Abstract
Insecticides can cause significant harm to both terrestrial and aquatic environments. The new insecticides derived from microbial sources are a good option with no environmental consequences. Metarhizium anisopliae (mycelia) ethyl acetate extracts were tested on larvae, pupae, and adult of Anopheles stephensi (Liston, 1901), Aedes aegypti (Meigen, 1818), and Culex quinquefasciatus (Say, 1823), as well as non-target species Eudrilus eugeniae (Kinberg, 1867) and Artemia nauplii (Linnaeus, 1758) at 24 h post treatment under laboratory condition. In bioassays, Metarhizium anisopliae extracts had remarkable toxicity on all mosquito species with LC50 values, 29.631 in Ae. aegypti, 32.578 in An. stephensi and 48.003 in Cx. quinquefasciatus disease-causing mosquitoes, in A. nauplii shows (5.33–18.33 %) mortality were produced by the M. anisopliae derived crude extract. The LC50 and LC90 values were, 620.481; 6893.990 μg/mL. No behavioral changes were observed. A low lethal effect was observed in E. eugeniae treated with the fungi metabolites shows a 14.0 % mortality. The earthworm E. eugeniae mid-gut histology revealed that M. anisopliae extracts had no more harmful effects on the epidermis, circular muscle, setae, mitochondrion, and intestinal lumen tissues than chemical pesticides. By Liquid chromatography mass spectrometry (LC-MS) analysis, camphor (25.4 %), caprolactam (20.68 %), and monobutyl phthalate (19.0 %) were identified as significant components of M. anisopliae metabolites. Fourier transform infrared (FT-IR) spectral investigations revealed the presence of carboxylic acid, amides, and phenol groups, all of which could be involved in mosquito toxicity. The M. anisopliae derived chemical constituents are effective on targeted pests, pollution-free, target-specific, and are an alternative chemical insecticide.
Keywords: Metarhizium anisopliae, Artemia nauplii, Eudrilus eugeniae, mosquitoes, target specific, green pesticides
1. Introduction
Mosquitoes are a major health problem because they transmit diseases such as malaria, dengue fever, yellow fever, and the Zika virus, which impact 700 million people each year and kill over one million people [1]. Adult mosquito control has been the primary approach for avoiding disease transmission, and it usually requires the use of synthetic pesticides and repellents, primarily organophosphates and pyrethroids [2]. Mosquitoes have evolved resistance to organophosphate and synthetic pyrethroids [3,4,5,6]. The chemical pesticides have been accumulated in our green ecosystem soil and waterbodies as well as food chains [5]. For insect pest management, entomopathogenic bacteria, fungi, and nematodes are considered effective microbial control methods for insect pests [7,8,9].
Entomopathogenic fungi produce secondary metabolites that could be used as a source for biopesticide development [10,11,12,13,14]. The entomopathogenic fungi Metarhizium anisopliae secondary metabolites, in particular, are known to be effective biopesticides for the control of Aedes aegypti (Meigen, 1818) mosquitoes [7,15], and other fungi such as Tolypocladium [13], Beauveria [9], Fusarium [10,11], and Lagenidium giganteum [14] have also been bioprospected as insect pests. Several biopesticides have been hampered by the fact that they are slow acting, taking anywhere from a few days to a week to exhibit action, which has hampered their commercialization [16]. Furthermore, their effects on non-target organisms are understudied [7].
The brine shrimp, Artemia sp. (Anostraca: Artemiidae), is a branchiopod crustacean that can tolerate salinities of up to 250 gL−1/L [17]. Artemia are commonly employed for the evaluation of marine contamination by synthetic chemicals because of their high sensitivity to chemicals or other toxicants [17], and Artemia nauplii (Linnaeus, 1758), an important component of the aquatic ecosystem, are regarded as indicators for environmental toxicity [17,18]. Earthworms, therefore, are considered to be bio-indicators of terrestrial ecosystems and are frequently used as biomarkers for assessing the environmental toxicity of chemical contaminants [19,20]. In the present study, we investigated the toxicity of secondary metabolites extracted from Metarhizium anisopliae (Metschn, 1879) strains and their toxicity effect was evaluated against disease-vector mosquitoes Aedes Aegypti (Meigen, 1818), Anopheles Stephensi (Liston, 1901), and Culex quinquefasciatus (Say, 1823), as well as their toxicity against non-target organisms, such as earthworm Eudrilus eugeniae (Kinberg, 1867) and brine shrimp Artemia nauplii (Linnaeus, 1758).
2. Materials and Methods
2.1. Fungal Cultures
M. anisopliae, was isolated and collected from a soil sample from the Eastern Ghats of Tamil Nadu, India (Latitudes 11°30′ and 22° N, and longitudes 76°50′ and 86°30′ E). Morphological and 18s rDNA sequencing was used to identify fungi cultures. The gene sequences were submitted to the National Center for Biotechnology Information (NCBI, Data Base Accession No is: MH165400.1).
2.2. Mass Culturing of M. anisopliae
Metarhizium anisopliae was cultured on Potato Dextrose Broth (PDB), as a medium for fungal growth. Sixteen 500 mL conical flasks, each containing 250 mL of PDB (dextrose 8 g, peptone 2 g and distilled water 250 mL), were autoclaved at 15 psi for 25 min. Chloramphenicol antibiotics (150 mg/mL) (Sigma-Aldrich Chemicals Private Limited, Bangalore) was added to the culture medium to prevent bacterial contamination. The cultures were allowed to grow for 20 days, and spore concentration was counted using a hemocytometer. A concentration of 1 × 107 spores/mL of M. anisopliae conidia were transferred to the culturing medium using an inoculation needle. The culture medium was maintained at the optimized culture conditions (pH 7.0, temperature 28 ± 5 °C) for 30 days.
2.3. Extraction of Secondary Metabolites
Fungus mycelial biomass was washed with distilled water after 20 days to eliminate culture medium components. Metarhizium anisopliae biomass was cold extracted with ethyl acetate to extract the biologically active chemical constituents under laboratory conditions. The ethyl acetate solvent was fully pooled with fungal biomass and left for 25 days. Then, the organic phase (light-yellow color) was separated after 25 days using a separating funnel, and the solvent was evaporated using a rotary evaporator at 45 °C.
2.4. Larval Collection and Maintenance
The Institute of Vector Control and Zoonoses at Hosur, Tamil Nadu, India. The mosquito egg masses per species were separately placed in a plastic tray (22 cm × 27 cm × 12 cm) (wonder, India) in dechlorinated tap water. The containers were transferred to room temperature with 28 ± 2 °C, 70–80% RH relative humidity and 12:12 (L:D) photoperiod and kept in it for 10–15 days. Each stage (larvae, pupae, and adult) of the mosquitos were taken for bioassay. During this process, mosquito larvae were fed with 0.5 g Tetra Bit (Pellet Fish Food) in each container, and adults were given a 10% sugar solution as a feeding source.
2.5. Non-Target Organisms
The Eudrilus eugeniae stock were maintained under laboratory condition at a room temperature of 27 ± 2 °C. Artemia nauplii larvae were kept in 1000 mL of saltwater with a salinity of 30 ppt in a culture medium with a pH range of (7–8). An aspirator was used to provide oxygen.
2.6. Mosquitocidal Bioassays
The fungal metabolites larvicidal and pupicidal efficacy was assessed using the World Health Organization protocol [21]. Stock solutions of fungal extract were dissolved in Dimethylsulfoxide (DMSO) (Sigma-Aldrich, India) at a concentration of 10% w/v (10 µg of extracts in 100 mL of DMSO) and diluted to five different concentrations: 10, 15, 30, 50, and 75 µg/mL. Twenty-five 4th instar larvae and pupae were each transferred to 249 mL of tap water with 1 mL of different concentrations of fungal extract and replicated three times. Dead insects were counted 24 h. As a negative control, DMSO at a concentration of 10% w/v was used.
The adulticidal activity was evaluated following methods described by the Centers for Disease Control and Prevention [22]. Twenty-five newly emerged adults of A. aegypti, A. stephensi and C. quinquefasciatus were exposed to different concentrations (25, 50, 100, 150 and 200 μg/mL) of M. anisopliae secondary metabolites. Metabolites solutions were dispensed to the screw cap bottle of 80 mL, and for solvent evaporation, it was air dried over-night. In the control treatment, adult mosquitoes were exposed to DMSO (0.1%). Mortality was recorded post 24 h of treatment. A cotton ball soaked with a 10% glucose solution was used as a food source for mosquitoes. Three replicates for each concentration were performed (n = 450).
2.7. Non-Target Bioassays
The effects of fungal metabolites on earthworms E. eugeniae was tested in an artificial soil composed of 15% sphagnum peat, 25% kaolinite clay, and 77 % fine sand. To keep the pH at 5.9, a few drops of CaCO3 were added. The water content was reduced to 30% of the dry weight. Fungi metabolites from M. anisopliae were put into the artificial soil at concentrations of 50 g/mL and 75 µg/mL. The 15 E. eugeniae larvae were then moved to a plastic container (375 mm × 300 mm × 75 mm) containing 1 kg of sterile artificial soil, which was then sealed with a plastic lid to keep the worms from escaping. Dead worms were counted 24–h after exposure. Monocrotophos was used as a positive control, while the negative control was free of fungal metabolites. Each treatment was replicated three times.
On brine shrimp A. nauplii, the toxicity of fungal secondary metabolites was determined as follows: Mature A. nauplii were collected with a hand pipette and utilized in toxicity tests on A. nauplii with different concentrations of M. anisopliae secondary metabolites (10, 15, 30, 50, and 75 µg/mL). As a negative control, the DMSO solution was employed. After 24 h of treatment, the A. nauplii dead mortality was calculated. Each concentration was tested three times, with each replicate containing 25 mature A. nauplii.
2.8. Fourier Transformed Infrared Spectroscopy Analysis
FT-IR analysis was conducted for the identification of the functional groups presents in the crude fungal metabolites. Two mg of fungi metabolites were properly mixed in 75 mg KBr; KBr acts as a binding agent on cleaned micro mortar and pestle. The mixed component was made into KBr pellets formed at low pressure. The KBr pellets were taken for FT-IR analysis using a BRUKER FT-IR spectrometer. FT-IR spectra scanning range was from 500 to 4000 cm−1.
2.9. Liquid Chromatography-Mass Spectrophotometer Analysis
The chemical components profiling of crude fungal extracts was completed through the use of a Bruker Daltonik Impact II ESI-Q-TOF system (Bremen, Germany), ready with a Bruker Daltonik Elute, Ultra High Performance Liquid Chromatography (UHPLC) system (Bremen, Germany), in each positive (M + H) and negative (M − H) electrospray ionisation modes. Chromatographic separation was carried out on a Bruker Daltonik (Bremen, Germany) C18 reversed segment column (2.1 mm, 1.8 m, 120) at 30 °C, with an autosampler temperature of 8 °C and a total run time of 20 min, using water/methanol (90:10%) as eluent with five mM ammonium formate and 0.1% formic acid. The crude extract was dissolved in 2.0 mL of DMSO, and the quantity was multiplied to 50 mL with acetonitrile prior to centrifugation at 4000 rpm for two min and injection. The composition of the samples became mounted with the aid of figuring out the m/z ratio when it comes to the retention length of the utilised standards.
2.10. Statistical Analysis
The mortality rate was corrected using Abbot formula [23]. The dead A. nauplii, mosquito larvae, pupae, and adults were counted separately 24 h after treatment, and LC50 and LC90 were estimated using probit analysis. The SPSS-16.00 programme [18] was used to conduct all of the analyses.
3. Results
3.1. M. anisopliae metabolites against Ae. aegypti, An. stephensi, Cx. quinquefasciatus Mosquitoes
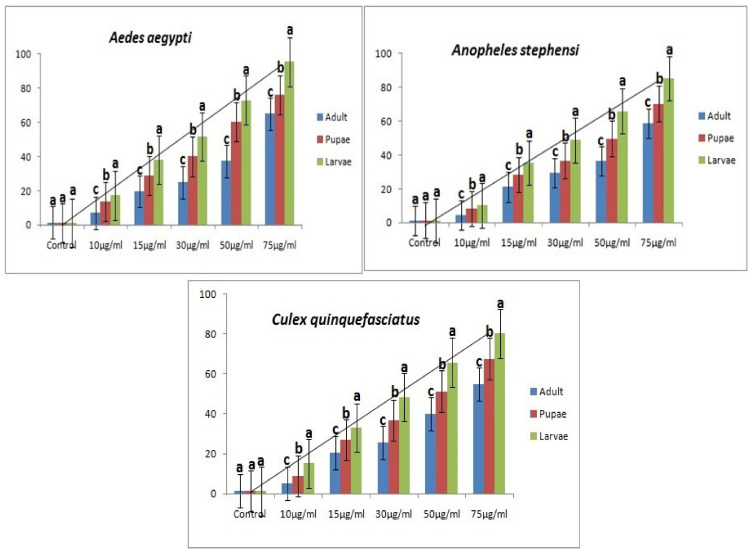
M. anisopliae crude metabolites treatments, at the tested concentrations (10, 15, 30, 50 and 75 μg/mL), caused significant mortality against Ae. aegypti larvae (ranging from 17.33 to 95.33%), pupae (ranging from 13.66 to 76.00%), and adults (ranging from 7.00 to 65.00%) (Figure 1; Table 1). The probit model indicated that, Ae. aegypti larvae are more susceptible to the M. anisopliae crude metabolites than the pupae and adults with an LC50-29.631, 45.530, and 62.589 µg/mL for larvae, pupae, and adults, respectively (Table 1).
Figure 1.
Larvicidal, pupicidal and adulticidal activities of M. anisopliae derived extract against larvae, pupae, and adult of Ae. aegypti, An. stephensi and Cx. quinquefasciatus vectors. Bars with the identical lower case letters do not differ significantly (p > 0.05).
Table 1.
Mosquitocidal activities of M. anisopliae ethyl acetate crude extract against larvae, pupae, and adults of three mosquito species at 24 h after treatments.
| Mosquito | Stage | N = Insect Number | LC50 (LCL-UCL) | LC90 (LCL-UCL) | χ2 (df = 12) |
|---|---|---|---|---|---|
| Ae. aegypti | Larvae | 450 | 29.631 (25.440–36.833) | 80.560 (74.910–87.001) | 5.673 |
| Pupae | 450 | 45.530 (39.920–51.532) | 103.430 (98.571–109.642) | 4.041 | |
| Adult | 450 | 62.589 (57.439–67.991) | 123.775 (115.679–129.002) | 6.090 | |
| An. stephensi | Larvae | 450 | 32.578 (27.871–35.900) | 88.003 (82.717–93.966) | 5.214 |
| Pupae | 450 | 52.491 (46.913–56.331) | 98.110 (95.332–105.88) | 1.287 | |
| Adult | 450 | 70.235 (66.057–75.339) | 150.921 (141.883–157.991) | 3.002 | |
| Cx. quinquefasciatus | Larvae | 450 | 48.003 (41.771–53.994) | 96.883 (93.880–103.439) | 6.454 |
| Pupae | 450 | 69.017 (64.771–74.000) | 158.881 (151.875–164.640) | 0.989 | |
| Adult | 450 | 73.937 (66.383–78.382) | 180.440 (176.003–189.337) | 7.046 |
na is total number of larvae, pupae and adult used per each species, 25 per replicate, three replicates were carried out, five concentrations were tested; LC50 = lethal concentration killing 50% of exposed organisms; LC90 = lethal concentration killing 90% of exposed organisms; LCL = 95% lower confidence limits; UCL = 95% upper confidence limits; χ2 = chi square; df = degrees of freedom; SD = Standard deviation.
Mortality of An. stephensi larvae varied from 10.33 to 85.33%, for pupae, 8.33 to 70.33%, and adult (from 4.33 to 58.66%) (Figure 1; Table 1). As for An. stephensi the susceptibility of the larvae to the M. anisopliae crude metabolites was higher than the pupae and adults (LC50 = 32.578, 52.491, and 70.235µg/mL for larvae, pupae, and adults, respectively) (Table 1). Similarly, larvae mortality of Cx. quinquefasciatus varied from 8.66 to 80.33%; pupal from 6.00 to 61.00%, for adult 21.00 to 54.66% (Figure 1; Table 1). The toxicity of the M. anisopliae crude metabolites was higher for the Cx. quinquefasciatus larvae, (LC50 = 48.003µg/mL) than it was for the pupae (LC50 = 69.017µg/mL) or for the adults (LC50, 73.937µg/mL) (Table 1).
3.2. Non-Target Organisms
Entomopathogenic fungi M. anisopliae constituents showed a minimal effect on non-targeted A. nauplii. This study clearly shows (5.33–18.33 %) mortality were produced by the M. anisopliae derived crude extract (Table 2; Figure 2). The LC50 and LC90 values were 620.481; 6893.990 μg/mL (Table 2). No behavioral changes were observed during the treatment with fungal extracts.
Table 2.
Toxicity of M. anisopliae secondary metabolites on A. nauplii at 24 h after treatments.
| Mosquito (na = 450) |
Concentration (µg/mL) | % Mortality ± SD | LC50 (LCL-UCL) | LC90 (LCL-UCL) | χ2 (df = 12) |
|---|---|---|---|---|---|
| M. anisopliae | Control | 1.33 ± 0.5 | 620.481 (612.550–635.779) | 6893.990 (6587.612–7432.900) | 1.599 |
| 10 | 5.33 ± 0.5 | ||||
| 15 | 12.66 ± 1.0 | ||||
| 30 | 15.0 ± 0.5 | ||||
| 50 | 13.33 ± 1.0 | ||||
| 75 | 18.33 ± 0.5 |
na = total number of A. nauplii used per each species, 25 per replicate, three replicates were carried out, five concentrations were tested; LC50 = lethal concentration killing 50% of exposed organisms; LC90 = lethal concentration killing 90 % of exposed organisms; LCL = 95 % lower confidence limits; UCL = 95 % upper confidence limits; χ2 = chi square; df = degrees of freedom; SD = Standard deviation.
Figure 2.
Morphological changes of A. nauplii exposed of M. anisopliae secondary metabolites at post 24 h of treatment. (A). Control (not treated fungal extract), (B). M. anisopliae secondary metabolites treated A. nauplii have no morphological changes were observed. (AN-1: Antennae 1, AN-2: Antennae 2, EYE: eye, EP: exopod, MA: mandible, GUT: gut, TE: telson, AN: anus, SS: swimming setae, ANT: antenna).
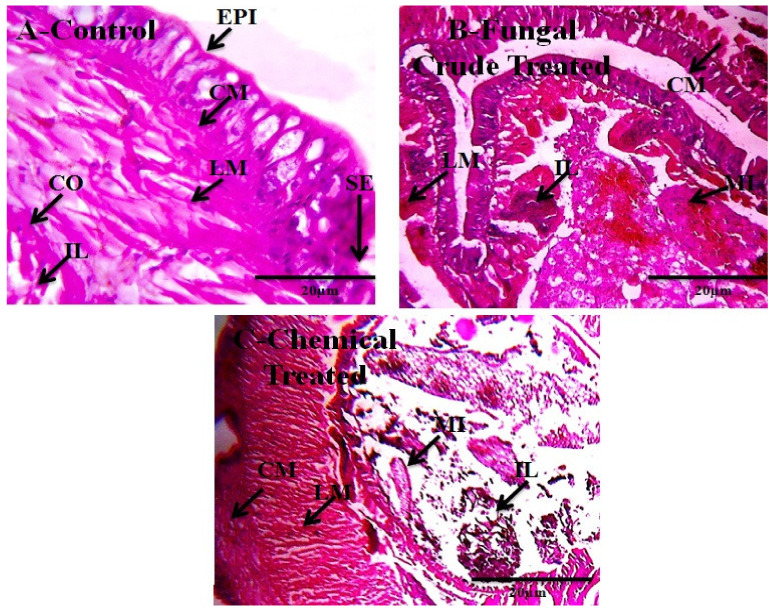
A low lethal effect was observed in E. eugeniae treated with the fungi metabolites; 14.0% mortality were observed in those treated with M. anisopliae secondary metabolites at 30 days after treatments. The highest earthworm mortality was observed in Monocrotophos pesticide treatment that shows 87.33 % mortality. Furthermore, the chemical treatment epidermis, intestinal and body wall thickness was reduced by the chemical (Figure 3; Table 3 and Table 4).
Figure 3.
The M. anisopliae secondary metabolites (200 µg/mL) were exposed E. eugeniae and after 30 days of treatment, the earthworm gut tissues were sectioned for histopathological evaluation and magnified at 40× under a light microscope. (A) is control (without fungal crude extract treatment); (B) is fungal secondary metabolites treated; and (C) is Monocrotophos 200 ppm/kg treated. In the control and entomopathogenic fungi crude extract treatments, no changes were observed, but chemical pesticide treatment of several gut tissues morphology and shapes changed in the lumen tissues was entirely spoiled compared with control (EPI-epidermis, SE-setae, IL-intestinal lumen, LM-longitudinal muscle, CO-coelom, CM-circular muscle, MI-mitochondrion).
Table 3.
Mortality of E. eugeniae after the treatment of M. anisopliae crude extract and Monocrotophos at post 24 h treatments. The identical lower case letters do not differ significantly (p > 0.05).
| Treatment | Concentration (µg/mL) | % Mortality ± SD |
|---|---|---|
| M. anisopliae | Control | 1.33 ± 0.5 a |
| 50 | 4.66 ± 1.0 b | |
| 75 | 14.00 ± 1.1 c | |
| Monocrotophos | Control | 1.33 ± 0.5 a |
| 50 | 50.00 ± 0.5 b | |
| 75 | 87.33 ± 0.5 c |
Table 4.
Thickness of the epidermis, intestinal epithelium, and body wall of earthworms after the 30 days treatment of M. anisopliae crude extract. The identical lower case letters do not differ significantly (p > 0.05).
| Treatments | E. eugeniae | ||
|---|---|---|---|
| Epidermis (µm) ± SD | Intestinal Epithelium (µm) ± SD | Body Wall (µm) ± SD | |
| Control | 37.13 ± 0.0 a | 71.14 ± 0.5 a | 280.12 ± 0.0 a |
| M. anisopliae | 36.51 ± 0.5 b | 70.55 ± 0.5 b | 279.10 ± 0.0 b |
| Monocrotophos | 23.32 ± 0.5 c | 55.15 ± 1.1 c | 210.12 ± 0.5 c |
3.3. LC-MS and FT-IR Analysis
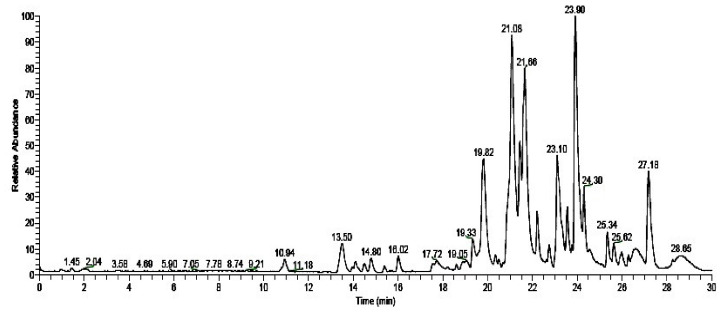
LC-MS analysis results of M. anisopliae extract showed the presence of two major chemical constituents, and retensition time namely Camphor (21.08), Caprolactam (21.66) and Monobutyl phthalate (23.90) (Figure 4; Table 5).
Figure 4.
Chemical constituents were identified from M. anisopliae secondary metabolites using LC-MS analysis.
Table 5.
The M. anisopliae ethyl acetate crude extract chemical constituents were identified using LC-MS analysis.
| S. No | Retention Time | Molecular Formula | Molecular Weight |
Compound Name | Compound Structure |
|---|---|---|---|---|---|
| 1 | 19.82 | C37H67NO13 | 733.46124 | (-)-Erythromycin |
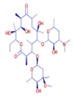
|
| 2 | 21.08 | C10H16O | 152.12012 | (-)-Camphor |
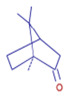
|
| 3 | 21.66 | C6H11NO | 113.08406 | Caprolactam |
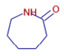
|
| 4 | 23.10 | C16H30O4 | 286.21441 |
2,2,4-Trimethyl-1,3-pentadienol diisobutyrate |
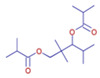
|
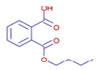
| 5 | 23.90 | C12H14O4 | 222.08921 | Monobutyl phthalate |
|
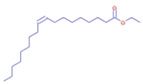
| 6 | 24.30 | C20H38O2 | 310.28718 | Ethyl oleate |
|
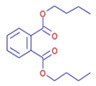
| 7 | 27.18 | C16H22O4 | 278.15181 | Dibutyl phthalate |
|
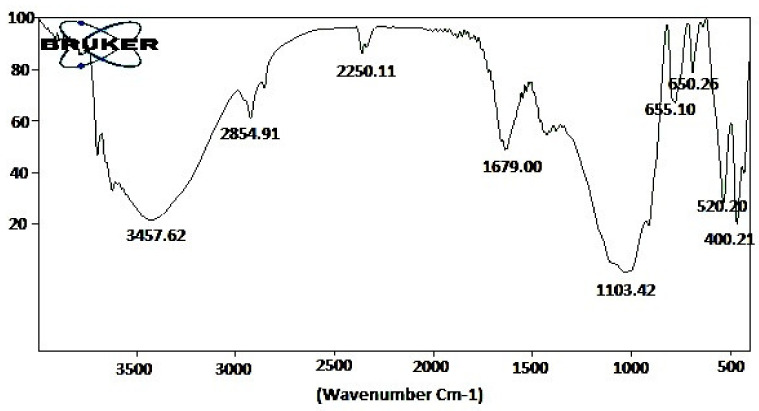
FT-IR showed the presence of functional groups such as, O–H stretching (3457.62 cm−1), O–H stretching (2854.91 cm−1) and the medium peak C=O stretching (1679.00 cm−1) (Figure 5; Table 6).
Figure 5.
The major functional group was identified from M. anisopliae secondary metabolites using FT-IR analysis.
Table 6.
The major functional group was identified from M. anisopliae ethyl acetate crude extract using FT-IR analysis.
| S. No | Observed Wavenumber (cm−1) | Functional Group | Bonding Pattern |
|---|---|---|---|
| 1 | 3457.62 | O–H stretch | Phenols |
| 2 | 2854.91 | O–H stretch | Carboxylic acids |
| 3 | 2250.11 | -C C- stretch | Alkynes |
| 4 | 1679.00 | C=O stretch | Aldehydes |
| 5 | 1103.42 | C-H wag | Alkyl halides |
| 6 | 655.10 | C-H bends | Aromatics |
| 7 | 650.25 | C-H bends | Aromatics |
| 8 | 520.20 | C-Br stretch | Alkyl halides |
| 9 | 400.21 | C-Br stretch | Alkyl halides |
4. Discussion
Recently, there has been a great interest in the use of biologically derived pesticides as an alternative to synthetic chemicals [9,10]. Entomopathogenic fungi-derived toxins have several advantages over synthetic pesticides in that they kill mosquitos at different stages in both laboratory and environmental conditions, have lower toxic effects on non-target organisms, and remain stable for several months in extreme cold and hot conditions [9,10,14]. In this study, we evaluated the toxic effects of secondary metabolites isolated from M. anisopliae strains against larvae, pupae, and adults of the disease-vector mosquitoes Ae. aegypti, An. stephensi and Cx. quinquefasciatus, and we assessed their target specificity and environmental safety by testing the extracts against the aquatic and terrestrial non-target species A. naupli L. and E. eugeniae.
Fungal secondary metabolites showed clear toxicity against all the tested instars of the mosquitoes and much lower toxicity against the non-target organisms. In the present study, M. anisopliae crude metabolites showed high toxicity towards the larvae, pupae, and adults of A. aegypti, A. stephensi and C. quinquefasciatus mosquitoes at 24 h post treatment under laboratory conditions (Figure 1; Table 1). In line with our results, previous studies on entomopathogenic fungal derived pesticides from several species of Metarhizium, Fusarium, Aspergillus, Trichoderma and Lecanicillium showed that they are effective against medical and agricultural insect pests [24]. Soni and Prakash [25] reported that Chrysosporium keratinophilum derived secondary metabolites have strong larvicidal activity against C. quinquefasciatus and A. stephensi mosquito larvae, while [26] reported that different fungal metabolites cause strong larvicidal activity against larvae of A. stephensi and C. quinquefasciatus. Similarly, Metarhiziumanisopliae, Aspergillus flavus, Fusarium oxysporum, Verticillium lecanii, Paecilomyces fumosoroseus, Beauveria bassiana, and Fusarium moniliforme and their toxins have been shown to produce remarkable mosquitocidal potential on larvae, pupae, and adult mosquitoes [9,10,11,27]. C. tropicum, C. clavisporus and F. oxysporum culture filtrates showed strong larvicidal activity against A. stephensi, A. aegypti and C. quinquefasciatus [10,11,22,28], and secondary metabolites of A. fumigatus showed strong larvicidal activity against larvae of A. aegypti [29].
On the contrary, in our study, we observed low toxicity of the fungal metabolites against non-target species such as A. nauplii and E. eugeniae (Table 2, Table 3 and Table 4; Figure 2 and Figure 3). Similarly, [30] reported few swimming speed alterations in Artemia adults after their treatment by different toxins. A similar study about the effects of the fungi secondary metabolites from Penicillium daleae on Artemia, observed morphological changes in eye shape, eye color, and eye fading [31]. These results suggest that secondary metabolites from different fungi may produce lower levels of toxicity to non-target organisms. For this reason, the assessment of the lower effects of fungal secondary metabolites in aquatic and terrestrial ecosystems on non-target species is of prime importance. The chemical composition of the secondary metabolites extracted from the M. anisopliae entomopathogenic fungi analysed in this study is in accord with previous research by [9,10] and by [32], who observed similar kinds of chemical constituents (Figure 4; Table 5). Previously, [9,10] reported that B. bassiana and F. oxysporum derived crude metabolites had the same chemical constituents showing a strong larvicidal activity on A. aegypti, A. stephensi and C. quinquefasciatus larvae. In this study, FT-IR analyses showed the presence of phenols, biogenic amines, and carboxylic acids, which may be involved in the toxic effects on mosquitoes (Figure 5; Table 6).
Similarly, previous studies showed that the metabolites of entomopathogenic fungi are constituted by components belonging to several chemical classes (phenols, alcohols, carboxylic acids, misc, aromatics, phosphoramide, and disulfides), which may be involved in the mosquitocidal effects [9,10,11,26,33,34,35,36]. The strong mosquitocidal activity and the low toxic effect on non-target organisms exhibited by M. anisopliae indicate that, besides entomopathogenic fungal conidia, their metabolites may also have a significant role in efficient microbial-derived mosquito control tools that can be used in mosquito control programmes as effective, cheaper, biodegradable, target-specific alternatives to chemical insecticides. Further research into the single crude metabolite chemical constituents under laboratory and semi-field conditions may result in the development of effective M. anisopliae derived bio-pesticides.
5. Conclusions
The strong mosquitocidal activity and the low toxic effect on non-target organisms exhibited by M. anisopliae indicate that, besides entomopathogenic fungal conidia, their metabolites may also have a significant role in efficient microbial-derived mosquito control tools that can be used in mosquito control programmes as effective, cheaper, biodegradable, target-specific alternatives to chemical insecticides. Further research into the single crude metabolite chemical constituents under laboratory and semi-field conditions may result in the development of effective M. anisopliae derived biopesticides.
Acknowledgments
Authors thank to Ananthanarayanan Yuvaraj, Department of Zoology for his valuable suggestions for histopathological studies and also thank to Periyar University, Tamil, Nadu, India for making available the infrastructure and resources for this study. This research was partially supported by Chiang Mai University, Thailand.
Author Contributions
Conceptualization: P.V., K.S. and P.K.; Data curation: P.V. and K.S.; Formal analysis: P.V., K.S., P.K. and A.C.M.; Funding acquisition: P.K.; Investigation: P.V.; Methodology: P.V.; Project administration: P.V., K.S. and P.K.; Resources: P.V. and K.S.; Software: K.S. and P.K.; Supervision: P.V. and K.S.; Validation: P.V., K.S. and P.K.; Visualization: K.S.; Writing—original draft: P.V., K.S. and P.K.; Writing—review & editing: P.V., K.S., P.K. and A.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable for this present studies and does not involving humans or animals.
Informed Consent Statement
Not applicable for this present studies and does not involving humans or animals.
Data Availability Statement
The data that support the findings of this present study are available from the corresponding author upon reasonable request.
Conflicts of Interest
All the authors state that they do not have any conflict of interest.
Ethical Statement
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang W., Wang S., Jacobs-Lorena M. Use of microbiota to fight mosquito-borne disease. Front. Genet. 2020;11:196. doi: 10.3389/fgene.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chareonviriyaphap T., Bangs M.J., Suwonkerd W., Kongmee M., Corbel V., Ngoen-Klan R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites Vectors. 2013;6:280. doi: 10.1186/1756-3305-6-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh A., Chowdhury N., Chandra G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012;135:581–598. [PMC free article] [PubMed] [Google Scholar]
- 4.Busvine J.R. Recommended Methods for Measurement of Pest Resistance to Pesticides. FAO; Rome, Italy: 1980. [Google Scholar]
- 5.Vivekanandhan P., Thendralmanikandan A., Kweka E.J., Mahande A.M. Resistance to temephos in Anopheles stephensi larvae is associated with increased cytochrome P450 and α-esterase genes overexpression. Int. J. Trop. Insect Sci. 2021;41:2543–2548. doi: 10.1007/s42690-021-00434-6. [DOI] [Google Scholar]
- 6.Vatandoost H., Hanafi-Bojd A.A. Indication of pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pac. J. Trop. Med. 2012;5:722–726. doi: 10.1016/S1995-7645(12)60114-X. [DOI] [PubMed] [Google Scholar]
- 7.Li Q.Q., Loganath A., Chong Y.S., Tan J., Obbard J.P. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health Part A. 2006;69:1987–2005. doi: 10.1080/15287390600751447. [DOI] [PubMed] [Google Scholar]
- 8.Sarwar M. Biopesticides: An effective and environmental friendly insect-pests inhibitor line of action. Int. J. Eng. Adv. Res. Technol. 2015;1:10–15. [Google Scholar]
- 9.Morales-Rodriguez A., Peck D.C. Synergies between biological and neonicotinoid insecticides for the curative control of the white grubs Amphimallon majale and Popillia japonica. Biol. Control. 2009;51:169–180. doi: 10.1016/j.biocontrol.2009.06.008. [DOI] [Google Scholar]
- 10.Ruiu L., Satta A., Floris I. Emerging entomopathogenic bacteria for insect pest management. Bull. Insectol. 2013;66:181–186. [Google Scholar]
- 11.Bojke A., Tkaczuk C., Stepnowski P., Gołębiowski M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018;214:129–136. doi: 10.1016/j.micres.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Rai D., Updhyay V., Mehra P., Rana M., Pandey A.K. Potential of entomopathogenic fungi as biopesticides. Indian J. Sci. Res. Technol. 2014;2:7–13. [Google Scholar]
- 13.Zhang L., Fasoyin O.E., Molnár I., Xu Y. Secondary metabolites from hypocrealean entomopathogenic fungi: Novel bioactive compounds. Nat. Prod. Rep. 2020;37:1181–1206. doi: 10.1039/C9NP00065H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darbro J.M., Thomas M.B. Spore persistence and likelihood of aeroallergenicity of entomopathogenic fungi used for mosquito control. Am. J. Trop. Med. Hyg. 2009;80:992–997. doi: 10.4269/ajtmh.2009.80.992. [DOI] [PubMed] [Google Scholar]
- 15.Islam W., Adnan M., Shabbir A., Naveed H., Abubakar Y.S., Qasim M., Tayyab M., Noman A., Nisar M.S., Khan K.A., et al. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021;159:105122. doi: 10.1016/j.micpath.2021.105122. [DOI] [PubMed] [Google Scholar]
- 16.Vyas N., Dua K.K., Prakash S. Efficacy of Lagenidium giganteum metabolites on mosquito larvae with reference to nontarget organisms. Parasitol. Res. 2007;101:385–390. doi: 10.1007/s00436-007-0496-9. [DOI] [PubMed] [Google Scholar]
- 17.Vivekanandhan P., Swathy K., Thomas A., Kweka E.J., Rahman A., Pittarate S., Krutmuang P. Insecticidal Efficacy of Microbial-Mediated Synthesized Copper Nano-Pesticide against Insect Pests and Non-Target Organisms. Int. J. Environ. Res. Public Health. 2021;18:10536. doi: 10.3390/ijerph181910536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivekanandhan P., Arunthirumeni M., Vengateswari G., Shivakumar M.S. Microbial Control of Vector-Borne Diseases. Taylor & Francis; Raton, FL, USA: 2018. 5 Bioprospecting of Novel Fungal Secondary Metabolites for Mosquito Control; pp. 61–89. [Google Scholar]
- 19.Lu Y., Yu J. Assessment and Management of Radioactive and Electronic Wastes. IntechOpen; London, UK: 2019. A Well-Established Method for the Rapid Assessment of Toxicity Using Artemia spp. Model; pp. 1–15. [DOI] [Google Scholar]
- 20.World Health Organization . Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Communicable Disease Control, Prevention and Eradication; WHO, Pesticide Evaluation Scheme. WHO; Geneva, Switzerland: 2005. pp. 1–219. [Google Scholar]
- 21.World Health Organization Global programme to eliminate lymphatic filariasis-progress report on mass drug administration in 2016. Wkly. Epidemiol. Rec. 2016;85:365–372. [Google Scholar]
- 22.Norris E.J., Bloomquist J.R. Nutritional status significantly affects toxicological endpoints in the CDC bottle bioassay. Pest Manag. Sci. 2022;78:743–748. doi: 10.1002/ps.6687. [DOI] [PubMed] [Google Scholar]
- 23.Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 24.Thakur N., Kaur S., Tomar P., Thakur S., Yadav A.N. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; Amsterdam, The Netherlands: 2020. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment; pp. 243–282. [Google Scholar]
- 25.Soni N., Prakash S. Effect of Chrysosporium keratinophilum metabolites against Culex quinquefasciatus after chromatographic purification. Parasitol. Res. 2010;107:1329–1336. doi: 10.1007/s00436-010-2003-y. [DOI] [PubMed] [Google Scholar]
- 26.Soni N., Prakash S. Entomopathogenic fungus generated Nanoparticles for enhancement of efficacy in Culex quinquefasciatus and Anopheles stephensi. Asian Pac. J. Trop. Dis. 2012;2:S356–S361. doi: 10.1016/S2222-1808(12)60181-9. [DOI] [Google Scholar]
- 27.Vivekanandhan P., Karthi S., Shivakumar M.S., Benelli G. Synergistic effect of entomopathogenic fungus Fusarium oxysporum extract in combination with temephos against three major mosquito vectors. Pathog. Glob. Health. 2018;112:37–46. doi: 10.1080/20477724.2018.1438228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivekanandhan P., Deepa S., Kweka E.J., Shivakumar M.S. Toxicity of Fusarium oxysporum-VKFO-01 Derived Silver Nanoparticles as Potential Inseciticide Against Three Mosquito Vector Species (Diptera: Culicidae) J. Clust. Sci. 2018;29:1139–1149. doi: 10.1007/s10876-018-1423-1. [DOI] [Google Scholar]
- 29.Balumahendhiran K., Vivekanandhan P., Shivakumar M.S. Mosquito control potential of secondary metabolites isolated from Aspergillus flavus and Aspergillus fumigatus. Biocatal. Agric. Biotechnol. 2019;21:101334. doi: 10.1016/j.bcab.2019.101334. [DOI] [Google Scholar]
- 30.Manfra L., Canepa S., Piazza V., Faimali M. Lethal and sublethal endpoints observed for Artemia exposed to two reference toxicants and an ecotoxicological concern organic compound. Ecotoxicol. Environ. Saf. 2016;123:60–64. doi: 10.1016/j.ecoenv.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Uwizeyimana H., Wang M., Chen W., Khan K. The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharmacol. 2017;55:20–29. doi: 10.1016/j.etap.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Dubey N.K., Shukla R., Kumar A., Singh P., Prakash B. Global scenario on the application of natural products in integrated pest management programmes. Nat. Prod. Plant Pest. Manag. 2011;1:1–20. [Google Scholar]
- 33.Libralato G., Prato E., Migliore L., Cicero A.M., Manfra L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016;69:35–49. doi: 10.1016/j.ecolind.2016.04.017. [DOI] [Google Scholar]
- 34.Libralato G. The case of Artemia spp. in nanoecotoxicology. Mar. Environ. Res. 2014;101:38–43. doi: 10.1016/j.marenvres.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Gao Q., Jin K., Ying S.H., Zhang Y., Xiao G., Shang Y., Duan Z., Hu X., Xie X.Q., Zhou G., et al. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011;7:e1001264. doi: 10.1371/journal.pgen.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasantha-Srinivasan P., Senthil-Nathan S., Thanigaivel A., Edwin E., Ponsankar A., Selin-Rani S., Pradeepa V., Sakthi-Bhagavathy M., Kalaivani K., Hunter W.B., et al. Developmental response of Spodoptera litura Fab. to treatments of crude volatile oil from Piper betle L. and evaluation of toxicity to earthworm, Eudrilus eugeniae Kinb. Chemosphere. 2016;155:336–347. doi: 10.1016/j.chemosphere.2016.03.139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this present study are available from the corresponding author upon reasonable request.