Abstract
β-carotene is widely available in plant-based foods, while the efficacy of β-carotene supplementation on cardiovascular disease (CVD) risk remains controversial. Hence, we performed a systematic review and meta-analysis on randomized controlled trials to investigate the associations between β-carotene supplementation and CVD risk as well as mortality. We conducted literature searches across eight databases and screened the publications from January 1900 to March 2022 on the topic of β-carotene treatments and cardiovascular outcomes. There were 10 trials and 16 reports included in the meta-analysis with a total of 182,788 individuals enrolled in the study. Results from the random-effects models indicated that β-carotene supplementation slightly increased overall cardiovascular incidence (RR: 1.04; 95% CI: 1.00, 1.08) and was constantly associated with increased cardiovascular mortality (RR: 1.12; 95% CI: 1.04, 1.19). Subgroup analyses suggested that, when β-carotene treatments were given singly, a higher risk of cardiovascular outcomes was observed (RR: 1.06; 95% CI: 1.01, 1.12). In addition, cigarettes smoking was shown to be a risk behavior associated with increased cardiovascular incidence and mortality in the β-carotene intervention group. In sum, the evidence of this study demonstrated that β-carotene supplementation had no beneficial effects on CVD incidence and potential harmful effects on CVD mortality. Further studies on understanding the efficacy of multivitamin supplementation in nutrient-deficient or sub-optimal populations are important for developing the tolerable upper intake level for β-carotene of different age and sex groups.
Keywords: β-carotene supplements, myocardial infarction, stroke, cardiovascular incidence, cardiovascular mortality
1. Introduction
Cardiovascular disease (CVD) is a major public health concern worldwide currently. It is the leading cause of death, accounting for about one-third of global death and premature death in 2019 [1]. Nutrient intake is an important modifiable risk factor for CVD prevention. Previous meta-analyses have consistently shown that vegetarian diets are beneficial for decreasing the risk of CVD mortality, especially for coronary disease mortality [2,3]. A high consumption of vegetables, fruits, whole grains, with low consumption of red meat, processed meat, and sodium reduces the incidence of heart failure and cerebrovascular disease, such as stroke [4,5]. Similar dietary patterns such as the Mediterranean diet also demonstrate protective effects towards lower death rates among patients with a history of myocardial infarction [6].
There are multiple nutrients which are highly available in plants and fruits, including vitamin C, folate, flavonoids, and β-carotene, which have been carefully examined in a few meta-analyses regarding their roles in CVD prevention and control [7,8,9,10,11]. β-carotene is a provitamin A carotenoid with antioxidant properties and the highest vitamin A activity. Nevertheless, the bioavailability of natural β-carotene in plants is low [12]. Some factors impacting bioavailability include change of cell wall structure when processing foods and interaction with other dietary ingredients and phytochemicals in the gastrointestinal tract [13,14]. Hence, more attention has been given to β-carotene supplementation, which has become an alternative for people to meet the recommended intake of β-carotene.
However, several studies have shown that β-carotene was associated with an increased risk of all-cause mortality [7,8,9]. The United States Preventive Services Task Force (USPSTF) in 2013 indicated a null effect of β-carotene on CVD prevention but an increased risk for lung cancer; thus, it was not recommended to use β-carotene supplements for prevention or treatment [7]. However, thus far, most previous studies have examined the combined effects of β-carotene with other antioxidants, and there are limited meta-analyses thoroughly discussing the effects of β-carotene treatment on different CVD outcomes specifically.
Previous studies have indicated a potential harmful effect of β-carotene acting as a co-carcinogen in different age and ethnic groups, while the conclusions were ambiguous when it comes to the single effects of β-carotene on CVD prevention [8,15]. Therefore, the aim of this study is to summarize the evidence from previous randomized controlled trials (RCTs), to exclusively investigate the efficacy and safety of β-carotene supplementation on CVD incidence and mortality among adults.
2. Materials and Methods
2.1. Data Sources and Searches
We conducted a systematic review and meta-analysis of RCTs regarding the effects of β-carotene on CVD, which were published in English and Chinese from January 1900 to March 2022. We systematically searched 8 databases, including four English databases: PubMed, Web of Science, EmBase, and Cochrane Library and four Chinese databases: China National Knowledge Internet, Wanfang Database, China Science and Technology Journal Database, and Chinese Biological Medicine disc. Search strategy and terms for PubMed are shown in Table A1.
2.2. Inclusion Criteria
RCTs of β-carotene in CVD risk were eligible for the following inclusion criteria: (1) RCTs comparing the effect of β-carotene supplementation alone or multi-vitamins including β-carotene supplements with untreated controls or placebo groups; (2) trials providing at least one outcome as follows: major CVD, major coronary heart disease, myocardial infarction, stroke, peripheral arterial disease, revascularization, angina pectoris, or ischemic CVD; (3) studies providing incidence or mortality rates.
2.3. Exclusion Criteria
Exclusion criteria were: (1) randomized trials without a nontreatment or placebo group; (2) trials involved with β-carotene fortified foods rather than supplements; and (3) no dosage information on β-carotene supplements being provided.
2.4. Data Collection and Extraction
Two reviewers independently conducted the search, using EndNote 20 (20.0). After importing the initial search results from eight databases into the reference manager, duplicates were removed. Then, based on the eligible criteria, reviewers screened all titles and abstracts and excluded the irrelevant search. The full texts of the remaining search were retrieved and examined to determine the included trials. The discrepancies during this screening process were discreetly discussed by two independent reviewers until a consensus was reached.
The selected studies went through the data extraction process. The following eligible criteria were extracted: (1) article characteristics (title, first author’s last name or study group’s name, publication year, country of origin); (2) study design (study population, sample size, participant’s mean age, sex, and health status, follow-up duration); (3) specification of intervention group (β-carotene dosage, antioxidant supplements dosage, mineral supplements dosage, treatment regimen); (4) specification of control group (placebo or no intervention); (5) health outcome (incidence, mortality, risk ratio, confidence interval). All variables were independently retrieved, and the disagreements were settled by the consensus of two reviewers.
2.5. Quality Assessment
The quality of each included trial was assessed using the Cochrane Collaboration risk of bias tool with Review Manager (5.4.1). Bias was addressed from (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and investigators, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting and (7) other bias. The risk of bias was categorized as “low risk of bias”, “unclear risk of bias”, or “high risk of bias”.
2.6. Grading of the Evidence
Based on the levels of risk of bias, the corresponding Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool was used to rate the overall quality of evidence based on the outcomes. Since RCTs provide high-quality evidence, the quality might have been downgraded when study limitations, inconsistencies, indirectness, imprecision, and publication bias were detected. GRADE assessments were performed by two researchers independently. Uncertainties were discussed during the review stage until the agreement had been met. The results of the GRADE assessments are presented in Table A2.
2.7. Statistical Analysis
The statistical analysis was performed by using R Studio (1.4.1717, Boston, MA, United States) and Review Manager (5.4.1, London, United Kingdom). Risk ratio (RR) and its associated 95% confidence interval (CI) were used to assess the comparison between outcomes reported by the studies. Random-effects model was used to present the data. A p value less than 0.05 was regarded as statistically significant. The I2 test determined the heterogeneity of results between RCTs. Low heterogeneity referred to an I2 between 25% and 50%, moderate heterogeneity referred to an I2 between 50% and 75%, and an I2 ≥ 75% was considered as high heterogeneous. When high heterogeneity was present, a sensitivity analysis excluding the ones with heterogeneity would be performed. The exclusion of studies was based on the baseline characteristics of participants. Finally, we generated sensitivity analyses on the following outcomes: stroke incidence and other-cause mortality. In addition, subgroup analyses were conducted based on β-carotene treatment regimen, sex, health status, and smoking behavior. Besides, we used funnel plots (Review Manager 5.4.1) and Egger’s test (R Studio 1.4.1717) to examine the potential publication bias. When there were 10 trials included in a meta-analysis, publication bias analyses were conducted.
3. Results
3.1. Study Identification
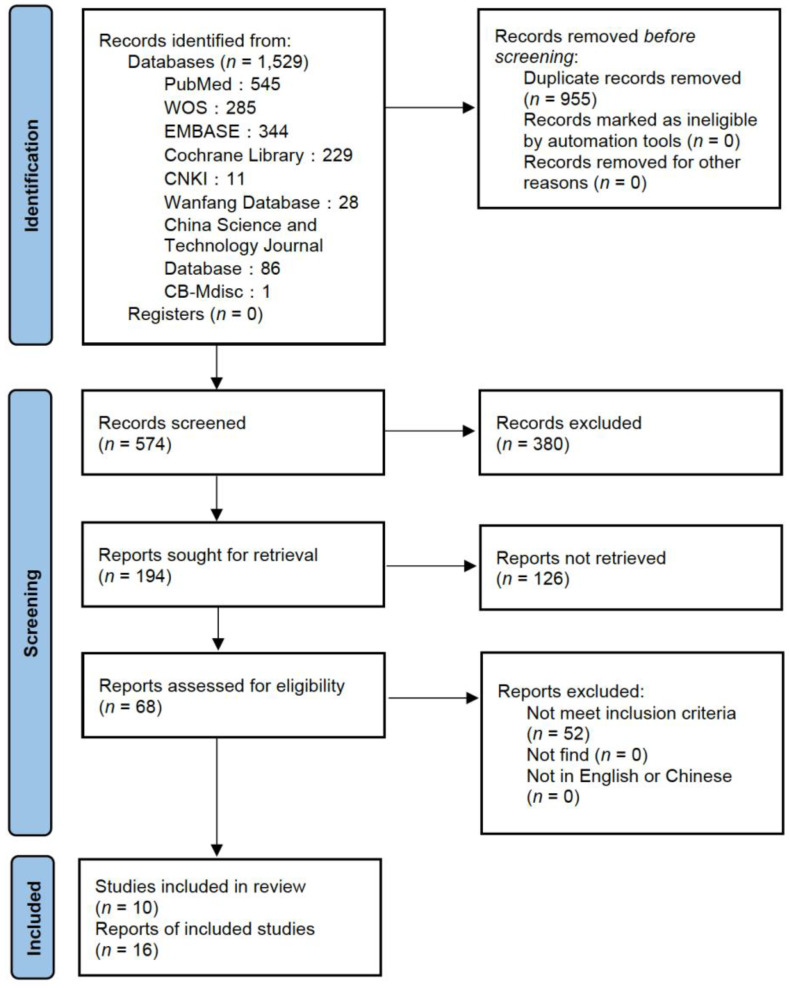
This meta-analysis of RCTs included 182,788 individuals in 16 papers with a broad range of baseline characteristics. A flow diagram is presented in Figure 1. The literature search in eight databases yielded 1529 publications in total. After removing the duplicates, 955 publications were excluded. There were 380 publications excluded due to irrelevant titles, and 126 publications were excluded after reviewing the abstracts. A total of 68 publications were retrieved and examined in full text. There were 52 publications which did not meet the inclusion criteria and were excluded. Finally, 16 publications reported on 10 unique studies were included in this systematic review, and the number of participants in the studies ranged from 366 to 39,876.
Figure 1.
PRISMA Flow Diagram of the literature review.
3.2. Study Characteristics
In 10 studies (16 publications), four took place in the United States, two were conducted in the United Kingdom, and the remaining four were conducted in China, France, Finland, and Italy, accordingly. All studies were published between 1996 and 2010. Regarding the study population, the age ranged from 27 to 84 years old. There were two studies that exclusively investigated the male population, and two studies that studied the female population solely; four studies enrolled health participants without a history of cancer (except nonmelanoma skin cancer) or CVD; two out of ten studies investigated the smoking population exclusively; four studies evaluated the combination effects of β-carotene and antioxidant vitamins (vitamin C, vitamin E) and minerals (selenium, zinc) compared with the placebo. The characteristics of the enrolled papers are listed in Table 1.
Table 1.
Characteristics of enrolled studies.
| First Author | Study | Country of Origin |
Sample Size | Population | Mean Age, y | Health Status | Treatment Regimen | Health Outcome | Follow-Up, y |
|---|---|---|---|---|---|---|---|---|---|
| Leppälä et al. (2000) [16] | Alpha Tocopherol, Beta Carotene Cancer Prevention (ATBC) Study | Finland | 28,519 | Current male smokers (≥5 cigarettes/d) | 50–69 | Stroke-free at baseline | 50 mg/d of α-Tocopherol (Vitamin E), 20 mg/d of β-carotene, both, or placebo | Total stroke, subarachnoid hemorrhage, intracerebral hemorrhage, cerebral infraction | 6.0 |
| Kataja-Tuomola et al.(2010) [17] | ATBC Study | Finland | 1700 | Current male smokers (≥5 cigarettes/d) | 58 | With type 2 diabetes | As described above | Total macrovascular outcomes, major coronary event, total stroke, peripheral arterial disease, total mortality | 19 |
| Rapola et al. (1996) [18] | ATBC Study | Finland | 22,269 | Current male smokers (≥5 cigarettes/d) | 57 | Free of coronary heart disease | As described above | Angina pectoris | 4.7 |
| Rapola et al. (1998) [19] | ATBC Study | Finland | 1795 | Current male smokers (≥5 cigarettes/d) | 58.8 | With angina pectoris at baseline, smoked 20 cigarettes a day | As described above | Recurrences of angina pectoris, major coronary events | 4–5.5 |
| Rapola et al. (1997) [20] | ATBC Study | Finland | 1862 | Current male smokers (≥5 cigarettes/d) | 59–60 | With previous myocardial infarction | As described above | Major coronary events, non-fatal myocardial infarction | 5.3 |
| Virtamo et al. (2003) [21] | ATBC Study | Finland | 25,563 | Current male smokers (≥5 cigarettes/d) | 63.5 | Participants who were still alive by 30 April 1993 | As described above | Cancer incidence, cause-specific mortality, total mortality | 6–8 |
| Lee et al. (1999) [22] | Women’s Health Study | United States | 39,876 | Female health professionals | ≥45 | Apparently healthy | Given on alternate days, 100 mg of aspirin, or 600 IU of vitamin E, or 50 mg of β-carotene | Myocardial infarction, stroke, death from cardiovascular causes, all-cause death | 4.1 |
| Cook et al. (2007) [23] | Women’s Antioxidant Cardiovascular Study |
United States | 8171 | Female health professionals | ≥40 | With a history of CVD or ≥3 CVD risk factors | Vitamin C (500 mg/d), vitamin E (600 IU every other day), β-carotene (50 mg every other day), or placebo | Myocardial infarction, coronary revascularization procedures, coronary heart disease, stroke, transient ischemic attack, CVD death | 9.4 |
| Hercberg et al. (2004) [24] | SU.VI.MAX Study | France | 13,017 | French adult volunteers | 49 | Free from diseases | A daily combined capsule of 120 mg of ascorbic acid, 30 mg of vitamin E, 6 mg of β-carotene, 100 g of selenium, and 20 mg of zinc, or a placebo | Cancer incidence, ischemic cardiovascular disease, mortality | 7.5 |
| Catalano et al. (2007) [25] | Critical Leg Ischemia Prevention Study (CLIPS) Group | Italy | 366 | Outpatients | 66 | Peripheral arterial disease | Oral aspirin (100 mg/d); oral antioxidant vitamins (600 mg vitamin E, 250 mg vitamin C and 20 mg β-carotene daily); both or neither (placebo) | Major vascular event, critical limb ischemia | 2 |
| Collins et al. (2002) [26] | Heart Protection Study Collaborative Group | United Kingdom | 20,536 | Patients from 69 hospitals | 40–80 | Coronary disease, other occlusive arterial disease, or diabetes | Antioxidant vitamins (600 mg synthetic vitamin E, 250 mg vitamin C, and 20 mg β-carotene daily) or placebo | Major coronary event, major vascular event | 5 |
| Omenn et al. (1996) [27] | Beta-Carotene and Retinol Efficacy Trial (CARET) | United States | 18,314 | Smokers, former smokers, and workers exposed to asbestos | 57–58 | At least 15 years exposure to asbestos, asbestos-related lung disease, or 5-year work in high-risk trades | A combination of 30 mg of β-carotene per day and 25,000 IU of retinol (vitamin A) | CVD death, all-cause death, lung cancer death | 4 |
| Goodman et al. (2004) [28] | CARET | United States | 18,140 | As described above | 62 | Postintervention | As described above | CVD death, all-cause death, lung cancer death, other-cause death | 6 |
| Blot et al. (1993) [29] | Linxian Study | China | 29,584 | Residents in Linxian communes | 40–69 | No debilitating diseases or prior esophageal or stomach cancer | Retinol and zinc; riboflavin and niacin; vitamin C and molybdenum; β-carotene (15 mg), vitamin E (30 mg), and selenium (50 µg) | Cerebrovascular disease death, cancer death, total death | 5.25 |
| Hennekens et al. (1996) [30] | Physicians’ Health Study | United States | 22,071 | Male physicians | 40–84 | No history of cancer (except nonmelanoma skin cancer), myocardial infarction, stroke, or transient cerebral ischemia | Aspirin (325 mg on alternate days) plus β-carotene placebo, β-carotene (50 mg on alternate days) plus aspirin placebo, both active agents, or both placebos | Myocardial infarction, stroke, all important cardiovascular events, malignant neoplasm | 12 |
| Greenberg et al. (1996) [31] | Skin Cancer Prevention Study | United Kingdom | 1720 | Treated patients | 63.2 | At least one biopsy-proved basal cell or squamous cell skin cancer treated | 50 mg of β-carotene or placebo | CVD death, cancer death, all death | 8.2 |
3.3. Risk of Bias
In the 16 included articles, all of them indicated a low risk of selection bias for random-sequence generation. In total, 65–90% of them indicated a low risk of for allocation concealment (selection bias), blinding of outcome assessments (detection bias), selective reporting (reporting bias), and other bias. About half of the studies indicated a low risk of attrition bias for incomplete outcome data, while 45% of the studies showed a high risk for indicating losses to follow-up or treatment withdrawals. In addition, there were 65% of included articles that indicated an unknown risk of bias for blinding of participants and personnel, where the blinding status of them was not explicitly reported in the studies. The risk of bias is presented in Figure 2.
Figure 2.
Risk of bias of included articles.
3.4. Incidence
3.4.1. Cardiovascular Disease Incidence
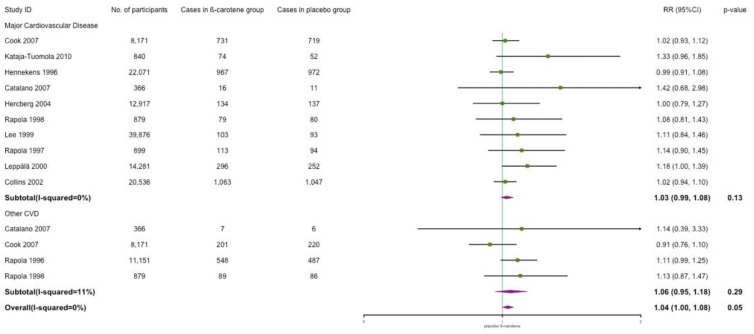
Major CVD includes myocardial infarction, stroke, peripheral arterial disease, ischemic CVD, and revascularization procedure. The results indicated no effect of β-carotene supplementation on major cardiovascular incidence when compared with placebo (RR: 1.03; 95% CI: 0.99, 1.08; p = 0.13; I2 = 0%). Other CVD includes angina pectoris, transient ischemic attack, and critical limb ischemia. No effects were observed in other CVD either (RR: 1.06; 95% CI: 0.95, 1.18; p = 0.29; I2 = 11%). However, overall, we observed a positive association between β-carotene supplementation and risk of CVD (RR: 1.04; 95% CI: 1.00, 1.08; p = 0.05; I2 = 0%). The results are shown in Figure 3.
Figure 3.
Association of β-carotene supplementation on CVD incidence.
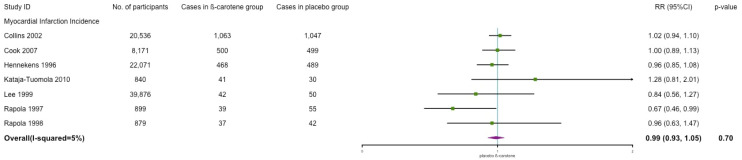
3.4.2. Myocardial Infarction Incidence
We categorized the cases of major coronary event and coronary heart disease into myocardial infarction. Similarly, no significant association between β-carotene and myocardial infarction incidence was observed (RR: 0.99; 95% CI: 0.93, 1.05; p = 0.70; I2 = 5%) (see Figure 4).
Figure 4.
Association of β-carotene supplementation on myocardial infarction incidence.
3.4.3. Stroke Incidence
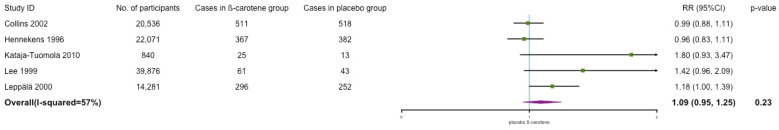
The incidence of stroke included cerebral infarction, intracerebral hemorrhage, and subarachnoid hemorrhage. As shown in Figure 5, the results indicated that β-carotene was associated with a significant increased risk of stroke with moderate to high heterogeneity (RR: 1.17; 95% CI: 1.00, 1.37; p = 0.05; I2 = 72%). After dropping one trial (Cook 2007) which studied female high-risk population, the significance disappeared, and the heterogeneity decreased significantly (RR: 1.09; 95% CI: 0.95, 1.25; p = 0.23; I2 = 57%) (the results are shown in Figure A1.
Figure 5.
Association of β-carotene supplementation on stroke incidence.
3.5. Mortality
Cardiovascular, All-Cause, and Other Mortality
β-carotene supplementation was associated with a significant increased risk of cardiovascular mortality (RR: 1.12; 95% CI: 1.04, 1.19; p = 0.002; I2 = 24%, Figure 6). Besides cardiovascular death, other causes included lung cancer, other cancer, malignant neoplasm, respiratory diseases, and the unknown. The results showed that, compared with placebo, the effect of β-carotene indicated a risk increment for all-cause mortality with low to moderate heterogeneity (RR: 1.08; 95% CI: 1.00, 1.16; p = 0.04; I2 = 48%, Figure 6). However, no effect was observed in other-cause mortality (RR: 1.23; 95% CI: 0.98, 1.53; p = 0.07; I2 = 84%, Figure 6). After removing the study (Hennekens 1996) focused on healthy individuals only, a significant risk increment was observed in the smoking population (RR: 1.36; 95% CI: 1.16, 1.59; p = 0.0001; I2 = 50%, Figure A2). In sum, a potential harmful effect of β-carotene was observed in overall mortality.
Figure 6.
Association of β-carotene supplementation on mortality.
3.6. Subgroup and Sensitivity Analyses
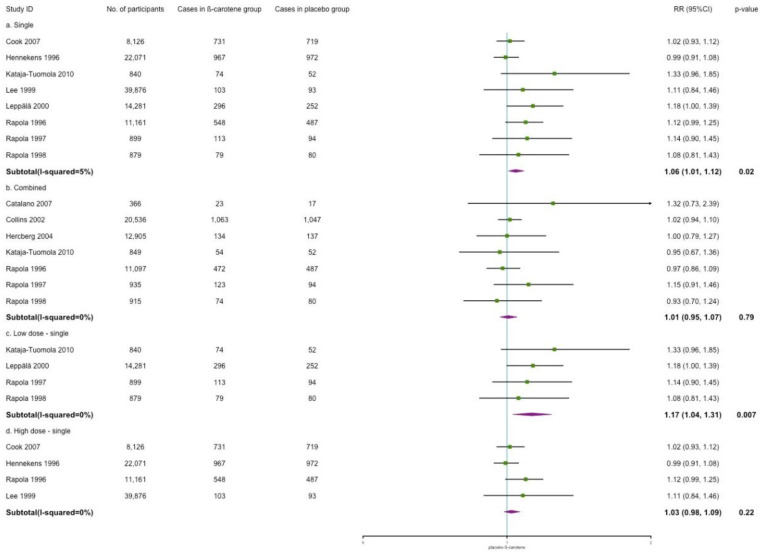
Figure 7 shows the subgroup analyses of the effects of different β-carotene treatment regimens on cardiovascular incidence. When considering the distinguishing effects of other antioxidant vitamins or minerals, subgroup analyses indicated that β-carotene as the only treatment was significantly associated with a higher risk of cardiovascular incidence (RR: 1.06; 95% CI: 1.01, 1.12; p = 0.02; I2 = 5%). However, no significant trend was identified with combined treatment of β-carotene and antioxidant vitamins or minerals (RR: 1.01; 95% CI: 0.95, 1.07; p = 0.79; I2 = 0%). Regarding the dose of β-carotene, we categorized supplements ≤30 mg as low dose and >30 mg as high dose. Low dose of single β-carotene treatment increased cardiovascular incidence (RR: 1.17; 95% CI: 1.04, 1.31; p = 0.007; I2 = 0%). However, no effect was shown in the high-dose group.
Figure 7.
Subgroup analysis for the efficacy of single/combined/dose of β-carotene on cardiovascular incidence.
In Figure 8, the effects of the different β-carotene treatment regimens on cardiovascular mortality are shown. We noted that the effect of single β-carotene supplementation on cardiovascular mortality showed a risk increment as well (RR: 1.10; 95% CI: 1.02, 1.19; p = 0.01; I2 = 11%). No effect was observed in the combined treatment group. In addition, different dosages did not have an impact on cardiovascular mortality either.
Figure 8.
Subgroup analysis for the efficacy of single/combined/dose of β-carotene on cardiovascular mortality.
There were two trials exclusively focused on male individuals, and two trials were solely conducted in female populations. In Figure A3, the subgroup analyses suggest a higher incidence of CVD in the male population (RR: 1.09; 95% CI: 1.01, 1.17; p = 0.03; I2 = 24%), while no effect is shown in the female group (RR: 1.03; 95% CI: 0.94, 1.12; p = 0.57; I2 = 0%). We observed no effects of β-carotene on cardiovascular mortality in male or female groups (Figure A4).
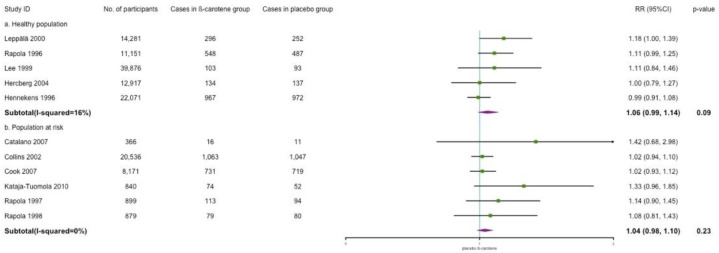
We defined healthy individuals as people without CVD histories, CVD risk factors, or current diseases. At-risk populations were defined as the opposite. Subgroup analyses suggested that, in both healthy and at-risk cohorts, there were no associations between β-carotene and cardiovascular incidence. Results are shown in Figure A5. Moreover, we noted no effect of β-carotene on cardiovascular mortality among healthy populations (RR: 1.05; 95% CI: 0.92, 1.19; p = 0.47; I2 = 0%) but a significant risk effect of β-carotene in at-risk populations (RR: 1.15; 95% CI: 1.05, 1.26; p = 0.003; I2 = 45%), as shown in Figure A6.
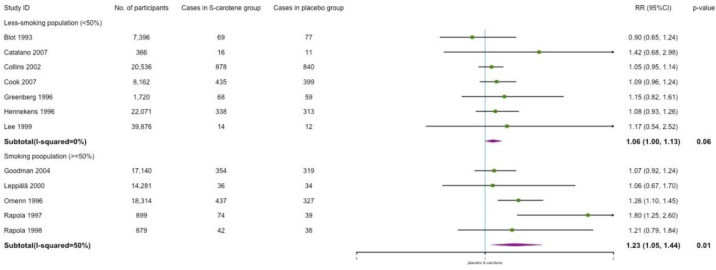
Concerning the modification effects of smoking behavior, we set the smoking rate of 50% at baseline as the threshold to distinguish the smoking status of different populations. In the less-smoking population (smoking rate < 50%), the data indicated no effects of β-carotene supplementation on cardiovascular incidence (RR: 1.01; 95% CI: 0.97, 1.06; p = 0.60; I2 = 0%), while a significant higher risk was shown in the smoking population (smoking rate ≥ 50%) (RR: 1.14; 95% CI: 1.05, 1.24; p = 0.001; I2 = 0%) (Figure A7). In terms of effects on cardiovascular mortality, we observed a borderline-significant risk in the less-smoking group (RR: 1.06; 95% CI: 1.00, 1.13; p = 0.06; I2 = 0%) and a positive association with β-carotene in the smoking group (RR: 1.23; 95% CI: 1.05, 1.44; p = 0.01; I2 = 50%) (Figure A8).
3.7. Publication Bias
Funnel plots for the outcomes of major cardiovascular incidence, overall CVD incidence, CVD mortality, and overall mortality are presented as Figures S1–S4. Egger’s linear regression test showed no evidence of publication bias for the outcomes of overall CVD incidence (p = 0.0506), CVD mortality (p = 0.4998), and overall mortality (p = 0.4937), while it did indicate some evidence of publication bias for major CVD incidence (p = 0.0071). A trim-and-fill analysis was conducted to correct the funnel plot asymmetry arising from the publication bias. There were studies being added, and the trend did not change (RR = 1.01; 95% CI: 0.97, 1.06; p = 0.54; I2 = 20%). The trim-and-fill funnel plot is shown in Figure S5.
4. Discussion
This review involved 16 reports and 182,788 individuals from 10 unique trials. Participants were all adults with different baseline characteristics. Overall, the evidence of this study suggested that β-carotene had no beneficial effects on the CVD incidence, and a risk increment was observed for CVD mortality. The subgroup analyses showed population heterogeneity, while β-carotene might be a risk factor in the smoking population and high-risk group.
4.1. Effects of β-Carotene on CVD Incidence
We first observed that β-carotene showed a 4% increased risk on overall CVD incidence, and we noted a 17% risk increment of β-carotene supplements for total stroke among adults compared with the placebo or controlled group. However, no effects were shown for major CVD events, other CVD, or myocardial infarction separately, which were in accordance with previous meta-analyses [7,9,10,11]. We also observed a 9% increased risk of CVD in the male population, while no effect was shown among female individuals; thus, we speculated that gender might play a role in β-carotene’s efficacy on cardiovascular incidence. The ATBC study found an increased risk of total stroke among male smokers and intracerebral hemorrhage among male heavy drinkers [16,17]. However, in the Women’s Health Study, no significant benefit or harm on stroke was observed among smokers (13% of female population at the baseline) [22]. Still, there was a possibility of random findings because of the small sample size. In addition, the gender differences may also be caused by the different health behaviors between men and women. Both cigarette smoking and heavy alcohol intake are established risk factors for stroke. Tobacco use facilitates the development of free radicals and atherosclerotic process [32]. It also increases the stroke risk by decreasing cerebral blood flow [32]. Studies also showed that, under certain conditions such as high oxygen concentration, β-carotene switched to a pro-oxidant effect [33,34,35]. This pro-oxidant mechanism generates the β-carotene radical cation, which requires vitamin C to repair. However, due to the low serum level of vitamin C in smokers, the β-carotene radical may lead to an increased risk of cardiovascular disease [34]. Heavy alcohol consumption (more than 3 to 4 drinks per day) causes harmful physiological responses and is associated with higher cardiovascular risk, which is apparent in both men and women [33]. Since smoking and drinking rates among male individuals are generally higher than female, these differences in proportion may lead to the discrepancy of β-carotene’s efficacy on stroke or CVD incidence in sex groups. In general, we observed a 14% risk increment of CVD among smoking populations.
Results also indicated an increased risk of CVD in the subgroups of low-dose and single treatment of β-carotene. This result conflicts with previous meta-analyses which showed null effects [9]. However, this finding can be explained by previous literature. A previous study manifested that, besides the oxygen tension, the β-carotene concentration and interactions with other antioxidants also influenced the pro-oxidant effect of β-carotene [35]. Animal studies suggested that excess dietary intake of β-carotene facilitated the peroxidation in vivo, especially in an α-tocopherol-deficient diet since the presence of other antioxidants in the body might attenuate the pro-oxidant effect of β-carotene [35,36,37]. Our results were consistent with these findings that we only observed increased CVD risk in β-carotene given-singly group. We also observed increased risk in the low-dose but not in the high-dose group. Since the trials we analyzed did not collect information on nutrient status or dietary intake at baseline of populations, it is possible that these associations could be random findings. Overall, our analyses failed to detect any protective effects of β-carotene against CVD incidence in any subgroups.
In nutrient-deficient populations, the effect of β-carotene supplementation is unclear. A study conducted in rural Nepal indicated beneficial effects of maternal β-carotene supplementation on decreased risk of hypertension among their undernourished children with a high waist circumference, while no overall benefits on cardiovascular risk factors were observed [38]. To provide better recommended daily β-carotene intake in different populations, we suggest conducting further research focused on vitamin interventions in malnourished populations at different ages. In addition, thus far, although both China and the United States have set the tolerable upper intake level (UL) for preformed vitamin A, which is 3000 μg/day for adults [39,40], there is a lack of consideration regarding the specific effects of β-carotene intake. Knowing the populations’ nutrient status and cardiovascular risk at baseline is essential for understanding the necessity of setting the UL for β-carotene.
4.2. Effects of β-Carotene on CVD Mortality
In line with the Cochrane database of systematic reviews and USPSTF report on antioxidant supplements [7,8], this study showed that β-carotene was consistently associated with increased risk of mortality, including CVD mortality and all-cause mortality. Harmful effects were also observed in the single-treatment subgroup. Previous studies also indicated that high-dose, single or combined intervention of β-carotene increased the risk of all-cause mortality [8,9]. In at-risk and smoking populations, we noticed positive associations between β-carotene and CVD mortality. The USPSTF report also identified an increased risk of lung cancer in the high-risk population or smokers and hypothesized that the single supplementation of vitamins affected the physiologic system in an implicated way which could be either ineffective or could dose-harm to a certain disease risk [7].
4.3. Strengths and Limitations
To the best of our knowledge, this is the first study fully examining the effects of β-carotene on cardiovascular outcomes. We only included RCTs involved with β-carotene treatments to increase the efficacy and exchangeability of the study. However, due to the potential harmful effects of β-carotene, as illustrated in the USPSTF report, it was difficult to find recent RCTs conducted in the past decade. Still, our study conducted a comprehensive literature search on β-carotene trials, included a large number of individuals, and incorporated the analyses of multiple clinical outcomes of cardiovascular diseases and subgroups.
Limitations of this study include the small number of studies in each subgroup analysis due to the discouraged use of single or paired β-carotene treatments. Second, the results of this study have limited generalizability to populations with nutrient deficiencies. The trials we included did not assess the nutrient status of the participants at baseline. Third, the follow-up duration of the selected trials was generally short. Only two trials had over ten years of follow-up time, while CVD is a chronic condition which requires a longer time to find the health outcomes. Fourth, potential publication bias was detected in the meta-analysis of major CVD incidence. Although a trim-and-fill analysis was performed and the trend did not change, the results should still be treated with caution. Finally, the inability to conduct a more comprehensive analysis for multiple clinical endpoints of CVD was due to some original data that were not available in details in publications or the inability to retrieve unpublished data in progress.
5. Conclusions
The evidence of this study showed that β-carotene had no beneficial effects on CVD incidence and had potential harmful effects on CVD mortality. Since low-dose, high-dose, and single-use indicated increased risk of cardiovascular outcomes, we do not recommend the use of β-carotene given singly for prevention purposes. Both previous and current meta-analyses found harmful effects among at-risk or smoking populations. Hence, the daily supplemental use of β-carotene among individuals with CVD histories, cigarettes smokers, and heavy drinkers should be avoided. In future studies, it is useful to further explore the combination effects of β-carotene use and antioxidants in multivitamin treatments in suboptimal populations with nutrient deficiencies and investigate the effects among different sex and age groups.
Acknowledgments
We acknowledge the revision panel of Chinese Dietary Reference Intake 2023 for supporting our work. Special thanks to Zhixu Wang for his idea of developing the current study.
Abbreviations
The following abbreviations are used in this manuscript:
| CVD | Cardiovascular disease |
| USPSTF | US Preventive Services Task Force |
| RCT | Randomized Controlled Trials |
| RR | Risk Ratio |
| CI | Confidence Interval |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| UL | Tolerable upper intake level |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14061284/s1, Figure S1: Major cardiovascular incidence funnel plot; Figure S2: Overall cardiovascular incidence funnel plot; Figure S3: Cardiovascular mortality funnel plot; Figure S4: Overall mortality funnel plot; Figure S5: Trim-and-fill funnel plot for major cardiovascular disease incidence.
Appendix A
Table A1.
Keywords used for the literature search for PubMed.
| Number | Keywords |
|---|---|
| 1 | (“beta carotene”(tiab) OR “beta carotene” (mesh) OR “beta-carotene” (tiab) OR “β carotene” (tiab) OR “β-carotene” (tiab)) AND |
| 2 | (“cardiovascular disease *” (tiab) OR “cardiovascular diseases” (mesh) OR CVD (tiab) OR CVDs(tiab) OR angiocardiopathy(tiab) OR angiocardiopathies (tiab) OR “coronary heart disease *” (tiab) OR CHD (tiab) OR “coronary disease *” (tiab) OR “coronary disease” (mesh) OR “coronary artery disease *” (tiab) OR “coronary artery disease” (mesh) OR CAD (tiab) OR “high blood pressure” (tiab) OR hypertension (tiab) OR hypertension (mesh) OR arrhythmia (tiab) OR arrhythmia (tiab) OR “cardiac failure” (tiab) OR “heart failure” (tiab) OR “heart failure”(mesh) OR HF (tiab) OR “viral myocarditis”(tiab) OR VMC(tiab) OR angina (tiab) OR “Angina Pectoris” (tiab) OR “Angina Pectoris” (mesh) OR stenocardia (tiab) OR “angor pectoris” (tiab) OR “rheumatic heart disease *” (tiab) OR “rheumatic heart disease” (mesh) OR “pulmonary heart disease *” (tiab) OR “pulmonary heart disease” (mesh) OR “myocardial infarction” (tiab) OR “myocardial infarction” (mesh)) AND |
| 3 | (“controlled clinical trial” (pt) OR “randomized controlled trial” (pt) OR “clinical trial” (pt) OR random * (tiab) OR trial * (tiab)) |
Table A2.
GRADE Evidence and Summary of Findings (SoF) Table.
| Certainty Assessment | No. of Patients | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistancy | Indirectness | Imprecision | Other Considerations | Experimental | Control | Relative (95% CI) | |
| Major Cardiovascular Disease | ||||||||||
| 10 | Randomized trials | Not serious a | Not serious | Serious c | Not serious | Publicaiton bias strongly suspected d | 3576/60,337 (5.9%) | 3457/60,499 (5.7%) | RR 1.03 (0.99 to 1.08) |
⨁⨁◯◯ Low |
| Myocardial Infarction | ||||||||||
| 7 | Randomized trials | Not serious a | Not serious | Serious c | Not serious | None | 2190/46,643 (4.7%) | 2212/46,629 (4.7%) | RR 0.99 (0.93 to 1.05) |
⨁⨁⨁◯ Moderate |
| Stroke | ||||||||||
| 6 | Randomized trials | Not serious a | Serious b | Serious c | Not serious | None | 1461/52,890 (2.8%) | 1345/52,885 (2.5%) | RR 1.17 (1.00 to 1.37) |
⨁⨁◯◯ Low |
| Other CVD | ||||||||||
| 4 | Randomized trials | Not serious a | Not serious | Serious c | Not serious | None | 845/10,291 (8.2%) | 799/10,276 (7.8%) | RR 1.06 (0.95 to 1.18) |
⨁⨁⨁◯ Moderate |
| CVD Mortality | ||||||||||
| 12 | Randomized trials | Not serious a | Not serious | Not serious | Not serious | None | 2761/76,244 (3.6%) | 2468/75,396 (3.3%) | RR 1.12 (1.04 to 1.19) |
⨁⨁⨁⨁ High |
| All-cause Mortality | ||||||||||
| 6 | Randomized trials | Not serious a | Serious b | Serious c | Not serious | None | 3516/41,836 (8.4%) | 3193/41,105 (7.8%) | RR 1.08 (1.00 to 1.16) |
⨁⨁◯◯ Low |
| Other Mortality | ||||||||||
| 3 | Randomized trials | Not serious | Serious b | Serious c | Not serious | None | 1144/29,200 (3.9%) | 907/28,325 (3.2%) | RR 1.23 (0.98 to 1.53) |
⨁⨁◯◯ Low |
a Several trials indicated some concerns for the incomplete accounting of patients and outcome events, such as loss to follow-up; however, we did not downgrade the grade level, as the proportion of loss to follow-up was low compared to the event rates, and there were not large differences between controlled and intervention groups. b We downgraded the grade level as the unexplained substantial heterogeneity in the results. c All the included trials in the meta-analysis were from high-income countries. The baseline nutrients status of the populations might differ from people from low- and middle-income countries. This could possibly limit the applicability of the results. d The Egger’s test (p value = 0.0071) detected potential publication bias in the meta-analysis.
Figure A1.
Stroke incidence after excluding the trial with female high-risk participants.
Figure A2.
Other-cause mortality in the smoking population.
Figure A3.
Cardiovascular incidence in male/female populations.
Figure A4.
Cardiovascular mortality in male/female populations.
Figure A5.
Cardiovascular incidence in healthy/at-risk populations.
Figure A6.
Cardiovascular mortality in healthy/at-risk populations.
Figure A7.
Cardiovascular incidence in less-smoking/smoking populations.
Figure A8.
Cardiovascular mortality in less-smoking/smoking populations.
Author Contributions
A.Z. provided support for ideation and methodology; X.N. developed the search strategy; J.Y. and Y.Z. performed the literature search, data extraction, data analysis, and data validation; J.Y. prepared the writing of the original draft; A.Z. and J.Y. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the inserted articles.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Cardiovascular Diseases (CVDS) 2021. [(accessed on 1 February 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2.Huang T., Yang B., Zheng J., Li G., Wahlqvist M.L., Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: A meta-analysis and systematic review. Ann. Nutr. Metab. 2012;60:233–240. doi: 10.1159/000337301. [DOI] [PubMed] [Google Scholar]
- 3.Kwok C.S., Umar S., Myint P.K., Mamas M.A., Loke Y.K. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: A systematic review and meta-analysis. Int. J. Cardiol. 2014;176:680–686. doi: 10.1016/j.ijcard.2014.07.080. [DOI] [PubMed] [Google Scholar]
- 4.Djoussé L., Driver J.A., Gaziano J.M. Relation between modifiable lifestyle factors and lifetime risk of heart failure. Jama. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Micha R., Peñalvo J.L., Cudhea F., Imamura F., Rehm C.D., Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. Jama. 2017;317:912–924. doi: 10.1001/jama.2017.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sikalidis A.K., Kelleher A.H., Kristo A.S. Mediterranean Diet. Encyclopedia. 2021;1:31. doi: 10.3390/encyclopedia1020031. [DOI] [Google Scholar]
- 7.Fortmann S.P., Burda B.U., Senger C.A., Lin J.S., Whitlock E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the US Preventive Services Task Force. Ann. Intern. Med. 2013;159:824–834. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- 8.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012;18:3. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwingshackl L., Boeing H., Stelmach-Mardas M., Gottschald M., Dietrich S., Hoffmann G., Chaimani A. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: A systematic review and meta-analysis of primary prevention trials. Adv. Nutr. 2017;8:27–39. doi: 10.3945/an.116.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Y., Li J., Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: A meta-analysis of randomized controlled trials. PLoS ONE. 2013;8:e56803. doi: 10.1371/journal.pone.0056803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins D.J., Spence J.D., Giovannucci E.L., Kim Y.i., Josse R., Vieth R., Blanco Mejia S., Viguiliouk E., Nishi S., Sahye-Pudaruth S., et al. Supplemental vitamins and minerals for CVD prevention and treatment. J. Am. Coll. Cardiol. 2018;71:2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Donhowe E.G., Kong F. Beta-carotene: Digestion, microencapsulation, and in vitro bioavailability. Food Bioprocess Technol. 2014;7:338–354. doi: 10.1007/s11947-013-1244-z. [DOI] [Google Scholar]
- 13.Derrick S.A., Kristo A.S., Reaves S.K., Sikalidis A.K. Effects of Dietary Red Raspberry Consumption on Pre-Diabetes and Type 2 Diabetes Mellitus Parameters. Int. J. Environ. Res. Public Health. 2021;18:9364. doi: 10.3390/ijerph18179364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rein M.J., Renouf M., Cruz-Hernandez C., Actis-Goretta L., Thakkar S.K., da Silva Pinto M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013;75:588–602. doi: 10.1111/j.1365-2125.2012.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivekananthan D.P., Penn M.S., Sapp S.K., Hsu A., Topol E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 16.Leppälä J.M., Virtamo J., Fogelholm R., Albanes D., Taylor P.R., Heinonen O.P. Vitamin E and beta carotene supplementation in high risk for stroke: A subgroup analysis of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Arch. Neurol. 2000;57:1503–1509. doi: 10.1001/archneur.57.10.1503. [DOI] [PubMed] [Google Scholar]
- 17.Kataja-Tuomola M.K., Kontto J.P., Männistö S., Albanes D., Virtamo J.R. Effect of alpha-tocopherol and beta-carotene supplementation on macrovascular complications and total mortality from diabetes: Results of the ATBC Study. Ann. Med. 2010;42:178–186. doi: 10.3109/07853890903508887. [DOI] [PubMed] [Google Scholar]
- 18.Rapola J.M., Virtamo J., Haukka J.K., Heinonen O.P., Albanes D., Taylor P.R., Huttunen J.K. Effect of vitamin E and beta carotene on the incidence of angina pectoris: A randomized, double-blind, controlled trial. Jama. 1996;275:693–698. doi: 10.1001/jama.1996.03530330037026. [DOI] [PubMed] [Google Scholar]
- 19.Rapola J., Virtamo J., Ripatti S., Haukka J., Huttunen J., Albanes D., Taylor P., Heinonen O. Effects of α tocopherol and β carotene supplements on symptoms, progression, and prognosis of angina pectoris. Heart. 1998;79:454–458. doi: 10.1136/hrt.79.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapola J.M., Virtamo J., Ripatti S., Huttunen J.K., Albanes D., Taylor P.R., Heinonen O.P. Randomised trial of α-tocopherol and β-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349:1715–1720. doi: 10.1016/S0140-6736(97)01234-8. [DOI] [PubMed] [Google Scholar]
- 21.Virtamo J., Pietinen P., Huttunen J.K., Korhonen P., Malila N., Virtanen M.J., Albanes D., Taylor P.R., Albert P. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: A postintervention follow-up. Jama. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 22.Lee I.M., Cook N.R., Manson J.E., Buring J.E., Hennekens C.H. β-Carotene supplementation and incidence of cancer and cardiovascular disease: The Women’s Health Study. J. Natl. Cancer Inst. 1999;91:2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 23.Cook N.R., Albert C.M., Gaziano J.M., Zaharris E., MacFadyen J., Danielson E., Buring J.E., Manson J.E. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant Cardiovascular Study. Arch. Intern. Med. 2007;167:1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hercberg S., Galan P., Preziosi P., Bertrais S., Mennen L., Malvy D., Roussel A.M., Favier A., Briançon S. The SU. VI. MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004;164:2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 25.Group C.L.I.P.S.C. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: Randomized, double-blind trial. J. Intern. Med. 2007;261:276–284. doi: 10.1111/j.1365-2796.2006.01763.x. [DOI] [PubMed] [Google Scholar]
- 26.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 27.Omenn G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A., Keogh J.P., Meyskens F.L., Jr., Valanis B., Williams J.H., Jr., et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 28.Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Meyskens Jr F.L., Omenn G.S., Valanis B., Williams Jr J.H. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping β-carotene and retinol supplements. J. Natl. Cancer Inst. 2004;96:1743–1750. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 29.Blot W.J., Li J.Y., Taylor P.R., Guo W., Dawsey S., Wang G.Q., Yang C.S., Zheng S.F., Gail M., Li G.Y., et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. JNCI J. Natl. Cancer Inst. 1993;85:1483–1491. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 30.Hennekens C.H., Buring J.E., Manson J.E., Stampfer M., Rosner B., Cook N.R., Belanger C., LaMotte F., Gaziano J.M., Ridker P.M., et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg E.R., Baron J.A., Karagas M.R., Stukel T.A., Nierenberg D.W., Stevens M.M., Mandel J.S., Haile R.W. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. Jama. 1996;275:699–703. doi: 10.1001/jama.1996.03530330043027. [DOI] [PubMed] [Google Scholar]
- 32.Shah R.S., Cole J.W. Smoking and stroke: The more you smoke the more you stroke. Expert Rev. Cardiovasc. Ther. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton G.W., Ingold K. Beta-carotene: An unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 34.Truscott T. β-Carotene and disease: A suggested pro-oxidant and anti-oxidant mechanism and speculations concerning its role in cigarette smoking. J. Photochem. Photobiol. B Biol. 1996;35:233–235. doi: 10.1016/S1011-1344(96)07299-5. [DOI] [PubMed] [Google Scholar]
- 35.Palozza P. Prooxidant actions of carotenoids in biologic systems. Nutr. Rev. 1998;56:257–265. doi: 10.1111/j.1753-4887.1998.tb01762.x. [DOI] [PubMed] [Google Scholar]
- 36.Andersen H.R., Andersen O. Effects of dietary α-tocopherol and β-carotene on lipid peroxidation induced by methyl mercuric chloride in mice. Pharmacol. Toxicol. 1993;73:192–201. doi: 10.1111/j.1600-0773.1993.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 37.Lomnitski L., Bergman M., Schön I., Grossman S. The effect of dietary vitamin E and ß-carotene on oxidation processes in the rat testis. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1991;1082:101–107. doi: 10.1016/0005-2760(91)90305-2. [DOI] [PubMed] [Google Scholar]
- 38.Stewart C., Christian P., Katz J., Schulze K., Wu L., LeClerq S., Shakya T., Khatry S., West K. Maternal supplementation with vitamin A or β-carotene and cardiovascular risk factors among pre-adolescent children in rural Nepal. J. Dev. Orig. Health Dis. 2010;1:262–270. doi: 10.1017/S2040174410000255. [DOI] [PubMed] [Google Scholar]
- 39.CNS . Chinese Dietary Reference Intakes (2013) Science Press; Beijing, China: 2014. [Google Scholar]
- 40.Trumbo P., Yates A.A., Schlicker S., Poos M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the inserted articles.