Abstract
Background
Even with optimal disease‐modifying treatment and good control of disease activity, persistent pain due to structural damage is common in people with inflammatory arthritis and therefore additional treatment for pain might be required. Because comorbidity is highly prevalent in people with inflammatory arthritis, it is important to consider comorbidities such as gastrointestinal or liver diseases in deciding upon optimal pharmacologic pain therapy.
Objectives
To assess the efficacy and safety of pharmacological pain treatment in patients with inflammatory arthritis who have gastrointestinal or liver comorbidities, or both.
Search methods
We searched Cochrane CENTRAL, MEDLINE and EMBASE for studies, to June 2010. We also searched the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) conference abstracts (2007 to 2010) and performed a handsearch of reference lists of articles.
Selection criteria
All randomised or quasi‐randomised controlled trials (RCTs or CCTs) were considered for inclusion, for the assessment of efficacy. For safety, we also considered single arm trials, controlled before‐after studies, interrupted time series, cohort and case‐control studies, and case series of 10 or more consecutive cases. Pain therapy comprised paracetamol, non‐steroidal anti‐inflammatory drugs (NSAIDs), opioids, opioid‐like drugs (tramadol) and neuromodulators (antidepressants, anticonvulsants and muscle relaxants). The study population comprised adults (≥ 18 years) with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis or other spondyloarthritis who had gastrointestinal or hepatic, or both, comorbid conditions. Outcomes of interest were pain, adverse effects, function and quality of life. Studies that included a mixed population of inflammatory arthritis and other conditions were included only if results for inflammatory arthritis were reported separately.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed risk of bias and extracted data.
Main results
From 2869 identified articles only one single arm open trial fulfilled our inclusion criteria. This trial assessed the safety and efficacy of naproxen (dosage not specified) in 58 patients with active rheumatoid arthritis and gastrointestinal comorbidities for up to 52 weeks. Thirteen participants (22%) remained on gold therapy, four participants (10%) remained on hydroxychloroquine, 27 (47%) remained on corticosteroids, 12 (21%) remained on salicylates and all participants continued on antacids and a bland diet. The presence of faecal occult blood was reported in 1/58 participants tested between weeks 1 to 26 and 2/32 participants tested between weeks 27 to 52. Over the course of the study, seven participants (12.1%) withdrew due to adverse events but, of these, only two participants withdrew due to gastrointestinal side effects (abdominal pain n = 1, nausea n = 1) and no serious adverse events were reported. It was noteable that out of 14 studies excluded due to inclusion of a mixed population (osteoarthritis or other rheumatic conditions) or an intervention that was already withdrawn, five trials reported a higher risk of developing gastrointestinal events in patients with prior gastrointestinal events when treated with NSAIDs.
Authors' conclusions
On the basis of the current review, there is scant evidence to guide clinicians about how gastrointestinal or liver comorbidities should influence the choice of pain treatment in patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis or other spondyloarthritis. Based upon additional studies that included a mixed population of participants with a range of rheumatic conditions, NSAIDs should be used cautiously in patients with inflammatory arthritis and a history of gastrointestinaI comorbidity as there is consistent evidence that they may be at increased risk.
Keywords: Female; Humans; Male; Middle Aged; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/adverse effects; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Arthritis; Arthritis/complications; Arthritis/drug therapy; Gastrointestinal Diseases; Gastrointestinal Diseases/complications; Gastrointestinal Diseases/therapy; Liver Diseases; Liver Diseases/complications; Liver Diseases/therapy; Naproxen; Naproxen/adverse effects; Naproxen/therapeutic use; Pain; Pain/drug therapy; Pain Management; Pain Management/methods
Plain language summary
Pain in patients with inflammatory arthritis and gastrointestinal or liver problems
This summary of a Cochrane review presents what we know from research about the effect of pain relieving drugs for people with inflammatory arthritis plus stomach or gut disease, or liver disease.
The review shows the following in people with inflammatory arthritis plus stomach or gut disease, such as hernia or stomach or intestinal ulcers or previous bleeding from the stomach or intestine, or patients with liver diseases such as hepatitis or fatty liver:
‐ We are uncertain if naproxen (Aleve®, Naprosyn®) produced more side effects in people with inflammatory arthiritis plus stomach or intestine disease, compared with people with inflammatory arthritis without stomach or intestine disease, because only a single study giving low quality evidence was available.
‐ No studies were found that looked at pain relief.
‐ No studies were found that looked at other pain‐relieving drugs.
‐ No studies were found in people with conditions other than rheumatoid arthritis.
‐ No studies were found in people with inflammatory arthritis plus liver disease.
We do not have precise information about side effects and complications, particularly for rare but serious side effects. Possible side effects associated with high dose paracetamol include liver problems. Aspirin and NSAIDs may cause stomach, kidney or heart problems.
What is inflammatory arthritis and what is pain management
Inflammatory arthritis is a group of diseases that includes rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and other types of spondyloarthritis. When you have inflammatory arthritis your immune system, which normally fights infection, attacks your joints. This makes your joints swollen, stiff and painful. In rheumatoid arthritis, the small joints of your hands and feet are usually affected first. In contrast, in ankylosing spondylitis the joints of the spine are the most affected. There is no cure for inflammatory arthritis at present, so the treatments aim to relieve pain and stiffness and improve your ability to move.
Paracetamol, or acetaminophen, is used to relieve pain but does not affect swelling; non‐steroidal anti‐inflammatory drugs (NSAIDs) such as ibuprofen, diclofenac, naproxen and COX‐2s (for example celecoxib) are used to decrease pain and swelling. Opioids, such as codeine‐containing Tylenol®, hydromorphone (Dilaudid®), oxycodone (Percocet®, Percodan®), morphine and the opioid‐like drug tramadol are powerful pain‐relieving substances. Other drugs have some pain‐relieving properties and can therefore be used to control pain. This is the case of the so‐called neuromodulators, such as antidepressants (for example fluoxetine, paroxetine, amitriptyline), anticonvulsants (for example gabapentine, pregabaline) or muscle relaxants (for example diazepam).
Background
Description of the condition
Chronic inflammatory arthritis (IA) of various types are common, affecting about 3% of the population (Bergmann 2006). The spectrum of IA includes diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS) and other forms of spondyloarthritis (SpA). Typically IA is characterized by a progressive course of synovial inflammation leading to joint destruction (Drossaers‐Bakker 1999), which can be effectively treated by disease modifying antirheumatic drugs. However, despite significant progress in the development of new therapeutics, including the biologics, pain that is either related or unrelated to disease activity persists in many patients causing substantial impairment of physical function and quality of life (Bergmann 2006; Minnrock 2003).
Description of the intervention
Although suppressing inflammation is the primary target for treatment of IA, maintaining functional integrity is its ultimate goal (Smolen 2010). Treating pain is thus a crucial step to achieve this goal and drugs that may be used for this purpose include paracetamol, non‐steroidal anti‐inflammatory drugs (NSAIDs), opioids and opioid‐like drugs (such as tramadol) and neuromodulators (including antidepressants, anticonvulsants and muscle relaxants). Some of these therapies, such as the NSAIDs, have been a standard treatment in IA as they can improve symptoms, although they have limited impact on disease progression (Emery 2006).
While the regular use of these medications in otherwise healthy individuals is not without risk, the potential for toxicity is likely to be increased in patients with pre‐existing comorbidities. Patients with IA are at an increased risk of developing extensive comorbid conditions (Gabriel 1999; Michaud 2007) not only due to the long course of the inflammatory disease process but also because of advanced age over time. For example, data from an Australian observational registry of IA indicates that about two thirds of patients starting biologic therapy for RA or AS have comorbidities at baseline and approximately one third self‐report the presence of gastrointestinal disease (Briggs 2009; Oldroyd 2009). Therefore, the consideration of comorbidities and the risk associated with them is important when prescribing medications to alleviate pain for patients with IA.
How the intervention might work
Two common comorbidities to consider include gastrointestinal and hepatic disease. Many drugs have an oral route of application and gastrointestinal adverse events are common causes for stopping these medications (Boswell 2010; Ng 2010). In addition, many drugs are metabolised by the liver, which might therefore increase the risk of adverse events, especially in patients with known liver disease.
Why it is important to do this review
In patients with IA, regardless of disease activity, pain management is a central issue. It is identified by patients to be among their highest priorities (da Silva 2001; Heiberg 2002). This review is one of two reviews that aim to summarise the existing data on the efficacy and safety of pharmacological pain treatment in patients with IA who also have comorbidities. This review focuses on the coexistence of gastrointestinal or hepatic comorbidity while a second review focuses on cardiovascular and renal comorbidities in patients with rheumatoid arthritis (Marks 2010). The issue of pain management in IA patients with gastric or liver comorbidities has not yet been addressed in a systematic review, hence reviewing existing evidence should provide important information for clinicians as well as patients.
Objectives
To assess the efficacy and safety of pharmacologic pain therapy in patients with IA (RA, PsA, AS, SpA) who have concomitant gastrointestinal or hepatic disease, or both.
Methods
Criteria for considering studies for this review
Types of studies
All published randomised controlled trials (RCTs) or controlled clinical trials with pseudo‐randomised methods of allocating treatment (CCTs) were considered for inclusion, for assessment of efficacy.
To assess safety, we also considered controlled before‐after studies (CBA), interrupted time series (ITS), single arm trials, cohort studies, case‐control studies and case series. The case series had to have at least 10 cases followed consecutively to be included.
Only studies that were published as full articles or were available as a full trial report were included. There were no language restrictions.
Types of participants
We selected studies including adults of at least 18 years of age with a clinical diagnosis of either RA, AS, PsA or SpA, and a reported concomitant gastrointestinal or hepatic comorbid condition. For controlled studies we included studies that included participants with and without gastrointestinal or hepatic comorbidities. Studies that included a mixed population of IA and other conditions were included only if the results for IA were reported separately.
Types of interventions
Interventions that were included were paracetamol (acetaminophen), NSAIDs, opioids or opioid‐like drugs (tramadol) and neuromodulators, including antidepressants, anticonvulsants and muscle relaxants. These drugs were considered irrespective of route, dosage, frequency or duration of treatment. Drugs that had been withdrawn from the marketplace were excluded.
Types of outcome measures
Major outcomes
Efficacy: participant‐reported pain relief of 50% or greater, reported on visual analogue scales, numerical rating scale, verbal rating scales.
Safety: number of withdrawals due to adverse events.
Minor outcomes
The following outcomes were also included.
-
Safety:
worsening of comorbid condition (for instance recurrence of ulcer bleeding, worsening of endoscopy grade of ulcers, increase in serum transaminases);
any adverse events;
withdrawals due to inadequate analgesia.
-
Pain:
participant‐reported pain relief of 30% or greater;
participant‐reported global impression of clinical change (PGIC) 'much' or 'very much' improved;
proportion of patients achieving a pain score below 30/100 mm on a visual analogue scale; and
mean pain score on a visual analogue scale or numerical rating scale.
Function: for RA, for example, as measured by the Health Assessment Questionnaire (HAQ) or modified HAQ (Fries 1980; Pincus 1983), or for AS by the Bath Ankylosing Spondylitis Functional Index (BASFI) (Calin 1994);
Quality of life: as measured by either generic instruments, such as the Short‐Form‐36 (SF‐36) (Ware 1992), or disease‐specific tools such as the Rheumatoid Arthritis Quality of Life instrument (RAQoL) (Doward 2003) and Ankylosing Spondylitis Quality of Life Instrument (ASQoL) (Jong 1997).
The pain outcomes were chosen based upon those currently recommended by the Cochrane Pain, Palliative and Supportive Care Review Group editors and others for systematic reviews on chronic pain (Moore 2010). However, the primary efficacy endpoint of participant‐reported pain relief of 50% or greater was chosen to be consistent with the recommendations of the Cochrane Musculoskeletal Group for choice of summary of findings outcomes to present for reviews in RA.
The outcomes were evaluated for the following endpoints if available in the included studies: (a) short‐term (1 to 6 weeks), (b) intermediate‐term (> 6 to 12 weeks), and (c) long‐term (> 12 weeks) follow‐up. These intervals were chosen based upon the recommendations of Moore et al (Moore 2010).
The short and long‐term outcomes for the proportion reporting pain relief of 50% or greater, withdrawals due to adverse effects, adverse events, and worsening of the comorbid condition were chosen a priori to be presented in the summary of findings table.
Search methods for identification of studies
Electronic searches
The following electronic databases were used to identify relevant studies: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 6); MEDLINE (from 1950); and EMBASE (from 1980), without language restrictions. The literature search was last updated on 18 June, 2010.
Specific MeSH headings and additional keywords were used to identify all relevant studies. The complete search strategies for the database searches are provided in the Appendices (MEDLINE and CENTRAL search strategy: Appendix 1, EMBASE search strategy: Appendix 2).
Searching other resources
To ensure that all potential studies were identified we also performed a search in the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE). We manually searched the bibliographies of all included papers for information on any other relevant studies. We also handsearched the conference proceedings for the American College of Rheumatology (ACR) and the European League against Rheumatism (EULAR) (2007 to 2010) to identify unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (HR, SR) independently screened each title and abstract for suitability of the study for inclusion in the review. They decided, independent of each other, the eligibility of the article according to the predetermined selection criteria (see Criteria for considering studies for this review). If more information was needed to decide upon the eligibility of the studies, the full text of the paper was obtained. Reasons for excluding studies were documented and disagreement between the review authors was resolved by consensus, or after the input of the third author (DA) who had the final decision.
Data extraction and management
Two independent review authors (HR and SR) extracted data from the included studies onto a standardised form. Information extracted included study design, characteristics of study population, characteristics of comorbid condition, treatment regimen and duration, outcomes including adverse events and timing of outcome assessment. The raw data (means and standard deviations for continuous outcomes and number of events or participants for dichotomous outcomes) were extracted for the outcomes of interest.
Differences in data extraction were resolved by referring back to the original articles and establishing consensus. A third review author (DA) was consulted to help resolve discrepancies, as needed.
For studies published in languages other than English, German, Portuguese, French, Spanish or Dutch, the help of a native speaker or translator with content and methodological expertise was obtained, as necessary.
Assessment of risk of bias in included studies
The potential for bias in included RCTs and CCTs was to be assessed independently by two review authors (HR, SR) using methods recommended by The Cochrane Collaboration (Higgins 2011b). The following items were to be assessed:
random sequence generation;
allocation concealment;
blinding of participants, care provider and outcome assessor for each outcome measure (see Types of outcome measures);
incomplete outcome data;
selective reporting; and
other potential sources of bias.
To determine the risk of bias of a study, for each criterion the presence of sufficient information and the likelihood of potential bias were to be evaluated. Each criterion was to be rated as low risk of bias, high risk of bias or as unclear (either lack of information or uncertainty over the potential for bias). Disagreements among the review authors were to be discussed and resolved in a consensus meeting. If consensus could not be reached, a third review author (RB) was to make the final decision.
For CBAs and ITS, we planned to use the criteria described by the Cochrane Effective Practice and Organisation of Care (EPOC) Group, which describes seven standard criteria for each of these study types, scored as done, not done or not clear (EPOC 2008).
For cohort and case‐control studies, we planned to use the Newcastle‐Ottawa Scale (NOS) (Wells 2008), which assesses bias according to selection, comparability and outcome.
For case series, the guidance recommended by the Centre for Reviews and Dissemination (CRD 2001) was to be used. This assesses the following criteria.
Is the study based on a representative sample selected from a relevant population?
Are the criteria for inclusion explicit?
Did all individuals enter the survey at a similar point in their disease progression?
Was follow‐up long enough for important events to occur?
Were outcomes assessed using objective criteria or was blinding used?
If comparisons of subseries are being made, was there sufficient description of the series and the distribution of prognostic factors?
Measures of treatment effect
Raw data for outcomes of interest (means and standard deviations for continuous outcomes and number of events for dichotomous outcomes) as well as number of participants were to be extracted if available from the published reports. The results of the studies were to be analysed and collated using Review Manager 5. Summarising the data in a meta‐analysis was planned only if the data from the different studies were sufficiently clinically and statistically homogeneous.
For continuous data, results were planned to be analysed as mean differences between the intervention and comparator groups (MD), with corresponding 95% confidence intervals. The mean difference between the treated group and control group is weighted by the inverse of the variance in the pooled treatment estimate. However, if different scales were used to measure the same conceptual outcome (for example functional status or pain), we planned to calculate standardised mean differences (SMD) with corresponding 95% confidence intervals. SMDs were to be calculated by dividing the MD by the standard deviation, resulting in a unitless measure of treatment effect. A 95% CI would be computed for the SMD and, upon completion of the analysis, the SMD would be translated back into a mean difference. For dichotomous data, a relative risk (RR), odds ratios (OR) or hazard ratio (HR) with corresponding 95% confidence interval was planned to be calculated, as appropriate.
Unit of analysis issues
Unit of analysis problems were not expected in this review. In the event that cross‐over trials were identified in which the reporting of continuous outcome data precluded paired analysis, these data were not to be included in a meta‐analysis in order to avoid unit‐of‐analysis error. Where carry‐over effects were thought to exist, and where sufficient data existed, only data from the first period were planned to be included in the analysis (Higgins 2011c).
Dealing with missing data
If any important data were missing, we planned to contact the study authors.
For dichotomous outcomes that measured adverse events (for example number of withdrawals due to adverse events), the number of patients that received treatment was to be used for the denominator (worst case analysis). For dichotomous outcomes that measured benefits (for example proportion of participants achieving 30% or more reduction in pain), the worst case analysis was to be calculated using the number of participants as the denominator.
For continuous outcomes (for example pain), we planned to calculate the MD or SMD based on the number of patients analysed at the time point. If the number of patients analysed was not presented for each time point, the number of randomised patients in each group at baseline was planned to be used.
Where possible, missing standard deviations were to be computed from other statistics such as standard errors, confidence intervals or P values, according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If standard deviations could not be calculated, they were to be imputed (for example from other studies) in the meta‐analysis (Higgins 2011c).
Assessment of heterogeneity
As we included non‐randomised studies in this review to assess safety, we considered that heterogeneity was likely to be greater than if RCTs alone were included, and we would be less likely to be able to pool our findings quantitatively. We planned to only pool studies with the same design.
Prior to meta‐analysis, we planned to assess studies for clinical homogeneity with respect to type of therapy, control group and the outcomes. For any studies judged as clinically homogeneous, statistical heterogeneity was planned to be assessed using the I2 statistic (Deeks 2011) using the following as a rough guide for interpretation: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% considerable heterogeneity. In cases of considerable heterogeneity (defined as I2 ≥ 75%), we planned to explore the data further, including subgroup analyses, in an attempt to explain the heterogeneity.
Assessment of reporting biases
In order to determine whether reporting bias was present, we planned to identify if the protocols of any included RCTs were published before recruitment of patients into the study was started. For studies published after 1 July 2005, we planned to screen the Clinical Trials Register at the International Clinical Trials Registry Platform of the World Health Organization (http://apps.who.int/trialssearch) (DeAngelis 2004). We planned to evaluate whether selective reporting of outcomes was present (outcome reporting bias).
We planned to compare the fixed‐effect model estimate against the random‐effects model to assess the possible presence of small sample bias in the published literature (that is where the intervention effect is more beneficial in smaller studies). In the presence of small sample bias, the random‐effects model estimate of the intervention is more beneficial than the fixed‐effect model estimate (Sterne 2011).
The potential for reporting bias was planned to be further explored by funnel plots if ≥ 10 studies were available.
We did not identify sufficient trials to assess reporting bias as planned.
Data synthesis
Where studies were sufficiently homogeneous that it was clinically meaningful for them to be pooled, meta‐analysis was planned using a random‐effects model, regardless of the I2 statistic results. However, we did not identify sufficient studies to perform meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Sufficient data were not available to undertake planned subgroup analyses:
differences between different comorbid conditions and their severity (e.g. ulcer bleeding versus dyspepsia);
differences between type of IA (RA versus AS, PsA, SpA);
Patients' ages (< 65 years versus ≥ 65 years).
Sensitivity analysis
Sufficient studies were not identified to perform planned sensitivity analyses: to assess the impact of any bias attributable to inadequate or unclear treatment allocation (including studies with pseudo‐randomised designs).
Summary of findings table
The planned summary of findings table could not be produced due to a lack of studies. The table would provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes (number of withdrawals due to adverse events, short and long‐term outcomes for pain, function and worsening of comorbid conditions), as recommended by The Cochrane Collaboration (Schünemann 2011a). It would include an overall grading of the evidence related to each of the main outcomes, using the GRADE approach (Schünemann 20011b).
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies
Results of the search
A total of 2869 articles were identified by the search strategy (Figure 1). After a title and abstract screen, 2795 articles were excluded. Of 73 articles retrieved for detailed review, only one study fulfilled the inclusion criteria (Roth 1975) (see Characteristics of included studies).
1.
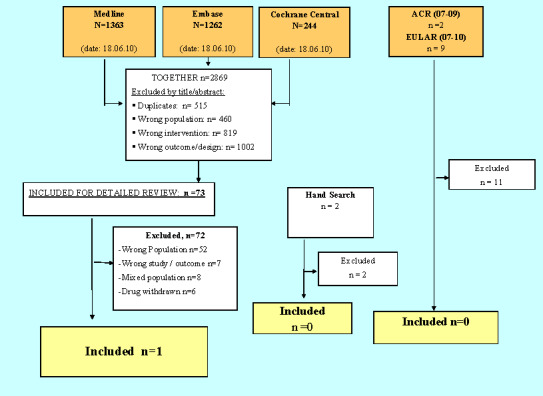
Flow chart of the results of the search strategy, outlining the main reasons for study exclusion.
Included studies
Roth 1975 included 58 patients with active RA and concomitant upper gastrointestinaI comorbidities in an open single arm trial. The gastrointestinaI comorbidities were defined as 'gastrointestinal dysfunction of varying degrees of severity'. In addition, the patients were reported to have an intolerance to anti‐inflammatory medication. To assess the safety of naproxen in this cohort of patients, participants in the trial received naproxen (dosage not specified) for up to 52 weeks. Participants were allowed to continue on stable maintenance doses of corticosteroids, gold or antimalarials. All other NSAIDs were discontinued but salicylates, while discouraged, were permitted provided their use was reported. In addition, patients continued on antacids and a bland diet.
The main outcomes of the study, as identified in the results, were gastrointestinal side effects assessed by faecal occult blood testing, side effects and side effects leading to discontinuation of treatment. Response to treatment was also evaluated according to the number of painful or tender joints, number of swollen joints, number of hot or red joints, number of clinically active joints; therapeutic response to naproxen on a scale of poor, fair, good or excellent was also reported. There was no indication of who performed the outcome assessment and the exact timing of outcome assessment was not specified but it was reported as occurring at baseline and in weeks 1 to 26 and weeks 27 to 52.
The mean ages of females and males were reported to be 50.8 and 52.0 years respectively, mean disease duration was reported to be 170 months or more, and 62.1% of the study population were female. Thirteen participants (22%) remained on gold therapy, four participants (10%) remained on hydroxychloroquine, 27 (47%) remained on corticosteroids and 12 (21%) remained on salicylates. The types of gastrointestinaI dysfunction in the cohort included hiatus hernia (as determined by upper gastrointestinal x‐ray examination at baseline) (n = 40), gastric ulcer (n =12), duodenal ulcer (n = 23), gastric hypersecretion (n = 25), pylorospasm (n = 12), gastritis (n = 8) and gastric resection (n = 6). Twenty‐six participants had both a hiatus hernia and either a gastric or duodenal ulcer while 10 participants had all three. A number of participants' were reported to have had upper gastrointestinaI bleeding problems prior to entering the study.
Excluded studies
The main reason for exclusion of studies after detailed review was that the studies did not include participants with hepatic or gastrointestinaI comorbidities (Adam 1991; Alvart 1983; Ammitzboll 1979; Andrada 1991; Appelboom 1994; Ashcroft 2001; Bernstein 1992; Burry 1980; Busson 1986; Cardoe N 1977; Caruso 1994; Castellsague 2009; de Melo Gomes 1992; Doggrell 2005; Emery 1999; Eversmeyer 1993; Fenton 1988; Fossgreen 1976; Franssen MJ; Furst 2002; Goldstein 2000; Goldstein 2004; Graziano 1991; Grennan 1977; Hernández‐Diaz 2010; Howard 1978; Hunter 1996; Huskisson 1991; Huskisson 1996; Jajic 2005; Kean 1993; Kennedy 1991; Koppes 1974; Krueger 2008; Laine 2006; Laine 2007; Lehtinen 1987; Lisse JR; Lussier 1976; Magaro 1989; Muller‐Fassbender 1975; Niculescu 2009; O´Brien 1976; Perez‐Ruiz 1996; Porkodi 1987; Richy F; Rooney 1974; Rostom 2005; Roth 1976; Schmid 1976; Seidman 1988; Simon 1987; Simpson 1989; Singh 1996; Soni 2009; Tausch 1976; Teule 1986; Turner 1988; Wollheim 1978; Zutshi 1976).
A further six articles were excluded as they concerned drugs that had been withdrawn from the market (Bombardier 2000; Eisen 2005; Goldstein 2004; Laine 2002; Reinisch 2003; Watson 2004). Although all studies other than Watson 2004 also included data concerning NSAIDs that had not been withdrawn, data that could address our research question were not presented separately.
One study (El‐Serag 1997) was excluded due to reporting the wrong outcome, and six studies (Geis 1993; Koppes 1974; Lazzaroni 2007; Nielsen 2006; Rahme 2007; Wong 2008) were excluded as they were narrative reviews not providing any data for extraction.
Finally, eight studies that included a mixed population of osteoarthritis (OA), IA and other rheumatic conditions such as low back pain were excluded as it was not possible to extract separate results for patients with IA (Chan 2002; Chan 2004; Chan 2010; Degner 2000; Laine 2008; Manniche 1987; Roth 1995; Seissiger 1987). We tried unsuccessfully to contact the first authors to obtain this data but received no response.
Of 11 meeting abstracts from the annual ACR and EULAR conferences (2007 to 2010) that were identified, only one appeared to meet the inclusion criteria, but it was excluded as no full paper was available and the study included a mixed population (Karateev 2008).
A summary of the search results and main reason for exclusion are depicted in Figure 1.
Risk of bias in included studies
As the included study was an open single arm trial it was at high risk of bias (see Table 1).
1. Risk of bias assessment.
| Quality Assessment | Authors judgment | Explanation |
| Is the study based on a representative sample selected from a relevant population? | NO | only 58 patients with active rheumatoid arthritis and upper GI dysfunction |
| Are the criteria for inclusion explicit? | NO | only described: patient with active RA but not according to which disease activity score or who decided whether active; or not; GI dysfunction of varying degree and severity assessed by whom? |
| Did all individuals enter the survey at a similar point in their disease progression? | NO | only quoted 58 patients with active rheumatoid arthritis and upper GI dysfunction, GI‐ comorbidities of varying degree and severity; disease activity of RA unclear |
| Was follow‐up long enough for important events to occur? | YES | 52 weeks |
| Were outcomes assessed using objective criteria or was blinding used? | NO | outcome was assessed 'between week 1‐26' or 'between weeks 27‐52' |
| If comparisons of subseries are being made, was there sufficient description of the series and the distribution of prognostic factors? |
No subseries were made |
Effects of interventions
People with RA and concomitant gastrointestinal comorbidities treated with NSAIDs
Efficacy
No data suitable for meta‐analysis could be extracted from the open single arm trial (Roth 1975). Roth and Boost reported significant reductions in the mean number of painful or tender joints (21.6; 13.5; 7.6), swollen joints (16.5; 11.4; 6.9), hot or red joints (8.7; 3.0; 0.3) and clinically active joints (21.9; 14.8; 9.8), from baseline to weeks 1 to 26 and weeks 26 to 52 respectively. At weeks 1 to 26, 12 participants were reported to have an excellent therapeutic response, 17 a good response, 12 a fair response and 17 a poor response. At weeks 27 to 52 weeks, 13, 17, three and two participants had an excellent, good, fair and poor response respectively. Fourteen participants (24.1%) were reported to have withdrawn from the study due to lack of efficacy, although the time point was not specified.
Safety
No data suitable for meta‐analysis could be extracted from the open single arm trial (Roth 1975). Roth and Boost reported the presence of faecal occult blood in 0/54 participants tested at baseline, 1/58 participants tested between weeks 1 to 26 and 2/32 participants tested between weeks 27 to 52.
Over the course of the study, seven participants (12.1%) withdrew due to adverse events but of these only two participants withdrew due to gastrointestinaI side effects (abdominal pain: n = 1, nausea: n = 1) and no serious adverse events were reported (see Characteristics of included studies). Over the duration of follow‐up, a variety of side effects were reported including nausea (n = 4), diarrhoea (n = 3), constipation (n = 1), abdominal pain (n = 6), dyspepsia (n = 5), melena (n = 1), skin eruption (n = 2), itching (n = 2), insomnia (n = 2), blurred vision (n = 2) and proteinuria (n = 1). For weeks 1 to 26 weeks only nine participants reported side effects of mild to moderate severity, and for weeks 27 to 52 only three participants reported mild to moderate side effects.
Due to lack of data, subgroup and sensitivity analyses could not be performed and a 'Summary of findings' table could not be constructed.
Discussion
The intention of this systematic review was to summarise the evidence for the efficacy and safety of pharmacological pain therapies in patients with IA and gastrointestinal or liver comorbidities. Despite the fact that a considerable percentage of patients with IA who are seen in daily practice will have comorbid disease, including gastrointestinal and hepatic conditions (Gabriel 1999; Michaud 2007; Mikulis 2003), these patients are often excluded from clinical trials. We were only able to identify a single study at high risk of bias that fulfilled our inclusion criteria and it provided limited data about the efficacy and safety of NSAIDs in RA patients with pre‐existing gastrointestinal comorbidity.
Summary of main results
Only one open single arm trial at high risk of bias, investigating the safety and efficacy of naproxen taken for up to a year in 58 patients with active RA and concomitant gastrointestinal comorbidity, could be included. In terms of safety, the presence of faecal occult blood was found in 3/58 participants. Furthermore, seven participants (12.1%) withdrew due to adverse effects, including only two who withdrew due to gastrointestinal adverse effects. No serious adverse events were reported. Regarding efficacy in terms of pain management, the authors reported a significant reduction in the number of painful and tender joints. Over the course of the year just under a quarter (24.1%) of participants withdrew due to lack of efficacy.
Overall completeness and applicability of evidence
There is a paucity of evidence regarding the safety and efficacy of pharmacological pain therapies in patients with IA and concomitant gastrointestinal or hepatic comorbidities.
Only one open single arm trial addressing the safety of NSAIDs in RA patients with gastrointestinal comorbidity was found to be eligible for our review and this study yielded limited data.
We found no evidence regarding the efficacy or safety of other pharmacological pain therapies in patients with RA and concomitant gastrointestinal comorbidity.
We found no evidence regarding the efficacy and safety of any pharmacological pain therapies in patients with PsA, AS or SpA and concomitant gastrointestinal comorbidity, and no evidence regarding the efficacy and safety of any pharmacological therapies in people with RA, PsA, AS or SpA and concomitant hepatic comorbidities.
Quality of the evidence
The included study was at high risk of bias (Table 1). As there was only one trial, which was susceptible to bias, it was difficult to apply the GRADE levels of evidence.
Potential biases in the review process
We performed a thorough search of the literature that comprised a search of all major databases without language restriction. We therefore believe we have identified all relevant studies. Two review authors assessed studies for inclusion in the review and their risk of bias, with a third review author adjudicating if there was any discrepancy. The protocol for this review has been published and all analyses were specified a priori.
Agreements and disagreements with other studies or reviews
Pre‐existing gastrointestinal comorbidity and NSAIDs
It is well known that NSAIDs are associated with an increased risk of gastrointestinal adverse events (Akarca 2005; Henry 2003; Singh 1999; Wolfe 1999) and several studies have specifically evaluated the risk of gastrointestinal toxicity in patients with inflammatory arthritis (Silverstein 2000; Simon 1999). However, patients with pre‐existing gastrointestinal comorbidities have been explicitly excluded from these studies.
Several studies that included a mixed population of participants appeared to address our research question, although we excluded them as they did not report results for the IA participants separately.
Laine 2008 performed an RCT to compare the risk of lower gastrointestinal clinical events in 34,701 patients with either RA or osteoarthritis who were receiving either etoricoxib (60 or 90 mg) or diclofenac (150 mg) (mean duration of therapy was 18 months). In a post hoc multivariable analysis they found that a prior lower gastrointestinal clinical event was the most important predictor of future events, with approximately a four‐fold increase in risk (hazard ratio = 4.06, 95% confidence interval (CI) 2.93 to 5.62).
Degner 2000 performed an observational controlled cohort study to assess the efficacy and tolerability of meloxicam compared with comparator NSAIDs (that is diclofenac, ibuprofen, piroxicam, or indomethacin) in 4526 patients with a mixed diagnosis (RA, osteoarthritis and other rheumatic conditions including low back pain and AS) (doses of drugs varied). In those who had received meloxicam, there did not appear to be a significant difference in development of gastrointestinal toxicity or gastrointestinal bleeding in those with and without a history of perforation, ulceration, or gastrointestinal bleeding. However, in those who had received one of the comparator NSAIDs there appeared to be an increased risk of gastrointestinal toxicity in those with a history of perforation, ulceration, or gastrointestinal bleeding compared to those without this history (10/136 versus 53/1860, P value < 0.009), but no difference in risk of CI bleeding (1/126 versus 9/1851, P value > 0.05) (calculations made by the review authors from data presented in the paper).
Chan 2002 assessed the incidence of recurrent ulcer bleeding in participants with RA, osteoarthritis and other types of arthritis who had a healed bleeding ulcer and were randomised to receive either diclofenac (75 mg twice daily) plus omeprazole (a proton‐pump inhibitor) (20 mg daily) or celecoxib (200 mg twice daily) for six months. They reported that the probability of recurrent bleeding during the six‐month period did not differ between the two groups (4.9%, 95% CI 3.1 to 6.7, n = 7/144 for patients who received celecoxib; and 6.4%, 95% CI 4.3 to 8.4, n = 9/143, for patients who received diclofenac plus omeprazole; difference ‐1.5%; 95% CI ‐6.8 to 3.8).
In a follow‐up report of the same study Chan 2004 reported that recurring ulcers in six months occurred in 18.7% of the celecoxib group and 25.6% of the diclofenac plus omeprazole group (difference 6.7%, 95% CI 17.8% to 3.9%, P = 0.21). Combining bleeding and endoscopic ulcers, 24.1% in the celecoxib group and 32.3% in the diclofenac plus omeprazole group had recurrent ulcers in six months (difference 8.2%, 95% CI 19.5% to 2.9%, P = 0.15). They concluded that among patients with previous ulcer bleeding, neither celecoxib nor diclofenac plus omeprazole adequately prevent ulcer recurrence.
Chan 2010 performed a large multicentre RCT including 4484 patients with either osteoarthritis (85% of study population) or RA (15% of study population) and a history of gastroduodenal ulceration or gastrointestinal haemorrhage. Participants were randomised to receive either celecoxib (200 mg twice a day) or diclofenac slow release (75 mg twice a day) plus omeprazole (20 mg daily) for six months. In contrast to the findings of their previous study, fewer participants in the celecoxib group developed a clinically significant upper or lower gastrointestinal event (20/2238) compared with those in the diclofenac plus omeprazole group (81/2236; hazard ratio 4.3, 95% CI 2.6 to 7.0). They did not report whether, or not, the results differed by the type of arthritis.
One RCT including 67 patients with mixed diagnoses (RA: n = 38, osteoarthritis: n = 24, back pain: n = 3 , PsA: n = 1, AS: n = 1) who had been treated with NSAIDs and developed a gastric or duodenal ulcer, or both types of ulcers, randomised patients to one of four treatment groups comprising ranitidine 150 mg twice daily or sucralfate 1 g four times a day with or without withdrawal of NSAID (Manniche 1987). Although the proportion with healed ulcers favoured the NSAID withdrawal group (23/30, 77% versus 29/32, 91%) this was not statistically significant (P > 0.10).
Roth 1995 showed no significant differences of endoscopic grades of ulcers in patients with prior reported ulcer treated with naproxen (mean change of endoscopic grading = 2.07) or diclofenac (mean change = 0.35) when compared to patients without such a history (mean change naproxen = 1.46, diclofenac = 0.47). The proportion of patients with worsening of endoscopic grading was almost equal for those patients with and without a history when treated with naproxen (18/25 = 72% versus 49/78 = 62.8%, respectively) or diclofenac (6/23 = 26.1% versus 23/78 = 29.5%, respectively).
Several studies that were excluded due to inclusion of a withdrawn drug also appeared to be relevant to our research question. Laine 2002 re‐analysed data from an RCT of rofecoxib versus naproxen in patients with RA (Bombardier 2000) and demonstrated an increased risk of clinical upper gastrointestinal events (bleeding, perforation, obstruction and symptomatic ulcer) in people treated with naproxen (500 mg twice daily) or rofecoxib (50 mg daily) (whole data set combined) who had a prior uncomplicated gastrointestinal event (RR 3.08, 95% CI 1.98 to 4.77) or a prior complicated gastrointestinal event (RR 3.73, 95% CI 2.25 to 6.17). Eisen 2005 performed a systematic review comparing valdecoxib to non‐selective NSAIDs and reported that in both univariate and multivariate analyses a history of prior gastric or duodenal ulcer was significantly associated with an earlier time to develop moderate to severe upper gastrointestinal toxicity. In a similar study, Goldstein 2004 reported that a history of upper GI bleeding significantly increased the risk of GI ulcer complications in patients receiving non‐selective NSAIDs (P < 0.001); and non‐selective NSAID‐treated patients had an increased risk of upper gastrointestinal ulcer complications if they had a history of gastroduodenal ulcers or gastrointestinal‐related NSAID intolerance (P < 0.05).
Pre‐existing GI comorbidity and other pain therapies
There is a body of evidence that opioids are associated with an increased risk of gastrointestinal events including constipation, nausea and vomiting (Boswell 2010; Cook 2008). However, no evidence was found for patients with pre‐existing gastrointestinal comorbidity and opioids or the other pain therapies included in the protocol for this review.
Pre‐existing hepatic comorbidity and pain therapies
In a small case series including 11 patients with rheumatic conditions (RA, vertebral syndrome) and hepatic comorbidities treated with acemetacin for 10 days, Seissiger 1987 reported an improvement of pain and no withdrawals due to adverse events.
Soni 2009 performed a systematic review including 41 RCTs to assess the hepatic safety of celecoxib in patients with osteoarthritis, RA, AS, chronic low back pain and Alzheimer's disease. They described a low incidence of serious hepatic adverse events in patients treated with celecoxib as well as in patients treated with diclofenac, a drug known for its hepatotoxicity. However, patients with pre‐existing hepatic diseases were excluded from the included trials.
Paracetamol is know to induce liver failure when taken in high dosage, but there is also evidence that when combined with an intake of alcohol or in the presence of acute viral hepatitis a lower dosage of paracetamol was associated with higher serum transaminases and could lead to hepatotoxicity (Prescott 2000). However, several published studies report no higher risk of hepatotoxicity in patients with chronic liver disease who were treated with paracetamol (Benson 1983; Benson 2005). No evidence was found for patients with IA and hepatic comorbidities taking paracetamol.
Authors' conclusions
Implications for practice.
On the basis of the current review, there is scant evidence to guide clinicians about how gastrointestinal or liver comorbidities should influence the choice of pain treatment in inflammatory arthritis. Based upon additional studies that included a mixed population of participants with a range of rheumatic conditions, NSAIDs should be used cautiously in patients with IA and a pre‐existing history of gastrointestinal comorbidity as there is consistent evidence that these patients may be at increased risk.
Implications for research.
As patients with pre‐existing comorbidities make up a considerable proportion of the patients with IA seen in clinical practice, but are often excluded from RCTs, further research is needed to determine whether special precautions are required when treating patients with IA and comorbid gastrointestinal or hepatic comorbidity with other pain therapies.
What's new
| Date | Event | Description |
|---|---|---|
| 5 July 2010 | Amended | CMSG ID A051‐P |
Acknowledgements
The authors thank Louise Falzon, Trials Search Coordinator of the Cochrane Musculoskeletal Group, for assisting with the design of the search strategy.
Appendices
Appendix 1. MEDLINE and CENTRAL search strategy
MEDLINE (Ovid format)
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. (felty$ adj2 syndrome).tw.
4. (caplan$ adj2 syndrome).tw.
5. (sjogren$ adj2 syndrome).tw.
6. (sicca adj2 syndrome).tw.
7. still$ disease.tw.
8. bechterew$ disease.tw.
9. exp Spondylarthropathies/
10. (ankylos$ or spondyl$).tw.
11. (bekhterev$ or bechterew$).tw.
12. (Marie adj struempell$).tw.
13. Arthritis, Psoriatic/
14. (psoria$ adj (arthriti$ or arthropath$)).tw.
15. ((arthriti$ or arthropath$) adj psoria$).tw.
16. exp Arthritis, Infectious/
17. reactive arthritis.tw.
18. (reiter$ adj (disease or syndrome)).tw.
19. ((sexual$ or chlamydia or yersinia or postyersinia or postdysenteric or salmonella or shigella or b27 or postinfectious or post infectious) adj5 arthrit$).tw.
20. reactive enthesitis.tw.
21. undifferentiated oligoarthritis.tw.
22. or/1‐21
23. exp Inflammatory Bowel Diseases/
24. exp Arthritis/
25. 23 and 24
26. ((inflamm$ or ibd or crohn$ or enteropath$) adj5 (arthrit$ or arthrop$)).tw.
27. (granulomatous colitis adj5 (arthrit$ or arthrop$)).tw.
28. (ulcerative colitis adj5 (arthrit$ or arthrop$)).tw.
29. (granulomatous enteritis adj5 (arthrit$ or arthrop$)).tw.
30. (regional enteritis adj5 (arthrit$ or arthrop$)).tw.
31. (Ileocolitis adj5 (arthrit$ or arthrop$)).tw.
32. (terminal ileitis adj5 (arthrit$ or arthrop$)).tw.
33. (regional ileitis adj5 (arthrit$ or arthrop$)).tw.
34. or/22,25‐33
35. exp analgesics, opioid/
36. exp narcotics/
37. narcotic$.tw.
38. opiate$.tw.
39. opioid$.tw.
40. acemethadone.tw.
41. acetylmehtadol.tw.
42. alfentanil.tw.
43. alphaprodine.tw.
44. anileridine.tw.
45. benzomorphan$.tw.
46. buprenorphin$.tw.
47. butorphanol.tw.
48. carfentanil.tw.
49. codeine.tw.
50. dextropropoxyphene.tw.
51. dextromoramide.tw.
52. dezocine.tw.
53. diacetyl morphine.tw.
54. diamorphine.tw.
55. dihydroetorphine.tw.
56. dimepheptanol.tw.
57. dionine.tw.
58. diprenorphine.tw.
59. dihydromorphinone.tw.
60. dynorphin.tw.
61. endomorphin.tw.
62. eseroline.tw.
63. ethylketocyclazocine.tw.
64. ethylmorphine.tw.
65. fenoperidine.tw.
66. fentany.tw.
67. heroin.tw.
68. hydrocodon$.tw.
69. hydromorphon$.tw.
70. isocodeine.tw.
71. isonipecain.tw.
72. isopromedol.tw.
73. kaolin‐pectin.tw.
74. ketobemidone.tw.
75. levallorphan.tw.
76. levodroman.tw.
77. levomethadryl.tw.
78. levorphan$.tw.
79. meperidine.tw.
80. meptazinol.tw.
81. methadol.tw.
82. methadone.tw.
83. methadyl acetate.tw.
84. morphia.tw.
85. morphine.tw.
86. exp morphine derivatives/
87. methylnaloxone.tw.
88. nalbuphine.tw
89. nocistatin.tw.
90. opium.tw.
91. oxycodein$.tw.
92. oxycodone.tw.
93. oxymorph$.tw.
94. pantopon.tw.
95. papaveretum.tw.
96. paracymethadol.tw.
97. paregoric.mp.
98. pentazocine.tw.
99. pethidine.tw.
100. phenazocine.tw.
101. phenbenzorphan.tw.
102. phenethylazocine.tw.
103. phenoperidine.tw.
104. pirinitramide.tw.
105. propoxyphene.tw.
106. protopine.tw.
107. pyrrolamidol.tw.
108. remifentanil.tw.
109. sufentanil.tw.
110. sufentanyl.tw.
111. talwin.tw.
112. tapentadol.tw.
113. thebaine.tw.
114. theocodin.tw.
115. tilidine.tw.
116. tramadol.tw.
117. trimeperidine.tw.
118. dihydromorphine.tw.
119. hydroxycodeinone.tw.
120. levomethadyl.tw.
121. dihydrocodein$.tw.
122. dihydroxycodeinone.tw.
123. dipipanone.tw.
124. or/35‐123
125. exp anti‐inflammatory agents, non‐steroidal/
126. ((anti‐inflammator$.tw. or anti.mp.) adj inflammator$.tw.) or antiinflammator$.tw.
127. nsaid$.tw.
128. ampyrone.tw.
129. antipyrine.tw.
130. apazone.tw.
131. aspirin.tw.
132. bufexamac.tw.
133. clofazimine.tw.
134. clonixin.tw.
135. curcumin.tw.
136. diclofenac.tw.
137. diflunisal.tw.
138. dipyrone.tw.
139. epirizole.tw.
140. etodolac.tw.
141. fenoprofen.tw.
142. flurbiprofen.tw.
143. ibuprofen.tw.
144. indomethacin.tw.
145. ketoprofen.tw.
146. ketorolac.tw.
147. meclofenamic acid.tw.
148. mefenamic acid.tw.
149. mesalamine.tw.
150. naproxen.tw.
151. niflumic acid.tw.
152. oxyphenbutazone.tw.
153. phenylbutazone.tw.
154. piroxicam.tw.
155. prenazone.tw.
156. salicylate$.tw.
157. sulfasalazine.tw.
158. sulindac.tw.
159. suprofen.tw.
160. tolmetin.tw.
161. or/125‐160
162. exp acetaminophen/
163. acet?aminophen.tw.
164. paracetamol.tw.
165. acetamidophenol.tw.
166. acephen.tw.
167. acetaco.tw.
168. tylenol.tw.
169. anacin$.tw.
170. datril.tw.
171. panadol.tw.
172. acamol.tw.
173. algotropyl.tw.
174. or/162‐173
175. exp muscle relaxants, central/
176. exp neuromuscular nondepolarizing agents/
177. exp neuromuscular blocking agents/
178. exp benzodiazepines/
179. benzodiazepine$.tw.
180. muscle relaxant$.tw.
181. (alprazolam or xanax or xanor or tafil or alprox or frontal).tw.
182. (bromazepam or lexotanil or lexotan or lexomil or somalium or bromam).tw.
183. (chlordiazepoxide or librium or tropium or risolid or klopoxid).tw.
184. (cinolazepam or gerodorm).tw.
185. (clonazepam or klonopin or rivotril or iktorivil).tw.
186. (cloxazolam or olcadil).tw.
187. (clorazepate or tranxene).tw.
188. (diazepam or valium or pax or apzepam or stesolid).tw.
189. (estazolam or prosom).tw.
190. (flunitrazepam or rohypnol or fluscand or flunipam or rona or rohydorm).tw.
191. (flurazepam or dalmadorm or dalmane).tw.
192. (flutoprazepam or restas).tw.
193. (halazepam or paxipam or ketazolam or anxon or loprazolam or dormonoct or lorazepam or ativan or temesta or tavor or lorabenz or lormetazepam or loramet or noctamid or pronoctan or medazepam or nobrium or midazolam or dormicum or versed or hypnovel or dormonid or nimetazepam or erimin or nitrazepam or mogadon or alodorm or pacisyn or dumolid or nordazepam or madar or stilny or oxazepam or seresta or serax or serenid or serepax or sobril or pinazepam or domar or prazepam or lysanxia or centrax or quazepam or doral or temazepam or restoril or normison or euhypnos or tenox or tetrazepam or mylostan or triazolam or halcion or rilamir).tw.
194. orphenadrine or norflex or mephenamin or disipal or banflex or flexon or tizanidine or zanaflex or sirdalud or flupirtine or dantrolene or dantrium or dantrolen or baclofen or kemstro or lioresal).tw.
195. exp neurotransmitter agents/
196. neuromodulator$.tw.
197. neurohumor$.tw.
198. exp anticonvulsants/
199. anticonvulsant$.tw.
200. gabapentin.tw.
201. neurontin.tw.
202. (carbamazepine or tegretol).tw.
203. (clonazepam or klonopin or rivotril or rivatril).tw.
204. (lamotrigine or lamictal).tw.
205. (oxcarbazepine or trileptal or oxaleptal).tw.
206. (phenytoin or phenytek or dilantin or eptoin or epanutin or diphenin or dipheninum or phydum).tw.
207. (pregabalin or lyrica).tw.
208. (topiramate or topamax).tw.
209. (valproic acid or valproate or epilim or depakote).tw.
210. (ketamine or ketalar).tw.
211. (capsaicin or zostrix or capsin or capzasin‐p).tw.
212. exp cannabinoids/
213. endocannabinoid$.tw.
214. (cannabinoids$ or rimonabant or SR141716 or acomplia or bethin or monaslim or remonabent or riobant or slimona or rimoslim or zimulti or riomont).tw.
215. exp capsaicin/
216. exp ketamine/
217. exp bupropion/
218. (bupropion or wellbutrin or zyban).tw.
219. exp methylphenidate/
220. (dexmethylphenidate or focalin).tw.
221. (methylphenidate or ritalin or concerta).tw.
222. antidepressive agents/
223. (anti‐depressant$ or antidepressant$).tw.
224. exp serotonin uptake inhibitors/
225. exp serotonin antagonists/
226. SSRI$.tw.
227. (citalopram or celexa).tw.
228. (fluoxetine or prozac).tw.
229. (paroxetine or paxil or seroxat).tw.
230. (sertraline or zoloft or lustral).tw.
231. (escitalopram or lexapro or cipralex).tw.
232. (fluvoxamine or luvox).tw.
233. (desvenlafaxine or pristiq).tw.
234. (venlafaxine or effexor).tw.
235. (duloxetine or cymbalta).tw.
236. (milnacipran or ixel or savella).tw.
237. (reboxetine or edronax).tw.
238. (viloxazine or vivalan).tw.
239. amitriptyline/
240. (amitriptyline or elavil or endep).tw.
241. (clomipramine or anafranil).tw.
242. (desipramine or norpramin or pertofrane).tw.
243. (dosulepin or dothiepin or prothiaden).tw.
244. (doxepin or adapin or sinequan).tw.
245. (imipramine or tofranil).tw.
246. (lofepramine or feprapax or gamanil or lomont).tw.
247. (nortriptyline or pamelor).tw.
248. (protriptyline or vivactil).tw.
249. (trimipraminie or surmontil).tw.
250. (amoxapine or asendin).tw.
251. (loxapine or loxapac or loxitane).tw.
252. (maprotiline or deprilept or ludiomil or psymion).tw.
253. (mazindol or mazanor or sanorex).tw.
254. (mianserin or bolvidon or norval or tolvon).tw.
255. (mirtazapine or remeron or avanza or zispin).tw.
256. (setiptiline or tecipul).tw.
257. exp monoamine oxidase inhibitors/
258. (isocarboxazid or marplan or moclobemide or aurorix or manerix or phenelzine or nardil or selegiline or l‐deprenyl or eldepryl or zelapar or emsam or tranylcypromine or parnate or moclobemide or aurorix or manerix).tw.
259. phenelzine/
260. moclobemide/
261. selegiline/
262. tranylcypromine/
263. isocarboxazid/
264. monoamine oxidase inhibitor$.tw.
265. serotonin‐norepinephrine reuptake inhibitor$.tw.
266. norepinephrine reuptake inhibitor$.tw.
267. serotonin‐noradrenaline reuptake inhibitor$.tw.
268. noradrenaline reuptake inhibitor$.tw.
269. (duloxetine or cymbalta or milnacipran or ixel or savella).tw.
270. (reboxetine or edronax or viloxazine or vivalan).tw.
271. or/175‐270
272. exp gastrointestinal disease/
273. exp esophageal disease/
274. ((gastrointestinal$ or gastro?intestinal$ or gastr$ or intestinal$) adj2 (disease or disorder or disorder$)).tw.
275. ((esophageal or ?sophageal) adj2 (disease$ or disorder$)).tw.
276. barrett$ syndrome.tw.
277. esophageal diverticulosis.tw.
278. esophagitis.tw.
279. ((reflux or peptic) adj2 (esophagitis or esophagitides)).tw.
280. exp Gastroenteritis/
281. colitis.tw.
282. colitides.tw.
283. ((colitis or colitides) adj2 (ulcerative or ischemic or microscopic)).tw.
284. diverticulitis.tw.
285. enteritis.tw.
286. exp diverticulitis/
287. exp enteritis/
288. duodenitis.tw.
289. ileitis.tw.
290. exp enterocolitis/
291. exp gastritis/
292. ((gastritis or gastritides or gastric$) adj2 (atrophic or hypertrophic)).tw.
293. menetrier disease.tw.
294. exp inflammatory bowel disease/
295. ((colitis or ileitis or enteritis or ileocolitis) adj (ulcerative or ulcerosa)).tw.
296. crohn$ disease$.tw.
297. proctitis.tw.
298. exp peptic ulcer/
299. ((peptic or duodenal or gastric or stomach or curling$ or gastroduodenal or marginal) adj2 ulcer$).tw.
300. exp stomach disease$/
301. ((gastric or stomach) adj disease$).tw.
302. ((diverticulitis or diverticulosis) adj2 (stomach or gastric)).tw.
303. exp duodenogastric reflux/
304. ((duodenal or duodenogastric or duodeno?gastic or gastric or gastral) adj2 reflux$).tw.
305. exp peptic ulcer bleeding/
306. peptic ulcer bleeding.tw.
307. gastric ulcer.tw.
308. exp gastric ulcer/
309. gastric bleeding.tw.
310. gastr$ bleeding.tw.
311. ulc$ bleeding.tw.
312. ulcus perforation.tw.
313. ulc$ perforation.tw.
314. exp duodenal ulcer/
315. gastrointestinal bleeding.tw.
316. (bleeding or bloodloss or hemorrhage or h?morrhag$).tw.
317. (gastric or gastrointestinal or gastro‐intestinal or gastral or gastr$ or stomache or intestinal or bowel or intestine).tw.
318. 316 and 317
319. (perforation or penetration or permeability).tw.
320. 317 and 319
321. ((history or hist$ or reported or previous) adj2 (gastrointestinal or gastro‐intestinal or gastric or gastr$ or intestinal or bowel or ulcus or ulcer)).tw.
322. or/272‐315
323. 318 or 320‐322
324. exp liver disease/
325. ((liver or hepatic) adj2 (disease$ or dysfunction$ or condition$)).tw.
326. chiari$ syndrome.tw.
327. budd chiari syndrome.tw.
328. drug induced liver injury.tw.
329. (drug‐induced adj2 (hepatitis or hepititdes or liver disease$ or liver injury or liver injuries)).tw.
330. exp fatty liver/
331. fatty liver.tw.
332. steatosis hepatis.tw.
333. steatohepatitis.tw.
334. ((reye or reye$) adj (syndrome or johnson syndrom or like syndrome)).tw.
335. exp hepatitis/
336. hepatitis alcoholic.tw.
337. exp chronic hepatitis/
338. chronic hepatits.tw.
339. exp autoimmune hepatitis/
340. exp hepatitis A/
341. exp hepatitis B/
342. exp hepatitis C/
343. exp hepatitis D/
344. exp hepatitis E/
345. exp hepatitis viral/
346. exp viral hepatitis/
347. (hepatitis adj (viral or infectious or transmitted or epidemic)).tw.
348. exp hepatomegaly/
349. hepatomegaly.tw.
350. enlarged liver.tw.
351. exp liver cirrhosis/
352. ((hepatic or liver) adj (cirrhosis or cirrhoses or cirrose or cirhos?s or fibros?s)).tw.
353. exp alcoholic liver disease/
354. exp parasitic liver disease/
355. toxic liver disease.tw.
356. ((toxic or alcoholic or drug‐induced or drug?induced) adj2 (liver disease or hepatitis or hepatitides)).tw.
357. toxi* hepatitis.tw.
358. elevat* liver enzym*.tw.
359. high* liver enzym*.tw.
360. or/324‐359
361. randomized controlled trial.pt.
362. controlled clinical trial.pt.
363. randomized.ab.
364. placebo.ab.
365. drug therapy.fs.
366. randomly.ab.
367. trial.ab.
368. groups.ab.
369. or/361‐368
370. humans.sh.
371. 369 and 370
372. exp cohort studies/
373. cohort$.tw.
374. controlled clinical trial.pt.
375. exp epidemiologic methods/
376. limit 376 to yr=1966‐1989
377. exp case‐control studies/
378. (case$ and control$).tw.
379. or/372‐374,376‐378
380. 368 or 379
381. 380 and 369
382. 323 or 360
383. 323 and 360
384. 382 or 383
385. 124 or 161 or 174 or 271
386. 34 and 384 and 385
386. 386 and 379
Appendix 2. EMBASE search strategy
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. (felty$ adj2 syndrome).tw.
4. (caplan$ adj2 syndrome).tw.
5. (sjogren$ adj2 syndrome).tw.
6. (sicca adj2 syndrome).tw.
7. still$ disease.tw.
8. bechterew$ disease.tw.
9. exp spondyloarthropathy/
10. (ankylos$ or spondyl$).tw.
11. (bekhterev$ or bechterew$).tw.
12. (Marie adj struempell$).tw.
13. psoriatic arthritis/
14. (psoria$ adj (arthriti$ or arthropath$)).tw.
15. ((arthriti$ or arthropath$) adj psoria$).tw.
16. exp infectious arthritis/
17. reactive arthritis.tw.
18. (reiter$ adj (disease or syndrome)).tw.
19. ((sexual$ or chlamydia or yersinia or postyersinia or postdysenteric or salmonella or shigella or b27 or postinfectious or post infectious) adj5 arthrit$).tw.
20. reactive enthesitis.tw.
21. undifferentiated oligoarthritis.tw.
22. or/1‐21
23. exp enteritis/
24. exp arthritis/
25. 23 and 24
26. ((inflamm$ or ibd or crohn$ or enteropath$) adj5 (arthrit$ or arthrop$)).tw.
27. (granulomatous colitis adj5 (arthrit$ or arthrop$)).tw.
28. (ulcerative colitis adj5 (arthrit$ or arthrop$)).tw.
29. (granulomatous enteritis adj5 (arthrit$ or arthrop$)).tw.
30. (regional enteritis adj5 (arthrit$ or arthrop$)).tw.
31. (Ileocolitis adj5 (arthrit$ or arthrop$)).tw.
32. (terminal ileitis adj5 (arthrit$ or arthrop$)).tw.
33. (regional ileitis adj5 (arthrit$ or arthrop$)).tw.
34. or/22,25‐33
35. narcotic analgesic agent/
36. narcotic$.tw.
37. opiate$.tw.
38. opioid$.tw.
39. acemethadone.tw.
40. acetylmehtadol.tw.
41. alfentanil.tw.
42. alphaprodine.tw.
43. anileridine.tw.
44. benzomorphan$.tw.
45. buprenorphin$.tw.
46. butorphanol.tw.
47. carfentanil.tw.
48. codeine.tw.
49. dextropropoxyphene.tw.
50. dextromoramide.tw.
51. dezocine.tw.
52. diacetyl morphine.tw.
53. diamorphine.tw.
54. dihydroetorphine.tw.
55. dimepheptanol.tw.
56. dionine.tw.
57. diprenorphine.tw.
58. dihydromorphinone.tw.
59. dynorphin.tw.
60. endomorphin.tw.
61. eseroline.tw.
62. ethylketocyclazocine.tw
63. ethylmorphine.tw.
64. fenoperidine.tw.
65. fentanyl.tw.
66. heroin.tw.
67. hydrocodon$.tw.
68. hydromorphon$.tw.
69. isocodeine.tw.
70. isonipecain.tw.
71. isopromedol.tw.
72. kaolin‐pectin.tw.
73. ketobemidone.tw.
74. levallorphan.tw.
75. levodroman.tw.
76. levomethadryl.tw.
77. levorphan$.tw.
78. meperidine.tw.
79. meptazinol.tw.
80. methadol.tw.
81. methadone.tw.
82. methadyl acetate.tw.
83. morphia.tw.
84. morphine.tw.
85. exp morphine derivatives/
86. methylnaloxone.tw.
87. nalbuphine.tw.
88. nocistatin.tw.
89. opium.tw.
90. oxycodein$.tw.
91. oxycodone.tw.
92. oxymorph$.tw.
93. pantopon.tw.
94. papaveretum.tw.
95. paracymethadol.tw.
96. paregoric.mp.
97. pentazocine.tw.
98. pethidine.tw.
99. phenazocine.tw.
100. phenbenzorphan.tw.
101. phenethylazocine.tw.
102. phenoperidine.tw.
103. pirinitramide.tw.
104. propoxyphene.tw.
105. protopine.tw.
106. pyrrolamidol.tw.
107. remifentanil.tw.
108. sufentanil.tw.
109. sufentanyl.tw.
110. talwin.tw.
111. tapentadol.tw.
112. thebaine.tw.
113. theocodin.tw.
114. tilidine.tw.
115. tramadol.tw.
116. trimeperidine.tw.
117. dihydromorphine.tw.
118. hydroxycodeinone.tw.
119. levomethadyl.tw.
120. dihydrocodein$.tw.
121. dihydroxycodeinone.tw.
122. dipipanone.tw
123. or/35‐122
124. exp nonsteroid antiinflammatory agent/
125. anti‐inflammator$.tw.
126. (Anti adj Inflammator$).tw.
127. AntiInflammator$.tw.
128. nsaid$.tw.
129. ampyrone.tw.
130. antipyrine.tw.
131. apazone.tw.
132. aspirin.tw.
133. bufexamac.tw.
134. clofazimine.tw.
135. clonixin.tw.
136. curcumin.tw.
137. diclofenac.tw.
138. diflunisal.tw.
139. dipyrone.tw.
140. epirizole.tw.
141. etodolac.tw.
142. fenoprofen.tw.
143. flurbiprofen.tw.
144. ibuprofen.tw.
145. indomethacin.tw.
146. ketoprofen.tw.
147. ketorolac.tw.
148. meclofenamic acid.tw.
149. mefenamic acid.tw.
150. mesalamine.tw.
151. naproxen.tw.
152. niflumic acid.tw.
153. oxyphenbutazone.tw
154. phenylbutazone.tw.
155. piroxicam.tw.
156. prenazone.tw.
157. salicylate$.tw.
158. sulfasalazine.tw.
159. sulindac.tw.
160. suprofen.tw.
161. tolmetin.tw.
162. or/124‐161
163. paracetamol.tw.
164. paracetamol/
165. acet?minophen.tw.
166. acetamidophenol.tw.
167. acephen.tw.
168. acetaco.tw.
169. tylenol.tw.
170. anacin$.tw.
171. datril.tw.
172. panadol.tw.
173. acamol.tw.
174. algotropyl.tw.
175. or/163‐174
176. exp muscle relaxant agent/
177. exp benzodiazepine derivative/
178. benzodiazepine$.tw.
179. muscle relaxant$.tw.
180. (alprazolam or xanax or xanor or tafil or alprox or frontal).tw.
181. (bromazepam or lexotanil or lexotan or lexomil or somalium or bromam).tw.
182. (chlordiazepoxide or librium or tropium or risolid or klopoxid).tw.
183. (cinolazepam or gerodorm).tw.
184. (clonazepam or klonopin or rivotril or iktorivil).tw.
185. (cloxazolam or olcadil).tw.
186. (clorazepate or tranxene).tw.
187. (diazepam or valium or pax or apzepam or stesolid).tw.
188. (estazolam or prosom).tw.
189. (flunitrazepam or rohypnol or fluscand or flunipam or rona or rohydorm).tw.
190. (flurazepam or dalmadorm or dalmane).tw.
191. (flutoprazepam or restas).tw.
192. (halazepam or paxipam or ketazolam or anxon or loprazolam or dormonoct or lorazepam or ativan or temesta or tavor or lorabenz or lormetazepam or loramet or noctamid or pronoctan or medazepam or nobrium or midazolam or dormicum or versed or hypnovel or dormonid or nimetazepam or erimin or nitrazepam or mogadon or alodorm or pacisyn or dumolid or nordazepam or madar or stilny or oxazepam or seresta or serax or serenid or serepax or sobril or pinazepam or domar or prazepam or lysanxia or centrax or quazepam or doral or temazepam or restoril or normison or euhypnos or tenox or tetrazepam or mylostan or triazolam or halcion or rilamir).tw.
193. (orphenadrine or norflex or mephenamin or disipal or banflex or flexon or tizanidine or zanaflex or sirdalud or flupirtine or dantrolene or dantrium or dantrolen or baclofen or kemstro or lioresal).tw.
194. neuromodulation/
195. neuromodulator$.tw.
196. neurohumor$.tw.
197. exp anticonvulsive agent/
198. anticonvulsant$.tw.
199. gabapentin.tw.
200. neurontin.tw.
201. (carbamazepine or tegretol).tw.
202. (clonazepam or klonopin or rivotril or rivatril).tw.
203. (lamotrigine or lamictal).tw.
204. (oxcarbazepine or trileptal or oxaleptal).tw.
205. (phenytoin or phenytek or dilantin or eptoin or epanutin or diphenin or dipheninum or phydum).tw.
206. (pregabalin or lyrica).tw.
207. (topiramate or topamax).tw.
208. (valproic acid or valproate or epilim or depakote).tw.
209. (ketamine or ketalar).tw.
210. (capsaicin or zostrix or capsin or capzasin‐p).tw.
211. exp cannabinoids/
212. endocannabinoid$.tw.
213. (cannabinoids$ or rimonabant or SR141716 or acomplia or bethin or monaslim or remonabent or riobant or slimona or rimoslim or zimulti or riomont).tw.
214. exp capsaicin/
215. exp ketamine/
216. (bupropion or wellbutrin or zyban).tw.
217. exp methylphenidate
218. (dexmethylphenidate or focalin).tw.
219. (methylphenidate or ritalin or concerta).w.
220. exp antidepressant agent/
221. (anti‐depressant$ or antidepressant$).tw.
222. exp serotonin antagonist/
223. SSRI$.tw.
224. (citalopram or celexa).tw.
225. (fluoxetine or prozac).tw.
226. (paroxetine or paxil or seroxat).tw.
227. (sertraline or zoloft or lustral).tw.
228. (escitalopram or lexapro or cipralex).tw.
229. (fluvoxamine or luvox).tw.
230. (desvenlafaxine or pristiq).tw.
231. (venlafaxine or effexor).tw.
232. (duloxetine or cymbalta).tw.
233. (milnacipran or ixel or savella).tw.
234. (reboxetine or edronax).tw.
235. (viloxazine or vivalan).tw.
236. (amitriptyline or elavil or endep).tw.
237. (clomipramine or anafranil).tw.
238. (desipramine or norpramin or pertofrane).tw.
239. (dosulepin or dothiepin or prothiaden).tw.
240. (doxepin or adapin or sinequan).tw.
241. (imipramine or tofranil).tw.
242. (lofepramine or feprapax or gamanil or lomont).tw.
243. (nortriptyline or pamelor).tw.
244. (protriptyline or vivactil).tw.
245. (trimipraminie or surmontil).tw.
246. (amoxapine or asendin).tw.
247. (loxapine or loxapac or loxitane).tw.
248. (maprotiline or deprilept or ludiomil or psymion).tw.
249. (mazindol or mazanor or sanorex).tw.
250. (mianserin or bolvidon or norval or tolvon).tw.
251. (mirtazapine or remeron or avanza or zispin).tw.
252. (setiptiline or tecipul).tw.
253. (isocarboxazid or marplan or moclobemide or aurorix or manerix or phenelzine or nardil or selegiline or l‐deprenyl or eldepryl or zelapar or emsam or tranylcypromine or parnate or moclobemide or aurorix or manerix).tw.
254. monoamine oxidase inhibitor$.tw.
255. serotonin‐norepinephrine reuptake inhibitor$.tw.
256. norepinephrine reuptake inhibitor$.tw.
257. serotonin‐noradrenaline reuptake inhibitor$.tw.
258. noradrenaline reuptake inhibitor$.tw.
259. (duloxetine or cymbalta or milnacipran or ixel or savella).tw.
260. (reboxetine or edronax or viloxazine or vivalan).tw.
261. or/176‐260
262. exp gastrointestinal disease/
263. exp esophageal disease/
264. ((gastrointestinal$ or gastro?intestinal$ or gastr$ or intestinal$) adj2 (disease or disorder or disorder$)).tw.
265. ((esophageal or ?sophageal) adj2 (disease$ or disorder$)).tw.
266. barrett$ syndrome.tw.
267. esophageal diverticulosis.tw.
268. esophagitis.tw.
269. ((reflux or peptic) adj2 (esophagitis or esophagitides)).tw.
270. exp Gastroenteritis/
271. colitis.tw.
272. colitides.tw.
273. ((colitis or colitides) adj2 (ulcerative or ischemic or microscopic)).tw.
274. diverticulitis.tw.
275. enteritis.tw.
276. exp diverticulitis/
277. exp enteritis/
278. duodenitis.tw.
279. ileitis.tw.
280. exp enterocolitis/
281. exp gastritis/
282. ((gastritis or gastritides or gastric$) adj2 (atrophic or hypertrophic)).tw.
283. menetrier disease.tw.
284. exp inflammatory bowel disease/
285. ((colitis or ileitis or enteritis or ileocolitis) adj (ulcerative or ulcerosa)).tw.
286. crohn$ disease$.tw.
287. proctitis.tw.
288. exp peptic ulcer/
289. ((peptic or duodenal or gastric or stomach or curling$ or gastroduodenal or marginal) adj2 ulcer$).tw.
290. exp stomach disease$/
291. ((gastric or stomach) adj disease$).tw.
292. ((diverticulitis or diverticulosis) adj2 (stomach or gastric)).tw.
293. exp duodenogastric reflux/
294. ((duodenal or duodenogastric or duodeno?gastic or gastric or gastral) adj2 reflux$).tw.
295. exp peptic ulcer bleeding/
296. peptic ulcer bleeding.tw.
297. gastric ulcer.tw.
298. exp gastric ulcer/
299. gastric bleeding.tw.
300. gastr$ bleeding.tw.
301. ulc$ bleeding.tw.
302. ulcus perforation.tw.
303. ulc$ perforation.tw.
304. exp duodenal ulcer/
305. gastrointestinal bleeding.tw.
306. (bleeding or bloodloss or hemorrhage or h?morrhag$).tw.
307. (gastric or gastrointestinal or gastro‐intestinal or gastral or gastr$ or stomache or intestinal or bowel or intestine).tw.
308. 306 and 307
309. (perforation or penetration or permeability).tw.
310. 307 and 309
311. ((history or hist$ or reported or previous) adj2 (gastrointestinal or gastro‐intestinal or gastric or gastr$ or intestinal or bowel or ulcus or ulcer)).tw.
312. or/262‐305
313. 308 or 310‐312
314. exp liver disease/
315. ((liver or hepatic) adj2 (disease$ or dysfunction$ or condition$)).tw.
316. chiari$ syndrome.tw.
317. budd chiari syndrome.tw.
318. drug induced liver injury.tw.
319. (drug‐induced adj2 (hepatitis or hepititdes or liver disease$ or liver injury or liver injuries)).tw.
320. exp fatty liver/
321. fatty liver.tw.
322. steatosis hepatis.tw.
323. steatohepatitis.tw.
324. ((reye or reye$) adj (syndrome or johnson syndrom or like syndrome)).tw.
325. exp hepatitis/
326. hepatitis alcoholic.tw.
327. exp chronic hepatitis/
328. chronic hepatits.tw.
329. exp autoimmune hepatitis/
330. exp hepatitis A/
331. exp hepatitis B/
332. exp hepatitis C
333. exp hepatitis D/
334. exp hepatitis E/
335. exp hepatitis viral/
336. exp viral hepatitis/
337. (hepatitis adj (viral or infectious or transmitted or epidemic)).tw.
338. exp hepatomegaly/
339. hepatomegaly.tw.
340. enlarged liver.tw.
341. exp liver cirrhosis/
342. ((hepatic or liver) adj (cirrhosis or cirrhoses or cirrose or cirhos?s or fibros?s)).tw.
343. exp alcoholic liver disease/
344. exp parasitic liver disease/
345. toxic liver disease.tw.
346. ((toxic or alcoholic or drug‐induced or drug?induced) adj2 (liver disease or hepatitis or hepatitides)).tw.
347. toxi* hepatitis.tw.
348. elevat* liver enzym*.tw.
349. high* liver enzym*.tw.
350. or/314‐349
351. randomized controlled trial.pt.
352. controlled clinical trial.pt.
353. randomized.ab.
354. placebo.ab.
355. drug therapy.fs.
356. randomly.ab.
357. trial.ab.
358. groups.ab.
359. or/361‐368
360. humans.sh.
361. 369 and 370
362. exp cohort studies/
363. cohort$.tw.
364. controlled clinical trial.pt.
365. exp epidemiologic methods/
366. limit 376 to yr=1966‐1989
367. exp case‐control studies/
368. (case$ and control$).tw.
369. or/362‐364,366‐368
370. 358 or 369
371. 370 and 359
372. 313 or 350
373. 313 and 350
374. 372 or 373
375. 123 or 162 or 175 or 261
376. 34 and 374 and 375
377. 376 and 369
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Roth 1975.
| Methods | Single arm open trial Study duration: 52 weeks |
|
| Participants | 58 patients with active rheumatoid arthritis Inclusion criteria: RA and concomitant upper gastrointestinal dysfunction of varying degrees and severity and history of intolerance to antiinflammatory medication Exclusion criteria: not reported Mean age, years: females 50.8, males 52.0 (SD not reported) Female gender: 62% Mean disease duration: 170 'or more' months (SD not reported) Stable maintenance doses of DMARDs and corticosteroids were allowed: 13 patients (22.%) gold; 4 patients (10%) hydroxychloroquine; 27 patients (47%) corticosteroids. 12 patients (21%) took salicylates during the study. All participants continued antacids and a bland diet. The types of GI dysfunction in the cohort included hiatus hernia (as determined by upper gastrointestinal x‐ray examination at baseline) (n=40), gastric ulcer (n=12), duodenal ulcer (n=23), gastric hypersecretion (n=25), pylorospasm (n=12), gastritis (n=8) and/or gastric resection (n=6). Twenty‐six participants had both a hiatus hernia and either a gastric or duodenal ulcer while 10 participants had all three. A 'number of participants' were reported to have had upper GI bleeding problems prior to entering the study. |
|
| Interventions | Naproxen ‐ dosage not specified, taken for up to 52 weeks. (It was reported that the 35 participants (60.3%) who had remained in the study and had taken naproxen for longer than 6 months had taken dosages ranging from 500 to 750mg daily.) | |
| Outcomes | There was no indication of who performed the outcome assessment. Timing of outcome assessment was not specified but reported as occurring at baseline and in weeks 1‐26 and weeks 27‐52. According to the results, the following outcomes were assessed: SAFETY 1) Number of patients with positive occult faecal blood 2) Adverse effects 3) Adverse effects leading to discontinuation of treatment EFFICACY: 1) Mean number of painful or tender joints 2) Mean number of swollen joints 3) Mean number of hot and/or red joints 4) Mean number of clinically active joints 5) Therapeutic response (indicated on a categorical scale of either exellent, good, fair or poor response) |
|
| Notes | SAFETY: There was no control group. 1) Faecal occult blood was found in 0/54 participants tested at baseline, 1/58 participants tested between weeks 1‐26 and 2/32 participants tested between weeks 27‐52. 2) Over the duration of follow up a variety of side effects were reported including nausea (n=4), diarrhoea (n=3), constipation (n=1), abdominal pain (n=6), dyspepsia (n=5), melena (n=1), skin eruption (n=2), itching (n=2), insomnia (n=2), blurred vision (n=2) and proteinuria (n=1). For weeks 1‐26 9 participants reported side effects of mild to moderate severity and for weeks 27‐52 three participants reported mild to moderate severity side effects. 3) Seven participants withdrew due to AE: lightheadedness and weakness n=1, nausea n=1, proteinuria n=1, sweating and flushes n=1, inability to concentrate n=1, skin eruption and pruritus n=1, abdominal pain n=1. EFFICACY: There was no control group, no measures of variance were reported for continuous outcomes, for both continuous and categorical outcomes results were only reported as Weeks 1‐26 and Weeks 27‐52, and results were only presented descriptively. As presented in the paper, results at baseline (n=57), 1‐26 weeks (n=58) and 27‐52 weeks (n=35) were as follows: 1) Mean number of tender or swollen joints: 21.6: 13.5; 7.6. 2) Mean number of swollen joints: 16.5; 11.4; 6.9. 3) Mean number of hot and/or red joints: 8.7; 3.0; 0.3. 4) Mean number of clinically active joints: 21.9; 14.8; 9.8. Therapeutic response at weeks 1‐26 (n=58): excellent n=12; good n=17; fair n=12; poor n=17. Therapeutic response at weeks 27‐52 (n=35): excellent n=13; good n=17; fair n=3; poor n=2 (n=35). Fourteen participants (24.1%) were reported to have withdrew from the study due to lack of efficacy although the time point was not specified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Single arm trial; no randomisation was performed |
| Allocation concealment (selection bias) | High risk | Open single arm trial; all participants received active treatment |
| Blinding (performance bias and detection bias) All outcomes | High risk | All participants received active treatment and no attempt at blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It appears that all participants have been accounted for in the results although the timing of outcome was only reported as 1‐26 weeks and 27‐52 weeks. |
| Selective reporting (reporting bias) | Unclear risk | The authors did not present a list of outcomes to be assessed in the Methods section so it is unclear whether or not there has been selective reporting of outcomes. |
| Other bias | High risk | The lack of a control group means that it is not possible to know whether the reported outcomes would differ for people with RA without concomitant GI comorbidity. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1991 | Wrong population; no patients included with GI or hepatic comorbidities |
| Alvart 1983 | Wrong population; no patients included with GI or hepatic comorbidities |
| Ammitzboll 1979 | Wrong population; no patients included with GI or hepatic comorbidities |
| Andrada 1991 | Wrong population; no patients included with GI or hepatic comorbidities |
| Appelboom 1994 | Wrong population; no patients included with GI or hepatic comorbidities |
| Ashcroft 2001 | Wrong population; no patients included with GI or hepatic comorbidities |
| Bernstein 1992 | Wrong population; no patients included with GI or hepatic comorbidities |
| Bombardier 2000 | Intervention rofecoxib already withdrawn |
| Burry 1980 | Wrong population; no patients included with GI or hepatic comorbidities |
| Busson 1986 | Wrong population; no patients included with GI or hepatic comorbidities |
| Cardoe N 1977 | Wrong population; no patients included with GI or hepatic comorbidities |
| Caruso 1994 | Wrong population; no patients included with GI or hepatic comorbidities |
| Castellsague 2009 | Wrong population: healthcare database; no subanalyses for IA patients |
| Chan 2002 | Wrong population, mixed population no data retrieved separately for patients with IA |
| Chan 2004 | Wrong population, mixed population no data retrieved separately for patients with IA |
| Chan 2010 | Wrong population, mixed population no data retrieved separately for patients with IA |
| de Melo Gomes 1992 | Wrong population; no patients included with GI or hepatic comorbidities |
| Degner 2000 | Wrong population, mixed population no data retrieved separately for patients with IA |
| Doggrell 2005 | Wrong population; no patients included with GI or hepatic comorbidities |
| Eisen 2005 | Intervention: valdecoxib already withdrawn |
| El Miedany 2006 | Wrong population, mixed population no data retrieved separately for patients with IA |
| El‐Serag 1997 | Wrong outcome: risk for developing esophagitis |
| Emery 1999 | Wrong population; no patients included with GI or hepatic comorbidities |
| Eversmeyer 1993 | Wrong population; no patients included with GI or hepatic comorbidities |
| Fenton 1988 | Wrong population; no patients included with GI or hepatic comorbidities |
| Fossgreen 1976 | Wrong population; no patients included with GI or hepatic comorbidities |
| Franssen MJ | Wrong population; no patients included with GI or hepatic comorbidities |
| Furst 2002 | Wrong population; no patients included with GI or hepatic comorbidities |
| Geis 1993 | Narrative review |
| Goldstein 2000 | Wrong population; no patients included with GI or hepatic comorbidities |
| Goldstein 2004 | Intervention: valdecoxib already withdrawn |
| Graziano 1991 | Wrong population; no patients included with GI or hepatic comorbidities |
| Grennan 1977 | Wrong population; no patients included with GI or hepatic comorbidities |
| Hernández‐Diaz 2010 | Wrong population; no patients included with GI or hepatic comorbidities |
| Howard 1978 | Wrong population; no patients included with GI or hepatic comorbidities |
| Hunter 1996 | Wrong population; no patients included with GI or hepatic comorbidities |
| Huskisson 1991 | Wrong population; no patients included with GI or hepatic comorbidities |
| Huskisson 1996 | Wrong population; no patients included with GI or hepatic comorbidities |
| Jajic 2005 | Wrong population; no patients included with GI or hepatic comorbidities |
| Karateev 2008 | Wrong population, mixed population no data retrieved separately for patients with IA |
| Kean 1993 | Wrong population; no patients included with GI or hepatic comorbidities |
| Kennedy 1991 | Wrong population; no patients included with GI or hepatic comorbidities |
| Koppes 1974 | Narrative review |
| Krueger 2008 | Wrong population; no patients included with GI or hepatic comorbidities |
| Laine 2002 | Intervention rofecoxib already withdrawn |
| Laine 2006 | Wrong population; no patients included with GI or hepatic comorbidities |
| Laine 2007 | Wrong population; no patients included with GI or hepatic comorbidities |
| Laine 2008 | Wrong population, mixed population no data retrieved separately for patients with IA |
| Lazzaroni 2007 | Narrative review |
| Lehtinen 1987 | Wrong population; no patients included with GI or hepatic comorbidities |
| Lisse JR | Wrong population; no patients included with GI or hepatic comorbidities |
| Lussier 1976 | Wrong population; no patients included with GI or hepatic comorbidities |
| Magaro 1989 | Wrong population; no patients included with GI or hepatic comorbidities |
| Manniche 1987 | Wrong population, mixed population no data retrieved separately for patients with IA |
| Muller‐Fassbender 1975 | Wrong Population; No patients included with GI or hepatic comorbidities |
| Niculescu 2009 | Wrong Population; No patients included with GI or hepatic comorbidities |
| Nielsen 2006 | Narrative Review |
| O´Brien 1976 | Wrong population; no patients included with GI or hepatic comorbidities |
| Perez‐Ruiz 1996 | Wrong population; no patients included with GI or hepatic comorbidities |
| Porkodi 1987 | Wrong Population; No patients included with GI or hepatic comorbidities |
| Rahme 2007 | Narrative review |
| Reinisch 2003 | Intervention rofecoxib already withdrawn |
| Richy F | Wrong population; no patients included with GI or hepatic comorbidities |
| Rooney 1974 | Wrong population; no patients included with GI or hepatic comorbidities |
| Rostom 2005 | Wrong population; no patients included with GI or hepatic comorbidities |
| Roth 1976 | Wrong population; no patients included with GI or hepatic comorbidities |
| Roth 1995 | Wrong population, mixed population no data retrieved separately for patients with IA |
| Schmid 1976 | Wrong population; no patients included with GI or hepatic comorbidities |
| Seidman 1988 | Wrong population; no patients included with GI or hepatic comorbidities |
| Seissiger 1987 | Wrong population, mixed population no data retrieved separately for patients with IA |
| Simon 1987 | Wrong population; no patients included with GI or hepatic comorbidities |
| Simpson 1989 | Wrong population; no patients included with GI or hepatic comorbidities |
| Singh 1996 | Wrong population; no patients included with GI or hepatic comorbidities |
| Soni 2009 | Wrong population; no patients included with GI or hepatic comorbidities |
| Tausch 1976 | Wrong population; no patients included with GI or hepatic comorbidities |
| Teule 1986 | Wrong population; no patients included with GI or hepatic comorbidities |
| Turner 1988 | Wrong population; no patients included with GI or hepatic comorbidities |
| Watson 2004 | Intervention rofecoxib already withdrawn |
| Wollheim 1978 | Wrong population; no patients included with GI or hepatic comorbidities |
| Wong 2008 | Narrative review |
| Zutshi 1976 | Wrong population; no patients included with GI or hepatic comorbidities |
Differences between protocol and review
As only one open label single arm trial was included in our review, we were only able to describe the results of this study. We were unable to perform our planned analyses and no summary of findings table could be constructed.
Contributions of authors
HR wrote the first draft of the protocol.
SR, DA, RL, DvH, and RB contributed to the final version of the protocol by providing comments and suggestions on draft versions of the protocol.
All authors approved the final version of the protocol.
Sources of support
Internal sources
-
Department of Internal Medicine 3, Mecial University Vienna, Austria.
In kind
-
Deapartment of Clinical Epidemiology, Cabrini Hospital, Australia.
In kind
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Roth 1975 {published data only}
- Roth SH, Boost G. An open trial of naproxen in rheumatoid arthritis patients with significant esophageal, gastric, and duodenal lesions. Journal of Clinical Pharmacology 1975;15:378‐84. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1991 {published data only}
- Adam W. Experiences with 800 mg ibuprofen retard in ambulatory patients with activated osteoarthrosis and extra‐articular rheumatic manifestations [Erfahrungen mit Ibuprofen 800mg retard bei ambulanten Patienten mit aktivierter Osteoarthrose und extraartikularen rheumatischen Beschwerden]. Zeitschrift für Rheumatologie 1991;50:69‐76. [PubMed] [Google Scholar]
Alvart 1983 {published data only}
- Alvart R. Multicentre trial of piroxicam in ambulant rheumatology. European Journal of Rheumatology and Inflammation 1983;6:99‐105. [PubMed] [Google Scholar]
Ammitzboll 1979 {published data only}
- Ammitzboll F. Fenbufen and Indomethacin in the treatment of rheumatoid arthritis. Scandinavian Journal of Rheumatology 1979;23:5‐10. [PubMed] [Google Scholar]
Andrada 1991 {published data only}
- Andrada SS, Rodriguez Valverde V. A double blind randomised controlled trial of droxicam versus indomehtacin in rheumatoid arthritis. European Journal of Rheumatology and Inflammation 1991;11:15‐20. [PubMed] [Google Scholar]
Appelboom 1994 {published data only}
- Appelboom T, Franchimont P. Proglumetacin versus indomethacin in rheumatoid arthritis: A double‐blind multicenter study. Advances in Therapy 1994;11(5):228‐34. [Google Scholar]
Ashcroft 2001 {published data only}
- Ashcroft DM, Chapman SR, Clark WK, Millson DS. Upper gastroduodenal ulceration in arthritis patients treated with celecoxib. The Annals of Pharmacotherapy 2001;35(7‐8):829‐34. [DOI] [PubMed] [Google Scholar]
Bernstein 1992 {published data only}
- Bernstein RM, Calin HJ, Calin A, Ollier S. A comparison of the efficacy and tolerability of lornoxicam and indomethacin in ankylosing spondylithis. European Journal of Rheumatology and Inflammation 1992;12:6‐13. [Google Scholar]
Bombardier 2000 {published data only}
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos‐Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. The New England Journal of Medicine 2000;343(21):1520‐8. [DOI] [PubMed] [Google Scholar]
Burry 1980 {published data only}
- Burry HC, Sibers R. A comparison of flurbiprofen with naproxen in ankylosing spondylitis. The New Zealand Medical Journal 1980;92:309‐11. [PubMed] [Google Scholar]
Busson 1986 {published data only}
- Busson M. A long‐term study of flurbiprofen in rheumatological disorders: I Rheumatoid arthritis. Journal of International Medical Research 1986;14:1‐6. [PubMed] [Google Scholar]
Cardoe N 1977 {published data only}
- Cardoe N, Fowler PD. Butacote: A six‐year follow‐up of patients with gastric intolerance to other medications. Journal of International Medical Research 1977;5(2):59‐66. [PubMed] [Google Scholar]
Caruso 1994 {published data only}
- Caruso I, Monrone F, Boari L, Davoli C, Fiorentini F. Lornoxicam versus diclofenac in rheumtoid arthritis: A double‐blind multicenter study. Advances in Therapy 1994;11(3):133‐8. [Google Scholar]
Castellsague 2009 {published data only}
- Castellsagugue J, Holick CN, Hoffmann CC, Perez‐Gutthann. Risk of upper gastrointestinal complications associated with cyclooxygenase‐2‐selective and nonselective nonsteroidal antiinflammatory drugs. Pharmacotherapy 2009;29:1397‐407. [DOI] [PubMed] [Google Scholar]
Chan 2002 {published data only}
- Chan FKL, Hung LCT, Suen BY, Wu JCY, Lee KC, Leung VKS, Hui AJ, To KF, W.K Leung WK, Wong VKS, Chung S, Sung JYY. Celecoxib versus diclofenac and omeprazol in reducing the risk of recurrent ulcer bleeding in patients with arthritis. The New England Journal of Medicine 2002;347(26):2104‐10. [DOI] [PubMed] [Google Scholar]
Chan 2004 {published data only}
- Chan FKL, Hung LCT, Suen BY, Wong VWS, Hui AJ, Wu JCY, Leung WK, Lee YT, To KF, Chung SCS, Sung JJY. Celecoxib versus diclofenac plus omeprazole in high risk arthritis patients: Results of a randomized double‐blind trial. Gastroenterology 2004;127:1038‐43. [DOI] [PubMed] [Google Scholar]
Chan 2010 {published data only}
- Chan FKL, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet 2010;376(9736):173‐9. [DOI] [PubMed] [Google Scholar]
Degner 2000 {published data only}
- Degner F, Sigmund R, Zeidler H. Efficacy and tolerability of meloxicam in an observational, controlled cohort study in patients with rheumatic disease. Clinical Therapeutics 2000;22(4):400‐10. [DOI] [PubMed] [Google Scholar]
de Melo Gomes 1992 {published data only}
- Melo Gomes JA. The safety of arthrotec in patients with rheumatoid arthritis or osteoarthritis: An assessment of the upper gastrointestinal tract by endoscopy. Scandinavian Journal of Rheumatology 1992;96:23‐31. [DOI] [PubMed] [Google Scholar]
Doggrell 2005 {published data only}
- Doggrell SA. The safety of lumiracoxib when used in the treatment of arthritis. Expert Opinion on Pharmacotherapy 2005;6:347‐50. [DOI] [PubMed] [Google Scholar]
Eisen 2005 {published data only}
- EIsen GM, Goldstein JL, Hanna DB, Rublee DA. Meta‐analysis: upper gastrointestinal tolerability of valdecoxib, a cyclooxygenase‐2‐specific inhibitor, compared with nonspecific nonsteroidal anti‐inflammatory drugs among patients with osteoarthritis and rheumatoid arthritis. Alimentary Pharmacology and Therapeutics 2005;21:591‐8. [DOI] [PubMed] [Google Scholar]
El Miedany 2006 {published data only}
- Miedany Y, Yousef S, Ahmed I, Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox‐2 inhibitor in inflammatory bowel disease. American Journal of Gastroenterology 2006;101:311‐7. [DOI] [PubMed] [Google Scholar]
El‐Serag 1997 {published data only}
- El‐Serag HB, Sonnenber A. Association of esophagitis and esophageal strictures with diseases treated with nonsteroidal anti‐inflammatory drugs. The American Journal of Gastroenerology 1997;92(1):52‐6. [PubMed] [Google Scholar]
Emery 1999 {published data only}
- Emery P, Zeidler H, Kvien TK, Geis GS. Celecoxib versus diclofenac in long‐term management of rheumatoid arthritis: randomised double‐blind comparison. Lancet 1999;354:2106‐11. [DOI] [PubMed] [Google Scholar]
Eversmeyer 1993 {published data only}
- Eversmeyer W, Poland M, DeLapp RE, Jensen CP. Safety experience with nabumetone versus diclofenac, naproxen, ibuprofen, and piroxicam in osteoarthritis and rheumatoid arthritis. The American Journal of Medicine 1993;95:10‐8. [DOI] [PubMed] [Google Scholar]
Fenton 1988 {published data only}
- Fenton SF, Ryan JP, Bensen WG. A double‐blind, crossover, multicenter study of piroxicam and naproxen in rheumatoid arthritis. Current Therapeutic Research 1988;66(6):1058‐70. [Google Scholar]
Fossgreen 1976 {published data only}
- Fossgreen J, Kirchheiner B, Pertersen FO, Tophoj E, Zachariae E. Clinical evaluation of ketoprofen in rheumatoid arthritis. Scandinavian Journal of Rheumatology 1976;14 Suppl:93‐8. [PubMed] [Google Scholar]
Franssen MJ {published data only}
- Franssen MJAM, Grinbnau FWJ, Putte LBA. A comparison of diflunisal and phenylbutazone in the treatment of ankylosing spondylitis. Clinical Rheumatology 1986;5(2):210‐20. [DOI] [PubMed] [Google Scholar]
Furst 2002 {published data only}
- Furst DE, Karen S, Kolba KS, Fleischmann R, Roszko PJ. Dose response and safety study of meloxicam up to 22.5mg daily in rheumatoid arthritis: A 12 week mulitcenter, double blind, dose response study versus placebo and diclofenac. The Journal of Rheumatology 2002;29(3):436‐46. [PubMed] [Google Scholar]
Geis 1993 {published data only}
- Geis GS. Arthrotec: A therapeutic option in the management of arthritis. European Journal of Rheumatology and Inflammation 1993;13:25‐32. [PubMed] [Google Scholar]
Goldstein 2000 {published data only}
- Goldstein JL, Fred E, Silverstein MD, et al. Reduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX‐2‐inhibitor. The American Journal of Gastroenterology 2000;95(7):1681‐90. [DOI] [PubMed] [Google Scholar]
Goldstein 2004 {published data only}
- Goldstein JL, Eisen GM, Agrawal N, Stenson WF, Kent JD, Verburg KM. Reduced incidence of upper gastrointestinal ulcer complications with the COX‐2 selective inhibitor, valdecoxib. Alimentary Pharmacology and Therapy 2004;20:527‐38. [DOI] [PubMed] [Google Scholar]
Graziano 1991 {published data only}
- Graziano FM. Once‐daily or twice daily administration of naproxen in patients with rheumatoid arthritis. Clinical Therapeutics 1991;13:20‐5. [Google Scholar]
Grennan 1977 {published data only}
- Grennan DM. Therapeutic uses of azapropazone. Scottish Medical Journal 1977;22:22‐4. [DOI] [PubMed] [Google Scholar]
Hernández‐Diaz 2010 {published data only}
- Hernández‐Diaz S, Garcia Rodriguez A. Association between nonsteroidal anti‐inflammatory drugs and upper gastrointestinal tract bleeding/perforation. Archives of Internal Medicine 2010;160:2093‐9. [DOI] [PubMed] [Google Scholar]
Howard 1978 {published data only}
- Howard DLG. A double‐blind crossover evaluation of ketoprofen and placebo in rheumatoid arthritis with assessment of long‐term tolerance. The Journal of International Medical Research 1978;6:300‐5. [DOI] [PubMed] [Google Scholar]
Hunter 1996 {published data only}
- Hunter JA, Parnham MJ, Balaguer I. Aceclofenac in rheumatoid arthritis: a useful and novel anti‐Inflammatory. Clinical Rheumatology 1996;15(4):329‐34. [DOI] [PubMed] [Google Scholar]
Huskisson 1991 {published data only}
- Huskisson EC, Scott DL. A clinical comparison of two leading non‐steroidal anti‐inflammatory drugs. European Journal of Rheumatology and Inflammation 1991;11:4‐7. [PubMed] [Google Scholar]
Huskisson 1996 {published data only}
- Huskisson EC, Ghozlan R, Kurthen R, Degners FL, Bluhmki E. A long‐term study to evaluate the safety and efficacy of meloxicam therapy in patients with rheumatoid arthritis. British Journal of Rheumatology 1996;35 Suppl 1:29‐34. [DOI] [PubMed] [Google Scholar]
Jajic 2005 {published data only}
- Jajic Z, Malaise M. Nekam K, Koó E, Dankó K, Kovacs M, Scarpignato C. Gastrointestinal safety of amtolmetin guacyl in comparison with celecoxib in patients with rheumatoid arthritis. Clinical and Experimental Rheumatology 2005;23:809‐18. [PubMed] [Google Scholar]
Karateev 2008 {published data only}
- Karateev AE. Relapses of NSAID‐induced ulcers in rheumatologic patients who continue treatment with NSAIDs (meeting abstract). Annals of Rheumatic Disease 2008;67 Suppl II:170. [Google Scholar]
Kean 1993 {published data only}
- Kean W, Buchanan W, Pelletier JP, Haraoui B, Choquette D, Senecal JL, Gascon J, Germain H, Menard H, Huang S, Elie R, Gerecz‐Simon E. Open study on the efficacy and safety of long‐term use of surgam‐sr (sustained‐release form of tiaprofenic acid) in rheumatoid arthritis patients. Inflammopharmacololgy 1993;2:63‐70. [Google Scholar]
Kennedy 1991 {published data only}
- Kennedy AC, Germain BF, Goldman AL, Vreede PD. A double‐blind crossover compariosn of ketoprofen and indomethacin in ankylosing spondylitis. Advances in Therapy 1991;8(3):148‐56. [Google Scholar]
Koppes 1974 {published data only}
- Koppes GM, Arnett FC. Salicylate hepatotoxicity. Postgraduate Medicine 1974;56:193‐5. [DOI] [PubMed] [Google Scholar]
Krueger 2008 {published data only}
- Krueger K, Lino L, Dore R. Gastrointestinal tolerability of etoricoxib in rheumatoid arthritis patients: results of the etoricoxib vs. diclofenac sodium gastrointestinal tolerability and effectiveness trial (EDGE II). Annals of Rheumatic Diseases 2008;67:315‐22. [DOI] [PubMed] [Google Scholar]
Laine 2002 {published data only}
- Laine L, Bombardier C, Hawkey CJ, Davis B, Shapiro D, Brett C, Reicin A. Stratifying the risk of NSAID‐related upper gastrointestinal clinical events: Results of a double‐blind outcomes study in patients with rheumatoid arthritis. Gastroenterology 2002;123:1006‐12. [DOI] [PubMed] [Google Scholar]
Laine 2006 {published data only}
- Laine L, Smith R, Min K, Chen Dubois RW. Systematic review: the lower gastrointestinal adverse effects of non‐steroidal anti‐inflammatory drugs. Alimentary Pharmacology and Therapy 2006;24:751‐67. [DOI] [PubMed] [Google Scholar]
Laine 2007 {published data only}
- Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Mulinational Etoricoxib and Diclofenac Arthritis Long‐term (MEDAL) programme: a randomised comparison. Lancet 2007;369:465‐73. [DOI] [PubMed] [Google Scholar]
Laine 2008 {published data only}
- Laine L, Curtis SP, Langman M, Jensen DM, Cryer B, Kaur A, Cannon CP. Lower gastrointestinal events in a double‐blind trial of the cyclo‐oxygenase‐2‐selective inhibitor etoricoxib and the traditional nonsteroidal anti‐inflammatory crug diclofenac. Gastroenterology 2008;135:1517‐25. [DOI] [PubMed] [Google Scholar]
Lazzaroni 2007 {published data only}
- Lazzaroni M, Battocchia A, Bianchi Porro G. COXIBs and non‐selective NSAIDs in the gastroenterological setting: what should patients and physicians do?. Digestive and Liver Disease 2007;39:589‐96. [DOI] [PubMed] [Google Scholar]
Lehtinen 1987 {published data only}
- Lehtinen K. A six‐month follow‐up study of a slow‐release indomehtacin tablet in rheumatic diseases. Clinical Rheumatology 1987;6:606‐7. [DOI] [PubMed] [Google Scholar]
Lisse JR {published data only}
- Lisse JR. Clinical efficacy and safety of naprelan versus naprosyn in the treatment of rheumatoid arthritis. American Journal of Orthopedics 1996;25:21‐9. [PubMed] [Google Scholar]
Lussier 1976 {published data only}
- Lussier A, Camerlain M, Menard H, Myhal D, Wehner S. A double blind cross‐over evaluation of ketorprofen and aspirin in rheumatoid arthritis. Scandinavian Journal of Rheumatology 1976;14 Suppl:99‐104. [PubMed] [Google Scholar]
Magaro 1989 {published data only}
- Magaro M, Altomonte L, Zoli A, Mirone L, Berchicci MC, Tricerri A, Corvino G. Asymptomatic and symptomatic gastroduodenal patients affected by rheumatic disease treated with non‐steroidal anti‐inflammatory drugs. Panminerva Medica 1989;31:16‐8. [PubMed] [Google Scholar]
Manniche 1987 {published data only}
- Manniche C, Malchow‐Moller A, Andersen JR, Pedersen C, Hansen TM, Jess P, et al. Randomised study of the influence of non‐steroidal anti‐inflammatory drugs on the treatment of peptic ulcer in patients with rheumatic disease. Gut 1987;28(2):226‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller‐Fassbender 1975 {published data only}
- Muller‐Fassbender A. A long‐term study of benorylate in patients with rheumatoid arthritis. Scandinavian Journal of Rheumatology 1976;13:21‐4. [DOI] [PubMed] [Google Scholar]
Niculescu 2009 {published data only}
- Niculescu L, Li C, Huang J, Mallen S. Pooled analysis of GI tolerability of 21 randomized controlled trials of celecoxib and nonselective NSAIDs. Current Medical Research and Opinions 2009;25(3):729‐40. [DOI] [PubMed] [Google Scholar]
Nielsen 2006 {published data only}
- Nielsen OH, Ainsworht M, Csillag C, Rask‐Madsen J. Systematic review: coxibs, non‐steroidal anti‐inflammatory drugs or non cyclo‐oxygenase inhibitors in gastroenterological high risk patients. Alimentary Pharmacology and Therapy 2006;23:27‐33. [DOI] [PubMed] [Google Scholar]
O´Brien 1976 {published data only}
- O'Brien W, Grunwaldt E. Ketoprofen in the treatment of rheumatoid arthritis: double blind cross‐over placebo comparison. Scandinavian Journal of Rheumatology 1976;14 Suppl:105‐10. [PubMed] [Google Scholar]
Perez‐Ruiz 1996 {published data only}
- Perez‐Ruiz F, Alonso‐Ruiz A, Ansoleaga JJ. Comparative study of the efficacy and safety of aceclofenac and tenoxicam in rheumatoid arthritis. Clinical Rheumatology 1996;15:473‐7. [DOI] [PubMed] [Google Scholar]
Porkodi 1987 {published data only}
- Porkodi R, Raghuram K, Parthiban M, Chandrasekaran AN. Therapeutic efficacy and gastric tolerance of ibuprofen at 1600‐2400 mg dosage in rheumatoid arthritis. The British Journal of Clinical Practice 1987;41(12):1037‐40. [PubMed] [Google Scholar]
Rahme 2007 {published data only}
- Rahme E, Nedjar H. Risk and benefits of COX‐2‐inhibitors vs non‐selective NSAIDs: does their cardiovascular risk exceed their gastrointestinal benefit? A retrospective cohort study. Rheumatology 2007;46:435‐8. [DOI] [PubMed] [Google Scholar]
Reinisch 2003 {published data only}
- Reinisch W, Miehsler W, Dejaco C, Harrer M, Waldhofer T, Lichtenberger, Vogelsang H. An open‐label trial of the selective cyclo‐oxygenase 2 inhibitor rofecoxib in inflammatory bowel disease associated peripheral arthritis and arthralgia. Alimentary Pharmacology and Therapy 2003;17:1371‐80. [DOI] [PubMed] [Google Scholar]
Richy F {published data only}
- Richy F, Scarpignato C, Lanas A. Efficacy and safety of piroxicam revisited: a global meta‐analysis of randomised control trials. Pharmacological Research 2009;60:254‐63. [DOI] [PubMed] [Google Scholar]
Rooney 1974 {published data only}
- Rooney PJ, Watkins C, Ahola SJ, Gray GIL, Carson DW. A short‐term double blind controlled trial of prenazone (DA2370) in rheumatoid arthritis. Current Medical Research and Opinion 1974;2:43. [DOI] [PubMed] [Google Scholar]
Rostom 2005 {published data only}
- Rostom A, Goldkind L, Laine L. Nonsteroidal anti‐inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clinical Gastroenterology and Hepatology 2005;3:489‐98. [DOI] [PubMed] [Google Scholar]
Roth 1976 {published data only}
- Roth SH, Englund DW, Harris BK. Longterm fenoprofen therapy in patients with rheumtoid arthritis. Journal of Rheumatology 1976;2:43‐8. [PubMed] [Google Scholar]
Roth 1995 {published data only}
- Roth SH, Bennett SE, Caldron PH, Mitchell CS, Swenson CM. Endoscopic evaluation of the long term effects of diclofenac sodium and naproxen in elderly patients with arthritis. Clinical Drug Investigation 1995;9(3):171‐7. [Google Scholar]
Schmid 1976 {published data only}
- Schmid FR, Culic DD. Anti‐inflammatory drugs and gastrointestinal bleeding: a comparison of aspirin and ibuprofen. Journal of Clinical Pharmacology 1976;16:418‐25. [DOI] [PubMed] [Google Scholar]
Seidman 1988 {published data only}
- Seideman P, Eriksson LO. Indomethacin in rheumatoid arthritis. Clinical Trials Journal 1988;25(5):349‐58. [Google Scholar]
Seissiger 1987 {published data only}
- Seissiger L, Dell HD. Acetaminophen in patients with rheumatic disease with concomitant liver diseases: pharmacokinetics, effectiveness and tolerance [Acemetacin bei rheumapatienten mit leberbegleiterkrankungen pharmakokinetik, wirksamkeit und verträglichkeit]. Zeitschrift für Rheumatologie 1987;46(Suppl 1):65‐9. [PubMed] [Google Scholar]
Simon 1987 {published data only}
- Simon B, Dammann HG, Müller P. Stomach tolerance of nonsteroidal antirheumatic drugs: comparative endoscopic study [Magenverträglichkeit nichtsteroidaler antirheumatika: endoskopische vergleichsstudien]. Zeitschrift für Rheumatologie 1987;46(Suppl.1):40‐6. [PubMed] [Google Scholar]
Simpson 1989 {published data only}
- Simpson J, Golding DN, Freeman AM, Cooke D, Hooper PA, Jamieson V, Osborne C. A large multicentre, parallel group, double‐blind study comparing tenoxicam and piroxicam in the treatment of osteoarthritis and rheumatoid arthritis. British Journal of Clinical Practice 1989;43:328‐33. [PubMed] [Google Scholar]
Singh 1996 {published data only}
- Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal tract complications of nonsteroidal anti‐inflammatory drug treatment in rheumatoid arthritis: a prospective observational cohort study. Archives of Internal Medicine 1996;156(14):1530‐6. [PubMed] [Google Scholar]
Soni 2009 {published data only}
- Soni P, Shell B, Cawkwell G, Li C, Ma H. The hepatic safety and tolerability of the cyclooxgenase‐2 selective NSAID celecoxib: pooled analysis of 41 randomized controlled trials. Current Medical Research and Opinion 2009;25(8):1841‐51. [DOI] [PubMed] [Google Scholar]
Tausch 1976 {published data only}
- Tausch G, Bröll H, Dunky A, Eberl R. Comparative clinical trial with a new substance, diftalone, versus indomethacin in 30 patients with rheumatoid arthritis. Scandanavian Journal of Rheumatology 1976;5:145‐50. [DOI] [PubMed] [Google Scholar]
Teule 1986 {published data only}
- Teule M. Double‐blind comparative multicenter study of the efficacy and safety of a slow release tablet of 200 mg ketoprofen once daily and a 50mg ketoprofen capsule four times daily. Current Therapeutic Research 1986;40(6):1129‐46. [Google Scholar]
Turner 1988 {published data only}
- Turner R. Hepatic and renal tolerability of long‐term naproxen treatment in patients with rheumatoid arthritis. Seminars in Arthritis and Rheumatism 1988;17(3):29‐35. [DOI] [PubMed] [Google Scholar]
Watson 2004 {published data only}
- Watson DJ, Yu Q, Bolognese JA, Reicin AS, Simon TJ. The upper gastrointestinal safety of rofecoxib versus NSAIDs: an updated combined analysis. Current Medical Research and Opinion 2004;20(10):1539‐48. [DOI] [PubMed] [Google Scholar]
Wollheim 1978 {published data only}
- Wollheim FA, Lindroth Y, Sjöblom K. A comparison of ketoprofen and naproxen in rheumatoid arthritis. Rheumatology and Rehabilitation 1978;Suppl:78‐83. [DOI] [PubMed] [Google Scholar]
Wong 2008 {published data only}
- Wong WSV, Chan FKL. Cyclooxygenase‐2‐inhibitors in patients with high gastrointestinal risk: are we there yet?. Journal of Gastroenterololgy 2009;44 Suppl XIX:53‐6. [DOI] [PubMed] [Google Scholar]
Zutshi 1976 {published data only}
- Zutshi D, Mason M. Ketorprofen in rheumatoid arthritis: its tolerance and therapeutic effect. Scandinavian Journal of Rheumatology 1976;14 Suppl:77‐84. [PubMed] [Google Scholar]
Additional references
Akarca 2005
- Akarca US. Gastrointestinal effects of selective and non‐selective non‐steroidal anti‐inflammatory drugs. Current Pharmaceutical Design 2005;11(14):1779‐93. [DOI] [PubMed] [Google Scholar]
Benson 1983
- Benson GD. Hepatotoxicity following the therapeutic use of antipyretic analgesics. American Journal of Medicine 1983;14(75):85‐93. [DOI] [PubMed] [Google Scholar]
Benson 2005
- Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. American Journal of Therapeutics 2005;12(2):133‐41. [DOI] [PubMed] [Google Scholar]
Bergmann 2006
- Bergmann MJ. Social and economic impact of inflammatory arthritis. Postgraduate Medicine 2006;Spec No:5‐11. [PubMed] [Google Scholar]
Boswell 2010
- Boswell K, Kwong WJ, Kavanagh S. Burden of opioid‐associated gastrointestinal side effects from clinical and economic perspectives: a systematic literature review. Journal of Opioid Management 2010;6(4):269‐89. [DOI] [PubMed] [Google Scholar]
Briggs 2009
- Briggs A, March L, Reid C, Lassere M, Reid C, Henderson L, et al. Baseline comorbidities in an Australian population‐based cohort of rheumatoid arthritis patients receiving biological therapy: Data from the Australian Rheumatology Association Database (ARAD). International Journal of Rheumatology. 2009. [DOI] [PMC free article] [PubMed]
Calin 1994
- Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, Jenkinson T. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. Journal of Rheumatology 1994;21(12):2281‐5. [PubMed] [Google Scholar]
Cook 2008
- Cook SF, Lanza L, Zhou X, Sweeney CT, Goss D, Hollis K, Mangel AW, Fehnel SE. Gastrointestinal side effects in chronic opioid users: results from a population‐based survey. Alimentary Pharmacology and Therapy 2008;27(12):1224‐32. [DOI] [PubMed] [Google Scholar]
CRD 2001
- Khan KS, ter Riet G, Popay J, Nixon N, Kleijnen J. Study quality assessment. Undertaking Systematic Reviews of Research on Effectiveness. CRD's guidance for those carrying out or commissioning reviews. Report 4. York Publishing Services Ltd, March 2001.
da Silva 2001
- Silva JA, Ramiro S, Pedro S, Rodrigues A, Vasconcelos JC, Benito‐Garcia E. Patients‐ and physicians‐ priorities for improvement. The case of rheumatic diseases. Acta Reumatologica 2001;35(2):192‐9. [PubMed] [Google Scholar]
DeAngelis 2004
- DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004;292(11):1363‐4. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester (UK): John Wiley & Sons.
Doward 2003
- Doward LC, Spoorenberg A, Cook SA, Whalley D, Helliwell PS, Kay LJ, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Annals of Rheumatic Disease 2003;62:20‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drossaers‐Bakker 1999
- Drossaers‐Bakker KW, Buck M, Zeben D, Zwinderman AH, Breedveld FC, Hazes JMW. Long‐term course and outcome of functional capacity in rheumatoid arthritis ‐ The effect of disease activity and radiologic damage over time. Arthritis and Rheumatism 1999;42:1854‐60. [DOI] [PubMed] [Google Scholar]
Emery 2006
- Emery P. Treatment of rheumatoid arthritis. BMJ 2006;332:152‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2008
- EPOC data collection checklist. http://epoc.cochrane.org/epoc‐resources‐review‐authors.
Fries 1980
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis and Rheumatism 1980;23(2):137‐45. [DOI] [PubMed] [Google Scholar]
Gabriel 1999
- Gabriel SE. Comorbidity in arthritis. Journal of Rheumatology 1999;26:2475‐9. [PubMed] [Google Scholar]
Heiberg 2002
- Heiberg T, Kvien TK. Preferences for improved health examined in 1,024 patients with rheumatoid arthritis: pain has highest priority. Arthritis and Rheumatism 2002;47(4):391‐7. [DOI] [PubMed] [Google Scholar]
Henry 2003
- Henry D, McGettigan P. Epidemiology overview of gastrointestinal and renal toxicity of NSAIDs. International Journal of Clinical Practice 2003;135:43‐9. [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editors, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011 ). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Jong 1997
- Jong Z, Heijde D, McKenna SP, Whalley D. The reliability and construct validity of the RAQoL: a rheumatoid arthritis‐specific quality of life instrument. British Journal of Rheumatology 1997;36(8):878‐83. [DOI] [PubMed] [Google Scholar]
Marks 2010
- Marks JL, Colebatch AN, Buchbinder R, Edwards CJ. Pain management for rheumatoid arthritis and cardiovascular or renal comorbidity. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008952.pub2] [DOI] [PubMed] [Google Scholar]
Michaud 2007
- Michaud K. Comorbidities in rheumatoid arthritis. Best Practice & Research in Clinical Rheumatology 2007;21(5):885‐906. [DOI] [PubMed] [Google Scholar]
Mikulis 2003
- Mikuls TR. Co‐morbidity in rheumatoid arthritis. Best Practice & Research in Clinical Rheumatology 2003;17(5):729‐52. [DOI] [PubMed] [Google Scholar]
Minnrock 2003
- Minnock P, FitzGerald O, Bresnihan B. Women with established rheumatoid arthritis perceive pain as the predominant impairment of health status. Rheumatology 2003;42(8):995‐1000. [DOI] [PubMed] [Google Scholar]
Moore 2010
- Moore AR, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, McQuay H. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief; Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain‐‐establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Ng 2010
- Ng SC, Chan FK. NSAID‐induced gastrointestinal and cardiovascular injury. Current Opinion in Gastroenterology 2010;26(6):611‐7. [DOI] [PubMed] [Google Scholar]
Oldroyd 2009
- Oldroyd J, Schachna L, Buchbinder R, Staples M, Briggs A, Lassere M, Reid C, March L. Baseline characteristics in an Australian population‐based cohort of patients with ankylosing spondylitis commencing biological therapy: data from the Australian Rheumatology Association Database (ARAD). International Journal of Rheumatology. 2009. [DOI] [PMC free article] [PubMed]
Pincus 1983
- Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis and Rheumatism 1983;26(11):1346‐53. [DOI] [PubMed] [Google Scholar]
Prescott 2000
- Prescott LF. Paracetamol, alcohol and the liver. British Journal of Clinical Pharmacology 2000;49(4):291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 20011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Silverstein 2000
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study. JAMA 2000;284(13):1247‐55. [DOI] [PubMed] [Google Scholar]
Simon 1999
- Simon LS, Weaver AL, Graham DY, Kivitz AJ, Lipsky PE, Hubbard RC, et al. Anti‐inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial.. JAMA 1999;282(20):1921‐8. [DOI] [PubMed] [Google Scholar]
Singh 1999
- Singh G, Triadafilopoulos G. Epidemiology NSAID induced gastrointestinal complications. Journal of Rheumatology 1999;26 Suppl 56:18‐24. [PubMed] [Google Scholar]
Smolen 2010
- Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs. Annals of Rheumatic Disease 2010;69(6):964‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Ware 1992
- Ware JE, Sherbourne CD. The Mos 36‐Item Short‐Form Health Survey (SF‐36). 1. Conceptual‐Framework and Item Selection. Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
Wells 2008
- Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta‐analyses. Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm 2009.
Wolfe 1999
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. New England Journal of Medicine 1999;340:1888‐99. [DOI] [PubMed] [Google Scholar]


