Abstract
Background
Once used by mountaineers to facilitate rapid adaptations to altitude and by athletes to improve their aerobic capacity, exposure to hypoxia has been proven to affect various physiological, clinically relevant parameters. A form of conditioning known as Intermittent Hypoxia Conditioning (IHC) consists of repeated exposures to intermittent hypoxia, combined with normoxia and hyperoxia, which has been shown to have potential as a treatment to improve cardio-metabolic risks profile in cardiac patients but results across studies are inconsistent. This systematic review and meta-analysis aimed to evaluate the clinical effectiveness of IHC.
Methods
Four electronic databases (PubMed, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials) were searched (from inception to December 2019) to retrieve all studies focused on IHC in elderly patients with cardiovascular disease. A meta-analysis of functional, efficacy and safety outcomes in cardiac patients was completed to compare IHC to sham treatments.
Results
Fourteen studies with 320 patients in the Interval Hypoxia-normoxia Group (IHNG) or Interval Hypoxia-hyperoxia training Group (IHHG) and 111 patients in the control group were included in our meta-analysis. IHNT and IHHT were associated with significant reduction in heart rate, SBP, and DBP at rest after treatment [MD= -5.35 beat/min, 95% CI (-9.19 to -1.50), p=0.006], [MD= -13.72 mmHg, 95% CI (-18.31 to -9.132), p<0.001], and [MD= -7.882 mmHg, 95% CI (-13.163 to -2.601), p=0.003], respectively. There were no significant complications or serious adverse events related to IHNT/IHHT.
Conclusion
The current evidence suggested that the use of the IHNT/IHHT program in elderly patients with CVDs can be safe and effective in terms of heart rate and elevated blood pressure. However, currently, there is no supporting evidence that IHNT/IHHT can significantly improve hematological parameters or lipid profile. Exercise tolerance increased at the end of the course of hypoxic conditioning within IHC group, but did not differ from controls. Further research is needed.
Keywords: Co-morbid cardiac patients, intermittent hypoxic conditioning, interval hypoxia-normoxia/hyperoxia training, exercise tolerance, hypertension, cardiovascular diseases
1. INTRODUCTION
The leading cause of death and disability in both men and women worldwide is Cardiovascular Diseases (CVDs), especially Coronary Artery Disease (CAD) and Hypertension (HPT). Physical inactivity is one of the main risk factors of CVDs [1, 2]. It has been reported that regular physical activity and cardiorespiratory fitness (CRF) are significantly related. Moreover, it improves cardiometabolic risk profiles [3].
Intermittent Hypoxic Conditioning (IHC) is a noninvasive method, based on repeated short exposures at rest (not while exercising) to a gas mixture with oxygen deficiency (up to 14-10% of O2), interspersed by intervals of normoxic (21% of O2) or hyperoxic (30-35% of O2) gas mixture [4]. IHC includes two approaches: Intermittent hypoxic-normoxic training (IHNT) and Intermittent hypoxic-hyperoxic training (IHHT). IHNT/IHHT technique has many beneficial effects, such as optimizing mitochondrial metabolism, preventing adverse events of excess mitochondrial reactive oxygen species generation and stimulating endothelial Nitric Oxide (NO) to provide vasodilation and enhance endothelial proliferation [5-7]. In preliminary studies focused on exercise performance in athletes with the overtraining syndrome and aged patients with coronary artery disease, normoxia replacement with hyperoxia in intermediate exposure to hypoxia proved significant efficiency [8, 9]. IHHT, in a study with male Wistar rats, has been reported to be more effective in increasing membrane stability on cells in the heart, liver, and brain compared to IHNT [10]. Many physiological adaptations such as improved endothelial function, better total antioxidant capacity, and reduced sympathetic drive were associated with IHHT compared to IHNT [11, 12]. Exercise combined with IHHT was reported to be more effective in the clearance of metabolites, negatively impacting neuronal metabolism and improving cognitive function in the elderly [13, 14]. In patients who cannot exercise due to CVDs (e.g., chronic heart failure), rehabilitation is the main treatment option for those patients; however, IHNT/IHHT could be a viable conditioning option [8]. Therefore, we aimed to conduct this systematic review and meta-analysis to synthesize the current evidence regarding the safety and efficacy of IHC in elderly patients with cardiac diseases.
2. METHODS
All steps of our systematic review and meta-analysis were implemented according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) checklist [15].
2.1. Search Strategy
Four electronic databases, PubMed, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials, were searched from inception to December 2019. We exported the research records into Endnote (Windows version X8). Articles were identified using the following search terms: (intermittent hypoxia or hypoxic conditioning or hypoxic-hyperoxic training or normobaric hypoxia or hypobaric hypoxia or interval hypoxia training or adaptation to intermittent hypoxia or adaptation to hypobaric hypoxia) and (cardiovascular disease or CVD or cardiovascular or cardiovascular abnormalities or heart diseases and vascular diseases or coronary artery disease or Hypertension or Chronic Heart Failure). Reference lists in each selected article were manually searched.
2.2. Selection of Studies
Two review authors (SYK and MAZ) independently assessed the retrieved records against inclusion criteria. Eligibility screening was performed in two steps: the first step was to screen abstracts for eligibility, and in the second step, inclusion criteria were checked in full-text articles of eligible abstracts. Disagreement regarding study eligibility was resolved by discussion and consensus with a third reviewer (OSG).
2.3. Inclusion and Exclusion Criteria
We included all articles eligible for these criteria:
-Population: studies that enrolled patients with any cardiovascular disease, such as coronary artery disease, essential hypertension, heart rhythm problems (arrhythmias), heart defects (congenital heart defects), and chronic heart failure.
-Intervention or exposure: IHC (IHNT or IHHT).
-Comparator(s)/control: Standard rehabilitation programs/ OR Placebo (sham) group.
-Types of study include clinical trials (randomized or non-randomized) and prospective observational studies.
-Outcomes: Safety and efficacy of IHNT or IHHT in patients with CVDs. We indicated the safety of IHC based on the absence of reported complications or adverse effects, and in studies reporting adverse effects or complications, we evaluated the incidence or severity of adverse events to determine the level of safety.
We excluded: 1) Studies whose population consisted of healthy volunteers; 2) Studies whose data were not available for extraction and analysis; 3) Thesis and conference papers; 4) Studies on animal models; 5) Studies published in Non-English and non-Russian languages.
2.4. Data Extraction
Two authors (SYK and MAZ) extracted the relevant data independently using an offline data extraction form (Microsoft Excel 2016, windows version). The extracted data include the following: 1) characters of study design (Study design, interventional, population and sample size); 2) the risk of bias domains (Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias); and 3) efficacy and safety outcomes: Heart rate (beat/min), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), VO2 peak/kg of body mass (ml/min/kg), exercise time to fatigue/exhaustion (min), hemoglobin (g/dL), reticulocytes (%), erythrocytes (102/L), total cholesterol (mg/dL), and low-density lipoprotein (mg/dL), adverse effects. Disagreement regarding data eligibility was resolved by discussion and consensus with a third reviewer (OSG).
2.5. Risk of Bias and Quality Assessment
We used the Cochrane Risk of Bias (ROB) assessment tool to assess our included studies' quality. This tool is used to screen against seven types of bias that might occur while implementing a randomized controlled trial. Those bias domains are Random sequence generation (selection bias), Allocation concealment (selection bias), Blinding of participants and personnel (performance bias), Blinding of outcome assessment (detection bias), Incomplete outcome data (attrition bias), Selective reporting (reporting bias), and other biases [16].
2.6. Data Synthesis and Heterogeneity
We assessed and interpreted data heterogeneity by visual inspection of the forest plots, and we measured it by I-square and Chi-Square tests, according to the Cochrane Handbook recommendations for Systematic Reviews and Meta-analysis (Chapter 9) [17]. In the case of significant heterogeneity (I2 >50%), the random-effects model was used. The effect estimates of efficacy and safety outcomes were pooled as Mean Difference (MD) in the random effect model meta-analysis (Der-Simonian Liard method). Significance was set at p<0.05. Statistical analyses were performed using Open Meta [Analyst].
3. RESULTS
3.1. Search Results
Our search of four databases returned 10,044 items (PubMed= 4611, Scopus= 2450, Web of Science=1054, Cochrane =129). After removing duplicates, 7339 records were screened against our inclusion and exclusion criteria by checking the title and abstract. A total of 7326 studies were excluded, while 18 full-text studies were assessed against inclusion criteria. Fourteen studies matched our inclusion criteria and were eligible to be included in our systematic review and meta-analysis [8, 12, 18-29]. Four studies did not match our criteria; Julian et al. (2004) and Harrison et al. (2002) were performed on athletes [30, 31], while Netzer et al. (2008) and Oltmanns et al. (2004) reported irrelevant outcomes [32, 33]. The study flow diagram for the study selection process can be seen in Fig. (1).
Fig. (1).
PRISMA flow chart.
We included five randomized controlled trials [19, 22-25] with a total number of 177 patients (Intervention= 90 patients, control= 87 patients). Moreover, we included nine non-randomized interventional studies [8, 12, 18, 20, 21, 26-29] with a total number of 439 patients (Intervention= 257 patients, control= 182 patients).
3.2. Demographics and Characteristics
The vast majority of the included patients were old, hypertensive, and current smokers. Baseline data of enrolled patients in included studies are illustrated in Table 1.
Table 1.
Summary of included studies.
| Study | Group | N | Males n (%) | Average Age, y (range) or Mean ± SD | Current Smoker, n (%) | Hypertension, n (%) | Diabetes, n (%) | Exertional Angina, II FC n (%) | Exertional Angina, III FC n (%) | Previous MI, n (%) | Paroxysmal AF, n (%) | COPD, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glazachev 2017 | IHHG | 27 | 9 (33%) | 63.9 (52-77) | 5 (18.5%) | 22 (81.5%) | 8 (29.6%) | 20 (74.1%) | 7 (25.9%) | 8 (29.6%) | 5 (18.5%) | 2 (7.4%) |
| CTRL | 19 | 9 (47%) | 63.2 (43-83) | 4 (18.5%) | 17 (89.5%) | 3 (15.8%) | 17 (89.5%) | 2 (10.5%) | 8 (42.1%) | 2 (10.5%) | 2 (10.5%) | |
| Dudnik 2018 | IHHG | 15 | - | 66.7 ± 5.7 | - | 12 (80%) | 7 (46.7%) | - | - | 5 (33.3%) | - | 2 (13.3%) |
| CTRL | 14 | - | 65.0 ± 6.2 | - | 10 (71.4%) | 6 (42.8%) | - | - | 6 (42.8%) | - | 2 (14.3%) | |
| Tuter 2018 | IHHG | 40 | 30 (75%) | 63±8.4 | 28 (70%) | 38 (95%) | 11 (27.5%) | 12 (30%) | 15 (37.5%) | 5 (12.5%) | 5 (12.5%) | 8 (20%) |
| CTRL | 40 | 31 (77.5%) | 64±7.6 | 25 (62.5%) | 37 (92.5%) | 10 (25%) | 12 (30%) | 17 (42.5%) | 4 (10%) | 6 (15%) | 5 (12.5%) | |
| Bayer 2017 | IHHG | 18 | 5 (28%) | 80.9 (7.9) | - | - | - | - | - | - | - | - |
| CTRL | 16 | 2 (12.5%) | 83.4 (5.5) | - | - | - | - | - | - | - | - | |
| Burtscher 2004 | IHHG | 8 | 8(100%) | 59.3 (5.4) | 1 (13) | 3 (38) | 0 (0) | - | - | 4 (50) | - | - |
| CTRL | 8 | 8(100%) | 61.3 (5.0) | 2 (25) | 3 (38) | 1 (13) | - | - | 4 (50) | - | - | |
| Lyamina 2011 | IHHG | 37 | 37(100%) | 32 years | - | Primary hypertension | - | - | - | - | - | - |
| CTRL | 20 | 20(100%) | 32 years | - | Primary hypertension | - | - | - | - | - | - | |
| Ishchuk 2011 | IHT | 45 | 25(55%) | 68.7±1.1 | 0 | 45 (100%) | 0 | - | - | - | - | - |
| CTRL | 20 | 12(60%) | 69.0±1.2 | 0 | 20 (100%) | 0 | - | - | - | - | - | |
| Yelchaninova 2005 | IHT | 30 | 9(30%) | 44.2±1 | - | - | - | - | - | - | - | - |
| CTRL | 30 | 10(33%) | 40.4±4 | - | - | - | - | - | - | - | - | |
| Rachok 2011 | IHT | 30 | 30(100%) | 57.25±2.11 | 0 | 90% | 0 | 100% | 0 | 100% | 13.3 | 0 |
| CTRL | 30 | 30(100%) | 56.88±1.42 | 0 | 86.6% | 0 | 100% | 0 | 100% | 16.6 | 0 | |
| Makhova 2005 | IHT | 40 | 40(100%) | 30-70 | - | - | - | 18.64% | - | 32.2% | - | - |
| Oxygen therapy | 49 | 49(100%) | 30-70 | - | - | - | 25.42% | - | 31.36% | - | - | |
| Dry carbon baths | 30 | 30(100%) | 30-70 | - | - | - | 13.56% | - | 23.73% | - | - | |
| Kalachev 2004 | IHT | 22 | - | 51.2±3.9 | - | - | - | - | - | - | - | - |
| CTRL | 28 | - | 46.4±3.2 | - | - | - | - | - | - | - | - | |
| Burtscher 2009 | IHHG | 9 | 5(55%) | 51.0±8.5 | 3 (33%) | - | - | - | - | - | - | 100% |
| CTRL | 9 | 5(55%) | 52.4±12.2 | 4 (44%) | - | - | - | - | - | - | 100% | |
| Saeed 2012 | Altitude Exposure Protocol | 12 | 9(75%) | 52.5 (42-70) | - | - | - | - | - | - | - | - |
| Shatlio 2008 | IHHG-Exercise | 14 | 14(100%) | 94± 3.7 | - | - | - | - | - | - | - | - |
| IHHG-no Exercise | 21 | 21(100%) | 66 ± 3.1 | - | - | - | - | - | - | - | - |
Abbreviations: AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; CTRL, control group; FC, functional class NYHA; IHHG, intermittent hypoxia-hyperoxia training group; MI, myocardial infarction.
3.3. Risk of Bias Assessment
Our included studies reached a level of moderate quality according to the Cochrane Risk of Bias assessment tool. Five studies used a reliable method to generate a random sequence for their enrolled patients, producing less bias within their data [22-25, 34]. Studies of Glazachev et al., Tuter et al., and Bayer et al. [8, 23, 24] used a low risk of bias technique in allocating patients to their experimental groups. When using a reliable method for patient allocation, no patient would know which group he is allocated to, so avoiding bias based on the allocation process. Only three studies used a blinding method during the allocation and intervention processes [8, 24, 25]. All included studies did not state that they used a clear outcome assessment domain [8, 12, 22-25, 34]. Our included studies did not have a loss of patients on follow-up or analyzed their data based on intention to treat the analysis method [8, 12, 22-25, 34]. All studies reported their data based on a pre-specified registered protocol without selective reporting of specific data [8, 12, 22-25, 34]. Included studies seem to be free of other sources of bias. Quality assessment of included studies is tabulated with justification for the decision taken in Supp. File 1. Based on our included studies' quality, our systematic review and meta-analysis are of moderate quality. The risk of bias graph and summary can be seen in Fig. (2). Only two of the included studies [8, 12] reported adverse events among participants.
Fig. (2).
Risk of Bias Summary. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4. Outcomes
3.4.1. Heart Rate at Rest (Beat/min)
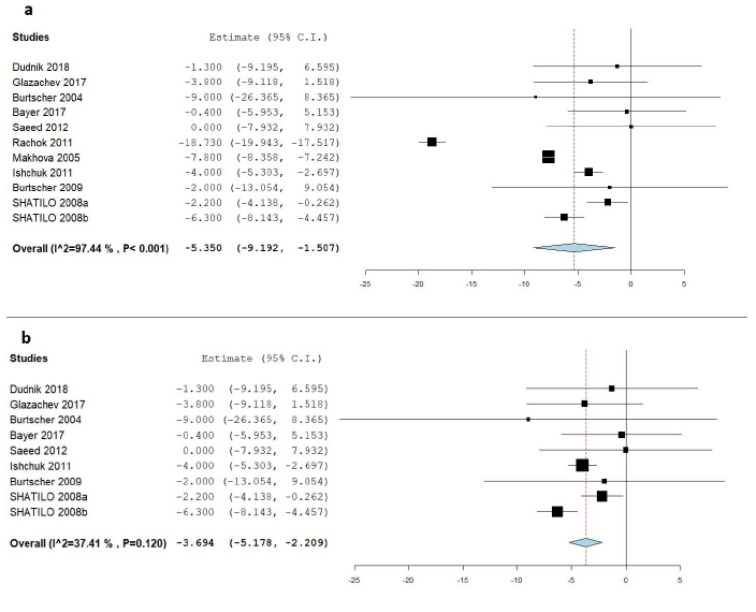
Ten studies reported a change in heart rate at rest values in the IHC group (repeated intermittent hypoxic exposures at rest) and the control group [8, 19-22, 24-26, 28, 29]. IHC was associated with a significant decrease in heart rate at rest after treatment [MD= -5.35 beat/min, 95% CI (-9.19 to -1.50), p=0.006], which reflects that IHC is more effective than the control treatment. Pooled studies were heteroge-neous (I2 = 97.44%; p<0.001), and the random-effects model was applied, using the Der-Simonian Liard method, (Fig. 3a). Sensitivity analysis was applied to solve the heterogeneity; we removed Rachok et al. [28] and Makhova et al. [29] from the analysis, and the heart rate reduction was still significant [MD= -3.69 beat/min, 95% CI (-5.17 to -2.209), p<0.001] and homogenous (I2 = 37.40, p=0.120), (Fig. 3b).
Fig. (3).
a) Forest plot of the pooled mean difference between IHC and control groups in the term of heart rate (beat/min); b) Forest plot of the pooled mean difference between IHC and control groups in the term of heart rate (beat/min) after the application of sensitivity analysis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4.2. Systolic Blood Pressure (mmHg)
Nine studies reported a change in systolic blood pressure in the IHC and control groups [8, 12, 19, 20, 22, 24-27]. The decrease in systolic blood pressure was significant in the IHC group [MD= -13.72 mmHg, 95% CI (-18.31 to -9.132), p<0.001], which reflects that IHC is more effective than the control treatment. Pooled studies were not homogenous, with the level of heterogeneity being significant (I2=98.61%; p < 0.001), and the random-effects model was applied using the Der-Simonian Liard method. Forest plot for change in systolic blood pressure parameter after treatment is seen in Fig. (4a). Heterogeneity could not be resolved by sensitivity analysis or any other means.
Fig. (4).
a) Forest plot of the pooled mean difference between IHC and control groups in the term of systolic blood pressure (mmHg); b) Forest plot of the pooled mean difference between IHC and control groups in the term of diastolic blood pressure (mm Hg). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4.3. Diastolic Blood Pressure (mmHg)
Eight studies reported a change in diastolic blood pressure in the IHC group in the passive and control groups [8, 12, 19, 20, 22, 24, 26, 27]. The decrease in diastolic blood pressure was significant in the IHC group [MD= -7.882 mmHg, 95% CI (-13.163 to -2.601), p=0.003], which reflects that IHC is more effective than the control treatment. Pooled studies were not homogenous with a marked degree of heterogeneity (I2 =99.68%; p < 0.001), and the random-effects model was applied, using the Der-Simonian Liard method. Forest plot for change in diastolic blood pressure after treatment is seen in Fig. (4b). Heterogeneity could not be resolved by sensitivity analysis or any other means.
3.4.4. Peak Oxygen Consumption: VO2peak, mL O2/min/kg
Seven studies reported changes in peak oxygen consumption in the IHC and control groups [8, 19, 21, 22, 25-27]. There was quite a significant increase in peak oxygen consumption in the IHC group at the end of interval hypoxia in comparison to the data at the baseline, but no significant difference in VO2peak, between the IHC group and the sham group after the study [MD= -0.035 mL O2/min/kg, 95% CI (-2.473 to 2.404), p= 0.97], reflecting that the efficacy of IHC is similar to control treatment in terms of VO2peak. Pooled studies were not homogenous, with the degree of heterogeneity being high (I2 = 96.61%; p< 0.001), and the random-effects model was applied, using the Der-Simonian Liard method. The Forest plot for change in peak oxygen consumption after the intervention is seen in Fig. (5). By doing a sensitivity analysis test by omitting one study every time, heterogeneity could only be decreased in a small amount by omitting the study of Dudnik et al. [25], (I2 = 85.38%; p< 0.001). By doing a forest plot visual analysis, there was a skewness produced by two studies. By excluding Dudnik et al. [25], and Ishchuk et al. [26], we could regain homogeneity (I2 = 5.81%; p= 0.379), with no significant difference between the intervention and control arms [MD= -0.170 mL O2/min/kg, 95% CI (-0.610 to 0.270), p= 0.449].
Fig. (5).
Forest plot of the pooled mean difference between IHC and control groups in the term of peak oxygen consumption: VO2 peak, mL O2/min/kg. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4.5. Exercise Time to Exhaustion
Four studies reported a change in exercise time in the IHC group and the control group [8, 19, 21, 25]. Despite reported significant increment in exercise time to fatigue in IHNT/IHHT group at the end of the IHC course, there was no significant difference between the study groups in term of change in time of exercise after the end of the intervention [MD= 0.879 min, 95% CI (-2.495 to 4.252), p=0.610], indicating that the efficacy of IHC is similar to control treatment in this regard. Pooled studies were homogenous (I2= 20.78%; p= 0.285).
3.4.6. Hemoglobin (g/dL)
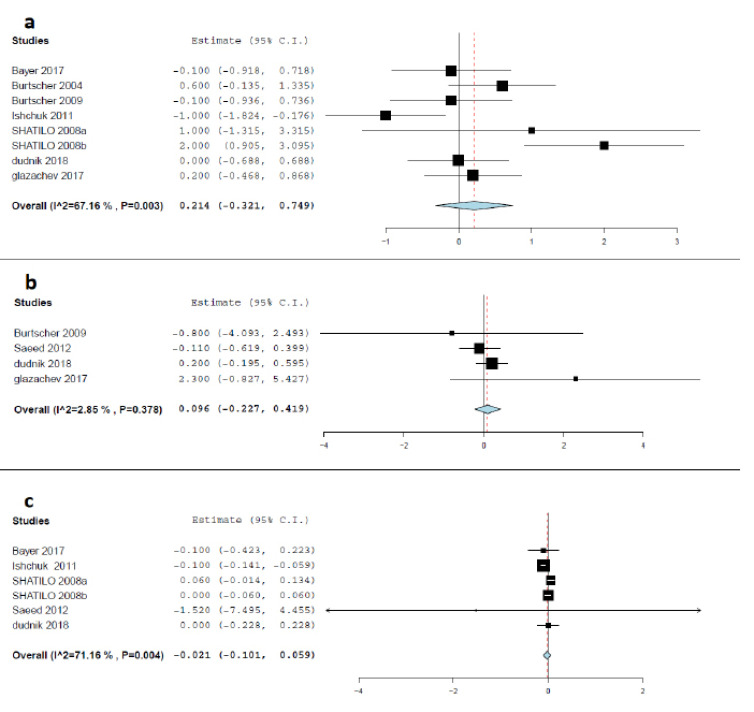
Seven studies reported a change in Hemoglobin level in the IHC and control groups [8, 19, 20, 22, 24-26]. There was no significant change in hemoglobin levels between the IHC group and the sham group after the treatment [MD= 0.214 g/dL, 95% CI (-0.321 to 0.749), p= 0.433], indicating that the efficacy of IHC and control treatment are comparable in terms of hemoglobin level. Pooled studies were heterogeneous (I2 = 67.16%; p=0.003), and the random-effects model was applied, using the Der-Simonian Liard method. Forest plot for change in hemoglobin level after the intervention is seen in Fig. (6a). Heterogeneity was resolved by discarding the reports of Shatilo et al. [20], (I2 = 42.29%; p= 0.123), with the effect estimate being consistent [MD= -0.039 g/dL, 95% CI (-0.445 to 0.368), p=0.851].
Fig. (6).
a) Forest plot of the pooled mean difference between IHC and control groups in the term of hemoglobin (g/dL); b) Forest plot of the pooled mean difference between IHC and control groups in the term of reticulocytes (%); c) Forest plot of the pooled mean difference between IHC and control groups in the term of erythrocyte (%). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4.7. Reticulocytes (%)
Four studies reported a change in the percentage of reticulocytes in the IHC group and the control group [8, 19, 21, 22]. There was no significant change in reticulocyte levels between the IHC group and the sham group after the treatment [MD= 0.096%, 95% CI (-0.227 to 0.419), p= 0.561)], indicating that the efficacy of IHC and control treatment are comparable in terms of reticulocytes. Pooled studies were homogenous (I2=2.85%; p= 0.378), and the random-effects model was applied using the Der-Simonian Liard method. Forest plot for change in reticulocytes level after the intervention is seen in Fig. (6b).
3.4.8. Erythrocytes (102/L)
Five studies reported a change in erythrocyte levels in the IHC and control groups [20-22, 24, 26]. Pooled analysis of the results of these five studies led to finding that there was no significant change in the level of erythrocytes after IHC and sham interventions by the end of the study [MD= -0.021 102/L, 95% CI (-0.101 to 0.059), p=0.610], reflecting that the efficacy of IHC and control treatment are comparable. Pooled studies were not homogenous (I2=71.16%; p= 0.004), and the random-effects model was applied using the Der-Simonian Liard method. Forest plot for change in reticulocytes level after the intervention is seen in Fig. (6c). We could resolve heterogeneity by removing the results of Ishchuk et al. (26), (I2= 0%; p= 0.669), with the results being consistent [MD= 0.021 102/L, 95% CI (-0.025 to 0.066), p=0.375].
3.4.9. Total Serum Cholesterol (mg/dL)
Five studies reported a change in total serum cholesterol level in the IHC and control groups [8, 19, 20, 24, 26]. There was a significant reduction in the level of total serum cholesterol in the IHC group [MD= -0.248 mg/dL, 95% CI (-0.348 to -0.147), p< 0.001] compared to the sham group, indicating that IHC is more effective than the control treatment. Pooled studies were homogenous (I2=11.92%; p= 0.339), and the random-effects model was applied using the Der-Simonian Liard method. Forest plot for change in total cholesterol level after the intervention is seen in Fig. (7a).
Fig. (7).
a) Forest plot of the pooled mean difference between IHC and control groups in the term of total cholesterol (mg/dL); b) Forest plot of the pooled mean difference between IHC and control groups in the term of low-density lipoprotein (mg/dL). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4.10. Low Density Lipoproteins (mg/dL)
The change in Low-density Lipoproteins (LDL) was reported in two studies [8, 24]. There was no significant change in LDL level between the IHC group and the control group [MD= -9.319 mg/dL, 95% CI (-25.745 to 7.107) p=0.266], indicating that IHC is similar to control treatment. Pooled studies were homogenous (I2=0%; p= 0.777), and the random-effects model was applied using the Der-Simonian Liard method. Forest plot for change in LDL level after the intervention is seen in Fig. (7b).
3.5. Safety of IHC Program
Only two of the included studies reported complications/adverse effects in the intervention group [8, 23]. There were no significant complications or serious adverse events related to IHC. In addition, Intraoperative and early postoperative complications did not differ significantly between treatment arms [23]. There were reported incidence of dizziness, palpitations, headache, and dyspnea in the IHC group participants during the first 2 to 5 sessions, but these symptoms disappeared after increasing the inhaled O2 concentration without interrupting the hypoxia-hyperoxia session. Also, angina attacks (without electrocardiogram abnormalities) occured in 6 out of 408 IHC sessions, but this occurred only during three patients' hypoxia exposure. Otherwise, there were no other reported adverse events in the study participants [8]. The other included studies did not report any adverse events, suggesting that IHC is safe.
4. DISCUSSION
In this systematic review and meta-analysis, we summarized the evidence regarding the safety and efficacy of IHC programs in CVDs patients trained in passive/resting state. IHNT/IHHT were found to be associated with significant reduction in heart rate (MD= -5.35 beat/min, p=0.006), SBP (MD= -13.72, p<0.001) mmHg, and DBP (MD= -7.882 mmHg, p=0.003). Moreover, it showed a statistically significant reduction in the total serum cholesterol and LDL. On the other hand, there was no significant effect for the IHNT/IHHT in terms of hematological parameters such as hemoglobin (p= 0.433), erythrocytes (p=0.610), and reticulocytes (p= 0.561). Regarding the peak oxygen consumption and exercise time to fatigue, despite the increase of both parameters at the end of the hypoxic conditioning course within the IHC group, there was no significant change in VO2 peak between the IHC group and the sham-IHC group (p=0.97 and p=0.610, respectively). However, the authors point out that the comparison groups were very heterogeneous at baseline, with better cardiorespiratory fitness scores in controls.
In Dudnik et al. study, there was no significant change in white blood cells or platelets between the intermittent hypoxia-hyperoxia training group and the control group [22]. In Glazachev et al. study [8], the authors followed the patients for one month. There were no differences between groups after their respective treatments and at the one-month follow-up regarding cardiovascular adaptations. Glucose measured in mmol/L was not significantly different between IHNT/IHHT and control groups at the end of treatment. During the follow-up, glycemia was similar in the IHHG and control groups at both measurement times. Glazachev et al. [8] used the Seattle Angina Questionnaire (SAQ) for assessment of the quality of life and angina grades of included patients within the IHHT group and the control group. There was a statistically significant improvement after IHHT and at 1-month follow-up as well in values of all five domains of SAQ: physical limitation, angina stability, angina frequency, treatment satisfaction, and disease perception. IHHT as a very short (4 treatments daily) course was used for myocardial preconditioning in patients undergoing Coronary Artery Bypass Grafting (CABG) surgery in the study performed by Tuter et al. [23]. While mean levels of serum troponin I and serum lactate were not significantly different between IHHT and control groups (IHHT-sham group and the group of patients underwent remote ischemic preconditioning - RIP) after CABG, authors found statistically significant differences of serum troponin I value twenty-four hours after surgery between IHHT and control groups. Similarly, mean lactate values 24 hours after surgery were significantly lower in the IHHT group than in the control groups. Moreover, there was a less total number of intra- and postoperative complications after a short IHHT program than both controls and RIP group [23].
The application of the IHHT program was also reported in elderly cognition and Alzheimer's disease. A pilot study was conducted by Serebrovskaya et al. to assess the effect of IHHT on elderly patients with mild cognitive impairment. They reported that IHHT could significantly improve the neuropsychological, electrophysiological, and biomarker in those patients [35]. Moreover, Bayer et al. reported that combining the IHHT with multimodal training intervention (MTI) was associated with a notable increase in cognitive function [24]. However, yet subsequently, they suggested that IHHT compared with MTI alone does not improve the quality of life (QoL) of the elderly [34]. In a very recent paper concerning the treatment of prediabetic patients, IHHT showed a positive effect in improving the level of glucose, total cholesterol, and LDL [36].
To the best of our knowledge, this is the first systematic review and meta-analysis, which evaluates the safety and efficacy of IHC programs (hypoxic interval exposures at rest in both regimes - IHNT and IHHT) in CVDs patients. This review enrolled all available studies in this regard without any resections. We analyzed all related and available parameters in order to assess the safety and efficacy of IHNT/IHHT. However, the study shows some limitations: 1) the large heterogeneity between the enrolled studies, 2) the small sample size of included participants, 3) meta-regression analysis could not be done, and 4) there was a lack of data in many studies regarding the presence/incidence of adverse effects and/or IHC treatment safety’.
CONCLUSION
In conclusion, our systematic review and meta-analysis provide the scientific community with moderate-quality evidence that IHC is useful to lower heart rate at rest and blood pressure at rest. In contrast, the currently available evidence on IHC safety is limited by poor reporting. Further well-controlled clinical studies are required 1) to identify hypoxic resistance/sensitivity indices for individual tailoring of IHC regimens, 2) to search for additional reliable clinical indicators of IHHT/IHNT effectiveness, 3) to determine the most effective protocols of intermittent hypoxia training (total effective hypoxic dose, the number of sessions in the course, etc.), as well as 4) to reveal the effects of the combined action of hypoxia and mild exercising in comparison with IHC at rest in older patients suffering from CVDs. Nevertheless, current research provides promising results and raises hope that IHC could be used in the future as therapy with no/very few side effects. In light of the high incidence and economic costs of cardiovascular pathology in elderlies, future efforts in this area are feasible from a medical and economic perspective.
ACKNOWLEDGEMENTS
The authors would like to thank the reviewers for their effort in reviewing this manuscript.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines have been followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
The supplementary material is available on the publisher's website along with the published article.
REFERENCES
- 1.Centers for Disease Control and Prevention C. Heart Disease Fact Sheet|Data & Statistics|DHDSP|CDC. 2019. Available from: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_disease.htm.
- 2.Abushouk A.I., El-Husseny M.W.A., Bahbah E.I., Elmaraezy A., Ali A.A., Ashraf A., Abdel-Daim M.M. Peroxisome proliferator-activated receptors as therapeutic targets for heart failure. Biomed. Pharmacother. 2017;95:692–700. doi: 10.1016/j.biopha.2017.08.083. http://linkinghub. elsevier.com/retrieve/pii/S0753332217326604 . [DOI] [PubMed] [Google Scholar]
- 3.Myers J., Kokkinos P., Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11(7):1652. doi: 10.3390/nu11071652. https://www.mdpi.com/2072-6643/11/7/1652 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neubauer J.A. Invited review: Physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol (1985) 2001;90(4):1593–1599. doi: 10.1152/jappl.2001.90.4.1593. http://www.ncbi.nlm.nih.gov/pubmed/11247965 [DOI] [PubMed] [Google Scholar]
- 5.Thompson J.W., Dave K.R., Young J.I., Perez-Pinzon M.A. Ischemic preconditioning alters the epigenetic profile of the brain from ischemic intolerance to ischemic tolerance. Neurotherapeutics. 2013;10(4):789–797. doi: 10.1007/s13311-013-0202-9. http://link.springer.com/10.1007/s13311-013-0202- 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El’chaninova S.A., Koreniak N.A., Pavlovskaia L.I., Smagina I.V., Makarenko V.V. The effect of interval hypoxic hypoxia on the vascular endothelial growth factor and basic fibroblast growth factor concentrations in the peripheral blood. Fiziol Cheloveka. 30(6):93–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/ 15624728. [PubMed] [Google Scholar]
- 7.Elhelaly A.E., AlBasher G., Alfarraj S., et al. Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ Sci Pollut Res. 2019. Available from: http://link.springer.com/10.1007/s11356-019- 06660-3. [DOI] [PubMed]
- 8.Glazachev O., Kopylov P., Susta D., Dudnik E., Zagaynaya E. Adaptations following an intermittent hypoxia-hyperoxia training in coronary artery disease patients: a controlled study. Clin. Cardiol. 2017;40(6):370–376. doi: 10.1002/clc.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susta D., Dudnik E., Glazachev O.S. A programme based on repeated hypoxia-hyperoxia exposure and light exercise enhances performance in athletes with overtraining syndrome: a pilot study. Clin. Physiol. Funct. Imaging. 2017;37(3):276–281. doi: 10.1111/cpf.12296. http://doi.wiley.com/ 10.1111/cpf.12296 . [DOI] [PubMed] [Google Scholar]
- 10.Arkhipenko Y.V., Sazontova T.G., Zhukova A.G. Adaptation to periodic hypoxia and hyperoxia improves resistance of membrane structures in heart, liver, and brain. Bull. Exp. Biol. Med. 2005;140(3):278–281. doi: 10.1007/s10517-005-0466-0. http://link.springer.com/10.1007/s10517-005-0466-0 [DOI] [PubMed] [Google Scholar]
- 11.Sazontova T.G., Glazachev O.S., Bolotova A.V., Dudnik E.N., Striapko N.V., Bedareva I.V., Anchishkina N.A., Arkhipenko IuV. Adaptation to hypoxia and hyperoxia improves physical endurance: the role of reactive oxygen species and redox-signaling. Ross. Fiziol. Zh. Im. I M Sechenova. 2012;98(6):793–807. http://www.ncbi.nlm.nih.gov/ pubmed/23013017 . [PubMed] [Google Scholar]
- 12.Lyamina N.P., Lyamina S.V., Senchiknin V.N., Mallet R.T., Downey H.F., Manukhina E.B. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J. Hypertens. 2011;29(11):2265–2272. doi: 10.1097/HJH.0b013e32834b5846. [DOI] [PubMed] [Google Scholar]
- 13.Schega L., Peter B., Törpel A., Mutschler H., Isermann B., Hamacher D. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study. Gerontology. 2013;59(4):316–323. doi: 10.1159/000350927. https://www.karger.com/Article/FullText/350927 [DOI] [PubMed] [Google Scholar]
- 14.Liguori C., Stefani A., Sancesario G., Sancesario G.M., Marciani M.G., Pierantozzi M. CSF lactate levels, τ proteins, cognitive decline: a dynamic relationship in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2015;86(6):655–659. doi: 10.1136/jnnp-2014-308577. http://www.ncbi.nlm.nih.gov/pubmed/25121572 . [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Altman D.G., Gotzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–d5928. doi: 10.1136/bmj.d5928. Available from: http://www.bmj.com/cgi/doi/10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P., Green S. Cochrane Handbook for Systematic Reviews of interventions. The Cochrane Collaboration. 2008;Version 5 doi: 10.1002/9780470712184. [DOI] [Google Scholar]
- 18.Kalachev A. The use of prolonged hypoxic training for secondary prevention of coronary heart disease. Vestn Arith. 2004:30–31. [Google Scholar]
- 19.Burtscher M., Gatterer H., Szubski C., Pierantozzi E., Faulhaber M. Effects of interval hypoxia on exercise tolerance: special focus on patients with CAD or COPD. Sleep Breath. 2010;14(3):209–220. doi: 10.1007/s11325-009-0289-8. [DOI] [PubMed] [Google Scholar]
- 20.Shatilo V.B., Korkushko O.V., Ischuk V.A., Downey H.F.S.T., Serebrovskaya T.V. Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Alt. Med. Biol. 2008;9(1):43–52. doi: 10.1089/ham.2007.1053. [DOI] [PubMed] [Google Scholar]
- 21.Saeed O., Bhatia V., Formica P., Browne A., Aldrich T.K., Shin J.J., Maybaum S. Improved exercise performance and skeletal muscle strength after simulated altitude exposure: a novel approach for patients with chronic heart failure. J. Card. Fail. 2012;18(5):387–391. doi: 10.1016/j.cardfail.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Dudnik E., Zagaynaya E., Glazachev O.S., Susta D. Intermittent hypoxia-hyperoxia conditioning improves cardiorespiratory fitness in older comorbid cardiac outpatients without hematological changes: A randomized controlled trial. High Alt. Med. Biol. 2018;19(4):339–343. doi: 10.1089/ham.2018.0014. [DOI] [PubMed] [Google Scholar]
- 23.Tuter D.S., Kopylov P.Y., Syrkin A.L., Glazachev O.S., Komarov R.N., Katkov A.I., Severova L.P., Ivanova E.V., Zhang Y., Saner H. Intermittent systemic hypoxic-hyperoxic training for myocardial protection in patients undergoing coronary artery bypass surgery: first results from a single-centre, randomised controlled trial. Open Heart. 2018;5(2):e000891. doi: 10.1136/openhrt-2018-000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayer U., Likar R., Pinter G., Stettner H., Demschar S., Trummer B., Neuwersch S., Glazachev O., Burtscher M. Intermittent hypoxic-hyperoxic training on cognitive performance in geriatric patients. Alzheimers Dement. (N. Y.) 2017;3(1):114–122. doi: 10.1016/j.trci.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burtscher M., Pachinger O., Ehrenbourg I., Mitterbauer G., Faulhaber M., Pühringer R., Tkatchouk E. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int. J. Cardiol. 2004;96(2):247–254. doi: 10.1016/j.ijcard.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Ischuk V. The use of interval normobaric hypoxic training in elderly patients with coronary heart disease. Ukr J Cardiol. 2011:75–89. [Google Scholar]
- 27.Yelchaninova S., Korenyak N., Pavlovskaya L., Makarenka V., Dryagina I., Simonova O. On the mechanisms of the hypotensive effect of intermittent normobaric hypoxia in arterial hypertension. Reports Int Acad Hypoxia Probl. 2005:33–38. [Google Scholar]
- 28.Rachok L.V., Dubovik T.A., Bulgak A.G., Ostrovsky Y.P.K.M., Belskaya M.I., Zhujko E.N.R.I. The effects of using normobaric intermittent hypoxia training as a method of preoperative preparation for coronary bypass surgery of the ischemic cardiomyopathy patients. Cardiol Belarus. 2011;17:28–45. [Google Scholar]
- 29.Makhova G. Intermittent normobaric hypoxic therapy is a promising and effective non-medication method to rehabilitate patients suffering from transmural miocardial infraction. Intermittent normobaric hypoxic Ther Reports Int Acad Hypoxia Probl. 2005:75–89. [Google Scholar]
- 30.Julian C.G., Gore C.J., Wilber R.L., Daniels J.T., Fredericson M., Stray-Gundersen J., Hahn A.G., Parisotto R., Levine B.D. Intermittent normobaric hypoxia does not alter performance or erythropoietic markers in highly trained distance runners. J Appl Physiol (1985) 2004;96(5):1800–1807. doi: 10.1152/japplphysiol.00969.2003. [DOI] [PubMed] [Google Scholar]
- 31.Harrison C.C., Fleming J.M., Giles L.C. Does interval hypoxic training affect the lung function of asthmatic athletes. New Zealand Journal of Sports Medicine. 2002;Vol. 30:P64–p67. Available from: http://articles.sirc.ca/search.cfm?id=S-867547%5Cnhttp://search.ebscohost.com/login.aspx?direct=true&db=sph&AN=SPHS-867547&site=ehost-live%5Cnhttp://www.sportsmedicine.co.nz. [Google Scholar]
- 32.Netzer N.C., Chytra R., Küpper T. Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. 2008;12(2):129–134. doi: 10.1007/s11325-007-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oltmanns K.M., Gehring H., Rudolf S., Schultes B., Rook S., Schweiger U., Born J., Fehm H.L., Peters A. Hypoxia causes glucose intolerance in humans. Am. J. Respir. Crit. Care Med. 2004;169(11):1231–1237. doi: 10.1164/rccm.200308-1200OC. [DOI] [PubMed] [Google Scholar]
- 34.Bayer U., Likar R., Pinter G., Stettner H., Demschar S., Trummer B., Neuwersch S., Glazachev O., Burtscher M. Effects of intermittent hypoxia-hyperoxia on mobility and perceived health in geriatric patients performing a multimodal training intervention: a randomized controlled trial. BMC Geriatr. 2019;19(1):167. doi: 10.1186/s12877-019-1184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serebrovska Z.O., Serebrovska T.V., Kholin V.A., Tumanovska L.V., Shysh A.M., Pashevin D.A., Goncharov S.V., Stroy D., Grib O.N., Shatylo V.B., Bachinskaya N.Y., Egorov E., Xi L., Dosenko V.E. Intermittent hypoxia-hyperoxia training improves cognitive function and decreases circulating biomarkers of Alzheimer’s disease in patients with mild cognitive impairment: A pilot study. Int. J. Mol. Sci. 2019;20(21):E5405. doi: 10.3390/ijms20215405. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Serebrovska T.V., Grib O.N., Portnichenko V.I., Serebrovska Z.O., Egorov E., Shatylo V.B. Intermittent Hypoxia/Hyperoxia Versus Intermittent Hypoxia/Normoxia: Comparative Study in Prediabetes. High Alt Med Biol. 2019. Available from: https://www.liebertpub.com/doi/10.1089/ham.2019.0053. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material is available on the publisher's website along with the published article.