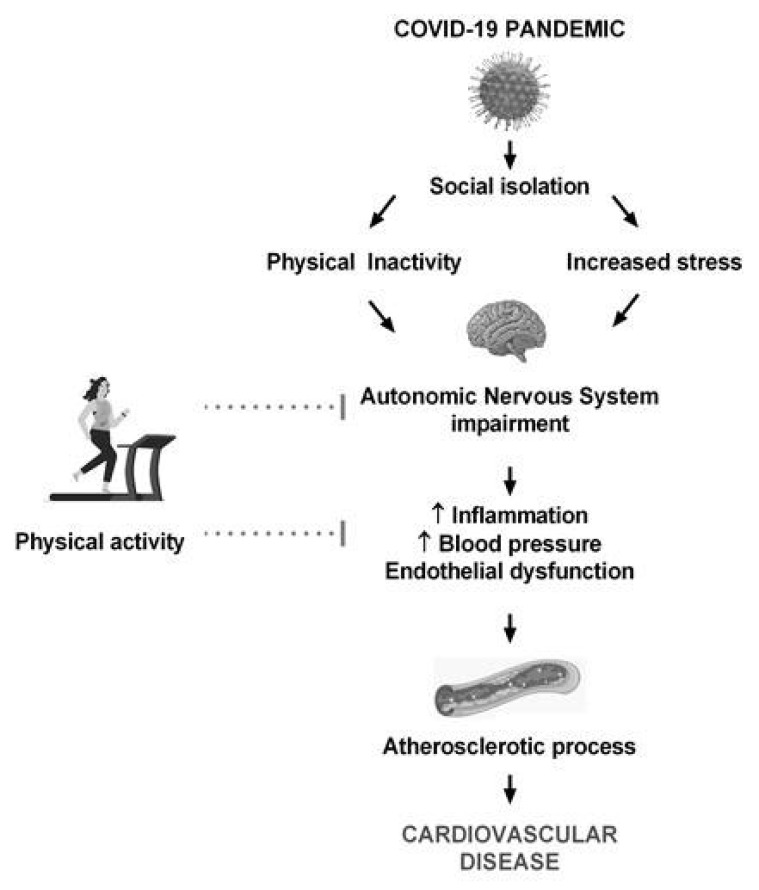
Quarantine during the COVID-19 period resulted in some long-term effects on cardiovascular disease, mainly related to unhealthy lifestyle, anxiety and stress [ 52 ]. In fact, COVID-19 patients presented a high level of post-traumatic stress symptoms and studies with the general public revealed lower psychological well-being during the quarantine period [ 53 ]. The literature points out several mechanisms that associate stress with cardiovascular risk [15]. Stress culminates in increased cortisol and decreased heart rate, suggesting an important effect on the autonomic and neuroendocrine systems [15, 16]. The associated mechanisms include ANS hyperactivity and humoral changes, resulting in endothelial dysfunction, sympathetic activation and related increases in blood pressure and pro-inflammatory and procoagulant responses [15]. Especially the peripheral sympathetic nervous system plays an important role in the cardiovascular response to stress, innervating the heart, vasculature and adrenal medulla. This response occurs through the adrenal medulla, which systemically releases catecholamines (e.g., epinephrine) and through the sympathetic nerve terminals, which innervate the vasculature and release norepinephrine. In this sense, the sympathetic nervous system is responsible for chronotropic and inotropic effects via β1-adrenergic receptors and pressor effects via α1-adrenergic receptors. In addition, the sympathetic nervous system also has immunological effects, including the release of interleukin-6 [54]. The inflammatory cascade related to oxidative imbalance may also explain the cardiovascular changes induced by stress [55]. In summary, the autonomic imbalance and the consequent increases in blood pressure and inflammation might favor the atherosclerotic process and cardiovascular diseases [15, 54]. Therefore, some clinical guidelines already point to stress as a target for cardiovascular disease prevention [51]. The relationship between physical inactivity, stress and cardiovascular diseases is illustrated in Fig. (1).
Fig. (1).
Relationship between physical inactivity, stress and cardiovascular risk. Image created with Freepik.com.
On the other hand, studies show that increased physical activity culminates in reductions in distress [56]. Additionally, both aerobic and resistance training results in reductions in psychological distress [57]. In this sense, it is clear that better control of stress and associated cardiovascular risks are crucial for preventing cardiovascular diseases.
4. IMPORTANCE OF PHYSICAL TRAINING ON CARDIOVASCULAR HEALTH AND STRESS MANAGEMENT
Several studies have shown the beneficial influence of physical exercise in the prevention and treatment of cardiovascular diseases [24, 58] such as improved exercise capacity, heart rate, endothelial function, autonomic nervous system function and blood pressure, reduced inflammatory cytokines, and reduced mortality [24, 45, 58]. Physical exercise is also associated with a 28% lower incidence of atrial fibrillation [59, 60]. Aerobic training is a common type of intervention that includes walking, running, cycling, and swimming. It has been showed that these training modalities increase peak work capacity and reduce all-cause mortality. For example, in patients with stable coronary artery disease, 3 × 1-hour aerobic training sessions at 75-80% of the peak heart rate for 6 weeks can increase the peak work capacity by 21% [61]. Aerobic training also reduces blood pressure, which is one of the main risk factors for cardiovascular events. It has been shown that aerobic training performed 90-150 min/week at 65% -75% heart rate reserve can decrease 5-8 mmHg of systolic blood pressure in hypertensive patients and 2-4 mmHg of systolic blood pressure in normotensive patients. On the other hand, resistance training performed 90-150 min/week at 50% -80% of 1 maximal repetition, 6 exercises, 3 sets/exercise, 10 repetitions/set can decrease 4mmHg of systolic blood pressure in hypertensive patients and 2mmHg of systolic pressure in normotensive patients [24].
Given the benefits above mentioned, guidelines for the prevention and rehabilitation of cardiovascular diseases from leading international societies suggest that healthy individuals and cardiovascular patients should undergo physical training [61]. According to the American College of Cardiology / American Heart Association, aerobic training is generally very safe, and sedentary individuals should start an exercise program at lower intensities and gradually progress to the recommended levels [61]. The European Society of Cardiology indicates moderate to vigorous intensities lasting 30 min/session for 3-5 days/week, reaching at least 150 min / week [62]. The Report of the American College of Cardiology / American Heart Association pointed out that in order to decrease cardiovascular risk, adults should perform at least 150 minutes per week of moderate-intensity aerobic training or 75 minutes per week of vigorous-intensity aerobic training [24]. Specifically, aerobic training for at least 3 days/week for 20-60 min/session with 10 to 15 min warm and cold, at intensities between 40 and 80% HR reserve is recommended [63]. On the other hand, resistance training improves muscle strength, which is also a significant predictor of risk factors and prognosis for cardiovascular diseases. Thus, including 15 to 20 minutes of resistance training twice a week for large muscle groups, in combination with aerobic training is recommended [63]. Elderly adults who are unable to meet physical training recommendations are encouraged to be as physically active as their conditions allow [11].
Another potential ally for improving health in pandemic COVID-19 times as it culminates in benefits for cardiovascular and lung health is inspiratory muscle training. It was showed that 12 weeks of inspiratory muscle training at 30% of the maximal inspiratory pressure increased autonomic modulation, and expiratory, inspiratory, and exercise capacity in patients with the chronic obstructive pulmonary disease [64]. Besides, a systematic review pointed out that inspiratory muscle training performed at low intensities can chronically increase the parasympathetic modulation and reduce sympathetic cardiac modulation in patients with diabetes, hypertension, and chronic heart failure [65]. Also, inspiratory muscle training set at 50% of maximum inspiratory pressure, every day for 4 weeks, increased the heart rate variation and improved the 6-minute walk test and the resting heart rate in older women [66].
Physical training (aerobic and resistance training) is also related to the reduction of general psychological suffering. The study of LeBouthillier & Asmundson (2017) randomized 48 participants with anxiety-related into 3 groups: aerobic training group, resistance training group, and waitlist. Traning sessions were performed three times a week for 4 weeks [57]. The aerobic training consisted of 40 minutes spin cycling with an intensity of 60-80% age-adjusted maximum heart rate reserve, while resistance training consisted of 2-3 sets of 10-12 repetitions to exhaustion. This study showed improved general psychological distress and anxiety with aerobic training, while resistance training improved anxiety sensitivity and distress tolerance [57]. Similarly, the critical influence of physical exercise on the perceived stress has been observed. A study with a heterogeneous group of 988 individuals looked at relationships between exercise frequency and the Perceived Stress Scale and showed that the reduced perceived stress was modest but significantly associated with aerobic exercise frequency [67].
It is noteworthy that other strategies can help controlling stress. Complementary and alternative medicine (CAM) practices can be an important strategy for stress control and cardiovascular health during the isolation period [68, 69]. Yoga increases vagal tone, reducing arrhythmia burden and improving hemodynamic parameters [68]. This practice is also associated with reduced evening and waking cortisol, systolic blood pressure, resting heart rate, and high-frequency heart rate variability [69]. In addition, Yoga, can increase the positive mental health of adults from non-clinical populations. Therefore, this practice contributes to a significant increase in psychological well-being when compared to no intervention [70]. Corroborating this data, a meta-analysis showed that Mind-Body exercises (i.e., Tai Chi / Yoga) can be an alternative method to reduce stress in people who live under high stress or negative emotions and this reduction may be attributed to sympathetic-vagal balance [71]. Finally, mindfulness-based stress reduction is also able to decrease the perceived stress scale of healthy individuals [72].
Regarding the effects of exercise on cardiovascular parameters associated with stress, a study aimed to evaluate the effects of exercise training and weight loss on blood pressure associated with emotional stress during daily life. Interestingly, exercise, especially when combined with weight loss, reduces blood pressure levels at rest in situations that typically elevate blood pressure, such as intense emotional distress. These participants performed a supervised exercise program 3-4 times/week at 70-85% of their heart rate reserve. The protocol consisted of 10 minutes of warm-up exercises, 35 minutes of cycling and walking, and 10 minutes of relaxation exercises. In addition, this group participated in a behavioral weight management program, which consisted of approximately 26 weekly meetings, aiming at weight loss of 1 to 2 lb/week by reducing caloric and fat intake [73]. Additionally, 11 weeks of aerobic training performed 3 times/week, 30 minutes/day, reduces heart rate in situations of psychological stress [74].
Although these data indicate that particular importance must be given to physical exercise during the COVID- pandemic [1], the difficulty in keeping physically active in isolation and stress might result in cardiovascular damage and increased risk factors for other comorbidities [19, 50]. Therefore, what strategies could be taken to maintain the level of physical activity safely with social isolation measures?
In times of pandemics, when gyms are closed and contact with other people should be avoided in some countries, a home-based exercise strategy can be an exciting alternative to maintaining/improve cardiovascular health. It has been shown that home-based exercise training can improve several cardiovascular variables such as resting blood pressure, cardiac output, and heart rate in health normotensive males [75]. A long-term home-based exercise program improves the functional capacity, blood metabolic profile, and blood pressure of hypertensive patients [76]. Finally, inspiratory muscle training can reduce resting blood pressure and increase inspiratory muscle strength, lung capacity, and arm exercise duration in older people with well-controlled isolated systolic hypertension [77]. Nevertheless, we emphasize the need for further studies to evaluate home-based training proposals for specific populations (hypertensive patients, heart disease patients, diabetic patients, among others) who need to maintain social isolation to reduce the risk of COVID-19 morbidity.
5. HOME-BASED EXERCISE PROPOSAL FOR CARDIOVASCULAR RISK PREVENTION DURING THE COVID-19 PANDEMIC
As previously mentioned, some detrimental effects of lockdown on health (i.e., psychological distress and physical inactivity) during the COVID-19 pandemic period can induce maladaptation to the cardiovascular system [ 7, 10]. Thus, home-based physical activities that are feasible even in lockdown moments and that promise to help patients deal with the situation can be an important strategy for the pandemic period [7]. In this last session, we provide a suggestion of a feasible physical training recommendation for cardiovascular risk prevention during the lockdown period.
5.1. Aerobic Training
We propose aerobic training of moderate-intensity (12-16 on the Borg scale or 50-70% of heart rate reserve for sedentary individuals or 60-70% of heart rate reserve for trained individuals), which can include walking/running on the treadmill or in large places in the house and/or cycling on the exercise bike if the individual has this device. Jumping rope is also an interesting activity to do indoors. The suggested frequency is 3-5 times/week, totaling at least 150 minutes/week. It is also suggested that the individual start the training with 30 minutes/day, being able to progress up to 40-60 min a day, increasing 5 - 10 minutes every 1-2 weeks Table 1. In addition, stairs can be used to exercise the lower limbs and, depending on the speed, can be used for aerobic training [7].
Table 1.
Home-based exercise protocol to improve cardiovascular health during COVID-19 pandemic.
| Type | Aerobic Training | Resistance Training | Inspiratory Muscle Training |
|---|---|---|---|
| Exercises | Walking, running cycling, jumping rope |
8-10 exercises for large muscle groups including: squat, push-up, sit and stand, split squat, straight abdominal, oblique abdominal, exercises with elastic band | Threshold Inspiratory Muscle Training (or others advices) |
| Frequency | 3-5 times / week | 2-3 times/week | 3 times/week |
| Intensity | Moderate intensity | 2-16 on the Borg scale | 30% of maximal inspiratory pressure |
| Duration | At least 30 minutes/day | 2-3 sets | - |
5.2. Resistance Training
We suggest dynamic resistance training with body weight, free weight, elastic band or machine exercises if the individual has. The recommended frequency is 2-3 times/week, 2-3 sets, 8-10 exercises for large muscle groups (squat, push-up, sit and stand, split squat, straight abdominal, oblique abdominal, exercises with elastic band, among others) with an intensity of 12-16 on the Borg scale.
5.3. Inspiratory Muscle Training
We also suggest the inspiratory muscle training at 30% of maximal inspiratory pressure, 3 times a week, using a Threshold Inspiratory Muscle Training device. If the individual does not have this device at home, other alternatives devices (i.e., EMST 150, Threshold Pep, Pflex Inspiratory Muscle Trainer) easily found in pharmacies or purchased online can be used.
5.4. How to Control the Training Intensity at Home
One way is to use the subjective effort scale (Borg scale), ranging from 0 to 20, where 0 represents rest and 20 represents exhaustion.
If the individual has a smartwatch or a frequency meter can use it to measure the heart rate. The 6-minute walk test and monitoring of resting heart rates are also interesting strategies. In addition, the fact that the individual can speak during the activity suggests that the intensity is moderate.
5.5. Care During Training
Individuals should avoid extremes of temperature and take days off. Importantly, individuals who show symptoms of COVID-19 must rest for ≥ 10 days from the onset of symptoms and another 7 days from the “cure” of the symptoms. Extra care should be taken by hypertensive patients: to avoid Valsalva maneuver, to avoid concentric fatigue, and to avoid apnea.
Individuals should also beware of excessive exercise. When the balance between high-load exercise sessions and the appropriate recovery period is interrupted, overtraining can lead to increased production and release of pro-inflammatory cytokines (i.e., interleukin 6, tumor necrosis factor-alpha and interleukin 1beta [ 78 ]. Overtraining can also result in pathologic cardiac hypertrophy [ 79 ] and arrhythmias (atrial fibrillation) [ 80 ].
During exercises on the stairs, individuals with compromised balance should hold on to the rails. For this practice, the use of gloves is recommended and hand washing is essential [ 7 ]. Lastly, we also emphasize the importance of continuing medical monitoring and follow-up by a physical education professional, even if remotely.
CONCLUSION
Sars-COV-2 pandemic is a health crisis culminated in high mortality and morbidity. In an attempt to reduce viral spread, governments adopted preventive measures that included social isolation. A home-based training protocol could be an interesting and effective strategy for the population who need to remain physically active and safe at home.
ACKNOWLEDGEMENTS
Declared none.
ABBREVIATION
- COVID-19
Coronavirus Disease 2019
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [FAPESP, grants numbers: #2017/21320/4; 2019/02975-5] and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [grant number: 88887.466715/2019-00]. BR is fellowship from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq-BPQ).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ranasinghe C., Ozemek C., Arena R. Exercise and well-being during COVID 19 - time to boost your immunity. Expert Rev. Anti Infect. Ther. 2020;18(12):1195–1200. doi: 10.1080/14787210.2020.1794818. [DOI] [PubMed] [Google Scholar]
- 2.WHO. World Health Organization - COVID-19 Weekly Epidemiological Update, 2020 [Google Scholar]
- 3.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020;55(5):105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González K., Fuentes J., Márquez J.L. Physical inactivity, sedentary behavior and chronic diseases. Korean J. Fam. Med. 2017;38(3):111–115. doi: 10.4082/kjfm.2017.38.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandrasekaran B., Ganesan T.B. Sedentarism and chronic disease risk in COVID 19 lockdown - a scoping review. Scott. Med. J. 2020:36933020946336. doi: 10.1177/0036933020946336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto A.J., Dunstan D.W., Owen N., Bonfá E., Gualano B. Combating physical inactivity during the COVID-19 pandemic. Nat. Rev. Rheumatol. 2020;16(7):347–348. doi: 10.1038/s41584-020-0427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Füzéki E., Groneberg D.A., Banzer W. Physical activity during COVID-19 induced lockdown: recommendations. J. Occup. Med. Toxicol. 2020;15:25. doi: 10.1186/s12995-020-00278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werneck A.O., Silva D.R., Malta D.C., Souza-Júnior P.R.B., Azevedo L.O., Barros M.B.A., Szwarcwald C.L. Changes in the clustering of unhealthy movement behaviors during the COVID-19 quarantine and the association with mental health indicators among Brazilian adults. Transl. Behav. Med. 2020:ibaa095. doi: 10.1093/tbm/ibaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuentes-García J.P., Martínez Patiño M.J., Villafaina S., Clemente-Suárez V.J. The effect of COVID-19 confinement in behavioral, psychological, and training patterns of chess players. Front. Psychol. 2020;11:1812. doi: 10.3389/fpsyg.2020.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingram J., Maciejewski G., Hand C.J. Changes in diet, sleep, and physical activity are associated with differences in negative mood during COVID-19 lockdown. Front. Psychol. 2020;11:588604. doi: 10.3389/fpsyg.2020.588604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roschel H., Artioli G.G., Gualano B. Risk of increased physical inactivity during COVID-19 outbreak in older people: A call for actions. J. Am. Geriatr. Soc. 2020;68(6):1126–1128. doi: 10.1111/jgs.16550. [DOI] [PubMed] [Google Scholar]
- 12.Dunton G.F., Do B., Wang S.D. Early effects of the COVID-19 pandemic on physical activity and sedentary behavior in children living in the U.S. BMC Public Health. 2020;20(1):1351. doi: 10.1186/s12889-020-09429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kono Y, Kawajiri H, Kamisaka K, Kamiya K, Akao K, Asai C, et al. Predictive impact of daily physical activity on new vascular events in patients with mild ischemic stroke. Int. J. Stroke. 2015;10(2):219–23. doi: 10.1111/ijs.12392. [DOI] [PubMed] [Google Scholar]
- 14.Lechner K., von Schacky C., McKenzie A.L., Worm N., Nixdorff U., Lechner B., Kränkel N., Halle M., Krauss R.M., Scherr J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020;27(4):394–406. doi: 10.1177/2047487319869400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steptoe A., Kivimäki M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012;9(6):360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 16.Chandola T., Britton A., Brunner E., Hemingway H., Malik M., Kumari M., Badrick E., Kivimaki M., Marmot M. Work stress and coronary heart disease: what are the mechanisms? Eur. Heart J. 2008;29(5):640–648. doi: 10.1093/eurheartj/ehm584. [DOI] [PubMed] [Google Scholar]
- 17.Martinez E.Z., Silva F.M., Morigi T.Z., Zucoloto M.L., Silva T.L., Joaquim A.G., Dall’Agnol G., Galdino G., Martinez M.O.Z., Silva W.R.D. Physical activity in periods of social distancing due to COVID-19: a cross-sectional survey. Cien. Saude Colet. 2020;25(Suppl. 2):4157–4168. doi: 10.1590/1413-812320202510.2.27242020. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y-Y., Ma Y-T., Zhang J-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narici M., De Vito G., Franchi M., Paoli A., Moro T., Marcolin G., Grassi B., Baldassarre G., Zuccarelli L., Biolo G., di Girolamo F.G., Fiotti N., Dela F., Greenhaff P., Maganaris C. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2020:1–22. doi: 10.1080/17461391.2020.1761076. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrini M., Ponzo V., Rosato R., Scumaci E., Goitre I., Benso A., Belcastro S., Crespi C., De Michieli F., Ghigo E., Broglio F., Bo S. Changes in Weight and nutritional habits in adults with obesity during the “lockdown” period caused by the COVID-19 virus emergency. Nutrients. 2020;12(7):E2016. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler M.J., Barrientos R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020;87:53–54. doi: 10.1016/j.bbi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall G., Laddu D.R., Phillips S.A., Lavie C.J., Arena R. A tale of two pandemics: How will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog. Cardiovasc. Dis. 2020:3. doi: 10.1016/j.pcad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisafulli A., Pagliaro P. Physical activity/inactivity and COVID-19. Eur. J. Prev. Cardiol. 2020:2047487320927597. [Google Scholar]
- 24.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., Michos E.D., Miedema M.D., Muñoz D., Smith S.C., Jr, Virani S.S., Williams K.A., Sr, Yeboah J., Ziaeian B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du X., Dong J., Ma C. Is atrial fibrillation a preventable disease? J. Am. Coll. Cardiol. 2017;69(15):1968–1982. doi: 10.1016/j.jacc.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Pišot R., Marusic U., Biolo G., Mazzucco S., Lazzer S., Grassi B., Reggiani C., Toniolo L., di Prampero P.E., Passaro A., Narici M., Mohammed S., Rittweger J., Gasparini M., Gabrijelčič Blenkuš M., Šimunič B. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J Appl Physiol (1985) 2016;120(8):922–929. doi: 10.1152/japplphysiol.00858.2015. [DOI] [PubMed] [Google Scholar]
- 27.Schwendinger F., Pocecco E. Counteracting physical inactivity during the COVID-19 pandemic: Evidence-based recommendations for home-based exercise. Int. J. Environ. Res. Public Health. 2020;17(11):3909. doi: 10.3390/ijerph17113909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodama S., Saito K., Tanaka S., Maki M., Yachi Y., Asumi M., Sugawara A., Totsuka K., Shimano H., Ohashi Y., Yamada N., Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 29.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., Zhang C., Yue J., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaffalon Júnior J.R., Viana A.O., de Melo G.E.L., De Angelis K. The impact of sedentarism on heart rate variability (HRV) at rest and in response to mental stress in young women. Physiol. Rep. 2018;6(18):e13873. doi: 10.14814/phy2.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehrwein E.A., Orer H.S., Barman S.M. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr. Physiol. 2016;6(3):1239–1278. doi: 10.1002/cphy.c150037. [DOI] [PubMed] [Google Scholar]
- 32.Abboud F.M., Singh M.V. Autonomic regulation of the immune system in cardiovascular diseases. Adv. Physiol. Educ. 2017;41(4):578–593. doi: 10.1152/advan.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heymans C., Bouckaert J.J. Sinus caroticus and respiratory reflexes: I. Cerebral blood flow and respiration. Adrenaline apnoea. J. Physiol. 1930;69(2):254–266. doi: 10.1113/jphysiol.1930.sp002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francica J.V., Bigongiari A., Mochizuki L., Scapini K.B., Moraes O.A., Mostarda C., Caperuto E.C., Irigoyen M.C., De Angelis K., Rodrigues B. Cardiac autonomic dysfunction in chronic stroke women is attenuated after submaximal exercise test, as evaluated by linear and nonlinear analysis. BMC Cardiovasc. Disord. 2015;15:105. doi: 10.1186/s12872-015-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irigoyen M-C., De Angelis K., Dos Santos F., Dartora D.R., Rodrigues B., Consolim-Colombo F.M. Hypertension, blood pressure variability, and target organ lesion. Curr. Hypertens. Rep. 2016;18(4):31. doi: 10.1007/s11906-016-0642-9. [DOI] [PubMed] [Google Scholar]
- 36.Mravec B. The role of the vagus nerve in stroke. Auton. Neurosci. 2010;158(1-2):8–12. doi: 10.1016/j.autneu.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Zderic T.W., Hamilton M.T. Identification of hemostatic genes expressed in human and rat leg muscles and a novel gene (LPP1/PAP2A) suppressed during prolonged physical inactivity (sitting). Lipids Health Dis. 2012;11:137. doi: 10.1186/1476-511X-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilbur J., Shian B. Diagnosis of deep venous thrombosis and pulmonary embolism. Am. Fam. Physician. 2012;86(10):913–919. [PubMed] [Google Scholar]
- 39.Shah S., Shah K., Patel S.B., Patel F.S., Osman M., Velagapudi P., Turagam M.K., Lakkireddy D., Garg J. Elevated D-dimer levels are associated with increased risk of mortality in coronavirus disease 2019: A systematic review and meta-analysis. Cardiol. Rev. 2020;28(6):295–302. doi: 10.1097/CRD.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weitz J.I., Fredenburgh J.C., Eikelboom J.W. A test in context: D-dimer. J. Am. Coll. Cardiol. 2017;70(19):2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 41.Tracey K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009;9(6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raizada M.K., Paton J.F.R. Recent advances in the renin-angiotensin system: Angiotensin-converting enzyme 2 and (pro)renin receptor. Exp. Physiol. 2008;93(5):517–518. doi: 10.1113/expphysiol.2008.042861. [DOI] [PubMed] [Google Scholar]
- 43.Zubcevic J., Waki H., Raizada M.K., Paton J.F.R. Autonomic-immune-vascular interaction: An emerging concept for neurogenic hypertension. Hypertension (Dallas, Tex : 1979) 2011;57(6) doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 45.Ruberti O.M., Sousa A.S., Viana L.R., Pereira Gomes M.F., Medeiros A., Gomes Marcondes M.C.C., et al. Aerobic training prevents cardiometabolic changes triggered by myocardial infarction in ovariectomized rats. J. Cell. Physiol. 2020 doi: 10.1002/jcp.29919. [DOI] [PubMed] [Google Scholar]
- 46.Rajkumar R.P. COVID-19 and mental health: A review of the existing literature. Asian J. Psychiatr. 2020;52:102066. doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shuja K.H., Aqeel M., Jaffar A., Ahmed A. COVID-19 pandemic and impending global mental health implications. Psychiatr. Danub. 2020;32(1):32–35. doi: 10.24869/psyd.2020.32. [DOI] [PubMed] [Google Scholar]
- 48.Ozamiz-Etxebarria N., Dosil-Santamaria M., Picaza-Gorrochategui M., Idoiaga-Mondragon N. Stress, anxiety, and depression levels in the initial stage of the COVID-19 outbreak in a population sample in the northern Spain. Cad. Saude Publica. 2020;36(4):e00054020. doi: 10.1590/0102-311x00054020. [DOI] [PubMed] [Google Scholar]
- 49.Grant N., Hamer M., Steptoe A. Social isolation and stress-related cardiovascular, lipid, and cortisol responses. Ann. Behav. Med. 2009;37(1):29–37. doi: 10.1007/s12160-009-9081-z. [DOI] [PubMed] [Google Scholar]
- 50.Burtscher J., Burtscher M., Millet G.P. (Indoor) isolation, stress, and physical inactivity: Vicious circles accelerated by COVID-19? Scand. J. Med. Sci. Sports. 2020;30(8):1544–1545. doi: 10.1111/sms.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kivimäki M., Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018;15(4):215–229. doi: 10.1038/nrcardio.2017.189. [DOI] [PubMed] [Google Scholar]
- 52.Mattioli A.V., Sciomer S., Cocchi C., Maffei S., Gallina S. Quarantine during COVID-19 outbreak: Changes in diet and physical activity increase the risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020;30(9):1409–1417. doi: 10.1016/j.numecd.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brotman D.J., Golden S.H., Wittstein I.S. The cardiovascular toll of stress. Lancet. 2007;370(9592):1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 55.Golbidi S., Frisbee J.C., Laher I. Chronic stress impacts the cardiovascular system: Animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015;308(12):H1476–H1498. doi: 10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- 56.Awick E.A., Ehlers D.K., Aguiñaga S., Daugherty A.M., Kramer A.F., McAuley E. Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. Gen. Hosp. Psychiatry. 2017;49:44–50. doi: 10.1016/j.genhosppsych.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LeBouthillier D.M., Asmundson G.J.G. The efficacy of aerobic exercise and resistance training as transdiagnostic interventions for anxiety-related disorders and constructs: A randomized controlled trial. J. Anxiety Disord. 2017;52:43–52. doi: 10.1016/j.janxdis.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Lavie C.J., Arena R., Swift D.L., Johannsen N.M., Sui X., Lee D.C., Earnest C.P., Church T.S., O’Keefe J.H., Milani R.V., Blair S.N. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015;117(2):207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mozaffarian D., Furberg C.D., Psaty B.M., Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118(8):800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Middeldorp M.E., Ariyaratnam J., Lau D., Sanders P. Lifestyle modifications for treatment of atrial fibrillation. Heart. 2020;106(5):325–332. doi: 10.1136/heartjnl-2019-315327. [DOI] [PubMed] [Google Scholar]
- 61.Adams V., Reich B., Uhlemann M., Niebauer J. Molecular effects of exercise training in patients with cardiovascular disease: focus on skeletal muscle, endothelium, and myocardium. Am. J. Physiol. Heart Circ. Physiol. 2017;313(1):H72–H88. doi: 10.1152/ajpheart.00470.2016. [DOI] [PubMed] [Google Scholar]
- 62.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corrà U., Cosyns B., Deaton C., Graham I., Hall M.S., Hobbs F.D., Løchen M.L., Löllgen H., Marques-Vidal P., Perk J., Prescott E., Redon J., Richter D.J., Sattar N., Smulders Y., Tiberi M., van der Worp H.B., van Dis I., Verschuren W.M., De Backer G., Roffi M., Aboyans V., Bachl N., Bueno H., Carerj S., Cho L., Cox J., De Sutter J., Egidi G., Fisher M., Fitzsimons D., Franco O.H., Guenoun M., Jennings C., Jug B., Kirchhof P., Kotseva K., Lip G.Y., Mach F., Mancia G., Bermudo F.M., Mezzani A., Niessner A., Ponikowski P., Rauch B., Rydén L., Stauder A., Turc G., Wiklund O., Windecker S., Zamorano J.L. Authors/task force members; Additional contributor: Simone Binno (Italy); Document reviewers. 2016 european guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. J. Prev. Cardiol. 2016;23(11):NP1–NP96. doi: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
- 63.Haskell W.L., Lee I.M., Pate R.R., Powell K.E., Blair S.N., Franklin B.A., Macera C.A., Heath G.W., Thompson P.D., Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 64.Cutrim A.L.C., Duarte A.A.M., Silva-Filho A.C., Dias C.J., Urtado C.B., Ribeiro R.M., Rigatto K., Rodrigues B., Dibai-Filho A.V., Mostarda C.T. Inspiratory muscle training improves autonomic modulation and exercise tolerance in chronic obstructive pulmonary disease subjects: A randomized-controlled trial. Respir. Physiol. Neurobiol. 2019;263:31–37. doi: 10.1016/j.resp.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 65.de Abreu R.M., Rehder-Santos P., Minatel V., Dos Santos G.L., Catai A.M. Abreu RMd. Effects of inspiratory muscle training on cardiovascular autonomic control: A systematic review. Auton. Neurosci. 2017;208:29–35. doi: 10.1016/j.autneu.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Rodrigues G.D., Gurgel J.L., Gonçalves T.R., da Silva Soares P.P. Inspiratory muscle training improves physical performance and cardiac autonomic modulation in older women. Eur. J. Appl. Physiol. 2018;118(6):1143–1152. doi: 10.1007/s00421-018-3844-9. [DOI] [PubMed] [Google Scholar]
- 67.Knab A.M., Nieman D.C., Sha W., Broman-Fulks J.J., Canu W.H. Exercise frequency is related to psychopathology but not neurocognitive function. Med. Sci. Sports Exerc. 2012;44(7):1395–1400. doi: 10.1249/MSS.0b013e31824795f4. [DOI] [PubMed] [Google Scholar]
- 68.Akella K., Kanuri S.H., Murtaza G., G Della Rocca D., Kodwani N., K Turagam M., Shenthar J., Padmanabhan D., Basu Ray I., Natale A., Gopinathannair R., Lakkireddy D. Impact of yoga on cardiac autonomic function and arrhythmias. J. Atr. Fibrillation. 2020;13(1):2408. doi: 10.4022/jafib.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pascoe M.C., Thompson D.R., Ski C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology. 2017;86:152–168. doi: 10.1016/j.psyneuen.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Hendriks T., de Jong J., Cramer H. The effects of yoga on positive mental health among healthy adults: A systematic review and meta-analysis. J. Altern. Complement. Med. 2017;23(7):505–517. doi: 10.1089/acm.2016.0334. [DOI] [PubMed] [Google Scholar]
- 71.Zou L., Sasaki J.E., Wei G.X., Huang T., Yeung A.S., Neto O.B., Chen K.W., Hui S.S. Effects of mind body exercises (Tai Chi/Yoga) on heart rate variability parameters and perceived stress: A systematic review with meta-analysis of randomized controlled trials. J. Clin. Med. 2018;7(11):E404. doi: 10.3390/jcm7110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simkin D.R., Black N.B. Meditation and mindfulness in clinical practice. Child Adolesc. Psychiatr. Clin. N. Am. 2014;23(3):487–534. doi: 10.1016/j.chc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Steffen P.R., Sherwood A., Gullette E.C., Georgiades A., Hinderliter A., Blumenthal J.A. Effects of exercise and weight loss on blood pressure during daily life. Med. Sci. Sports Exerc. 2001;33(10):1635–1640. doi: 10.1097/00005768-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Holmes A.P., Ray C.J., Kumar P., Coney A.M. A student practical to conceptualize the importance of Poiseuille’s law and flow control in the cardiovascular system. Adv. Physiol. Educ. 2020;44(3):436–443. doi: 10.1152/advan.00004.2019. [DOI] [PubMed] [Google Scholar]
- 75.Wiles J.D., Goldring N., Coleman D. Home-based isometric exercise training induced reductions resting blood pressure. Eur. J. Appl. Physiol. 2017;117(1):83–93. doi: 10.1007/s00421-016-3501-0. [DOI] [PubMed] [Google Scholar]
- 76.Farinatti P., Monteiro W.D., Oliveira R.B. Long Term Home-Based Exercise is Effective to Reduce Blood Pressure in Low Income Brazilian Hypertensive Patients: A Controlled Trial. High Blood Press. Cardiovasc. Prev. 2016;23(4):395–404. doi: 10.1007/s40292-016-0169-9. [DOI] [PubMed] [Google Scholar]
- 77.Ublosakka-Jones C., Tongdee P., Pachirat O., Jones D.A. Slow loaded breathing training improves blood pressure, lung capacity and arm exercise endurance for older people with treated and stable isolated systolic hypertension. Exp. Gerontol. 2018;108:48–53. doi: 10.1016/j.exger.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 78.da Rocha A.L., Pinto A.P., Kohama E.B., Pauli J.R., de Moura L.P., Cintra D.E., Ropelle E.R., da Silva A.S.R. The proinflammatory effects of chronic excessive exercise. Cytokine. 2019;119:57–61. doi: 10.1016/j.cyto.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 79.da Rocha A.L., Teixeira G.R., Pinto A.P., de Morais G.P., Oliveira L.D.C., de Vicente L.G., da Silva L.E.C.M., Pauli J.R., Cintra D.E., Ropelle E.R., de Moura L.P., Mekary R.A., de Freitas E.C., da Silva A.S.R. Excessive training induces molecular signs of pathologic cardiac hypertrophy. J. Cell. Physiol. 2018;233(11):8850–8861. doi: 10.1002/jcp.26799. [DOI] [PubMed] [Google Scholar]
- 80.Turagam M.K., Flaker G.C., Velagapudi P., Vadali S., Alpert M.A. Atrial fibrillation in athletes: Pathophysiology, clinical presentation, evaluation and management. J. Atr. Fibrillation. 2015;8(4):1309. doi: 10.4022/jafib.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]