Abstract
Flowering is an important link in the life process of angiosperms, and it is also an important sign of the transformation of plants from vegetative to reproductive growth. Although the flowering regulation network of Arabidopsis is well-understood, there has been little research on the molecular mechanisms of perennial woody plant flower development regulation. Populus tomentosa is a unique Chinese poplar species with fast growth, strong ecological adaptability, and a long lifecycle. However, it has a long juvenile phase, which seriously affects its breeding process. Nuclear factor-Y (NF-Y) is an important type of transcription factor involved in the regulation of plant flowering. However, there are few reports on PtoNF-Y gene flowering regulation, and the members of the PtNF-YC subfamily are unknown. In this study, four key genes were cloned and analyzed for sequence characteristics, gene structure, genetic evolution, expression patterns, and subcellular localization. The plant expression vector was further constructed, and transgenic Arabidopsis and P. tomentosa plants were obtained through genetic transformation and a series of molecular tests. The flowering time and other growth characteristics were analyzed. Finally, the expression level of flowering genes was detected by quantitative PCR, the interaction between PtoNF-YC and PtoCOL proteins was measured using the yeast two-hybrid system to further explain the flowering regulation mechanism, and the molecular mechanisms by which PtNF-YC6 and PtNF-YC8 regulate poplar flowering were discussed. These results lay the foundation for elucidating the molecular regulation mechanism of PtoNF-YC in flowering and furthering the molecular design and breeding of poplar, while providing a reference for other flowering woody plants.
Keywords: flowering time, genome-wide analysis, NF-YC, Populus tomentosa, transgenic plant
1. Introduction
The transition from vegetative growth to reproductive growth is an important step in the plant lifecycle, and flowering is a key sign of this transition [1]. Compared with annual plants, most woody plants need a longer juvenile period before entering the flowering period, and many traits can only be expressed after reaching maturity. However, economic forests and timber forests have the problems of long a juvenile period and late flowering, which seriously limit the improvement of their economic benefits and production development, as well as their breeding process [2,3,4]. Therefore, promoting early flowering, shortening the juvenile period, and accelerating the breeding cycle of trees are of great significance to the development of forestry science and the study of the molecular mechanisms of plant sexual reproduction.
Nuclear factor-Y transcription factors also called the heme activator protein (HAP) or CCAAT-binding transcription factor, are ubiquitous in animals, plants, and other eukaryotes [5,6,7]. NF-Y complexes bind to the CCAAT motif in the promoters of many genes [8,9]. NF-Y transcription factors are a heterotrimeric complex composed of three subunits: NF-YA, NF-YB, and NF-YC. Among them, NF-YB and NF-YC form dimers in the cytoplasm, and further combine with the NF-YA protein in the nucleus to form heterotrimers [10]. In yeast and mammals, each NF-Y subunit is encoded by one gene [11]. By contrast, each NF-Y subfamily in plants consists of multiple members. At present, NF-Y family members have been identified in many species, including Arabidopsis [12], rice [13], maize [14], tomato [15], poplar [16], and Pinus tabuliformis [17]. Some species members expanded, resulting in functional redundancy and functional differences, while also helping to form a transcription factor network to regulate plant growth and development [18,19].
NF-Y is involved in multiple growth and development processes of plants, including embryogenesis, seed germination, flowering, fruit ripening, and other growth processes [15,20,21,22,23,24]. Furthermore, NF-Y plays an important role in responding to abiotic stress, such as drought, high salt, and low temperature [25,26,27]. Notably, NF-Y plays an important role in the process of flowering regulation, especially the NF-YC subfamily, which participates in flowering regulation in different ways. For example, in the photoperiod-dependent flowering pathway, Arabidopsis AtNF-YB2 and AtNF-YB3 bind to AtNF-YC3, AtNF-YC4, and AtNF-YC9, and the heterodimers further interact with CONSTANS to induce FT expression [22]. In the aging-regulated flowering pathway, CmNF-YB8 regulates flowering time by regulating the expression of the cmo-MIR156 gene in the senescence pathway [23]. In addition, the NF-Y complex can also act as an epigenetic regulator in the gibberellic acid pathway [28]. However, little is known about the poplar NF-Y gene in flowering. In recent years, studies have shown that overexpression of poplar PtNF-YB2 in Arabidopsis and tomato can induce earlier flowering [3], and overexpression of PtNF-YA9 can delay flowering in Arabidopsis [29]. However, research on the flowering of poplar NF-YC is lacking.
Populus tomentosa is a unique Chinese poplar species with rapid growth, strong ecological adaptability, and a long lifecycle. It plays an important role in forestry economy, ecological construction, and urban greening [2,4,16]. However, P. tomentosa has a long juvenile phase, which seriously affects its breeding process. Although NF-Y is an important transcription factor involved in regulating plant flowering, there are few reports on NF-Y gene flowering regulation in poplar, and the members of the PtNF-YC subfamily are unknown.
In this study, four PtoNF-YC genes were cloned from P. tomentosa. We confirmed the function of the PtoNF-Y6 and PtoNF-Y8 in regulating flowering timing using transgenic Arabidopsis and P. tomentosa. Based on the result, we proposed a potential molecular mechanism model of PtoNF-Y6 and PtoNF-Y8 in flowering regulation. The results of this study lay the foundation for elucidating the molecular regulation mechanism of PtNF-YC in flowering, and provide a reference for research on flowering regulation in other woody plants.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
In this study, roots, stems, leaves, leaf buds, flower buds, and flowers of P. tomentosa were collected as previously described [16]. For poplar genetic transformation, 2-month-old P. tomentosa (TC1521, female clone) seedlings were used as described by Li [30]. All plants were grown in the Beijing Forestry University greenhouse (Beijing, China) and maintained at 23 ± 1 °C and 60–70% humidity. For Arabidopsis transformation, wild-type (WT) ecotype Columbia (Col) was used as the experimental material. It was grown in long-day conditions (16 h light/8 h dark) at 20–22°C.
2.2. Cloning and Sequence Analysis of the Four PtoNF-YC Genes
The total RNA was extracted from tissue culture seedlings of P. tomentosa using the TRIzol Total RNA Extraction Kit (Promega, Madison, WI, USA). RNA quality was measured using a NanoDrop 2000 spectrophotometer (Implen, Inc., Westlake Village, CA, USA). RQ1 DNase was used to remove genomic DNA, and the Reverse Transcription System (Promega, Madison, WI, USA) was used to synthesize cDNA according to the manufacturer’s instructions. Using the Arabidopsis NF-YC protein sequences as the query sequences, those with high similarity to Arabidopsis NF-YC were obtained from the Phytozome v12.1 database using BLAST. All cloning primers were designed using the Primer Premier 5.0 software [31], and are presented in Table S1. The PCR program was as follows: 95 °C for 3 min, 35 cycles of 30 s each at 95 °C, 30 s at 60 °C, and 1 min at 72 °C, and a final elongation step of 72 °C for 5 min. The PCR products were detected by 1.5% agarose gel and the target bands were purified as previously described [32]. Then, the purified band was cloned into the pGEM-T Easy Vector (Promega) for sequencing. The physicochemical parameters of the PtoNF-YC proteins were predicted by Expasy (http://cn.expasy.org/, accessed on 3 January 2022) [33]. Multiple alignment analysis of the sequences was performed with ClustalX2.1 and GenDoc software [34]. The phylogenetic tree was generated by MEGA7.0 using the neighbor-joining method based on the NF-Y protein sequences [35].
2.3. PtoNF-YC Expressing Vector Construction and Subcellular Localization Assay
To express the PtoNF-YC protein, we ligated the four cloned PtoNF-YC5, PtoNF-YC6, PtoNF-YC7, and PtoNF-YC8 genes into pSuper1300-GFP with the eGFP gene to produce pSuper1300-GFP-PtoNF-YC5, pSuper1300-GFP-PtoNF-YC6, pSuper1300-GFP-PtoNF-YC7, and pSuper1300-GFP-PtoNF-YC8 constructs, respectively. The constructed vectors were verified by PCR and double enzymatic digestion, and transferred into the Agrobacterium expression strain GV3101. For subcellular localization analysis, Agrobacterium containing the expression vector was cultured to an optical density value of 1.0, harvested, resuspended in agroinfiltration buffer (150 μM acetosyringone, 10 mM MgCl2, and 10 mM MES), and injected using a syringe into tobacco leaves. Three days after the injection, a Leica TCS SP8 confocal microscope was used to observe the results.
2.4. Analysis of the Interaction Mode of PtoNF-YCs and PtoCOL
We performed yeast two-hybrid (Y2H) experiments using the Gal4-based two-hybrid system according to the manufacturer’s instructions (Clontech Laboratories, San Jose, CA, USA). The open reading frames (ORFs) of poplar PtoNF-YC6 and PtoNF-YC8 were inserted into the bait vector pGBKT7, and finally pGBKT7-PtoNF-YC6 and pGBKT7-PtoNF-YC8 were formed as bait, respectively. The PtoNF-COL1 and PtoNF-COL2 ORFs were cloned into the vector pGADT7. The primers used are listed in Table S1. Then, the pGBKT7-PtNF-YC6/8 constructs and pGADT7-PtNFCOL1 and pGADT7-PtNF-COL2 were respectively transformed into the yeast strain Y2HGold in pairs. Then pGBKT7-53 and pGBKT7-Lam were used as positive and negative control plasmids, respectively. Finally, positive colonies were selected using synthetic defined medium without Trp-Leu-His-Ade (SD-Trp-Leu-His-Ade medium).
2.5. Arabidopsis and Poplar Transformation
For the transformation of Arabidopsis, PtoNF-Y6, and PtoNF-Y8 overexpression constructs were introduced into Col using the floral dip method with Agrobacterium GV3101 strain [36]. The seeds of the PtoNF-YC6 and PtoNF-YC8 transgenic lines were harvested separately and sown again, and finally the homozygous transgenic lines were used for further research. For poplar transformation, we used 2-month-old P. tomentosa TC1521 tissue culture seedlings as explant material, and further transformed explants through Agrobacterium-mediated leaf disc [30].
2.6. Extraction of RNA and Quantitative PCR Analysis
Roots, stems, leaves, leaf bud, flower bud, and flower were obtained for tissue-specific expression pattern analysis. The PtoNF-YCs overexpressing plants and wild-type plants were used to detect the expression level of PtoNF-YC6 and PtoNF-YC8 in poplar and Arabidopsis. All samples were immediately frozen in liquid nitrogen and then stored at −80 °C until further use. Next, the total RNA of different tissues and transgenic plants were extracted and reverse transcribed as described above. The quantitative PCR (qPCR) primers were designed using Primer Premier 5.0, and the PtACTIN gene was used as the internal control [2,37,38]. All primers are listed in Table S1. The expression levels of the target genes were detected using SYBR® Premix ExTaq™ (TaKaRa Bio, Shiga, Japan) on an ABI PRISM 7500 Fast Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The PCR program used was previously described [16]. Data were analyzed using the 2−ΔΔCT method [39].
2.7. Phenotypic Analysis and Biomass Measurements
The phenotypes of transgenic Arabidopsis and P. tomentosa plants were recorded, including total height, stem diameter, flowering time, and floral organ variation.
2.8. Statistical Analyses
In the study, three wild-type and transgenic plant lines were selected (except the overexpressing PtoNF-YC8 P. tomentosa plants were two lines), with three plants per line, and each experiment was repeated five times. All presented data are from the mean of three experiments. The data are presented as the mean ± standard error of the mean and were analyzed using the SPSS 19.0 software (IBM Inc., Armonk, NY, USA). The error bars were calculated according to Tukey’s multiple range test, and with * (p < 0.05), ** (p < 0.01) being used to indicate statistically significant effects.
3. Results
3.1. Cloning and Sequence Analysis of the Four PtoNF-YC Genes
Previous research by our group showed that the four PtNF-YC5/6/7/8 proteins form an independent subfamily with the Arabidopsis flowering-related proteins AtNF-YC3, AtNF-YC4, and AtNF-YC9 (Figure S1). In addition, the expression patterns of flower bud development indicated that PtoNF-YC5 and PtoNF-YC6 have higher expression levels in the dormancy and sporulation phases, while PtoNF-YC7 and PtoNF-YC8 have higher expression levels in the early stages of flower bud development [16]. Thus, we speculated that PtoNF-YC5/6/7/8 members play an important role in flower development.
To obtain the four NF-YC genes of P. tomentosa, we used the cDNA of P. tomentosa TC1521 as a template to perform PCR, and finally obtained four fragments of 753 bp, 711 bp, 681 bp, and 777 bp, respectively. The PCR product was purified and ligated into the pGEM-T Easy Vector for sequencing. Finally, the four genes that were sequenced correctly were named PtoNF-YC5, PtoNF-YC6, PtoNF-YC7, and PtoNF-YC8 (Figure S2).
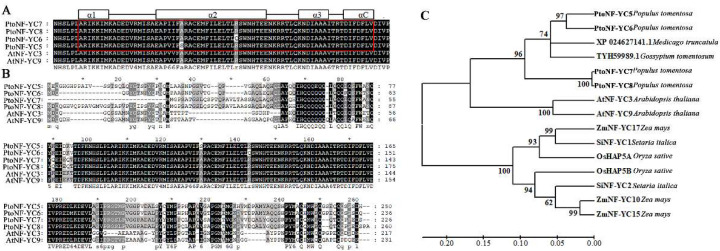
To further analyze the evolutionary relationship between the PtoNF-YC5/6/7/8 protein and NF-YC proteins in other species, a comprehensive phylogenetic tree was constructed using NF-YC proteins from a variety of monocot and dicot plants. The results showed that these proteins were divided into two major branches. Among them, the NF-YC protein of monocots Zea mays, Setaria italica, and Oryza sativa formed one branch, while dicots formed another branch. The dicot branch was further divided into two sub-branches: Arabidopsis thaliana formed one sub-branch, and Populus, Gossypium, Medicago, and other plants formed the other sub-branch. PtoNF-YC5 protein showed the closest relationship with PtoNF-YC6, while PtoNF-YC7 had the closest relationship with PtoNF-YC8. This also showed that the functions of the two pairs of members of PtoNF-YC5/6 and PtoNF-YC7/8 are similar or redundant (Figure 1C). The conserved domain analysis showed that the four proteins PtoNF-YC5/6/7/8 all have a core histone sequence, indicating that they belong to the PtoNF-YC family of transcription factors (Figure 1A,B).
Figure 1.
Comparison of the four PtoNF-YC proteins with NF-YC protein sequences from other species. (A) Comparison of conserved sequence regions of P. tomentosa PtoNF-YC5/6/7/8 and Arabidopsis homologous protein. (B) Comparison of P. tomentosa PtoNF-YC5/6/7/8 and Arabidopsis homologous protein sequence. (C) Phylogenetic analysis of PtoNF-YC5/6/7/8 homologous protein sequence in P. tomentosa.
3.2. Tissue-Specific Expression and Subcellular Localization Analysis of the Four PtoNF-YC Genes
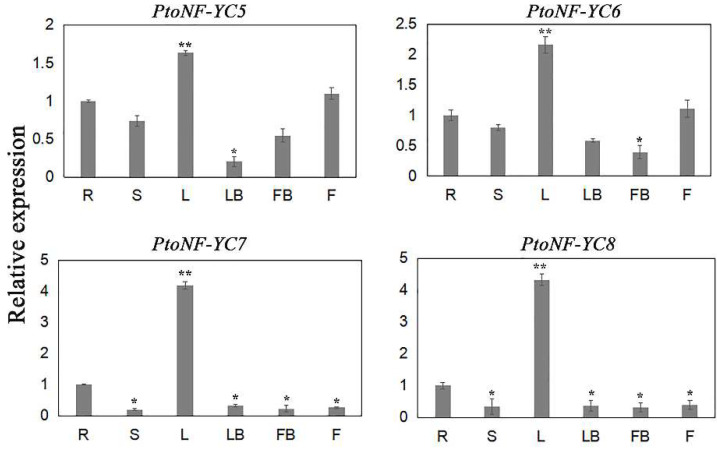
To further investigate the potential functions of PtoNF-YC genes in different tissues and organs of P. tomentosa, we analyzed the expression patterns of PtoNF-YC5/6/7/8 using qPCR. The four PtoNF-YC genes were expressed in different tissues in P. tomentosa, with different expression levels. In the vegetative growth stage, expression levels were the highest in leaves. In the reproductive growth stage, the expression levels of PtoNF-YC5 and PtoNF-YC6 in flower buds and flower were higher than those of PtoNF-YC7 and PtoNF-YC8. Moreover, all four genes showed the highest expression in leaves, indicating that they may be redundant (Figure 2).
Figure 2.
Tissue-specific expression patterns of PtoNF-YC5/6/7/8 in P. tomentosa. R, S, L, LB, FB, and F represent roots, stems, leaves, leaf buds, flower buds, and flowers, respectively. The data was presented as mean ± SE (n = 3 independent replicates), * (p < 0.05) and ** (p < 0.01) indicate significant differences.
The function of a gene is related not only to its expression pattern but also to its location. Therefore, cellular localization has important significance for the study of proteins with unknown functions. To explore the location of the PtoNF-YC5/6/7/8 proteins, we fused the four proteins of PtoNF-YC with the GFP protein and transformed them into Agrobacterium GV3101 (Figure S3). The Agrobacterium solution of pSuper1300-GFP vector and pSuper1300-GFP-PtoNF-YC5/6/7/8 vector were injected into tobacco leaves and expressed transiently, and localization was analyzed by fluorescence. All four proteins were located in the cell membrane and nucleus. Compared with the pSuper1300-GFP vector, pSuper1300-GFP-PtoNF-YC8 exhibited a stronger fluorescence signal on the cell membrane, while the fluorescence signals of the other three proteins did not differ significantly from that of pSuper1300-GFP (Figure 3).
Figure 3.
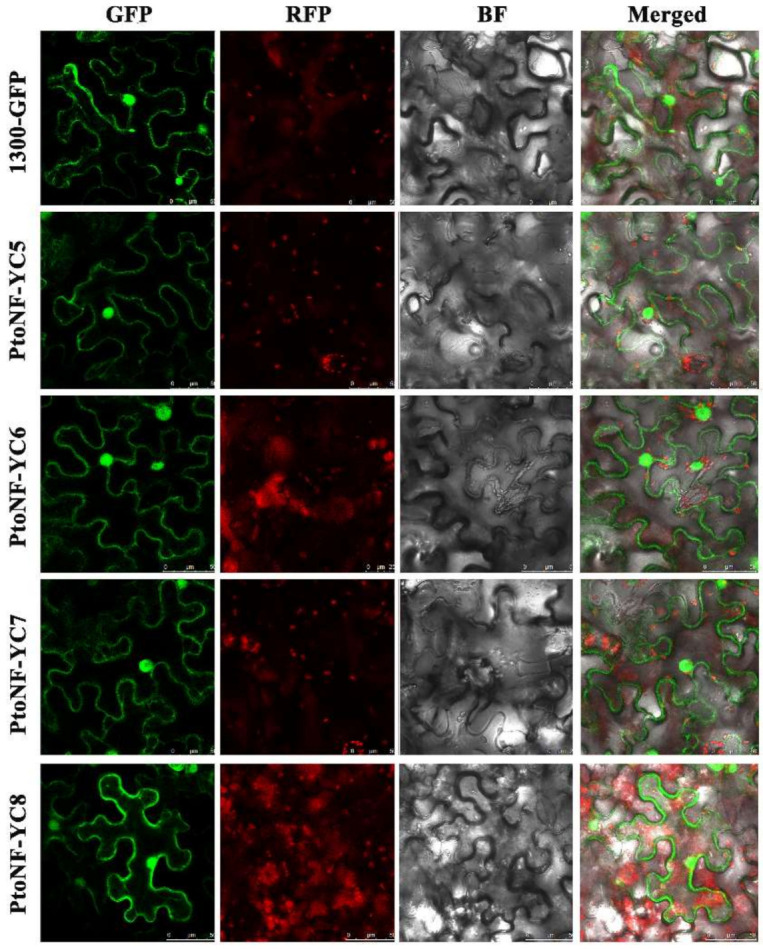
Subcellular localization of pSuper1300-GFP-PtoNF-YC5/6/7/8 proteins. From left to right, GFP, RFP, BF, and Merged represent the GFP signal, chloroplast spontaneous signal, bright field, and superimposed signal, respectively, bar 50 μm.
3.3. Effect of Ectopic Expression of PtoNF-YC6/8 on Early Flowering in Transgenic Arabidopsis
The sequence analysis showed that the homology of PtoNF-YC5 and PtoNF-YC6 reached 85.39%, and the homology of PtoNF-YC7 and PtoNF-YC8 was 83.53%. Compared with PtoNF-Y5 and PtoNF-YC7, PtoNF-YC6 and PtoNF-YC8 had higher similarity with AtNF-YC3 and AtNF-YC9 (Table S2). Therefore, PtoNF-YC6 and PtoNF-YC8 were selected for further research.
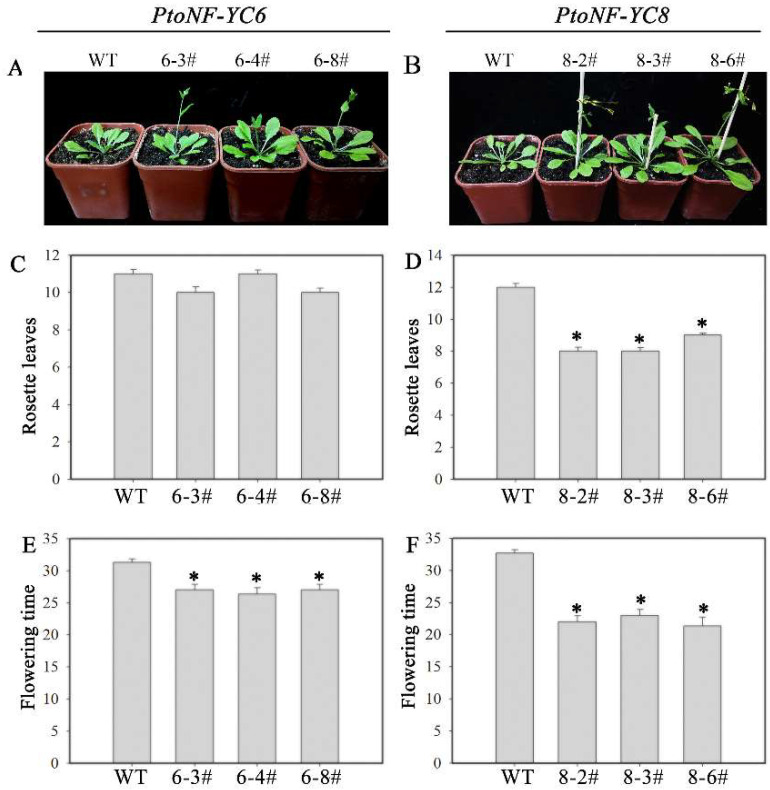
In this study, to determine the effects of the PtoNF-YC6 and PtoNF-YC8 genes on flowering time, we obtained 10 lines of PtoNF-YC6 and 9 lines of PtoNF-YC8 transgenic Arabidopsis through multi-level identification. The positive plants were continued to be cultivated and the seeds were collected from a single plant, screened again, and the seeds of the T3 generation were collected for subsequent experiments. The T3 generation plants were grown under long-day conditions (16 h light/8 h dark) and their phenotypes were observed and counted. The analysis of transcriptional expression levels showed that 6–3#, 6–4#, 6–8#, 8–2#, 8–3#, and 8–6# had the highest expression levels (Figures S4 and S5), and their flowering time were most significantly earlier than the WT. In addition, the number of rosette leaves showed differences. The results showed that the expression of PtoNF-YC6 and PtoNF-YC8 significantly shortened the flowering time of transgenic Arabidopsis, and PtoNF-YC8 had a stronger effect than PtoNF-YC6 (Figure 4).
Figure 4.
Flowering time and phenotype analysis of PtoNF-YC6/8 transgenic Arabidopsis. (A,B) Images of flowering phenotype. (C,D) Analysis of rosette leaf number. (E,F) Analysis of flowering time. * (p < 0.05) indicate significant differences.
3.4. Analysis of the Effects of PtoNF-YC6 and PtoNF-YC8 on the Floral Organs of Arabidopsis
Next, we determined whether the PtoNF-YC6 and PtoNF-YC8 genes affected the morphology of Arabidopsis floral organs. Compared with WT plants, 6–3# transgenic plants had significantly more branches, but there was no difference in flower morphology between WT and transgenic Arabidopsis (Figure 5D). They both had four petals, and the petals and calyx fell off as the pod grew (Figure 5C,F). For PtoNF-YC8 transgenic Arabidopsis plants, the secondary stems of line 8–6# significantly increased (Figure 6A,B,G). The PtoNF-YC8 transgenic plants also showed a reduction in the number of petals and elongated petals (Figure 6I–K). The petals and calyx also fell off with pod growth (Figure 6E,F). In addition, some secondary branches showed floral organ abortion (Figure 6H). Overall, the results indicate that PtoNF-YC8 plays an important role in regulating flower morphology and floral organ development.
Figure 5.
Phenotypes of floral organs in PtoNF-YC6 transgenic Arabidopsis. (A) WT plants. (B) WT petals. (C) WT seeds. (D) PtoNF-YC6 gene transgenic plants. (E) PtoNF-YC6 transgenic plant petals. (F) PtoNF-YC6 transgenic plant seeds.
Figure 6.
Phenotypes of floral organs in PtoNF-YC8 transgenic Arabidopsis. (A,G) WT and PtoNF-YC8 transgenic Arabidopsis plants, respectively. (B–D). WT plant floral morphology. (H–K) PtoNF-YC8 transgenic Arabidopsis floral morphology. (E) WT plant seeds. (F) PtoNF-YC8 transgenic plant seeds.
3.5. Effects of PtoNF-YC6 and PtoNF-YC8 on the Growth of P. tomentosa
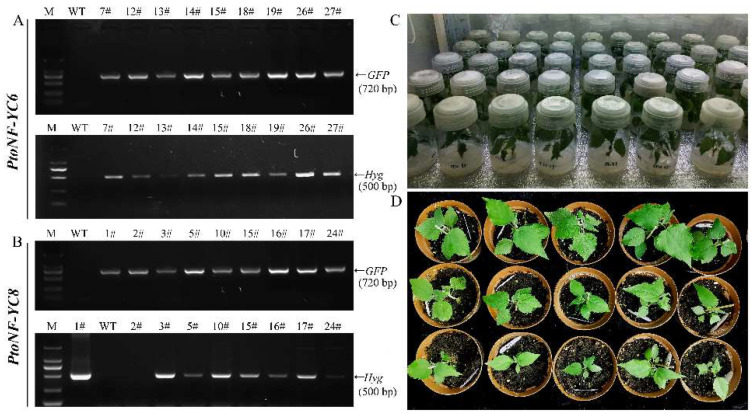
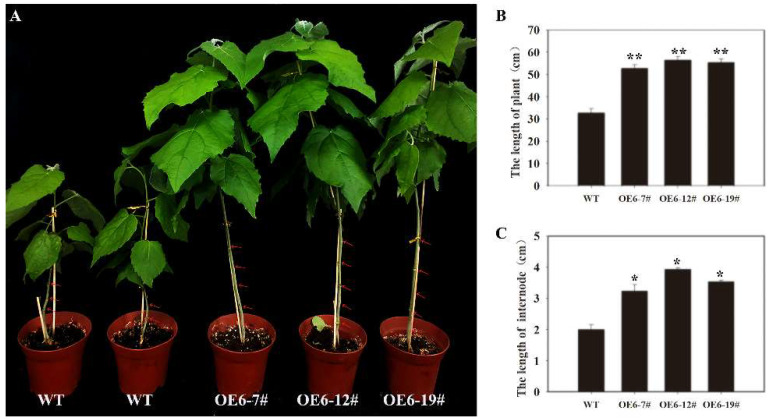
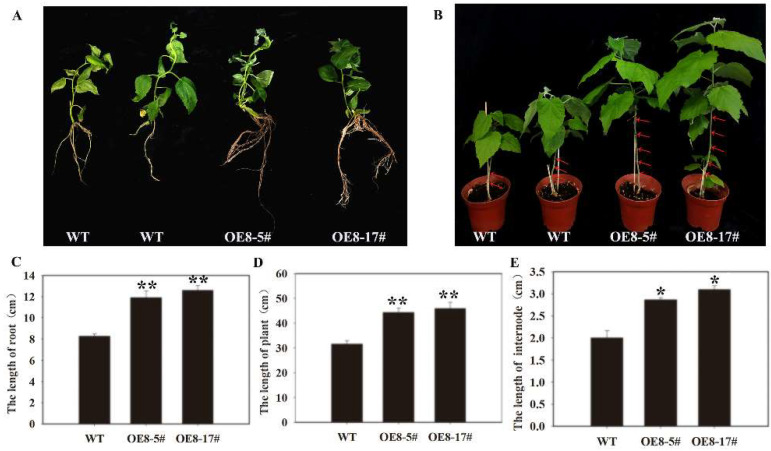
To verify the functions of PtoNF-YC6 and PtoNF-YC8 in promoting early flowering, we also obtained 12 lines of PtoNF-YC6 and 8 lines of PtoNF-YC8 transgenic P. tomentosa by PCR identification, respectively, and they were propagated and cultured for subsequent experiments (Figure 7). Subsequently, the expression levels of the transgenic plants were analyzed by RT-qPCR, the results showed that the expression levels of PtoNF-YC6 and PtoNF-YC8 transgenic P. tomentosa lines were generally 1.5–5.3 times higher than the wild-type plants, and the five plants (6–7#, 6–12#, 6–19#, 8–5# and 8–17#)with the highest expression levels were selected for subsequent phenotypic experiments (Figures S6A and S7A). In the same growth environment, we analyzed the plants grown for 6 months, and the results showed that the growth rate of the PtNF-YC6/8-overexpressing P. tomentosa lines was significantly faster than that of the wild-type (WT) plants. For example, when the height of wild-type plants was 33 cm, the height of PtoNF-YC6 and PtoNF-YC8 transgenic poplar was 45–55 cm. The height of the plant mainly depends on the internode distance of the plant. Therefore, the internode distance was measured and counted. The results showed that the internode distance of the transgenic plant was significantly larger than that of the wild type. However, the plants obtained so far do not show early flowering, and further cultivation and observation are needed to determine their function (Figure 8 and Figure 9).
Figure 7.
PCR identification and propagation of PtoNF-YC6 and PtoNF-YC8 transgenic P. tomentosa. (A) Identification of PtoNF-YC6 transgenic lines. (B) Identification of PtoNF-YC8 transgenic lines. (C) Transgenic plant propagation. (D) The transgenic plants transplanted into soil in greenhouse.
Figure 8.
The phenotype analysis of PtoNF-YC6 transgenic P. tomentosa. (A) PtoNF-YC6 transgenic P. tomentosa plants with different growth phenotype. (B) Plant height analysis of PtoNF-YC6 transgenic P. tomentosa plants. (C) Internode statistical analysis of PtoNF-YC6 transgenic P. tomentosa plants. * (p < 0.05) and ** (p < 0.01) indicate significant differences. Subfigure A red arrows represent internodes.
Figure 9.
The phenotype analysis of PtoNF-YC8 transgenic P. tomentosa. (A) The phenotypic traits of PtoNF-YC8 transgenic P. tomentosa. (B) PtoNF-YC8 transgenic P. tomentosa plants with different growth phenotype. (C) Root length analysis. (D) Plant height analysis. (E) Internode statistical analysis. * (p < 0.05) and ** (p < 0.01) indicate significant differences. Subfigure B red arrows represent internodes.
3.6. The Possible Molecular Mechanism of PtoNF-YC6/8 Expression in P. tomentosa Promoting Early Flowering
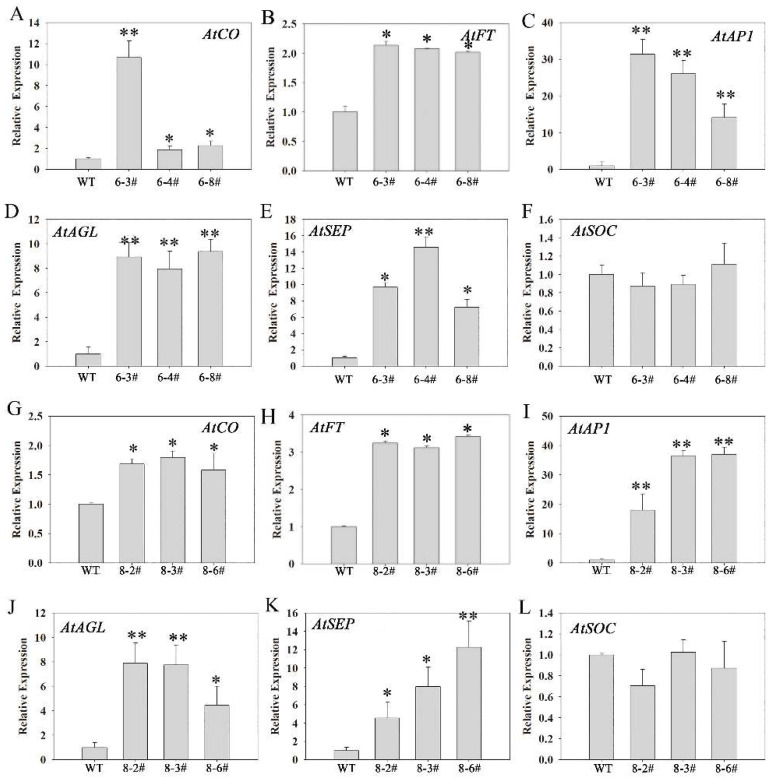
To further analyze the mechanism of how PtoNF-YC6 and PtoNF-YC8 regulate plant early flowering, we used the leaves of WT (Col), PtoNF-YC6, and PtoNF-YC8 transgenic plants before bolting as samples and analyzed the expression levels of flowering genes by qRT-PCR. In PtoNF-YC6 transgenic Arabidopsis lines, except for AtSOC, the expression levels of AtCO, AtFT, AtAP, AtAGL, and AtSEP3 were higher than those of the WT (Figure 10A–F). For PtoNF-YC8 transgenic plants, the expression pattern of flowering-related genes was similar to that of PtoNF-YC6 transgenic plants (Figure 10G–L). Thus, we speculate that overexpression of PtoNF-YC6 and PtoNF-YC8 genes can upregulate the expression level of AtCO, and then activate the downstream expression of flowering genes such as AtFT, and ultimately advance flowering in Arabidopsis. Meanwhile, we also analyzed the expression levels of flowering-related genes such as PtoCO, PtoFT, PtoSOC, PtoAP1, and PtoAGL by qRT-PCR. The analysis results showed that PtoNF-YC6 and PtoNF-YC8 were similar, and PtoCO, PtoFT, PtoAP1, and PtoAGL were generally up-regulated in transgenic P. tomentosa lines, especially 6–7#, 6–12#, 6–19#, and 8–17# (Figures S6B–F and S7B–F).
Figure 10.
Analysis of the expression patterns of flowering time-related genes in regulated PtoNF-YC6 and PtoNF-YC8 transgenic Arabidopsis. (A–F) represent the expression levels of AtCO, AtFT, AtAP1, AtAGL, AtSEP and AtSOC in PtNF-YC6 transgenic Arabidopsis, respectively. (G–L) represent the expression levels of AtCO, AtFT, AtAP1, AtAGL, AtSEP and AtSOC in PtNF-YC8 transgenic Arabidopsis, respectively. * (p < 0.05) and ** (p < 0.01) indicate significant differences.
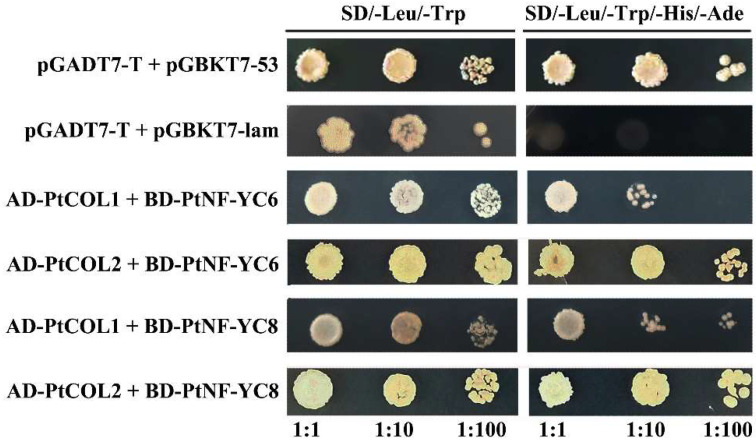
CO plays an important role in photoperiod regulation of the flowering pathway of Arabidopsis [40]. Studies have shown that PtoCOL1 and PtoCOL2 have similar expression patterns to AtCOL1, AtCOL2, and AtCO in Arabidopsis. Meanwhile, the genetic relationship also showed that PtCOL1/2 and AtCOL1, AtCOL2, and AtCO protein form a branch [41]. Therefore, PtoCOL1/2 may have similar functions to AtCOL1, AtCOL2, and AtCO. We further used the Y2H system to verify the interaction between PtoNF-YC6/8 and PtoCO1/CO2 proteins. The results indicated that pGADT7-PtoCOL1 and pGBKT7-PtoNF-YC6/8, as well as the combination of pGADT7-PtoCOL2 and pGBKT7-PtoNF-YC6/8, can grow normally on SD-Trp-Leu-His-Ade medium (Figure 11). Thus, we speculate that PtoNF-YC6 and PtoNF-YC8 can promote flowering by interacting with PtoCOL1 and PtoCOL2 proteins.
Figure 11.
Y2H analysis of the interactions between PtoNF-YCs and PtoCOLs; 1:1, 1:10, and 1:100 represent the interaction of undiluted bacterial solution, 10-fold dilution and 100-fold dilution, respectively.
4. Discussion
The NF-Y gene has been isolated and analyzed in a variety of plants. We previously identified the poplar NF-Y gene family through bioinformatics methods, and initially screened four PtNF-YC genes related to flowering [16]. In this study, four PtoNF-YC genes were cloned from P. tomentosa TC1521. The multiple alignment analysis showed that all four members of PtoNF-YC have three α-helix structures and αC structures, and are highly conserved with AtNF-YC3 and AtNF-YC9 proteins (Figure 1A,B). In addition, the phylogenetic analysis showed that all NF-YC proteins were divided into two groups, while the four members of PtoNF-YC formed a branch with dicot plants and were more closely related to cotton and alfalfa (Figure 1C). Phylogenetic analysis showed that after the differentiation of angiosperms into monocots and dicots plants, many gene families have undergone gene duplication events [42], resulting in various differences in many aspects such as flower formation and flowering time. Although research has investigated the floral organs and flowering time of dicot plants, little is known about the regulation mechanism of flower development in poplar, an important dicot plant and woody plant model. Therefore, we studied the function of the PtoNF-YC gene of P. tomentosa, providing an important theoretical basis for improving the molecular regulation of flowering in dicots and woody plants.
The subcellular localization of the NF-Y gene has been reported in many species, and has indicated that the protein is mainly localized in the nucleus. For instance, nuclear localization has been observed for the PdNF-YB protein [43] and the PtNF-YA6 and PtNF-YA9 proteins of poplar [29]. However, NF-Y proteins are also localized elsewhere. For example, the Cdt-NF-YC1 fusion protein is localized in the nucleus and the periphery of the cell [43], and the TaNF-YA10 protein of wheat is localized in the nucleus and cytoplasm [44]. Recent studies have shown that poplar NF-YA3 is localized in the nucleus and cell membrane under normal conditions, but when exposed to external stress, NF-YA3 mainly accumulates in the nucleus [45]. In this study, the four pSuper1300-GFP-PtoNF-YC5/6/7/8 proteins were localized in the nucleus and cell membrane (Figure 3). However, in PtoNF-YC6/8 transgenic Arabidopsis, the fluorescence signal was only located in the nucleus and guard cells (Figure S5). In addition, the CO protein is localized in the nucleus. Thus, we speculate that PtoNF-YC6/8 mainly accumulates in the cell nucleus and interacts with CO protein to induce the expression of downstream flowering genes and regulate flowering.
To further study the role of PtoNF-YC in flowering regulation, we genetically transformed the PtoNF-YC6 and PtoNF-YC8 genes. Because poplar has a long juvenile period and Arabidopsis as a model plant has the characteristics of a short lifecycle and mature genetic transformation system, there is a need to study the gene function of species with a long juvenile period. The two genes PtoNF-YC6 and PtoNF-YC8 were transformed into Arabidopsis, and their phenotypes were observed and counted. We observed that the transgenic Arabidopsis with PtoNF-YC6 and PtoNF-YC8 showed early flowering (Figure 4), consistent with the expected results. AtNF-YC3/4/9 and AtNF-YB2/3 first form a dimer, and then complex with CO in the nucleus, thereby promoting Arabidopsis early flowering [22]. In addition, AtNF-YC2, the first AtNF-YC member isolated from plants, can upregulate FT expression, thereby promoting early flowering in Arabidopsis [46].Tomato HAP5a can also trigger early flowering in Arabidopsis [47]. Moreover, the five members of TaNF-YC5/8/9/11/12 in wheat are regulated by light signals to participate in the regulation of flowering time [48]. In general, homologous genes usually have the same or similar functions. Here, we further analyzed the expression levels of genes related to the flowering pathway of PtoNF-YC6 and PtoNF-YC8 transgenic Arabidopsis plants, and observed significantly upregulated expression levels of AtCO, AtFT, AtAP1, AtAGL, and other related genes (Figure 10). This suggests that PtoNF-YC6 and PtoNF-YC8 not only interact with CO to promote target gene expression, but also promote CO expression itself in A. thaliana. Therefore, it is further speculated that PtoNF-YC6 and PtoNF-YC8 have similar regulatory mechanisms to AtNF-YC3, AtNF-YC4, and AtNF-YC9 in flowering regulation. In addition, studies have shown that in Arabidopsis, NF-Y can regulate plant flowering through aging and gibberellic acid pathways [23,28], but whether poplar NF-Y members play a role in these pathways needs to be further explored.
Poplar has been regarded as the model plant among woody plants. As an important native tree species in China, P. tomentosa plays an important role in rural greening and ecological protection. P. tomentosa has a long juvenile phase, which seriously affects the poplar breeding cycle. Therefore, it is very important to choose P. tomentosa as the research object to study the molecular mechanism of its flowering regulation, and it also provides a reference value for the flowering regulation of other woody plants. In this study, transgenic PtoNF-YC6 and PtoNF-YC8 plants were also obtained, and it was observed that transgenic P. tomentosa plants grew better than WT plants, which may have caused the transgenic plants to enter the reproductive growth stage earlier. CO, as an important transcription factor in the photoperiod response pathway, is usually up-regulated under long-day conditions, further promoting the expression of FT and activating the key gene AP1 in floral meristems, and finally making plants flower early [40]. In this study, the expression level of PtoCO gene was up-regulated 2–7 times in both PtoNF-YC6 and PtoNF-YC8 transgenic lines, and key flowering integrons such as PtoFT and PtoAGL and key genes of floral meristem in transgenic lines are also up-regulated (Figures S6 and S7), indicating that this will have an impact on the flowering time and flowering development of P. tomentosa. In addition, Y2H experiments also showed that PtoCOL1 and PtoCOL2 proteins can interact with PtoNF-YC6 and PtoNF-YC8 proteins (Figure 11). Research showed that PtoCOL1 and PtoCOL2 have similar expression patterns and are closely related to AtCOL1, AtCOL2, and AtCO in Arabidopsis [41,49]. Therefore, we further speculated that PtoNF-YC6 and PtoNF-YC8 are similar to AtNF-YC3, AtNF-YC4, and AtNF-YC9 in flowering regulation. The regulation mechanism also involves interactions with the CO protein, which then regulates the expression of downstream flowering genes, and ultimately promotes flowering (Figure 12). However, the obtained transgenic P. tomentosa plants did not show the expected early flowering (Figure 8 and Figure 9). There are three possible reasons for this. First, NF-Y transcription factors are important regulators of epigenetic marks that control flowering. Second, as a perennial woody plant, poplar has a long juvenile period. Third, NF-Y usually functions in a trimeric form. Therefore, whether PtoNF-YA and PtoNF-YB are involved in the formation of the PtoNF-Y complex that regulates poplar flowering requires further analysis through epigenetics and proteomics [50,51]. In addition, whether the polymer formed by the PtoNF-YC protein of poplar is bound to the promoter region of key flowering genes requires further analysis to clarify the downstream genes and specific mechanisms regulated by PtoNF-YC.
Figure 12.
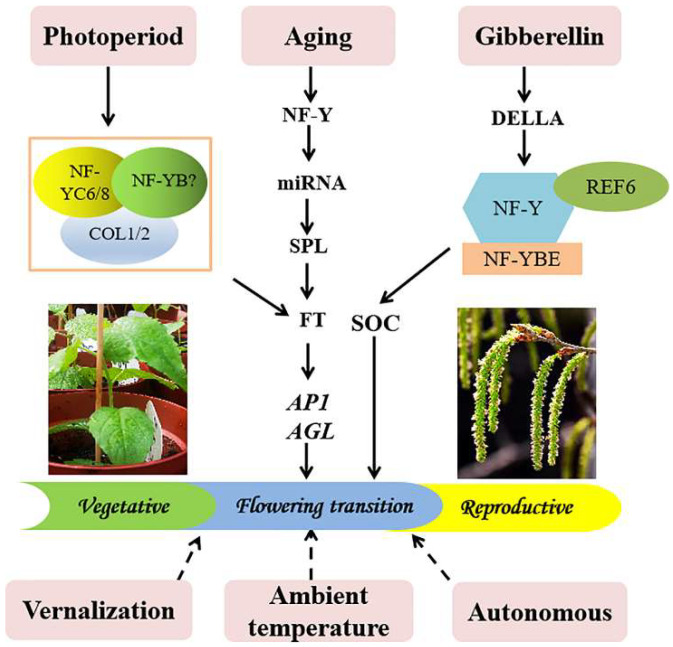
Flowering time network regulation by NF-Y.
5. Conclusions
In this study, four PtoNF-YC genes were cloned from P. tomentosa, and their characterization and function were analyzed. Overall, our results showed that although these genes are highly similar in sequence, there are some differences in their functions. In addition, the mechanism of PtoNF-YC6 and PtoNF-YC8 involved in plant flowering regulation was analyzed, laying a theoretical foundation for follow-up studies on the molecular regulation network of poplar NF-Y regulating flowering, and providing a reference for research on the flowering regulation of other woody plants.
Acknowledgments
All of experiments in this study had been completed in National Engineering Research Center of Tree Breeding and Ecological Restoration, College of Biological Sciences and Technology, Beijing Forestry University.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23063116/s1.
Author Contributions
J.L. and X.A. designed the experiments and wrote the manuscript. J.L., K.G., Y.X., S.H. and B.G. performed the experiments. J.L., D.M. and X.Y. analyzed the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China National Key R&D Program during the 14th Five-year Plan Period “Forest Tree Genome Editing Technology” (2021YFD2200101), and the National Natural Science Foundation of China (No. 31870652 and No.31570661).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have declared no conflicting or competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hsu C.Y., Adams J.P., Kim H., No K., Ma C., Strauss S.H., Drnevich J., Vandervelde L., Ellis J.D., Rice B.M., et al. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc. Natl. Acad. Sci. USA. 2011;108:10756–10761. doi: 10.1073/pnas.1104713108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An X.M., Wang D.M., Wang Z.L., Li B., Bo W.H., Cao G.L., Zhang Z.Y. Isolation of a LEAFY homolog from Populus tomentosa: Expression of PtLFY in P. tomentosa floral buds and PtLFY-IR-mediated gene silencing in tobacco (Nicotiana tabacum) Plant Cell Rep. 2011;30:89–100. doi: 10.1007/s00299-010-0947-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang R., Zhu L., Zhang Y., Fan J., Li L. Genome-wide analysis of poplar NF-YB gene family and identified PtNF-YB1 important in regulate flowering timing in transgenic plants. BMC Plant Biol. 2019;19:251. doi: 10.1186/s12870-019-1863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z., Rao P., Yang X., Su X., Zhao T., Gao K., Yang X., An X. A Global view of transcriptome dynamics during male floral bud development in Populus tomentosa. Sci. Rep. 2018;8:722. doi: 10.1038/s41598-017-18084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucher P., Trifonov E.N. CCAAT box revisited: Bidirectionality, location and context. J. Biomol. Struct. Dyn. 1988;5:1231–1236. doi: 10.1080/07391102.1988.10506466. [DOI] [PubMed] [Google Scholar]
- 6.Roberto M. A survey of 178 NF-Y binding CCAAT boxes. Nucleic Acids Res. 1998;5:1135–1143. doi: 10.1093/nar/26.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNabb D.S., Xing Y., Guarente L. Cloning of yeast HAP5: A novel subunit of a heterotrimeric complex required for CCAAT binding. Genes Dev. 1995;9:47–58. doi: 10.1101/gad.9.1.47. [DOI] [PubMed] [Google Scholar]
- 8.Edwards D., Murray A.H., Smith A.G. Multiple genes encoding the conserved CCAAT-Box transcription factor complex are expressed in Arabidopsis. Plant Physiol. 1998;117:1015–1022. doi: 10.1104/pp.117.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruyuma K., Todaka D., Mizoi J., Yoshida T., Kidokoro S., Matsukura S., Takasaki H., Sakurai T., Yamamoto Y.Y., Yoshiwawa K. Identification of Cis-acting promoter elements in cold and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA Res. 2011;19:37–49. doi: 10.1093/dnares/dsr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laloum T., Mita D.S., Gamas P., Baudin M., Niebel A. CCAAT-box binding transcription factors in plants: Y so many? Trends Plant Sci. 2013;18:157–166. doi: 10.1016/j.tplants.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Li X.Y., Huijsduijnen R., Mantovani R., Benoist C., Mathis D. Intron-exon organization of the NF-Y genes. Tissue-specific splicing modifies an activation domain. J. Biol. Chem. 1992;267:8984. doi: 10.1016/S0021-9258(19)50377-5. [DOI] [PubMed] [Google Scholar]
- 12.Siefers N., Dang K.K., Kumimoto R.W., Bynum W.E., IV, Tayrose G., Holt B.F., III Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 2008;149:625–641. doi: 10.1104/pp.108.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thirumurugan T., Ito Y., Kubo T., Serizawa A., Kurata N. Identification, characterization and interaction of HAP family genes in rice. J. Recept. Res. 2008;7:279–289. doi: 10.1007/s00438-007-0312-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z., Li X., Zhang C., Zou H., Wu Z. Isolation, structural analysis, and expression characteristics of the maize nuclear factor Y gene families. Biochem. Biophys. Res. Commun. 2016;478:752–758. doi: 10.1016/j.bbrc.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Li K., Ju Z., Cao D., Fu D., Zhu H., Zhu B., Luo Y. Genome-wide analysis of tomato NF-Y factors and their role in fruit ripening. BMC Genom. 2016;17:36. doi: 10.1186/s12864-015-2334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Gao K., Khan W.U., Yang X., Yang X., Zhao T., Chen Z., An X. Genome-wide analysis of the poplar NF-Y gene family and its expression in floral bud development of Populus tomentosa. Trees. 2019;34:285–296. doi: 10.1007/s00468-019-01917-3. [DOI] [Google Scholar]
- 17.Guo Y., Niu S., El-Kassaby Y.A., Li W. Transcriptome-wide isolation and expression of NF-Y gene family in male cone development and hormonal treatment of Pinus tabuliformis. Physiol. Plant. 2021;171:34–47. doi: 10.1111/ppl.13183. [DOI] [PubMed] [Google Scholar]
- 18.Calvenzani V., Testoni B., Gusmaroli G., Lorenzo M., Gnesutta N., Petroni K., Mantovani R., Tonelli C. Interactions and CCAAT-Binding of Arabidopsis thaliana NF-Y Subunits. PLoS ONE. 2012;7:e42902. doi: 10.1371/journal.pone.0042902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petroni K., Kumimoto R.W., Gnesutta N., Calvenzani V., Fornari M., Tonelli C., Holt B.F., Mantovani R. The promiscuous life of plant NUCLEAR FACTOR Y transcription factors. Plant Cell. 2012;24:4777–4792. doi: 10.1105/tpc.112.105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fornari M., Calvenzani V., Masiero S., Tonelli C., Petroni K.J.P.O. The Arabidopsis NF-YA3 and NF-YA8 genes are functionally redundant and are required in early embryogenesis. PLoS ONE. 2013;8:e82043. doi: 10.1371/journal.pone.0082043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Hu P., Huang M., Tang Y., Li Y., Li L., Hou X. The NF-YC-RGL2 module integrates GA and ABA signalling to regulate seed germination in Arabidopsis. Nat. Commun. 2016;7:12768. doi: 10.1038/ncomms12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumimoto R.W., Zhang Y., Siefers N., Holt B.F. NF-YC3, NF-YC4 and NF-YC9 are required for CONSTANS-mediated, photoperiod-dependent flowering in Arabidopsis thaliana. Plant J. 2010;63:379–391. doi: 10.1111/j.1365-313X.2010.04247.x. [DOI] [PubMed] [Google Scholar]
- 23.Wei Q., Ma C., Xu Y., Wang T., Chen Y., Lü J., Zhang L., Jiang C.-Z., Hong B., Gao J. Control of chrysanthemum flowering through integration with an aging pathway. Nat. Commun. 2017;8:829. doi: 10.1038/s41467-017-00812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Li G., Li C., Zhang C., Cui L., Ai G., Wang X., Zheng F., Zhang D., Larkin R.M., et al. NF-Y plays essential roles in flavonoid biosynthesis by modulating histone modifications in tomato. New Phytol. 2021;229:3237–3252. doi: 10.1111/nph.17112. [DOI] [PubMed] [Google Scholar]
- 25.Li W.X., Oono Y., Zhu J., He X.J., Wu J., Iida K., Lu X.Y., Cui X., Jin H., Zhu J.K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell. 2008;20:2238–2251. doi: 10.1105/tpc.108.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Xu W., Chen Z., Han B., Haque M.E., Liu A. Gene structure, expression pattern and interaction of Nuclear Factor-Y family in castor bean (Ricinus communis) Planta. 2018;247:559–572. doi: 10.1007/s00425-017-2809-2. [DOI] [PubMed] [Google Scholar]
- 27.Sato H., Suzuki T., Takahashi F., Shinozaki K., Yamaguchi-Shinozaki K.J.P.P. NF-YB2 and NF-YB3 have functionally diverged and differentially induce drought and heat stress-specific genes. Plant Physiol. 2019;80:1677–1690. doi: 10.1104/pp.19.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou X. Nuclear factor Y-mediated H3K27me3 demethylation of the SOC1 locus orchestrates flowering responses of Arabidopsis. Nat. Commun. 2014;5:4601. doi: 10.1038/ncomms5601. [DOI] [PubMed] [Google Scholar]
- 29.Lian C., Li Q., Yao K., Zhang Y., Meng S., Yin W., Xia X. Populus trichocarpa PtNF-YA9, a multifunctional transcription factor, regulates seed germination, abiotic stress, plant growth and development in Arabidopsis. Front. Plant Sci. 2018;9:954. doi: 10.3389/fpls.2018.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., Gao K., Lei B., Zhou J., Guo T., An X. Altered sucrose metabolism and plant growth in transgenic Populus tomentosa with altered sucrose synthase PtSS3. Transgenic Res. 2020;29:125–134. doi: 10.1007/s11248-019-00184-9. [DOI] [PubMed] [Google Scholar]
- 31.Singh V.K., Mangalam A., Dwivedi S., Naik S.J.B. Primer premier: Program for design of degenerate primers from a protein sequence. Biotechniques. 1998;24:318–319. doi: 10.2144/98242pf02. [DOI] [PubMed] [Google Scholar]
- 32.Liu D., Li J., Lu J., Tian B., Liu X., Yang G., Pei Y. Cloning and functional analysis of four O-Acetylserine (thiol) lyase family genes from foxtail millet. Plant Physiol. Biochem. 2019;139:325–332. doi: 10.1016/j.plaphy.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Krogh A., Larsson B., Heijne G., Sonnhammer E.L.L. Bioinformatics SJJoMB. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 34.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;7:1870. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 2010;6:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q., Zhang Z.Y., Lin S.Z., Zheng H.Q., Lin Y.Z., An X.M., Li Y., Li H.X. Characterization of resistance gene analogs with a nucleotide binding site isolated from a triploid white poplar. Plant Biol. 2008;10:310–322. doi: 10.1111/j.1438-8677.2008.00029.x. [DOI] [PubMed] [Google Scholar]
- 38.Zheng H., Lin S., Zhang Q., Lei Y., Zhang Z. Functional analysis of 5′untranslated region of a TIR-NBS-encoding gene from triploid white poplar. Mol. Gen. Genet. 2009;282:381–394. doi: 10.1007/s00438-009-0471-5. [DOI] [PubMed] [Google Scholar]
- 39.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Putterill J., Robson F., Lee K., Simon R., Coupland G.J.C. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 41.Li J., Gao K., Yang X., Khan W.U., Guo B., Guo T., An X. Identification and characterization of the CONSTANS-like gene family and its expression profiling under light treatment in Populus. Int. J. Biol. Macromol. 2020;161:999–1010. doi: 10.1016/j.ijbiomac.2020.06.056. [DOI] [PubMed] [Google Scholar]
- 42.Ferrandiz C., Gu Q., Martienssen R., Yanofsky M.F.J.D. Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development. 2000;127:725–734. doi: 10.1242/dev.127.4.725. [DOI] [PubMed] [Google Scholar]
- 43.Miao C., Zhao Y., Zhuo C., Lu S., Guo Z.J.P.B.J. Overexpression of a NF-YC transcription factor from bermudagrass confers tolerance to drought and salinity in transgenic rice. Plant Biotechnol. J. 2015;13:482–491. doi: 10.1111/pbi.12270. [DOI] [PubMed] [Google Scholar]
- 44.Ma X., Li C., Wang M.J.B. Wheat NF-YA10 functions independently in salinity and drought stress. Bioengineered. 2015;6:245–247. doi: 10.1080/21655979.2015.1054085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu R., Wu M., Liu H.L., Gao Y.M., Chen J., Yan H.W., Xiang Y. Genome-wide identification and expression analysis of the NF-Y transcription factor family in Populus. Physiol. Plant. 2021;171:309–327. doi: 10.1111/ppl.13084. [DOI] [PubMed] [Google Scholar]
- 46.Hackenberg D., Keetman U., Grimm B. Homologous NF-YC2 subunit from Arabidopsis and tobacco is activated by photooxidative stress and induces flowering. Int. J. Mol. Sci. 2012;13:3458–3477. doi: 10.3390/ijms13033458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Naim O., Eshed R., Parnis A., Teper-Bamnolker P., Shalit A., Coupland G., Samach A., Lifschitz E. The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J. 2006;46:462–476. doi: 10.1111/j.1365-313X.2006.02706.x. [DOI] [PubMed] [Google Scholar]
- 48.Stephenson T.J., McIntyre C.L., Collet C., Xue G.P. TaNF-YC11, one of the light-upregulated NF-YC members in Triticum aestivum, is co-regulated with photosynthesis-related genes. Funct. Integr. Genom. 2010;10:265–276. doi: 10.1007/s10142-010-0158-3. [DOI] [PubMed] [Google Scholar]
- 49.Cheng X.F., Wang Z.Y. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005;43:758–768. doi: 10.1111/j.1365-313X.2005.02491.x. [DOI] [PubMed] [Google Scholar]
- 50.Zicola J., Liu L., Tänzler P., Turck F. Targeted DNA methylation represses two enhancers of FLOWERING LOCUS T in Arabidopsis thaliana. Nat. Plants. 2019;5:300–307. doi: 10.1038/s41477-019-0375-2. [DOI] [PubMed] [Google Scholar]
- 51.Myers Z.A., Holt B.F. NUCLEAR FACTOR-Y: Still complex after all these years? Curr. Opin. Plant Biol. 2018;45:96–102. doi: 10.1016/j.pbi.2018.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.