Abstract
Background: Since the outbreak of the COVID-19 pandemic, a growing number of evidence suggests that COVID-19 presents sex-dependent differences in clinical course and outcomes. Nevertheless, there is still an unmet need to stratify the risk for poor outcome at the beginning of hospitalization. Since individual C2HEST components are similar COVID-19 mortality risk factors, we evaluated sex-related predictive value of the score. Material and Methods: A total of 2183 medical records of consecutive patients hospitalized due to confirmed SARS-CoV-2 infections were analyzed. Subjects were assigned to one of two of the study arms (male vs. female) and afterward allocated to different stratum based on the C2HEST score result. The measured outcomes included: in-hospital-mortality, three-month- and six-month-all-cause-mortality and in-hospital non-fatal adverse clinical events. Results: The C2HEST score predicted the mortality with better sensitivity in female population regarding the short- and mid-term. Among secondary outcomes, C2HEST-score revealed predictive value in both genders for pneumonia, myocardial injury, myocardial infarction, acute heart failure, cardiogenic shock, and acute kidney injury. Additionally in the male cohort, the C2HEST value predicted acute liver dysfunction and all-cause bleeding, whereas in the female arm-stroke/TIA and SIRS. Conclusion: In the present study, we demonstrated the better C2HEST-score predictive value for mortality in women and illustrated sex-dependent differences predicting non-fatal secondary outcomes.
Keywords: risk factors, COVID-19, SARS-CoV-2, predicting value, mortality, C2HEST score, gender differences
1. Introduction
Since the outbreak in 2019 in China of the coronavirus disease (COVID-19), the pandemic has revealed an unprecedented impact on the global health care system, with over 450 million confirmed cases resulting in approximately 6 million of deaths reported worldwide [1]. From the initial phase of the pandemic, a growing number of evidence [2] suggests that COVID-19 presents significant sex-dependent differences in clinical course and mortality.
The clinical manifestation of COVID-19 remains unpredictable and varies from asymptomatic to severe or lethal [3,4,5]. Hence, there is an urgent need to introduce a simple and fast triage tool to clinical practice aimed at supporting the decision-making process for the clinicians in terms of appropriate management and optimized use of limited resources.
The C2HEST score was originally designed [6] to predict the potential development of atrial fibrillation (AF) in the general population. Lately, a growing body of evidence has appeared, illustrating that the C2HEST score can predict poor outcomes of patients in severe clinical conditions. Our previous study demonstrated the usefulness of the C2HEST-score in predicting the adverse COVID-19-outcomes in hospitalized subjects with type 2 diabetes mellitus. Since male sex is postulated to be an independent risk factor of an unfavorable COVID-19 outcome, we aimed to assess the sex-dependent predictive value of the C2HEST-score.
2. Materials and Methods
2.1. Study Design and Population
The study population consisted of 2183 consecutive patients with confirmed by reverse transcription-polymerase chain reaction (RT-PCR) infection of SARS-CoV-2 admitted to the Medical University COVID-19 Center. All subjects were hospitalized between February 2020 and June 2021. The study protocol has been approved by the Institutional Review Board and Ethics Committee at the Wroclaw Medical University, Wroclaw, Poland (No: KB-444/2021). All medical data were fully anonymized and retrospectively analyzed. Due to the character of the study protocol written informed consent from participants was not required. Subjects were assigned to one of two of the study arms male vs. female. Subsequently, all patients were assigned into one C2HEST score stratum. The C2HEST score value was calculated depending on originally proposed variables; coronary artery disease (CAD) (1 point), chronic obstructive pulmonary disease (COPD) (1 point), hypertension (1 point), elderly (age ≥ 75 years, 2 points), systolic heart failure (HF) (2 points), and thyroid disease (1 point). Based on the calculated score subjects were allocated to one of three stratum -low-risk 0 or 1 point, medium-risk 2 or 3 points, and high-risk 4 and more points.
2.2. Follow-Up and Outcomes
The primary clinical outcome was an in-hospital, three-month-, and six-month-all-cause mortality. Other clinical outcomes focused on in-hospital: end of hospitalization other than death (discharge, deterioration or recovery with subsequent transfer to another hospital) advanced mechanical ventilation support, shock, multiple organ dysfunction syndrome (MODS), systemic inflammatory response syndrome (SIRS), sepsis. Also, other clinical features were collected symptomatic bleeding, pneumonia, pulmonary embolism, acute heart failure, myocardial injury, stroke, acute kidney injury, acute liver dysfunction.
2.3. Statistical Analysis
Statisticians with experience in medical academic research performed the analyses to this manuscript. The R language version 4.0.4 with additional packages-pROC and time-ROC [7], survival [8], coin [9], and odds ratio was used for the purpose of data analysis [10] A level of 0.05 was set as significance value.
Descriptive data regarding categorical variables are shown as numbers and percentages, whereas for numerical variables as mean with standard deviation, range (minimum-maximum) along with the number of non-missing values. The omnibus and chi-square tests were performed for categorical variables which exceeded five expected cases in each group. The Fisher exact test was performed for subjects with fewer cell counts. The Welch’s ANOVA was set up for continuous variables in order to adjust for unequal variances between the risk-strata and sample size large sufficient for appropriateness of asymptotic results. For continuous variables, the Games-Howell’s variant of Tukey correction was performed as a part of a post-hoc analysis. On the other hand, for categorical variables, the post-hoc test was analogous to the omnibus test. However, it was performed in subgroups with a Bonferroni correction. Due to a fact that the in-hospital mortality along with the all-cause mortality were available as right-censored data, the time-dependent ROC analysis with inverse probability of censoring weighting (IPCW) was used to estimate them. The time-dependent area under the curve (AUC) was used to assess the C2HEST score and additionally a confirmation of differences in survival curves among risk strata was obtained by a Log-rank test. Proportional hazard assumption was verified using the Grambsch-Therneau test. During analysis of the hazard ratio (HR) in the C2HEST score, its components, as well as risk strata, a Cox proportional hazard model was used. Dichotomic nature of secondary outcomes resulted in the use of a logistic regression model during their analysis. In order to assess predictive capability, the classical receiver operating characteristic (ROC) analysis with an AUC measure was performed. Odds ratio (OR) was presented as a size effect for the influence of the C2HEST score, its components and risk strata.
3. Results
3.1. Baseline Demographical and Clinical Features of the Studied Population
The study population was composed of 2183 subjects at mean age 60.1 ±18.8 [17–100] A total of 1101 women at mean age 59.3 ± 21.1 [17–100] were enrolled to this study, who were subsequently assigned to the low-risk n = 682 subjects, medium-risk n = 284 patients, and high-risk n = 135 C2HEST strata, respectively. Simultaneously, a total of 1082 males at mean age of 60.8 ± 16.1 [17–99], were assigned to the low-risk (n = 735), medium-risk (n = 208) and high-risk(n = 139). The baseline clinical data of both study cohorts is presented in Table 1. In both cohorts, higher C2HEST risk was related to a higher number of comorbidities and more advanced age.
Table 1.
Baseline demographics and clinical characteristics.
| Variables Units |
Low Risk [0–1] |
Medium [2–3] |
High Risk [≥4] |
OMNIBUS p-Value |
p Value for Post-Hoc Analysis |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females N = 682 |
Males N = 735 |
Females N = 284 |
Males N = 208 |
Females N = 135 |
Males N = 139 |
Females | Males | Females | Males | |
| Demographics | ||||||||||
|
Age, years mean ± SD/min-max |
47.8 ± 17.1 17–74 |
54.2 ± 14.0 17–74 |
76.7 ± 12.0 29–100 |
74.0 ± 1.2 37–99 |
81.0 ± 8.7 47–100 |
76.2 ± 9.4 38–92 |
<0.0001 | <0.0001 |
0.0 a,b
0.0001 c |
0.0 a <0.0001 b 0.115 c |
|
Age ≥ 65 years n/n(%) |
165 (24.2) |
211 (28.7) |
247 (87.0) |
172 (82.7) |
129 (95.6) |
123 (88.5) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0339 c |
<0.0001 a,b 0.5515 c |
|
BMI, kg/m2 mean ± SD/min-max/N |
28.3 ±5.3 17.1–45.7 199 |
28.2 ± 4.8 15.4–49.4 198 |
30.1 ±5.9 18.6–47.8 48 |
28.3 ±5.2 20.9–46.7 42 |
27.1 ±6.7 16.4–45.8 17 |
28.0 ± 5.6 17.3–48.2 50 |
0.1255 | 0.9609 | N/A | N/A |
| Co-morbidities | ||||||||||
|
Hypertension n/n(%) |
179 (26.2) |
236 (32.1) |
213 (75.0) |
144 (69.2) |
126 (93.3) |
123 (88.5) |
<0.0001 | <0.0001 | <0.0001 a,b,c |
<0.0001 a,b
0.0002 c |
|
Dyslipidaemia n/n(%)/N |
74 (59.2) 125 |
138 (57.3) 241 |
37 (44.6) 83 |
32 (39.0) 82 |
29 (48.3) 60 |
17 (29.8) 57 |
0.0932 | 0.00011 | N/A |
0.0191 a 0.001 b 1.0 c |
|
Atrial fibrilation/flutter n/n(%) |
14 (2.1) |
35 (4.8) |
60 (21.1) |
46 (22.1) |
65 (48.1) |
70 (50.4) |
<0.0001 | <0.0001 | <0.0001 a,b,c | <0.0001 a,b,c |
|
Previous coronary revascularisation n/n(%) |
0 (0.0) |
6 (0.8) |
9 (3.2) |
28 (13.5) |
35 (25.9) |
76 (54.7) |
<0.0001 | <0.0001 | <0.0001 a,b,c | <0.0001 a,b,c |
|
Previous myocardial infarction n/n(%) |
1 (0.1) |
10 (1.4) |
18 (6.3) |
45 (21.6) |
37 (27.4) |
80 (57.6) |
<0.0001 | <0.0001 | <0.0001 a,b,c | <0.0001 a,b,c |
|
Heart failure n/n(%) |
0 (0.0) |
0 (0.0) |
20 (7.0) |
33 (15.9) |
91 (67.4) |
111 (79.9) |
<0.0001 | <0.0001 | <0.0001 a,b,c | <0.0001 a,b,c |
|
Moderate/severe valvular heart disease or previous valve heart surgery n/n(%) |
7 (1.0) |
6 (0.8) |
14 (4.9) |
18 (8.7) |
26 (19.3) |
25 (18.0) |
<0.0001 | <0.0001 |
0.0012 a
<0.0001 b,c |
<0.0001 a,b
0.0467 c |
|
Peripheral artery disease n/n(%) |
7 (1.0) |
19 (2.6) |
14 (4.9) |
17 (8.2) |
11 (8.1) |
32 (23.0) |
<0.0001 | <0.0001 |
0.0012 a <0.0001 b 0.5813 c |
0.0014 a
<0.0001 b 0.0006 c |
|
Previous stroke/TIA n/n(%) |
17 (2.5) |
30 (4.1) |
33 (11.6) |
26 (12.5) |
24 (17.8) |
34 (24.5) |
<0.0001 | <0.0001 |
<0.0001 a,b 0.3522 c |
<0.0001 a,b
0.0183 c |
|
Chronic kidney disease n/n(%) |
33 (4.8) |
37 (5.0) |
26 (9.2) |
44 (21.2) |
39 (28.9) |
52 (37.4) |
<0.0001 | <0.0001 |
0.0486 a
<0.0001 b,c |
<0.0001 a,b
0.0042 c |
|
Haemodialysis n/n(%) |
11 (1.6) |
8 (1.1) |
5 (1.8) |
15 (7.2) |
8 (5.9) |
11 (7.9) |
0.01467 | <0.0001 | 1.0 a 0.0204 b 0.0963 c |
<0.0001 a,b 1.0 c |
|
Asthma n/n(%) |
32 (4.7) |
22 (3.0) |
17 (6.0) |
3 (1.4) |
7 (5.2) |
4 (2.9) |
0.7053 | 0.4996 | N/A | N/A |
|
COPD n/n(%) |
1 (0.1) |
5 (0.7) |
9 (3.2) |
16 (7.7) |
16 (11.9) |
28 (20.1) |
<0.0001 | <0.0001 |
0.0003 a
<0.0001 b 0.0041 c |
<0.0001 a,b
0.0035 c |
|
Hypothyroidism n/n(%) |
65 (9.5) |
11 (1.5) |
56 (19.7) |
12 (5.8) |
52 (38.5) |
12 (8.6) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0002 c |
0.004 a <0.0001 b 1.0 c |
|
Hyperthyroidism n/n(%) |
3 (0.4) |
1 (0.1) |
7 (2.5) |
3 (1.4) |
3 (2.2) |
4 (2.9) |
0.0083 | 0.0009 |
0.0272 a 0.1807 b 1.0 c |
0.1065 a 0.0081 b 1.0 c |
Continuous variables are presented as: mean ± SD, range (minimum–maximum) and number of non-missing values. Categorized variables are presented as: a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: N, valid measurements; n, number of patients with parameter above cut-off point; SD, standard deviation; BMI, body mass index; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; OMNIBUS, analysis of variance; N/A, non-applicable; a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red color text = statistically significant values.
Data regarding the relationship between the C2HEST score result and treatment applied before hospitalization is shown in the Table 2. In the both cohorts along with increased C2HEST score, we observed an increasing prevalence drug commonly used in cardiovascular disorders such as angiotensin-converting-enzyme inhibitors (ACEI), mineralocorticoid receptor antagonists (MRA), b-blockers, calcium channel blockers, diuretics, statins, vitamin K antagonists (VKA), novel oral anticoagulants (NOAC), acetylsalicylic acid, P2Y12 inhibitor, metformin, and insulin.
Table 2.
Baseline characteristics of the study cohort-treatment applied before hospitalization.
| Variables Units |
Low Risk [0–1] |
Medium [2–3] |
High Risk [≥4] |
OMNIBUS p-Value |
p Value for Post-Hoc Analysis |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females N = 682 |
Males N = 735 |
Females N = 284 |
Males N = 208 |
Females N = 135 |
Males N = 139 |
Females | Males | Females | Males | |
| Treatment applied before hospitalization | ||||||||||
|
ACEI n/n(%) |
47 (6.9) |
69 (9.4) |
57 (20.1) |
63 (30.3) |
54 (40.0) |
62 (44.6) |
<0.0001 | <0.0001 | <0.0001 a,b,c |
<0.0001 a,b
0.0273 c |
|
ARB n/n(%) |
33 (4.8) |
43 (5.9) |
26 (9.2) |
12 (5.8) |
14 (10.4) |
16 (11.5) |
0.0087 | 0.0413 |
0.04855 a 0.0611 b 1.0 c |
1.0 a 0.0724 b 0.2546 c |
|
MRA n/n(%) |
3 (0.4) |
15 (2.0) |
13 (4.6) |
20 (9.6) |
20 (14.8) |
29 (20.9) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0021 c |
<0.0001 a,b
0.0158 c |
|
β-blocker n/n(%) |
78 (11.4) |
119 (16.2) |
102 (35.9) |
77 (37.0) |
76 (56.3) |
81 (58.3) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0004 c |
<0.0001 a,b
0.0005 c |
|
Calcium channel blocker dihydropiridines n/n(%) |
37 (5.4) |
66 (9.0) |
48 (16.9) |
36 (17.3) |
34 (25.2) |
40 (28.8) |
<0.0001 | <0.0001 |
<0.0001 a,b 0.1863 c |
0.003 a
<0.0001 b 0.0493 c |
|
α-adrenergic blocker n/n(%) |
10 (1.5) |
35 (4.8) |
6 (2.1) |
28 (13.5) |
8 (5.9) |
31 (22.3) |
0.0113 | <0.0001 | 1.0 a 0.0137 b 0.2272 c |
<0.0001 a,b 0.1358 c |
|
Amiodarone n/n(%) |
1 (0.1) |
0 (0.0) |
1 (0.4) |
1 (0.5) |
0 (0.0) |
1 (0.7) |
0.6165 | 0.1027 | N/A | N/A |
|
Thiazide or thiazide-like diuretic n/n(%) |
29 (4.3) |
39 (5.3) |
36 (12.7) |
11 (5.3) |
16 (11.9) |
19 (13.7) |
<0.0001 | 0.0008 |
<0.0001 a 0.0026 b 1 c |
1.0 a 0.0017 b 0.0345 c |
|
Loop diuretic n/n(%) |
13 (1.9) |
26 (3.5) |
25 (8.8) |
40 (19.2) |
33 (24.4) |
48 (34.5) |
<0.0001 | <0.0001 | <0.0001 a,b,c | <0.0001 a,b 0.0061 c |
|
Statin n/n(%) |
40 (5.9) |
63 (8.6) |
56 (19.7) |
65 (31.3) |
49 (36.3) |
77 (55.4) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0012 c |
<0.0001 a, b, c |
|
Acetylsalicylic acid n/n(%) |
35 (5.1) |
46 (6.3) |
44 (15.5) |
51 (24.5) |
33 (24.4) |
49 (35.3) |
<0.0001 | <0.0001 |
<0.0001 a,b 0.1137 c |
<0.0001 a,b 0.1234 c |
|
The second antiplatelet drug n/n(%) |
1 (0.1) |
6 (0.8) |
5 (1.8) |
5 (2.4) |
4 (3.0) |
18 (12.9) |
0.0009 | <0.0001 |
0.0292 a 0.0094 b 1.0 c |
0.2154 a <0.0001 b 0.0007 c |
|
LMWH n/n(%) |
32 (4.7) |
42 (5.7) |
23 (8.1) |
18 (8.7) |
11 (8.1) |
15 (10.8) |
0.0674 | 0.0535 | N/A | N/A |
|
VKA n/n(%) |
4 (0.6) |
6 (0.8) |
6 (2.1) |
8 (3.8) |
10 (7.4) |
13 (9.4) |
<0.0001 | <0.0001 | 0.2172 a <0.0001 b 0.038 c |
0.0129 a <0.0001 b 0.1213 c |
|
NOAC n/n(%) |
6 (0.9) |
12 (1.6) |
22 (7.7) |
15 (7.2) |
23 (17.0) |
29 (20.9) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0207 c |
0.0002 a
<0.0001 b 0.001 c |
|
Insulin n/n(%) |
23 (3.4) |
39 (5.3) |
14 (4.9) |
15 (7.2) |
22 (16.3) |
18 (12.9) |
<0.0001 | 0.0038 | 1.0 a <0.0001 b 0.0007 c |
1.0 a 0.0047 b 0.3296 c |
|
Metformin n/n(%) |
40 (5.9) |
64 (8.7) |
35 (12.3) |
32 (15.4) |
22 (16.3) |
29 (20.9) |
<0.0001 | <0.0001 |
0.0031 a 0.0002 b 1.0 c |
0.022 a 0.0001 b 0.7261 c |
|
SGLT2 inhibitor n/n(%) |
4 (0.6) |
7 (1.0) |
4 (1.4) |
3 (1.4) |
3 (2.2) |
6 (4.3) |
0.12658 | 0.018 | N/A | 1.0 a 0.0286 b 0.4938 c |
|
Oral antidiabetics other than SGLT2 inhibitor and metformin n/n(%) |
10 (1.5) |
17 (2.3) |
20 (7.0) |
14 (6.7) |
11 (8.1) |
17 (12.2) |
<0.0001 | <0.0001 |
<0.0001 a,b 1.0 c |
0.01 a <0.0001 b 0.3507 c |
|
Proton pump inhibitor n/n(%) |
31 (4.5) |
58 (7.9) |
39 (13.7) |
36 (17.3) |
37 (27.4) |
49 (35.3) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0034 c |
0.0003 a
<0.0001 b 0.0007 c |
|
Oral corticosteroid n/n(%) |
31 (4.5) |
31 (4.2) |
17 (6.0) |
7 (3.4) |
5 (3.7) |
1 (0.7) |
0.5164 | 0.125 | N/A | N/A |
|
Immuno-suppression other than oral corticosteroid n/n(%) |
24 (3.5) |
25 (3.4) |
12 (4.2) |
10 (4.8) |
2 (1.5) |
0 (0.0) |
0.3606 | 0.0185 | N/A | 1.0 a 0.0686 b 0.0209 c |
Categorized variables are presented as: a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: N, valid measurements; n, number of patients with parameter above the cut-off point; ACEI, angiotensin-converting-enzyme inhibitors; ARBs, angiotensin receptor blockers; MRAs, mineralocorticoid receptor antagonists; LMWH, low molecular weight heparin; VKA, vitamin K antagonists; NOAC, novel oral anticoagulants; SGLT2 inhibitors, sodium glucose co-transporter-2 inhibitors; OMNIBUS, analysis of variance; N/A, non-applicable; a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red color text = statistically significant values.
Table 3 shows the sex-specific baseline characteristics of patient-reported symptoms, and vital signs during the hospital admission in the studied cohort. The female but not male cohort, had significant differences between the C2HEST strata regarding the prevalence of cough, smell dysfunction, body temperature, and systolic blood pressure, which were decreasing as the score raised. Opposite findings were observed regarding dyspnoea, heart rate, and the diastolic blood pressure.
Table 3.
Patient-reported symptoms, vital signs and abnormalities measured during physical examination at hospital admission in the studied cohort.
| VariablesUnits | Low Risk [0–1] |
Medium [2–3] |
High Risk [≥4] |
OMNIBUS p Value |
p Value for Post-Hoc Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females N = 682 |
Males N = 735 |
Females N = 284 |
Males N = 208 |
Females N = 135 |
Males N = 139 |
Females | Males | Females | Males | |
| Patient-reported symptoms | ||||||||||
|
Cough n/n(%) |
219 (32.1) |
236 (32.1) |
71 (25.0) |
53 (25.5) |
27 (20.0) |
42 (30.2) |
0.0047 | 0.1859 | 0.102 a 0.0208 b 0.9427 c |
n/A |
|
Dyspnoea n/n(%) |
244 (35.8) |
325 (44.2) |
110 (38.7) |
96 (46.2) |
63 (46.7) |
83 (59.7) |
0.0551 | 0.0035 | N/A | 1.0 a 0.0033 b 0.0538 c |
|
Chest pain n/n(%) |
49 (7.2) |
53 (7.2) |
18 (6.3) |
16 (7.7) |
11 (8.1) |
16 (11.5) |
0.7855 | 0.2237 | N/A | N/A |
|
Smell dysfunction n/n(%) |
26 (3.8) |
35 (4.8) |
3 (1.1) |
7 (3.4) |
0 (0.0) |
5 (3.6) |
0.0039 | 0.6142 | 0.0656 a 0.0414 b 1.0 c |
N/A |
|
Diarrhoea n/n(%) |
37 (5.4) |
38 (5.2) |
22 (7.7) |
11 (5.3) |
11 (8.1) |
8 (5.8) |
0.2667 | 0.9606 | N/A | N/A |
|
Nausea/Vomiting n/n(%) |
36 (5.3) |
21 (2.9) |
18 (6.3) |
9 (4.3) |
11 (8.1) |
3 (2.2) |
0.4065 | 0.4662 | N/A | N/A |
| Measured vital signs | ||||||||||
|
Body temperature, °C mean ± SD/min-max/N |
37.1 ± 0.8 35.0–40.5 416 |
37.1 ± 0.9 34.4–40.0 393 |
36.9 ± 0.9 35.8–40.0 131 |
36.9 ± 1.0 35.0–40.0 104 |
36.8 ± 0.9 35.2–40.0 63 |
37.1 ± 0.8 35.5–40.0 78 |
0.0456 | 0.3888 | 0.3 a 0.07 b 0.588 c |
N/A |
| Heart rate, beats/minute mean ± SD/min-max/N | 85.9 ± 14.6 48–150 490 |
86.9 ± 16.5 48–160 555 |
84.6 ± 17.2 50–160 217 |
83.5 ± 15.5 52–140 170 |
87.4 ± 21.3 36–170 116 |
82.3 ± 15.8 58–140 124 |
0.4159 | 0.0035 | N/A |
0.045 a 0.012 b 0.773 c |
|
Respiratory rate breaths/minute mean ± SD/min-max/N |
17.9 ± 5.9 12–50 107 |
18.9 ± 5.7 12–50 97 |
17.8 ± 3.8 12–31 34 |
19.6 ± 6.7 12–45 34 |
19.0 ± 4.1 12–29 22 |
19.6 ± 7.6 12–50 24 |
0.5185 | 0.8014 | N/A | N/A |
|
Systolic blood pressure mmHg mean ± SD/min-max/N |
128.6 ± 21.3 74–240 488 |
132.6 ± 21.1 60–220 552 |
133.2 ± 24.2 50–210 216 |
135.6 ± 26.7 50–270 169 |
135.6 ± 25.5 70–210 117 |
133.5 ± 24.0 85–200 127 |
0.004 | 0.4149 |
0.042 a 0.018 b 0.687 c |
N/A |
|
Diastolic blood pressure, mmHg mean ± SD/min-max/N |
77.4 ± 12.5 40–150 487 |
79.5 ± 12.7 40–130 550 |
77.1 ± 13.7 40–157 214 |
79.3 ± 13.5 45–150 166 |
7.5 ± 15.5 40–143 117 |
75.1 ± 15.2 40–120 127 |
0.8167 | 0.0091 | N/A | 0.986 a 0.007 b 0.034 c |
|
SpO2 on room air, % (FiO2 = 21%) mean ± SD/min-max/N |
94.4 ± 5.9 56–100 421 |
91.1 ± 7.9 48–99 393 |
90.8 ± 8.5 50–100 160 |
88.2 ± 10.9 50–99 121 |
91.2 ± 6.9 64–99 84 |
89.2 ± 9.9 50–99 83 |
<0.0001 | 0.0102 |
<0.0001 a 0.0003 b 0.934 c |
0.018 a 0.205 b 0.79 c |
| Abnormalities detected during physical examination | ||||||||||
|
Cracles n/n(%) |
62 (9.1) |
92 (12.5) |
47 (16.5) |
52 (25.0) |
30 (22.2) |
36 (25.9) |
<0.0001 | <0.0001 |
0.0038 a <0.0001 b 0.6164 c |
<0.0001 a 0.0002 b 1.0 c |
|
Wheezing n/n(%) |
32 (4.7) |
62 (8.4) |
23 (8.1) |
33 (15.9) |
32 (23.7) |
37 (26.6) |
<0.0001 | <0.0001 | 0.1611 a <0.0001 b,c |
0.0078 a <0.0001 b 0.0628 c |
|
Pulmonarycongestion n/n(%) |
70 (10.3) |
114 (15.5) |
51 (18.0) |
54 (26.0) |
37 (27.4) |
41 (29.5) |
<0.0001 | <0.0001 |
0.0044 a <0.0001 b 0.1096 c |
0.0022 a 0.0004 b 1.0 c |
Categorized variables are presented as: a number with a percentage. Continuous variables are presented as: mean ± SD, range (minimum -maximum) and number of non-missing values. Information about the numbers with valid values is provided in the left column. Abbreviations: N, valid measurements; n, number of patients with parameter above the cut-off point; SD, standard deviation. OMNIBUS, analysis of variance; N/A, non-applicable, a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red color text = statistically significant values.
The detailed characteristics of the laboratory parameters measured during the hospitalisation in the study cohort were pooled in Table 4 and Table 5.
Table 4.
Patient initial and on discharge laboratory assay in the studied cohort after C2HEST risk stratification.
| Parameter Time of Assessment | Units | Low Risk [0–1] |
Medium [2–3] |
High Risk [≥4] |
p-Value OMNIBUS |
p-Value for Post-Hoc Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | ||
| Morphology | |||||||||||
|
Leucocytes n/n(%)/N On admission |
>12 × 103/µL | 85 (13.8) 615 |
116 (16.9) 686 |
52 (18.8) 277 |
32 (15.8) 203 |
23 (17.7) 130 |
29 (212) 137 |
0.3085 | 0.3279 | N/A | N/A |
| 4–12× 103/µL | 467 (75.9) 615 |
504 (73.5) 686 |
198 (71.5) 277 |
147 (72.4) 203 |
91 (70.0) 130 |
100 (73.0) 137 |
|||||
| <4 × 103/µL | 63 (10.2) 615 |
66 (9.6) 686 |
27 (9.7) 277 |
24 (11.8) 203 |
16 (12.3) 130 |
8 (5.8) 137 |
|||||
| On discharge | >12 × 103/µL | 81 (13.2) 615 |
119 (17.3) 686 |
55 (19.9) 277 |
48 (23.6) 203 |
36 (27.7) 130 |
28 (20.4) 137 |
0.0008 | 0.0028 | 0.0971 a 0.0006 b 0.5375 c |
0.002 a 1.0 b 0.1331 c |
| 4–12× 103/µL | 487 (79.2) 615 |
530 (77.3) 686 |
205 (74.0) 277 |
132 (65.0) 203 |
85 (65.4) 130 |
103 (75.2) 137 |
|||||
| <4 × 103/µL | 47 (7.6) 615 |
37 (5.4) 686 |
17 (6.1) 277 |
23 (11.3) 203 |
9 (6.9) 130 |
6 (4.4) 137 |
|||||
|
Haemoglobin n/n(%)/N On admission |
<12 g/dL females <13 g/dL males anaemia | 172 (28.0) 615 |
173 (25.2) 686 |
91 (32.9) 277 |
104 (51.2) 203 |
63 (48.5) 130 |
84 (61.3) 137 |
<0.0001 | <0.0001 |
0.4836 a <0.0001 b 0.0106 c |
<0.0001 a,b 0.2546 c |
| On discharge | 266 (43.3) 615 |
244 (35.6) 686 |
122 (44.0) 277 |
136 (67.0) 203 |
79 (60.8) 130 |
92 (67.2) 137 |
0.0011 | <0.0001 |
1.0 a 0.0012 b 0.0071 c |
<0.0001 a,b 1.0 c |
|
|
Platelets mean ± SD/min-max/N On admission |
×103/µL | 244.8 ± 115.7 4.0–1356 615 |
227.4 ± 101.0 0.0–746.0 686 |
244.9 ± 115.8 41.0–740.0 277 |
209.8 ± 108.3 3.0–730.0 203 |
236.9 ± 98.7 8.0–537.0 130 |
198.9 ± 83.6 15.0–578.0 137 |
0.7077 | 0.001 | N/A | 0.099 a 0.002 b 0.548 c |
| On discharge | 267.7 ± 122.9 2.0–929.0 614 |
273.6 ± 133.0 6.0–1101.0 685 |
259.6 ± 117.1 27.0–694.0 277 |
225.7 ± 124.3 3.0–606.0 203 |
225.6 ± 102.3 4.0–592.0 130 |
203.3 ± 92.3 15.0–472.0 137 |
0.0003 | <0.0001 |
0.614 a 0.0002 b 0.009 c |
<0.0001 a,b 0.139 c |
|
| Acid -base balance in the arterial blood gas | |||||||||||
|
PH mean ± SD/min-max/N On admission |
7.42 ± 0.08 7.19–7.58 48 |
7.43 ± 0.09 7.04–7.57 73 |
7.43 ± 0.07 7.24–7.53 37 |
7.43 ± 0.07 7.10–7.54 51 |
7.39 ± 0.08 7.09–7.52 32 |
7.42 ± 0.07 7.28–7.54 35 |
0.2287 | 0.8496 | N/A | N/A | |
| On discharge | 7.43 ± 0.07 7.22–7.54 48 |
7.42 ± 0.09 7.06–7.54 73 |
7.43 ± 0.06 7.27–7.53 37 |
7.42 ± 0.09 7.01–7.55 51 |
7.44 ± 0,06 7.26–7.56 32 |
7.40 ± 0.06 7.25–7.52 35 |
0.8782 | 0.5746 | N/A | N/A | |
|
PaO2 mean ± SD/min-max/N On admission |
mmHg | 75.3 ± 33.0 12.8–207.0 48 |
70.2 ± 22.8 23.5–136.0 73 |
80.7 ± 54.2 28.3–286.0 37 |
73.2 ± 42.5 28.6–298.0 51 |
70.7 ± 25.7 32.8–134.0 32 |
70.5 ± 41.4 23.7–222.0 35 |
0.562 | 0.9031 | N/A | N/A |
| On discharge | 74.8 ± 27.7 12.8–207..0 48 |
75.7 ± 26.0 23.5–165.0 73 |
81.9 ± 55.0 23.3–286.0 37 |
74.6 ± 43.5 28.6–298.0 51 |
69.5 ± 27.6 28.5–134.0 32 |
63.6 ± 20.5 28.5–129.0 35 |
0.4499 | 0.0316 | N/A | 0.985 a 0.028 b 0.268 c |
|
|
PaCO2 mean ± SD/min-max/N On admission |
mmHg | 38.3 ± 8.2 20.2–58.0 48 |
37.8 ± 11.5 25.7–82.4 73 |
37.2 ± 9.3 26.9–79.4 37 |
36.3 ± 9.6 20.9–67.0 51 |
38.6 ± 13.6 25.0–88,4 32 |
38.7 ± 8.0 19.7–61.0 35 |
0.8084 | 0.4415 | N/A | N/A |
| On discharge | 38.3 ± 8.4 20.2–62.2 48 |
38.5 ± 10.7 24.1–75.5 73 |
38.5 ± 10.0 27.8–84.4 37 |
37.5 ± 11.7 20.9–88.4 51 |
37.4 ± 11.5 25.0–88.4 32 |
39.9 ± 8.7 26.8–67.8 35 |
0.9071 | 0.5398 | N/A | N/A | |
|
HCO3 standard mean ± SD/min-max/N On admission |
mmol/L | 25.0 ± 3.7 12.5–32.9 47 |
24.9 ± 3.8 12.1–32.8 73 |
24.9 ± 4.4 16.9–39.5 36 |
24.0 ± 4.0 14.3–32.4 49 |
23.4 ± 4.6 13.5–32.3 32 |
24.8 ± 4.5 17.5–38.6 35 |
0.2666 | 0.4967 | N/A | N/A |
| On discharge | 25.3 ± 3.4 12.5–35.7 47 |
24.8 ± 4.0 12.1–33.6 73 |
25.7 ± 4.8 16.9–40.3 36 |
25.0 ± 6.1 13.7–51.7 49 |
25.1 ± 4.3 17.4–35.8 32 |
24.7 ± 3.7 19.4–36.7 35 |
0.8862 | 0.9539 | N/A | N/A | |
|
BE mean ± SD/min-max/N On admission |
mmol/L | 0.63 ± 5.06 [−]15.7–5.9 16 |
1.12 ± 4.67 [−]9.1–10.5 25 |
2.96 ± 4.72 [−]3.3–15.7 17 |
0.88 ± 5.59 [−]12.5–9.7 26 |
[−]0.1 ± 4.75 [−]7.4–7.9 7 |
2.92 ± 5.21 [−]3.3–14.6 17 |
0.2745 | 0.4315 | N/A | N/A |
| On discharge | 1.21 ± 5.91 [−]15.7–11.9 16 |
0.46 ± 5.21 [−]11.0–8.3 25 |
3.54 ± 4.99 [−]3.3–17.1 17 |
1.62 ± 6.58 [−]14.7–11.8 26 |
0.91 ± 4.58 [−]7.4–7.9 7 |
1.65 ± 5.0 [−]5.3–13.2 17 |
0.363 | 0.6978 | N/A | N/A | |
|
Lactates mean ± SD/min-max/N On admission |
mmol/L | 2.0 ± 0.8 0.6–4.3 38 |
2.7 ± 1.9 1.1–12.8 67 |
2.0 ± 1.0 0.6–5.7 32 |
2.0 ± 0.7 0.5–3.8 47 |
2.9 ± 2.1 0.8–12.0 31 |
2.1 ± 1.4 0.6–5.7 30 |
0.1027 | 0.0291 | N/A | 0.02 a 0.199 b 0.913 c |
| On discharge | 2.1 ± 0.8 0.7–4.9 38 |
2.7 ± 1.9 1.0–12.8 67 |
2.0 ± 0.9 0.6–5.7 32 |
2.2 ± 1.1 0.5–6.4 47 |
2.6 ± 1.3 0.8–6.0 31 |
2.2 ± 1.1 0.8–4.3 30 |
0.0544 | 0.239 | N/A | N/A | |
| Electrolytes, inflammatory and iron biomarkers | |||||||||||
|
Na mean ± SD/min-max/N On admission |
mmol/L | 138.3 ± 3.8 106.0−155.0 605 |
138.2 ± 4.8 109.0−159.0 683 |
137.7 ± 7.6 101.0−175.0 272 |
137.7 ± 6.1 105.0−158.0 203 |
138.3 ± 7.7 108.0−174.0 130 |
137.6 ± 5.9 112.0−158.0 137 |
0.4803 | 0.3745 | N/A | N/A |
| On discharge | 138.9 ± 3.7 113.0−167.0 605 |
139.3 ± 4.8 109.0−175.0 683 |
139.0 ± 7.4 101.0−172.0 272 |
139.4 ± 7.2 105.0−165.0 203 |
140.7 ± 7.1 124.0−172.0 130 |
139.8 ± 6.3 120.0–157.0 137 |
0.0179 | 0.6389 | 0.977 a 0.013 b 0.062 c |
N/A | |
|
K mean ± SD/min-max/N On admission |
mmol/L | 3.99 ± 0.54 2.33–6.5 609 |
4.13 ± 0.61 2.0–7.5 684 |
4.06 ± 0.7 2.42 ± 5.9 275 |
4.25 ± 0.69 2.4–7.0 202 |
4.14 ± 0.74 2.53–6.6 130 |
4.43 ± 0.87 3.0–8.7 137 |
0.0403 | 0.0002 | 0.325 a 0.059 b 0.479 c |
0.072 a 0.0005 b 0.1 c |
| On discharge | 4.13 ± 0.56 2.47–7.4 609 |
4.33 ± 0.6 2.0–6.9 684 |
4.26 ± 0.75 2.28–6.32 275 |
4.5 ± 0.77 2.4–7.0 202 |
4.36 ± 0.69 2.53–6.5 130 |
4.51 ± 0.69 2.76–6.64 137 |
0.0004 | 0.0011 | 0.033 a 0.002 b 0.373 c |
0.015 a,b 0.983 c |
|
|
CRP mean ± SD/min-max/N On admission |
mg/L | 60.49 ± 72.41 0.13−531.58 597 |
90.54 ± 91.63 0.32−496.98 677 |
74.25 ± 84.61 0.4−538.55 275 |
95.36 ± 88.06 0.29–487.38 202 |
64.75 ± 72.93 0.4–344.95 130 |
87.45 ± 87.37 0.4–390.94 137 |
0.0674 | 0.69258 | N/A | N/A |
| On discharge | 36.85 ± 64.5 0.13–494.73 597 |
58.33± 88.96 0.25–496.98 677 |
62.6 ± 89.56 0.22–538.55 275 |
86.23± 99.39 0.46–447.61 202 |
63.78± 80.7 0.4–431.9 130 |
83.42± 90.91 0.42–390.94 137 |
<0.0001 | 0.0001 | <0.0001 a 0.001 b 0.99 c |
0.001 a 0.01 b 0.961 c |
|
|
Procalcitonin mean ± SD/min-max/N On admission |
ng/mL | 0.33 ± 1.55 0.01–24.95 404 |
1.24 ± 5.79 0.01–61.28 514 |
2.0 ± 15.13 0.01–196.04 188 |
1.62 ± 6.6 0.01–72.61 156 |
1.36 ± 6.46 0.01–60.77 98 |
1.59 ± 5.81 0.01–49.83 113 |
0.0993 | 0.7214 | N/A | N/A |
| On discharge | 0.57 ± 3.26 0.01–41.32 404 |
1.16 ± 6.14 0.01–75.16 514 |
0.86 ± 3.62 0.01–30.67 188 |
2.49 ± 8.44 0.01–81.09 156 |
1.11 ± 6.17 0.01–60.77 98 |
1.19 ± 3.68 0.01–27.61 113 |
0.5044 | 0.1807 | N/A | N/A | |
|
IL-6 mean ± SD/min-max/N On admission |
pg/mL | 85.5 ± 660.2 2.0–9099.0 192 |
45.2 ± 98.7 2.0–1000.0 288 |
34.3 ± 52.7 2.0–398.0 84 |
55.9 ± 75.3 2.0–499.0 59 |
55.2 ± 94.1 2.0–421.0 38 |
69.2 ± 97.8 2.0–369.0 40 |
0.2692 | 0.2811 | N/A | N/A |
| On discharge | 90.3 ± 672.0 2.0–9099.0 192 |
42.0 ± 111.0 2.0–1000.0 288 |
28.5 ± 53.5 2.0–398.0 84 |
56.5 ± 94.3 2.0–499.0 59 |
67.6 ± 170.4 2.0–1000.0 38 |
82.3 ± 150.6 2.0–804.0 40 |
0.1877 | 0.1939 | N/A | N/A | |
|
D-dimer mean ± SD/min-max/N On admission |
µg/mL | 2.60 ± 8.39 0.15–-118.32 444 |
4.63 ± 14.46 0.18–132.82 558 |
5.40 ± 12.57 0.2–107.65 206 |
7.84 ± 20.75 0.23–127.24 167 |
3.78 ± 11.48 0.24–107.54 100 |
7.01 ± 21.41 0.22–128.0 103 |
0.0133 | 0.1192 | 0.011 a 0.596 b 0.501 c |
N/A |
| On discharge | 3.17 ± 11.99 0.15–128.0 444 |
3.25 ± 9.63 0.21–115.13 558 |
4.38 ± 8.28 0.21–74.28 206 |
7.2 ± 17.51 0.23–106.02 167 |
3.65 ± 11.23 0.21–107.54 100 |
3.72 ± 6.9 0.22–46.72 103 |
0.3287 | 0.0215 | N/A | 0.016 a 0.821 b 0.059 c |
|
|
INR mean ± SD/min-max/N On admission |
1.07 ± 0.2 0.82–3.6 580 |
1.19 ± 0.63 0.83–15.2 647 |
1.25 ± 0.69 0.87–7.8 257 |
1.27 ± 0.44 0.89–4.37 188 |
1.58 ± 1.75 0.9–18.74 127 |
1.99 ± 2.98 0.89–21.1 124 |
<0.0001 | 0.0031 |
0.0002 a 0.005 b 0.112 c |
0.136 a 0.01 b 0.023 c |
|
| On discharge | 1.1 ± 0.4 0.82–9.2 580 |
1.17 ± 0.33 0.87–6.82 647 |
1.2 ± 0.8 0.88–13.1 257 |
1.32 ± 0.7 0.92–7.85 188 |
1.4 ± 0.8 0.9–8.0 127 |
1.53 ± 1.88 0.87–21.1 124 |
0.0003 | 0.0019 | 0.048 a 0.001 b 0.251 c |
0.011 a 0.082 b 0.452 c |
|
|
APTT n/n(%)/N On admission |
>60 s | 6 1.1 561 |
22 3.5 630 |
3 1.2 247 |
4 2.2 184 |
6 4.8 124 |
5 4.2 120 |
0.0243 | 0.5704 | 1.0 a 0.0337 b 0.1964 c |
N/A |
| On discharge | 14 2.5 561 |
32 5.1 630 |
3 1.2 247 |
5 2.7 184 |
4 3.2 124 |
8 6.7 120 |
0.3472 | 0.2518 | N/A | N/A | |
|
Fibrinogen mean ± SD/min-max/N On admission |
g/dL | 4.69 ± 1.53 0.35–9.04 153 |
5.11 ± 2.14 0.44–10.0 132 |
4.34 ± 1.4 0.35–6.72 29 |
4.93 ± 2.0 0.37–9.2 52 |
3.62 ± 1.06 1.78–5.51 24 |
5.31 ± 1.71 2.54–9.1 29 |
0.0004 | 0.6765 | 0.441 a 0.0003 b 0.096 c |
N/A |
| On discharge | 4.58 ± 1.8 0.44–10.0 153 |
4.95 ± 2.13 0.6–10.0 132 |
5.01 ± 2.11 0.35–9.4 29 |
4.98 ± 2.3 0.37–11.3 52 |
3.84 ± 1.21 1.53–5.75 24 |
5.71 ± 2.07 2.2–9.04 29 |
0.0184 | 0.2055 |
0.561 a 0.037 b 0.04 c |
N/A | |
Continuous variables are presented as: mean ± SD. range (minimum -maximum) and number of non-missing values. Categorized variables are presented as: a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: N, valid measurements; n, number of patients with parameter above cut-off point; SD, standard deviation. OMNIBUS, analysis of variance; N/A, non-applicable, a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red text—statistically significant values.
Table 5.
Patient initial and on discharge laboratory assay in the studied cohort after C2HEST risk stratification.
| Parameter Time of Assessment | Units | Low Risk [0–1] |
Medium [2–3] |
High Risk [≥4] |
p-Value OMNIBUS |
p-Value for Post-Hoc Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | ||
| Biochemistry | |||||||||||
|
Glucose mean ± SD/min-max/N On admission |
mg/dL | 128.1 ± 67.0 61.0–671.0 425 |
139.3 ± 79.5 28.0–933.0 638 |
144.1 ± 74.9 54.0–662.0 257 |
160.5 ± 110.3 47.0–1026.0 192 |
149.1 ± 86.5 70–685 120 |
152.0 ± 109.4 37.0–1064.0 126 |
0.0035 | 0.0315 | 0.014 a 0.039 b 0.849 c |
0.038 a 0.433 b 0.779 c |
| On discharge | 119.0 ± 56.0 37.0–595.0 425 |
127.3 ± 78.8 50.0–1444.0 638 |
136.4 ± 75.3 54.0–596.0 257 |
150.7 ± 92.2 47.0–578.0 192 |
144.8 ± 90.4 14.0–685.0 120 |
143.5 ± 63.1 37.0–406.0 126 |
0.0003 | 0.0012 | 0.004 a 0.01 b 0.653 c |
0.005 a 0.033 b 0.688 c |
|
|
Glycated hemoglobin (HbA1c) mean ± SD/min-max/N On admission |
% | 7.1 ± 1.9 4.2–12.2 47 |
7.9 ± 2.5 4.9–14.9 80 |
7.9 ± 2.7 4.9–16.6 39 |
7.2 ± 1.4 4.8–12.2 36 |
7.2 ± 1.7 5.1–11.4 33 |
7.4 ± 1.9 5.1–13.7 28 |
0.3182 | 0.1497 | N/A | N/A |
| On discharge | 7.0 ± 1.8 4.2–12.2 47 |
7.8 ± 2,4 4.9–14.9 80 |
7.9 ± 2.7 4.9–16.8 39 |
7.1 ± 1.4 4.7–12.2 36 |
7.2 ± 1.7 5.1–11.4 33 |
7.4 ± 1.9 5.1–13.7 28 |
0.2299 | 0.1563 | N/A | N/A | |
|
Urea mean ± SD/min-max/N On admission |
mg/dL | 36.3 ± 35.1 7.0–301.0 481 |
47.6 ± 35.8 5.0–307.0 664 |
60.2 ± 50.6 8.0–353.0 256 |
69.9 ± 47.5 15.0–271.0 199 |
69.5 ± 48.9 12.0–336.0 124 |
84.4 ± 57.1 17.0–369.0 133 |
<0.0001 | <0.0001 |
<0.0001a,b 0.197 c |
<0.0001a,b
0.042 c |
| On discharge | 35.5 ± 29.6 7.0–231.0 481 |
44.9 ± 32.9 5.0–307.0 664 |
59.0 ± 48.2 10.0–353.0 256 |
75.6 ± 59.8 12.0–396.0 199 |
66.9 ± 41.7 15.0–204.0 124 |
88.9 ± 58.6 21.0–342.0 133 |
<0.0001 | <0.0001 |
<0.0001a,b 0.236 c |
<0.0001a,b 0.11 c |
|
|
Creatinine mean ± SD/min-max/N On admission |
mg/dL | 1.0 ± 0.99 0.34–11.99 533 |
1.26 ± 1.3 0.26–14.87 683 |
1.22 ± 0.97 0.48–9.56 275 |
1.76 ± 1.6 0.58–12.66 203 |
1.58 ± 1.27 0.44–8.46 130 |
2.02 ± 1.81 0.49–11.3 137 |
<0.0001 | <0.0001 |
0.008 a
< 0.0001 b 0.012 c |
0.0002 a < 0.0001 b 0.369 c |
| On discharge | 0.96 ± 0.86 0.34–9.11 533 |
1.16 ± 1.18 0.26–14.87 683 |
1.16 ± 0.92 0.45–9.06 275 |
1.81 ± 1.72 0.43–12.35 203 |
1.42 ± 1.21 0.43–7.66 130 |
1.89 ± 1.58 0.43–9.27 137 |
<0.0001 | <0.0001 |
0.009 a 0.0002 b 0.084 c |
<0.0001a,b 0.877 c |
|
|
eGFR mean ± SD/min-max/N On admission |
mL/min/1.73 m2 | 84.6 ± 32.1 0.0–207.0 531 |
85.3 ± 35.9 3.0–433.0 680 |
60.8 ± 25.0 4.0–136.0 275 |
63.7 ± 33.1 4.0–149.0 203 |
49.7 ± 26.4 5.0–145.0 130 |
55.3 ± 32.0 5.0–180.0 137 |
<0.0001 | <0.0001 |
0.0 a
< 0.0001 b 0.0002 c |
0.0 a
0.0 b 0.054 c |
| On discharge | 86.6 ± 32.1 0.0–207.0 531 |
91.5 ± 36.5 3.0–433.0 680 |
65.0 ± 26.6 4.0–148.0 275 |
66.0 ± 36.1 4.0–208,0 203 |
58.2 ± 30.3 5.0–147.0 130 |
58.6 ± 35.7 6.0–209.0 137 |
<0.0001 | <0.0001 |
0.0 a < 0.0001 b 0.076 c |
<0.0001a,b 0.147 c |
|
|
Total protein mean ± SD/min-max/N On admission |
g/L | 6.1 ± 0.8 3.9–8.2 145 |
6.1 ± 0.8 3.5–8.1 186 |
5.8 ± 0.8 3.6–8.2 78 |
6.0 ± 1.0 4.2–9.5 74 |
5.7 ± 0.9 3.3–8.1 62 |
5.7 ± 0.9 3.4–8.2 61 |
0.0235 | 0.0555 | 0.148 a 0.033 b 0.741 c |
N/A |
| On discharge | 6.0 ± 0.9 3.9–8.2 145 |
6.0 ± 0.9 3.0–8.1 186 |
5.7 ± 0.9 3.7–8.2 78 |
5.9 ± 0.9 4.3–9.1 74 |
5.5 ± 1.0 3.3–8.1 62 |
5.7 ± 0.9 3.4–7.8 61 |
0.0012 | 0.0162 |
0.049 a 0.002 b 0.388 c |
0.799 a 0.012 b 0.158 c |
|
|
Albumin mean ± SD/min-max/N On admission |
g/L | 3.1 ± 0.6 1.6–4.6 152 |
3.2 ± 0.6 1.5–5.1 222 |
3.0 ± 0.5 1.1–4.3 78 |
3.2 ± 0.6 2.1–4.4 82 |
2.9 ± 0.6 0.7–3.7 62 |
3.1 ± 0.6 1.5–4.9 67 |
0.0134 | 0.3087 | 0.287 a 0.011 b 0.307 c |
N/A |
| On discharge | 3.1 ± 0.6 1.1–4.6 152 |
3.0 ± 0.7 0.4–5.1 222 |
3.0 ± 0.5 1.9–4.2 78 |
3.1 ± 0.6 1.7–4,4 82 |
2.8 ± 0.5 1.4–3.7 62 |
2.8 ± 0.7 0.9–4..5 67 |
0.005 | 0.0549 | 0.64 a 0.004 b 0.277 c |
N/A | |
|
AST mean ± SD/min-max/N On admission = |
IU/L | 56.8 ± 139.7 6.0–2405.0 384 |
62.7 ± 89.4 5.0–1261.0 499 |
72.7 ± 343.6 8.0–4776 193 |
58.8 ± 49.5 7.0–323.0 154 |
113.5 ± 450.8 8.0–3866.0 104 |
60.2 ± 101.8 10.0–731.0 107 |
0.3869 | 0.7844 | N/A | N/A |
| On discharge | 123.4 ± 1244.4 10.0–23,896.0 384 |
68.3 ± 255.1 5.0–3761.0 499 |
43.3 ± 46.5 8.0–380.0 193 |
107.5 ± 537.6 11.0–6591.0 154 |
148.9 ± 702.4 8.0–6088.0 104 |
97.4 ± 402.4 7.0–4019.0 107 |
0.1438 | 0.5525 | N/A | N/A | |
|
ALT mean ± SD/min-max/N On admission |
IU/L | 47.0 ± 87.7 5.0–1411.0 435 |
61.4 ± 96.4 4.0–1278.0 537 |
52.2 ± 251.2 5.0–3700.0 219 |
45.0 ± 43.2 4.0–270.0 172 |
57.1 ± 183.6 5.0–1361.0 112 |
46.7 ± 88.2 6.0–612.0 113 |
0.8212 | 0.0081 | N/A |
0.006 a 0.256 b 0.98 c |
| On discharge | 65.5 ± 265.4 6.0–5163.0 435 |
74.3 ± 105.0 4.0–1217.0 537 |
38.5 ± 46.1 5.0–449.0 219 |
65.1 ± 124.7 7.0–1247.0 172 |
74.4 ± 308.8 5.0–2985.0 112 |
71.4 ± 207.3 9.0–1570.0 113 |
0.0624 | 0.6835 | N/A | N/A | |
|
Bilirubin mean ± SD/min-max/N On admission |
mg/dL | 0.78 ± 1.68 0.1–19.1 363 |
0.88 ± 1.24 0.1–15.1 489 |
0.85 ± 0.88 0.2–9.2 195 |
0.80 ± 0.49 0.2–3.1 157 |
0.77 ± 0.51 0.1–4.2 100 |
0.98 ± 0.84 0.3–6.6 103 |
0.5771 | 0.1292 | N/A | N/A |
| On discharge | 0.77 ± 1.65 0.1–19.0 363 |
0.95 ± 1.91 0.1–25.9 489 |
0.95 ± 2.55 0.2–35.3 195 |
0.76 ± 0.47 0.2–3.1 157 |
0.78 ± 0.67 0.3–6.1 100 |
1.06 ± 1.33 0.2–12.8 103 |
0.6611 | 0.0224 | N/A | 0.123 a 0.754 b 0.08 c |
|
|
LDH mean ± SD/min-max/N On admission |
U/L | 404.5 ± 478.5 50.0–7100.0 328 |
448.6 ± 282.2 120.0–3194.0 448 |
368.2 ± 189.8 44.0–1357.0 156 |
418.9 ± 212.9 134.0–1172.0 130 |
468.1 ± 1015.3 71.0–9505.0 83 |
416.9 ± 269.7 113.0–1863.0 86 |
0.3576 | 0.3427 | N/A | N/A |
| On discharge | 387.2 ± 739.3 50.0–11,227.0 328 |
389.2 ± 396.2 93.0–6577.0 448 |
340.3 ± 167.3 44.0–1357.0 156 |
407.1 ± 243.5 112.0–1584.0 130 |
474.0 ± 1028.1 106.0–9505.0 83 |
388.8 ± 215.4 97.0–1260.0 86 |
0.292 | 0.7848 | N/A | N/A | |
| Cardiacbiomarkers | |||||||||||
|
BNP mean ± SD/min-max/N On admission |
pg/mL | 152.5 ± 241.1 1.7–1130.8 54 |
254.1 ± 763.7 1.7–6924.2 107 |
455.4 ± 872.4 10.1–4890.6 50 |
433.3 ± 747.2 3.0–3153.2 50 |
711.7 ± 995.6 22.3–4993.0 56 |
1432.8 ± 2864.5 5.9–13,368.4 42 |
<0.0001 | 0.0206 | 0.054 a 0.0004 b 0.338 c |
0.35 a 0.031 b 0.082 c |
| On discharge | 177.7 ± 308.1 5.3–1877.0 54 |
239.8 ± 753.1 1.7–6924.2 107 |
536.1 ± 1562.6 10.1–10,622.8 50 |
396.2 ± 697.6 3.0–3153.2 50 |
592.3 ± 769.1 22.3–3729.8 56 |
1389.2 ± 2735.4 11.9–13,368.4 42 |
0.0008 | 0.0206 | 0.257 a 0.001 b 0.971 c |
0.412 a 0.027 b 0.067 c |
|
|
NT-proBNP mean ± SD/min-max/N On admission |
ng/mL | 1467.1± 3250.7 18.7–16,551.7 62 |
2126.5± 9426.7 12.0–70,000.0 110 |
6608.9± 12,708.7 49.6–70,000.0 54 |
10,323.4 ± 16,141.4 18.2–70,000.0 55 |
14,888.1 ± 18,982.5 119.6–70,000.0 43 |
13,522.6 ± 19,276.7 343.7–70,000.0 55 |
<0.0001 | <0.0001 |
0.015 a
0.0001 b 0.043 c |
0.002 a 0.0003 b 0.614 c |
| On discharge | 1694.0 ± 5047.8 28.5–35,000.0 62 |
1893.4 ± 7660.6 12.0–70,000.0 110 |
7852.3 ± 15,159.0 49.6–70,000.0 54 |
10,661.5 ± 16,202.2 18.2–70,000.0 55 |
13,084.8 ± 17,275.9 119.6–69,519.7 43 |
13,265.6 ± 17,873.3 391.3–70,000.0 55 |
<0.0001 | <0.0001 |
0.016 a 0.0003 b 0.267 c |
0.0009 a <0.0001 b 0.703 c |
|
|
Troponin I, mean ± SD/min-max/N On admission |
ng/mL | 53.1 ± 211.1 0.0–1994.8 263 |
189.6 ± 1015.9 1.3–11,758.2 415 |
658.5 ± 7215.3 1.9–94,365.5 171 |
3044.2 ± 15,485.9 1.0–125,592.6 134 |
988.4 ± 3316.8 3.3–21,022.9 94 |
542.0 ± 1724.6 4.8–14,128.8 97 |
0.015 | 0.0185 | 0.517 a 0.02 b 0.867 c |
0.087 a 0.133 b 0.156 c |
| On discharge | 105.7 ± 873.1 0.2–12,391.6 263 |
124.0 ± 797.8 0.8–11,758.2 415 |
692.7 ± 7243.6 1.9–94,365.5 171 |
3359.3 ± 18,244.2 0.8–174,652.6 134 |
838.2 ± 3666.2 1.8–29.828.3 94 |
493.1 ± 1504.8 4.8–12,657.2 97 |
0.0977 | 0.0095 | N/A | 0.104 a 0.055 b 0.17 c |
|
|
n/n(%)/N = F: >46.8 ng/mL M: >102.6 ng/mL |
>3-fold upper range | 46 17.5 263 |
67 16.1 415 |
51 29.8 171 |
47 35.1 134 |
49 52.1 94 |
38 39.2 97 |
<0.0001 | <0.0001 |
0.0113 a
<0.0001b 0.0017 c |
<0.0001a,b 1.0 c |
|
LDL-cholesterol mean ± SD/min-max/N On admission |
mg/dL | 106.8 ± 64.8 6.0–510.0 85 |
96.2 ± 40.5 27.0–242.0 147 |
93.9 ± 39.7 23.0–199.0 69 |
79.4 ± 40.6 17.0–230.0 60 |
83.3 ± 44.2 14.0–187.0 49 |
64.2 ± 37.6 6.0–210.0 39 |
0.0498 | <0.0001 | 0.283 a 0.038 b 0.381 c |
0.022 a <0.0001 b 0.142 c |
|
HDL-cholesterol mean ± SD/min-max/N On admission |
mg/dL | 43.9 ± 17.9 2.0–120.0 86 |
37.7 ± 14.5 10.0–101.0 150 |
44.5 ± 16.7 12.0–110.0 69 |
35.2 ± 11.9 7.0–66.0 60 |
39.8 ± 17.5 8.0–79.0 48 |
34.0 ± 10.3 17.0–61.0 38 |
0.303979 | 0.154387 | N/A | N/A |
|
Triglycerides mean ± SD/min-max/N On admission |
mg/dL | 189.4 ± 154.5 40.0–1100.0 122 |
173.7 ± 105.1 44.0–664.0 237 |
141.0 ± 94.5 48.0–595.0 83 |
148.0 ± 98.8 50.0–550.0 81 |
133.4 ± 56.7 46.0–282.0 60 |
124.8 ± 66.9 51.0–413.0 56 |
0.0022 | 0.0001 |
0.016 a 0.001 b 0.817 c |
0.117 a <0.0001 b 0.232 c |
| Hormones | |||||||||||
|
25-hydroxy-vitamin D mean ± SD/min-max/N On admission |
ng/mL | 27.4 ± 21.8 3.5–146.1 99 |
23.4 ± 15.0 3.5–126.4 206 |
26.1 ± 17.2 3.5–77.7 63 |
22.9 ± 15.4 5.1–75.6 45 |
22.4 ± 16.8 3.5–63.5 36 |
14.5 ± 9.6 3.5–39.1 25 |
0.3738 | 0.0006 | N/A | 0.974 a 0.0006 b 0.018 c |
|
TSH mean ± SD/min-max/N On admission |
mIU/L | 1.55 ± 2.0 0.01–18.6 186 |
1.2 ± 1.06 0.0–6.33 255 |
1.72 ± 2.98 0.01–28.81 137 |
1.31 ± 1.39 0.01–8.28 95 |
2.74 ±5.04 0.0–38.24 85 |
1.43 ± 1.25 0.0–6.36 62 |
0.1063 | 0.3834 | N/A | N/A |
Continuous variables are presented as: mean ± SD. range (minimum -maximum) and number of non-missing values. Categorized variables are presented as: a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: N, valid measurements; n, number of patients with parameter above cut-off point; SD, standard deviation. OMNIBUS, analysis of variance; N/A, non-applicable, a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red text = statistically significant values.
Both genders revealed significant differences between the C2HEST strata and complete blood count parameters along with ion parameters. Noteworthy, no significant differences between strata in terms of initial inflammatory markers (procalcitonin, IL-6, CRP) along with acid-base balance parameters were noted.
The parameters of kidney function, including urea, creatinine, eGFR maintained significantly worse in the high-risk C2HEST stratum for both genders, however baseline serum concentration of protein and albumin was significantly lower only in females with higher C2HEST score value. In both study cohorts we observed increasing level of cardiac injury markers including troponin T and NT-pro-BNP levels in patientsallocated higher-risk group depending on their C2HEST score value. Surprisingly, lipid disorders (level of LDL and triglycerides) noticed at the time of admission were less severe subjects from high-risk stratum in both study cohorts.
3.2. Specific Treatment Applied during Hospitalization
Differences in applied treatment during hospitalization between the C2HEST group among genders are highlighted in Table 6. Women in the higher C2HEST stratum were prone to receive convalescent plasma. We did not observe any differences among the male cohort. In both study arms, we observed changes in the prevalence of antibiotic application. Subjects from the high-risk stratum more often received this type of therapy.
Table 6.
Treatment applied during hospitalization.
| Variables, Units | Low Risk [0–1] |
Medium [2–3] |
High Risk [≥4] |
p-Value OMNIBUS |
p Value for Post-Hoc Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females N = 682 |
Males N = 735 |
Females N = 384 |
Males N = 208 |
Females N = 135 |
Males N = 139 |
Females |
Males |
Females |
Males |
|
| Applied treatment and procedures | ||||||||||
|
Systemic corticosteroid n/n(%) |
299 (43.8) |
409 (55.6) |
127 (44.7) |
119 (57.2) |
64 (47.4) |
78 (56.1) |
0.7456 | 0.9222 | N/A | N/A |
|
Convalescentplasma n/n(%) |
54 (7.9) |
113 (15.4) |
12 (4.2) |
29 (13.9) |
15 (11.1) |
16 (11.5) |
0.0274 | 0.4749 | 0.1599 a 0.8816 b 0.0406 c |
N/A |
|
Tocilizumab n/n(%) |
11 (1.6) |
11 (1.5) |
0 (0.0) |
2 (1.0) |
1 (0.7) |
0 (0.0) |
0.054 | 0.4308 | N/A | N/A |
|
Remdesivir n/n(%) |
83 (12.2) |
153 (20.8) |
37 (13.0) |
35 (16.8) |
12 (8.9) |
23 (16.5) |
0.4627 | 0.2822 | N/A | N/A |
|
Antibiotic n/n(%) |
338 (49.6) |
408 (55.5) |
157 (55.3) |
146 (70.2) |
88 (65.2) |
103 (74.1) |
0.0026 | <0.0001 | 0.3633 a
0.0038 b 0.2079 c |
0.0006 a
0.0002 b 1.0 c |
Continuous variables are presented as: mean ± SD, range (minimum–maximum) and number of non-missing values. Categorized variables are presented as: a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: N, valid measurements; n, number of patients with parameter above cut-off point; SD, standard deviation; OMNIBUS, analysis of variance; N/A, non-applicable; a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red text = statistically significant values.
The assignment to specific C2HEST stratum score correlated with the type of respiratory support applied during the hospitalization. Additionally, in the male cohort, it correlated with the prevalence of coronary revascularization procedures during index hospitalization along with the need for the catecholamine’s administration (Table 7).
Table 7.
Applied treatment and procedures.
| Variables | Low Risk [0–1] |
Medium [2–3] |
High Risk [≥4] |
p Value OMNIBUS |
p Value for Post-Hoc Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females N = 681 |
Males N = 734 |
Females N= 284 |
Males N = 207 |
Females N = 135 |
Males N = 139 |
Females | Males | Females | Males | |
| Applied treatment and procedures | ||||||||||
|
The most advanced respiratory support applied during the hospitalisation no oxygen n/n(%) |
409 (60.1) |
332 (45.2) |
140 (49.3) |
62 (30.0) |
50 (37.0) |
39 (28.1) |
<0.0001 | <0.0001 |
0.001a
<0.0001 b 0.0114 c |
0.0001 a 0.0007b 1.0 c |
|
low flow oxygen support n/n(%) |
199 (29.2) |
252 (34.3) |
103 (36.3) |
85 (41.1) |
65 (48.1) |
59 (42.4) |
||||
|
high flow nasal cannula non-invasive ventilation n/n(%) |
26 (3.8) |
56 (7.6) |
24 (8.5) |
28 (13.5) |
17 (12.6) |
22 (15.8) |
||||
|
invasive ventilation n/n(%) |
47 (6.9) |
94 (12.8) |
17 (6.0) |
32 (15.5) |
3 (2.2) |
19 (13.7) |
||||
|
Oxygenation parameters from the period of qualification for advanced respiratory support: SpO2, % mean ± SD/(min-max/N |
92.2 ± 6.8 (59–100) 221 |
88.8 ± 8.6 (50–100) 189 |
87.0 ± 11.0 (55–99) 64 |
86.0 ± 8.4 (60–99) 69 |
86.2 ± 9.3 (59–98) 40 |
85.1 ± 10.5 (60–99) 48 |
<0.0001 | 0.0159 |
0.002 a 0.0008 b 0.908 c |
0.057 a 0.072 b 0.87 c |
|
Therapy with catecholamines n/n(%)/N |
39 (5.7) 682 |
92 (12.5) 735 |
14 (4.9) |
31 (14.9) 208 |
9 (6.7) |
33 (23.7) |
0.7614 | 0.0025 | N/A | 1.0 a 0.0026 b 0.1576 c |
|
Coronary revascularisation or/and an indication for coronary revascularisation, n/n(%)/N |
1 (0.1) 682 |
7 (1.0) 735 |
3 (1.1) |
8 (3.8) 208 |
1 (0.7) |
6 (4.3) |
0.0795 | 0.0021 | N/A |
0.0225 a 0.0286 b 1.0 c |
|
Haemodialysis n/n(%)/N |
15 (2.2) 682 |
31 (4.2) 735 |
2 (0.7) |
11 (0.7) 208 |
4 (3.0) |
8 (5.8) |
0.1486 | 0.6417 | N/A | N/A |
Continuous variables are presented as: mean ± SD, range (minimum–maximum) and number of non-missing values. Categorized variables are presented as: a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: N, valid measurements; n, number of patients with parameter above cut-off point; SD, standard deviation; OMNIBUS, analysis of variance;N/A, non-applicable; a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red text = statistically significant values.
3.3. Association C2HEST Score with Results and Mortality
In the female cohort, the in-hospital and three-month andsix-month mortality rates were the highest in high-risk C2HEST stratum reaching 31.9%, 48.1%, and 61.4%. Noteworthy, mortality rates in the medium-risk stratum were significantly higher than in low-risk. All data regarding short and long-term mortality were presented in Table 8. Similarly, in the males’ cohort in-hospital, three-month and six-month mortality was also highest in the high-risk C2HEST stratum and come to 38.8%, 59.0%, and 68.8%. Also, in this study arm differences between all C2HEST groups were statistically significant.
Table 8.
Total and in-hospital all-cause mortality in the C2HEST risk strata in males’ and females’ cohort.
| Variables | Low Risk [0–1] |
Medium [2–3] |
High Risk [≥4] |
p Value OMNIBUS |
p Value for Post-Hoc Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females N = 682 |
Males N = 735 |
Females N = 284 |
Males N = 208 |
Females N = 135 |
Males N = 139 |
Females | Males | Females | Males | |
| All-cause mortality rate | ||||||||||
|
In-hospital mortality n/n(%) |
36 (5.3) |
83 (11.3) |
50 (17.6) |
60 (28.8) |
43 (31.9) |
54 (38.8) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0048 c |
<0.0001 a,b 0.2029 c |
|
3-month mortality n/n(%) |
68 (10.0) |
134 (18.2) |
95 (33.5) |
103 (49.5) |
65 (48.1) |
82 (59.0) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.016 c |
<0.0001 a,b 0.3134 c |
|
6-month mortality n/n(%/)/N |
72 (17.3) 415 |
142 (31.4) 452 |
104 (49.3) 211 |
104 (60.1) 173 |
70 (61.4) 114 |
86 (68.8) 125 |
<0.0001 | <0.0001 |
<0.0001 a,b 0.1454 c |
<0.0001 a,b 0.4696 c |
| Hospitalization | ||||||||||
|
Duration of hospitalization days mean ± SD/(min-max) |
10.4 ±12.7 (1–131) |
12.4 ± 14.4 (1–130) |
12.1 ± 11.9 (1–68) |
14.6 ± 15.6 (1–124) |
18.3 ±17.5 (1–87) |
13.9 ± 13.9 (1–121) |
<0.0001 | 0.1386 |
0.128 a
<0.0001 b 0.0007 c |
NA |
|
End of hospitalisation death n/n(%) |
36 (5.3) |
83 (11.3) |
50 (17.6) |
60 (28.8) |
43 (31.9) |
54 (38.8) |
<0.0001 | <0.0001 |
<0.0001 a,b
0.0143 c |
<0.0001 a,b 0.3663 c |
| discharge to home–full recovery | 515 (75.5) |
478 (65.0) |
141 (49.6) |
79 (38.0) |
57 (42.2) |
46 (33.1) |
||||
|
transfer to another hospital
–worsening) |
60 (8.8) |
79 (10.7) |
59 (20.8) |
38 (18.3) |
17 (12.6) |
27 (19.4) |
||||
|
transfer to another hospital
–in recovery |
71 (10.4) |
95 (12.9) |
34 (12.0) |
31 (14.9) |
18 (13.3) |
12 (8.6) |
||||
Continuous variables are presented as: mean ± SD, range (minimum–maximum) and number of non-missing values. Categorized variables are presented as: a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: N, valid measurements; n, number of patients with parameter above cut-off point; SD, standard deviation; OMNIBUS, analysis of variance; N/A, non-applicable; a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red text = statistically significant values.
3.4. The All-Cause Mortality Discriminatory Performance of the C2HEST Score
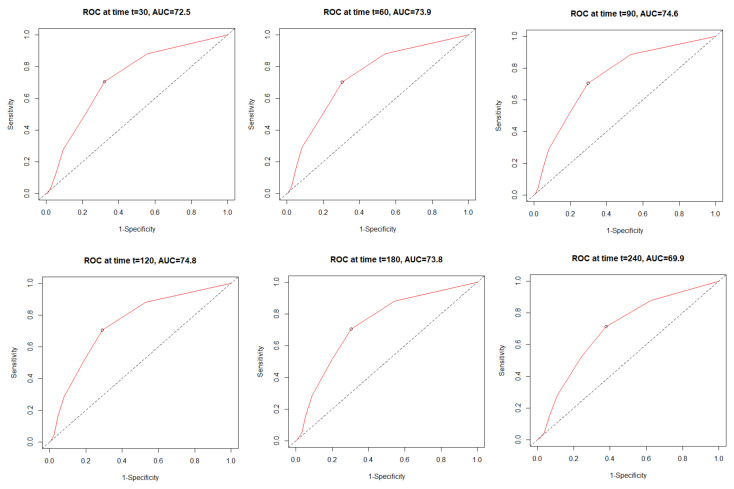
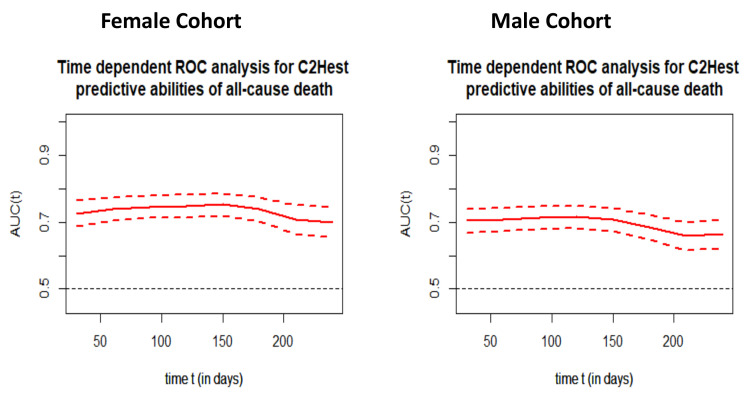
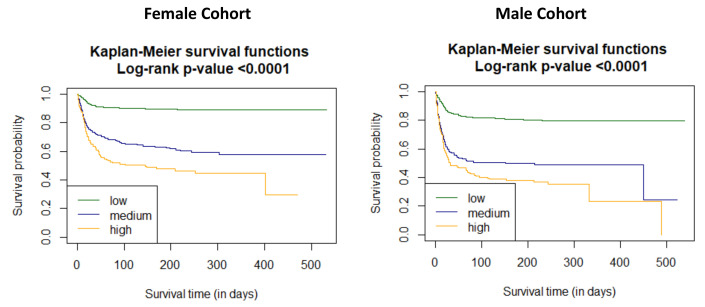
The time dependent receiver operating characteristic (ROC) analysis in both study cohorts revealed that the C2HEST scale is more sensitive in the female cohort (Figure 1). The C2HEST predicting AUC in women vs. man cohorts were higher at all calculated periods. Following the 1-month AUC = 72.5 vs. 70.3% 3-month AUC = 74.6 vs. 71.3%, six-month AUC = 73.8 vs. 68.4 %. All of the data were calculated for all-cause death without competing risk Figure 2 present ROC analysis in the male population. Figure 3 presented the time-dependent AUC for the C2HEST score in predicting the all-cause deaths in both cohort, slightly higher AUC value was observed in the female arm. The survival curves for the C2HEST stratum in both study cohorts were estimated using Kaplan-Meier functions. The p value for Log-rank test was <0.0001 (Figure 4). We have observed differences in estimated survival probability in both study cohorts. Practically, starting from admission time, the females were more likely to survive the COVID-19. Estimated six-month survival probability for high-risk subjects reached 0.5 in the female cohort, while for the male subject was below 0.4. Similarly, in medium-risk-stratum for women the survival probability was above 0.6 when compared to 0.5 in men. Additionally, the low-risk subjects in the female cohort maintained at the level of more than 0.9 for the whole observation period while in men reached 0.8, respectively.
Figure 1.
The time dependent receiver operating characteristic (ROC) for all-cause mortality in female cohort.
Figure 2.
The time dependent receiver operating characteristic (ROC) for all-cause mortality in male cohort.
Figure 3.
Time-dependent ROC analysis for the C2HEST predictive abilities of all cause death in both study cohorts.
Figure 4.
The survival curves for the C2HEST stratum in both study cohorts estimated by Kaplan-Meier function.
Subsequently, two Cox models were analyzed to assess the effect of the C2HEST score stratification on COVID-19 mortality. The overall model takes an uncategorized value of the C2HEST score, and it met the hazard proportional assumption in both study cohorts. An additional point in the C2HEST score resulted in increased the total-death intensity approximately in 42.8% in female subjects (HR 1.428, 95% CI 1.349–1.513 p < 0.0001) and respectively in male population 40.0% (HR 1.400, 95% CI 1.331–1.474 p < 0.0001). Furthermore, considering the categorized model, the change from the low to the medium category in the female population increased death expectation 4.267 times, and respectively; 3.289 times for males. Subsequently, transfer between the low-risk stratum to high-risk stratum raised all-cause death intensity 6.52 (female) and 4.476(male) times. The data are shown in Table 9 and Table 10.
Table 9.
The total all-cause-death hazard Ratios for C2HEST risk stratification in female cohort.
| Total Death | |||
| HR | 95%CI | p Value | |
| Overall | 1.428 | 1.349–1.513 | <0.0001 |
| Risk strata | |||
| Low risk vs. Medium risk | 4.267 | 3.170–5.732 | <0.0001 |
| Low risk vs. High risk | 6.524 | 4.714–9.031 | <0.0001 |
Red text—statistically significant values.
Table 10.
The total all-cause-death Hazard Ratios for C2HEST risk stratification in male cohort.
| Total Death | |||
| HR | 95%CI | p Value | |
| Overall | 1.400 | 1.331–1.474 | <0.0001 |
| Risk strata | |||
| Low risk vs. Medium risk | 3.289 | 2.559–4.227 | <0.0001 |
| Low risk vs.High risk | 4.476 | 3.438–5.827 | <0.0001 |
Red text = statistically significant values.
The associations of individual C2HEST score components with mortality in both study cohorts are presented in Table 11 and Table 12. The highest prognostic value for all-cause- death in both study groups was noticed for age (in women 2.750 vs. 3.059 in men, respectively). Interestingly, coronary artery disease was associated with higher HR for death only in men, whereas the COPD and hypertension only in woman.
Table 11.
Associations of individual C2HEST score components with mortality in female cohort.
| Component | HR | CI Min. | CI Max. | p Value | |
|---|---|---|---|---|---|
| All-causemortality | Coronaryarterydisease | 1.133 | 0.743 | 1.728 | 0.5627 |
| COPD | 2.083 | 1.299 | 3.532 | 0.0064 | |
| Age > 75 | 2.750 | 2.088 | 3.6216 | <0.0001 | |
| Thyroiddisease | 0.784 | 0.566 | 1.105 | 0.1649 | |
| Hypertension | 1.881 | 1.394 | 2.537 | <0.0001 | |
| HfrEF | 1.584 | 1.134 | 2.212 | 0.007 |
Abbreviations: COPD chronic obstructive pulmonary disease; HfrEF, heart failure with reduce ejection fraction. Red text = statistically significant values.
Table 12.
Associations of individual C2HEST score components with mortality in male cohort.
| Component | HR | CI Min. | CI Max. | p Value | |
|---|---|---|---|---|---|
| All-causemortality | Coronaryarterydisease | 1.568 | 1.180 | 2.084 | 0.0019 |
| COPD | 1.182 | 0.786 | 1.615 | 0.4227 | |
| Age > 75 | 3.0541 | 2.411 | 3.869 | <0.0001 | |
| Thyroiddisease | 1.126 | 0.688 | 1.842 | 0.6378 | |
| Hypertension | 1.200 | 0.952 | 1.513 | 0.1233 | |
| HfrEF | 1.415 | 1.055 | 1.899 | 0.0206 |
Abbreviations: COPD, chronic obstructive pulmonary disease; HfrEF, heart failure with reduce ejection fraction. Red text = statistically significant values.
Additionally, we verified whether the original cut-off values for particular C2HEST score risk (the low/medium/high-risk categories for 0–1/2–3/≥4 points, respectively) is potentially the best possible stratification system. Regarding the difference in Kaplan-Meier survival curves, all of the possible C2HEST intervals were analyzed in both study cohorts, and for each, we calculated the log-rank statistics (Table 13 and Table 14). The highest value of log-rank test statistics, presenting the best cut-off point for high (h) and medium (m) strata was obtained for the original C2HEST-score risk strata in the female population (m2 and h4, respectively). On the other hand, in male cohort the highest value of the Log-rank corresponded with m2 and h5, which reflects the following strata: 0–1 low, 2–4 medium, 5–8 high.
Table 13.
The log-rank statistics for matching the C2HEST risk strata for in-hospital mortality in female cohort.
| H2 | h3 | h4 | h5 | h6 | h7 | h8 | |
|---|---|---|---|---|---|---|---|
| m1 | 164.317 | 148.669 | 142.661 | 121.294 | 105.396 | 105.533 | 10.259 |
| m2 | 158.373 | 166.213 | 158.483 | 155.603 | 155.940 | 12.436 | |
| m3 | 122.464 | 116.484 | 116.367 | 116.190 | 10.699 | ||
| m4 | 79.813 | 86.505 | 82.846 | 8.919 | |||
| m5 | 45.423 | 40.946 | 6.156 | ||||
| m6 | 3.820 | 1.793 | |||||
| m7 | 0.139 |
Abbreviations: m, medium; h, high. Red text = statistically significant values.
Table 14.
The Log-rank statistics for matching the C2HEST risk strata for in-hospital mortality in male cohort.
| H2 | h3 | h4 | h5 | h6 | h7 | h8 | |
|---|---|---|---|---|---|---|---|
| m1 | 152.361 | 134.106 | 118.904 | 112.785 | 98.649 | 84.149 | 8.929 |
| m2 | 152.619 | 154.813 | 159.181 | 155.352 | 149.997 | 12.183 | |
| m3 | 116.694 | 121.473 | 118.900 | 115.004 | 10.673 | ||
| m4 | 84.079 | 82.389 | 79.865 | 8.909 | |||
| m5 | 58.586 | 58.244 | 7.628 | ||||
| m6 | 32.326 | 5.686 | |||||
| m7 | 2.769 |
Abbreviations: m, medium; h, high. Red text = statistically significant values.
3.5. Relationship of C2HEST Score with Non-Fatal Outcomes
Clinical non-fatal events in the C2HEST risk strata in both study arms are presented in Table 15. In both study cohorts, the subjects assigned to the C2HEST high-risk stratum were characterized by greater prevalence of pneumonia, acute kidney injury, and cardiovascular disorders during hospitalization. This observation regards myocardial injury, myocardial infarction, acute heart failure, and cardiogenic shock. Additional, female subjects with higher C2HEST values were more prone to subject a new episode of stroke/transient ischemic attack (TIA), and systemic inflammatory response syndrome (SIRS) during hospitalization. On the other hand, a high C2HEST score in the male subpopulation was associated with a higher probability of shock, acute liver dysfunction, and bleeding occurrence.
Table 15.
Clinical non-fatal events in the C2HEST risk strata in both study arms.
| Variables | Low Risk [0,1] |
Medium [2,3] |
High Risk [≥4] |
p-Value OMNIBUS |
p-Value for Post-Hoc Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females N = 682 |
Males N = 735 |
Females N= 284 |
Males N = 208 |
Females N = 135 |
Males N = 139 |
Females | Males | Females | Males | |
|
Shock n/n(%) |
34 (5.0) |
74 (10.1) |
15 (5.3) |
31 (14.9) |
11 (8.1) |
22 (15.8) |
0.3314 | 0.0443 | N/A | 0.2006 a 0.1958 b 1.0 c |
|
Hypovolemic shock n/n(%) |
9 (1.3) |
13 (1.8) |
4 (1.4) |
3 (1.4) |
5 (3.7) |
1 (0.7) |
0.1362 | 0.811 | N/A | N/A |
|
Cardiogenic shock n/n(%) |
2 (0.3) |
5 (0.7) |
1 (0.4) |
10 (4.8) |
5 (3.7) |
9 (6.5) |
0.0018 | <0.0001 |
1.0 a 0.0055 b 0.0439 c |
0.0007 a 0.0002 b 1.0 c |
|
Septic shock n/n(%) |
26 (3.8) |
62 (8.4) |
12 (4.2) |
18 (8.7) |
4 (3.0) |
18 (12.9) |
0.8198 | 0.2296 | N/A | N/A |
|
Venous thromboembolic disease n/n(%) |
30 (4.4) |
53 (7.2) |
18 (6.3) |
12 (5.8) |
8 (5.9) |
7 (5.0) |
0.4093 | 0.5447 | N/A | N/A |
|
Pulmonary embolism n/n(%) |
24 (3.5) |
44 (6.0) |
15 (5.3) |
11 (5.3) |
8 (5.9) |
5 (3.6) |
0.5516 | 0.8214 | N/A | N/A |
|
Myocardial infarction n/n(%) |
2 (0.3) |
6 (0.8) |
3 (1.1) |
7 (3.4) |
3 (2.2) |
5 (3.6) |
0.0251 | 0.0038 | 0.464 a 0.1026 b 1.0 c |
0.035 a 0.0586 b 1.0 c |
|
Myocardial injury, 3x, n/n(%)/N |
46 (17.5) 263 |
67 (16.1) 415 |
51 (29.8) 171 |
47 (35.1) 134 |
49 (52.1) 94 |
38 (39.2) 97 |
<0.0001 | <0.0001 |
0.0114 a
<0.0001 b 0.0017 c |
<0.0001 a,b 1.0 c |
|
Acute heart failure n/n(%) |
5 (0.7) |
3 (0.4) |
8 (2.8) |
14 (6.7) |
24 (17.8) |
22 (15.8) |
<0.0001 | <0.0001 | 0.0777 a <0.0001 b,c |
<0.0001 a,b
0.0329 c |
|
Stroke/TIA n/n(%) |
4 (0.6) |
14 (1.9) |
12 (4.2) |
7 (3.4) |
4 (3.0) |
3 (2.2) |
0.0002 | 0.4167 |
0.0006 a 0.0872 b 1.0 c |
N/A |
|
Pneumonia n/n(%) |
268 (39.3) |
414 (56.3) |
164 (57.4) |
141 (67.8) |
88 (65.2) |
98 (70.5) |
<0.0001 | 0.0004 |
<0.0001 a, b 0.5343 c |
0.0117 a 0.0076 b 1.0 c |
|
Complete respiratory failure n/n(%)/N |
23 (47.9) 48 |
34 (46.6) 73 |
16 (43.2) 37 |
30 (58.8) 51 |
20 (62.5) 32 |
23 (65.7) 35 |
0.2528 | 0.1348 | N/A | N/A |
|
SIRS n/n(%)/N |
53 (8.2) 647 |
89 (12.6) 705 |
22 (7.8) 283 |
20 (9.7) 206 |
21 (15.7) 134 |
15 (10.8) 139 |
0.0158 | 0.4818 | 1.0 a 0.0343 b 0.0636 c |
N/A |
|
Sepsis n/n(%)/N |
3 (1.0) 288 |
6 (2.1) 288 |
3 (2.9) 104 |
4 (5.1) 79 |
3 (5.3) 57 |
4 (5.9) 68 |
0.053 | 0.1334 | N/A | N/A |
|
Acute kidney injury n/n(%) |
37 (5.4) |
73 (9.9) |
30 (10.6) |
37 (17.8) |
28 (20.7) |
31 (22.3) |
<0.0001 | <0.0001 |
0.0193 a
<0.0001 b 0.0229 c |
0.0083 a 0.0002 b 1.0 c |
|
Acute liver dysfunction n/n(%)/N |
11 (1.9) 592 |
19 (2.9) 664 |
12 (4.5) 268 |
10 (5.1) 197 |
5 (4.0) 126 |
9 (7.1) 127 |
0.0619 | 0.0458 | N/A | 0.5214 a 0.0936 b 1.0 c |
|
Multiple organ dysfunction syndrome n/n(%) |
7 (1.0) |
14 (1.9) |
3 (1.1) |
5 (2.4) |
4 (3.0) |
4 (2.9) |
0.1674 | 0.6162 | N/A | N/A |
|
Bleedings n/n(%) |
27 (4.0) |
37 (5.0) |
13 (4.6) |
12 (5.8) |
9 (6.7) |
16 (11.5) |
0.3758 | 0.0128 | N/A | 1.0 a 0.0184 b 0.2545 c |
Continuous variables are presented as: mean ± SD range (minimum-maximum) and number of non-missing values. Categorized variables are presented as: a number with a percentage. Abbreviations: N, valid measurements; n, number of patients with parameter above cut-off point; SD, standard deviation; OMNIBUS, analysis of variance; TIA, transient ischemic attack; SIRS, systemic inflammatory response syndrome; N/A, non-applicable; a low risk vs. medium risk, b low risk vs. high risk, c medium risk vs. high risk. Red color text = statistically significant values.
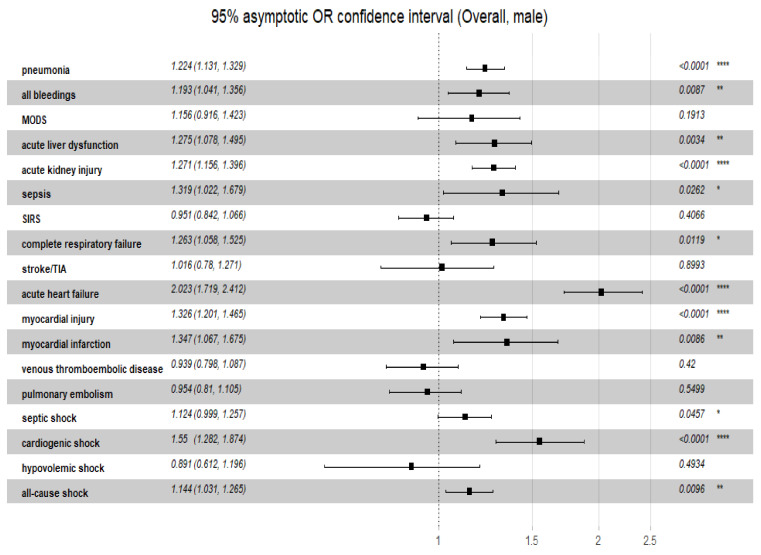
Additionally, the overall odds ratio for the discriminatory performance of the C2HEST score on the clinical non-fatal events was summarized in Figure 5 (female) and Figure 6 (male). Noteworthy, the highest predictive of C2HEST score value in the female cohort was achieved for, acute heart failure (ORoverall = 2.180, 95%CI 1.778–2.724, p = 0.0034). Similar findings were observed in the male cohort -the highest value was observed for acute heart failure (ORoverall = 1.861, 95%CI 1.574–2.229, p < 0.0001).
Figure 5.
The overall odds ratio for the discriminatory performance of the C2HEST score on the clinical non-fatal events in female cohort. Abbreviations: MODS, multiple organ dysfunction syndrome; TIA, transient ischemic attack; SIRS, systemic inflammatory response syndrome. Significance code: * <0.05; ** <0.01; *** <0.001; **** <0.0001.
Figure 6.
The overall odds ratio for the discriminatory performance of the C2HEST score on the clinical non-fatal events in female cohort. Abbreviations: MODS, multiple organ dysfunction syndrome; TIA, transient ischemic attack; SIRS, systemic inflammatory response syndrome. Significance code: * <0.05; ** <0.01; *** <0.001; **** <0.0001.
4. Discussion
Several studies demonstrated [11] no significant differences regarding the susceptibility to the SARS-CoV-2 infection between biological genders. Nevertheless, male gender is an independent risk factor for the poor outcome of COVID-19 including higher severity and fatality rates [12]. Various biological factors may play a role in sex-dependent different responses to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Biological sex affects the initial phase of infection mainly by sex-based differences in the expression of the ACE2 receptor responsible for the entry of the SARS-CoV-2 into the cells [13]. Sex differences affect also an immune response to viral infection. Females tend to have a lower potency to develop an uncontrolled inflammatory response process [14] with coexisting decreased viral load during the infection. The physiological mechanism of this process is multifactorial [15,16] and includes the sex-specific transcriptional regulatory network, various gen variants especially connected with chromosome X, epigenetic modifications, transcription factors, and sex steroids. Noteworthy, different social, behavioral, and comorbid factors are also postulated [17] to worsen the prognosis in men.
The previously observed sex-dependent dichotomy in the COVID-19 mortality was also confirmed in our study. For all of the three C2HEST strata, greater fatality rate in the male cohort compared to the female one was noted. Independently, we confirmed the previously reported usefulness of the C2HEST score in predicting the adverse COVID-19 outcomes, including the mortality in both genders. However, despite lower mortality observed in women, the ROC analysis revealed that the C2HEST-score is a more sensitive tool in women regarding the short- and mid-term (up to 6 month-) mortality (for 1-month the AUC = 72.5 vs. 70.3%and for 6-month AUC = 73.8 vs. 68.4 % in men, respectively). Gender is often considered among the variables defining the probability of a severe clinical outcome of infection.
Analysis of individual C2HEST score variables in both cohorts revealed differences between gender in features significantly affecting mortality. Beyond age and previously diagnosed heart failure common for both sexes, in the female group, only hypertension and COPD reached statistical significance. On the other hand, in the male cohort such observation was made for coronary artery disease. Although the pathophysiology underlying severe COVID-19 course remains not fully understood, it can be hypothesized that endothelial dysfunction induced by hypertension [18] might abolish the initial favorable female immune response [14] to SARS-CoV-2 infection. Moreover, the endothelial dysfunction promotes microvascular thrombi and pro-thrombotic state associated with respiratory failure and fatal outcome in COVID-19 [19]. On the other hand, the increased mortality rate of COVID-19 male patients with CAD is probably related to the presence of multiple comorbidities [20] or direct myocardial injury connected with enhanced platelet activation induced by SARS-CoV-2 infection [21].
It is noteworthy that, besides observed in both genders significant differences in mortality between the C2HEST strata, a similar relationship was noticed in the prevalence of pneumonia and cardiovascular non-fatal secondary outcomes (myocardial infarction, myocardial injury, acute heart failure, cardiogenic shock, and acute kidney failure). Our study revealed that in the male cohort alongside with higher C2HEST stratum, a greater rate of acute liver injury (ALI), bleedings and shock was present. This observation supports the previously described relationships between male gender and liver impairment in COVID subjects [22]. Although the mechanism of liver injury in SARS-CoV-2 infection remains unclear, a combination of direct viral inclusion of hepatocytes, as well as the result of uncontrolled immune, may be responsible for the damage, which interestingly, have also been associated with poor outcomes in COVID patients [23].
Furthermore, some data [5,24] suggests that individuals with gastrointestinal problems particularly those with earlier stages of liver impairment are more prone to develop severe COVID-19 disease with advanced respiratory failure. Concerning epidemiological data a higher prevalence of liver disorders [25] with coexisting higher susceptibility for endothelial dysfunction [26,27] may be important factors affecting outcomes in the male population.
It is possible that acute liver injury in the male cohort may be also partially responsible for the higher rate of bleedings as a result of coagulation systems disorders (mild elevations of INR, activated partial thromboplastin time (APTT), and thrombin time (TT)) observed in patients with ALI in course of COVID-19 [23,28]. Initial higher level of INR in males high-risk C2HEST score stratum seems to support this thesis. Although the principal clinical manifestation of severe COVID-19 is a respiratory failure with a coexisting uncontrolled immune reaction, subjects with COVID-19 show a high incidence of thromboembolic events [29], particularly in fatal cases [30], however antithrombotic treatment prior to COVID-19 infection is unlikely to have a protective effect [31]. Bleeding complications in subjects with COVID-19 give rise to justifiable concerns [32,33] and should always be considered before applying anticoagulation in patients with SARS-CoV-2 infection. Therefore several predictive scores [34] focused on identifying patients at increased risk for major bleeding have been recently proposed. Results of our study suggest that the C2HEST score might be also useful in the identification of the “high-risk for bleeding” subpopulations. However, subsequent studies are needed to define predictive value of the C2HEST score in terms of bleedings.
Limitations
We have observed several limitations of this study including the retrospective, single-center, character. These factors could affect the validity of our conclusions. Additionally, the study population was homogeneous and consisted of hospitalized patients and not involved ambulatory subjects. Furthermore, all hospitalizations were carried out in the face of limited resources (global COVID-19 pandemic) probably these extraordinary circumstances could partially affect the clinical outcomes.
5. Conclusions
To the best of our knowledge, this study is the first demonstration of the sex-dependent differences in the predictive value of the C2HEST score in subjects admitted to hospital due to SARS-CoV-2 infection. This simple risk score evaluated during the hospital admission could predict adverse outcomes in both including in-hospital and six-month-mortality and other clinical events such as acute kidney injury, myocardial injury acute heart failure, myocardial infarction, and cardiogenic shock. Additionally in the male cohort, it well correlated with acute liver injury and prevalence of all kinds of bleeding. The simplicity of this scale allows assuming that C2HEST-score might become a useful triage tool for risk stratification in both genders with COVID-19.
Acknowledgments
The authors are grateful to all the staff and the patients at the study center who contributed to this work.
Author Contributions
Conceptualization, P.R., A.D., M.T., E.A.J. and K.M.; methodology, P.R., A.D., M.T., E.A.J. and K.M.; software, K.G., K.K. (Krzysztof Kujawa) and M.S.; validation, K.G., K.K. (Krzysztof Kujawa) and M.S.; formal analysis, P.R., K.G., K.K. (Krzysztof Kujawa), M.S., A.D., E.A.J., and K.M.; investigation, P.R., A.D., M.T., J.G., T.M., E.S.-K., D.G., B.A., K.K. (Krzysztof Kaliszewski), K.K.-P., K.L., A.M.-W., M.P. (Michał Pomorski), M.P. (Marcin Protasiewicz), M.M., J.S., E.A.J. and K.M.; resources, P.R., A.D., M.T., J.G., T.M., E.S.-K., D.G., B.A., K.K. (Krzysztof Kaliszewski), K.K.-P., K.L., A.M.-W., M.P. (Michał Pomorski), M.P. (Marcin Protasiewicz), M.M., U.C., J.R.-Z., A.R., A.Z., W.P., J.S., E.A.J. and K.M.; data curation, P.R., A.D., M.T., J.G., T.M., E.S.-K., D.G., B.A., K.K. (Krzysztof Kaliszewski), K.K.-P., K.L., A.M.-W., M.P. (Michał Pomorski), M.P. (Marcin Protasiewicz), M.B., J.S., E.A.J. and K.M.; writing—original draft preparation, P.R., A.D., M.T., E.A.J. and K.M.; writing—review and editing, A.D., E.A.J. and K.M.; visualization, K.G., K.K. (Krzysztof Kujawa), M.S., P.R., A.D., M.T., M.B., E.A.J. and K.M.; supervision, A.D., M.T., E.A.J. and K.M.; project administration, A.D., M.T., E.A.J. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was not supported by any funds.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of Wroclaw Medical University, Wroclaw, Poland (Signature number: KB-444/2021).
Informed Consent Statement
The routine data were collected retrospectively. Therefore, written informed consent to participate in the study was not required. The Bioethics Committee approved the publication of anonymized data.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. [(accessed on 12 March 2022)]. Available online: https://covid19.who.int/
- 2.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. [(accessed on 1 December 2021)];Nat. Rev. Immunol. 2020 20:442–447. doi: 10.1038/s41577-020-0348-8. Available online: https://www.nature.com/articles/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendiola-Pastrana I.R., López-Ortiz E., Río de la Loza-Zamora J.G., González J., Gómez-García A., López-Ortiz G. SARS-CoV-2 Variants and Clinical Outcomes: A Systematic Review. Life. 2022;12:170. doi: 10.3390/life12020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukagoshi H., Shinoda D., Saito M., Okayama K., Sada M., Kimura H., Saruki N. Relationships between Viral Load and the Clinical Course of COVID-19. Viruses. 2021;13:304. doi: 10.3390/v13020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahban M., Stanek A., Hooshmand A., Khamineh Y., Ahi S., Kazim S.N., Ahmad F., Muronetz V., Samy Abousenna M., Zolghadri S., et al. Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients. J. Clin. Med. 2021;10:4802. doi: 10.3390/jcm10214802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y.G., Pastori D., Farcomeni A., Yang P.S., Jang E., Joung B., Wang Y.T., Guo Y.T., Lip G. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in Asian subjects: Derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest. 2019;155:510–518. doi: 10.1016/j.chest.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.-C., Müller M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Therneau T. A Package for Survival Analysis in R. R Package Version 3.2-7. [(accessed on 1 November 2021)]. Available online: https://CRAN.R-project.org/package=survival.
- 9.Hothorn T., Hornik K., Van De Wiel M.A., Zeileis A. A lego system for conditional inference. Am. Stat. 2006;60:257–263. doi: 10.1198/000313006X118430. [DOI] [Google Scholar]
- 10.Schratz P. R Package ‘Oddsratio’: Odds Ratio Calculation for GAM(M)s & GLM(M)s, Version: 1.0.2. [(accessed on 1 November 2021)]. Available online: [DOI]
- 11.Mukherjee S., Pahan K. Is COVID-19 Gender-sensitive? J. Neuroimmune Pharmacol. 2021;16:38–47. doi: 10.1007/s11481-020-09974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., Rosser E.C., Webb K., Deakin C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aleksova A., Gagno G., Sinagra G., Beltrami A.P., Janjusevic M., Ippolito G., Zumla A., Fluca A.L., Ferro F. Effects of SARS-CoV-2 on Cardiovascular System: The Dual Role of Angiotensin-Converting Enzyme 2 (ACE2) as the Virus Receptor and Homeostasis Regulator-Review. Int. J. Mol. Sci. 2021;22:4526. doi: 10.3390/ijms22094526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J. Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maleki Dana P., Sadoughi F., Hallajzadeh J., Asemi Z., Mansournia M.A., Yousefi B., Momen-Heravi M. An Insight into the Sex Differences in COVID-19 Patients: What are the Possible Causes? Prehosp. Disaster Med. 2020;35:438–441. doi: 10.1017/S1049023X20000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gemmati D., Bramanti B., Serino M.L., Secchiero P., Zauli G., Tisato V. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int. J. Mol. Sci. 2020;21:3474. doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haitao T., Vermunt J., Abeykoon J., Ghamrawi R., Gunaratne M., Jayachandran M., Narang K., Parashuram S., Suvakov S., Garovic V. COVID-19 and sex differences: Mechanisms and biomarkers. Mayo Clin. Proc. 2020;95:2189–2203. doi: 10.1016/j.mayocp.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doroszko A., Andrzejak R., Szuba A. Role of the nitric oxide metabolic pathway and prostanoids in the pathogenesis of endothelial dysfunction and essential hypertension in young men. Hypertens. Res. 2011;34:79–86. doi: 10.1038/hr.2010.169. [DOI] [PubMed] [Google Scholar]
- 19.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. NEJM. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 21.Sheth A.R., Grewal U.S., Patel H.P., Thakkar S., Garikipati S., Gaddam J., Bawa D. Possible mechanisms responsible for acute coronary events in COVID-19. Med. Hypotheses. 2020;143:110125. doi: 10.1016/j.mehy.2020.110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei F., Liu Y.M., Zhou F., Qin J.J., Zhang P., Zhu L., Zhang X.J., Cai J., Lin L., Ouyang S., et al. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phipps M.M., Barraza L.H., LaSota E.D., Sobieszczyk M.E., Pereira M.R., Zheng E.X., Fox A.N., Zucker J., Verna E.C. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U. S. Cohort. Hepatol. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahlawat S., Sharma K.K. Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res. 2020;286:198103. doi: 10.1016/j.virusres.2020.198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon A.M., Singal A.G., Tapper E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2020;18:2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doroszko A., Szahidewicz-Krupska E., Janus A., Jakubowski M., Turek A., Ilnicka P., Szuba A., Mazur G., Derkacz A. Endothelial dysfunction in young healthy men is associated with aspirin resistance. Vasc. Pharmacol. 2015;67–69:30–37. doi: 10.1016/j.vph.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Stanek A., Fazeli B., Bartuś S., Sutkowska E. The Role of Endothelium in Physiological and Pathological States: New Data. Biomed Res. Int. 2018;2018:1098039 doi101155/2018/1098039. doi: 10.1155/2018/1098039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin X., Duan Y., Bao T., Gu J., Chen Y., Li Y., Mao S., Chen Y., Xie W. The values of coagulation function in COVID-19 patients. PLoS ONE. 2020;15:e0241329. doi: 10.1371/journal.pone.0241329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondal S., Quintili A.L., Karamchandani K., Bose S. Thromboembolic disease in COVID-19 patients: A brief narrative review. J. Intensive Care. 2020;8:70. doi: 10.1186/s40560-020-00483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiese A., Manetti A.C., La Russa R., Di Paolo M., Turillazzi E., Frati P., Fineschi V. Autopsy findings in COVID-19-related deaths: A literature review. Forensic Sci. Med. Pathol. 2021;17:279–296. doi: 10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Protasiewicz M., Reszka K., Kosowski W., Adamik B., Bombala W., Doroszko A., Gajecki D., Gawryś J., Guziński M., Jedrzejczyk M., et al. Anticoagulation Prior to COVID-19 Infection Has No Impact on 6 Months Mortality: A Propensity Score–Matched Cohort Study. J. Clin. Med. 2022;11:352. doi: 10.3390/jcm11020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorgalaleh A. Bleeding and Bleeding Risk in COVID-19. Semin. Thromb Hemost. 2020;46:815–818 doi101055/s. doi: 10.1055/s-0040-1713434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayakrishnan T., Haag A., Mealy S., Minich C., Attah A., Turk M., Alrifai N., Alhuneafat L., Khoury F., Nasrullah A., et al. Novel Coronavirus Infection (COVID-19) Related Thrombotic and Bleeding Complications in Critically Ill Patients: Experience from an Academic Medical Center. J. Clin. Med. 2021;10:5652. doi: 10.3390/jcm10235652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demelo-Rodriguez P., Galeano-Valle F., Ordieres-Ortega L., Siniscalchi C., Martín Del Pozo M., Fidalgo Á., Gil-Díaz A., Lobo J.L., De Ancos C., Monreal M. For the RIETE-Bleeding Investigators. Validation of a Prognostic Score to Identify Hospitalized Patients with COVID-19 at Increased Risk for Bleeding. Viruses. 2021;13:2278. doi: 10.3390/v13112278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.