Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a member of the Coronavirus family which caused the worldwide pandemic of human respiratory illness coronavirus disease 2019 (COVID-19). Presumably emerging at the end of 2019, it poses a severe threat to public health and safety, with a high incidence of transmission, predominately through aerosols and/or direct contact with infected surfaces. In 2020, the search for vaccines began, leading to the obtaining of, to date, about twenty COVID-19 vaccines approved for use in at least one country. However, COVID-19 continues to spread and new genetic mutations and variants have been discovered, requiring pharmacological treatments. The most common therapies for COVID-19 are represented by antiviral and antimalarial agents, antibiotics, immunomodulators, angiotensin II receptor blockers, bradykinin B2 receptor antagonists and corticosteroids. In addition, nutraceuticals, vitamins D and C, omega-3 fatty acids and probiotics are under study. Finally, drug repositioning, which concerns the investigation of existing drugs for new therapeutic target indications, has been widely proposed in the literature for COVID-19 therapies. Considering the importance of this ongoing global public health emergency, this review aims to offer a synthetic up-to-date overview regarding diagnoses, variants and vaccines for COVID-19, with particular attention paid to the adopted treatments.
Keywords: COVID-19, repositioning, repurposing, pandemic, SARS-CoV-2, vaccines, therapies, drugs, nutraceuticals
1. Introduction
Coronavirus disease 2019 (COVID-19), the highly contagious infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a catastrophic effect on the world’s demographics, resulting in more than 5.8 million deaths worldwide and emerging as the most consequential global health crisis since the era of the influenza pandemic of 1918 [1]. The outbreak of this novel disease has become a global health emergency and continues to rapidly spread around the world. Up to 15 February 2022, more than 410,000,000 cases with COVID-19 have been diagnosed and about 5800,000 death cases have been recorded [2]. Italy was “hit by nothing short of a tsunami of unprecedented forces, punctuated by an incessant stream of deaths. [...] Italy’s biggest crisis since World War II” [3]. Vaccination has been a very effective public health intervention, and different types of vaccines are used worldwide. As of 14 February 2022, a total of 10,227,670,521 vaccine doses have been administered around the world. However, in underdeveloped countries, most people must wait until 2023 for vaccination [4]; according to the World Health Organization (WHO)’s Africa Office, just over 3% of Africans are fully vaccinated against COVID-19 [5]. Despite the use of COVID-19 vaccines in many countries, the crisis is far from being resolved [6]. The ongoing evolution of SARS-CoV-2, the behavior of citizens, the decisions of governments in response to the pandemic and progress made in vaccine development and treatments will undoubtedly affect human life in the future. It is impossible to imagine when the world will return to “business as usual”, but what will remain is the lesson that this pandemic has provided to humankind, mostly in terms of prevention and behaviors. Nowadays, two years on from the beginning of the pandemic, new studies are aimed at post-acute COVID-19 syndrome (PCS), health-related quality of life (HRQoL) [7], “Long-COVID” [8], neurological complications [9,10], and so on. The structure, function, epidemiology and transmission of SARS-CoV-2 have been widely described in adults [11,12]. Fewer studies have reported on children, newborns [13] and pregnant women [14]. Newly arising SARS-CoV-2 variants are now a threat to global health and are creating COVID-19 surges in different countries. To date, there is limited knowledge about these emerging variants, which are now circulating globally [15]. At the time of writing, Europe is facing a fourth wave of COVID-19, while the third wave is not yet over, particularly due to low vaccination coverage. Increasingly transmissible variants (e.g., Delta and Omicron) are proliferating, and the risk of new and immunity-escaping variants is higher when populations are only partially vaccinated [16]. The Omicron variant is now dominant in at least 85 countries, raising concerns about its potentially high transmissibility [17]. It is yet been clearly understood whether vaccines will be effective against all of these variants [18]. Vaccine top-ups, rolled out in autumn 2021, might boost the immune system and block new coronavirus variants, but there are many complexities [19,20]. In most countries, contacts of confirmed COVID-19 cases are asked to quarantine for 14 days after exposure, to limit asymptomatic onward transmission even though, at the individual level, the 14-day quarantine may cause strain on mental health. There are recent studies aimed at reducing the length of quarantine for uninfected contacts [21], considering the difficulty to maintain the quarantine for a prolonged period. Another issue relating to the COVID-19 pandemic is also represented by increased alcohol consumption, which was observed during lockdown in people in quarantine and studying or working at home [22,23]. Besides the vaccination procedure, the primary examined therapies to treat COVID-19 patients include: antiviral regimens, such as remdesivir, ritonavir, lopinavir; antimalarials, such as chloroquine and hydroxychloroquine; antibacterials, such as azithromicin; immunomodulating drugs, such as corticosteroids and interleukin (IL) inhibitors; and other therapies including convalescent plasma infusion [24]. In particular, dexamethasone therapy plays a major role in the treatment of patients with COVID-19 pneumonia requiring supplemental oxygen or on mechanical ventilation [25]. Molnupiravir (MK-4482, EIDD-2801) is an oral antiviral drug working through the mechanism of “error catastrophe” against SARS-CoV-2 [26]. It increases the frequency of viral RNA mutations and impairs SARS-CoV-2 replication in animal models and in humans. Molnupiravir was authorized for COVID-19 treatment in the UK on 4 November 2021 [27]. PaxlovidTM (nirmatrelvir + ritonavir), developed by Pfizer Inc., has been proposed by the U.S. Food and Drug Administration (FDA) for emergency use in infected adults at high risk of severe illness who are unvaccinated [28]. The advantage of these two pills over other antiviral compounds is that they are given at home [29]. If COVID-19 is diagnosed early, it has been suggested that the use of a combination of antivirals and an immunomodulator (e.g., a drug potentiating IFNα/β) may lower the risk of cytokine storm manifestation. When the disease becomes severe, the new combination should prioritize targeting the cytokine storm [30]. Lastly, vitamin D supplementation has been suggested for the prevention and/or treatment of COVID-19 [31], because of its well-known immunomodulatory role. Another aspect of the current COVID-19 outbreak is the rise of germaphobes that rarely leave the house without a bottle of hand sanitizer. However, it would be better to be more cautious and to think of possible consequences before occurrence. Indeed, there are broad-spectrum antibacterial agents used in sanitizers, such as triclosan and triclocarban, which are highly absorbed by the skin and at high doses have shown toxicity [32]. Moreover, some hand sanitizer products may contain unacceptable active ingredients (especially methanol) other than the recommended alcohols (ethanol or isopropyl alcohol), whose toxicity after dermal and respiratory exposure is known: if swallowed, it may lead to death. The COVID-19 pandemic is considered the largest methanol mass poisoning event around the world in recent decades [33]. Finally, because of the high antibiotic use in COVID-19 patients, there has been a rapid increase in antimicrobial resistance [34,35,36], which poses collateral damage in the COVID-19 pandemic. Overall, this review aims to provide a quick update of the drugs, vaccines, and therapeutic treatments used today for this devastating disease.
2. Diagnosis, Variants and Vaccines
2.1. Diagnosis
COVID-19 is generally diagnosed by reverse transcription (RT-PCR) or serological assays. Antibody testing, using a rapid immunochromatographic assay, is reliable in the diagnosis of SARS-CoV-2 infection; however, RT-PCR testing on oropharyngeal or nasopharyngeal swabs is an important and more sensitive tool with respect to antigenic testing even though several parameters can affect both assays. Recently, some concerns have been raised about the performance of RT-PCR testing [37], since patients who are ultimately proven to have COVID-19 may have a negative RT-PCR test, particularly early in the course of their disease, while false positive antibody tests are also a concern [38]. Thus, the combination of two methods, such as RT-PCR and the IgG assay, has been proposed to be more trustworthy [39]. In severe cases, clinicians need to rapidly and reliably diagnose COVID-19 for proper risk stratification, isolation strategies, and treatment decisions. Chest computed tomography (CT) has been demonstrated as being a high-performance test for diagnosing COVID-19 [40]. Recently, the combined use of chest X-ray images (CXI) and chest computer tomography images (CCTI) was proposed, by using the Fourier–Bessel series expansion-based decomposition (FBSED) method [41].
2.2. Mutations and Variants
As has been assessed, SARS-CoV-2 is genetically variable and easily adaptable to various hosts, including humans. Indeed, SARS-CoV-2 has accumulated around two mutations per genome each month, and several other variants have emerged [42]. The terminology of viral variation can be confusing, as the media and even scientific papers often use the terms variant, strain, and lineage interchangeably. Fortunately, the WHO is working on a systematic nomenclature that does not require a geographic reference, since viral variants can spread rapidly and globally [43]. Three nomenclature systems for naming and tracking SARS-CoV-2 genetic lineages are currently in use: Pango [44,45] (available online) [46], Global Initiative on Sharing All Influenza Data (GISAID) [47,48] and NextStrain [49,50]. The last two databases focus on broader phylogenetic ‘clades’ and incorporate criteria for minimum prevalence and persistence. Among the numerous variants, the WHO, CDC (USA) and European Centre for Disease Prevention (ECDC) (Europe) highlight some as significant ones and categorize them into two subgroups: variants of concern (VOCs) and variants of interest (VOIs), which are updated when required [51]. VOCs emerged as a greater threat to public health, with higher infectivity and transmissibility, and an increased virulence pattern. They can alter COVID-19’s clinical manifestations, decrease the efficacy of available vaccines and therapeutics and may obstruct the present ability of RT-PCR assays to detect the virus. The existence of different nomenclature systems means that the same variant has multiple names, often at the same time and without regard for the properties of VOIs and VOCs. By this time, a continuously enriched spectrum of SARS-CoV-2 genomic variants has been evidenced and categorized by the WHO using the Greek alphabet (Alpha to Omicron). In Table 1, the known SARS-CoV-2 variants, to date, are summarized.
Table 1.
Variants of SARS-CoV-2.
| Variant Name (Pango Lineage) | GISAID Database | Nextstrain Database | WHO Names | Variant Name (Pango Sublineage) | WHO Designation | Date of Designation | |
|---|---|---|---|---|---|---|---|
| B.1.525 | G/484K.V3 | 20A/S:484K | Eta | η | – | VOI | UK/Nigeria December 2020 |
| B.1.526 | GH | 20C/S:484K | Iota | ι | – | VOI | New York, NY, (USA) November 2020 |
| – | – | 20C | – | – | B.1.526.1 | VOI | USA October 2020 |
| B.1.617 | G/452R.V3 | 20A | |||||
| 20A/S:154K | Kappa | κ | B.1.617.1 | VOI | India December 2020 |
||
| – | G/452R.V3 | 20A/S:478K | Delta | δ | B.1.617.2 | VOC | India October 2020 |
| – | – | 20A | – | – | B.1.617.3 | VOI | India October 2020 |
| C.37 | – | – | Lambda | λ | – | VOI | Lima November 2020 |
| B.1.621 | – | – | Mu | μ | – | VOI | Colombia January 2021 |
| P.2 | GR | 20B/S:484K | Zeta | ζ | B.1.1.28.2 | VOI | Rio de Janeiro (Brazil) April 2020 |
| P.3 | GR | 20B/S:265C | Theta | θ | B.1.1.28.3 | VOI | Japan/Philippines, January 2021 |
| B.1.616 | – | 20C | – | – | – | VOI | France January 2021 |
| B1.1.7 | GRY (formerly GR/501Y.V1) | 20I/S:501Y.V1 | Alpha | α | – | VOC | South-East England (UK), September 2020 |
| B1.351 | GH/501Y.V2 | 20H/501Y.V2 | Beta | β | – | VOC | South Africa, May 2020 |
| P.1 | GR/501Y.V3 | 20J/S:501Y.V3 | Gamma | γ | B.1.1.28.1 or P.1 | VOC | Japan/Amazonas (Brazil), November 2020 |
| B.1.427 | GH/452R.V1 | 20C/S:452R | Epsilon | ε | – | VOI a | Southern California (USA) December 2020 |
| B.1.429 | GH/452R.V1 | 20C/S:452R | Formerly Epsilon | Formerly ε | – | VOI a | Southern California, CA, (USA) |
| B.1.620 | – | – | – | – | B1.177 | VOC | Lithuania April 2021 |
| – | – | – | – | B.1.258D | Other | Czech Republic/Slovakia | |
| – | – | – | – | B.1.1.298 | Other | Denmark | |
| BA.1/BA.2 | – | – | Omicron | o | B.1.1.529 | VOC | South Africa (early Nov. 2021) |
2.3. Vaccines for COVID-19
By 1 September 2020, an enormous number of vaccines (more than 200) were in preclinical development, some of which had entered clinical trials [59]. The approved vaccines are summarized in Table 2. Four vaccines have been used in Italy: two mRNA vaccines, Pfizer-BioNTech mRNA BNT162b2 (Comirnaty) (USA) [60] and Moderna mRNA 1273 (USA) [61], and two recombinant viral vector adenoviruses, Astra-Zeneca ChAdOx1-S, belonging to Oxford University (UK) and Pomezia (Italy), and Janssen, belonging to Johnson & Johnson (USA) [62,63]. Firstly, two doses were needed to induce satisfactory immunity; however, the administration of third doses, called “booster doses”, of COVID-19 vaccines started in autumn 2021. The rapid development and deployment of vaccines against the SARS-CoV-2 virus has been an unprecedented scientific achievement. Thus, not all the side effects evidenced were predictable [64]. The viral vector-based vaccines are associated with a higher prevalence of systemic side effects (e.g., headache/fatigue), whereas the mRNA-based vaccines are associated with a higher prevalence of local side effects (e.g., injection site pain, muscle pain, malaise, chills, and joint pain). The vast majority of side effects resolve themselves within 1–3 days after vaccination [65]; however, severe side effects do also sometimes occur. The most frequent neurological side effects of SARS-CoV-2 vaccines are Guillain–Barre syndrome (GBS), venous sinus thrombosis (VST), and transverse myelitis, with VST particularly occurring after vaccination with a vector-based vaccine [66]. Cutaneous reactions after COVID-19 vaccination have also been commonly reported [67], as well as cases of erythema nodosum and pityriasis rosea after Oxford–AstraZeneca COVID-19 vaccine administration [68]. Moreover, reactivation of varicella zoster virus (VZV), as well as herpes zoster (HZ), after COVID-19 vaccination with both viral vectors and mRNA vaccines has been reported in a series of adult patients [69,70]. The new onset and exacerbation of psoriasis after COVID-19 vaccination have also been described with mRNA vaccines [71]. In children, the phenomena of encephalopathy and myocarditis following COVID-19 mRNA vaccination have been described [72]. In Italy, after the first dose, vaccine brands were mixed for people who received Johnson & Johnson and AstraZeneca vaccines, as these two vaccines were retired because of the severe side effects that occurred. Mixing adenoviral and mRNA vaccines has been demonstrated to be safe and tolerable [73]. Currently, only Pfizer and Moderna vaccines are administered in Italy. The recent emergence of the SARS-CoV-2 Omicron variant has become a global concern and the vaccine protection against this variant is still under debate [74].
Table 2.
COVID-19 vaccines.
| Manufacturer/WHO EUL Holder | Name of Vaccine | Platform | NRA of Record | Recommendation Issued |
|---|---|---|---|---|
| Pfizer Biontech | BNT162b2/COMIRNATY® Tozinameran (INN) | Nucleoside modified mRNA | EMA FDA |
31 December 20 16 July 21 |
| Astra Zeneca | AZD1222 VAXZEVRIA |
Recombinant ChAdOx1 adenoviral vector encoding the Spike protein antigen of the SARS-CoV-2 | EMA MFDS KOREA Japan MHLW PMDA Australian TGA |
15 April 21 15 February 21 9 July 21 |
| Serum Institute of India | CovishieldTM (ChAdOx1_nCoV-19) | Recombinant ChAdOx1 adenoviral vector encoding the Spike protein antigen of the SARS-CoV-2 | DCGI | 15 February 21 |
| Janssen | Ad26.COV2.S | Recombinant, replication-incompetent adenovirus type 26 (Ad26) vectored vaccine encoding the SARS-CoV-2 Spike (S) protein | EMA | 12 March 21 |
| Moderna | mRNA-1273 SPIKEVAX |
mRNA-based vaccine encapsulated in lipid nanoparticle (LNP) | EMA MFDS FDA |
30 April 21 23 December 21 6 August 21 |
| Sinopharm (Beijing, Wuhan)/BIBP | SARS-CoV-2 Vaccine (Vero Cell), Inactivated (lnCoV) | Inactivated, produced in Vero cells | NMPA | 7 May 21 |
| Sinovac | SARS-CoV-2 Vaccine (Vero Cell), Inactivated (lnCoV) CoronaVac |
Inactivated, produced in Vero cells | NMPA | 1 June 21 |
| The Gamaleya National Center of Epidemiology and Microbiology | Sputnik V | Human adenovirus vector-based COVID-19 vaccine | Russian NRA | 11 August 20 |
| CanSinoBIO- Beijing Institute of Biotechnology | Ad5-nCoV | Inactivated, produced in Vero cells | NMPA | – |
| Bharat Biotech, India | SARS-CoV-2 Vaccine, Inactivated (Vero Cell)/COVAXIN® | Whole-virion inactivated Vero cell | DCGI/CDSCO | 3 November 21 |
| Novavax | NVX-CoV2373/Covovax NUVAXOVID™ |
Recombinant nanoparticle prefusion spike protein formulated with Matrix-M™ adjuvant | EMA | 21 December 21 |
| CureVac | Zorecimeran (INN) concentrate and solvent for dispersion for injection; Company code: CVnCoV/CV07050101 | mRNA-based vaccine encapsulated in lipid nanoparticle (LNP) | EMA | – |
| Sanofi Pasteur | CoV2 preS dTM-AS03 vaccine | Recombinant, adjuvanted | EMA | – |
Adapted from refs. [75,76] Abbreviations: NRA, National Regulatory Agency; EMA, European Medicines Agency; FDA, Food and Drug Administration; MFDS, Ministry of Food and Drug Safety; MHLW, Ministry of Health, Labour, and Welfare; PMDA, Pharmaceuticals and Medical Devices Agency; TGA, Therapeutic Goods Administration; DCGI, Drugs Controller General of India; NMPA, National Medical Products Administration; CDSCO, Central Drugs Standard Control Organization.
3. Current Therapies
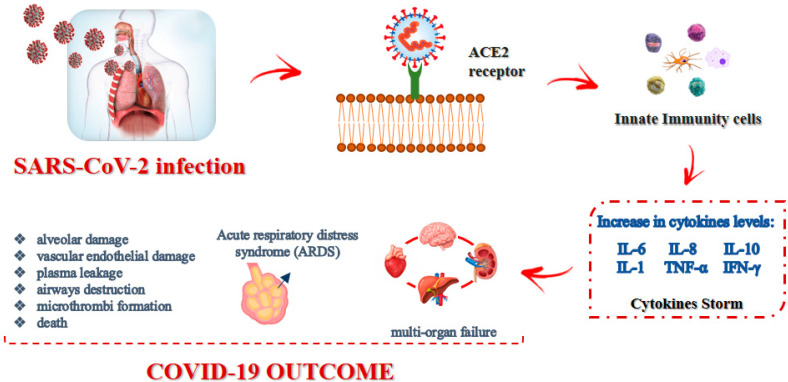
Current therapies used for COVID-19 target the so-called “cytokine storm” (CS), also known as cytokine release syndrome (CRS) [77]. Indeed, COVID-19 is characterized by dramatically elevated levels of inflammatory cytokines, mainly IL-6, IL-8, IL-10, IL-1, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), as depicted in Figure 1. These pro-inflammatory mediators can provoke systemic inflammatory response syndrome, resulting in acute respiratory distress syndrome (ARDS).
Figure 1.
SARS-CoV-2 infection modality and effects.
The pathological changes that generally occur in COVID-19 patients include diffuse alveolar damage, due to immunological injury and viral infection, as well as multiorgan failure, such as airway destruction, vascular endothelial damage, plasma leakage, and extensive microthrombi formation [78]. Pneumonia, frequently occurring in COVID-19 patients, may result as a direct consequence of the viral infection in the lung or arise because of secondary bacterial infections after the viral episode. Targeting the appropriate cytokine is one of the main objectives of current studies, and one of the first approaches to COVID-19 cytokine storm syndrome treatment was targeting the IL-6, since early during the pandemic, IL-6 concentrations were noted to be elevated. Unfortunately, most randomized controlled trials have not documented an improved survival rate using agents targeting IL-6; by contrast, targeting IL-1 was largely successful, as reported by a retrospective cohort study [79]. SARS-CoV-2, as with other viruses, triggers an antiviral response that relies on the immediate production of IFNβ in the host, which binds to its receptor (human interferon alpha-receptor, IFNAR) and triggers the production of IFNα. If the production of IFNα/β occurs immediately and is intense enough, the infection can be stopped, as often happens in asymptomatic or paucisymptomatic patients, especially in children. If the response is weak, the virus replicates, causing a second inflammatory/immune response, which may become explosive and potentially result in a cytokine storm and ARDS.
Beyond therapies targeting cytokines, other treatments have been extensively reviewed recently [80], namely antiviral agents, antimalarial agents, antibacterials, immunomodulators, angiotensin II receptor blockers, bradykinin B2 receptor antagonists, corticosteroids, anthelmintic, antiprotozoal, H2 blockers and anticoagulants. However, alternative and effective therapies to overcome the already known and/or the unforeseen possible manifestations of SARS-CoV-2 in the near future may be needed. The most important therapeutic drugs available for the treatment of COVID-19 are summarized in Figure 2.
Figure 2.
SARS-CoV-2 adopted medical strategies.
Remdesivir, a broad-spectrum antiviral agent able to inhibit SARS-CoV-2 replication, was the first available therapeutic drug for COVID-19. It is a small-molecule monophosphoramidate prodrug, as it is metabolized into the nucleoside triphosphate (active drug form) inside the cell through sequential reactions by ester-mediated hydrolysis, and an adenosine analog, blocking the RNA-dependent RNA-polymerase (RdRp) through its nucleoside component after the virus’ entry into the host cell. On 1 May 2020, on the basis of preliminary results from phase 3 trials, the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) allowing the use of Remdesivir for the treatment of suspected or laboratory-confirmed COVID-19 in adult and pediatric patients (over 12 years old and weighing at least 40 kg or more) hospitalized with severe disease [81]. An intravenous nucleotide prodrug of Remdesivir (Veklury®) is currently approved by the FDA for the treatment of SARS-CoV-2 infection, but its limitations and controversial efficacy make it difficult to be widely used in hospitalized patients [82].
Chloroquine and hydroxychloroquine are antimalarial drugs that inhibit the lysosomes’ vital functions by increasing the pH, which results in blocking endosome-mediated entry, and can interfere with nucleic acid replication, viral protein glycosylation, virus assembly, and release. In particular, Chloroquine possesses an immune-modulatory activity, which could enhance its antiviral effects, with some limitations because of the potential for overdosing, acute poisoning, and death. Even though hydroxychloroquine has been demonstrated to be much less toxic (by about 40%) [83], both are responsible for the onset of side effects, such as gastrointestinal disorders, headaches, retinopathy, and arrhythmia. Meanwhile, a large randomized clinical trial demonstrated that these drugs were not associated with mortality rate reduction, nor with recovery rate improvement [84]. After 15 June 2020, the FDA finally indicated that hydroxychloroquine and chloroquine were not beneficial for the treatment of COVID-19 [85].
Another old antimalarial drug derived from Chinese herbs, artemisinin, together with other artemisinin-related drugs, has been reported to have multiple pharmacological activities, including antiviral, anticancer and immune modulation ones [86]. Cao et al. (2020) [87] systematically explored the antiviral activities of several artemisinins against SARS-CoV-2 in vitro. Among them, artesunate and arteannuin B were found to be good anti-SARS-CoV-2 agents but, interestingly, the authors found that the antimalarial drug lumefantrine, even though it is structurally distant from artemisinins, could inhibit SARS-CoV-2 in vitro as well.
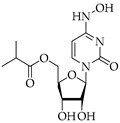
Molnupiravir (Lagevrio, Merck (Branchburg, NJ, USA)) is the first oral antiviral drug authorized for COVID-19 treatment in the UK starting from the 4 November 2021 for adults with a positive COVID test who have at least one risk factor for developing severe illness [27]. Molnupiravir prevents SARS-CoV-2 replication by causing multiple mutations in its genome, and demonstrated a significant benefit in reducing hospitalization or death in mild COVID-19 patients. However, its role in moderate to severe COVID-19 is still questionable [88].
Paxlovid (Pfizer (New York, NY, USA)) is a combination of ritonavir with nirmatrelvir, a SARS-CoV-2 protease inhibitor currently being assessed in phase 3 trials for its safety and efficacy in the treatment of non-hospitalized adult patients with COVID-19 who are not at risk of developing severe illness. Its clinical efficacy meant that it was able to reduce hospitalization by 80%, and it is also being explored as a post-exposure prophylaxis agent in patients previously exposed to SARS-CoV-2 [89].
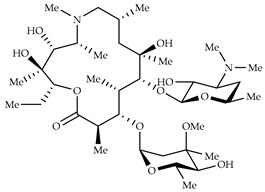
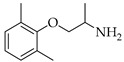
Azithromycin is a macrolide antimicrobial agent which acts against a broad range of Gram-positive and Gram-negative bacteria and plays an immunomodulatory role and has been widely used for the treatment of COVID-19 patients with moderate to severe symptoms [90]. It is also used in combination with hydroxychloroquine, even though an increased risk of malignant arrhythmia and sudden cardiac death has been reported [91]. Mexiletine, a well-known anti-arrhythmic drug used in congenital long QT syndrome (LQTS) patients was proposed in association with hydroxychloroquine and azithromycin for the treatment of COVID-19 patients to limit excessive QT prolongation. In subclinical conditions, the model suggests that mexiletine may limit the deleterious effects of azithromycin and hydroxychloroquine [92,93].
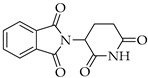
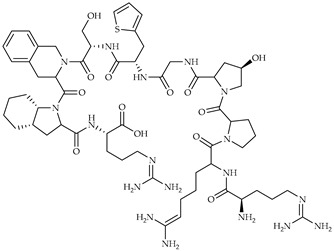
As a monoclonal antibody, bevacizumab acts against the vascular endothelial growth factor (VEGF) and is usually indicated for cancer therapy; however, numerous investigations have supported the fundamental role of VEGF in acute lung injury (ALI) and ARDS, suggesting a potential role in the management of COVID-19 patients. Indeed, several clinical trials on the efficacy of bevacizumab in severe patients with COVID-19 are in different phases of study [94]. Again, thalidomide, an anti-inflammatory and immunomodulating drug that has demonstrated antiproliferative activity in cancer, has been proposed, since it significantly accelerated the negative conversion of SARS-CoV-2 and shortened the hospital stay length of affected patients [95]. It also reduced the requirement for mechanical ventilation in patients with critical COVID-19. Most importantly, the combination therapy of thalidomide with low-dose glucocorticoid was effective in improving the prognosis of patients with critical COVID-19.
Angiotensin II receptor (ACE2) antagonists, belonging to the class of sartans, are known antihypertensive agents and are now under study for COVID-19 treatment, starting from the early consideration that coronaviruses transfer their genetic material to the host cell, binding the ACE2 receptors. Losartan inhibits ACE2 binding to the AT1 receptor, thereby counteracting the physiological effects of angiotensin II [96]. ACE2 is also related to bradykinin, a potent inflammatory mediator, and hydrolyzes the active bradykinin metabolite [des-Arg973] BK (DABK). As a side effect, with the reduction in the amount of ACE2 in the body, COVID-19 activates the bradykinin system, leading to fluid extravasation and leukocyte recruitment to the lung, which persists in pulmonary edema subsequently. For this purpose, icatibant, a bradykinin B2 receptor inhibitor, has been introduced as a potential candidate for the treatment of COVID-19 [97].
Finally, the use of corticosteroids in patients with COVID-19 is still controversial, since the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) generally advised the use of glucocorticoids against COVID-19 pneumonia, unless in specific comorbid clinical conditions, e.g., exacerbation of chronic obstructive pulmonary disease. Indeed, it seems that clinical phenotypes are associated with a differential response: for critical COVID-19 patients, corticosteroid therapy was associated with a decrease in 28-day mortality, but the use of corticosteroids showed significant survival benefits in patients with the hyperinflammatory phenotype [98]. In hospitalized hypoxic COVID-19 patients, methylprednisolone demonstrated better results compared to dexamethasone [99]. Structures for described compounds are reported in Table 3. The fight against COVID-19 is not finished yet, and new research focused on investigations of further drugs use/association in the management of the most severe effects is needed.
Table 3.
Structure of current drugs used for COVID-19 treatment.
| Structure | Name | Class | Ref. |
|---|---|---|---|
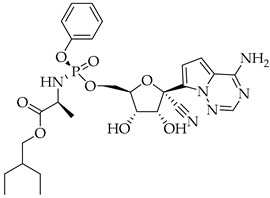
|
Remdesivir | Antiviral Nucleoside analogue |
[81,82] |
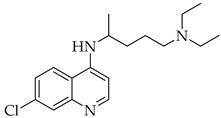
|
Chroloquine | Antimalarial | [83,84,85] |
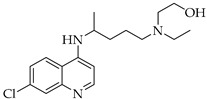
|
Hydroxychloroquine | Antimalarial | [83,84,85] |
|
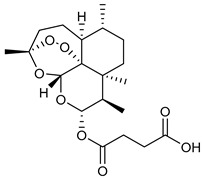
Artesunate | Antimalarial | [86,87] |
|
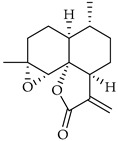
Arteannuin B | Antimalarial | [86,87] |
|
Lumefantrine | Antimalarial | [86,87] |
|
Molnupiravir | Antiviral Nucleoside analogue |
[27,88] |
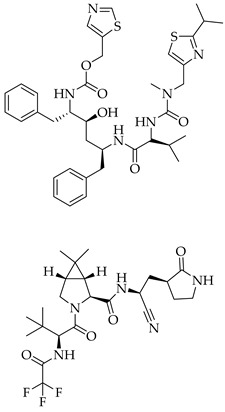
|
Paxlovid (ritonavir + nirmatrelvir) |
Antiviral Viral protease inhibitor |
[89] |
|
Azithromycin | Antimicrobial | [91] |
|
Mexiletine | Antiarrhythmic | [92,93] |
| Monoclonal antibody | Bevacizumab | Angiogenesis inhibitor | [94] |
|
Thalidomide | Immunomodulator | [95] |
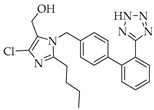
|
Losartan | Angiotensin II receptor blockers | [96] |
|
Icatibant | Bradykinin B2 receptor antagonist | [97] |
|
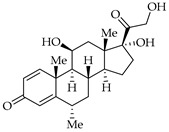
Methylprednisolone | Corticosteroid | [98,99] |
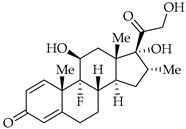
|
Dexamethasone | Corticosteroid | [98,99] |
4. Alternative Therapies
Several studies on phytonutrients, dietary supplements and nutraceuticals for the treatment and prevention of COVID-19 have been reported [100]. Such products include vitamin C, vitamin D, polyphenols, omega 3 polyunsaturated fatty acids, probiotics, and zinc, all of which are currently under clinical investigation [101]. Several studies on the potential role of vitamin D in COVID-19 in adults, older people and children are ongoing [102,103]. A recent study reported that the administration of high-dose intravenous vitamin C (HDIVC) is beneficial for critically ill COVID-19 [104]. However, other authors found no significantly positive outcomes in patients with severe COVID-19 disease treated with HDIVC [105]. Polyphenols, such as epigallocatechin gallate (EGCG) and gallocatechingallate (GCG), which are common constituents of green tea, quercetin (found abundantly in apples) and hesperetin (in citric foods) are currently under study [106,107,108]. Spices such as turmeric, thyme, rosemary, and garlic are also under investigation for the prevention and treatment of COVID-19 [109]. Five selected flavonoids, namely curcumin, kaempferol, quercetin, apigenin and monolaurin, have been critically examined for use as adjuvant therapeutic agents against viral infections, including SARS-CoV-2 [110]. Quercetin was suggested as a reasonably potent inhibitor of SARS-CoV-2 3CLpro protease [111]. Curcumin supplementation was demonstrated to decrease common symptoms, the duration of hospitalization and deaths, through the partial restoration of pro-/anti-inflammatory balance [112]. Studies on Mentha piperita demonstrated the existence of active compounds in mint leaves (M. piperita L.), including rutin, hesperidin, and isorhoifolin [113,114]. Currently, a double-blinded clinical trial (participant, researcher) on pomegranate juice is underway [115,116]. Probiotics, such as lactobacillus and bifidobacterium, have numerous benefits such as balancing the composition of human gut microflora, strengthening gut barrier function, and protective immune responses. They may serve as lead prophylactic and immune-boosting probiotics in COVID-19 management [117]. Recently, melatonin, the so-called ’hormone of darkness’, due to its synthesis at night-time, has attracted the interest of infectious diseases specialists and epidemiologists for its adjuvant role in treating patients with COVID-19 [118]. It has been recently given as part of a US Food and Drug Administration emergency authorized cocktail, REGEN-COV2, for the management of COVID-19 progression [119].
Staying at home for quarantine and consequently being largely inactive is associated with unintended consequences. These measures can actually raise the infection risk and exacerbate poor health conditions involving the immune system. Physical activity and yoga may represent a feasible way of improving health, particularly physical and mental health [120,121]. Irisin, a muscle-contraction-induced immunomodulatory myokine that is released from myocytes during physical activity, has been proposed as a possible strategy to tackle COVID-19 [122,123], even though, to date, only a few articles have reported the use of irisin in COVID-19 [124]. Finally, the combination of active natural products with approved antiviral drugs has recently been suggested as an alternative to improve the biological effect and/or produce a synergistic outcome as supportive agents to minimize clinical symptoms [125].
5. Repositioning Drugs
The discovery of new drugs is a long, costly, and rigorous scientific process; therefore, a more effective approach could be represented by the search for effective anti-SARS-CoV-2 therapies from existing drug databases. Thus, drug repositioning, also known as drug repurposing, has emerged as a successful strategy for uncovering the potential of drugs in the management of diverse diseases. Since it concerns the investigation of existing drugs for new therapeutic target indications, the major benefits are represented by the reduced costs and expedited approval procedures. The urgency to find drugs fighting COVID-19 has tremendously pushed this kind of research [126,127]; indeed, in the last year, computational drug repositioning represented one of the most rapid and winning strategies applied for discovering SARS-CoV-2 drugs [128]. A total of 1553 FDA-approved drugs, as well as another 7012 investigational or off-market drugs in DrugBank, were proposed [129]. As an emblematic example, the first treatment available for COVID-19, remdesivir, was proposed as a repositioned drug [130]. Virtual screening studies showed that broad-spectrum antiviral agents (BSAAs) revealed potential activity against the SARS-CoV-2 spike glycoprotein, RdRp, the main protease (Mpro) and the helicase enzyme of SARS-CoV-2. In particular, imatinib (a tyrosine kinase inhibitor), suramin (an anti-parasitic), glycyrrhizin (an anti-inflammatory) and bromocriptine (a dopamine agonist) revealed good affinity towards multiple viral targets [131].
Recently, in silico screening followed by wet-lab validation indicated a poly-ADP-ribose polymerase 1 (PARP1) inhibitor, CVL218, currently in phase I clinical trial, for repositioning to treat COVID-19. CVL218 was able to inhibit SARS-CoV-2 replication without exhibiting any obvious cytopathic effects; moreover, CVL218 can interact with the nucleocapsid protein of SARS-CoV-2 and suppress the LPS-induced production of several inflammatory cytokines that are highly relevant to the prevention of the immunopathology induced by SARS-CoV-2 infection [132]. Several other compounds, endowed with different biological activities, have been proposed for repositioning [133], such as ivermectin (IVM), an FDA-approved antiparasitic drug for onchocerciasis and strongyloidiasis [134], fibrates, diarylureas [135,136], Schiff bases [137,138,139] and transition metal complexes [140,141]. Moreover, a recent article indicated the use of repositioning microbial biotechnology for studies against COVID-19, particularly for flavonoids. The idea is that the adoption of new systems and synthetic biological tools could provide an optimal framework for the guided and targeted expansion of flavonoid chemical diversity [142]. Finally, repositioning drugs may be used to counteract the side effects of currently used drugs, such as drug-induced LQTS. The common cause of LQTS induced by drugs is due to the impairing of human ether-ago-go-related gene (hERG) channels. For instance, among COVID-19 treatment drugs, chloroquine and hydroxychloroquine were demonstrated to block hERG potassium channels, whereas azithromycin and remdesivir did not [143]. Thus, the addition of hERG potassium channels inhibitors, such as lubeluzole, to the conventional therapies may represent an interesting strategy to overcome LQTS [144,145].
6. Recent Preclinical Studies
A recent study was focused on aminothiol cysteamine, a human existing drug, and its disulfide product of oxidation, cystamine, which have anti-infective properties targeting viruses, bacteria, and parasites. The antiviral effects against SARS-CoV-2 were evaluated using different in vitro viral-infected cell-based assays. Moreover, the ability of cysteamine to modulate the SARS-CoV-2-specific immune response was studied in vitro in blood samples from COVID-19 patients. Both compounds were shown to decrease SARS-CoV-2-induced cytopathic effects (CPE) in Vero E6 cells. Cysteamine and cystamine significantly decreased the viral production in Vero E6 and Calu-3 cells, and their antiviral action did not depend on the treatment time with respect to SARS-CoV-2 infection. Additionally, they showed an anti-inflammatory effect by lowering the SARS-CoV-2 specific IFN-γ production in vitro, using blood samples from COVID-19 patients [146].
Recent studies addressed the most feared novel Omicron variant. Nirmatrelvir, molnupiravir, and remdesivir were tested in vitro against a panel of SARS-CoV-2 variants in live-virus antiviral assays. It was demonstrated that nirmatrelvir, as well as other clinically relevant antivirals, maintains its activity against all variants tested, including Omicron [147], confirming what was previously reported [148,149]. The isolation of the Omicron variant was reported for the first time by Yadav et al. (2022) [150] by using an in vivo followed by in vitro method of SARS-CoV-2 virus culture; more specifically, it was obtained from infected Syrian hamsters and the subsequent passage into Vero CCL-81 cells, since the Omicron variant’s isolation in Vero CCL-81 from the clinical specimens of COVID-19 cases failed. Recent in vitro studies have been carried out to evaluate therapeutic antibodies against a SARS-CoV-2 Omicron B.1.1.529 isolate. The authors found that a dose of 500 mg of sotrovimab retained a significant level of neutralizing activity against the Omicron compared to previous variants. The activity of evusheld 300 mg represented about 10% of the activity of sotrovimab 500 mg [151].
Finally, a high-throughput screening test was performed by some authors to explore the in vitro antiviral activity of itraconazole and its metabolite hydro-itraconazole, already known for the feline coronavirus and influenza A virus [152]. The positive pharmacological and pharmacokinetic properties led the authors to adopt a hamster SARS-CoV-2 infection model to study the possible activity of itraconazole in acute infection and viral transmission models. However, the results were disappointing, given that the administration of itraconazole in hamsters was unable to reduce the viral RNA load in the lungs, ileum or stools and did not mitigate pulmonary inflammation, despite sufficient exposure.
7. Recent Clinical Studies
A recent interesting study described the production of the first chicken egg yolk-derived anti-index SARS-CoV-2 receptor-binding domain (RBD) immunoglobulin Y (IgY) polyclonal antibodies as an intranasal drop product for humans, with equal in vitro activity against all VOCs. The major advantages are the ease and fast production and the low cost; moreover, the antibodies were developed as nasal drops, in order to capture the virus directly on the nasal mucosa. In this double-blinded, randomized, placebo-controlled phase 1 study, single-ascending and multiple doses of anti-SARS-CoV-2 RBD IgY were administered intranasally for 14 days in 48 healthy participants, demonstrated an excellent safety and tolerability profile, and the absence of systemic absorption of topically administered IgY. Moreover, no evidence of a systemic inflammatory immune response triggered by the topical treatment with anti-SARS-CoV-2 RBD IgY in humans was observed [153]. Recently, the valuable beneficial effects of beta glucans, derived from two strains AFO-202 and N-163 of a black yeast Aureobasidium pullulans, were described. They were evaluated on the biomarkers for cytokine storm and coagulopathy in COVID-19 patients, in a randomized pilot clinical study, where the supplementation with these beta glucans helped to maintain the major biomarkers of clinical severity and mortality of COVID-19, such as IL-6, D-Dimer, neutrophil to lymphocyte ratio (NLR) over 15 and 30 days, compared to those who underwent standard care alone [154]. Another recent pilot study, an open label, randomized clinical trial, investigated the potential benefit of calcitriol therapy given to 50 patients hospitalized with COVID-19 indicating an improvement in oxygenation, suggesting the need for a larger randomized trial [155]. Studies in IVM are debated. A randomized, double-blinded, multicenter, phase II, dose-finding, proof-of-concept clinical trial showed that high-dose IVM led to a not significant reduction in viral load when compared to placebo. Whether this drug might have clinical efficacy at lower doses remains debated. The recommendation of the WHO suggests that it is currently advisable to refrain from administrating ivermectin for the treatment of COVID-19 outside clinical trials [156]. These studies are summarized in Table 4.
Table 4.
Recent clinical studies.
| Type of Clinical Studies | Treatments | Participants | Administration | Results |
|---|---|---|---|---|
| Double-blind, randomized, placebo-controlled phase 1 study | Chicken egg yolk-derived anti-index SARS- CoV-2 RBD IgY polyclonal antibodies as an intranasal drop product | 48 healthy adults | Intranasally single-ascending doses of 2, 4, and 8 mg for 14 days | Excellent safety and tolerability profile and absence of systemic absorption |
| Randomized multiple-arm pilot clinical study | Beta glucans derived from two strains AFO-202 and N-163 of a black yeast Aureobasidium pullulans | 24 RT-PCR positive COVID-19 patients | Additional supplementation for 30 days Group 1 control Group 2: AFO-202 beta glucan Group 3: a combination of AFO-202 and N-163 beta glucans |
Significant control of IL6, D-Dimer and NLR, a significant increase in LCR, LeCR and marginal control of ESR in COVID-19 patients |
| Open label, randomized clinical trial | Calcitriol for the treatment of COVID-19 | 50 patients hospitalized with COVID-19 | 0.5 μg daily for 14 days | Improvement in oxygenation among hospitalized |
| Randomized, double-blind, multicenter, phase II, dose-finding, proof-of-concept clinical trial | Ivermectin for the treatment of COVID-19 | 89 adults recently diagnosed with asymptomatic/oligosymptomatic SARS-CoV-2 infection | (A) placebo (B) single-dose 600 μg/kg plus placebo for 5 days (C) single-dose 1200 μg/kg for 5 days |
No significant reduction in viral load between ivermectin and placebo groups |
8. Conclusions
As a matter of fact, the pandemic is still far from over. In this paper, we summarized the most important features regarding COVID-19, the human respiratory illness caused by the virus SARS-CoV-2. Studies concerning diagnosis, treatment and vaccines for this disease have been addressed, together with the worth of rapid diagnostics, which is fundamental. This aspect is only partially satisfied by the available tests, in our opinion, considering that the viral genomic detection has been exclusively based on molecular tests, mainly RT-PCR, and that the more rapid antibody (serology) tests, necessary for a wider immune screening, cannot confirm with certainty the virus’ presence. The COVID-19 pandemic prompted scientists from around the world to design anti-SARS-CoV-2 vaccines and try several therapies, most of them obtained by exploiting the approach of drug repositioning, a field of drug research whose importance has increased in recent years, due to several advantages, such as the possibility to shorten the clinical trials and also the life extension of old drugs by finding a new therapeutic target. In spite of these efforts, proper and effective therapies for COVID-19 have still not been discovered, mostly because of the presence of different forms and variants of this disease. However, among the therapies currently used, antibiotics are at the forefront, and, for this reason, particular attention must be paid to the emergence of multidrug-resistant bacteria, a very important issue that must be taken into account to avoid the so-called “jumping out of the frying pan into the fire”. Appropriate prescriptions, optimized antibiotic use and aggressive infection control may help to prevent the occurrence of multidrug resistance (MDR). Additionally, efficient and early diagnosis, combined with effective treatments, might significantly lessen the burden of other pandemic waves.
There is also the need to develop additional safe, effective, easy-to-produce, and inexpensive preventive tools to reduce the risk of acquiring SARS-CoV-2 infection. New preclinical studies, i.e., those with intranasal delivery of anti-SARS-CoV-2 IgY, have been suggested to provide passive immunization, including for use as an add-on to personal protective equipment and other preventive measures for the general population until vaccination, which is hopefully highly effective against prevalent variants, becomes available worldwide or herd immunity is achieved. This IgY may also provide short-term protection, in addition to vaccines, in less well-ventilated environments, including trains, airplanes, lecture halls, etc. To conclude, it is also necessary to emphasize that the recently described VOC Omicron (B.1.1.529) variant has rapidly spread worldwide and is now responsible for the majority of COVID-19 cases in many countries, in which less severe clinical diseases in humans are registered. Finally, it is desirable that, although many knowledge gaps exist about the epidemiology, clinical severity, and disease course of the Omicron variant, its high transmissibility could potentially contribute to the achievement of the desired herd immunity.
Abbreviations
| ACE2 | Angiotensin II receptor |
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| BSAAs | Broad-spectrum antiviral agents |
| CCTI | Chest computer tomography image |
| CDC | Centers for Disease Control and Prevention |
| CDSCO | Central Drugs Standard Control Organization |
| COVID-19 | Coronavirus Disease 2019 |
| CPE | Cytopathic effects |
| CRS | Cytokine release syndrome |
| CS | “Cytokine storm” |
| CT | Computed tomography |
| CXI | Chest X-ray image |
| DABK | Bradykinin metabolite [des-Arg973] BK |
| DCGI | Drugs Controller General of India |
| EGCG | Epigallocatechin gallate |
| ECDC | European Centre for Disease Prevention |
| EMA | European Medicines Agency |
| EUA | Emergency Use Authorization |
| FBSED | Fourier-Bessel series expansion-based decomposition |
| FDA | Food and Drug Administration |
| GBS | Guillain-Barre syndrome |
| GCG | Gallocatechingallate |
| GISAID | Global Initiative on Sharing All Influenza Data |
| HDIVC | High-dose intravenous vitamin C |
| hERG | Human ether-ago-go-related gene |
| HRQoL | Health-related quality of life |
| HZ | Herpes zoster |
| IFNAR | Human interferon alpha-receptor |
| IFN | Interferon |
| IgY | Immunoglobulin Y |
| IL | Interleukin |
| IVM | Ivermectin |
| LQTS | Long QT syndrome |
| MDR | Multidrug resistance |
| MFDS | Ministry of Food and Drug Safety |
| MHLW | Ministry of Health, Labour, and Welfare |
| Mpro | Main protease |
| NLR | Neutrophil to lymphocyte ratio |
| NMPA | National Medical Products Administration |
| NRA | National Regulatory Agency |
| PARP1 | Poly-ADP-ribose polymerase 1 |
| PCS | Post-acute COVID-19 syndrome |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| RBD | Receptor-binding domain |
| RdRp | RNA-dependent RNA polymerase |
| RT-PCR | Reverse transcriptase-polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TGA | Therapeutic Goods Administration |
| TNF-α | Tumor necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
| VOCs | Variants of concern |
| VOIs | Variants of interest |
| VST | Venous sinus thrombosis |
| VZV | Varicella zoster virus |
| WHO | World Health Organization |
Author Contributions
Conceptualization, M.S.S. and C.S.; literature review and data analysis, A.C. and A.M.; writing—original draft preparation, D.I. and J.C.; writing—review and editing, A.C. and M.P.; supervision, P.L. and S.A.; funding acquisition, S.A. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by PRIN (Progetti di Rilevante Interesse Nazionale) Grant 2017M8R7N9_004 and 2020KSY3KL_005 from MUR, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Features, evaluation and treatment of Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dashboard. [(accessed on 1 February 2022)]. Available online: http://covid19.who.int.
- 3.Pisano G.P., Sadun R., Zanini M. Lessons from Italy’s Response to Coronavirus. [(accessed on 24 January 2022)]. Available online: https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
- 4.Padma T.V. COVID Vaccines to Reach Poorest Countries in 2023—Despite Recent Pledges. Nature. 2021;595:342–343. doi: 10.1038/d41586-021-01762-w. [DOI] [PubMed] [Google Scholar]
- 5.Burki T. Booster shots for COVID-19—The debate continues. Lancet Infect. Dis. 2021;21:1359–1360. doi: 10.1016/S1473-3099(21)00574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahase E. COVID-19: What new variants are emerging and how are they being investigated? BMJ. 2021;372:158. doi: 10.1136/bmj.n158. [DOI] [PubMed] [Google Scholar]
- 7.Malik P., Patel K., Pinto C., Jaiswal R., Tirupathi R., Pillai S., Patel U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—A systematic review and meta-analysis. J. Med. Virol. 2022;94:253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cascella M., De Blasio E. Features and Management of Acute and Chronic Neuro-COVID. Springer; Cham, Switzerland: 2022. Neurological, psychological, and cognitive manifestations of long-COVID; pp. 137–158. [Google Scholar]
- 9.Baig A.M. Counting the neurological cost of COVID-19. Nat. Rev. Neurol. 2022;18:5–6. doi: 10.1038/s41582-021-00593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore P., Esmail F., Qin S., Nand S., Berg S. Hypercoagulability of COVID-19 and neurological complications: A review. J. Stroke Cerebrovasc. Dis. 2022;31:106163. doi: 10.1016/j.jstrokecerebrovasdis.2021.106163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uddin M., Mustafa F., Rizvi T.A., Loney T., Al Suwaidi H., Al-Marzouqi A.H.H., Eldin A.K., Alsabeeha N., Adrian T.E., Stefanini C., et al. SARS-CoV-2/COVID-19: Viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses. 2020;12:526. doi: 10.3390/v12050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liguoro I., Pilotto C., Bonanni M., Ferrari M.E., Pusiol A., Nocerino A., Vidal E., Cogo P. SARS-CoV-2 infection in children and newborns: A systematic review. Eur. J. Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Principi N., Esposito S. Is the Immunization of Pregnant Women against COVID-19 Justified? Vaccines. 2021;9:970. doi: 10.3390/vaccines9090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahase E. COVID-19: Where are we on vaccines and variants? BMJ. 2021;372:597. doi: 10.1136/bmj.n597. [DOI] [PubMed] [Google Scholar]
- 16.Wang C., Han J. Will the COVID-19 pandemic end with the Delta and Omicron variants? Environ. Chem. Lett. 2022:1–11. doi: 10.1007/s10311-021-01369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baraniuk C. COVID-19: How effective are vaccines against the Delta variant? BMJ. 2021;374 doi: 10.1136/bmj.n1960. [DOI] [PubMed] [Google Scholar]
- 18.Rebold N., Holger D., Alosaimy S., Morrisette T., Rybak M. COVID-19: Before the fall, an evidence-based narrative review of treatment options. Infect. Dis. Ther. 2021;10:93–113. doi: 10.1007/s40121-021-00399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawton G. Are booster shots coming? New Sci. 2021;250:8–9. doi: 10.1016/S0262-4079(21)00808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahase E. COVID-19 booster vaccines: What we know and who’s doing what. BMJ. 2021;374:n2082. doi: 10.1136/bmj.n2082. [DOI] [PubMed] [Google Scholar]
- 21.Quilty B.J., Clifford S., Hellewell J., Russell T.W., Kucharski A.J., Flasche S., Edmunds W.J., Atkins K.E., Foss A.M., Waterlow N.R., et al. Quarantine and testing strategies in contact tracing for SARS-CoV-2: A modelling study. Lancet Public Health. 2021;6:e175–e183. doi: 10.1016/S2468-2667(20)30308-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killgore W.D., Cloonan S.A., Taylor E.C., Vanuk J.R., Dailey N.S. Morning drinking during COVID-19 lockdowns. Psychiatry Res. 2022;307:114320. doi: 10.1016/j.psychres.2021.114320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt R.A., Genois R., Jin J., Vigo D., Rehm J., Rush B. The early impact of COVID-19 on the incidence, prevalence, and severity of alcohol use and other drugs: A systematic review. Drug Alcohol Depend. 2021;228:109065. doi: 10.1016/j.drugalcdep.2021.109065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feuillet V., Canard B., Trautmann A. Combining antivirals and immunomodulators to fight COVID-19. Trends Immunol. 2021;42:31–44. doi: 10.1016/j.it.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romanou V., Koukaki E., Chantziara V., Stamou P., Kote A., Vasileiadis I., Koutsoukou A., Rovina N. Dexamethasone in the Treatment of COVID-19: Primus Inter Pares? J. Pers. Med. 2021;11:556. doi: 10.3390/jpm11060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malone B., Campbell E.A. Molnupiravir: Coding for catastrophe. Nat. Struct. Mol. Biol. 2021;28:706–708. doi: 10.1038/s41594-021-00657-8. [DOI] [PubMed] [Google Scholar]
- 27.Cave J.A., Phizackerley D. Molnupiravir: Evidence by press release. Drug Ther. Bull. 2022;60:2. doi: 10.1136/dtb.2021.000064. [DOI] [PubMed] [Google Scholar]
- 28.Dyer O. COVID-19: Doctors will refuse to limit use of antiviral drug to unvaccinated patients, say ethicists. Br. Med. J. Publ. Group. 2021;375:n2855. doi: 10.1136/bmj.n2855. [DOI] [PubMed] [Google Scholar]
- 29.Ledford H. COVID antiviral pills: What scientists still want to know. Nature. 2021;599:358–359. doi: 10.1038/d41586-021-03074-5. [DOI] [PubMed] [Google Scholar]
- 30.Hariyanto T.I., Intan D., Hananto J.E., Harapan H., Kurniawan A. Vitamin D supplementation and COVID-19 outcomes: A systematic review, meta-analysis and meta-regression. Rev. Med. Virol. 2021:e2269. doi: 10.1002/rmv.2269. [DOI] [Google Scholar]
- 31.Thacher T.D. Evaluating the Evidence in Clinical Studies of Vitamin D in COVID-19. Nutrients. 2022;14:464. doi: 10.3390/nu14030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacopetta D., Catalano A., Ceramella J., Saturnino C., Salvagno L., Ielo I., Drommi D., Scali E., Plutino M.R., Rosace G., et al. The different facets of triclocarban: A review. Molecules. 2021;26:2811. doi: 10.3390/molecules26092811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mousavi-Roknabadi R.S., Arzhangzadeh M., Safaei-Firouzabadi H., Mousavi-Roknabadi R.S., Sharifi M., Fathi N., Jelyani N.Z., Mokdad M. Methanol poisoning during COVID-19 pandemic; A systematic scoping review. Am. J. Emerg. Med. 2022;52:69–84. doi: 10.1016/j.ajem.2021.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizvi S.G., Ahammad S.Z. COVID-19 and antimicrobial resistance: A cross-study. Sci. Total Environ. 2022;807:150873. doi: 10.1016/j.scitotenv.2021.150873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catalano A., Iacopetta D., Ceramella J., Scumaci D., Giuzio F., Saturnino C., Aquaro S., Rosano C., Sinicropi M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules. 2022;27:616. doi: 10.3390/molecules27030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pozzi C., Ferrari S., Cortesi D., Luciani R., Stroud R.M., Catalano A., Costi M.P., Mangani S. The structure of Enterococcus faecalis thymidylate synthase provides clues about folate bacterial metabolism. Acta Crystallogr. D Biol. Crystallogr. 2012;68:1232–1241. doi: 10.1107/S0907444912026236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lascarrou J.-B., Colin G., Le Thuaut A., Serck N., Ohana M., Sauneuf B., Geri G., Mesland J.-B., Ribeyre G., Hussenet C., et al. Predictors of Negative First SARS-CoV-2 RT-PCR despite Final Diagnosis of COVID-19 and Association with Outcome. Sci. Rep. 2021;11:2388. doi: 10.1038/s41598-021-82192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia X., Xiao L., Liu Y. False negative RT-PCR and false positive antibody tests–concern and solutions in the diagnosis of COVID-19. J. Infect. 2021;82:414–451. doi: 10.1016/j.jinf.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoki K., Takai K., Nagasawa T., Kashiwagi K., Mori N., Matsubayashi K., Satake M., Tanaka I., Kodama N., Shimodaira T., et al. Combination of a SARS-CoV-2 IgG assay and RT-PCR for improved COVID-19 diagnosis. Ann. Lab. Med. 2021;41:568–576. doi: 10.3343/alm.2021.41.6.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Y., Zheng S., Li L., Zhang X., Zhang X., Huang Z., Chen J., Zhao H., Jie Y., Wang R. Deep learning enables accurate diagnosis of novel coronavirus (COVID-19) with CT images. medRxiv. 2020;18:2775–2780. doi: 10.1109/TCBB.2021.3065361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhary P.K., Pachori R.B. FBSED based automatic diagnosis of COVID-19 using X-ray and CT images. Comput. Biol. Med. 2021;134:104454. doi: 10.1016/j.compbiomed.2021.104454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaqroun A., Hartard C., Schvoerer E. Anti-SARS-CoV-2 vaccines and monoclonal antibodies facing viral variants. Viruses. 2021;13:1171. doi: 10.3390/v13061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascola J.R., Graham B.S., Fauci A.S. SARS-CoV-2 Viral Variants—Tackling a Moving Target. JAMA. 2021;325:1261–1262. doi: 10.1001/jama.2021.2088. [DOI] [PubMed] [Google Scholar]
- 44.Rambaut A., Holmes E.C., O’Toole Á., Hill V., McCrone J.T., Ruis C., du Plessis L., Pybus O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rambaut A., Holmes E.C., O’Toole Á., Hill V., McCrone J.T., Ruis C., du Plessis L., Pybus O.G. Addendum: A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2021;6:415. doi: 10.1038/s41564-021-00872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.PANGO Lineages. [(accessed on 1 February 2022)]. Available online: https://cov-lineages.org.
- 47.Shu Y., McCauley J. GISAID: Global initiative on sharing all influenza data—From vision to reality. Eurosurveillance. 2017;22:30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clade and Lineage Nomenclature Aids in Genomic Epidemiology Studies of Active hCoV-19 Viruses. GISAID. [(accessed on 1 February 2022)]. Available online: https://go.nature.com/3pgSIt6.
- 49.Hadfield J., Megill C., Bell S.M., Huddleston J., Potter B., Callender C., Sagulenko P., Bedford T., Neher R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bedford T., Hodcroft E.B., Neher R.A. Updated Nextstrain SARS-CoV-2 Clade Naming Strategy. Nextstrain. [(accessed on 1 February 2022)]. Available online: https://go.nature.com/3c9Riep.
- 51.WHO COVID-19 Weekly Epidemiological Update—25 February 2021. [(accessed on 1 February 2022)]. Available online: https://go.nature.com/3uEtXIj.
- 52.Chakraborty C., Bhattacharya M., Sharma A.R. Present variants of concern and variants of interest of severe acute respiratory syndrome coronavirus 2: Their significant mutations in S-glycoprotein, infectivity, re-infectivity, immune escape and vaccines activity. Rev. Med. Virol. 2021:e2270. doi: 10.1002/rmv.2270. [DOI] [Google Scholar]
- 53.Das I. Vigilância genômica E. Monitoramento.; do, variantes. SARS-CoV-2. [(accessed on 1 February 2022)]. Available online: https://amazonia.fiocruz.br/wp-content/uploads/2021/09/Vigilancia_Genomica_do_SARS-CoV-2_no_Estado_do_Amazonas_No_02.pdf.
- 54.Swift C.L., Isanovic M., Velez K.E.C., Norman R.S. Community-level SARS-CoV-2 sequence diversity revealed by wastewater sampling. Sci. Total Environ. 2021;801:149691. doi: 10.1016/j.scitotenv.2021.149691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Focosi D., Tuccori M., Baj A., Maggi F. SARS-CoV-2 variants: A synopsis of in vitro efficacy data of convalescent plasma, currently marketed vaccines, and monoclonal antibodies. Viruses. 2021;13:1211. doi: 10.3390/v13071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wahid M., Jawed A., Mandal R.K., Dailah H.G., Janahi E.M., Dhama K., Somvanshi P., Haque S. Variants of SARS-CoV-2, their effects on infection, transmission and neutralization by vaccine induced antibodies. Eur. Rev. Med. Pharmacol. Sci. 2021;25:5857–5864. doi: 10.26355/eurrev_202109_26805. [DOI] [PubMed] [Google Scholar]
- 57.Dudas G., Hong S.L., Potter B.I., Calvignac-Spencer S., Niatou-Singa F.S., Tombolomako T.B., Fuh-Neba T., Vickos U., Ulrich M., Leendertz F.H., et al. Emergence and spread of SARS-CoV-2 lineage B. 1.620 with variant of concern-like mutations and deletions. Nat. Commun. 2021;12:5769. doi: 10.1038/s41467-021-26055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jhun H., Park H.Y., Hisham Y., Song C.S., Kim S. SARS-CoV-2 Delta (B. 1.617. 2) variant: A unique T478K mutation in receptor binding motif (RBM) of spike gene. Immune Netw. 2021;21:e32. doi: 10.4110/in.2021.21.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Regulatory Affairs Professionals Society COVID-19 Vaccine Tracker. [(accessed on 18 February 2022)]. Available online: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker.
- 60.Thomas S.J., Moreira E.D., Jr., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N. Engl. J. Med. 2021;383:2603–2615. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knoll M.D., Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Livingston E.H., Malani P.N., Creech C.B. The Johnson & Johnson vaccine for COVID-19. JAMA. 2021;325:1575. doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- 64.Miller E. Rapid evaluation of the safety of COVID-19 vaccines: How well have we done? Clin. Microbiol. Infect. 2022. in press . [DOI] [PMC free article] [PubMed]
- 65.Klugar M., Riad A., Mekhemar M., Conrad J., Buchbender M., Howaldt H.-P., Attia S. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among german healthcare workers. Biology. 2021;10:752. doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finsterer J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol. Scand. 2022;145:5–9. doi: 10.1111/ane.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMahon D.E., Kovarik C.L., Damsky W., Rosenbach M., Lipoff J.B., Tyagi A., Chamberlin G., Fathy R., Nazarian R.M., Desai S.R., et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J. Am. Acad. Dermatol. 2022;86:113–121. doi: 10.1016/j.jaad.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehta H., Handa S., Malhotra P., Patial M., Gupta S., Mukherjee A., Chatterjee D., Takkar A., Mahajan R. Erythema nodosum, zoster duplex and pityriasis rosea as possible cutaneous adverse effects of Oxford–AstraZeneca COVID-19 vaccine: Report of three cases from India. J. Eur. Acad. Dermatol. Venereol. 2022;36:e16–e18. doi: 10.1111/jdv.17678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iwanaga J., Fukuoka H., Fukuoka N., Yutori H., Ibaragi S., Tubbs R.S. A narrative review and clinical anatomy of herpes zoster infection following COVID-19 vaccination. Clin. Anatom. 2022;35:45–51. doi: 10.1002/ca.23790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koh S., Kim H.N., Kim Y.S., Kim T.J. Varicella zoster virus reactivation in central and peripheral nervous systems following COVID-19 vaccination in an immunocompetent patient. J. Clin. Neurol. 2022;18:99–101. doi: 10.3988/jcn.2022.18.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei N., Kresch M., Elbogen E., Lebwohl M. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74–77. doi: 10.1016/j.jdcr.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poussaint T.Y., LaRovere K.L., Newburger J.W., Chou J., Nigrovic L.E., Novak T., Randolph A.G. Multisystem Inflammatory-like Syndrome in a Child Following COVID-19 mRNA Vaccination. Vaccines. 2022;10:43. doi: 10.3390/vaccines10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stuart A.S., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399:36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dolgin E. Omicron is supercharging the COVID vaccine booster debate. Nature. 2021;10 doi: 10.1038/d41586-021-03592-2. [DOI] [PubMed] [Google Scholar]
- 75.BIO S. Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process. Assessment. Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process. 2021. [(accessed on 28 February 2022)]. Available online: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_29June2021.pdf.
- 76.COVID-19 Vaccines WHO EUL issued|WHO—Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control) [(accessed on 28 February 2022)]. Available online: http://extranet.who.it/pqweb/
- 77.Elekhnawy E., Kamar A.A., Sonbol F. Present and future treatment strategies for coronavirus disease 2019. Fut. J. Pharm. Sci. 2021;7:1–9. doi: 10.1186/s43094-021-00238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peiris S., Mesa H., Aysola A., Manivel J., Toledo J., Borges-Sa M., Aldighieri S., Reveiz L. Pathological findings in organs and tissues of patients with COVID-19: A systematic review. PLoS ONE. 2021;16:e0250708. doi: 10.1371/journal.pone.0250708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cron R.Q. COVID-19 cytokine storm: Targeting the appropriate cytokine. Lancet Rheumatol. 2021;3:e236–e237. doi: 10.1016/S2665-9913(21)00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tarighi P., Eftekhari S., Chizari M., Sabernavaei M., Jafari D., Mirzabeigi P. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur. J. Pharmacol. 2021;895:173890. doi: 10.1016/j.ejphar.2021.173890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubin D., Chan-Tack K., Farley J., Sherwat A. FDA Approval of remdesivir—A Step in the Right Direction. N. Engl. J. Med. 2020;383:2598–2600. doi: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- 82.Clerc E.D. Remdesivir: Quo vadis? Biochem. Pharmacol. 2021;193:114800. doi: 10.1016/j.bcp.2021.114800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perez J., Roustit M., Lepelley M., Revol B., Cracowski J.L., Khouri C. Reported adverse drug reactions associated with the use of hydroxychloroquine and chloroquine during the COVID-19 pandemic. Ann. Intern. Med. 2021;74:878–880. doi: 10.7326/M20-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fuzimoto A.D. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J. Integr. Med. 2021;19:375–388. doi: 10.1016/j.joim.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao R., Hu H., Li Y., Wang X., Xu M., Liu J., Zhang H., Yan Y., Zhao L., Li W., et al. Anti-SARS-CoV-2 Potential of Artemisinins in vitro. ACS Infect. Dis. 2020;6:2524–2531. doi: 10.1021/acsinfecdis.0c00522. [DOI] [PubMed] [Google Scholar]
- 88.Singh A.K., Singh A., Singh R., Misra A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:102329. doi: 10.1016/j.dsx.2021.102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heskin J., Pallett S.J., Mughal N., Davies G.W., Moore L.S., Rayment M., Jones R. Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management. Lancet. 2022;399:21–22. doi: 10.1016/S0140-6736(21)02657-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.U.S. Food and Drug Administration. [(accessed on 28 February 2022)]; Available online: https://www.fda.gov/media/138945/download.
- 91.Echeverria-Esnal D., Martin-Ontiyuelo C., Navarrete-Rouco M.E., De-Antonio Cusco M., Ferrandez O., Horcajada J.P., Grau S. Azithromycin in the treatment of COVID-19: A review. Expert Rev. Anti-Infect. 2021;19:147–163. doi: 10.1080/14787210.2020.1813024. [DOI] [PubMed] [Google Scholar]
- 92.Chorin E., Dai M., Shulman E., Wadhwani L., Bar-Cohen R., Barbhayia C., Aizer A., Holmes D., Bernstein S., Spinelli M., et al. The QT interval in patients with SARS-CoV-2 infection treated with hydroxychloroquine/azithromycin. Nat. Med. 2020;26:808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 93.Montnach J., Baró I., Charpentier F., De Waard M., Loussouarn G. Modelling sudden cardiac death risks factors in patients with coronavirus disease of 2019: The hydroxychloroquine and azithromycin case. Europace. 2021;23:1124–1136. doi: 10.1093/europace/euab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Pública. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iacopetta D., Carocci A., Sinicropi M.S., Catalano A., Lentini G., Ceramella J., Curcio R., Caroleo M.C. Old drug scaffold, new activity: Thalidomide-correlated compounds exert different effects on breast cancer cell growth and progression. ChemMedChem. 2017;12:381–389. doi: 10.1002/cmdc.201600629. [DOI] [PubMed] [Google Scholar]
- 96.Zeinalian M., Salari-Jazi A., Jannesari A., Khanahmad H. A potential protective role of losartan against coronavirus-induced lung damage. Infect. Control Hosp. Epidemiol. 2020;41:752–753. doi: 10.1017/ice.2020.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tolouian R., Vahed S.Z., Ghiyasvand S., Tolouian A., Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J. Ren. Inj. Prev. 2020;9:e19. doi: 10.34172/jrip.2020.19. [DOI] [Google Scholar]
- 98.Chen H., Xie J., Su N., Wang J., Sun Q., Li S., Jin J., Zhou J., Mo M., Wei Y., et al. Corticosteroid therapy is associated with improved outcome in critically ill patients with COVID-19 with hyperinflammatory phenotype. Chest. 2021;159:1793–1802. doi: 10.1016/j.chest.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ranjbar K., Moghadami M., Mirahmadizadeh A., Fallahi M.J., Khaloo V., Shahriarirad R., Erfani A., Khodamoradi Z., Saadi M.H.G. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial. BMC Infect. Dis. 2021;21:337. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mukherjee P.K., Efferth T., Das B., Kar A., Ghosh S., Singha S., Debnath P., Sharma N., Bhardwaj P., Haldar P.K. Role of medicinal plants in inhibiting SARS-CoV-2 and in the management of post-COVID-19 complications. Phytomedicine. 2022;98:153930. doi: 10.1016/j.phymed.2022.153930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lordan R., Rando H.M., Consortium C.-R., Greene C.S. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. Msystems. 2021;6:e00122-21. doi: 10.1128/mSystems.00122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baktash V., Hosack T., Patel N., Shah S., Kandiah P., Abbeele K.V.D., Mandal A.K.J., Missouris C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2021;97:442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Panfili F.M., Roversi M., D’Argenio P., Rossi P., Cappa M., Fintini D. Possible role of vitamin D in COVID-19 infection in pediatric population. J. Endocrinol. Investig. 2020;44:27–35. doi: 10.1007/s40618-020-01327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J., Rao X., Li Y., Zhu Y., Liu F., Guo G., Luo G., Meng Z., De Backer D., Xiang H., et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensiv. Care. 2021;11:5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.JamaliMoghadamSiahkali S., Zarezade B., Koolaji S., SeyedAlinaghi S., Zendehdel A., Tabarestani M., Moghadam E.S., Abbasian L., Manshadi S.A.D., Salehi M. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial. Eur. J. Med. Res. 2021;26:20. doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mhatre S., Srivastava T., Naik S., Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine. 2021;85:153286. doi: 10.1016/j.phymed.2020.153286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Derosa G., Maffioli P., D’Angelo A., Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19) Phytother. Res. 2021;35:1230–1236. doi: 10.1002/ptr.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdul-Hammed M., Adedotun I.O., Olajide M., Irabor C.O., Afolabi T.I., Gbadebo I.O., Rhymand L., Ramasami P. Virtual screening, ADMET profiling, PASS prediction, and bioactivity studies of potential inhibitory roles of alkaloids, phytosterols, and flavonoids against COVID-19 main protease (Mpro) Nat. Prod. Res. 2021:1–7. doi: 10.1080/14786419.2021.1935933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savant S., Srinivasan S., Kruthiventi A.K. Potential Nutraceuticals for COVID-19. Nutr. Diet. Suppl. 2021;13:25. doi: 10.2147/NDS.S294231. [DOI] [Google Scholar]
- 110.Pastor N., Collado M.C., Manzoni P. Phytonutrient and nutraceutical action against COVID-19: Current review of characteristics and benefits. Nutrients. 2021;13:464. doi: 10.3390/nu13020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abian O., Ortega-Alarcon D., Jimenez-Alesanco A., Ceballos-Laita L., Vega S., Reyburn H.T., Rizzuti B., Velázquez-Campoy A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020;164:1693–1703. doi: 10.1016/j.ijbiomac.2020.07.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vahedian-Azimi A., Abbasifard M., Rahimi-Bashar F., Guest P.C., Majeed M., Mohammadi A., Banach M., Jamialahmadi T., Sahebkar A. Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: A systematic review of clinical trials. Nutrients. 2022;14:256. doi: 10.3390/nu14020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Serlahwaty D., Giovani C. IAI CONFERENCE: In silico screening of mint leaves compound (Mentha piperita L.) as a potential inhibitor of SARS-CoV-2. Pharm. Educat. 2021;21:81–86. doi: 10.46542/pe.2021.212.8186. [DOI] [Google Scholar]
- 114.Rosato A., Carocci A., Catalano A., Clodoveo M.L., Franchini C., Corbo F., Carbonara G.G., Carrieri A., Fracchiolla G. Elucidation of the synergistic action of Mentha piperita essential oil with common antimicrobials. PLoS ONE. 2018;13:e0200902. doi: 10.1371/journal.pone.0200902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yousefi M., Sadriirani M., PourMahmoudi A., Mahmoodi S., Samimi B., Hosseinikia M., Saeedinezhad Z., Panahande S.B. Effects of pomegranate juice (Punica Granatum) on inflammatory biomarkers and complete blood count in patients with COVID-19: A structured summary of a study protocol for a randomized clinical trial. Trials. 2021;22:246. doi: 10.1186/s13063-021-05194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fazio A., Iacopetta D., La Torre C., Ceramella J., Muia N., Catalano A., Carocci A., Sinicropi M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate Akko peel extracts and β-glucan recovery. Food Funct. 2018;9:6618–6631. doi: 10.1039/C8FO01394B. [DOI] [PubMed] [Google Scholar]
- 117.Singh K., Rao A. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021;87:1–12. doi: 10.1016/j.nutres.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vlachou M., Siamidi A., Dedeloudi A., Konstantinidou S.K., Papanastasiou I.P. Pineal hormone melatonin as an adjuvant treatment for COVID-19. Int. J. Mol. Med. 2021;47:1. doi: 10.3892/ijmm.2021.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Camp O.G., Bai D., Gonullu D.C., Nayak N., Abu-Soud H.M. Melatonin interferes with COVID-19 at several distinct ROS-related steps. J. Inorg. Biochem. 2021;223:111546. doi: 10.1016/j.jinorgbio.2021.111546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khoramipour K., Basereh A., Hekmatikar A.A., Castell L., Ruhee R.T., Suzuki K. Physical activity and nutrition guidelines to help with the fight against COVID-19. J. Sports Sci. 2021;39:101–107. doi: 10.1080/02640414.2020.1807089. [DOI] [PubMed] [Google Scholar]
- 121.Ransing R., da Costa M.P., Adiukwu F., Grandinetti P., Teixeira A.L.S., Kilic O., Soler-Vidal J., Ramalho R. Yoga for COVID-19 and natural disaster related mental health issues: Challenges and perspectives. Asian J. Psych. 2020;53:102386. doi: 10.1016/j.ajp.2020.102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leustean L., Preda C., Teodoriu L., Mihalache L., Arhire L., Ungureanu M.C. Role of irisin in endocrine and metabolic disorders—Possible new therapeutic agent? Appl. Sci. 2021;11:5579. doi: 10.3390/app11125579. [DOI] [Google Scholar]
- 123.Catalano A. COVID-19: Could irisin become the handyman myokine of the 21st century. Coronaviruses. 2020;1:32–41. doi: 10.2174/2666796701999200617154655. [DOI] [Google Scholar]
- 124.De Oliveira M., De Sibio M.T., Mathias L.S., Rodrigues B.M., Sakalem M.E., Nogueira C.R. Irisin modulates genes associated with severe coronavirus disease (COVID-19) outcome in human subcutaneous adipocytes cell culture. Mol. Cell. Endocrinol. 2020;515:110917. doi: 10.1016/j.mce.2020.110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Loera D. Biomedical Innovations to Combat COVID-19. Academic Press; Cambridge, MA, USA: 2022. The role of traditional medicine in the fight against SARS-CoV-2; pp. 339–385. [Google Scholar]
- 126.Dotolo S., Marabotti A., Facchiano A., Tagliaferri R. A review on drug repurposing applicable to COVID-19. Brief. Bioinform. 2020;22:726–741. doi: 10.1093/bib/bbaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharma D., Kunamneni A. Recent progress in the repurposing of drugs/molecules for the management of COVID-19. Exp. Rev. Anti-Infect. Ther. 2021;19:889–897. doi: 10.1080/14787210.2021.1860020. [DOI] [PubMed] [Google Scholar]
- 128.Asrani P., Tiwari K., Eapen M.S., McAlinden K.D., Haug G., Johansen M.D., Hansbro P.M., Flanagan K.N., Hassan M.I., Sohal S.S. Clinical features and mechanistic insights into drug repurposing for combating COVID-19. Int. J. Biochem. Cell Biol. 2022;142:106114. doi: 10.1016/j.biocel.2021.106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao K., Nguyen D.D., Chen J., Wang R., Wei G.W. Repositioning of 8565 existing drugs for COVID-19. J. Phys. Chem. Lett. 2020;11:5373–5382. doi: 10.1021/acs.jpclett.0c01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nejadi Babadaei M.M., Hasan A., Vahdani Y., Haj Bloukh S., Sharifi M., Kachooei E., Haghighat S., Falahati M. Development of remdesivir repositioning as a nucleotide analog against COVID-19 RNA dependent RNA polymerase. J. Biomol. Struct. Dyn. 2021;39:3771–3779. doi: 10.1080/07391102.2020.1767210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bibi N., Farid A., Gul S., Ali J., Amin F., Kalthiya U., Hupp T. Drug repositioning against COVID-19: A first line treatment. J. Biomol. Struct. Dyn. 2021:1–15. doi: 10.1080/07391102.2021.1977698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ge Y., Tian T., Huang S., Wan F., Li J., Li S., Wang X., Yang H., Hong L., Wu N., et al. An integrative drug repositioning framework discovered a potential therapeutic agent targeting COVID-19. Signal. Transduct. Target. Ther. 2021;6:165. doi: 10.1038/s41392-021-00568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Serafin M.B., Bottega A., Foletto V.S., da Rosa T.F., Horner A., Horner R. Drug repositioning is an alternative for the treatment of coronavirus COVID-19. Int. J. Antimicrob. Agents. 2020;55:105969. doi: 10.1016/j.ijantimicag.2020.105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Low Z.Y., Yip A.J.W., Lal S.K. Repositioning Ivermectin for COVID-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication. Biochim. Biophys. Acta. 2022;1868:166294. doi: 10.1016/j.bbadis.2021.166294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Catalano A., Iacopetta D., Pellegrino M., Aquaro S., Franchini C., Sinicropi M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics. 2021;10:92. doi: 10.3390/antibiotics10010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Catalano A., Iacopetta D., Sinicropi M.S., Franchini C. Diarylureas as antitumor agents. Appl. Sci. 2021;11:374. doi: 10.3390/app11010374. [DOI] [Google Scholar]
- 137.Iacopetta D., Ceramella J., Catalano A., Saturnino C., Bonomo M.G., Franchini C., Sinicropi M.S. Schiff bases: Interesting scaffolds with promising antitumoral properties. Appl. Sci. 2021;11:1877. doi: 10.3390/app11041877. [DOI] [Google Scholar]
- 138.Mansour M.A., AboulMagd A.M., Abdel-Rahman H.M. Quinazoline-Schiff base conjugates: In silico study and ADMET predictions as multi-target inhibitors of coronavirus (SARS-CoV-2) proteins. RSC Adv. 2020;10:34033–34045. doi: 10.1039/D0RA06424F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ceramella J., Iacopetta D., Catalano A., Cirillo F., Lappano R., Sinicropi M.S. A review on the antimicrobial activity of schiff bases: Data collection and recent studies. Antibiotics. 2022;11:191. doi: 10.3390/antibiotics11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.El-Gammal O.A., El-Bindary A.A., Mohamed F.S., Rezk G.N., El-Bindary M.A. Synthesis, characterization, design, molecular docking, anti COVID-19 activity, DFT calculations of novel Schiff base with some transition metal complexes. J. Mol. Liq. 2021;346:117850. doi: 10.1016/j.molliq.2021.117850. [DOI] [Google Scholar]
- 141.Catalano A., Sinicropi M.S., Iacopetta D., Ceramella J., Mariconda A., Rosano C., Scali E., Saturnino C., Longo P. A review on the advancements in the field of metal complexes with Schiff bases as antiproliferative agents. Appl. Sci. 2021;11:6027. doi: 10.3390/app11136027. [DOI] [Google Scholar]
- 142.Goris T., Perez-Valero A., Martınez I., Dong Y., Fernandez-Calleja L., San Leon D., Bornscheuer U.T., Magadan-Corpas P., Lombo F., Nogales J. Repositioning microbial biotechnology against COVID-19: The case of microbial production of flavonoids. Microb. Biotechnol. 2021;14:94–110. doi: 10.1111/1751-7915.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Szendrey M., Guo J., Li W., Yang T., Zhang S. COVID-19 drugs chloroquine and hydroxychloroquine, but not azithromycin and remdesivir, block hERG potassium channels. J. Pharmacol. Exp. Ther. 2021;377:265–272. doi: 10.1124/jpet.120.000484. [DOI] [PubMed] [Google Scholar]
- 144.Bruno C., Carocci A., Catalano A., Cavalluzzi M.M., Corbo F., Franchini C., Lentini G., Tortorella V. Facile, alternative route to lubeluzole, its enantiomer, and the racemate. Chirality. 2006;18:227–231. doi: 10.1002/chir.20240. [DOI] [PubMed] [Google Scholar]
- 145.Gualdani R., Cavalluzzi M.M., Tadini-Buoninsegni F., Convertino M., Gailly P., Stary-Weinzinger A., Lentini G. Molecular insights into hERG potassium channel blockade by lubeluzole. Cell. Physiol. Biochem. 2018;45:2233–2245. doi: 10.1159/000488169. [DOI] [PubMed] [Google Scholar]
- 146.Alonzi T., Aiello A., Petrone L., Najafi Fard S., D’eletto M., Falasca L., Nardacci R., Rossin F., Delogu C., Castilletti C., et al. Cysteamine with in vitro antiviral activity and immunomodulatory effects has the potential to be a repurposing drug candidate for COVID-19 therapy. Cells. 2022;11:52. doi: 10.3390/cells11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vangeel L., De Jonghe S., Maes P., Slechten B., Raymenants J., André E., Neyts J., Jochmans D. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 2022;198:105252. doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rai D.K., Yurgelonis I., McMonagle P., Rothan H.A., Hao L., Gribenko A., Titova E., Kreiswirth B., White K.M., Zhu Y., et al. Nirmatrelvir, an orally active Mpro inhibitor, is a potent inhibitor of SARS-CoV-2 Variants of Concern. bioRxiv. 2022 doi: 10.1101/2022.01.17.476644. [DOI] [Google Scholar]
- 149.Rosales R., McGovern B.L., Rodriquez M.L., Rai D.K., Cardin R.D., Anderson A.S., PSP Study Group. Sordillo E.M., van Bakel H., Simon V., et al. Nirmatrelvir, molnupiravir, and remdesivir maintain potent in vitro activity against the SARS-CoV-2 Omicron variant. bioRxiv. 2022 doi: 10.1101/2022.01.17.476685. [DOI] [Google Scholar]
- 150.Yadav P.D., Gupta N., Potdar V., Mohandas S., Sahay R.R., Sarkale P., Shete A.M., Razdan A., Patil D.Y., Nyayanit D.A., et al. An in vitro and in vivo approach for the isolation of Omicron variant from human clinical specimens. bioRxiv. 2022 doi: 10.1101/2022.01.02.474750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Touret F., Baronti C., Bouzidi H.S., de Lamballerie X. In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron, B. 1.1. 529 isolate. bioRxiv. 2022 doi: 10.1101/2022.01.01.474639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liesenborghs L., Spriet I., Jochmans D., Belmans A., Gyselinck I., Teuwen L.-A., ter Horst S., Dreesen E., Geukens T., Engelen M.M., et al. Itraconazole for COVID-19: Preclinical studies and a proof-of-concept randomized clinical trial. EBioMedicine. 2021;66:103288. doi: 10.1016/j.ebiom.2021.103288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frumkin L.R., Lucas M., Scribner C.L., Ortega-Heinly N., Rogers J., Yin G., Hallam T.J., Yam A., Bedard K., Begley R., et al. Egg-derived anti-SARS-CoV-2 immunoglobulin Y (IgY) with broad variant activity as intranasal prophylaxis against COVID-19: Preclinical studies and randomized controlled phase 1 clinical trial. medRxiv. 2022 doi: 10.1101/2022.01.07.22268914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Raghavan K., Dedeepiya V.D., Suryaprakash V., Rao K.S., Ikewaki N., Sonoda T., Levy G.A., Iwasaki M., Senthilkumar R., Preethy S., et al. Beneficial effects of novel Aureobasidium pullulans strains produced Beta-1,3-1,6 Glucans on Interleukin-6 and D-Dimer levels in COVID-19 patients; results of a randomized multiple-arm pilot clinical study. Biomed. Pharmacother. 2022;145:112243. doi: 10.1016/j.biopha.2021.112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Elamir Y.M., Amir H., Lim S., Rana Y.P., Lopez C.G., Feliciano N.V., Omar A., Grist W.P., Via M.A. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone. 2021;154:116175. doi: 10.1016/j.bone.2021.116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Buonfrate D., Chesini F., Martini D., Roncaglioni M.C., Fernandez M.L.O., Alvisi M.F., De Simone I., Rulli E., Nobili A., Casalini G., et al. High dose ivermectin for the early treatment of COVID-19 (COVER study): A randomised, double-blind, multicentre, phase II, dose-finding, proof of concept clinical trial. Int. J. Antimicrob. Agents. 2022;59:106516. doi: 10.1016/j.ijantimicag.2021.106516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.