Abstract
Objective: We aimed to determine the relationship between vaccine-related adverse effects and antibody (Ab) titers from 3 to 6 months after the second dose of the BNT162b2 coronavirus disease 2019 (COVID-19) mRNA vaccine (Pfizer/BioNTech) in Japan. Methods: We enrolled 378 healthcare workers (255 women and 123 men) whose Ab titers were analyzed 3 and 6 months after the second dose in our previous study and whose characteristics and adverse effects were collected previously by using a structured self-report questionnaire. Results: The workers’ median age was 44 years. Although injection-site symptoms occurred with almost equal frequency between the first and second doses, systemic adverse effects, such as general fatigue and fever, were significantly more frequent after the second dose than after the first dose. Multivariate analysis showed that fever was significantly correlated with female participants for the second dose (odds ratio (OR), 2.139; 95% confidence interval (95% CI), 1.185–3.859), older age for the first dose (OR, 0.962; 95% CI, 0.931–0.994) and second dose (OR, 0.957; 95% CI, 0.936–0.979), and dyslipidemia for the first dose (OR, 8.750; 95% CI, 1.814–42.20). Age-adjusted Ab titers at 3 months after vaccination were 23.7% and 23.4% higher in patients with a fever than in those without a fever after the first and second dose, respectively. In addition, age-adjusted Ab titers at 3 and 6 months after the second dose were, respectively, 21.7% and 19.3% higher in the group in which an anti-inflammatory agent was used than in the group without the use of an anti-inflammatory agent. Conclusion: Participants with systemic adverse effects tend to have higher Ab titers from 3 to 6 months after the second dose of the BNT162b2 vaccine. Our results may encourage vaccination, even among people with vaccine hesitancy related to relatively common systemic adverse effects.
Keywords: SARS-CoV-2, viral infection, clinical epidemiology, adverse effect
1. Introduction
The BNT162b2 vaccine (Pfizer/BioNTech, NY, USA / Mainz, Germany) was selected as the first coronavirus disease 2019 (COVID-19) mRNA vaccine to be administered to healthcare professionals in Japan, beginning in February 2021. The vaccine helps to prevent not only the onset [1] but also the progression of COVID-19. However, some people hesitate in getting vaccinated due to fear of relatively common adverse effects that occur shortly after vaccination with the BNT162b2 vaccine [2,3,4].
If the maximum vaccine efficacy, including antibody (Ab) responses, is guaranteed, most people will accept a certain severity of vaccination-related adverse effects [2]. Although several reports concluded that there is no correlation or only low correlation between adverse effects and the Ab titers detected immediately after vaccination [5,6,7,8], a correlation is expected between adverse effects and the Ab titers detected several months after vaccination [9]. Antibody titers alone do not correlate perfectly with protection because the benefits of mRNA vaccines include not only B-cell/humoral responses but also T-cell/cell-mediated immunity. To examine the efficacy of vaccination, it is more important to analyze the moderate-term levels of Ab titers after vaccination rather than the peak Ab titer obtained shortly after vaccination. Indeed, some reports [6,10] have suggested that systemic adverse effects may be induced as a hypersensitive immune reaction relative to the accumulation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein itself immediately after injection of the first mRNA vaccine dose rather than to chemical additives in vaccines. The analysis of the response to the first vaccine dose in persons with a previous COVID-19 infection revealed that Ab titers were 10 to 45 times higher in individuals with preexisting immunity than in those without preexisting immunity at the same time points after the first vaccine dose [10]. Interestingly, systemic adverse effects, including fatigue, headache, chills, muscle pain, fever, and joint pain, were more common among participants with preexisting immunity whereas local adverse effects occurred with similar frequency among participants with and without preexisting immunity [10].
In our previous studies [11,12], we showed that the most important factors associated with lower Ab titers at 3 and 6 months were age and smoking, probably reflecting their effect on peak Ab titers, and that the only factor significantly associated with the attenuation in the Ab titer from 3 to 6 months was sex, which reduced the sex difference seen during the first 3 months. Against this background, we analyzed the relationship between adverse effects and Ab titers against the SARS-CoV-2 spike antigen from 3 to 6 months after vaccination with the BNT162b2 vaccine in Japan. This study is expected to contribute to maximizing the efficacy of vaccination while minimizing adverse effects as a step toward optimizing and individualizing the vaccination regimen.
2. Methods
2.1. Population and Study Design
In our previous single-center prospective observational study [11,12], we enrolled 378 healthcare workers (255 women and 123 men) at the National Hospital Organization Utsunomiya National Hospital in the Tochigi Prefecture, Japan, and analyzed their Ab titers at 3 and 6 months after the second of two BNT162b2 vaccine inoculations, which were administered 3 weeks apart in February and March 2021. The participants’ characteristics and adverse effects were collected by using a structured self-report questionnaire 2 months after the second dose of the vaccine, and in this study, we analyzed the relationships of adverse effects with Ab titers at 3 and 6 months.
In our previous study [11], we used the participants’ blood samples to measure total Ab titers against the SARS-CoV-2 spike antigen by using a commercially available electrochemiluminescence immunoassay (Elecsys® Anti-SARS-CoV-2 RUO; Roche Diagnostics, Basel, Switzerland) [13]. In addition to their clinical and demographic characteristics, adverse effects occurring shortly after the first and second doses were recorded in that study by using a structured self-report questionnaire [11]. In total, 365 healthcare workers (250 women and 115 men) remained after the exclusion of 10 participants whose blood samples were not obtained at 6 months and 3 participants whose blood samples confirmed the presence of Abs against the nucleocapsid proteins for SARS-CoV-2 [12]; the blood samples of these 365 participants were used to measure total Ab titers against the SARS-CoV-2 spike antigen 6 months after the second dose. The relationships between Ab titers against the SARS-CoV-2 spike antigen and clinical and lifestyle characteristics were analyzed. In the age-adjusted analysis, individual Ab titers were recalculated by subtracting the median Ab titer of the corresponding age group from an individual’s Ab titer. For example, an age-adjusted Ab titer for an individual in his/her 20s was calculated as follows: individual Ab titer − median Ab titer for participants in their 20s.
The Ethics Committee of the National Hospital Organization Utsunomiya National Hospital (No. 03-01; 19 April 2021) approved this study, and written informed consent was obtained from all participants before their enrollment.
2.2. Structured Self-Report Questionnaire to Obtain Individual Variables
Adverse effects in response to the first and second doses of the BNT162b2 vaccine, as well as clinical history and demographic characteristics, were collected by means of a structured self-report questionnaire. The maximum body temperature induced by the BNT162b2 vaccine was taken under armpits and included in the questionnaire. In addition, we asked participants about the use of an anti-inflammatory agent for adverse effects induced by the BNT162b2 vaccine. However, the preventive use of acetaminophen was excluded.
2.3. Data Analysis
Nonparametric continuous data were expressed as the median with the interquartile range (IQR). Categorical data were presented as absolute numbers (n) and relative frequencies (%). We used Microsoft® Excel® 2016 MSO (Microsoft Corp., Redmond, WA, USA). To calculate Spearman’s rank correlation coefficient and perform the Mann–Whitney U test, chi-square test, and multivariate logistic regression analysis, we used Statistical Package for Social Sciences (SPSS) version 28 (IBM Japan, Ltd., Tokyo, Japan). Univariate and multivariate logistic regression models were applied to analyze the relationship between SARS-CoV-2 Ab or fever as the dependent variable and adverse effects or clinical parameters as the independent variables.
3. Results
3.1. Incidence of Adverse Effects in Response to the BNT162b2 Vaccine
The participants’ baseline characteristics were reported in our previous publications [11,12]. In total, 378 healthcare workers (255 women and 123 men) were enrolled [11], and the median age (IQR) of the participants was 44 (32–54) years. Nurses (n = 177) and physicians (n = 38) comprised 56.9% of the study population. Overall, the vaccine had no adverse effects resulting in hospitalization. The prevalence of adverse effects and the anti-inflammatory agent used in response to the BNT162b2 vaccine is shown in Table 1. The prevalence of each was higher after the second vaccine dose than after the first dose (chi-square test, p < 0.01). Local adverse effects occurred with almost equal frequency for the first and second doses. The rates of concordance, in which the same reactions were seen for both the first and second doses, were higher for systemic reactions than for local reactions.
Table 1.
Prevalence of adverse effects induced by the BNT162b2 vaccine (n = 378).
| Adverse Effects | After First Dose | After Second Dose | Rate of Concordance between 1st and 2nd |
|---|---|---|---|
| Systemic reactions | |||
| Fever ≥ 37.0 °C | 17.0% (56/274/48) | 56.8% (191/145/42) | 83.9% (47/56) |
| General fatigue | 35.0% (132/245/1) | 73.3% (277/101/0) | 93.9% (124/132) |
| Headache | 20.7% (78/299/1) | 46.0% (173/203/2) | 79.5% (62/78) |
| Muscle pain | 18.6% (70/307/1) | 35.7% (135/243/0) | 80.0% (56/70) |
| Joint pain | 7.7% (29/348/1) | 33.2% (125/252/1) | 86.2% (25/29) |
| Nausea | 2.4% (9/368/1) | 7.0% (26/347/5) | 66.7% (6/9) |
| Diarrhea | 1.1% (4/373/1) | 3.7% (14/362/2) | 50.0% (2/4) |
| Local reactions | |||
| Pain | 89.6% (337/39/2) | 83.3% (310/62/6) | 87.2% (294/337) |
| Swelling | 31.6% (119/257/2) | 31.1% (117/259/2) | 74.8% (89/119) |
| Induration | 23.4% (88/288/2) | 21.0% (79/297/2) | 72.7% (64/88) |
| Itching | 16.5% (62/314/2) | 17.2% (65/313/0) | 72.6% (45/62) |
| Acetaminophen use | 21.1% (76/284/18) | 57.1% (210/158/10) | 76.3% (58/76) |
Data show the percentage of positive reactions (number of yes/no/unknown).
3.2. Relationship between Participants’ Characteristics and Vaccine-Related Systemic Adverse Effects
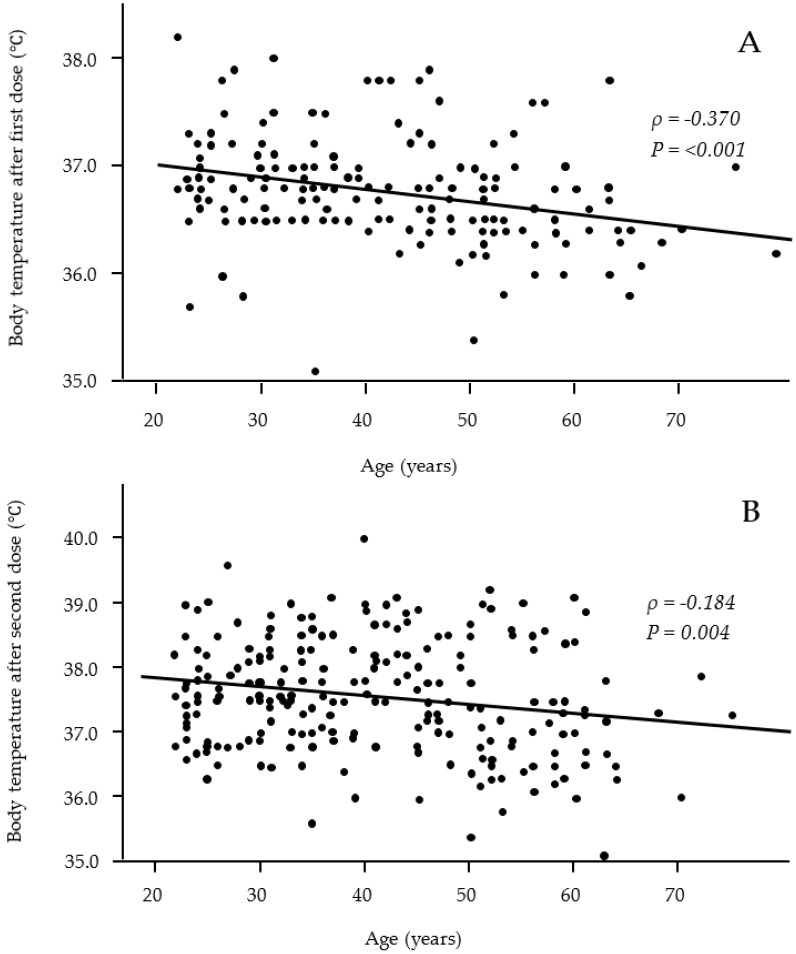
Fever is one of the most common systemic adverse effects, and we used multivariate logistic regression analysis to investigate whether participants’ characteristics were risk factors for the occurrence of fever (Table 2). Fever was defined as a maximum body temperature of 37.0 °C or higher. The multivariate analysis revelaed that the female sex (odds ratio (OR), 2.139; 95% confidence interval (95% CI), 1.185–3.859) was correlated with the occurrence of fever after the second dose. Patients of a younger age also correlated with the occurrence of fever after the first dose (OR, 0.962; 95% CI, 0.931–0.994) and second dose (OR, 0.957; 95% CI, 0.936–0.979). A scatter plot of the distribution of body temperature by age showed that younger participants had a significantly higher body temperature shortly after both the first and second doses (correlation coefficient ρ = −0.370 and −0.184, respectively) (Figure 1). Dyslipidemia was also correlated with fever after the first dose (OR, 8.750; 95%CI, 1.815–42.25), but no other clinical or lifestyle characteristics were correlated with fever.
Table 2.
Multivariate logistic regression analysis of risk factors associated with fever (≥37.0 °C) (n = 378).
| Risk Factors | After the First Dose | After the Second Dose | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Sex (female) | 1.862 | 0.789–4.392 | 2.139 | 1.185–3.859 |
| Age (older) | 0.962 | 0.931–0.994 | 0.957 | 0.936–0.979 |
| BMI (obesity) | 1.054 | 0.966–1.149 | 0.995 | 0.929–1.066 |
| Smoking | 0.677 | 0.303–1.510 | 1.616 | 0.913–2.863 |
| Drinking | 0.738 | 0.349–1.563 | 0.584 | 0.338–1.010 |
| Any allergic history | 0.702 | 0.268–1.842 | 1.312 | 0.629–2.736 |
| Food | 0.748 | 0.207–2.700 | 1.088 | 0.411–2.879 |
| Drug or chemical | 0.955 | 0.247–3.696 | 1.018 | 0.368–2.813 |
| Any allergic disease | 0.804 | 0.155–4.183 | 3.125 | 0.918–10.64 |
| Allergic rhinitis | 3.368 | 0.751–15.12 | 0.455 | 0.145–1.428 |
| Asthma | 1.695 | 0.602–4.768 | 0.955 | 0.389–2.347 |
| Atopic dermatitis/Urticaria | 1.869 | 0.703–4.968 | 0.757 | 0.323–1.774 |
| Diabetes mellitus | 5.979 | 0.953–37.50 | 1.457 | 0.287–7.411 |
| Hypertension | 0.522 | 0.085–3.193 | 0.525 | 0.159–1.732 |
| Dyslipidemia | 8.750 | 1.814–42.20 | 2.284 | 0.593–8.804 |
| Collagen diseases | 2.727 | 0.629–11.83 | 3.501 | 0.690–17.76 |
Figure 1.
Relationships between age and body temperature after the first (A) and second (B) vaccine doses. Significant negative correlations were observed after both the first and second vaccine doses. (A: n = 176; B: n = 243).
3.3. Association between Ab Titers and Vaccine-Related Adverse Effects
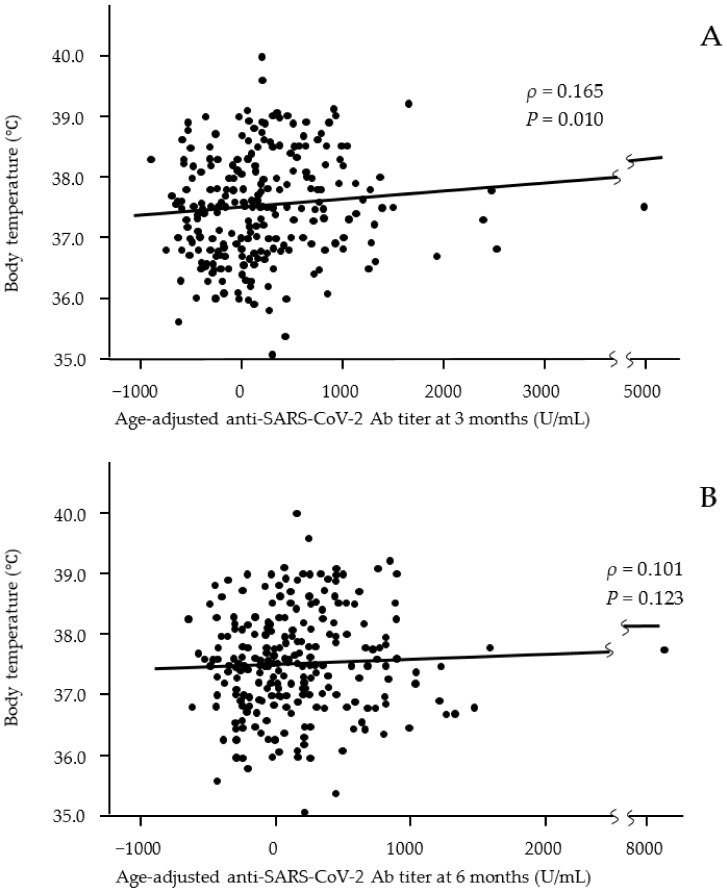
We analyzed the relationship between Ab titers at 3 and 6 months after vaccination and adverse effects after the first and second vaccine doses (Table 3 and Table 4). Age-adjusted Ab titers were used because the titers significantly differed between younger and older participants at 3 and 6 months after vaccination. Fever after both the first and second doses was associated with a significantly higher Ab titer at 3 months after vaccination. A scatter plot of the distribution of body temperature by Ab titer also showed that participants with a high body temperature as an adverse effect after the second dose had a significantly higher Ab titer at 3 months after vaccination but not at 6 months (correlation coefficients ρ = 0.166 and −0.102, respectively) (Figure 2). General fatigue and joint pain after the first and/or second vaccine doses were associated with significantly higher Ab titers at both 3 and 6 months after vaccination. Nausea after the second vaccine dose was associated with a significantly higher Ab titer at 6 months after vaccination. In addition, the use of an anti-inflammatory agent after the second vaccine dose was significantly associated with high Ab titers at both 3 and 6 months after vaccination. However, local reactions were not associated with Ab titers.
Table 3.
Relationship between adverse effects and age-adjusted antibody titers at 3 months after vaccination (n = 378).
| Adverse Effects | After the First Dose | After the Second Dose | ||
|---|---|---|---|---|
| Median (IQR) U/mL | p-Value | Median (IQR) U/mL | p-Value | |
| Systemic reactions | ||||
| Fever (≥37.0 °C) | 153 (−242 to 529)/−28 (−306 to 293) | 0.042 | 110 (−259 to 511)/−69 (−335 to 263) | 0.002 |
| General fatigue * | 22 (−337 to 369)/0 (−279 to 334) | 0.841 | 60 (−287 to 430)/−109 (−327 to 130) | 0.010 |
| Headache * | −30 (−416 to 334)/0 (−285 to 355) | 0.289 | 70 (−309 to 481)/−47 (−293 to 297) | 0.120 |
| Muscle pain * | 32 (−329 to 395)/0 (−293 to 341) | 0.770 | 90 (−246 to 340)/−39 (−336 to 352) | 0.058 |
| Joint pain * | 189 (−133 to 751)/−13 (−308 to 321) | 0.014 | 147 (−140 to 510)/−72 (−370 to 275) | <0.001 |
| Nausea * | 145 (−516 to 369)/0 (−299 to 340) | 0.926 | −249 (−441 to 171)/9 (−285 to 353) | 0.088 |
| Diarrhea * | 418(120 to 691)/0 (−299 to 340) | 0.295 | −91 (−413 to 376)/1 (−296 to 341) | 0.430 |
| Local reactions | ||||
| Pain * | 2 (−308 to 369)/−88 (−256 to 237) | 0.498 | −1 (−317 to 341)/26 (−226 to 335) | 0.528 |
| Swelling * | 0 (−307 to 395)/−3 (−299 to 317) | 0.822 | 0 (−312 to 411)/−11 (−293 to 322) | 0.780 |
| Induration * | 38 (−293 to 510)/−1 (−299 to 318) | 0.289 | −18 (−311 to 316)/0 (−299 to 339) | 0.902 |
| Itching * | 81 (−174 to 451)/−17 (−309 to 339) | 0.265 | 9 (−273 to 269)/0 (−301 to 364) | 0.896 |
| Acetaminophen use * | 108 (−281 to 632)/−17 (−299 to 292) | 0.136 | 82 (−256 to 517)/−84 (−371 to 226) | <0.001 |
* Yes/no are shown.
Table 4.
Relationship between adverse effects and age-adjusted antibody titers at 6 months after vaccination (n = 365).
| Adverse Effects | After the First Dose | After the Second Dose | ||
|---|---|---|---|---|
| Median (IQR) U/mL | p-Value | Median (IQR) U/mL | p-Value | |
| Systemic reactions | ||||
| Fever (≥37.0 °C) | 60 (−181 to 343)/−11 (−227to 256) | 0.187 | 57 (−201 to 359)/−25 (−229 to 207) | 0.065 |
| General fatigue * | 0 (−242 to 286)/1 (−223 to 263) | 0.853 | 35 (−217 to 328)/−117 (−241 to 174) | 0.015 |
| Headache * | −28 (−290 to 290)/8 (−214 to 263) | 0.278 | 30 (−240 to 336)/−20 (−224 to 254) | 0.425 |
| Muscle pain * | −4 (−256 to 248)/7 (−222 to 274) | 0.635 | 33 (−178 to 288)/−19 (−241 to 265) | 0.237 |
| Joint pain * | 142 (−71 to 461)/−7 (−240 to 256) | 0.023 | 54 (−92 to 339)/−58 (−264 to 244) | 0.001 |
| Nausea * | −56 (−339 to 314)/6 (−224 to 270) | 0.580 | −188 (−303 to −7)/14 (−221 to 283) | 0.013 |
| Diarrhea * | 695 (169 to 1036)/0 (−225 to 268) | 0.181 | −148 (−308 to 295)/1 (−224 to 271) | 0.294 |
| Local reactions | ||||
| Pain * | 9 (−240 to 274)/−55 (−186 to 144) | 0.441 | −9 (−317 to 341)/42 (−226 to 335) | 0.257 |
| Swelling * | 31 (−210 to 306)/−9 (−238 to 268) | 0.543 | −4 (−212 to 256)/3 (−232 to 273) | 0.785 |
| Induration * | 0 (−203 to 334)/6 (−233 to 268) | 0.697 | −49(−244 to 244)/11 (−226 to 280) | 0.476 |
| Itching * | 37 (−169 to 358)/−9 (−239 to 268) | 0.304 | 1 (−239 to 200)/0 (−224 to 281) | 0.735 |
| Acetaminophen use * | 78 (−224 to 446)/−8 (−233 to 251) | 0.191 | 46 (−208 to 334)/−58 (−246 to 209) | 0.019 |
* Yes/no are shown.
Figure 2.
Relationships between body temperature after the second vaccine dose and age-adjusted anti-SARS-CoV-2 Ab titers at 3 months (A) and 6 months (B) after the second dose of the vaccine. Significant positive correlations were observed at 3 months after vaccination but not at 6 months. (A: n = 243; B: n = 243).
The differences in age-adjusted Ab titers at 3 months after vaccination between individuals with and without fever after the first and second doses were 181 U/mL and 179 U/mL, respectively. These values were 23.7% and 23.4% of the median Ab titer of 746 U/mL at 3 months after vaccination. In addition, differences in the age-adjusted Ab titers between individuals with and without anti-inflammatory agent use after the second vaccine dose were 166 U/mL and 104 U/mL at 3 and 6 months after vaccination, respectively. These values were 21.7% and 19.3% of the median Ab titers of 746 U/mL and 539 U/mL at 3 and 6 months after vaccination, respectively.
4. Discussion
To our knowledge, this is the first study to report the relationship between adverse effects and Ab titers against the SARS-CoV-2 spike antigen from 3 to 6 months after vaccination with the BNT162b2 vaccine in Japan. Three important findings were obtained. First, although injection-site symptoms occurred with almost equal frequency for the first and second vaccine doses, systemic reactions were significantly more common after the second dose (Table 1). General fatigue and fever were the first and second most common systemic adverse effects. Second, multivariate analysis revealed significant correlations of fever with female patients for the second dose; younger patients for both the first and second doses; and dyslipidemia for the first dose (Table 2). No other clinical or lifestyle characteristics were correlated with fever. Third, regarding the relationship between systemic adverse effects and Ab titers (Table 3 and Table 4), participants with general fatigue after the second dose had significantly higher age-adjusted Ab titers at both 3 months and 6 months than those without general fatigue. Compared with participants without fever, those with fever after the first or second dose had significantly higher age-adjusted Ab titers at 3 months but not at 6 months. Participants with joint pain after the first or second dose had significantly higher age-adjusted Ab titers at both 3 months and 6 months than those without joint pain. Participants who used anti-inflammatory agents after the second dose showed a significantly higher Ab titer.
Not only high Ab responses against SARS-CoV-2 spike protein but also systemic adverse effects induced by the BNT162b2 vaccine were reported to be more common among participants with preexisting immunity due to COVID-19 infection [10], suggesting that some systemic adverse effects are induced as a more vigorous response to the SARS-CoV-2 spike protein itself produced by the BNT162b2 vaccine. Previous publications reported that adverse effects induced by the BNT162b2 vaccine had no correlation or only low correlation with Ab titers detected immediately after vaccination [5,6,7,8]. However, to evaluate the long-term effects of vaccination, research should consider Ab titers several months after vaccination and not only the peak level of Ab titers. Accordingly, we determined the relationship between adverse effects and Ab titers from 3 to 6 months after the second dose of the BNT162b2 vaccine. Few studies have addressed this issue, but we found one report from Estonia showing that the Ab response was negatively correlated with age and positively correlated with the total score for vaccination adverse effects [9].
For the second findings, we found significant correlations of the female sex, younger age, and dyslipidemia with fever (Table 2). Some studies have reported that women and younger people more commonly show adverse effects of the BNT162b2 vaccine [6], and our results support those findings. However, the reasons for why dyslipidemia would be a risk factor for fever are not known. Atherogenic dyslipidemia may have effects on the activity of collagen diseases, such as systemic lupus erythematosus and rheumatoid arthritis [14,15]. Diabetes mellitus, a known immune disorder, was also a near-significant risk factor for adverse effects after the first dose. These metabolic diseases complicate immune disorders, and these immune disorders may contribute to systemic reactions after the first vaccine dose.
Regarding the third finding, the occurrence of systemic adverse effects, including general fatigue, fever, and joint pain, and the use of an anti-inflammatory agent for adverse effects were significantly associated with a high Ab titer. In particular, body temperatures recorded as an adverse effect were significantly and positively correlated with the Ab titer at 3 months after vaccination, suggesting that the severity of systemic adverse effects may promote a stronger ability to maintain high Ab titers against the spike protein of this virus. Joint pain contributed to significantly higher Ab titers after both the first and second doses at both 3 and 6 months after vaccination. Joint pain was one of the most common reactions. However, we could not find previous publications that explained the relationship between Ab titer and joint pain, and we also are unable to speculate why Ab titers were more strongly reflected by joint pain than by fever or general fatigue. We speculate that participants taking anti-inflammatory drugs (acetaminophen) have more systemic inflammation and, thus, have higher titers because of greater reactogenicity; the higher titers would not be a result of anti-inflammatory medications.
Some limitations and possible sources of bias in this study include the following. First, participants were limited in number and were all healthcare workers vaccinated at a single national hospital in the Tochigi Prefecture. Therefore, the results obtained in this study might not be widely generalizable or even generalizable within Japan. Second, we may require additional analysis and discussion to determine whether age is an important factor independently associated with adverse effects. One possibility is that fever may be an independent factor associated with higher Ab titers but not age. In this case, because most of the participants with fever were younger, a younger age may not be independently associated with a higher Ab titer. The opposite is also a possibility. In addition, both fever and age may be important factors. Therefore, we cannot determine whether fever or young age is a more important factor. Third, individuals with systemic or local symptoms used drugs to counter them, resulting in bias whereby some other symptoms might have been prevented from occurring.
5. Conclusions
In conclusion, participants with systemic adverse effects had approximately 20% higher Ab titers at 3 and 6 months after the second dose of the BNT162b2 vaccine. Although the systemic adverse effects induced by the BNT162b2 vaccine are sometimes unpleasant, higher Ab titers should reduce the risk of a more severe COVID-19 infection. We hope that our results will encourage vaccine uptake.
Acknowledgments
The authors thank all staff at the National Hospital Organization Utsunomiya National Hospital for providing support during sample collection. We would like to thank Miwa Okada, Minako Yamagishi, Junko Shibayama, Yuko Tajima, Mami Ochiai, Midori Takahashi, Hiroko Ueno, Natsuka Suzuki, and Yoshino Iwaya for providing support in data analysis.
Abbreviations
COVID-19: coronavirus disease 2019; Ab: antibody; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; IQR: interquartile range; OR: odds ratio; CI: confidence interval.
Author Contributions
Conceptualization, M.S., K.S., R.K. and Y.N. (Yosikazu Nakamura); methodology and software, R.K., K.S., M.S. and Y.N. (Yosikazu Nakamura); validation, K.S., M.S. and Y.N. (Yosikazu Nakamura); formal analysis and investigation, K.S., R.K., M.S. and Y.N. (Yosikazu Nakamura); resources, T.T., K.S., N.M., R.K., M.S., O.K. and Y.N. (Yushi Nomura); data curation, R.K., K.S. and M.S.; writing original draft preparation, M.S., K.S., R.K. and Y.N. (Yosikazu Nakamura); writing review and editing, T.T., N.M., S.N., K.H., O.K. and Y.N. (Yushi Nomura); visualization, K.S., R.K. and M.S.; supervision, M.S., K.S., T.T., S.N., and K.H.; project administration, K.S. and T.T.; funding acquisition, T.T. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of National Hospital Organization Utsunomiya National Hospital (No. 03-01, 19 April 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., et al. COVID-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. C4591001 clinical trial group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozakai R., Kushida A., Moumouni P.F., Okuma S., Takahashi K., Hoshi K., Sato Y., Takahashi M., Chida N., Takahashi M., et al. Assessment of COVID-19 mRNA vaccination titer and side effects in healthy volunteers. J. Lab. Med. 2021 doi: 10.1515/labmed-2021-0156. [DOI] [Google Scholar]
- 4.Izumo T., Kuse N., Awano N., Tone M., Sakamoto K., Takada K., Muto Y., Fujimoto K., Saiki A., Ito Y., et al. Side effects and antibody titer transition of the BNT162b2 messenger ribonucleic acid coronavirus disease 2019 vaccine in Japan. Respir. Investig. 2021;59:635–642. doi: 10.1016/j.resinv.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coggins S.A., Laing E.D., Olsen C.H., Goguet E., Moser M., Jackson-Thompson B.M., Samuels E.C., Pollett S.D., Tribble D.R., Davies J., et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. Open Forum Infect. Dis. 2021;9:ofab575. doi: 10.1093/ofid/ofab575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debes A.K., Xiao S., Colantuoni E., Egbert E.R., Caturegli P., Gadala A., Milstone A.M. association of vaccine type and prior SARS-CoV-2 infection with symptoms and antibody measurements following vaccination among health care workers. JAMA Intern. Med. 2021;181:1660–1662. doi: 10.1001/jamainternmed.2021.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi M., Higa Y., Esaki A., Nabeshima Y., Nakazono A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLoS ONE. 2021;16:e0257668. doi: 10.1371/journal.pone.0257668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda K., Amano M., Uemura Y., Tsuchiya K., Matsushima T., Noda K., Shimizu Y., Fujiwara A., Takamatsu Y., Ichikawa Y., et al. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. Sci. Rep. 2021;11:22848. doi: 10.1038/s41598-021-01930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., Haljasmägi L., Rumm A.P., Maruste R., Kärner J., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura Y., Sawahata M., Nakamura Y., Kurihara M., Koike R., Katsube O., Hagiwara K., Niho S., Masuda N., Tanaka T., et al. Age and smoking predict antibody titres at 3 months after the second dose of the BNT162b2 COVID-19 vaccine. Vaccines. 2021;9:1042. doi: 10.3390/vaccines9091042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomura Y., Sawahata M., Nakamura Y., Koike R., Katsube O., Hagiwara K., Niho S., Masuda N., Tanaka T., Sugiyama K. Attenuation of antibody titers from 3 to 6 months after the second dose of the BNT162b2 vaccine depends on sex, with age and smoking risk factors for lower antibody titers at 6 months. Vaccines. 2021;9:1500. doi: 10.3390/vaccines9121500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkmann T., Perkmann-Nagele N., Breyer M.K., Breyer-Kohansal R., Burghuber O.C., Hartl S., Aletaha D., Sieghart D., Quehenberger P., Marculescu R., et al. Side-by-side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin. Chem. 2020;66:1405–1413. doi: 10.1093/clinchem/hvaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman M.J., Shanker B.A., Davis A., Lockshin M.D., Sammaritano L., Simantov R., Crow M.K., Schwartz J.E., Paget S.A., Devereux R.B., et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 15.Goodson N., Marks J., Lunt M., Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann. Rheum. 2005;64:1595–1601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]